Stefan Flow in Char Combustion: A Critical Review of Mass Transfer and Combustion Differences Between Air-Fuel and Oxy-Fuel Conditions
Abstract
1. Introduction
2. Influence of Stefan Flow on Mass Transfer in Char Combustion
2.1. Binary Diffusion Model
2.2. Multi-Component Diffusion Model
2.2.1. In the Absence of Homogeneous Oxidation of CO
2.2.2. In the Presence of Homogeneous Oxidation of CO
2.3. Summary
3. Influence of Stefan Flow on Combustion Characteristics
3.1. Influence of Stefan Flow on Combustion Characteristics in Air-Fuel Combustion
3.2. Influence of Stefan Flow on Combustion Characteristics in Oxy-Fuel Combustion
3.3. Summary
4. Discussion
- The char–CO2 gasification reaction becomes more prominent in oxy-fuel combustion; the endothermicity (172 kJ/mol C) of the gasification reaction is strong, which lowers the char combustion temperature and thus reduces the oxidation rate.
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Dukowicz, J.K. An exact solution for the drag of a sphere in low Reynolds number flow with strong uniform suction or blowing. Phys. Fluids 1982, 25, 1117. [Google Scholar] [CrossRef]
- Dukowicz, J.K. Drag of evaporating or condensing droplets in low Reynolds number flow. Phys. Fluids 1984, 27, 1351. [Google Scholar] [CrossRef]
- Jayawickrama, T.R.; Haugen, N.E.L.; Babler, M.U.; Chishty, M.A.; Umeki, K. The effect of Stefan flow on the drag coefficient of spherical particles in a gas flow. Int. J. Multiph. Flow 2019, 117, 130–137. [Google Scholar] [CrossRef]
- Vittori, O.A. Scavenging of atmospheric particles by ice crystals. J. Atmos. Sci. 1967, 24, 533–538. [Google Scholar] [CrossRef][Green Version]
- Prodi, F.; Santachiara, G.; Belosi, F.; Vedernikov, A.; Balapanov, D. Phoretic forces on aerosol particles surrounding an evaporating droplet in microgravity conditions. Atmos. Res. 2014, 142, 40–44. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Cui, L.; Yan, L.; Zhao, C.; Dong, Y. Cold condensing scrubbing method for fine particle reduction from saturated flue gas. Energy 2019, 171, 1193–1205. [Google Scholar] [CrossRef]
- Taylor, R.; Krishna, R. Multi-Component Mass Transfer; John Wiley and Sons: New York, NY, USA, 1993. [Google Scholar]
- Hayhurst, A.N. The mass transfer coefficient for oxygen reacting with a carbon particle in a fluidized or packed bed. Combust. Flame 2000, 121, 679–688. [Google Scholar] [CrossRef]
- Man, Y.H.; Choi, B.R. A numerical study on the combustion of a single carbon particle entrained in a steady flow. Combust. Flame 1994, 97, 1–16. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, M.C. Experimental and modeling study on char combustion. Energy Fuels 2009, 23, 2874–2885. [Google Scholar] [CrossRef]
- Essenhigh, R.H. Rate equations for the carbon-oxygen reaction: An evaluation of the Langmuir adsorption isotherm at atmospheric pressure. Energy Fuels 1991, 5, 41–46. [Google Scholar] [CrossRef]
- Niksa, S.; Liu, G.S.; Hurt, R.H. Coal conversion submodels for design applications at elevated pressures. Part I. devolatilization and char oxidation. Prog. Energy Combust. Sci. 2003, 29, 425–477. [Google Scholar] [CrossRef]
- Hurt, R.H.; Calo, J.M. Semi-global intrinsic kinetics for char combustion modeling. Combust. Flame 2001, 125, 1138–1149. [Google Scholar] [CrossRef]
- Campbell, P.A.; Mitchell, R.E. The impact of the distributions of surface oxides and their migration on characterization of the heterogeneous carbon–oxygen reaction. Genomics 2008, 154, 47–66. [Google Scholar] [CrossRef]
- Field, M.A. Measurements of the effect of rank on combustion rates of pulverized coal. Combust. Flame 1970, 14, 237–248. [Google Scholar] [CrossRef]
- Sotirchos, S.V.; Srinivas, B.; Amundson, N.R. Modelling of the combustion of single char particles. Rev. Chem. Eng. 1984, 2, 175–236. [Google Scholar] [CrossRef]
- Tomeczek, J. Kinetics of mineral matter transformation during coal combustion. Fuel 2002, 81, 1251–1258. [Google Scholar] [CrossRef]
- Nauze, R. Fundamentals of coal combustion in fluidized beds. Chem. Eng. Res. Des. 1985, 63, 3–33. [Google Scholar]
- Zolin, A.; Jensen, A.; Damjohansen, K. Kinetic analysis of char thermal deactivation. Proc. Combust. Inst. 2001, 28, 2181–2188. [Google Scholar] [CrossRef]
- Tognotti, L.; Longwell, J.P.; Sarofim, A.F. The products of the high temperature oxidation of a single char particle in an electrodynamic balance. Symp. Combust. 1991, 23, 1207–1213. [Google Scholar] [CrossRef]
- Yuan, C.; Sheng, C.D. Modeling of a single char particle burning in oxygen-enriched O2/N2 and O2/CO2 environment with single film model. Fuel 2016, 184, 905–914. [Google Scholar]
- Paterson, W.R. Mass transfer to, and reaction on, a sphere immersed in a stationary or flowing gas. Chem. Eng. Sci. 2000, 55, 3567–3570. [Google Scholar] [CrossRef]
- Fortsch, D.; Schnell, U.; Hein, K.R.G. The mass transfer coefficient for the combustion of pulverized carbon particles. Combust. Flame 2001, 126, 1662–1668. [Google Scholar] [CrossRef]
- Scala, F. Calculation of the mass transfer coefficient for the combustion of a carbon particle. Combust. Flame 2010, 157, 137–142. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, M.C. Mass transfer coefficients for the combustion of a char particle in O2/CO2. Energy Fuels 2009, 23, 5717–5724. [Google Scholar] [CrossRef]
- Yu, J.; Wei, O.; Zhou, K. Mass transfer coefficients considering boundary layer reaction in oxy-fuel combustion of coal char. Fuel 2014, 124, 173–182. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications; WCB/McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Frank, M.; Kuipers, J.; Versteeg, G.F.; Swaaij, W. Modelling of simultaneous mass and heat transfer with chemical reaction using the Maxwell-Stefan theory—I. Model development and isothermal study. Chem. Eng. Sci. 1995, 50, 1645–1659. [Google Scholar] [CrossRef]
- Mon, E.; Amundson, N.R. Diffusion and reaction in a stagnant boundary layer about a carbon particle. 3. Stability. Ind. Eng. Chem. Fundan. 1979, 18, 162–168. [Google Scholar] [CrossRef]
- Sundaresan, S.; Amundson, N.R. Diffusion and reaction in a stagnant boundary layer about a carbon particle. 6. Effect of water vapor on the pseudo-steady-state structure. Ind. Eng. Chem. Fundan. 1980, 19, 344–351. [Google Scholar] [CrossRef]
- Makino, A. An approximate explicit expression for the combustion rate of a small carbon particle. Combust. Flame 1992, 90, 143–154. [Google Scholar] [CrossRef]
- Chen, L.; Yong, S.Z.; Ghoniem, A.F. Oxy-fuel combustion of pulverized coal: Characterization, fundamentals, stabilization and CFD modeling. Prog. Energy Combust. Sci. 2012, 38, 156–214. [Google Scholar] [CrossRef]
- Murphy, J.J.; Shaddix, C.R. Combustion kinetics of coal chars in oxygen-enriched environments. Combust. Flame 2006, 144, 710–729. [Google Scholar] [CrossRef]
- Buhre, B.J.P.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Wall, T.F. Combustion processes for carbon capture. In Proceedings of the Thirty-First International Symposium on Combustion School of Engineering, Heidelberg, Germany, 6–11 August 2006; Volume 31, Pt. 1. [Google Scholar]
- Reginald, E.M.; Robert, J.K.; Peter, G.; Michael, E.C. The effect of CO conversion in the boundary layers surrounding pulverized-coal char particles. Symp. Combust. 1991, 23, 1169–1176. [Google Scholar]
- Cengel, Y.A.; Boles, M.A. Thermodynamics—An Engineering Approach, 5th ed.; McGraw-Hill Publishing Company: New York, NY, USA, 2005. [Google Scholar]
- Goel, S.; Lee, C.H.; Longwell, J.P.; Sarofim, A.F. Modeling of ignition and CO oxidation in the boundary layer of a single char particle. Energy Fuels 1996, 10, 1091–1098. [Google Scholar] [CrossRef]
- Higuera, F.J. Combustion of a coal char particle in a stream of dry gas. Combust. Flame 2008, 152, 230–244. [Google Scholar] [CrossRef]
- Hecht, E.S.; Shaddix, C.R.; Molina, A.; Haynes, B.S. Effect of CO2 gasification reaction on oxy-combustion of pulverized coal char. Proc. Combust. Inst. 2011, 33, 1699–1706. [Google Scholar] [CrossRef]
- Matsui, K.; Tsuji, H. An aerothermochemical analysis of solid carbon combustion in the stagnation flow accompanied by homogeneous CO oxidation. Combust. Flame 1987, 70, 79–99. [Google Scholar] [CrossRef]
- Gonzalo-Tirado, C.; Jiménez, S.; Johansson, R.; Ballester, J. Comparative study of four alternative models for CO oxidation around a burning coal char particle. Combust. Flame 2014, 161, 1085–1095. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, K.; Wei, O. Effects of Stefan flow and CO oxidation on char particle combustion in O2/CO2 atmosphere. Fuel 2013, 106, 576–585. [Google Scholar] [CrossRef]
- Rosner, D.E. Transport Processes in Chemically Reacting Flow Systems; Elsevier B.V.: Amsterdam, The Netherlands, 1986; Volume 33, p. iii. [Google Scholar]
- Wang, C.A.; Han, T.; Du, Y.B.; Liu, Y.H.; Che, D.H. Diffusional effects on differences of coal char reactivity between air and oxy-fuel combustion in thermogravimetric experiments. J. Therm. Anal. Calorim. 2016, 125, 897–904. [Google Scholar] [CrossRef]
- Edge, P.; Gharebaghi, M.; Irons, R.; Porter, R.; Pourkashanian, M. Combustion modelling opportunities and challenges for oxy-coal carbon capture technology. Chem. Eng. Res. Des. 2011, 89, 1470–1493. [Google Scholar] [CrossRef]
- Mulcahy, M.F.R.; Smith, I.W. Kinetics of combustion of pulverized fuel: A review of theory and experiment. Rev. Pure Appl. Chem. 1969, 19, 81–108. [Google Scholar]
- Sheng, C.; Moghtaderi, B.; Gupta, R. A computational fluid dynamics based study of the combustion characteristics of coal blends in pulverised coal-fired furnace. Fuel 2004, 83, 1543–1552. [Google Scholar] [CrossRef]
- Hurt, R.; Sun, J.K.; Lunden, M. A kinetic model of carbon burnout in pulverized coal combustion. Combust. Flame 1998, 113, 181–197. [Google Scholar] [CrossRef]
- Sun, J. Numerical Simulation of the Carbon Burnout Process in Solid Fuel Combustion; Brown University: Providence, RI, USA, 2000. [Google Scholar]
- Kalinchak, V.V.; Dubinskii, A.V. Influence of Stefan flow on heat and mass transfer and kinetics of chemical reactions of a carbon particle in air. J. Eng. Phys. Thermophys. 1995, 68, 470–475. [Google Scholar] [CrossRef]
- Gonzalo-Tirado, C.; Jimenez, S.; Ballester, J. Gasification of a pulverized sub-bituminous coal in CO2 at atmospheric pressure in an entrained flow reactor. Combust. Flame 2012, 159, 385–395. [Google Scholar] [CrossRef]
- Vorobiev, N.; Becker, A.; Kruggel-Emden, H.; Panahi, A.; Schiemann, M. Particle shape and stefan flow effects on the burning rate of torrefied biomass. Fuel 2017, 210, 107–120. [Google Scholar] [CrossRef]
- Maffei, T.; Khatami, R.; Pierucci, S.; Ranzi, E.; Levendis, Y.A. Experimental and modeling study of single coal particle combustion in O2/N2 and Oxy-fuel (O2/CO2) atmospheres. Combust. Flame 2013, 160, 2559–2572. [Google Scholar] [CrossRef]
- Hecht, E.S.; Shaddix, C.R.; Lighty, J. Analysis of the errors associated with typical pulverized coal char combustion modeling assumptions for oxy-fuel combustion. Combust. Flame 2013, 160, 1499–1509. [Google Scholar] [CrossRef]
- Ou, W.; Zhou, K.; Yu, J.; Bai, D. Effect of Stefan flow on heat balance of a char particle in Oxy-fuel combustion. Combust. Sci. Technol. 2013, 19, 452–456. [Google Scholar]
- Kalinchak, V.V. Influence of Stefan flow and convection on the kinetics of chemical reactions and heat and mass exchange of carbon particles with gases. J. Eng. Phys. Thermophys. 2001, 71, 323–330. [Google Scholar] [CrossRef]
- Kalinchak, V.V.; Orlovskaya, S.G.; Kalinchak, A.I. Influence of Stefan flow on the characteristics of heterogeneous combustion of a carbon particle in air. J. Eng. Phys. Thermophys. 1997, 70, 150–156. [Google Scholar] [CrossRef]
- Murphy, J.J.; Shaddix, C. Effects of stefan flow on heat transfer from reacting carbon particles. Sandia-Rep. 2003, 2003, 1–24. [Google Scholar]
- Le, W.; Yu, Q.; Hong, Y. Experimental and numerical study of pulverized bituminous coal ignition characteristics in O2/N2 and O2/CO2 atmospheres. Asia-Pac. J. Chem. Eng. 2012, 7, 195–200. [Google Scholar]
- Zhou, K.; Yu, J.; Ou, W. Effects of Stefan Flow on Ignition and Heat Transfer of a Char Particle in O2/CO2 Atmospheres. Energy Fuels 2013, 27, 3454–3459. [Google Scholar] [CrossRef]
- Huang, J.; Guo, R.; Tao, L.; Wang, Q.; Liu, Z. Effects of stefan flow on metallurgical coke gasification with CO2. Energy Fuels. 2020, 34, 2936–2944. [Google Scholar] [CrossRef]
- Anthony, E.J.; Symonds, R.T. Oxy-fuel firing technology for power generation and heat and steam production. In Handbook of Climate Change Mitigation and Adaptation; Springer: New York, NY, USA, 2025; Volume 1, pp. 1–27. [Google Scholar]
- Vaziri, P.; Rasaei, M.R.; Seyfoori, S.; Zamani, S.; Mahmoodi, M.; Sedaee, B. Advancing carbon capture technologies in CCS: A comprehensive review of pre-combustion processes. Gas Sci. Eng. 2024, 131, 205481. [Google Scholar] [CrossRef]
- Fu, Y.; Cai, L.; Liu, C.; Wu, M.; Guan, Y. Thermodynamic and economic performance comparison of biomass gasification oxy-fuel combustion power plant in different gasifying atmospheres using advanced exergy and exergoeconomic approach. Renew. Energy 2024, 226, 120290. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Pan, W. Exploration of efficient utilization of high-alkali coal based on combustion process and combustion parameter optimization. Sustain. Energy Technol. Assess. 2023, 60, 103554. [Google Scholar] [CrossRef]
- Wall, T.; Liu, Y.; Spero, C.; Elliott, L.; Khare, S.; Rathnam, R. An overview on oxy-fuel coal combustion—State of the art research and technology development. Chem. Eng. Res. Des. 2009, 87, 1003–1016. [Google Scholar] [CrossRef]
- Shaddix, C.R.; Murphy, J.J. Coal char combustion reactivity in oxy-fuel applications. In Proceedings of the 20th Pittsburgh Coal Conference, Pittsburgh, PA, USA, 15–19 September 2003. [Google Scholar]
- Yama, D.T.; Kiga, T.; Okawa, M.; Omata, K.; Kimura, N.; Arai, K. Characteristics of pulverized-coal combustion in CO2-recovery power plant applied O2/CO2 combustion. JSME. Int. J. B-Fluid. T. 2008, 41, 1017–1022. [Google Scholar] [CrossRef]
- Châtelpélage., F.; Macadam, S.; Perrin, N.; Marin, O.; Carty, R.; Philo, G. A pilot-scale demonstration of oxy-combustion with flue gas recirculation in a pulverized coal-fired boiler. In Proceedings of the 28th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FA, USA, 23 March 2003. [Google Scholar]
- Liu, H. A comparison of combustion of coal chars in O2/CO2 and O2/N2 mixtures—Isothermal TGA studies. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- Liu, H. Combustion of coal chars in O2/CO2 and O2/N2 mixtures: A comparative study with non-isothermal thermogravimetric analyzer tests. Energy Fuels 2019, 23, 4278–4285. [Google Scholar] [CrossRef]
- Bejarano, P.A.; Levendis, Y.A. Single-coal-particle combustion in O2/N2 and O2/CO2 environments. Combust. Flame 2008, 153, 270–287. [Google Scholar] [CrossRef]
- Zhang, L.; Binner, E.; Qiao, Y.; Li, C.Z. In situ diagnostics of Victorian brown coal combustion in O2/N2 and O2/CO2 mixtures in drop-tube furnace. Fuel 2010, 89, 2703–2712. [Google Scholar] [CrossRef]
- Khatami, R.; Stivers, C.; Joshi, K.; Levendis, Y.A.; Sarofim, A.F. Combustion behaviour of single particles from three different coal ranks and from sugar cane bagasse in O2/N2 and O2/CO2 atmospheres. Combust. Flame 2012, 159, 1253–1271. [Google Scholar] [CrossRef]
- Riaza, J.; Khatami, R.; Levendis, Y.A. Single particle ignition and combustion of anthracite, semi-anthracite and bituminous coals in air and simulated oxy-fuel conditions. Combust. Flame 2014, 161, 1096–1108. [Google Scholar] [CrossRef]
- Liu, H.; Zailani, R.; Gibbs, B.M. Comparisons of pulverized coal combustion in air and in mixtures of O2/CO2. Fuel 2005, 84, 833–840. [Google Scholar] [CrossRef]
- Rathnam, R.K.; Elliott, L.K.; Wall, T.F.; Liu, Y.; Moghtaderi, B. Differences in reactivity of pulverised coal in air (O2/N2) and oxy-fuel (O2/CO2) conditions. Fuel Process. Technol. 2009, 90, 797–802. [Google Scholar] [CrossRef]
- Shen, Z.J.; Zhang, L.Q.; Liang, Q.F.; Xu, J.L.; Lin, K.F.; Liu, H.F. In situ experimental and modeling study on coal char combustion for coarse particle with effect of gasification in air (O2/N2) and O2/CO2 atmospheres. Fuel 2018, 233, 177–187. [Google Scholar] [CrossRef]
- Shaddix, C.R.; Molina, A. Particle imaging of ignition and devolatilization of pulverized coal during oxy-fuel combustion. Proc. Combust. Inst. 2009, 32, 2091–2098. [Google Scholar] [CrossRef]
- Bu, C.S.; Leckner, B.; Chen, X.P.; Liu, D.Y.; Pallarès, D.; Gómez-Barea, A. Devolatilization of a single fuel particle in a fluidized bed under oxy-combustion conditions. Part A: Experimental results. Combust. Flame 2015, 162, 797–808. [Google Scholar] [CrossRef]
- Bartok, W.; Sarofim, A.F. Fossil Fuel Combustion: A Source Book; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Dh, A.; Ts, A.; Xl, B.; Xin, G.A. Reactive molecular dynamic simulations of the CO2 gasification effect on the oxy-fuel combustion of Zhundong coal char. Fuel Process. Technol. 2020, 199, 106305. [Google Scholar] [CrossRef]
- Kleinhansa, U.; Halamaa, S.; Spliethoffa, H. The role of gasification reactions during pulverized solid fuel combustion: A detailed char combustion model based on measurements of char structure and kinetics for coal and pre-treated biomass. Combust. Flame 2017, 184, 117–135. [Google Scholar] [CrossRef]
- Kim, D.; Choi, S.; Shaddix, C.R.; Geier, M. Effect of CO2 gasification reaction on char particle combustion in oxy-fuel conditions. Fuel 2014, 120, 130–140. [Google Scholar] [CrossRef]
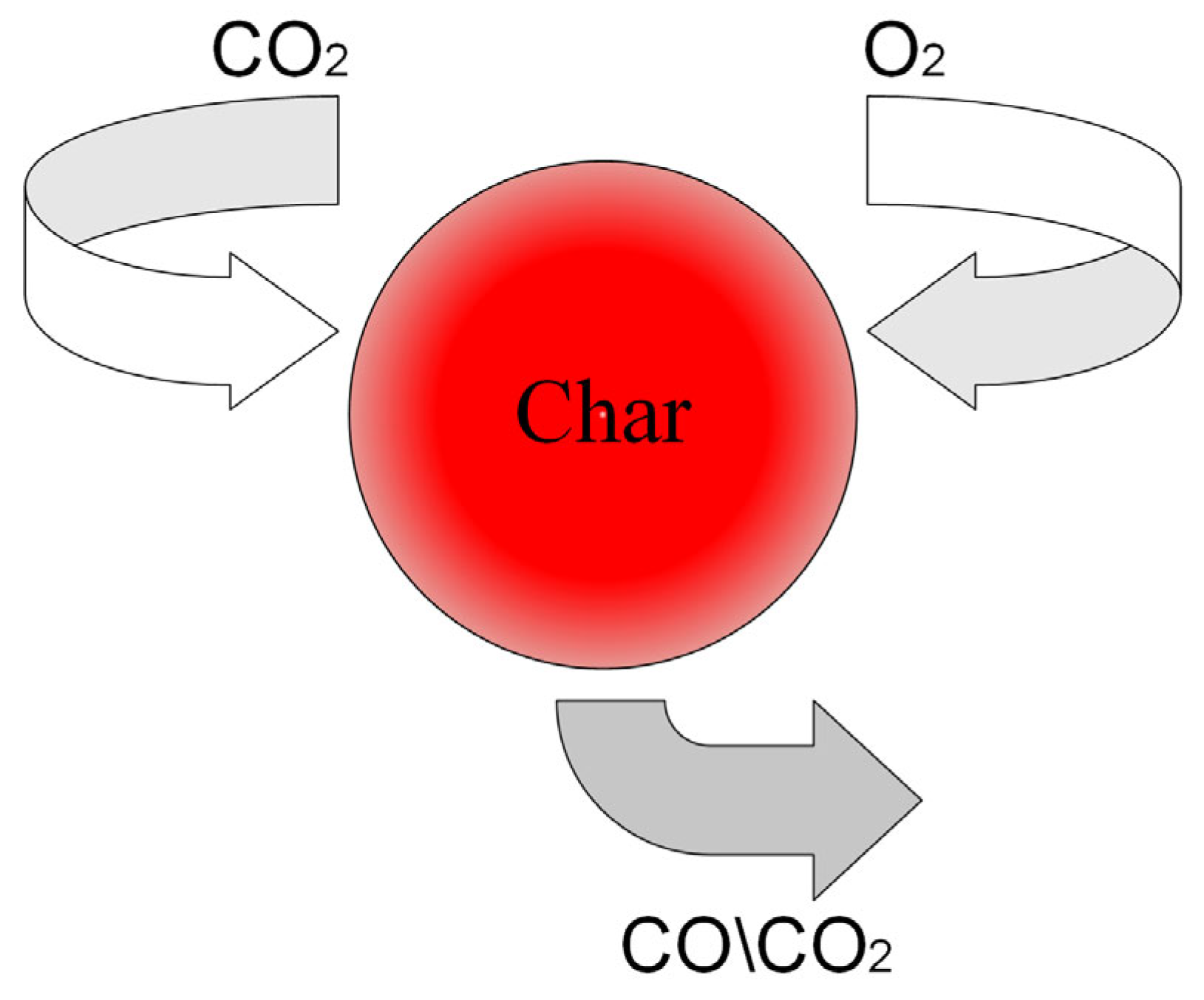
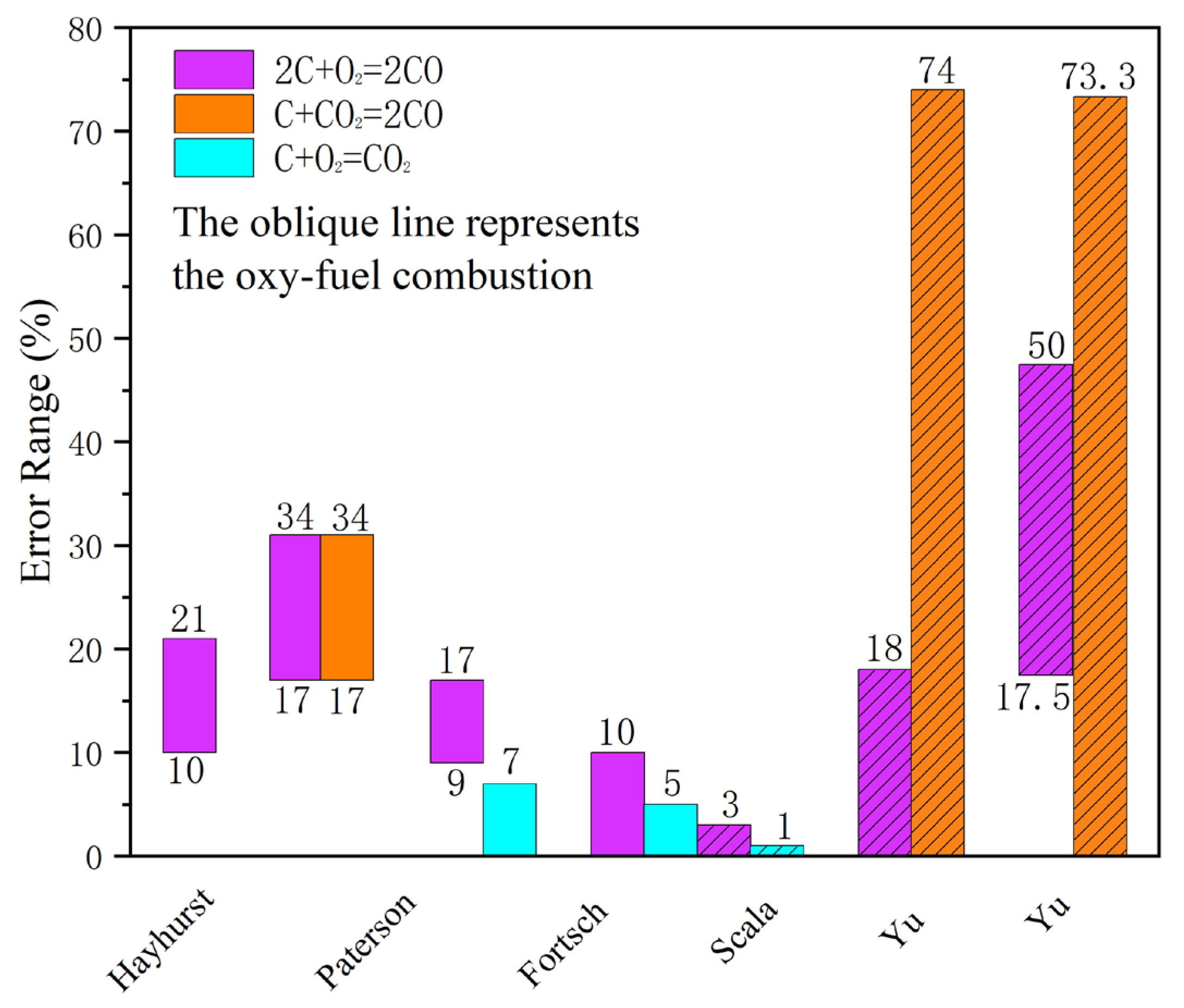
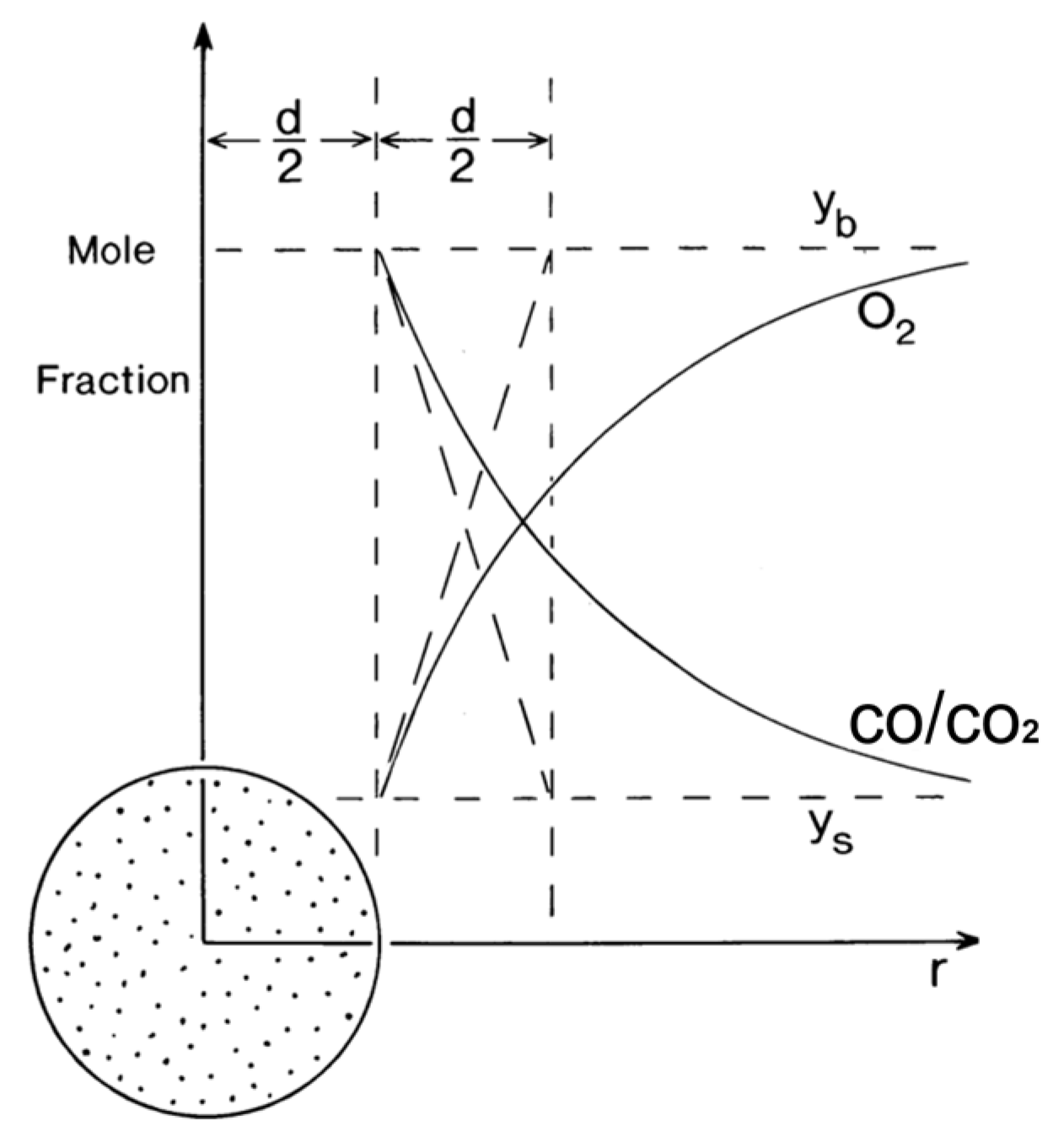


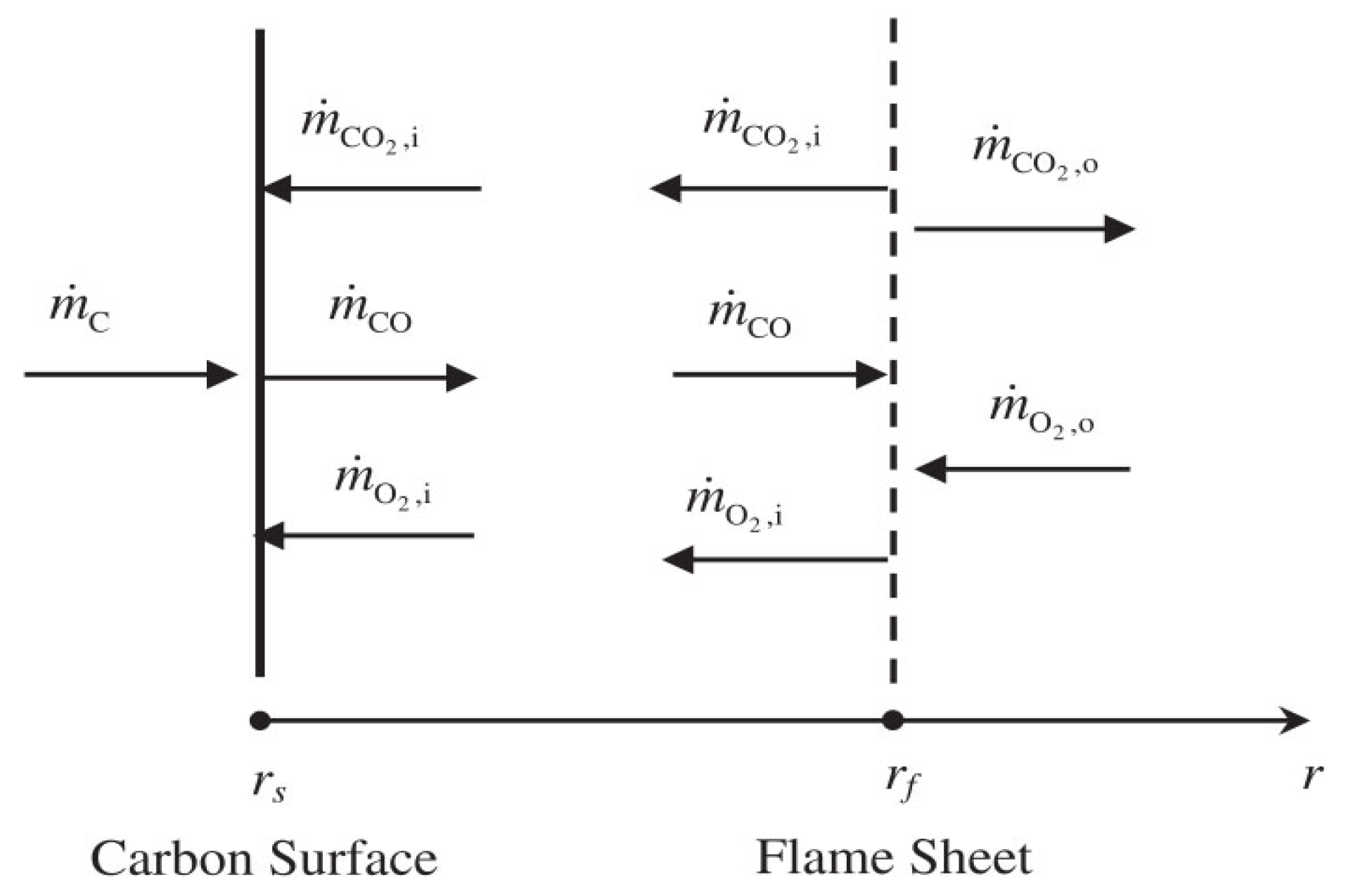
| Relevant Studies | Models | Dominant Reactions | The Influence Factors of the Mass Transfer |
|---|---|---|---|
| Hayhurst [8] | Single-film Double-film | Single-film model: 2C + O2 = 2CO Double-film model: C + CO2 = 2CO 2CO + O2 = 2CO2 (Homogeneous reaction) |
|
| Peterson [22] | — | 2C + O2 = 2CO 2C + CO2 = 2CO |
|
| Fortsch [23] | Single-film | C + O2 = CO2 2C + O2 = 2CO |
|
| Scala [24] | Single-film | C + O2 = CO2 2C + O2 = 2CO |
|
| Yu [25] | Single-film | 2C + O2 = 2CO C + CO2 = 2CO |
|
| Yu [26] | Continuous-film | 2C + O2 = 2CO C + CO2 = 2CO 2CO + O2 = 2CO2 (Homogeneous reaction) |
|
| Relevant Studies | Models | Combustion Atmosphere | Dominant Reactions | The Mentioned Influence Factors of the Stefan Flow | Research Conclusions |
|---|---|---|---|---|---|
| Kalinchak [51] | - | O2/N2 | C + O2 = CO2 2C + O2 = 2CO |
|
|
| Gonzalo-Tirado [52] | Single-film | O2/N2 | 2C + O2 = 2CO |
|
|
| Vorobiev [53] | Single-film | O2/N2 | C + O2 = CO2 2C + O2 = 2CO |
|
|
| Maffei [54] | Single-film | O2/N2 O2/CO2 | Char oxidation Char–CO2 gasification |
| Stefan flow has an unimportant effect on the combustion temperature and burnout time of the char particle. |
| Hecht [55] | Single-film | O2/CO2 | 5-step mechanism |
|
|
| Ou [56] | Single-film | O2/CO2 | 2C + O2 = 2CO C + CO2 = 2CO |
|
|
| Yu [43] | Continuous-film | O2/CO2 | 2C + O2 = 2CO C + CO2 = 2CO 2CO + O2 = 2CO2 (Homogeneous reaction) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, W.; Gan, Z.; Li, Y.; Ma, Y. Stefan Flow in Char Combustion: A Critical Review of Mass Transfer and Combustion Differences Between Air-Fuel and Oxy-Fuel Conditions. Energies 2025, 18, 4347. https://doi.org/10.3390/en18164347
Bao W, Gan Z, Li Y, Ma Y. Stefan Flow in Char Combustion: A Critical Review of Mass Transfer and Combustion Differences Between Air-Fuel and Oxy-Fuel Conditions. Energies. 2025; 18(16):4347. https://doi.org/10.3390/en18164347
Chicago/Turabian StyleBao, Wenfei, Zongwei Gan, Yuzhong Li, and Yan Ma. 2025. "Stefan Flow in Char Combustion: A Critical Review of Mass Transfer and Combustion Differences Between Air-Fuel and Oxy-Fuel Conditions" Energies 18, no. 16: 4347. https://doi.org/10.3390/en18164347
APA StyleBao, W., Gan, Z., Li, Y., & Ma, Y. (2025). Stefan Flow in Char Combustion: A Critical Review of Mass Transfer and Combustion Differences Between Air-Fuel and Oxy-Fuel Conditions. Energies, 18(16), 4347. https://doi.org/10.3390/en18164347





