Effect of Inlet Pressure on the Biodegradability Index of Cavitated Herbal Waste
Abstract
1. Introduction
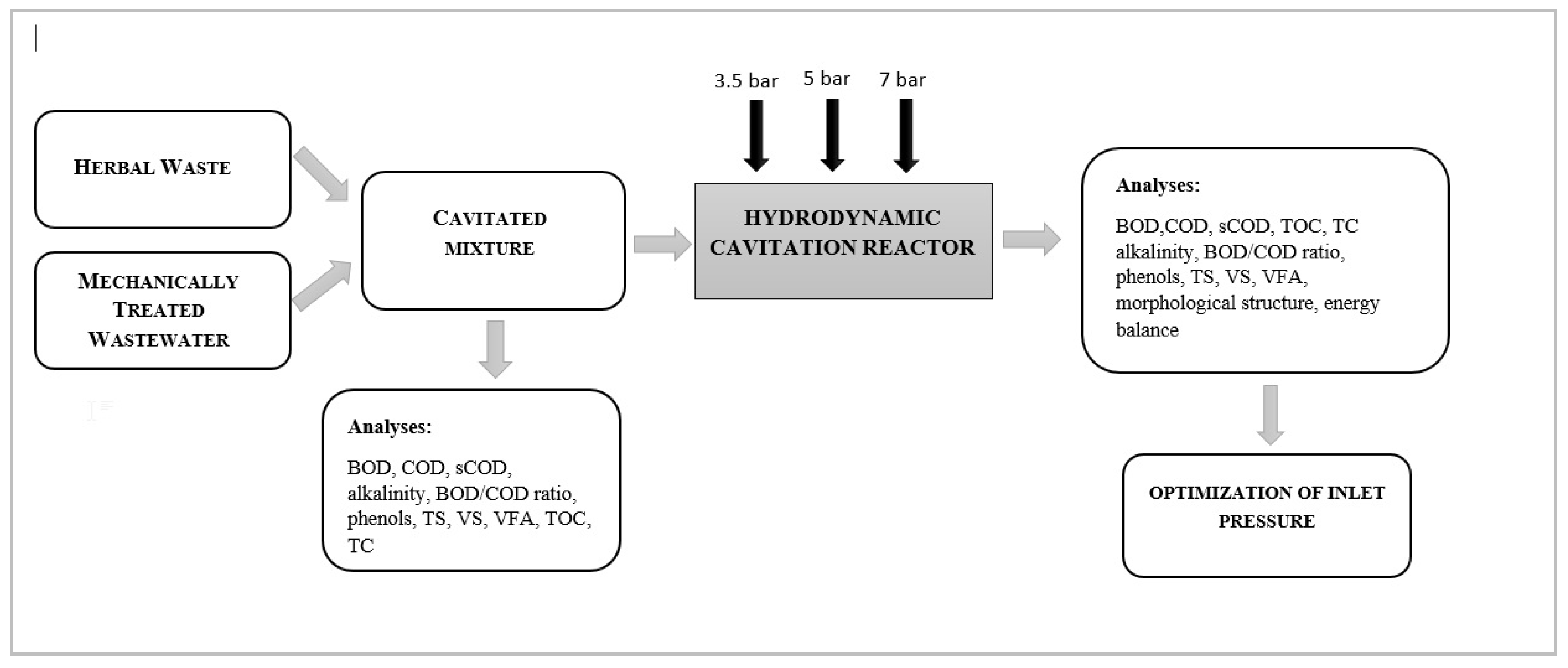
2. Materials and Methods
2.1. Materials
2.2. Operational Set-Up and Laboratory Installation
2.3. Analytical Methods
2.3.1. The Physicochemical Analyses
2.3.2. SEM Analysis
3. Results and Discussion
3.1. Effect of HC on the Characetristics of the Cavitated Mixture
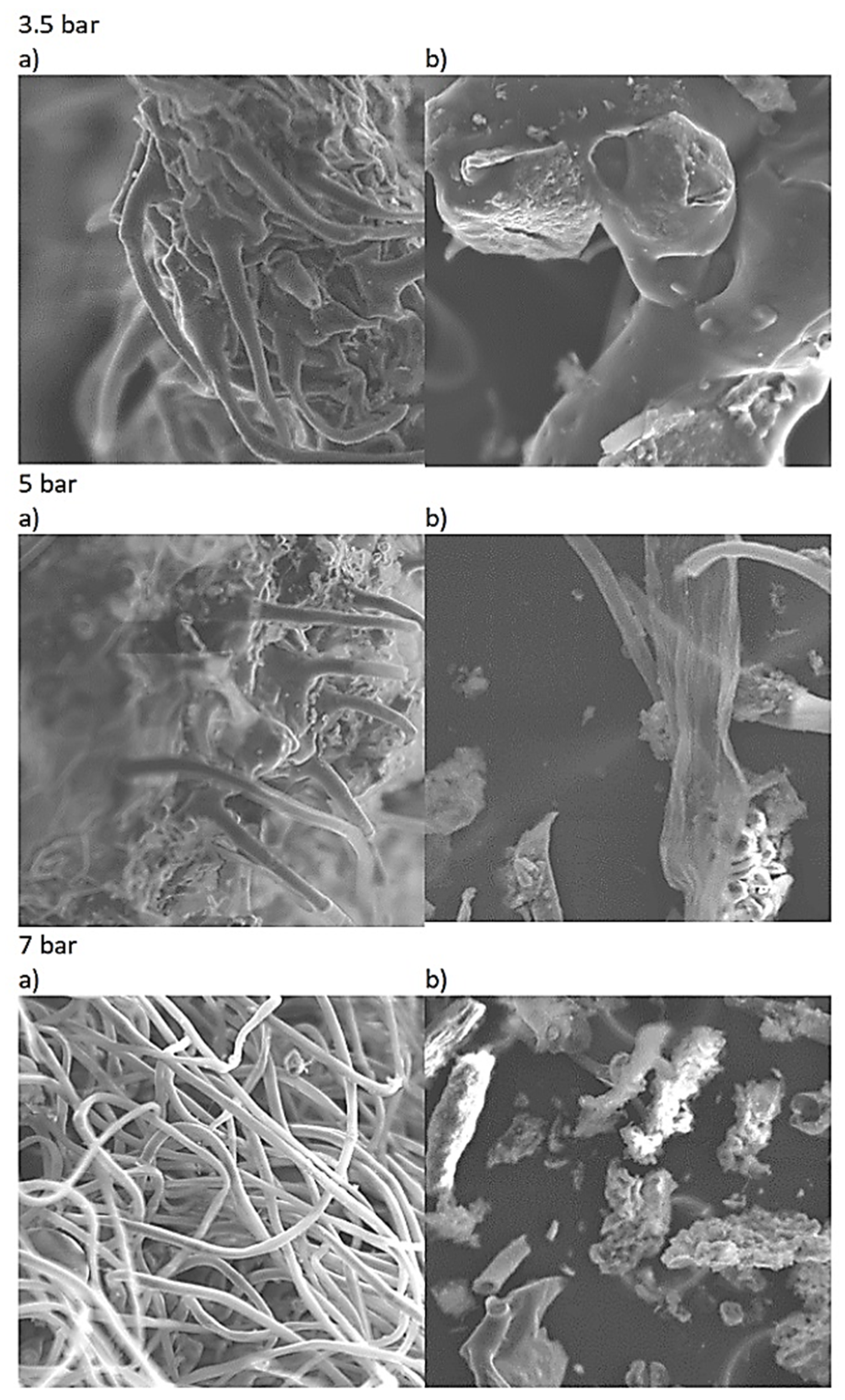
3.2. SEM Analysis—Morpological Structure
3.3. Energy Balance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-Tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.C.; et al. Lignocellulosic biorefineries: The current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.I. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dikshit, P.K.; Sherpa, K.C.; Singh, A.; Jacob, S.; Chandra Rajak, R. Recent nanobiotechnological advancements in lignocellulosic biomass valorization: A review. J. Environ. Manag. 2021, 297, 113422. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowski, A.L.; Dalmas Neto, C.J.; Porto de Souza, L.; Carvalho Neto, V.D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, R.; Sarangi, P.K.; Kovalev, A.A.; Vivekanand, V. Effect of physical and thermal pretreatment of lignocellulosic biomass on biohydrogen production by thermochemical route: A critical review. Bioresour. Technol. 2023, 369, 128458. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvan, R.; Selwynraj, A.I. Enhancing biogas generation from lignocellulosic biomass through biological pretreatment: Exploring the role of ruminant microbes and anaerobic fungi. Anaerobe 2024, 85, 102815. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Bharadwaj, A.V.S.L.S.; Dev, S.; Zhuang, J.; Wang, Y.; Yoo, C.G.; Jeon, B.-H.; Aggarwal, S.; Park, S.H.; Kim, T.H. Review of chemical pretreatment of lignocellulosic biomass using low-liquid and low-chemical catalysts for effective bioconversion. Bioresour. Technol. 2003, 368, 128339. [Google Scholar] [CrossRef] [PubMed]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass—A review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, B.N.; Sudakova, I.G.; Chudina, A.I.; Garyntseva, N.V.; Kazachenko, A.S.; Skripnikov, A.M.; Yuriy Malyar, Y.N.; Ivanov, I.P. Fractionation of birch wood biomass into valuable chemicals by the extraction and catalytic processes. Biomass Convers. Biorefin. 2024, 14, 2341–2355. [Google Scholar] [CrossRef]
- M’Arimi, M.M.; Mecha, C.A.; Kiprop, A.K.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: Review. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Prado, C.A.; Antunes, F.A.F.; Rocha, T.M.; Sánchez-Muñoz, S.; Barbosa, F.G.; Terán-Hilares, R.; Cruz-Santos, M.M.; Arruda, G.L.; da Silva, S.S.; Santos, J.C. A review on recent developments in hydrodynamic cavitation and advanced oxidative processes for pretreatment of lignocellulosic materials. Bioresour. Technol. 2022, 345, 126458. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, R.; Damizia, M.; De Filippis, P.; Patrizi, D.; Verdone, N.; Vilardi, G.; de Caprariis, B. Recent developments and future outlooks of hydrodynamic cavitation as an intensification technology for renewable biofuels production. J. Environ. Chem. Eng. 2023, 11, 110819. [Google Scholar] [CrossRef]
- Verdini, F.; Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Cravotto, G. Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Appl. Sci. 2021, 11, 4693. [Google Scholar] [CrossRef]
- Madison, M.J.; Coward-Kelly, G.; Liang, C.; Karim, M.N.; Falls, M.; Holtzapple, M.T. Mechanical pretreatment of biomass—Part I: Acoustic and hydrodynamic cavitation. Biomass Bioenergy 2017, 98, 135–141. [Google Scholar] [CrossRef]
- Song, X.; Hou, R.; Zhang, W.; Liu, J. Hydrodynamic cavitation as an efficient water treatment method for various sewage: A review. Water Sci. Technol. 2022, 86, 302–320. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Sánchez Vera, F.P.; Colina Andrade, G.J.; Tejada Meza, K.; García, J.C.; Pacheco Tanaka, D.A. Continuous Cultivation of Microalgae in Cattle Slaughterhouse Wastewater Treated with Hydrodynamic Cavitation. Water 2022, 14, 1288. [Google Scholar] [CrossRef]
- Patil, P.B.; Gogate, P.R.; Csoka, L.; Dregelyi-Kiss, A.; Horvath, M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason. Sonochem. 2016, 30, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Microalgal cell disruption by hydrodynamic cavitation for the production of biofuels. J. Appl. Phycol. 2015, 27, 1881–1889. [Google Scholar] [CrossRef]
- Mevada, J.; Devi, S.; Pandit, A. Large scale microbial cell disruption using hydrodynamic cavitation: Energy saving options. Biochem. Eng. J. 2019, 143, 151–160. [Google Scholar] [CrossRef]
- Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M.; Szaja, A.; Szymańska-Chargot, M. Hydrodynamic cavitation of brewery spent grain diluted by wastewater. Chem. Eng. J. 2017, 313, 946–956. [Google Scholar] [CrossRef]
- Gągol, M.; Cako, E.; Fedorov, K.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Hydrodynamic cavitation based advanced oxidation processes: Studies on specific effects of inorganic acids on the degradation effectiveness of organic pollutants. J. Mol. Liq. 2020, 307, 113002. [Google Scholar] [CrossRef]
- Mohod, A.V.; Silva Costa Teixeira, A.C.; Bagal, M.V.; Gogate, P.R.; Giudici, R. Degradation of organic pollutants from wastewater using hydrodynamic cavitation: A review. J. Environ. Chem. Eng. 2023, 11, 109773. [Google Scholar] [CrossRef]
- Kunz, P.; Wagner, S. Results and outlooks of investigations of sewage sludge disintegration. In Ergebnisse und Perspektive aus Untersuchungen zur Klärschlammdesintegration; AWT Abwassertechnik: Lauchhammer, Germany, 1994. [Google Scholar]
- Müller, J. Mechanical Disintegration of Sewage Sludge Mechanischer Klärschlammaufschluß-, Schriftenereihe “Berichte aus der Verfahrenstechnik” der Fakultät für Maschinenbau und Elektrotechnik der Universität Braunschweig; Shaker Verlag: Duren, Germany, 1996. [Google Scholar]
- Langone, M.; Ferrentino, R.; Trombino, G.; De Puiseau, D.W.; Andreottola, G.; Rada, E.C.; Ragazzi, M. Application of novel hydrodynamic cavitation system I wastewater treatment plants. UPB Sci. Bull. Ser. D 2015, 77, 225–234. [Google Scholar]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Nowicka, A.; Dudek, M. Application of Hydrodynamic Cavitation in the Disintegration of Aerobic Granular Sludge—Evaluation of Pretreatment Time on Biomass Properties, Anaerobic Digestion Efficiency and Energy Balance. Energies 2024, 17, 335. [Google Scholar] [CrossRef]
- Gutiérrez-Mosquera, L.F.; Arias-Giraldo, S.; Zuluaga-Meza, A. Landfill leachate treatment using hydrodynamic cavitation: Exploratory evaluation. Heliyon 2022, 8, e09019. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.P.; Gogate, P.R. Cavitation-based pre-treatment of wastewater and waste sludge for improvement in the performance of biological processes: A review. J. Environ. Chem. Eng. 2021, 9, 104743. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Nowicka, A.; Dudek, M.; Zieliński, M. The Use of Hydrodynamic Cavitation to Improve the Anaerobic Digestion of Waste from Dairy Cattle Farming—From Laboratory Tests to Large-Scale Agricultural Biogas Plants. Energies 2024, 17, 1409. [Google Scholar] [CrossRef]
- Lebiocka, M.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Szaja, A. Pretreatment of herbal waste using sonication. Bioresour. Technol. 2023, 377, 128932. [Google Scholar] [CrossRef] [PubMed]
- Padoley, K.V.; Saharan, V.K.; Mudliar, S.N.; Pandey, S.N.; Pandit, A.B. Cavitationally induced biodegradability enhancement of a distillery wastewater. J. Hazard. Mater. 2012, 219–220, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bis, M.; Montusiewicz, A.; Ozonek, J.; Pasieczna-Patkowska, S. Application of hydrodynamic cavitation to improve the biodegradability of mature landfill leachate. Ultrason. Sonochem. 2015, 26, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Dhanke, P.; Wagh, S.; Patil, A. Treatment of fish processing industry wastewater using hydrodynamic cavitational reactor with biodegradability improvement. Water Sci. Technol. 2019, 80, 2310–2319. [Google Scholar] [CrossRef]
- Thangavelu, K.; Desikan, R.; Taran, O.P.; Uthandi, S. Delignification of corncob via combined hydrodynamic cavitation and enzymatic pretreatment: Process optimization by response surface methodology. Biotechnol. Biofuels 2018, 11, 203. [Google Scholar] [CrossRef]
- Terán Hilares, R.; de Almeida, G.F.; Ahmed, M.A.; Antunes, F.A.F.; da Sliva, S.S.; Han, J.-I.; Santos, J.C.D. Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass: A parametric study. Bioresour. Technol. 2017, 235, 301–308. [Google Scholar] [CrossRef]
- Grimaldi, M.P.; Marques, M.P.; Laluce, C.; Cilli, E.M.; Sponchiado, S.R.P. Evaluation of lime and hydrothermal pretreatments for efcient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol. Biofuels 2015, 8, 205. [Google Scholar] [CrossRef]
| Parameter | Unit | MTW | HW | Raw Mixture of HW and MTW | ||
|---|---|---|---|---|---|---|
| 3.5 Bar | 5 Bar | 7 Bar | ||||
| Biochemical oxygen demand (BOD5) | mg L−1 | 275 ± 23.6 | 1204 ± 194.4 | 1380 ± 245.2 | 1269 ± 210.2 | 1280 ± 171.3 |
| Chemical oxygen demand (COD) | mg L−1 | 491 ± 35.1 | 6395 ± 249.4 | 3837 ± 215.1 | 4951 ± 298.1 | 5800 ± 205.2 |
| Soluble chemical oxygen demand (sCOD) | mg L−1 | 415 ± 38.6 | 2720 ± 241.8 | 2476 ± 212.2 | 2505 ± 199.6 | |
| Volatile fatty acids (VFAs) | mg L−1 | 129 ± 42.1 | 1133 ± 50.1 | 937 ± 32.2 | 890 ± 30.5 | |
| Phenols | mg L−1 | 2.76 ± 0.38 | 40.8 ± 1.57 | 37.5 ± 1.45 | 35.5 ± 1.38 | |
| Alkalinity | mgCaCO3 L−1 | 462.1 ± 51.4 | 452 ± 19.9 | 502 ± 25.6 | 601 ± 30.1 | |
| pH | - | 6.68 ± 0.12 | 6.16 ± 0.07 | 6.42 ± 0.11 | 6.57 ± 0.09 | |
| Total solids (TSs) | g kg−1 | 0.97 ± 0.03 | 962 ± 108 | 6.36 ± 0.11 | 8.62 ± 0.12 | 9.48 ± 0.12 |
| Volatile solids (VSs) | g kg−1 | 0.32 ± 0.03 | 818 ± 102 | 4.89 ± 0.09 | 6.43 ± 0.09 | 7.67 ± 0.09 |
| Total carbon (TC) | mg L−1 | 241 ± 27.7 | 903.2 ± 20.8 | 949 ± 30.1 | 835 ± 33.7 | |
| Total organic carbon (TOC) | mg L−1 | 60 ± 5.5 | 746 ± 25.5 | 824 ± 31.8 | 720 ± 21.1 | |
| Acid detergent lignin (ADL) | %TSs | 10.3 ± 0.34 | ||||
| Cellulose | %TSs | 16.2 ± 0.41 | ||||
| Hemicellulose | %TSs | 5.53 ± 0.17 | ||||
| Monosaccharides | %TSs | 11.3 ± 0.29 | ||||
| Parameter | Unit | Inlet Pressure [Bar] | ||
| 3.5 | 5.0 | 7.0 | ||
| p2 | Pa | 96,286 | 95,132 | 95,879 |
| pv | Pa | 2063 | 2063 | 2063 |
| v0 | ms−1 | 41.59 | 54.75 | 77.38 |
| cv | -- | 0.11 | 0.06 | 0.03 |
| Time | Unit | Passes through the Cavitation Zone | ||
| 2 | min | 1.18 | 1.55 | 2.19 |
| 5 | min | 2.94 | 3.87 | 5.47 |
| 10 | min | 5.88 | 7.74 | 10.94 |
| 30 | min | 17.64 | 23.22 | 32.82 |
| 45 | min | 26.46 | 34.83 | 49.23 |
| 60 | min | 35.28 | 46.44 | 65.64 |
| Time | COD | sCOD | BOD5 | BI | TSs | VSs | TC | TOC | pH | VFAs | Alkalinity | Phenols |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| min | mg L−1 | mg L−1 | mg L−1 | - | g kg−1 | g kg−1 | mg L−1 | mg L−1 | - | mg L−1 | mg L−1 | mg L−1 |
| 3.5 bar | ||||||||||||
| 0 | 3837 ± 515.1 | 2720 ± 241.8 | 1380 ± 245.2 | 0.36 | 6.36 ± 0.11 | 4.89 ± 0.09 | 903 ± 35.1 | 746 ± 25.5 | 6.16 ± 0.07 | 1133 ± 50.1 | 452 ± 19.9 | 40.8 ± 1.57 |
| 2 | 3803 ± 497.5 | 2990 ± 239.5 | 1391 ± 212.9 | 0.37 | 6.26 ± 0.09 | 4.31 ± 0.08 | 863 ± 29.7 | 741 ± 24.9 | 6.67 ± 0.09 | 1145 ± 49.7 | 476 ± 19.5 | 41.0 ± 1.68 |
| 5 | 3740 ± 480.5 | 3104 ± 228.1 | 1718 ± 238.9 | 0.46 | 5.36 ± 0.11 | 3.96 ± 0.09 | 888 ± 31.2 | 737 ± 28.7 | 6.8 ± 0.08 | 1174 ± 60.2 | 485 ± 20.2 | 42.4 ± 1.58 |
| 10 | 3640 ± 492.5 | 3216 ± 238.7 | 1699 ± 201.7 | 0.47 | 5.69 ± 0.10 | 3.22 ± 0.10 | 871 ± 33.4 | 715 ± 30.5 | 6.85 ± 0.09 | 1250 ± 70.5 | 508 ± 20.6 | 43.5 ± 1.79 |
| 30 | 3570 ± 452.1 | 3297 ± 249.1 | 1499 ± 198.8 | 0.42 | 5.66 ± 0.08 | 3.37 ± 0.11 | 883 ± 32.7 | 712 ± 26.7 | 6.97 ± 0.10 | 1290 ± 55.9 | 515 ± 17.9 | 43.9 ± 1.80 |
| 45 | 3437 ± 398.5 | 3313 ± 248.5 | 1452 ± 186.7 | 0.42 | 5.28 ± 0.12 | 3.09 ± 0.09 | 896 ± 31.0 | 711 ± 27.8 | 7.07 ± 0.11 | 1299 ± 56.7 | 565 ± 20.8 | 46.2 ± 1.75 |
| 60 | 3447 ± 397.6 | 3173 ± 229.6 | 1478 ± 182.2 | 0.43 | 4.80 ± 0.09 | 2.48 ± 0.08 | 870 ± 29.5 | 713 ± 30.9 | 7.2 ± 0.10 | 1372 ± 42.9 | 598 ± 19.8 | 46.7 ± 1.87 |
| 5 bar | ||||||||||||
| 0 | 4951 ± 298.1 | 2476 ± 212.2 | 1269 ± 210.2 | 0.26 | 8.62 ± 0.12 | 6.43 ± 0.09 | 949 ± 30.1 | 824 ± 31.8 | 6.42 ± 0.11 | 937 ± 32.2 | 502 ± 25.6 | 37.5 ±1.45. |
| 2 | 4858 ± 277.6 | 2444 ± 207.6 | 1329 ± 195.8 | 0.27 | 8.66 ± 0.10 | 6.87 ± 0.07 | 909 ± 29.7 | 760 ± 33.1 | 6.58 ± 0.09 | 945 ± 29.7 | 525 ± 18.9 | 37.8 ± 1.50 |
| 5 | 4863 ± 285.4 | 2554 ± 209.5 | 1303 ± 201.4 | 0.27 | 7.80 ± 0.08 | 5.33 ± 0.08 | 911 ± 28.5 | 797 ± 29.7 | 6.60 ± 0.10 | 964 ± 30.3 | 548 ± 20.8 | 39.9 ± 1.52 |
| 10 | 4813 ± 264.7 | 2568 ± 210.5 | 1352 ± 204.6 | 0.28 | 7.77 ± 0.09 | 5.36 ± 0.04 | 925 ± 29.6 | 778 ± 30.5 | 6.69 ± 0.09 | 994 ± 35.6 | 589 ± 19.8 | 41.9 ± 1.49 |
| 30 | 4767 ± 225.4 | 2689 ± 200.9 | 1317 ± 213.5 | 0.28 | 7.37 ± 0.12 | 5.20 ± 0.07 | 896 ± 27.8 | 743 ± 28.4 | 6.82 ± 0.08 | 909 ± 37.8 | 601 ± 17.8 | 43.8 ± 1.47 |
| 45 | 4744 ± 298.1 | 2651 ± 212.6 | 1345 ± 209.9 | 0.28 | 7.17 ± 0.11 | 5.56 ± 0.05 | 894 ± 30.2 | 730 ± 24.4 | 6.88 ± 0.08 | 1043 ± 40.1 | 612 ± 20.1 | 44.6 ± 1.39 |
| 60 | 4257 ± 278.9 | 2595 ± 215.9 | 1349 ± 210.1 | 0.32 | 6.97 ± 0.07 | 4.45 ± 0.07 | 859 ± 35.5 | 711 ± 28.7 | 6.99 ± 0.10 | 1110 ± 41.1 | 642 ± 19.8 | 45.1 ± 1.42 |
| 7 bar | ||||||||||||
| 0 | 5800 ± 205.2 | 2505 ± 199.6 | 1280 ± 171.3 | 0.23 | 9.48 ± 0.12 | 7.67 ± 0.09 | 835 ± 33.7 | 720 ± 21.1 | 6.57 ± 0.09 | 890 ± 30.5 | 601 ± 30.1 | 35.5 ± 1.38 |
| 2 | 5733 ± 207.9 | 2319 ± 197.5 | 1297 ± 118.5 | 0.23 | 9.28 ± 0.13 | 7.38 ± 0.11 | 763 ± 30.4 | 645 ± 19.8 | 6.76 ± 0.11 | 892 ± 29.9 | 612 ± 25.1 | 36.3 ± 1.35 |
| 5 | 5724 ± 210.1 | 2424 ± 195.7 | 1305 ± 116.9 | 0.23 | 9.13 ± 0.14 | 7.61 ± 0.12 | 769 ± 31.2 | 645 ± 20.1 | 6.83 ± 0.12 | 896 ± 31.7 | 628 ± 22.2 | 36.5 ± 1.40 |
| 10 | 5684 ± 209.5 | 2489 ± 198.2 | 1331 ± 202.2 | 0.23 | 8.79 ± 0.10 | 7.08 ± 0.10 | 750 ± 29.9 | 635 ± 22.9 | 6.91 ± 0.09 | 921 ± 32.5 | 638 ± 21.8 | 40.2 ± 1.65 |
| 30 | 5661 ± 204.5 | 2529 ± 189.5 | 1364 ± 199.5 | 0.24 | 8.60 ± 0.09 | 7.09 ± 0.09 | 759 ± 30.6 | 635 ± 23.7 | 7.07 ± 0.11 | 938 ± 33.5 | 651 ± 23.9 | 41.5 ± 1.35 |
| 45 | 5531 ± 203.1 | 2575 ± 192.4 | 1382 ± 189.5 | 0.25 | 8.53 ± 0.11 | 7.08 ± 0.11 | 761 ± 29.8 | 637 ± 25.4 | 7.20 ± 0.10 | 992 ± 35.8 | 678 ± 29.7 | 42.7 ± 1.39 |
| 60 | 5297 ± 201.9 | 2680 ± 189.9 | 1395 ± 190.5 | 0.26 | 8.48 ± 0.11 | 7.07 ± 0.12 | 738 ± 30.5 | 623 ± 20.7 | 7.26 ± 0.11 | 1016 ± 36.9 | 695 ± 29.1 | 44.5 ± 1.42 |
| Time | Electrical Power Consumption | Energy Efficiency—Total Energy Supplied | Power Density | COD Removed | Cavitation Yield |
|---|---|---|---|---|---|
| min | Js−1 | kJ | kJ ml−1 | mg mL−1 | mg kJ−1 |
| 3.5 bar | |||||
| 2 | 20 | 2.4 | 0.00008 | 0.067 | 837.5 |
| 5 | 44 | 13.2 | 0.00044 | 0.097 | 220.5 |
| 10 | 81.5 | 48.9 | 0.00163 | 0.197 | 120.9 |
| 30 | 259 | 466.2 | 0.01554 | 0.267 | 17.2 |
| 45 | 389 | 1050.3 | 0.03050 | 0.4 | 11.4 |
| 60 | 519 | 1868.4 | 0.06228 | 0.39 | 6.3 |
| 5 bar | |||||
| 2 | 25 | 3 | 0.0001 | 0.093 | 930.0 |
| 5 | 57 | 17.1 | 0.00057 | 0.088 | 154.4 |
| 10 | 114 | 68.4 | 0.0028 | 0.138 | 60.5 |
| 30 | 343 | 617.4 | 0.02058 | 0.184 | 8.9 |
| 45 | 515 | 1390.4 | 0.04635 | 0.207 | 4.5 |
| 60 | 687 | 2473.2 | 0.08244 | 0.694 | 8.4 |
| 7 bar | |||||
| 2 | 35 | 4.2 | 0.00014 | 0.067 | 478.6 |
| 5 | 77 | 23.1 | 0.00077 | 0.076 | 98.7 |
| 10 | 154 | 92.4 | 0.00308 | 0.116 | 37.7 |
| 30 | 463 | 833.4 | 0.02778 | 0.139 | 5.0 |
| 45 | 694 | 1873.8 | 0.06246 | 0.269 | 4.3 |
| 60 | 925 | 3330 | 0.11100 | 0.503 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebiocka, M.; Montusiewicz, A.; Szaja, A. Effect of Inlet Pressure on the Biodegradability Index of Cavitated Herbal Waste. Energies 2024, 17, 2023. https://doi.org/10.3390/en17092023
Lebiocka M, Montusiewicz A, Szaja A. Effect of Inlet Pressure on the Biodegradability Index of Cavitated Herbal Waste. Energies. 2024; 17(9):2023. https://doi.org/10.3390/en17092023
Chicago/Turabian StyleLebiocka, Magdalena, Agnieszka Montusiewicz, and Aleksandra Szaja. 2024. "Effect of Inlet Pressure on the Biodegradability Index of Cavitated Herbal Waste" Energies 17, no. 9: 2023. https://doi.org/10.3390/en17092023
APA StyleLebiocka, M., Montusiewicz, A., & Szaja, A. (2024). Effect of Inlet Pressure on the Biodegradability Index of Cavitated Herbal Waste. Energies, 17(9), 2023. https://doi.org/10.3390/en17092023






