A Systematic Review of Biopolymer Phase Change Materials for Thermal Energy Storage: Challenges, Opportunities, and Future Direction
Abstract
1. Introduction
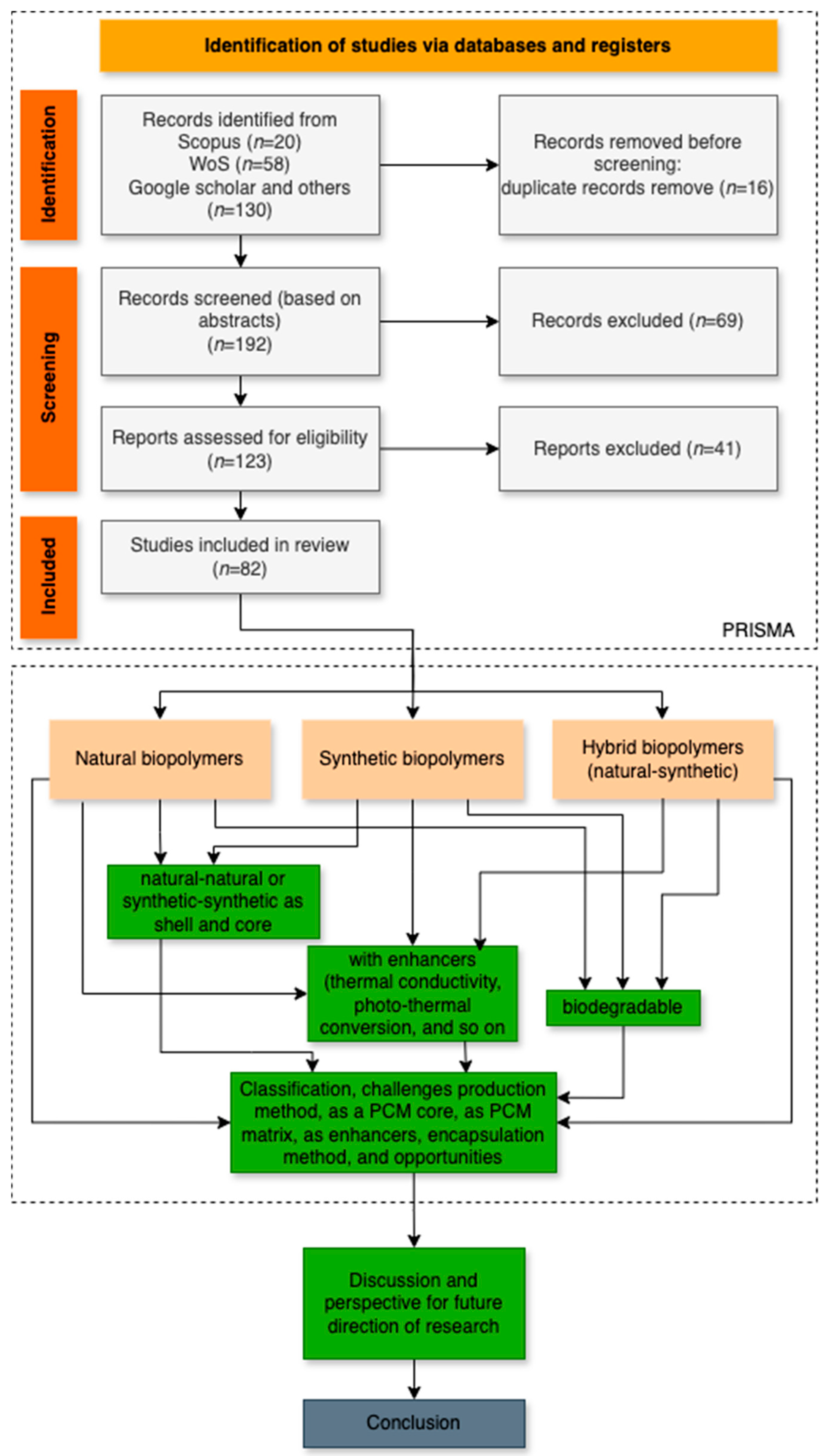
2. The Methodology of Review
3. Brief Description Related to Biopolymer Phase Change Materials and Their Classifications
3.1. Classification of Biopolymers
3.2. Biopolymer Materials
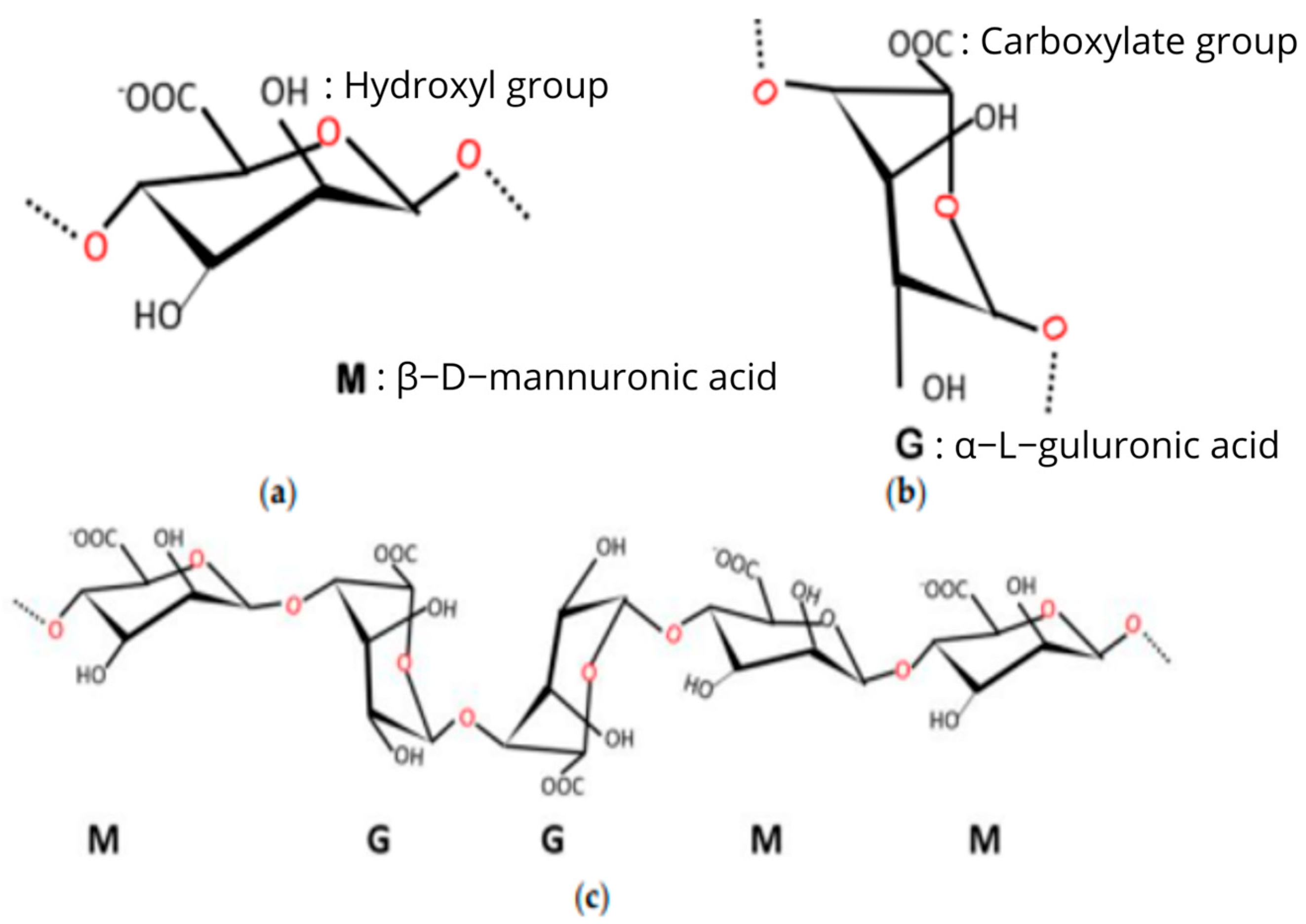
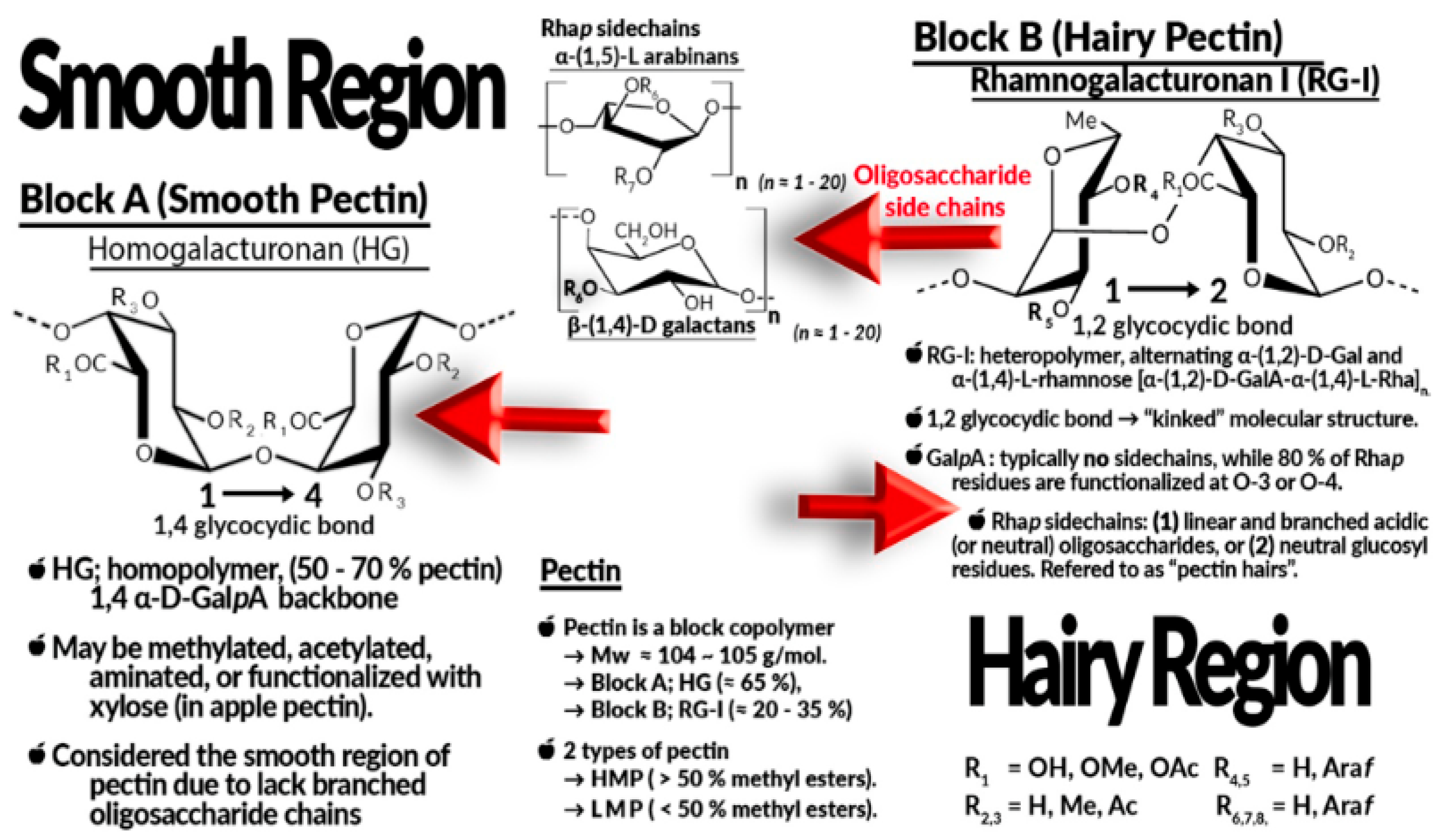
3.2.1. Natural Biopolymer Materials
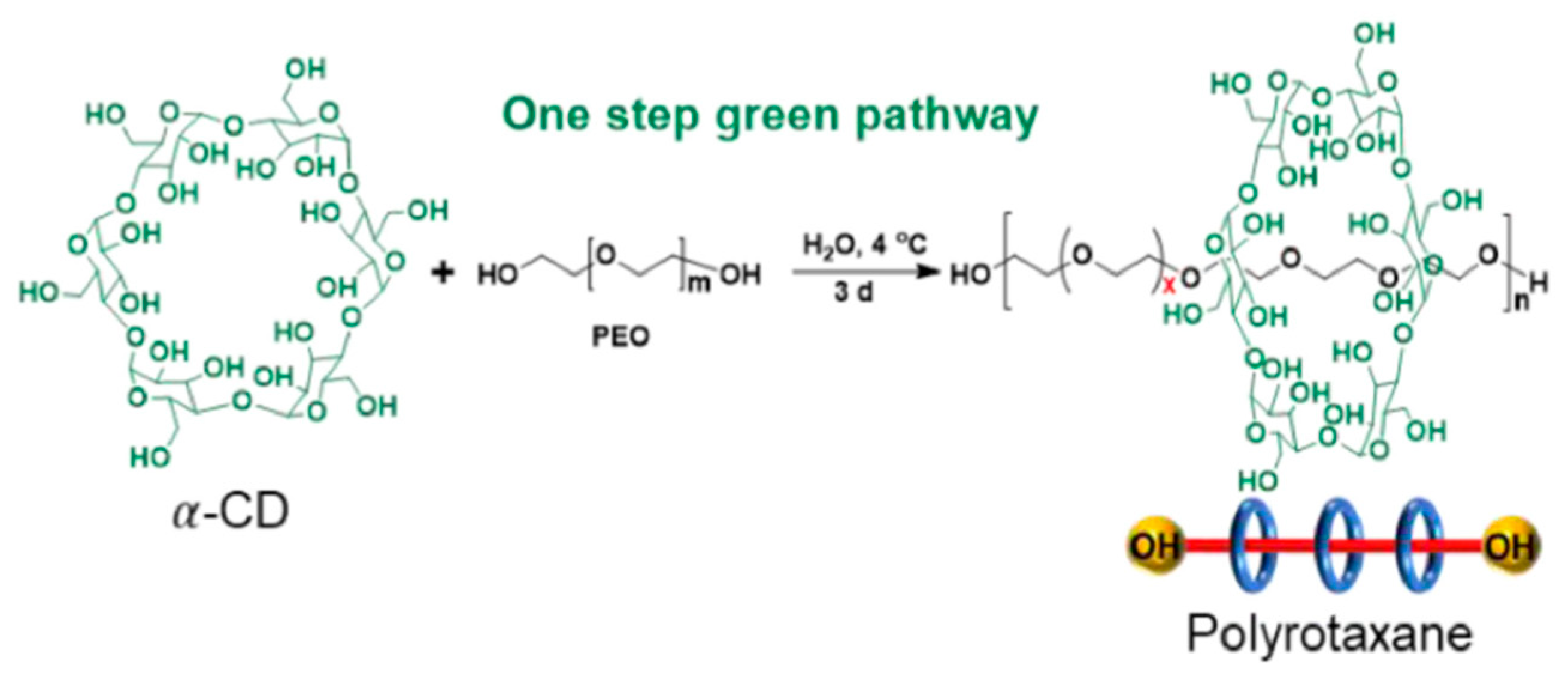
3.2.2. Synthetic Biopolymer Materials
3.2.3. Functional Roles of Biopolymers in PCM Systems
4. Natural Biopolymers PCM for TES
5. Synthetic Biopolymer PCMs for TES
6. Hybrid or Composite Biopolymer PCMs for TES
7. Discussion and Future Directions of Research
8. Conclusions
- -
- This article provides a comprehensive classification of natural and synthetic biopolymer materials, highlighting their suitability and potential in developing PCMs. The natural biopolymer materials include polysaccharides, polyphenolics, lignocellulose, proteins, lipids, and so on. Several naturally derived materials were described. The synthetic biopolymer materials include supramolecular, polyether, aliphatic polyester, polyolefin, and others. Several examples of materials based on the classification have been briefly described;
- -
- Several natural biopolymers, such as lipids, lignin, polysaccharides, protein, and others, offer a sustainable and promising approach for developing high-performance PCMs for TES. Some innovations, such as solvent-free synthesis of fatty acid amides, lignin-stabilized microencapsulation, electrospun core-shell fibers, and transparent wood composites, represent state-of-the-art approaches that improve thermal reliability, structural integrity, and multifunctionality. Despite challenges like limited thermal conductivity and long-term stability, these advancements highlight the growing potential and interest of biopolymers in next-generation applications, including smart textiles, passive building cooling, and industrial waste heat recovery;
- -
- Furthermore, synthetic biopolymer PCMs demonstrate significant potential for sustainable TES. Innovations, like supramolecular PLR–PEO networks, PLA–PCL blends, and PEG–MDI crosslinked systems achieve high latent heat, mechanical flexibility, and leakage resistance. The combination of synthetic PCMs also demonstrates promising solutions with enhanced thermal conductivity. However, challenges, such as supercooling, trade-offs between crystallinity and additive ratios, and the scalability of solvent-free methods, remain barriers in this case. More studies on synthetic biopolymer PCMs are still required for further improvements;
- -
- Hybrid biopolymer PCMs offer sustainability and high-performance solutions for TES by combining the structural and functional benefits of natural and synthetic polymers with conductive fillers. While they demonstrate enhanced energy retention, shape stability, and multifunctionality, challenges, such as leakage, low conductivity, and long-term reliability, still persist;
- -
- For comparison, hybrid biopolymer PCMs offer a balanced combination of high latent heat from natural polymers and enhanced thermal stability and durability from synthetic components. Their melting enthalpy may be slightly higher than some natural PCMs. In addition, their tunable properties and long-term reliability make them well-suited for demanding TES applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclatures
| a | Impact strength [kJ/m2] |
| E | Elastic modulus [MPa] |
| Eb | Bending modulus [MPa] |
| Ec | Compressive modulus [MPa] |
| hm | Melting enthalpy [J/g] |
| Tm | Melting temperature [°C] |
| k | Thermal conductivity k [W/m·K] |
| ρ | Density [kg/m3] |
| σb | Bending strength [MPa] |
| σc | Compressive strength [MPa] |
| σf | Fracture strength [MPa] |
| σflex | Flexural strength [MPa] |
| σm | Mechanical strength [MPa] |
| σt | Tensile strength [MPa] |
| σy | Yield stress/yield strength [MPa] |
| AC | Activated carbon |
| CMC | Carboxymethyl cellulose |
| G | Guluronic acid |
| LPB | Lignin-based polyols |
| M | Mannuronic acid |
| ORC | Organic Rankine cycle |
| PCM | Phase change material |
| PE | Polyethylene |
| PEG | Polyethylene glycol |
| PET | Polyethylene terephthalate |
| PLR | Polyrotaxane |
| PP | Polypropylene |
| PS | Polystyrene |
| RG-1 | Rhamnogalacturonan I |
| TES | Thermal energy storage |
| WoS | Web of Science |
References
- Alva, G.; Lin, Y.; Fang, G. An Overview of Thermal Energy Storage Systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Daniarta, S.; Nemś, M.; Kolasiński, P. A Review on Thermal Energy Storage Applicable for Low- and Medium-Temperature Organic Rankine Cycle. Energy 2023, 278, 127931. [Google Scholar] [CrossRef]
- Said, Z.; Pandey, A.K.; Tiwari, A.K.; Kalidasan, B.; Jamil, F.; Thakur, A.K.; Tyagi, V.V.; Sarı, A.; Ali, H.M. Nano-Enhanced Phase Change Materials: Fundamentals and Applications. Prog. Energy Combust. Sci. 2024, 104, 101162. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent Developments in Phase Change Materials for Energy Storage Applications: A Review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase Change Materials for Thermal Energy Storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of Solid–Liquid Phase Change Materials and Their Encapsulation Technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Dai, J.; Yang, K.; Wang, S.; Liu, X. Integration of Sustainable Polymers with Phase Change Materials. Prog. Mater. Sci. 2025, 151, 101447. [Google Scholar] [CrossRef]
- Prajapati, D.G.; Kandasubramanian, B. Biodegradable Polymeric Solid Framework-Based Organic Phase-Change Materials for Thermal Energy Storage. Ind. Eng. Chem. Res. 2019, 58, 10652–10677. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, J.; Lin, L.; Shi, J. A Review of Composite Phase Change Materials Based on Biomass Materials. Polymers 2022, 14, 4089. [Google Scholar] [CrossRef]
- Baylis, C.; Cruickshank, C.A. Review of Bio-Based Phase Change Materials as Passive Thermal Storage in Buildings. Renew. Sustain. Energy Rev. 2023, 186, 113690. [Google Scholar] [CrossRef]
- Dutta, S.; Sarkar, C.; Saha, S. Bio-Polymeric Green Composites for Thermal Energy Storage Applications. In Biodegradable Polymers and Their Emerging Applications; Saha, S., Sarkar, C., Eds.; Springer Nature: Singapore, 2023; pp. 213–234. ISBN 978-981-99-3307-5. [Google Scholar]
- Pielichowska, K.; Nowicka-Dunal, K.; Pielichowski, K. Bio-Based Polymers for Environmentally Friendly Phase Change Materials. Polymers 2024, 16, 328. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Peng, G.; Dou, G.; Hu, Y.; Sun, Y.; Chen, Z. Phase Change Material (PCM) Microcapsules for Thermal Energy Storage. Adv. Polym. Technol. 2020, 2020, 9490873. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, Characterization and Applications of Microencapsulated Phase Change Materials in Thermal Energy Storage: A Review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Mondal, S. Phase Change Materials for Smart Textiles—An Overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, D.; Dong, H.; Lv, J.; Wang, L.; Zhou, H.; Li, Z.; Liu, J.; He, Z. Magnetically Tightened Form-Stable Phase Change Materials with Modular Assembly and Geometric Conformality Features. Nat. Commun. 2022, 13, 1397. [Google Scholar] [CrossRef]
- Zeng, C.; Yuan, Y.; Cao, H.; Panchabikesan, K.; Haghighat, F. Stability and Durability of Microencapsulated Phase Change Materials (MePCMs) in Building Applications: A State of the Review. J. Energy Storage 2024, 80, 110249. [Google Scholar] [CrossRef]
- Nagar, S.; Sreenivasa, S. Polymer-Based Supporting Materials and Polymer-Encapsulated Phase Change Materials for Thermal Energy Storage: A Review on the Recent Advances of Materials, Synthesis, and Characterization Techniques. Polym. Eng. Sci. 2024, 64, 4341–4370. [Google Scholar] [CrossRef]
- Yazdani McCord, M.R.; Baniasadi, H. Advancements in Form-Stabilized Phase Change Materials: Stabilization Mechanisms, Multifunctionalities, and Applications—A Comprehensive Review. Mater. Today Energy 2024, 41, 101532. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation Methods for Phase Change Materials—A Critical Review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, A.; Zhao, Y.; Liu, Q.; Lei, J. Phase Change Material with Flexible Crosslinking for Thermal Energy Storage. J. Appl. Polym. Sci. 2020, 137, 48497. [Google Scholar] [CrossRef]
- Jing, Y.; Zhao, Z.; Cao, X.; Sun, Q.; Yuan, Y.; Li, T. Ultraflexible, Cost-Effective and Scalable Polymer-Based Phase Change Composites via Chemical Cross-Linking for Wearable Thermal Management. Nat. Commun. 2023, 14, 8060. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of Biopolymer Technology as an Alternative Option for Non-Degradable Plastics and Sustainable Management of Plastic Wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Oliver-Cuenca, V.; Salaris, V.; Muñoz-Gimena, P.F.; Agüero, Á.; Peltzer, M.A.; Montero, V.A.; Arrieta, M.P.; Sempere-Torregrosa, J.; Pavon, C.; Samper, M.D.; et al. Bio-Based and Biodegradable Polymeric Materials for a Circular Economy. Polymers 2024, 16, 3015. [Google Scholar] [CrossRef]
- Stoica, M.; Bichescu, C.I.; Crețu, C.-M.; Dragomir, M.; Ivan, A.S.; Podaru, G.M.; Stoica, D.; Stuparu-Crețu, M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods 2024, 13, 3027. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Zhang, Y.; Liu, Z. Natural Biopolymers for Flexible Sensing and Energy Devices. Chin. J. Polym. Sci. 2020, 38, 459–490. [Google Scholar] [CrossRef]
- Balart, R.; Garcia-Garcia, D.; Fombuena, V.; Quiles-Carrillo, L.; Arrieta, M.P. Biopolymers from Natural Resources. Polymers 2021, 13, 2532. [Google Scholar] [CrossRef] [PubMed]
- Flórez, M.; Cazón, P.; Vázquez, M. Selected Biopolymers’ Processing and Their Applications: A Review. Polymers 2023, 15, 641. [Google Scholar] [CrossRef]
- Ojo, A.O. An Overview of Lignocellulose and Its Biotechnological Importance in High-Value Product Production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium Species for Fermentative Hydrogen Production: An Overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Saraswat, R.; Shagun; Dhir, A.; Balan, A.S.S.; Powar, S.; Doddamani, M. Synthesis and Application of Sustainable Vegetable Oil-Based Polymers in 3D Printing. RSC Sustain. 2024, 2, 1708–1737. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef]
- Li, S.-L.; Xiao, T.; Lin, C.; Wang, L. Advanced Supramolecular Polymers Constructed by Orthogonal Self-Assembly. Chem. Soc. Rev. 2012, 41, 5950–5968. [Google Scholar] [CrossRef] [PubMed]
- Verjans, J.; Hoogenboom, R. Supramolecular Polymer Materials Based on Ureidopyrimidinone Quadruple Hydrogen Bonding Units. Prog. Polym. Sci. 2023, 142, 101689. [Google Scholar] [CrossRef]
- Peng, H.-Q.; Zhu, W.; Guo, W.-J.; Li, Q.; Ma, S.; Bucher, C.; Liu, B.; Ji, X.; Huang, F.; Sessler, J.L. Supramolecular Polymers: Recent Advances Based on the Types of Underlying Interactions. Prog. Polym. Sci. 2023, 137, 101635. [Google Scholar] [CrossRef]
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, A.; Semenova, A.; Nasonova, V.; Polishchuk, E.; Revutskaya, N.; Kozyrev, I.; Kotenkova, E. Approaches in Animal Proteins and Natural Polysaccharides Application for Food Packaging: Edible Film Production and Quality Estimation. Polymers 2021, 13, 1592. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the Most Important Methods of Improving the Processing Properties of Starch toward Non-Food Applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef] [PubMed]
- Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability 2022, 14, 6085. [Google Scholar] [CrossRef]
- Falua, K.J.; Pokharel, A.; Babaei-Ghazvini, A.; Ai, Y.; Acharya, B. Valorization of Starch to Biobased Materials: A Review. Polymers 2022, 14, 2215. [Google Scholar] [CrossRef]
- Pires, J.B.; dos Santos, F.N.; da Cruz, E.P.; Fonseca, L.M.; Pacheco, C.d.O.; da Rosa, B.N.; Santana, L.R.; de Pereira, C.M.P.; Carreno, N.L.V.; Diaz, P.S.; et al. Cassava, Corn, Wheat, and Sweet Potato Native Starches: A Promising Biopolymer in the Production of Capsules by Electrospraying. Int. J. Biol. Macromol. 2024, 281, 136436. [Google Scholar] [CrossRef]
- Zheng, Q.; Tian, Y.; Ye, F.; Zhou, Y.; Zhao, G. Fabrication and Application of Starch-Based Aerogel: Technical Strategies. Trends Food Sci. Technol. 2020, 99, 608–620. [Google Scholar] [CrossRef]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of Starch, Cellulose and Their Derivatives in the Development of Microparticle Drug-Delivery Systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Dalwadi, S.; Goel, A.; Kapetanakis, C.; Salas-de la Cruz, D.; Hu, X. The Integration of Biopolymer-Based Materials for Energy Storage Applications: A Review. Int. J. Mol. Sci. 2023, 24, 3975. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the Fermentation Process and Properties of Bacterial Cellulose: A Review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and Application of Chitin and Its Derivatives: A Review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Kumar, M.; Ahmad, N.; Ge, Y.; Mahmood, S. Recent Applications of Chitosan and Its Derivatives in Antibacterial, Anticancer, Wound Healing, and Tissue Engineering Fields. Polymers 2024, 16, 1351. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Chapter 1-Polymer Synthesis and Processing. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–31. ISBN 978-0-12-396983-5. [Google Scholar]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Tamilisai, R.; Palanisamy, P.N.; Selvasekarapandian, S.; Maheshwari, T. Sodium Alginate Incorporated with Magnesium Nitrate as a Novel Solid Biopolymer Electrolyte for Magnesium-Ion Batteries. J. Mater. Sci. Mater. Electron. 2021, 32, 22270–22285. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y. Sodium Alginate-Based Functional Materials toward Sustainable Applications: Water Treatment and Energy Storage. Ind. Eng. Chem. Res. 2023, 62, 11279–11304. [Google Scholar] [CrossRef]
- Dirpan, A.; Deliana, Y.; Ainani, A.F.; Irwan; Bahmid, N.A. Exploring the Potential of Pectin as a Source of Biopolymers for Active and Intelligent Packaging: A Review. Polymers 2024, 16, 2783. [Google Scholar] [CrossRef] [PubMed]
- Butler, I.P.; Banta, R.A.; Tyuftin, A.A.; Holmes, J.; Pathania, S.; Kerry, J. Pectin as a Biopolymer Source for Packaging Films Using a Circular Economy Approach: Origins, Extraction, Structure and Films Properties. Food Packag. Shelf Life 2023, 40, 101224. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Van Niekerk, J. Pectin and Alginate Functional Biopolymers: Factors Influencing Structural Composition, Functional Characteristics and Biofilm Development. Coatings 2024, 14, 987. [Google Scholar] [CrossRef]
- Matta, E.; Bertola, N. Development and Characterization of High Methoxyl Pectin Film by Using Isomalt as Plasticizer. J. Food Process. Preserv. 2020, 44, e14568. [Google Scholar] [CrossRef]
- Singh, B.G.; Das, R.P.; Kunwar, A. Protein: A Versatile Biopolymer for the Fabrication of Smart Materials for Drug Delivery. J. Chem. Sci. 2019, 131, 91. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. A Comprehensive Review on Types and Properties of Biopolymers as Sustainable Bio-Based Alternatives for Packaging. Food Biomacromol. 2024, 1, 58–87. [Google Scholar] [CrossRef]
- Du, Y.; Li, S.; Zhang, Y.; Rempel, C.; Liu, Q. Treatments of Protein for Biopolymer Production in View of Processability and Physical Properties: A Review. J. Appl. Polym. Sci. 2016, 133, 43351. [Google Scholar] [CrossRef]
- Gopinath, G.; Ayyasamy, S.; Shanmugaraj, P.; Swaminathan, R.; Subbiah, K.; Kandasamy, S. Effects of Biopolymers in Energy Storage Applications: A State-of-the-Art Review. J. Energy Storage 2023, 70, 108065. [Google Scholar] [CrossRef]
- Vidya, M.; Rajagopal, S. Silk Fibroin: A Promising Tool for Wound Healing and Skin Regeneration. Int. J. Polym. Sci. 2021, 2021, 9069924. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A Comprehensive Classification System for Lipids1. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Qurat-ul-Ain; Zia, K.M.; Zia, F.; Ali, M.; Rehman, S.; Zuber, M. Lipid Functionalized Biopolymers: A Review. Int. J. Biol. Macromol. 2016, 93, 1057–1068. [Google Scholar] [CrossRef]
- Ye, Z.; Xu, Y.-J.; Liu, Y. Influences of Dietary Oils and Fats, and the Accompanied Minor Content of Components on the Gut Microbiota and Gut Inflammation: A Review. Trends Food Sci. Technol. 2021, 113, 255–276. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Introduction to Fatty Acids and Lipids. Intraven. Lipid Emuls. 2014, 112, 1–16. [Google Scholar]
- Bakhtiari, S.; Nastaran, A.; Mahtab, J.; Mostafa, R.-T.; Somayeh, J.-S.; Rostami-Nejad, M. The Connection between Fatty Acids and Inflammation in Celiac Disease; a Deep Exploring. Tissue Barriers 2025, 13, 2342619. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Fraga-Corral, M.; Garcia-Oliveira, P.; Soria-Lopez, A.; Barba, F.J.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Aquaculture and Agriculture-by Products as Sustainable Sources of Omega-3 Fatty Acids in the Food Industry. eFood 2021, 2, 209–233. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Mulla, S.I.; Pant, D.; Sharma, T.; Kumar, A. Lignin as Potent Industrial Biopolymer: An Introduction. In Lignin: Biosynthesis and Transformation for Industrial Applications; Sharma, S., Kumar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–15. ISBN 978-3-030-40663-9. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.H.; Headrick, R.J.; Li, Z.; Spanu, L.; Loftus, D.J.; Lepech, M.D. Development of Biopolymer Composites Using Lignin: A Sustainable Technology for Fostering a Green Transition in the Construction Sector. Clean. Mater. 2024, 14, 100279. [Google Scholar] [CrossRef]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Teixeira Mendonça, R. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Yang, X.-M.; López, A.M.; Molleja, J.G.; Vázquez-López, A.; Wang, D.-Y. Highly Thermal Conductive Boron Nitride/Polyrotaxane Encapsulated PEG-Based Phase Change Materials. Eur. Polym. J. 2023, 199, 112431. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Shape-Stabilized Poly(Ethylene Glycol) (PEG)-Cellulose Acetate Blend Preparation with Superior PEG Loading via Microwave-Assisted Blending. Sol. Energy 2017, 144, 32–39. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal Analysis and Heat Capacity Study of Polyethylene Glycol (PEG) Phase Change Materials for Thermal Energy Storage Applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Shinde, V.V.; Shelke, S.D.; Celestine, A.-D.N.; Beckingham, B.S. Self-Healing in High Impact Polystyrene (HIPS) Composites via Embedded Non-Toxic Solvent-Filled Microcapsules. J. Appl. Polym. Sci. 2022, 139, 51463. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Hobson, J.; Duan, Y.; Wang, D.-Y. Polyrotaxane: New Generation of Sustainable, Ultra-Flexible, Form-Stable and Smart Phase Change Materials. Energy Storage Mater. 2021, 40, 347–357. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Saju, J.; Alcock, K.M.; Mavelil, A.T.; Markapudi, P.R.; Yu, H.; Manjakkal, L. Biodegradable Biopolymers for Electrochemical Energy Storage Devices in a Circular Economy. RSC Sustain. 2025, 3, 37–63. [Google Scholar] [CrossRef]
- Ramezani Dana, H.; Ebrahimi, F. Synthesis, Properties and Applications of Polylactic Acid-Based Polymers. Polym. Eng. Sci. 2023, 63, 22–43. [Google Scholar] [CrossRef]
- Radouane, N. A Comprehensive Review of Composite Phase Change Materials (CPCMs) for Thermal Management Applications, Including Manufacturing Processes, Performance and Applications. Energies 2022, 15, 8271. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Heo, J.; Jung, S.; Lee, H.; Cho, H. Shape Stabilized Phase Change Materials Based on Different Support Structures for Thermal Energy Storage Applications—A Review. Energy 2023, 262, 125463. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel Strategies and Supporting Materials Applied to Shape-Stabilize Organic Phase Change Materials for Thermal Energy Storage—A Review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, B.; Wu, J. Optimization of Filler Distribution for Organic Phase Change Material Composites: Numerical Investigation and Entropy Analysis. Appl. Energy 2014, 132, 543–550. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J. Fabrication of Porous Boron Nitride and Thermally Conductive Inorganic Phase Change Material Composites for Efficient Thermal Management. Ceram. Int. 2023, 49, 18363–18370. [Google Scholar] [CrossRef]
- Mert, H.H.; Ali, E.; Emine Hilal, M.; Mert, M.S. Preparation and Characterization of Shape-Stable Bio-Based Composite Phase Change Materials for Thermal Energy Storage: Coconut Oil/Activated Carbon from Cherry Stones Doped Composites. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 5381–5397. [Google Scholar] [CrossRef]
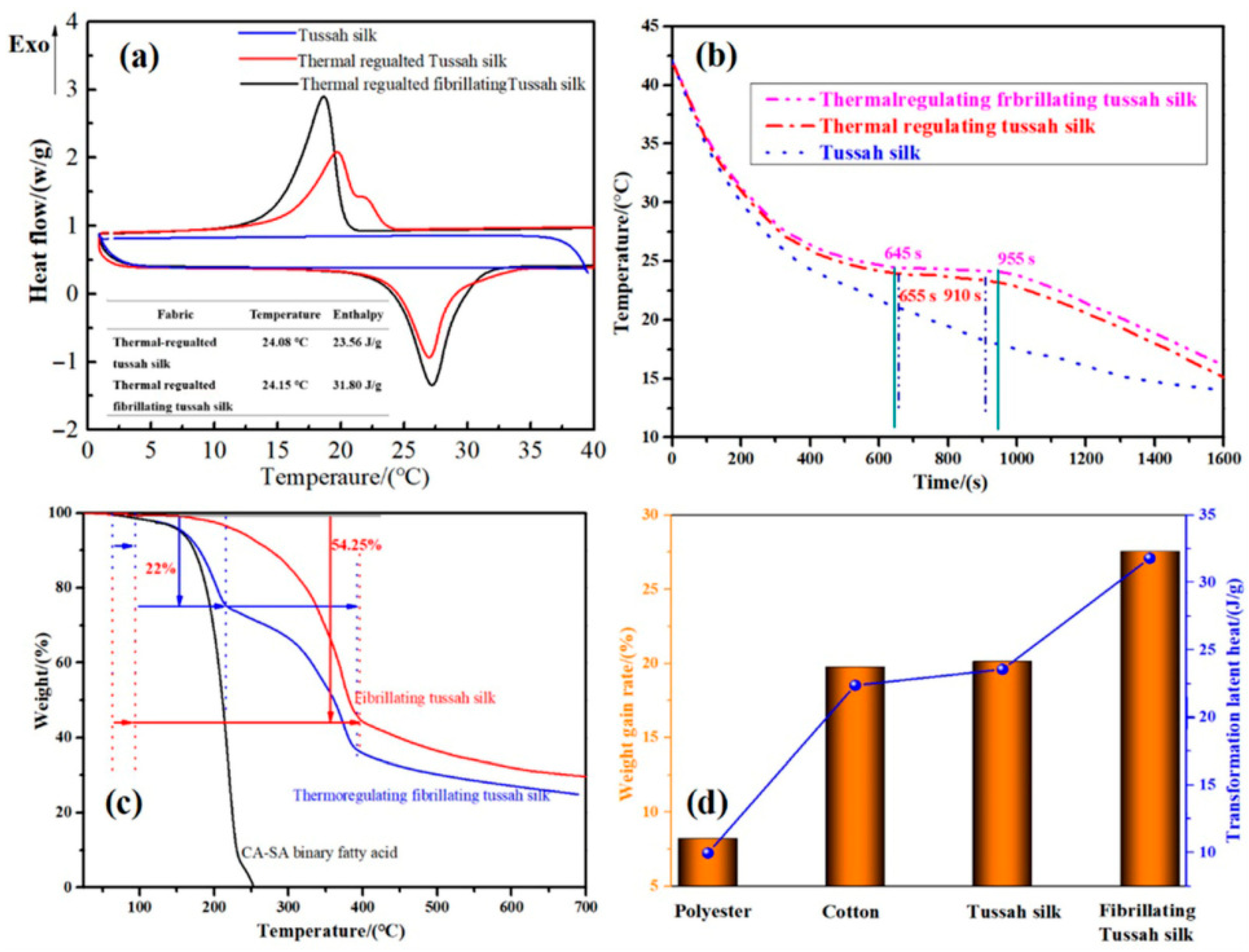
- Wang, W.; Zhang, Q.; Liu, L.; Sun, Y.; Wu, J.; Tao, J.; Lu, X. Fabrication of Air-Conditioning Tussah Silk with Capric–Stearic Eutectic Mixture for Effective Energy Storage and Thermal-Regulatory Applications. Polym. Int. 2023, 72, 217–229. [Google Scholar] [CrossRef]
- Montanari, C.; Chen, H.; Lidfeldt, M.; Gunnarsson, J.; Olsén, P.; Berglund, L.A. Sustainable Thermal Energy Batteries from Fully Bio-Based Transparent Wood. Small 2023, 19, 2301262. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Z.; Lang, Z.; Xie, Y.; Xiao, Z.; Wang, H.; Liang, D.; Li, J.; Zhang, K. Multifunctional Reversible Self-Assembled Structures of Cellulose-Derived Phase-Change Nanocrystals. Adv. Mater. 2021, 33, 2005263. [Google Scholar] [CrossRef]
- Cao, L.; Tang, Y.; Fang, G. Preparation and Properties of Shape-Stabilized Phase Change Materials Based on Fatty Acid Eutectics and Cellulose Composites for Thermal Energy Storage. Energy 2015, 80, 98–103. [Google Scholar] [CrossRef]
- Udangawa, W.M.R.N.; Willard, C.F.; Mancinelli, C.; Chapman, C.; Linhardt, R.J.; Simmons, T.J. Coconut Oil-Cellulose Beaded Microfibers by Coaxial Electrospinning: An Eco-Model System to Study Thermoregulation of Confined Phase Change Materials. Cellulose 2019, 26, 1855–1868. [Google Scholar] [CrossRef]
- Németh, B.; Németh, Á.S.; Ujhidy, A.; Tóth, J.; Trif, L.; Gyenis, J.; Feczkó, T. Fully Bio-Originated Latent Heat Storing Calcium Alginate Microcapsules with High Coconut Oil Loading. Sol. Energy 2018, 170, 314–322. [Google Scholar] [CrossRef]
- Németh, B.; Ujhidy, A.; Tóth, J.; Trif, L.; Feczkó, T.; Rauch, R. Gelation Elimination in Eco-Friendly Preparation of Double-Layered Calcium Alginate-Coconut Oil Latent Heat Energy Storing Microcapsules. Mater. Chem. Phys. 2023, 293, 126889. [Google Scholar] [CrossRef]
- Konuklu, Y.; Erzin, F.; Akar, H.B.; Turan, A.M. Cellulose-Based Myristic Acid Composites for Thermal Energy Storage Applications. Sol. Energy Mater. Sol. Cells 2019, 193, 85–91. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Microencapsulation and Thermal Properties of Myristic Acid with Ethyl Cellulose Shell for Thermal Energy Storage. Appl. Energy 2018, 231, 494–501. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Z.; Kapar, S.; Ataeian, P.; da Silva Bernardes, J.; Berry, R.; Zhao, W.; Zhou, G.; Tam, K.C. Microencapsulation of Phase Change Materials with Polystyrene/Cellulose Nanocrystal Hybrid Shell via Pickering Emulsion Polymerization. ACS Sustain. Chem. Eng. 2019, 7, 17756–17767. [Google Scholar] [CrossRef]
- Yoo, Y.; Martinez, C.; Youngblood, J.P. Synthesis and Characterization of Microencapsulated Phase Change Materials with Poly(Urea−urethane) Shells Containing Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 31763–31776. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.O. The Preparation and Characterization of Chitosan–Gelatin Microcapsules and Microcomposites with Fatty Acids as Thermal Energy Storage Materials. Energy Technol. 2015, 3, 503–508. [Google Scholar] [CrossRef]
- Singh, J.; Vennapusa, J.R.; Chattopadhyay, S. Protein-Polysaccharide Based Microencapsulated Phase Change Material Composites for Thermal Energy Storage. Carbohydr. Polym. 2020, 229, 115531. [Google Scholar] [CrossRef]
- Yazdani McCord, M.R.; Kankkunen, A.; Chatzikosmidou, D.; Seppälä, A.; Seppälä, J.; Baniasadi, H. Polypyrrole-Modified Flax Fiber Sponge Impregnated with Fatty Acids as Bio-Based Form-Stable Phase Change Materials for Enhanced Thermal Energy Storage and Conversion. J. Energy Storage 2024, 81, 110363. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Marta López, A.; Yang, X.-M.; Ye, W.; Xu, B.; Hobson, J.; Wang, D.-Y. Shape-Stable and Smart Polyrotaxane-Based Phase Change Materials with Enhanced Flexibility and Fire-Safety. Eur. Polym. J. 2022, 173, 111262. [Google Scholar] [CrossRef]
- Trigui, A.; Ben Aribia, W.; Akrouti, A.; Znaidia, S.; Alshammari, N.K.; Abdelmouleh, M. Latent Heat Storage Bio-Composites from Egg-Shell/PE/PEG as Feasible Eco-Friendly Building Materials. Polym. Compos. 2024, 45, 11416–11433. [Google Scholar] [CrossRef]
- Pereira, E.D.; de Souza, F.G.; Pal, K.; da Silveira Maranhão, F.; Filho, R.D.T.; Hasparyk, N.P.; de Melo Monteiro, V.; Dantas, M.C.N.; Rodrigues, J.G.P. Oligo(Butylene-Succinate) and Nanocatalyst Effect Prediction: Could a Neural Network Determine the Lowest Melting Temperature of This Phase-Changing Material Better than a Classic Approach? Top. Catal. 2022, 65, 1984–1993. [Google Scholar] [CrossRef]
- Botlhoko, O.J.; Ramontja, J.; Ray, S.S. A New Insight into Morphological, Thermal, and Mechanical Properties of Melt-Processed Polylactide/Poly(ε-Caprolactone) Blends. Polym. Degrad. Stab. 2018, 154, 84–95. [Google Scholar] [CrossRef]
- Lan, T.-Y.; Mao, H.-I.; Chen, C.-W.; Lee, Y.-T.; Yang, Z.-Y.; Luo, J.-L.; Li, P.-R.; Rwei, S.-P. A Rapid Thermal Absorption Rate and High Latent Heat Enthalpy Phase Change Fiber Derived from Bio-Based Low Melting Point Copolyesters. Polymers 2022, 14, 3298. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, J.; Wang, Y.; Zhang, X.; Liu, B.; Shi, H.; He, L. Phase Transition and Crystallization of Bio-Based Comb-like Polymers Based on Renewable Castor Oil-Derived Epoxides and CO2. Macromolecules 2021, 54, 8503–8511. [Google Scholar] [CrossRef]
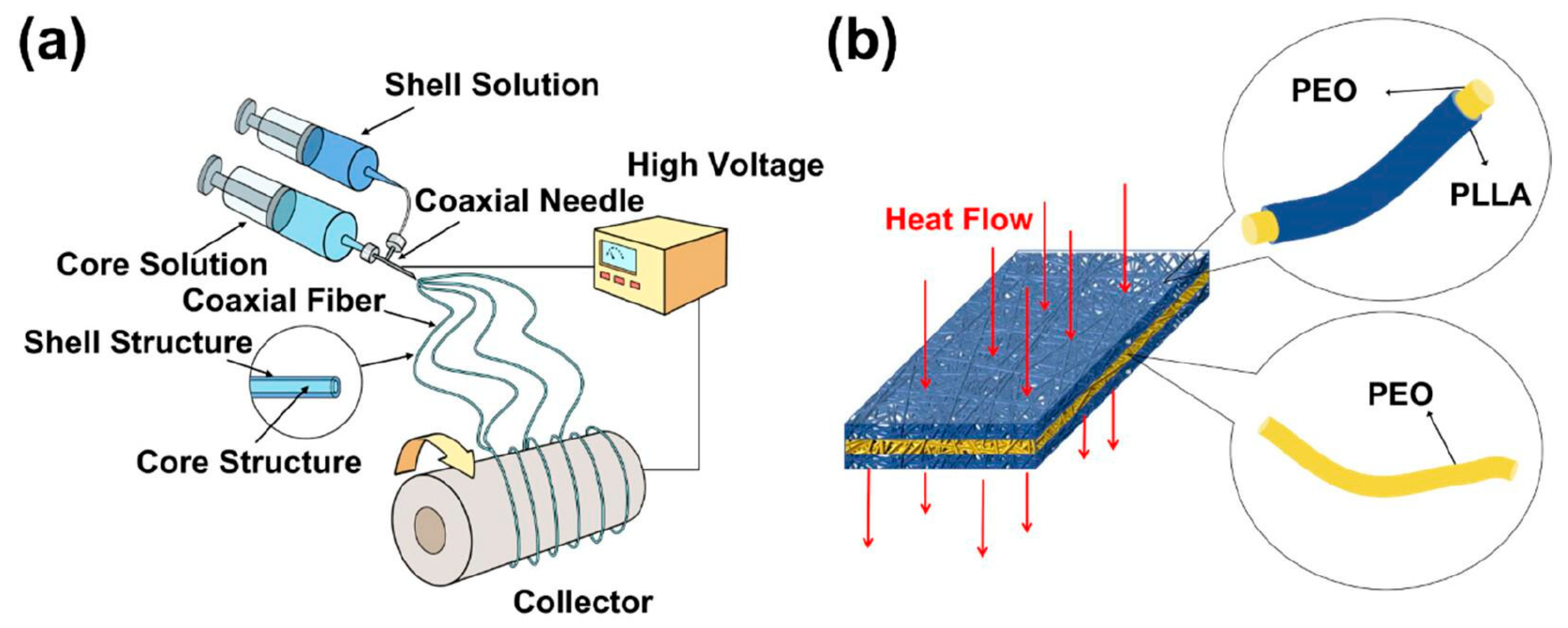
- Fredi, G.; Kianfar, P.; Dalle Vacche, S.; Pegoretti, A.; Vitale, A. Electrospun Shape-Stabilized Phase Change Materials Based on Photo-Crosslinked Polyethylene Oxide. Polymers 2021, 13, 2979. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Thermal Energy Storage Using Poly(Ethylene Glycol) Incorporated Hyperbranched Polyurethane as Solid–Solid Phase Change Material. Ind. Eng. Chem. Res. 2017, 56, 14401–14409. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H. Synthesis and Characterization of Biopolyurethane Crosslinked with Castor Oil-Based Hyperbranched Polyols as Polymeric Solid–Solid Phase Change Materials. Sci. Rep. 2022, 12, 14646. [Google Scholar] [CrossRef]
- Feng, L.; Ding, J.; Hu, H.; Lv, Z.; Zhang, Y.; Xu, B.; Quan, J.; Hao, S.; Fan, H.; Hang, Z. Preparation and Characterization of Bio-Based PLA/PEG/g-C3N4 Low-Temperature Composite Phase Change Energy Storage Materials. Polymers 2023, 15, 2872. [Google Scholar] [CrossRef]
- Jia, Y.; Liao, G.; Wu, Y.; Mykhaylyk, O.; Topham, P.D.; Dong, X.-H.; Chen, C.; Yu, Q.; Wang, L. Investigating the Effect of Crystallizability and Glass Transition Temperature of Supporting Materials for Preparing High Enthalpy Electrospun Poly(Lactic Acid)/Poly(Ethylene Glycol) Phase Change Fibers. Sol. Energy Mater. Sol. Cells 2023, 256, 112322. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhao, S.; Wang, X.; Zheng, J.; Zeng, W.; Yuan, M.; Zhao, N.; Li, Q.; Wang, Z.; et al. Biobased Phase Change Material with Reduced Thermal Conductivity: From Preparation to Analysis of Thermal Insulation Performance. ACS Appl. Polym. Mater. 2023, 5, 3728–3736. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Díaz Palencia, J.L.; Wang, D.-Y. Fully Bio-Based Poly (Glycerol-Itaconic Acid) as Supporter for PEG Based Form Stable Phase Change Materials. Compos. Commun. 2021, 27, 100893. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Muiruri, J.K.; Yeo, J.C.C.; Png, Z.M.; Sng, A.; Xie, H.; Ji, R.; Wang, S.; Liu, H.; Xu, J.; et al. Polyethylene Glycol/Polylactic Acid Block Co-Polymers as Solid–Solid Phase Change Materials. SmartMat 2023, 4, e1188. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.; Kang, B.; Yuan, T.; Qu, J. Bio-Based Poly (Lactic Acid)/High-Density Polyethylene Blends as Shape-Stabilized Phase Change Material for Thermal Energy Storage Applications. Sol. Energy Mater. Sol. Cells 2019, 192, 170–178. [Google Scholar] [CrossRef]
- Lu, X.; Tang, L.; Wang, L.; Zhao, J.; Li, D.; Wu, Z.; Xiao, P. Morphology and Properties of Bio-Based Poly (Lactic Acid)/High-Density Polyethylene Blends and Their Glass Fiber Reinforced Composites. Polym. Test. 2016, 54, 90–97. [Google Scholar] [CrossRef]
- Sarkar, J.; Samanta, D.; Chaudhuri, S.; Angeline, J.; Kumari, K.G.A.; Jaisankar, S.N. Harnessing Leather Waste in Polymer Matrix for Sustainable Smart Shape-Stable Phase Change Materials. J. Appl. Polym. Sci. 2024, 141, e55659. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wang, Z.; Peng, K.; Pan, W.; Xie, Q. Novel Form Stable Phase Change Materials Based on the Composites of Polyethylene Glycol/Polymeric Solid-Solid Phase Change Material. Sol. Energy Mater. Sol. Cells 2015, 134, 80–88. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, W.; Cai, X. Solvent-Free Preparation and Performance of Novel Xylitol Based Solid-Solid Phase Change Materials for Thermal Energy Storage. Energy Build. 2018, 158, 37–42. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, X.; Jiang, L.; Wu, B.; Wang, J.; Lei, J. Solvent-Free Synthesis and Properties of Novel Solid–Solid Phase Change Materials with Biodegradable Castor Oil for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2016, 147, 177–184. [Google Scholar] [CrossRef]
- Bragaglia, M.; Lamastra, F.R.; Berrocal, J.A.; Paleari, L.; Nanni, F. Sustainable Phase Change Materials (PCMs): Waste Fat from Cooking Pork Meat Confined in Polypropylene Fibrous Mat from Waste Surgical Mask and Porous Bio-Silica. Mater. Today Sustain. 2023, 23, 100454. [Google Scholar] [CrossRef]
- Zheng, Y.; Martinez, C.J.; Youngblood, J.P. Synthesis and Characterization of Long-Chain (C20~C48) Fatty Acid Amides (FAAms) from Soybean Oil and Alkyl Amines for Phase Change Material Applications. J. Appl. Polym. Sci. 2023, 140, e54675. [Google Scholar] [CrossRef]
- Perez-Arce, J.; Serrano, A.; Dauvergne, J.-L.; Centeno-Pedrazo, A.; Prieto-Fernandez, S.; Palomo Del Barrio, E.; Garcia-Suarez, E.J. Sustainable Lignin-Based Polyols as Promising Thermal Energy Storage Materials. J. Appl. Polym. Sci. 2021, 138, 51356. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Shen, C.; Mao, Z.; Xu, H.; Zhong, Y.; Sui, X.; Feng, X.; Wang, B. Lignin Assisted Pickering Emulsion Polymerization to Microencapsulate 1-Tetradecanol for Thermal Management. Int. J. Biol. Macromol. 2020, 146, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, K.K.; Padmanaban, R.; Sathishkumar, M.; Sarkar, J. Preparation of Waste-Incorporated Bio-Smart Shape-Stable Phase Change Material (FSSPCM) with Responsive Fluorescent Properties. New J. Chem. 2024, 48, 18656–18665. [Google Scholar] [CrossRef]
- Fashandi, M.; Leung, S.N. Preparation and Characterization of 100% Bio-Based Polylactic Acid/Palmitic Acid Microcapsules for Thermal Energy Storage. Mater. Renew. Sustain. Energy 2017, 6, 14. [Google Scholar] [CrossRef]
- Baştürk, E.; Kahraman, M.V. Photocrosslinked Biobased Phase Change Material for Thermal Energy Storage. J. Appl. Polym. Sci. 2016, 133, 43757. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, Y.; Liu, Q.; Zhou, C.; Zhang, X.; Lei, J. Form-Stable Phase Change Materials Based on Castor Oil and Palmitic Acid for Renewable Thermal Energy Storage. J. Therm. Anal. Calorim. 2019, 137, 1225–1232. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Tian, Y.; Zhou, K.; Cheng, J.; Zhang, J. Bio-Based Recyclable Form-Stable Phase Change Material Based on Thermally Reversible Diels–Alder Reaction for Sustainable Thermal Energy Storage. Chem. Eng. J. 2022, 448, 137749. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Luo, F.; Jin, Y.; Huang, B.; Qian, Q. Bio-Based Flexible Phase Change Composite Film with High Thermal Conductivity for Thermal Energy Storage. Compos. Part A Appl. Sci. Manuf. 2021, 151, 106638. [Google Scholar] [CrossRef]
- Jia, X.; Li, Q.; Ao, C.; Hu, R.; Xia, T.; Xue, Z.; Wang, Q.; Deng, X.; Zhang, W.; Lu, C. High Thermal Conductive Shape-Stabilized Phase Change Materials of Polyethylene Glycol/Boron Nitride@chitosan Composites for Thermal Energy Storage. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105710. [Google Scholar] [CrossRef]
- Wan, L.; Liu, C.; Cao, D.; Sun, X.; Zhu, H. High Phase Change Enthalpy Enabled by Nanocellulose Enhanced Shape Stable Boron Nitride Aerogel. ACS Appl. Polym. Mater. 2020, 2, 3001–3009. [Google Scholar] [CrossRef]
- Liu, L.; Fan, X.; Zhang, Y.; Zhang, S.; Wang, W.; Jin, X.; Tang, B. Novel Bio-Based Phase Change Materials with High Enthalpy for Thermal Energy Storage. Appl. Energy 2020, 268, 114979. [Google Scholar] [CrossRef]
- Wei, D.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; El Azab, I.H.; Sheng, X.; Huang, M.; El-Bahy, Z.M.; Huang, J. Development of Novel Biomass Hybrid Aerogel Supported Composite Phase Change Materials with Improved Light-Thermal Conversion and Thermal Energy Storage Capacity. Adv. Compos. Hybrid Mater. 2022, 5, 1910–1921. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Sheng, D.; Yang, Y. CsxWO3-Doped PEG/Sweet Potato Form-Stable Composites for Light-Thermal Conversion and Energy Storage. J. Polym. Eng. 2024, 44, 117–124. [Google Scholar] [CrossRef]
- Baniasadi, H.; Seppälä, J.; Kankkunen, A.; Seppälä, A.; Yazdani, M.R. Water-Resistant Gum-Based Phase Change Composite for Thermo-Regulating Insulation Packaging. J. Energy Storage 2023, 61, 106725. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Ajdary, R.; Kankkunen, A.; Rojas, O.J.; Seppälä, A. Cellulose Nanofibrils Endow Phase-Change Polyethylene Glycol with Form Control and Solid-to-Gel Transition for Thermal Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 6188–6200. [Google Scholar] [CrossRef]
- Chen, L.; Lv, J.; Ding, L.; Yang, G.; Mao, Z.; Wang, B.; Feng, X.; Zapotoczny, S.; Sui, X. A Shape-Stable Phase Change Composite Prepared from Cellulose Nanofiber/Polypyrrole/Polyethylene Glycol for Electric-Thermal Energy Conversion and Storage. Chem. Eng. J. 2020, 400, 125950. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, L.-S.; Tao, X.-F.; Yang, J.; Yang, M.-B.; Yang, W. Facile Fabrication of Shape-Stabilized Polyethylene Glycol/Cellulose Nanocrystal Phase Change Materials Based on Thiol-Ene Click Chemistry and Solvent Exchange. Chem. Eng. J. 2020, 396, 125206. [Google Scholar] [CrossRef]
- Cheng, M.; Hu, J.; Xia, J.; Liu, Q.; Wei, T.; Ling, Y.; Li, W.; Liu, B. One-Step in-Situ Green Synthesis of Cellulose Nanocrystal Aerogel Based Shape Stable Phase Change Material. Chem. Eng. J. 2022, 431, 133935. [Google Scholar] [CrossRef]
- Reyes, G.; Ajdary, R.; Yazdani, M.R.; Rojas, O.J. Hollow Filaments Synthesized by Dry-Jet Wet Spinning of Cellulose Nanofibrils: Structural Properties and Thermoregulation with Phase-Change Infills. ACS Appl. Polym. Mater. 2022, 4, 2908–2916. [Google Scholar] [CrossRef]
- Guo, C.; Miao, Y.; Li, L. Synthesis and Characterization of Lauric Acid/Carboxymethyl Cellulose Ester and Polylactic Acid Phase Change Material. J. Renew. Sustain. Energy 2018, 10, 64102. [Google Scholar] [CrossRef]
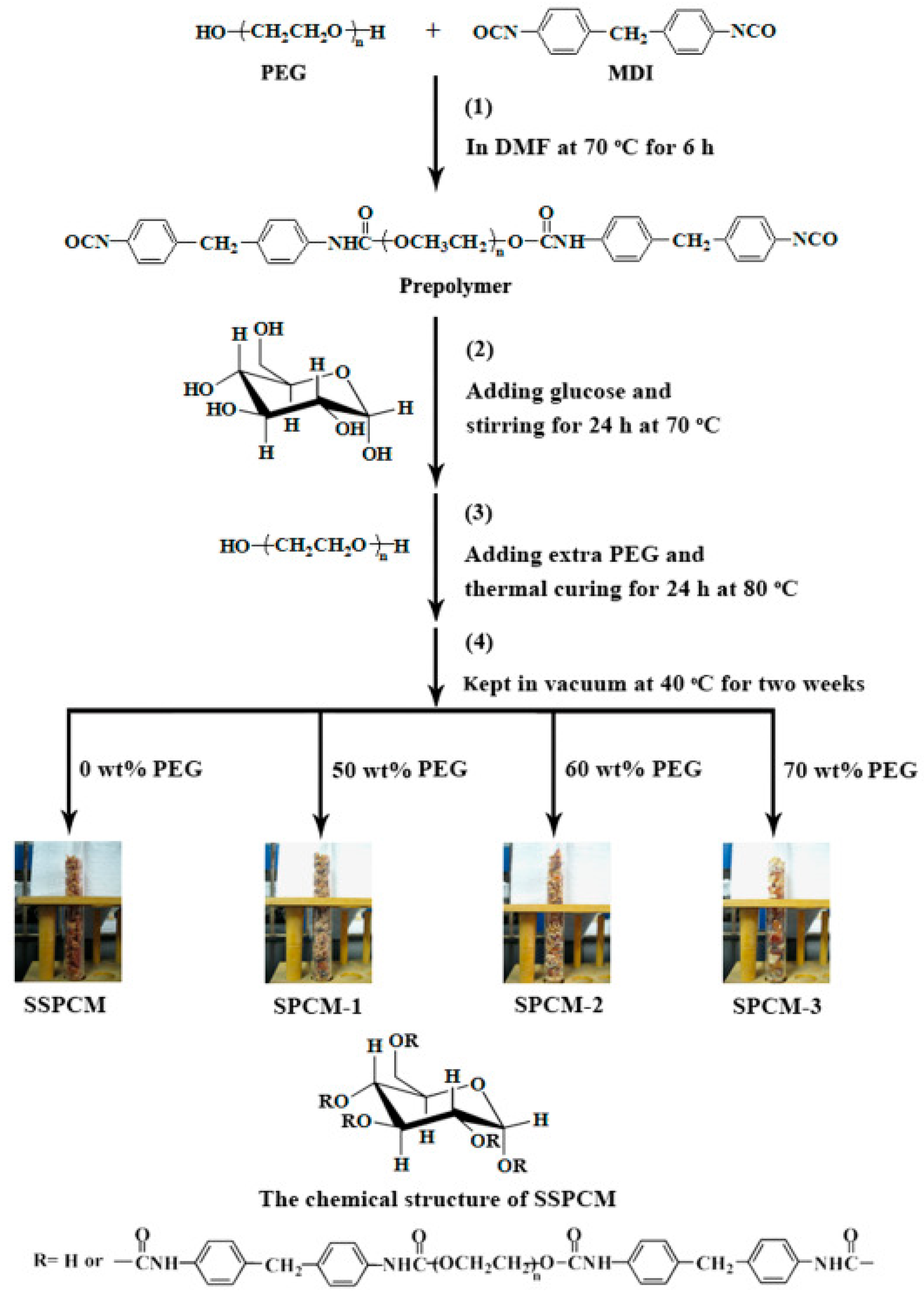
- Peng, K.; Chen, C.; Pan, W.; Liu, W.; Wang, Z.; Zhu, L. Preparation and Properties of β-Cyclodextrin/4,4′-Diphenylmethane Diisocyanate/Polyethylene Glycol (β-CD/MDI/PEG) Crosslinking Copolymers as Polymeric Solid–Solid Phase Change Materials. Sol. Energy Mater. Sol. Cells 2016, 145, 238–247. [Google Scholar] [CrossRef]
- Gao, Y.; Geng, X.; Wang, X.; Han, N.; Zhang, X.; Li, W. Synthesis and Characterization of Microencapsulated Phase Change Materials with Chitosan-Based Polyurethane Shell. Carbohydr. Polym. 2021, 273, 118629. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xue, F.; Qi, X.; Yang, J.; Zhou, Z.; Yuan, Y.; Wang, Y. Photo- and Electro-Responsive Phase Change Materials Based on Highly Anisotropic Microcrystalline Cellulose/Graphene Nanoplatelet Structure. Appl. Energy 2019, 236, 70–80. [Google Scholar] [CrossRef]
- Liu, L.; Zou, X.; Wang, Y.; Zhou, W.; Shi, J.; Ye, Y.; Zhao, Y.; Zhang, H.; Yu, Y.; Guo, J.; et al. Phase Change and Aerogel Dual Functionalized Composites Materials with Double Network Structure through One-Step Preparation of Polyacrylamide/Calcium Alginate/Polyethylene Glycol. Polymer 2021, 223, 123710. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Liu, R.; Huang, Y. Cellulose-Based Solid–Solid Phase Change Materials Synthesized in Ionic Liquid. Sol. Energy Mater. Sol. Cells 2009, 93, 1321–1328. [Google Scholar] [CrossRef]
- Fan, X.; Guan, Y.; Li, Y.; Yu, H.-Y.; Marek, J.; Wang, D.; Militky, J.; Zou, Z.-Y.; Yao, J. Shape-Stabilized Cellulose Nanocrystal-Based Phase-Change Materials for Energy Storage. ACS Appl. Nano Mater. 2020, 3, 1741–1748. [Google Scholar] [CrossRef]
- Zhou, L.; Tao, X.; Tang, L.; Yang, M.-B.; Yang, W. Waterproof Phase Change Material with a Facilely Incorporated Cellulose Nanocrystal/Poly(N-Isopropylacrylamide) Network for All-Weather Outdoor Thermal Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 53365–53375. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, Y.; Wu, F.; Jin, H.; Liu, Y.; Zheng, J. Facile In-Situ Fabrication of Latent Heat Enhanced Cellulose Aerogel-Based Form-Stable Composite Phase Change Materials Based on Dopamine Modification Strategy. Sol. Energy Mater. Sol. Cells 2021, 230, 111236. [Google Scholar] [CrossRef]
- Bao, D.; Liu, L.; Sun, T.; Han, Y.; Meng, F.; Zhao, M.; Yu, Y.; Guo, J.; Zhang, S. Solid Solid Phase Change (SSPC) Chitosan-g-MPEG Fiber with Improved Mechanical Performance via in-Situ Wet Spinning Process. Carbohydr. Polym. 2020, 240, 116313. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhao, X.; Feng, C.; Bai, L.; Yang, J.; Bao, R.; Liu, Z.; Yang, M.; Yang, W. Bacterial Cellulose/MXene Hybrid Aerogels for Photodriven Shape-Stabilized Composite Phase Change Materials. Sol. Energy Mater. Sol. Cells 2019, 203, 110174. [Google Scholar] [CrossRef]
- Moreno Balderrama, J.A.; Dourges, M.-A.; Magueresse, A.; Maheo, L.; Deleuze, H.; Glouannec, P. Emulsion-Templated Pullulan Monoliths as Phase Change Materials Encapsulating Matrices. Mater. Today Commun. 2018, 17, 466–473. [Google Scholar] [CrossRef]
- Liang, B.; Lu, X.; Li, R.; Tu, W.; Yang, Z.; Yuan, T. Solvent-Free Preparation of Bio-Based Polyethylene Glycol/Wood Flour Composites as Novel Shape-Stabilized Phase Change Materials for Solar Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110037. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, H.; Xu, L.; Xu, F. Production of Multifunctional Bamboo-Based Phase Change Encapsulating Material by Straightforward Dry Ball Milling. J. Energy Storage 2022, 46, 103630. [Google Scholar] [CrossRef]
- Gao, N.; Du, J.; Yang, W.; Li, Y.; Chen, N. Biomass-Based Shape-Stabilized Composite Phase-Change Materials with High Solar–Thermal Conversion Efficiency for Thermal Energy Storage. Polymers 2023, 15, 3747. [Google Scholar] [CrossRef]
- Hu, X.; Huang, H.; Hu, Y.; Lu, X.; Qin, Y. Novel Bio-Based Composite Phase Change Materials with Reduced Graphene Oxide-Functionalized Spent Coffee Grounds for Efficient Solar-to-Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2021, 219, 110790. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.; Wong, W.-Y.; Qu, J. A Novel Bio-Based Polyurethane/Wood Powder Composite as Shape-Stable Phase Change Material with High Relative Enthalpy Efficiency for Solar Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2019, 200, 109987. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Kang, B.; Sheng, X.; Lu, X. Novel Bio-Based Pomelo Peel Flour/Polyethylene Glycol Composite Phase Change Material for Thermal Energy Storage. Polymers 2019, 11, 2043. [Google Scholar] [CrossRef]
- Sheng, X.; Dong, D.; Lu, X.; Zhang, L.; Chen, Y. MXene-Wrapped Bio-Based Pomelo Peel Foam/Polyethylene Glycol Composite Phase Change Material with Enhanced Light-to-Thermal Conversion Efficiency, Thermal Energy Storage Capability and Thermal Conductivity. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106067. [Google Scholar] [CrossRef]
- Lee, J.J.C.; Sugiarto, S.; Ong, P.J.; Soo, X.Y.D.; Ni, X.; Luo, P.; Hnin, Y.Y.K.; See, J.S.Y.; Wei, F.; Zheng, R.; et al. Lignin-g-Polycaprolactone as a Form-Stable Phase Change Material for Thermal Energy Storage Application. J. Energy Storage 2022, 56, 106118. [Google Scholar] [CrossRef]
- Deshpande, M.; Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Microwave Assisted Preparation of Poly(Ethylene) Glycol/Lignin Blends for Thermal Energy Storage. J. Energy Storage 2021, 35, 102338. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Yang, X.-M.; López, A.M.; Wang, M.-T.; Ye, W.; Xu, B.; Wang, D.-Y. Sodium Alginate and Chitosan Aided Design of Form-Stable Polyrotaxane Based Phase Change Materials with Ultra-High Latent Heat. Int. J. Biol. Macromol. 2022, 222, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, W.; Huang, H.; Dong, D.; Zhang, X.; Zhang, L.; Chen, Y.; Sheng, X.; Lu, X. Bio-Based Radish@PDA/PEG Sandwich Composite with High Efficiency Solar Thermal Energy Storage. ACS Sustain. Chem. Eng. 2020, 8, 8448–8457. [Google Scholar] [CrossRef]
- Bao, J.; Tu, H.; Li, J.; Li, Y.; Yu, S.; Gao, J.; Lei, K.; Zhang, F.; Li, J. Applications of Phase Change Materials in Smart Drug Delivery for Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 991005. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, S.; Zhai, X.; Wu, Z.; Wang, J.; Ma, J.; Peng, X.; Peng, H. Chitosan-Based Bilayer Shell Phase Change Nano-Capsules with Excellent Anti-Permeability for Thermal Regulation Dressings. J. Energy Storage 2024, 99, 113496. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Xu, X.; Lin, X.; Zhao, Y.; Zou, L.; Wu, Y.; Zheng, H. Development of Low-Temperature Eutectic Phase Change Material with Expanded Graphite for Vaccine Cold Chain Logistics. Renew. Energy 2021, 179, 2348–2358. [Google Scholar] [CrossRef]






| Authors, Year | Focus Area of Studies | Research Gap |
|---|---|---|
| Prajapati et al., 2019 [8] | This review discussed the development and application of biodegradable polymers to stabilize PCM for TES. | A lack of systematic studies that compare different biopolymer PCMs in terms of their thermal performance, stability, mechanical integrity, and so on. There is limited exploration into the processing techniques, design, and flexibility of biopolymer PCMs. |
| Zhang et al., 2022 [9] | The study reviewed how biomass materials can be combined with conventional PCMs (like polyethylene glycols, paraffins, and fatty acids) to overcome common issues such as leakage during phase transition. | Although some additional functions, such as photothermal conversion, thermochromism, and magnetothermal conversion, were briefly discussed, there is a lack of studies on the flexibility of PCM based on biomass materials. There is limited work on integrating these additional features with biopolymer PCMs for TES. More studies are needed to improve encapsulation techniques for PCMs based on biomass materials. |
| Baylis and Cruickshank, 2023 [10] | The article discussed the biopolymer PCMs for passive TES in building applications. Some integration methods and performances were discussed. | There is a lack of studies in the experimental validation to assess the long-term stability, performance, and fire safety. The flexibility of biopolymer PCMs is not comprehensively discussed. |
| Dutta et al., 2023 [11] | The study briefly discussed developing and evaluating biopolymer PCMs for TES applications. A few techniques and composite designs were briefly reviewed. | There is a lack of comprehensive studies on biopolymer PCMs and their flexibility. The optimization of, and strategies to improve, biopolymer PCMs are not comprehensively discussed. |
| Pielichowska et al., 2024 [12] | The review focused on reviewing the state of the-art in applying biopolymers to develop more sustainable PCMs for TES. | Although many encapsulation and stabilization techniques have been developed at the laboratory level, methods to scale up these processes for industrial production and practical applications are still underexplored. There is also a lack of systematic studies on the flexibility offered by biopolymer PCMs. The compatibility between biopolymers and various additives is still underexplored. |
| Liu et al., 2025 [7] | The study integrates sustainable polymer with PCMs, focusing on preparation methods, encapsulation strategies, performance enhancement, advanced applications, and recyclable polymers. | There is a lack of information related to a comprehensive evaluation of how sustainable polymers can be optimally integrated with PCMs as TES. There is a lack of systematic studies on the flexibility offered by biopolymer PCMs. |
| PEG Molecular Weight | Tm [°C] | hm [J/g] | k [W/m·K] |
|---|---|---|---|
| PEG 1000 | 34.75 | 154.40 | 0.29 |
| PEG 1500 | 47.23 | 161.43 | 0.31 |
| PEG 2000 | 50.77 | 165.43 | 0.31 |
| PEG 4000 | 55.95 | 173.62 | 0.33 |
| PEG 6000 | 59.54 | 179.70 | 0.34 |
| PEG 8000 | 59.74 | 177.53 | 0.33 |
| PEG 10,000 | 58.01 | 182.86 | 0.33 |
| PEG 12,000 | 60.93 | 173.40 | 0.32 |
| PEG 20,000 | 62.27 | 168.50 | 0.32 |
| No | Biopolymer PCM | Ratio | Type of Polymer | hm [J/g] | Tm [°C] | k [W/m·K] | ρ [kg/m3] | Mechanical Properties | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Coconut oil/(activated carbon, another supporting matrix) | 31.34/68.66 wt% | lipid | 42.92 | 24.02 | n.a. | n.a. | Ec = 7.2 MPa and σc = 0.19 MPa | [95] |
| 2 | CA/SA | 92.89/7.11 wt% | lipid | 170.2 | 26.30 | n.a. | n.a. | n.a. | [96] |
| 3 | 1-Dodecanol/PLIM/succinylated birch wood | 52/23/24 wt% | lignocellulosic | 89.4 | 24.3 | n.a. | 1001 | σm = 86 MPa, σb = 39.4 MPa, and Ec = 9600 MPa | [97] |
| 4 | C18/UCNC | n.a. | polysaccharide | 40.8 | 60 | n.a. | n.a. | σf = 9.71 MPa | [98] |
| 5 | (LA+SA)/CMC | 70.4/29.6 wt% | lipid-polysaccharide | 114.6 | 32.2 | n.a. | n.a. | n.a. | [99] |
| 6 | Coconut oil/Cellulose | 76/24 wt% | lipid-polysaccharide | 134.9 | 22 | n.a. | n.a. | n.a. | [100] |
| 7 | Coconut oil/CA | 81.1/18.9 wt% | lipid-polysaccharide | 84.7 | 24.7 | n.a. | n.a. | n.a. | [101] |
| 8 | Coconut oil/CA | 75/25 wt% | lipid-polysaccharide | 81.8 | ~25 | n.a. | n.a. | n.a. | [102] |
| 9 | Myristic Acid/Methyl Cellulose | 50/50 wt% | lipid-polysaccharide | 71.3 | 53.2 | n.a. | n.a. | n.a. | [103] |
| 10 | Myristic Acid/Ethyl Cellulose | 66.7/33.3 wt% | lipid-polysaccharide | 122.61 | 55.3 | n.a. | n.a. | n.a. | [104] |
| 11 | Coconut oil/CNC | n.a. | lipid-polysaccharide | n.a. | 23.4 | n.a. | n.a. | n.a. | [105] |
| 12 | Methyl laurate/(CNC and other components) | 60/40 wt% | lipid-polysaccharide | 113.18 | 6.12 | n.a. | n.a. | E = 0.4–0.8 MPa | [106] |
| 13 | Caprylic Acid/(Gelatin+Chitosan) | 49.98/50.02 wt% | protein-polysaccharide | 79.18 | 11.53 | n.a. | n.a. | n.a. | [107] |
| 14 | Gelatin/Gum Arabic | 50/50 wt% | protein-polysaccharide | 86.4 | 31.74 | n.a. | n.a. | n.a. | [108] |
| 15 | Decanoic acid/(Flax fiber+SA+Polypyrrole) | 59.75/40.25 wt% | lipid-polysaccharide-lignocellulosic | 100.98 | 32.85 | 0.31 | 71.3 | σc30% strain = ~0.23 MPa and σc70% strain = ~1 MPa | [109] |
| 16 | Palmatic acid/(Flax fiber+SA+Polypyrrole) | 73.84/26.16 wt% | lipid-polysaccharide-lignocellulosic | 154.52 | 63.33 | 0.28 | 98.02 | n.a. | [109] |
| No | Biopolymer PCM | Ratio | Type of Polymer | hm [J/g] | Tm [°C] | k [W/m·K] | ρ [kg/m3] | Mechanical Properties | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PLR/Pentaerythritol Phosphate | 90/10 wt% | supramolecular | 81.86 | ~45.9 | 0.36 | n.a. | E = 1193 MPa and σt = 31.5 MPa | [110] |
| 2 | PEO/PLR | 70/30 wt% | supramolecular | 88.57 | 60.12 | n.a. | 1420 | E = 826.7 MPa and σt = 13.9 MPa | [86] |
| 3 | PEG/low-density PE/eggshell powder/graphite | 70/20/5/5 wt% | polyether | 120.1 | 62.5 | n.a. | n.a. | n.a. | [111] |
| 4 | Oligo(butylene succinate) | n.a. | aliphatic polyester | n.a. | 48 | n.a. | n.a. | n.a. | [112] |
| 5 | PLA/PCL | 60/40 wt% | aliphatic polyester | 32.53 and 17.23 | 176.42 and 56.13 | n.a. | n.a. | σt = ~66 MPa | [113] |
| 6 | PBHA/C5/PBT | 33/67 wt% | aliphatic polyester | 43.9 | 35.7 | n.a. | n.a. | σmax27% = ~2.07 cN/dtex, σmax30% = ~1.78 cN/dtex, and σmax33% = ~1.62 cN/dtex | [114] |
| 7 | Comb-like | n.a. | branched | 108.5 | 70.3 | n.a. | n.a. | n.a. | [115] |
| No | Biopolymer PCM | Ratio | Type of Polymer | hm [J/g] | Tm [°C] | k [W/m·K] | ρ [kg/m3] | Mechanical Properties | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PEG/PLR/BN | 66/22/12 wt% | supramolecular, polyether | 91.16 | 35.98 and 56.79 | 2.72 | n.a. | n.a. | [81] |
| 2 | PEO/Trimethylolpropane triacrylate | 75/25 wt% | polyether-thermosetting | 112.2 | 58.8 | n.a. | n.a. | σt = 0.74 MPa | [116] |
| 3 | PEG/supporting matrix | n.a. | polyether-thermosetting | 146.6 | 56.15 | n.a. | n.a. | n.a. | [117] |
| 4 | PEG/PU | 86/14 wt% | polyether-thermosetting | 126.5 | 52.54 | n.a. | n.a. | n.a. | [118] |
| 5 | PLA/PEG/g-C3N4 | 30/60/10 wt% | aliphatic polyester-polyether | 106.1 | 64.54 and 174.15 | 0.32 | n.a. | E = 3044 MPa and hardness at max load = 96 MPa | [119] |
| 6 | PLA/PEG | 50/50 wt% | aliphatic polyester-polyether | 100.3 | 63.2 | n.a. | n.a. | n.a. | [120] |
| 7 | PLA/PEG | 51/49 wt% | aliphatic polyester-polyether | 58.79 | 60.95 | ~0.026 | n.a. | E = 502 MPa and σy = 12 MPa | [121] |
| 8 | PEG/Poly(Glycerol-Itaconic acid) | 72.67/27.33 wt% | aliphatic polyester-polyether | 86.93 | 41.92 | n.a. | n.a. | n.a. | [122] |
| 9 | PLA/mPEG | 50/50 wt% | aliphatic polyester-polyether | 55.8 | 50.5 | n.a. | n.a. | n.a. | [123] |
| 10 | PLA/HDPE | 50/50 wt% | aliphatic polyester-polyolefin | 100.1 | 136.6 | n.a. | n.a. | n.a. | [124] |
| 11 | PLA/HDPE | 60/40 wt% | aliphatic polyester-polyolefin | PLA: 24.5 HDPE: 83.2 | PLA: 168.5 HDPE: 132.6 | n.a. | n.a. | σt = 36.9 MPa and a = ~4.4 kJ/m2 | [125] |
| No | Biopolymer PCM | Ratio | Type of Polymer | hm [J/g] | Tm [°C] | k [W/m·K] | ρ [kg/m3] | Mechanical Properties | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PU/leather waste/glycidyl methacrylate/AIBN | 60/10/30/0.001 wt% | protein-thermosetting | 36.73 | 46.9 | n.a. | n.a. | n.a. | [126] |
| 2 | PEG/(PEG+4,4’-diphenylmethane diisocyanate/glucose) | 70/30 wt% | polyether-glucose | 131.9 | 61.11 | n.a. | n.a. | n.a. | [127] |
| 3 | PEG/4,4’-diphenylmethane diisocyanate/Xylitol | n.a. | polyether-xylitol | 76.37 | 41.65 | n.a. | n.a. | n.a. | [128] |
| 4 | PEG/(Castor oil+HDI) | 85.43/14.57 wt% | polyether-thermosetting-lipid hybrid | 117.7 | 51.4 | n.a. | n.a. | n.a. | [129] |
| 5 | Waste cooking fat/PP | 75/25 wt% | lipid-synthetic polymer | 20.29 | 32 | 0.213 | n.a. | σt = 3 MPa | [130] |
| 6 | FHS/D4 | 66.67/33.33 mol% | lipid-siloxane | 202.3 | 151.75 | n.a. | n.a. | n.a. | [131] |
| 7 | Organosolv Lignin/Butylene 3Oxide/Co-polymerized Tetrahydrofuran | 15.7/5.1/79.2 wt% | synthetic polymer-polyphenolic | 53.7 | 19.9 | n.a. | n.a. | n.a. | [132] |
| 8 | (1-Tetradecanol/(PMMA + PETRA))/Lignin nanoparticles | (66.7/33.3)/3% wt% | synthetic polymer-polyphenolic | 190 | 43.84 | n.a. | n.a. | n.a. | [133] |
| 9 | PEG/Poly(glycidyl methacrylate)/leather waste/AIBN | 70/20/10/1.5 wt% | protein-polyether | 152.6 | 56.3 | n.a. | n.a. | σt = 3.08 MPa | [134] |
| 10 | PA/PLA | ~40/~60 wt% | lipid-aliphatic polyester | 70.1 | 62.1 | n.a. | n.a. | n.a. | [135] |
| 11 | (ASO+fatty acids)/Darocure 1173 | (67 + 33 wt%)/3 wt% | lipid-acrylate | 67.51 | 73.07 | n.a. | n.a. | n.a. | [136] |
| 12 | PA/COPUA | 70/30 wt% | lipid-thermosetting | 141.2 | 66.6 | n.a. | n.a. | n.a. | [137] |
| 13 | Beeswax/DGEM-18/FA/MA crosslinked polymer network | 52.4/47.6 wt% | lipid-thermosetting | 119.1 | 48.3 | n.a. | n.a. | σt = ~2 MPa | [138] |
| 14 | PEG/CNF/EG/BN | 63/15/7/15 wt% | polysaccharide-polyether | 79.46 | 59.51 | 10.83 | n.a. | n.a. | [139] |
| 15 | MA/PU | 30/70 wt% | polysaccharide-polyether | 136.9 | 64.6 | 2.78 | n.a. | n.a. | [140] |
| 16 | PEG/BNNSs-g | n.a. | polysaccharide-polyether | 150.1 | 45.2 | 0.59 | n.a. | n.a. | [141] |
| 17 | PEG/SA | 93/7 wt% | polysaccharide-polyether | 156.8 | 59 | n.a. | n.a. | n.a. | [142] |
| 18 | PEG/(SA+PDA@ZrP) | 92.75/7.25 wt% | polysaccharide-polyether | 159.8 | n.a. | n.a. | n.a. | n.a. | [143] |
| 19 | PEG/sweet potato foam/CsxWO3 | 70.1/29/0.99 wt% | polysaccharide-polyether | 137.7 | 61.9 | n.a. | n.a. | n.a. | [144] |
| 20 | PEG/Gum Tragacanth/biochar | 63/27/10 wt% | polysaccharide-polyether | 110.5 | 58.1 | 0.038 | 126 | σc = 0.702 MPa | [145] |
| 21 | PEG/Cellulose Acetate | 96.5/3.5 wt% | polysaccharide-polyether | 155.35 | 60.56 | n.a. | n.a. | n.a. | [82] |
| 22 | PEG/TOCNF | 75/25 wt% | polysaccharide-polyether | 137.2 | 58.8 | n.a. | n.a. | E = 3000 MPa and σm = 39 MPa | [150] |
| 23 | PEG/cellulose | 90.1/9.9 wt% | polysaccharide-polyether | 151.8-170.5 | 58.9-59 | 00.21 | n.a. | n.a. | [156] |
| 24 | PEG/CNC | 97/3 wt% | polysaccharide-polyether | 151.8 | 33.5 | 0.44 | n.a. | n.a. | [148] |
| 25 | PEG/CNF | 85/15 wt% | polysaccharide-polyether | 146.2 | 65.4 | 0.040 | n.a. | σt = 28 MPa | [146] |
| 26 | PEG/CNC | 86/14 wt% | polysaccharide-polyether | 140.3 | 30.6 | 0.42 | 35 | σc80% strain = 0.1145 MPa | [149] |
| 27 | PEG/Polypyrrole-coated CNF | 94/6 wt% | polysaccharide-polyether | 169.7 | 57.4 | n.a. | n.a. | σc = 4.5 MPa | [147] |
| 28 | PEG/CA | n.a. | polysaccharide-polyether | 91.5 | 37.9 | 0.068 | 200 | n.a. | [155] |
| 30 | PEG/CNC | n.a. | polysaccharide-polyether | 82.3 | 47.1 | n.a. | n.a. | n.a. | [157] |
| 31 | PEG/(CNC+PNIPAM) | 92/8 wt% | polysaccharide-polyether | 178.4 | 54.2 | n.a. | n.a. | σc = 0.0362 MPa and Ec = 0.1829 MPa | [158] |
| 32 | PEG/Cellulose/Dopmine | 90/9.4/0.6 wt% | polysaccharide-polyether | 194.3 | 59.3 | n.a. | n.a. | n.a. | [159] |
| 33 | mPEG+CHO/Chitosan | 75/25 wt% | polysaccharide-polyether | 49.03 | 46.42 | n.a. | n.a. | σt = 1.36 cN/dtex | [160] |
| 34 | PEG/(Bacterial cellulose+Mxene) | 97.9/2.1 wt% | polysaccharide-polyether | 196.7 | 67 | n.a. | n.a. | n.a. | [161] |
| 35 | PEG/Microcystalline Cellulose/Graphene Nanoplatelets | 97.47/1.02/1.51 wt% | polyether-polysaccharide | 182.6 | ~67.6 | 1.03 | n.a. | n.a. | [154] |
| 36 | PEG/4,4′-Diphenylmethane diisocyanate/β-CD | 28/56/16 mol% | cyclic oligosaccharide-polyether | 115.2 | 60.2 | n.a. | n.a. | n.a. | [152] |
| 37 | Butyl stearate/hexamethylene diisocyanate/Chitosan | 66.67/30.67/2.66 wt% | polysaccharide-thermosetting | 104.1 | ~24 | n.a. | n.a. | n.a. | [153] |
| 38 | (LA+CMC)/PLA | 85/15 wt% | polysaccharide-aliphatic polyester | 86.4 | 40.1 | n.a. | n.a. | σt = 27.61 MPa and σflex = 50.02 MPa | [151] |
| 39 | Butyl Stearate/crosslinked pullulan matrix | n.a. | aliphatic ester-polysaccharide | 33 | 26.2 | N/A | 880 | E = 15 MPa | [162] |
| 40 | Wood flour/PEG | 25/75 wt% | lignocellulosic-polyether | 108.6 | 47.7 | n.a. | n.a. | n.a. | [163] |
| 41 | Pomelo Peel Foam/PEG | 3.8/96.2 wt% | lignocellulosic-polyether | 158.1 | 62.7 | 0.35 | n.a. | n.a. | [169] |
| 42 | PEG/Wood powder | 97/3 wt% | lignocellulosic-polyether | 134.2 | 63.5 | n.a. | n.a. | n.a. | [167] |
| 43 | PCC/PEG/RPUF | 12.5/12.5/75 wt% | lignocellulosic-polyether | 51.99 | n.a. | 0.0542 | n.a. | σc = 1.154 MPa | [165] |
| 44 | SCGs/PEG | 39.7/60.3 wt% | lignocellulosic-polyether | 104.7 | 63 | 0.336 | n.a. | n.a. | [166] |
| 45 | Bamboo flour/PEG | 30/70 wt% | lignocellulosic-polyether | 113 | 64.19 | 0.5 | n.a. | n.a. | [164] |
| 46 | Pomelo Peel Flour/PEG | 10.1/89.9 wt% | lignocellulosic-polyether | 143.2 | 66.4 | n.a. | n.a. | n.a. | [168] |
| 47 | Lignin/PCL | 12.6/87.4 wt% | polyphenolic-aliphatic polyester | 61.16 | 51.33 | n.a. | n.a. | n.a. | [170] |
| 48 | Lignin/PEG | 30/70 wt% | polyphenolic-polyether | 100.91 | 60.33 | 0.31 | n.a. | n.a. | [171] |
| 49 | PEG/(SAT+PLR) | 95.22/4.78 wt% | polysaccharide-supramolecular-polyether | 178.4 | n.a. | n.a. | 1120 | n.a. | [172] |
| 50 | PEG/(Radish+PDA) | 95/5 wt% | polysaccharide-eumelanin-polyether | 161.52 | 64.77 | n.a. | n.a. | n.a. | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijanarko, N.P.; Daniarta, S.; Kolasiński, P. A Systematic Review of Biopolymer Phase Change Materials for Thermal Energy Storage: Challenges, Opportunities, and Future Direction. Energies 2025, 18, 4262. https://doi.org/10.3390/en18164262
Wijanarko NP, Daniarta S, Kolasiński P. A Systematic Review of Biopolymer Phase Change Materials for Thermal Energy Storage: Challenges, Opportunities, and Future Direction. Energies. 2025; 18(16):4262. https://doi.org/10.3390/en18164262
Chicago/Turabian StyleWijanarko, Nadia Parwaty, Sindu Daniarta, and Piotr Kolasiński. 2025. "A Systematic Review of Biopolymer Phase Change Materials for Thermal Energy Storage: Challenges, Opportunities, and Future Direction" Energies 18, no. 16: 4262. https://doi.org/10.3390/en18164262
APA StyleWijanarko, N. P., Daniarta, S., & Kolasiński, P. (2025). A Systematic Review of Biopolymer Phase Change Materials for Thermal Energy Storage: Challenges, Opportunities, and Future Direction. Energies, 18(16), 4262. https://doi.org/10.3390/en18164262








