Economical Operation and Hazardous Air Pollutant Emissions of Biodegradable Sludge Combustion Process in Commercial Fluidized Bed Plant
Abstract
1. Introduction
2. Facilities and Experimental Methods
2.1. Investigated Facility
2.2. Sampling and Analysis
3. Results and Discussion
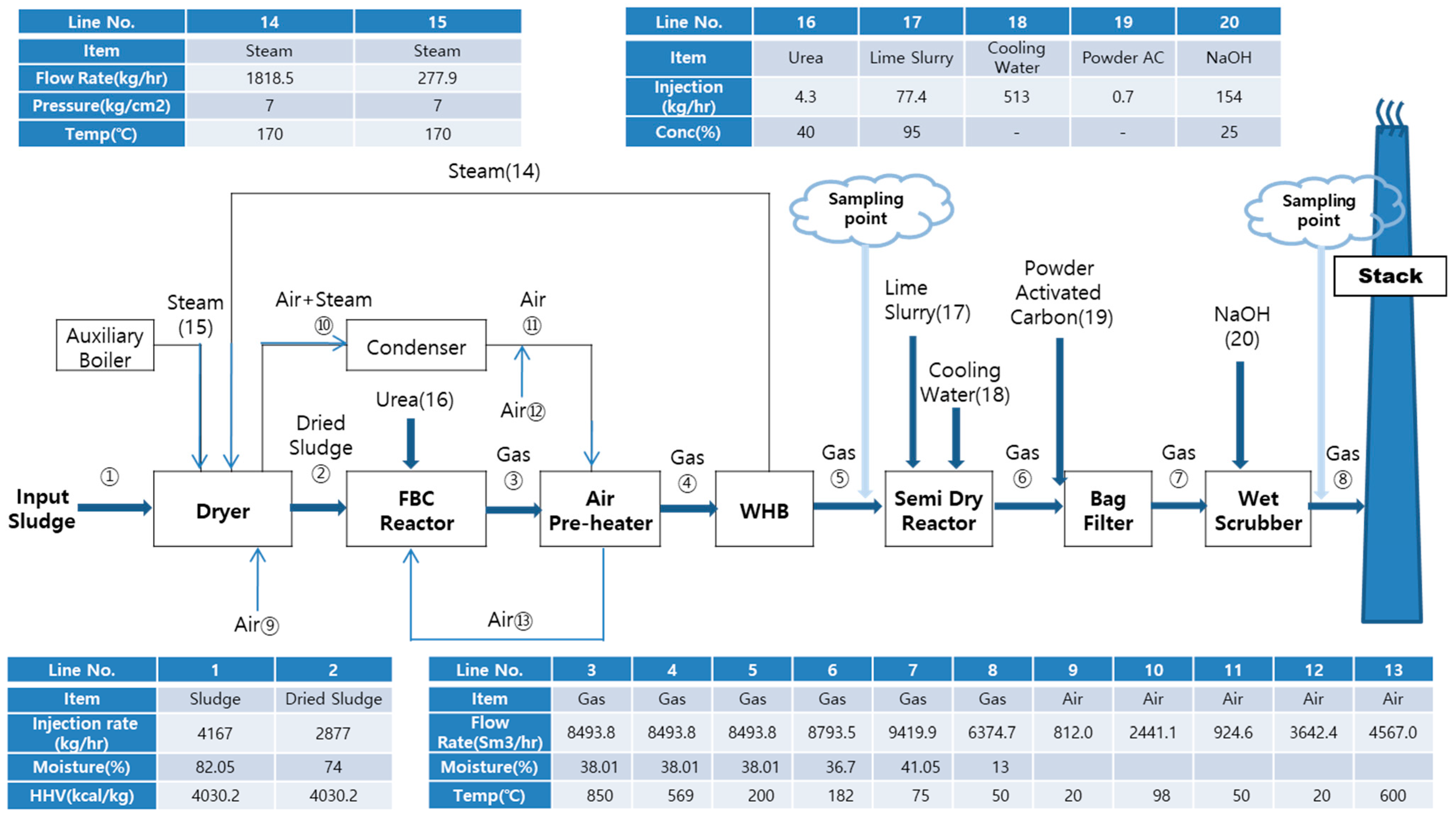
3.1. Mass Balance of the FBC Incineration Process
3.2. HAP Emission from Sewage Sludge Combustion
3.3. Determination of APCD Operation Factor for FBC Sludge Combustion
- DeNOx process:
- Desulfurization process: SDA (Equation (2)) + WS (Equation (3))
- HCl capture process: SDA (Equation (4)) + WS (Equation (5))
4. Conclusions
- Because of the high moisture and toxic compound levels in waste sludge from industrial plants, the heat and mass balance of the FBC combustion process should be monitored to ensure economical operation. After the drying process, the input sewage sludge decreased from 4167 kg/h to 2877 kg/h. After sludge combustion, the flue gas rate increased to 8493.8 Nm3/h and the flue gas temperature decreased to 200 °C.
- Since waste sludge contains abundant organic compounds and chloride, gaseous pollutants such as NOx, SOx, and HCl should be carefully treated in the process. The control efficiency of SOx and HCl for gaseous pollutants was 99% following additive injection into the SDA and WS, whereas the control efficiency of NOx was 41%, and thus an alternative capture process is needed.
- With the APCD configurations used, coarse and fine particulates were well captured with 100% control efficiency. At the stack, fine PM was more enriched with chromium and nickel than other heavy metals. It was found that fine PM at the outlet of the APCDs mostly consisted of toxic metals that were not captured by APCD configurations.
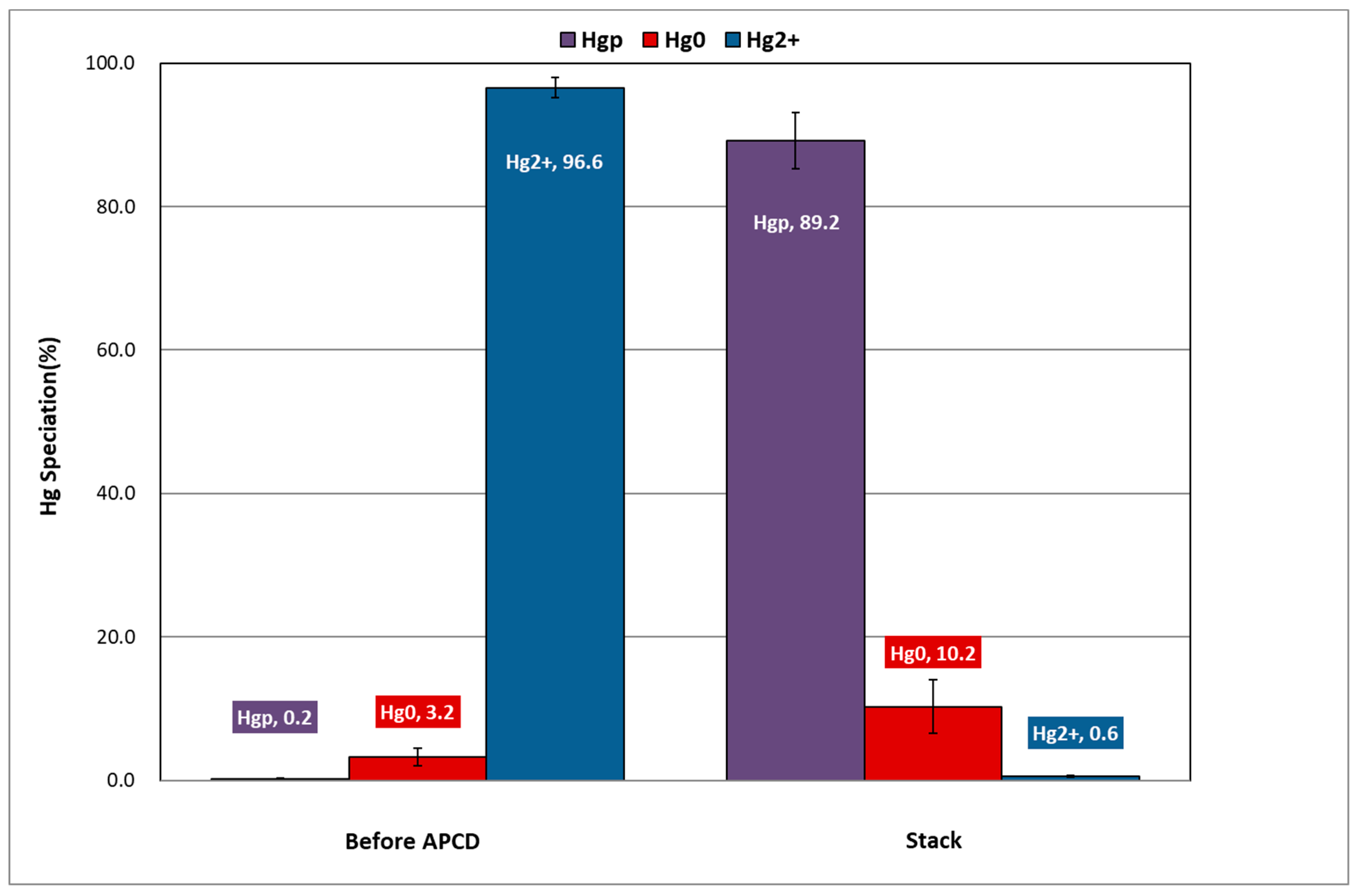
- At the inlet of the APCDs, oxidized mercury accounted for 96.4% of the total mercury, and thus was the dominant species among mercury compounds. After passing through the APCDs, the concentrations of elemental and oxidized mercury decreased, and particulate mercury was mostly distributed in the flue gas. It was observed that oxidized mercury was transformed into particulate mercury by reacting with additives in the SDA and finally penetrated the APCDs.
- For economical operation of an APCD, APCD operation factors and potential waste generation were determined based on the HAP test results. The additive injection and potential waste generation for gaseous pollutants were decreased by 30~40% compared with the predicted design injection rate in the SDA and WS. This should contribute to the efficient operation of APCDs and mitigate the operation costs of the combustion process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anthony, E. Fluidized bed combustion of alternative solid fuels; status, successes and problems of the technology. Prog. Energy Combust. Sci. 1995, 21, 239–268. [Google Scholar] [CrossRef]
- Ogada, T.; Werther, J. Combustion characteristics of wet sludge in a fluidized bed. Fuel 1996, 75, 617–626. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage Sludge Combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Rink, K.K.; Kozinski, J.A.; Lighty, J.S. Biosludge incineration in FBCs: Behavior of ash particles. Combust. Flame 1995, 100, 121–130. [Google Scholar] [CrossRef]
- Latva-Somppi, J.; Kauppinen, E.I.; Valmari, T.; Ahonen, P.; Gurav, A.S.; Kodas, T.T.; Johanson, B. The ash formation during co-combustion of wood and sludge in industrial fluidized bed boilers. Fuel Process. Technol. 1998, 54, 79–94. [Google Scholar] [CrossRef]
- Elled, A.-L.; Åmand, L.-E.; Leckner, B.; Åndersson, B.-A. Influence of Phosphorus on Sulphur Capture during Co-firing of Sewage Sludge with Wood or Bark in a Fluidized Bed. Fuel 2006, 85, 1671–1678. [Google Scholar] [CrossRef]
- Lapa, N.; Barbosa, R.; Lopes, M.H.; Mendes, B.; Abelha, P.; Gulyurtlu, I.; Oliveira, J.S. Chemical and Ecotoxicological Charac-terization of Ashes obtained from Sewage sludge combustion in a Fluidized Bed Reactor. J. Hazard. Mater. 2007, 147, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.; Pohořelý, M.; Trnka, O. Fluidization of Dried Wastewater Sludge. Powder Technol. 2007, 178, 166–172. [Google Scholar] [CrossRef]
- Khiari, B.; Marias, F.; Vaxelaire, J.; Zagrouba, F. Incineration of a small particle of wet sewage sludge: A numerical comparison between two states of the surrounding atmosphere. J. Hazard. Mater. 2007, 147, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Khiari, B.; Marias, F.; Zagrouba, F.; Vaxelaire, J. Transient mathematical modelling of a fluidized bed incinerator for sewage sludge. J. Clean. Prod. 2008, 16, 178–191. [Google Scholar] [CrossRef]
- Somppi, J.L.; Kauppinen, E.I.; Kurkela, J.; Tapper, U.; Ohman, M.; Nordin, A.; Johanson, B. Ultrafine Ash Particle Formation in Fluidized Bed Reactor. Combust. Sci. Technol. 1998, 134, 433–455. [Google Scholar] [CrossRef]
- Seames, W.S.; Fernandez, A.; Wendt, J.O.L. A Study of Fine Particulate Emissions from Combustion of Treated Pulverized Municipal Sewage Sludge. Environ. Sci. Technol. 2002, 36, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.H.; Abelha, P.; Lapa, N.; Oliveira, J.S.; Cabrita, I.; Gulyurtlu, I. The behavior of Ashes and Heavy Metals during the Co-combustion of Sewage Sludge in a Fluidized Bed. Waste Manag. 2003, 23, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Leckner, B.; Åmand, L.-E.; Lücke, K.; Werther, J. Gaseous emissions from co-combustion of sewage sludge and coal/wood in a fluidized bed. Fuel 2004, 83, 477–486. [Google Scholar] [CrossRef]
- Sanger, M.; Werther, J.; Ogada, T. NOx and N2O Emission Characteristics from Fluidized Bed Combustion of Semi-dried Municipal Sewage Sludge. Fuel 2001, 80, 167–177. [Google Scholar] [CrossRef]
- Shao, J.; Yan, R.; Chen, H.; Yang, H.; Lee, D.H.; Liang, D.T. Emission Characteristics of Heavy Metals and Organic Pollutants from the Combustion of Sewage Sludge in a Fluidized Bed Combustor. Energy Fuels 2008, 22, 2278–2283. [Google Scholar] [CrossRef]
- Lopes, M.H.; Gulyurtlu, I.; Cabrita, I. Control of Pollutants during FBC Combustion of Sewage Sludge. Ind. Eng. Chem. Res. 2004, 43, 5540–5547. [Google Scholar] [CrossRef]
- ISO 23210:2009; Stationary Source Emissions, Determination of PM10/PM2.5 Mass Concentration in Flue Gas, Measurement at Low Concentrations by Use of Impactors. ISO: Geneva, Switzerland, 2009.
- US EPA Method. Test Method for Evaluating Solid Waste, SW-846-3050B Acid Digestion of Sediments, Sludges, and Solis, 3rd ed.; US EPA, Office of Solid Waste and Emergency Response, US Government Printing Office: Washington, DC, USA, 1986.
- US EPA. Compilation of Air Pollutant Emission Factors AP-42. In Stationary Point and Area Sources, 5th ed.; AP-42 US EPA: Research Triangle Park, NC, USA, 1995; Volume 1. [Google Scholar]
- EMTIC TM-201A; Determination of PM10 Emissions. US EPA Method: Research Triangle Park, NC, USA, 1997.
- EMTIC TM-5; Determination of Particulate Matter Emissions from Stationary Sources. US EPA Method: Research Triangle Park, NC, USA, 2000.

| Incineration Condition | Sludge Element Analysis (wt.%) | ||
|---|---|---|---|
| Facility Capacity (ton/d) | 100 | Carbon | 34.07 |
| Temperature (°C) | 923 | Hydrogen | 5.58 |
| Sludge Injection | S + I | Nitrogen | 5.02 |
| Sludge Moisture (%) | 82.1 | Oxygen | 16.62 |
| Ash Production (kg/d) | 9140 | Sulfur | 3.19 |
| APCD Configuration | SDA + BF + WS | Chloride | 0.68 |
| HLV (kcal/kg) | 525.5 | - | - |
| H2O (%) | O2 (%) | CO (ppm) | NOx (ppm) | SOx (ppm) | HCl (ppm) | |
|---|---|---|---|---|---|---|
| Inlet SDR | 38.01 | 5.77 | 16.0 | 78.0 | 2368.0 | 355.0 |
| Inlet B/F | 36.70 | 6.29 | - | - | - | - |
| Outlet B/F | 41.05 | 6.29 | - | - | - | - |
| Stack | 13.0 | 9.0 | 46.0 | 45.59 | 1.59 | 3.94 |
| PM (mg/Sm3) | AS (μg/Sm3) | Cd (μg/Sm3) | ||||
| PM2.5↑ | PM2.5↓ | PM2.5↑ | PM2.5↓ | PM2.5↑ | PM2.5↓ | |
| Before B/F | 13,117.4 | 2564.6 | 142.0 | 73.0 | 90.0 | 38.0 |
| Stack | 1.0 | 1.9 | 1.0 | N.D. | N.D. | N.D. |
| Efficiency (%) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Cr (μg/Sm3) | Ni (μg/Sm3) | Pb (μg/Sm3) | ||||
| PM2.5↑ | PM2.5↓ | PM2.5↑ | PM2.5↓ | PM2.5↑ | PM2.5↓ | |
| Before B/F | 155,103.0 | 50,711.0 | 21,163.0 | 8495.0 | 4403.0 | 1904.0 |
| Stack | 6.0 | 56.0 | 2.0 | 97.0 | 8.0 | 1.0 |
| Efficiency (%) | 100.0 | 99.9 | 100.0 | 98.8 | 99.8 | 100.0 |
| Sampling Point | Sampling Method | Hg Conc. (μg/Sm3) | Hg Distribution (μg/Sm3) | Hg Speciation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Hgp | Hg0 | Hg2+ | Hgp | Hg0 | Hg2+ | |||
| Before B/F | OHM-1 | 907.1 | 0.7 | 17.7 | 888.6 | 0.1 | 2.0 | 98.0 |
| OHM-2 | 1069.9 | 3.6 | 47.9 | 1018.4 | 0.3 | 4.5 | 95.2 | |
| Average | 988.5 | 2.2 | 32.8 | 953.5 | 0.2 | 3.2 | 96.6 | |
| Stack | OHM-1 | 16.7 | 15.5 | 1.1 | 0.1 | 93.1 | 6.4 | 0.5 |
| OHM-2 | 9.5 | 8.1 | 1.3 | 0.1 | 85.3 | 14.0 | 1.0 | |
| Average | 13.1 | 11.8 | 1.2 | 0.1 | 90.3 | 9.1 | 0.7 | |
| Control Eff. | 98.7 | - | 96.4 | 100.0 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-N.; Choi, M.K.; Choi, H.S. Economical Operation and Hazardous Air Pollutant Emissions of Biodegradable Sludge Combustion Process in Commercial Fluidized Bed Plant. Energies 2024, 17, 542. https://doi.org/10.3390/en17020542
Jang H-N, Choi MK, Choi HS. Economical Operation and Hazardous Air Pollutant Emissions of Biodegradable Sludge Combustion Process in Commercial Fluidized Bed Plant. Energies. 2024; 17(2):542. https://doi.org/10.3390/en17020542
Chicago/Turabian StyleJang, Ha-Na, Myung Kyu Choi, and Hang Seok Choi. 2024. "Economical Operation and Hazardous Air Pollutant Emissions of Biodegradable Sludge Combustion Process in Commercial Fluidized Bed Plant" Energies 17, no. 2: 542. https://doi.org/10.3390/en17020542
APA StyleJang, H.-N., Choi, M. K., & Choi, H. S. (2024). Economical Operation and Hazardous Air Pollutant Emissions of Biodegradable Sludge Combustion Process in Commercial Fluidized Bed Plant. Energies, 17(2), 542. https://doi.org/10.3390/en17020542






