Feasibility Study on Production of Slush Hydrogen Based on Liquid and Solid Phase for Long Term Storage
Abstract
1. Introduction
2. Definition for Slush Hydrogen Production
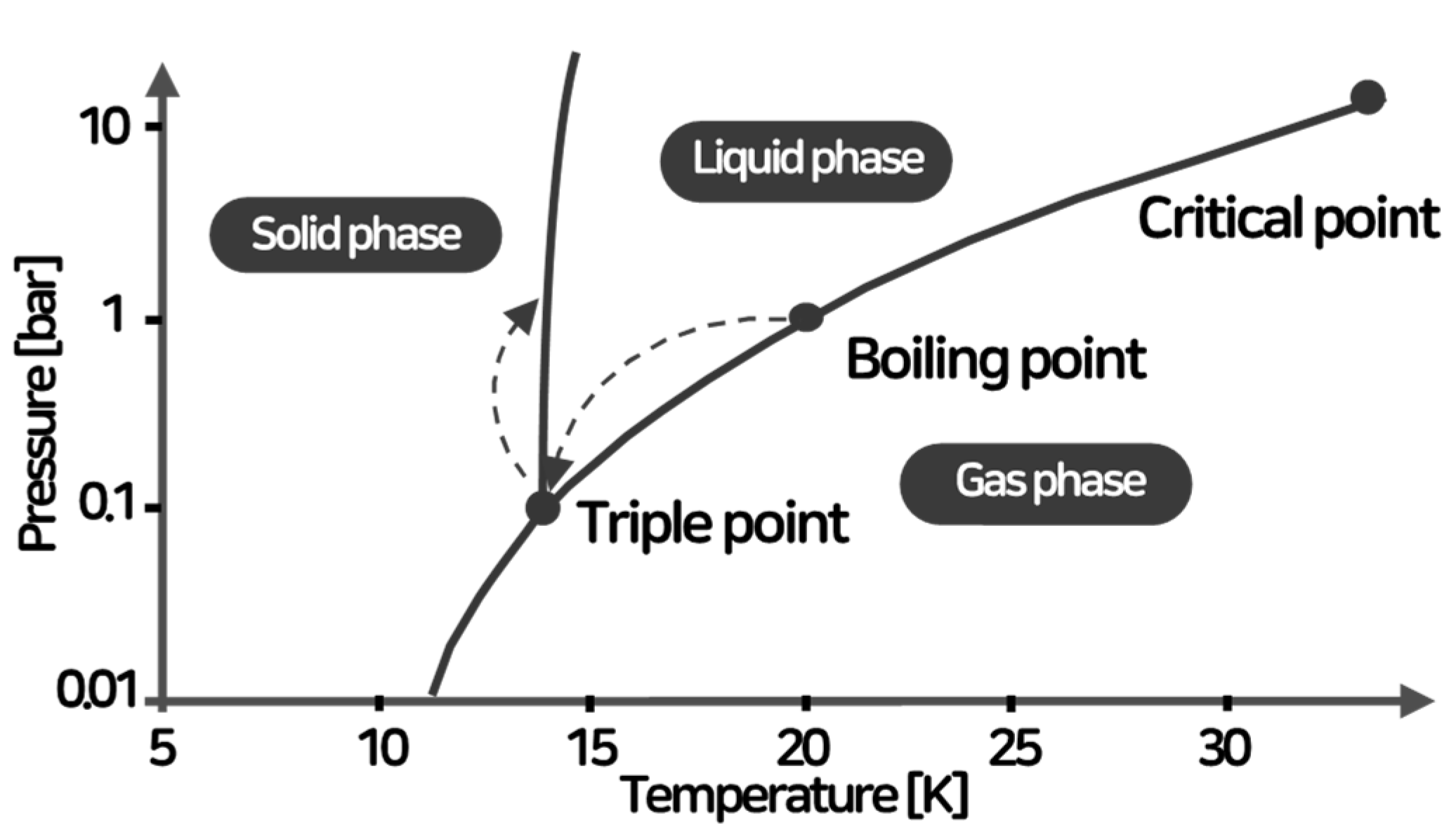
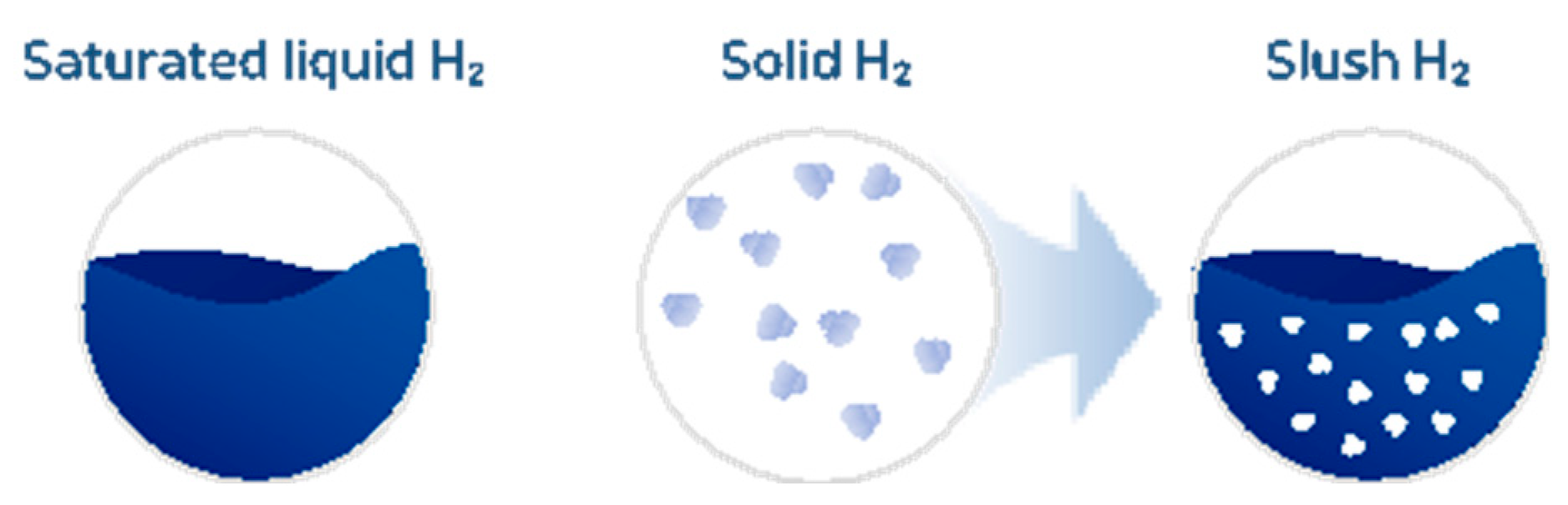
2.1. Slush Hydrogen
2.2. Physical Properties of Slush Hydrogen
2.3. Production Methods for Slush Hydrogen
3. Experimental Study on Slush Hydrogen Production
3.1. Experimental Facilities for Slush Hydrogen Production
3.2. Experimental Result for Slush Hydrogen Production
3.3. Verification of Slush Hydrogen Production
4. Computational Fluid Dynamics Simulation for Slush Hydrogen Production
4.1. Computational Model and Simulation Conditions
4.2. Simulation of Natural Convection and Boil-Off in a Slush Hydrogen Tank
5. Conclusions
- Slush hydrogen, a fluid where liquid and solid phases coexist, has a density approximately 15% higher than liquid hydrogen and helps suppress boil-off gas. This characteristic is expected to address challenges related to the transportation and storage of liquid hydrogen, which has a lower boiling point compared to conventional LNG.
- We designed and validated a facility capable of producing 2 kg/day of slush hydrogen using the freeze-thaw method. The production method was confirmed through experiments, and we recorded the slush hydrogen formation process. Additionally, we introduced and validated a new methodology to demonstrate that slush hydrogen consists of a 50% liquid and 50% solid mixture.
- In our 2 kg/day slush production facility, we verified that the slush hydrogen was approximately 50% liquid and 50% solid. The ratio of solid to liquid hydrogen was determined by comparing the boil-off rate to that of a standard amount of liquid hydrogen, estimating the solid hydrogen formed. The resulting ratio of solid hydrogen to liquid hydrogen was approximately 0.57, with a density of about 80.9 kg/m3.
- We performed simulations of natural convection and boil-off tests for slush hydrogen using STAR-CCM+ to verify the state of the slush hydrogen. The simulations utilized the VOF model to account for phase changes among gas, liquid, and solid states. The solid-to-liquid hydrogen ratio confirmed in the simulations was approximately 0.57. The BOR test results from the simulations aligned well with experimental values, indicating that the simulation environment accurately replicated experimental conditions.
- We aimed to control the particle size of slush hydrogen. Various approaches have been explored, as controlling its particle size would eliminate barriers to its application in various industrial fields. Although we successfully achieved nearly 50% solid hydrogen content, further development is needed to produce solid hydrogen with uniform particles. Future efforts will focus on applying additional techniques to achieve this goal in this study.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOG | Boil-off gas | LCOE | Levelized cost of electricity |
| COP | Coefficient of performance | LCOS | Levelized cost of LH2 storage and transportation |
| HDPE | High-density polyethylene | LHV | Lower heating value |
| HDSAM | Hydrogen delivery scenario analysis model | LOHC | Liquid organic hydrogen carriers |
| GODU | Ground operation demonstration unit | MOFs | Metal-organic frameworks |
| IEA | International energy agency | NASA | National aeronautics and space administration |
| IPCC | Intergovernmental panel on climate change | LNG | Liquefied natural gas |
| IRAS | Integrated refrigeration and storage | STP | Standard temperature and pressure |
References
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Adolf, J.; Balzer, C.H.; Louis, J.; Schabla, U.; Fischedick, M.; Arnold, K.; Pastowski, A.; Schüwer, D. Energy of the Future: Sustainable Mobility through Fuel Cells and H2; Shell Hydrogen Study; Shell Deutschland Oil: Hamburg, Germany, 2017. [Google Scholar]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The Hydrogen-Fueled Internal Combustion Engine: A Technical Review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The Survey of Key Technologies in Hydrogen Energy Storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal-Organic Framework Composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.; Zhu, Y. Porous Carbon-Based Materials for Hydrogen Storage: Advancement and Challenges. J. Mater. Chem. A Mater. 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Petit, J.F.; Miele, P.; Demirci, U.B. Ammonia Borane H3N-BH3 for Solid-State Chemical Hydrogen Storage: Different Samples with Different Thermal Behaviors. Int. J. Hydrogen Energy 2016, 41, 15462–15470. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-Free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen Storage: Recent Improvements and Industrial Perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Yersak, T.A.; Baker, D.R.; Yanagisawa, Y.; Slavik, S.; Immel, R.; Mack-Gardner, A.; Herrmann, M.; Cai, M. Predictive Model for Depressurization-Induced Blistering of Type IV Tank Liners for Hydrogen Storage. Int. J. Hydrogen Energy 2017, 42, 28910–28917. [Google Scholar] [CrossRef]
- Aceves, S.M.; Espinosa-Loza, F.; Ledesma-Orozco, E.; Ross, T.O.; Weisberg, A.H.; Brunner, T.C.; Kircher, O. High-Density Automotive Hydrogen Storage with Cryogenic Capable Pressure Vessels. Int. J. Hydrogen Energy 2010, 35, 1219–1226. [Google Scholar] [CrossRef]
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On Hydrogen and Hydrogen Energy Strategies I: Current Status and Needs. Renew. Sustain. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Sotsky, L.; Castillo, A.; Ramos, H.; Mitchko, E.; Heuvel-Horwitz, J.; Bick, B.; Mahajan, D.; Wong, S.S. Hydrogen Storage Properties of Metal-Modified Graphene Materials. Energies 2024, 17, 3944. [Google Scholar] [CrossRef]
- International Energy Agency. The Future of Hydrogen; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Retief, H.L.; Markgraaff, J. A Review of Hydrogen Storage for Vehicular Application and the Determination of the Effect of Extraction Boil-Off. Ph.D. Thesis, North-West University, Potchefstroom Campus, Potchefstroom, South Africa, 2012. [Google Scholar]
- Al Ghafri, S.Z.S.; Swanger, A.; Jusko, V.; Siahvashi, A.; Perez, F.; Johns, M.L.; May, E.F. Modelling of Liquid Hydrogen Boil-Off. Energies 2022, 15, 1149. [Google Scholar] [CrossRef]
- Aursand, E.; Odsæter, L.H.; Skarsvåg, H.; Reigstad, G.; Ustolin, F.; Paltrinieri, N. Risk and Consequences of Rapid Phase Transition for Liquid Hydrogen. In Proceedings of the 30th European Safety and Reliability Conference and the 15th Probabilistic Safety Assessment and Management Conference, Venice, Italy, 1–5 November 2020; Research Publishing: Singapore, 2020; pp. 1899–1906. [Google Scholar]
- Voth, R.O. Producing Liquid-Solid Mixtures of Hydrogen Using an Auger; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1978. [Google Scholar]
- Swanger, A.M.; Notardonato, W.U.; Fesmire, J.E.; Jumper, K.M.; Johnson, W.L.; Tomsik, T.M. Large Scale Production of Densified Hydrogen to the Triple Point and Below. IOP Conf. Ser. Mater. Sci. Eng. 2017, 278, 012013. [Google Scholar] [CrossRef]
- Daney, D.E.; Arp, V.D.; Voth, R.O. Hydrogen Slush Production with a Large Auger. Adv. Cryog. Eng. 1990, 35, 1767–1776. [Google Scholar]
- Fujiwara, H.; Yatabe, M.; Tamura, H.; Takahashi, M.; Mlyazakif, J.; Tsuruta, Y. Experiment on Slush Hydrogen Production with the Auger Method. Int. J. Hydrog. Energy 1998, 23, 333–338. [Google Scholar] [CrossRef]
- Ohira, K.; Matsuo, S.; Furumoto, H. An Experimental Investigation of Production and Density Measurement of Slush Hydrogen. Cryogenics 1994, 34, 397–400. [Google Scholar] [CrossRef]
- Brunnhofer, K.; Paragina, A.S.; Scheerer, M.; Dermouz, H. Slush Hydrogen and Slush Nitrogen Production and Characterization. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9 July 2006; pp. 1–19. [Google Scholar]
- Berstad, D.; Gardarsdottir, S.; Roussanaly, S.; Voldsund, M.; Ishimoto, Y.; Nekså, P. Liquid Hydrogen as Prospective Energy Carrier: A Brief Review and Discussion of Underlying Assumptions Applied in Value Chain Analysis. Renew. Sustain. Energy Rev. 2022, 154, 111772. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Gupta, N.; Zhang, K.; van Doorne, C.; Fesmire, J.; Dindoruk, B.; Balakotaiah, V. Hydrogen Supply Chain and Challenges in Large-Scale LH2 Storage and Transportation. Int. J. Hydrogen Energy 2021, 46, 24149–24168. [Google Scholar] [CrossRef]
- Ohira, K. Slush Hydrogen Production, Storage, and Transportation. In Compendium of Hydrogen Energy: Hydrogen Storage, Distribution and Infrastructure: Volume 2; Elsevier: Amsterdam, The Netherlands, 2015; pp. 53–90. ISBN 9781782423621. [Google Scholar]
- McCarty, R.D.; Hord, J.; Roder, H.M. Selected Properties of Hydrogen (Engineering Design Data); National Institute of Standards and Technology: Gaithersburg, MD, USA, 1981. [Google Scholar]
- Chung, S.; Ahn, H.; Park, J. Numerical approach to analyze fluid flow in a type C tank for liquefied hydrogen carrier (part 2: Thermal flow). J. Energy Storage 2024, 76, 109599. [Google Scholar] [CrossRef]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, C.; Chung, S.; Hwang, S.; Lim, J.; Chang, D. Feasibility Study on Production of Slush Hydrogen Based on Liquid and Solid Phase for Long Term Storage. Energies 2024, 17, 4415. https://doi.org/10.3390/en17174415
Park S, Lee C, Chung S, Hwang S, Lim J, Chang D. Feasibility Study on Production of Slush Hydrogen Based on Liquid and Solid Phase for Long Term Storage. Energies. 2024; 17(17):4415. https://doi.org/10.3390/en17174415
Chicago/Turabian StylePark, Sungho, Changhyeong Lee, Sohmyung Chung, Seonghyeon Hwang, Jongwoong Lim, and DaeJun Chang. 2024. "Feasibility Study on Production of Slush Hydrogen Based on Liquid and Solid Phase for Long Term Storage" Energies 17, no. 17: 4415. https://doi.org/10.3390/en17174415
APA StylePark, S., Lee, C., Chung, S., Hwang, S., Lim, J., & Chang, D. (2024). Feasibility Study on Production of Slush Hydrogen Based on Liquid and Solid Phase for Long Term Storage. Energies, 17(17), 4415. https://doi.org/10.3390/en17174415




