Biogas Potential of Food Waste-Recycling Wastewater after Oil–Water Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Analysis
2.2. Biochemical Methane Potential (BMP) Assay
2.3. DNA Extraction and High-Throughput Sequencing
3. Results
3.1. Sample Characterization
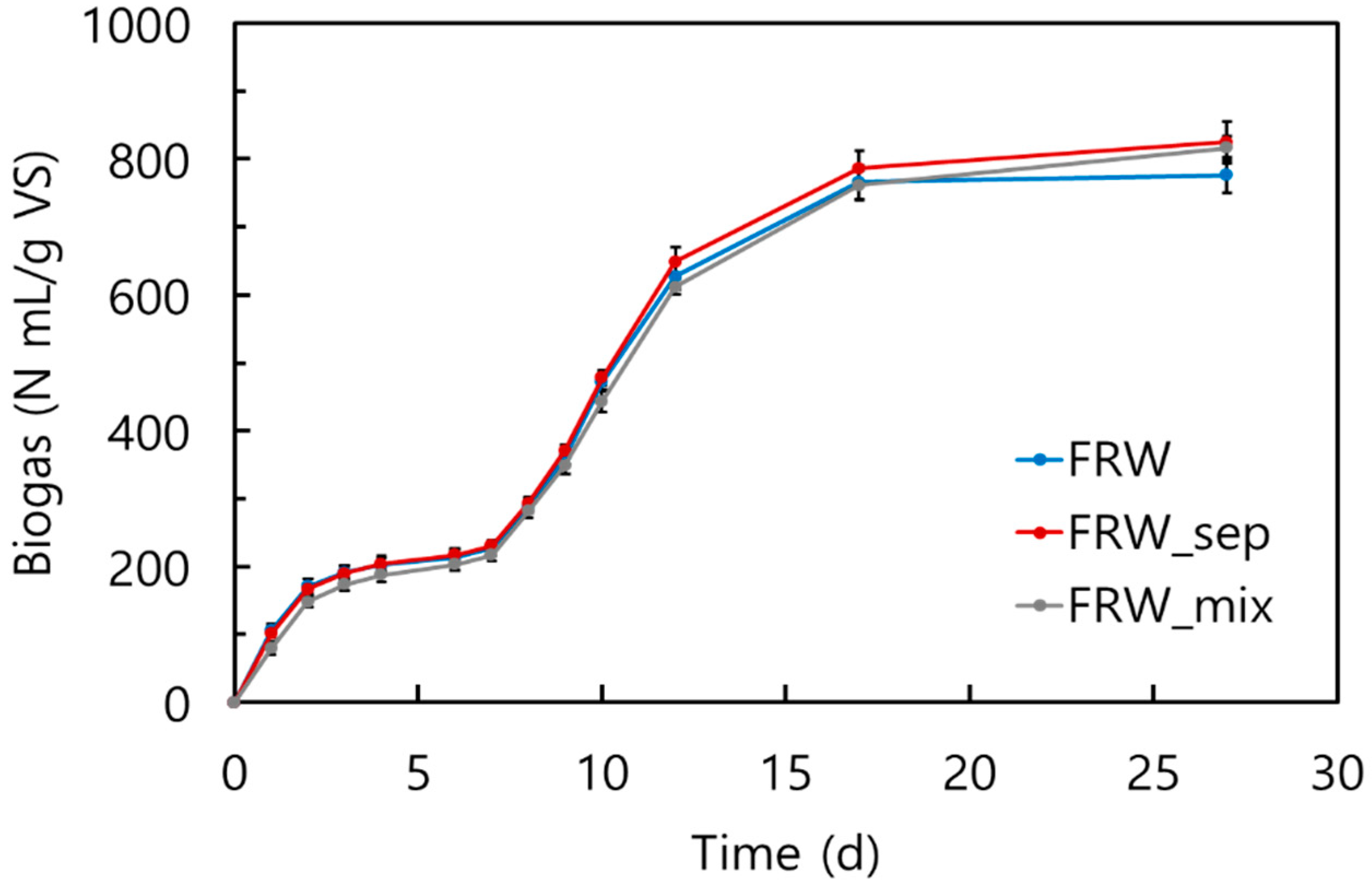
3.2. Biogas Potential Assessment through BMP Assay
3.3. Microbial Community Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sravani, A.; Patil, C.R.; Sharma, S. Value-Added Product Development Utilising the Food Wastes. In Valorization of Biomass Wastes for Environmental Sustainability: Green Practices for the Rural Circular Economy; Srivastav, A.L., Bhardwaj, A.K., Kumar, M., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 287–301. [Google Scholar]
- MoE. Statistical Data of Korean Waste Generation and Treatment; Ministry of Environment: Sejong, Republic of Korea, 2023.
- Lee, E.; Jin Min, K.; Choi, H.; Young Park, K. Impact of dewatering inorganic coagulants on anaerobic digestion treating food waste leachate. Bioresour. Technol. 2024, 393, 130136. [Google Scholar] [CrossRef]
- Bae, J.S.; Yoon, Y.M.; Shin, S.K.; Lee, D.J.; Seo, D.C. Biogas potential and methanogenic community shift in in-situ anaerobic sewage sludge digestion with food waste leachate additions. Appl. Biol. Chem. 2020, 63, 62. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Ahn, J.; Kim, J.; Lee, S.; Lee, I.; Kim, S.; Chang, S.; Chung, W. Volatile fatty acid production from food waste leachate using enriched bacterial culture and soil bacteria as co-digester. Sustainability 2021, 13, 9606. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Z.; Guan, D.; Zhao, J.; Wang, Y.; Zhang, Q.; Xie, J.; Sun, Y.; Guo, L.; Wang, D. A comprehensive review on food waste anaerobic co-digestion: Current situation and research prospect. Process Saf. Environ. Prot. 2023, 179, 546–558. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Karmee, S.K. Liquid biofuels from food waste: Current trends, prospect and limitation. Renew. Sustain. Energy Rev. 2016, 53, 945–953. [Google Scholar] [CrossRef]
- Heo, H.S.; Kim, S.G.; Jeong, K.-E.; Jeon, J.-K.; Park, S.H.; Kim, J.M.; Kim, S.-S.; Park, Y.-K. Catalytic upgrading of oil fractions separated from food waste leachate. Bioresour. Technol. 2011, 102, 3952–3957. [Google Scholar] [CrossRef]
- Farahdiba, A.U.; Warmadewanthi, I.D.A.A.; Fransiscus, Y.; Rosyidah, E.; Hermana, J.; Yuniarto, A. The present and proposed sustainable food waste treatment technology in Indonesia: A review. Environ. Technol. Innov. 2023, 32, 103256. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L. Study on the comprehensive utilization of city kitchen waste as a resource in China. Energy 2019, 173, 263–277. [Google Scholar] [CrossRef]
- Batool, F.; Kurniawan, T.A.; Mohyuddin, A.; Othman, M.H.D.; Aziz, F.; Al-Hazmi, H.E.; Goh, H.H.; Anouzla, A. Environmental impacts of food waste management technologies: A critical review of life cycle assessment (LCA) studies. Trends Food Sci. Technol. 2024, 143, 104287. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; pp. 2–56. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ribadeau-Dumas, B.; Grappin, R. Milk protein analysis. Le Lait 1989, 69, 357–416. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, O.; Sommer, S.G.; Shin, S.G.; Triolo, J.M. Residual biochemical methane potential (BMP) of concentrated digestate from full-scale biogas plants. Fuel 2014, 132, 44–46. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Wiley and Sons: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef]
- Rhee, C.; Park, S.-G.; Yu, S.I.; Dalantai, T.; Shin, J.; Chae, K.-J.; Shin, S.G. Mapping microbial dynamics in anaerobic digestion system linked with organic composition of substrates: Protein and lipid. Energy 2023, 275, 127411. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Mukherjee, G. Present scenario and future scope of food waste to biofuel production. J. Food Process Eng. 2021, 44, e13594. [Google Scholar] [CrossRef]
- Economou, F.; Voukkali, I.; Papamichael, I.; Phinikettou, V.; Loizia, P.; Naddeo, V.; Sospiro, P.; Liscio, M.C.; Zoumides, C.; Țîrcă, D.M.; et al. Turning Food Loss and Food Waste into Watts: A Review of Food Waste as an Energy Source. Energies 2024, 17, 3191. [Google Scholar] [CrossRef]
- Lelicińska-Serafin, K.; Manczarski, P.; Rolewicz-Kalińska, A. An Insight into Post-Consumer Food Waste Characteristics as the Key to an Organic Recycling Method Selection in a Circular Economy. Energies 2023, 16, 1735. [Google Scholar] [CrossRef]
- Kim, S.I.; Aghasa, A.; Choi, S.; Hong, S.; Park, T.; Hwang, S. Variations in Lipid Accumulation and Methanogenic Predominance in Full-Scale Anerobic Digestors Treating Food Waste Leachate. Waste Biomass Valorization 2023, 14, 3223–3234. [Google Scholar] [CrossRef]
- Ansari, K.; Goga, G.; Mohan, R. Utilization of food waste into ethanol and biodiesel. Mater. Today Proc. 2022, 65, 3596–3601. [Google Scholar] [CrossRef]
- Jeon, D.; Chung, K.; Shin, J.; Min Park, C.; Shin, S.G.; Mo Kim, Y. Reducing food waste in residential complexes using a pilot-scale on-site system. Bioresour. Technol. 2020, 311, 123497. [Google Scholar] [CrossRef] [PubMed]
- Buendia-Kandia, F.; Rondags, E.; Framboisier, X.; Mauviel, G.; Dufour, A.; Guedon, E. Diauxic growth of Clostridium acetobutylicum ATCC 824 when grown on mixtures of glucose and cellobiose. AMB Express 2018, 8, 85. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.H. Minimization of diauxic growth lag-phase for high-efficiency biogas production. J. Environ. Manag. 2017, 187, 456–463. [Google Scholar] [CrossRef]
- Gomes, C.S.; Strangfeld, M.; Meyer, M. Diauxie Studies in Biogas Production from Gelatin and Adaptation of the Modified Gompertz Model: Two-Phase Gompertz Model. Appl. Sci. 2021, 11, 1067. [Google Scholar] [CrossRef]
- Sophonsiri, C.; Morgenroth, E. Chemical composition associated with different particle size fractions in municipal, industrial, and agricultural wastewaters. Chemosphere 2004, 55, 691–703. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Lyu, X.; Zhao, N.; Song, J.; Wang, X.; Yang, G. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Gao, F.; Hang, W.-J.W.; Yang, H.-L.; Jin, W.-H.; Li, C. Effects of organic matters in domestic wastewater on lipid/carbohydrate production and nutrient removal of Chlorella vulgaris cultivated under mixotrophic growth conditions. J. Chem. Technol. Biotechnol. 2019, 94, 3578–3584. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production—A review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef] [PubMed]

| Parameter | FRW | FRW_sep | FRW_mix | |||
|---|---|---|---|---|---|---|
| A * | B * | A | B | A | B | |
| pH | 4.20 | 4.28 | 4.04 | 4.04 | 3.89 | 3.83 |
| TS (g/L) | 73.3 ± 0.2 ** | 73.5 ± 0.4 | 57.6 ± 0.1 | 59.0 ± 0.4 | 47.2 ± 0.3 | 49.7 ± 0.9 |
| VS (g/L) | 63.3 ± 0.2 | 63.4 ± 0.3 | 49.7 ± 0.1 | 51.0 ± 0.3 | 40.5 ± 0.2 | 42.9 ± 0.9 |
| COD (g/L) | 94.7 ± 1.0 | 100.8 ± 1.9 | 87.9 ± 1.0 | 94.7 ± 8.2 | 72.2 ± 1.9 | 72.9 ± 1.0 |
| Carbohydrate (g/L) | 21.0 ± 0.7 | 21.8 ± 0.3 | 13.7 ± 0.0 | 14.9 ± 0.6 | 10.5 ± 0.4 | 9.7 ± 0.7 |
| Protein (g/L) | 18.9 ± 0.0 | 18.2 ± 0.1 | 16.7 ± 0.0 | 16.0 ± 0.1 | 14.2 ± 0.1 | 14.6 ± 0.3 |
| Lipid (g/L) | 14.3 ± 0.0 | 14.7 ± 0.4 | 10.4 ± 0.3 | 11.0 ± 0.1 | 9.4 ± 0.5 | 10.3 ± 0.8 |
| Bacteria (%) | Blank | FRW | FRW_sep | FRW_mix |
|---|---|---|---|---|
| Firmicutes | 32.08 | 61.91 | 63.84 | 60.32 |
| Cloacimonetes | 18.28 | 11.50 | 11.17 | 12.04 |
| Proteobacteria | 14.52 | 6.51 | 6.14 | 6.93 |
| Bacteroidetes | 3.00 | 3.65 | 3.91 | 3.63 |
| Chloroflexi | 2.01 | 1.72 | 1.22 | 1.46 |
| Armatimonadetes | 0.47 | 0.86 | 0.82 | 0.85 |
| Planctomycetes | 1.19 | 0.36 | 0.35 | 0.43 |
| Actinobacteria | 1.13 | 0.30 | 0.32 | 0.56 |
| Verrucomicrobia | 0.25 | 0.29 | 0.33 | 0.31 |
| Acidobacteria | 0.71 | 0.26 | 0.20 | 0.32 |
| Synergistetes | 0.17 | 0.11 | 0.10 | 0.06 |
| Unclassified | 26.19 | 12.89 | 11.74 | 13.06 |
| Archaea (%) | Blank | FRW | FRW_sep | FRW_mix |
|---|---|---|---|---|
| Methanolinea | 78.11 | 63.88 | 61.08 | 60.73 |
| Methanoculleus | 3.56 | 22.71 | 25.20 | 25.90 |
| Methanothrix | 11.75 | 9.12 | 9.79 | 9.15 |
| Methanospirillum | 6.31 | 4.19 | 3.85 | 4.07 |
| Methanobrevibacter | 0.25 | 0.05 | 0.04 | 0.06 |
| Unclassified | 0.01 | 0.04 | 0.03 | 0.08 |
| Parameter | Unit | Scenario 1 | Scenario 2 | Remark |
|---|---|---|---|---|
| FRW treatment | m3/day | 100 | 100 | |
| FRW used for biogas | m3/day | 100 | 95 | 5% loss during oil–water separation |
| VS concentration | kg/m3 | 63.38 | 50.37 | Obtained from the characterization |
| VS used for biogas | kg VS/day | 6338 | 4785 | [FRW used] × [VS concentration] |
| CH4 productivity | Nm3/kg VS | 0.465 | 0.541 | Obtained from the BMP assay |
| CH4 production | Nm3/day | 2947 | 2589 | [VS used] × [CH4 productivity] |
| Energy value of CH4 | MJ/Nm3 | 35.8 | 35.8 | |
| CH4 production (energy) | MJ/day | 105,500 | 92,678 | [CH4 production] × [Energy value] |
| Biodiesel (BD) production | m3/day | - | 0.5 | 0.5% yield |
| Energy value of BD | MJ/m3 | - | 32 | Reference from [27] |
| BD production (energy) | MJ/day | - | 16,000 | [BD production] × [Energy value] |
| BD operation cost (energy) | MJ/day | - | 360 | Oil–water separator, 3.6 MJ per ton of FRW |
| Net energy gain * | MJ/day | 105,500 | 108,318 | [CH4 energy] + [BD energy] − [BD operation] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Shin, J.; Lee, M.-E.; Shin, S.G. Biogas Potential of Food Waste-Recycling Wastewater after Oil–Water Separation. Energies 2024, 17, 4428. https://doi.org/10.3390/en17174428
Han G, Shin J, Lee M-E, Shin SG. Biogas Potential of Food Waste-Recycling Wastewater after Oil–Water Separation. Energies. 2024; 17(17):4428. https://doi.org/10.3390/en17174428
Chicago/Turabian StyleHan, Gyuseong, Juhee Shin, Myoung-Eun Lee, and Seung Gu Shin. 2024. "Biogas Potential of Food Waste-Recycling Wastewater after Oil–Water Separation" Energies 17, no. 17: 4428. https://doi.org/10.3390/en17174428
APA StyleHan, G., Shin, J., Lee, M.-E., & Shin, S. G. (2024). Biogas Potential of Food Waste-Recycling Wastewater after Oil–Water Separation. Energies, 17(17), 4428. https://doi.org/10.3390/en17174428







