Scalable Ni12P5-Coated Carbon Cloth Cathode for Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
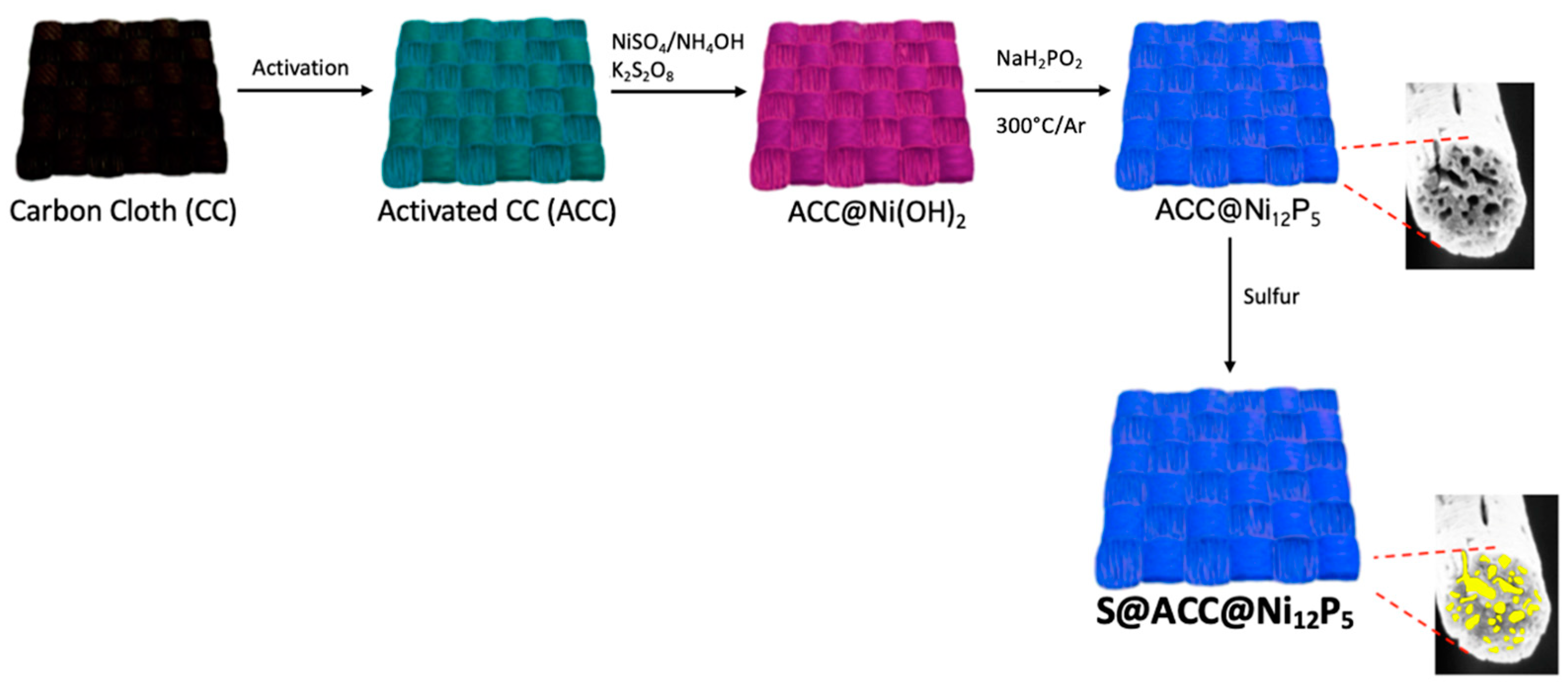
2.2. Activation of CC and Preparation of ACC@Ni(OH)2
2.3. Preparation of ACC@Ni12P5 and S@ACC@Ni12P5
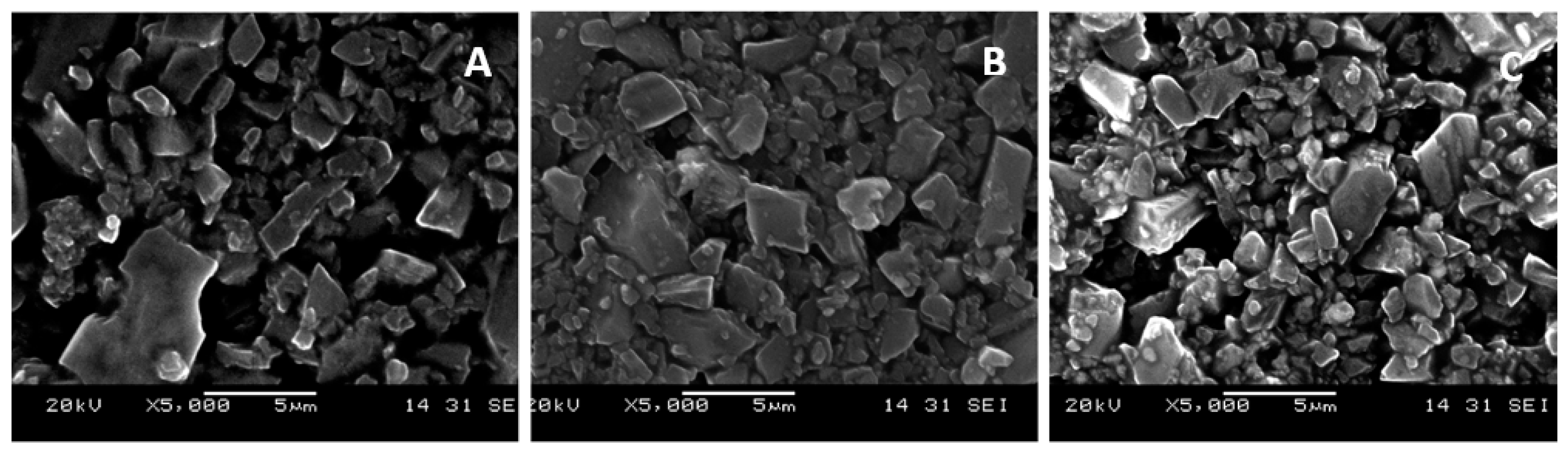
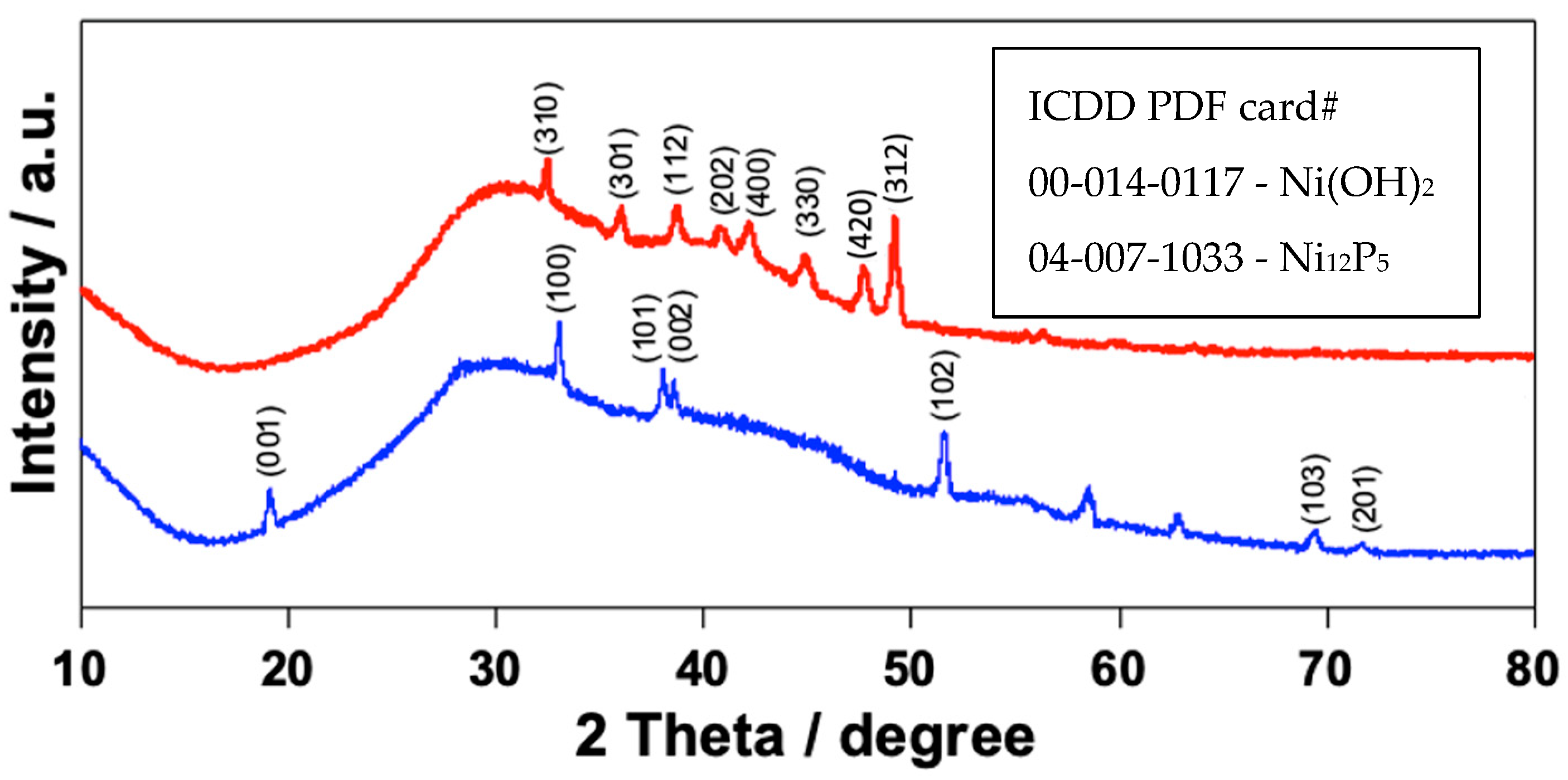
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashuri, M.; Dunya, H.; Yue, Z.; Alramahi, D.; Mei, X.; Kucuk, K.; Aryal, S.; Segre, C.U.; Mandal, B.K. Enhancement in Electrochemical Performance of Lithium-Sulfur Cells through Sulfur Encapsulation in Hollow Carbon Nanospheres Coated with Ultra-Thin Aluminum Fluoride Layer. ChemistrySelect 2019, 4, 12622–12629. [Google Scholar] [CrossRef]
- Dunya, H.; Ashuri, M.; Alramahi, D.; Yue, Z.; Kucuk, K.; Segre, C.U.; Mandal, B.K. MnO2-Coated Dual Core–Shell Spindle-Like Nanorods for Improved Capacity Retention of Lithium–Sulfur Batteries. ChemEngineering 2020, 4, 42. [Google Scholar] [CrossRef]
- Ye, H.; Li, M.; Liu, T.; Li, Y.; Lu, J. Activating Li2S as the Lithium-Containing Cathode in Lithium–Sulfur Batteries. ACS Energy Lett. 2020, 5, 2234–2245. [Google Scholar] [CrossRef]
- Pang, Q.; Shyamsunder, A.; Narayanan, B.; Kwok, C.Y.; Curtiss, L.A.; Nazar, L.F. Tuning the electrolyte network structure to invoke quasi-solid state sulfur conversion and suppress lithium dendrite formation in Li–S batteries. Nat. Energy 2018, 3, 783–791. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Capacity fade analysis of sulfur cathodes in lithium–sulfur batteries. Adv. Sci. 2016, 3, 101. [Google Scholar] [CrossRef] [PubMed]
- Periodictable.com. Available online: https://periodictable.com/Elements/016/data.html (accessed on 5 January 2021).
- Yue, Z.; Dunya, H.; Kucuk, K.; Aryal, S.; Ma, Q.; Antonov, S.; Ashuri, M.; Alabbad, B.; Lin, Y.; Segre, C.U.; et al. MnO2-Coated Sulfur-Filled Hollow Carbon Nanosphere-Based Cathode Materials for Enhancing Electrochemical Performance of Li-S Cells. J. Electrochem. Soc. 2019, 166, A1355–A1362. [Google Scholar] [CrossRef]
- Dunya, H.; Ashuri, M.; Yue, Z.; Kucuk, K.; Lin, Y.; Alramahi, D.; Segre, C.U.; Mandal, B.K. Rational design of titanium oxide-coated dual Core–Shell sulfur nanocomposite cathode for highly stable lithium–sulfur batteries. J. Phys. Chem. Solids 2021, 149, 109791. [Google Scholar] [CrossRef]
- Dunya, H.; Yue, Z.; Ashuri, M.; Mei, X.; Lin, Y.; Kucuk, K.; Aryal, S.; Segre, C.U.; Mandal, B.K. A new graphitic carbon nitride-coated dual Core–Shell sulfur cathode for highly stable lithium–sulfur cells. Mater. Chem. Phys. 2020, 246, 122842. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Q.; Han, J.; Tao, Y.; Liu, D.; Zhang, C.; Lv, W.; Yang, Q.H. Catalyzing polysulfide conversion by g-C3N4 in a graphene network for long-life lithium-sulfur batteries. Nano Res. 2018, 11, 3480–3489. [Google Scholar] [CrossRef]
- Wang, R.; Wang, K.; Tao, H.; Zhao, W.; Jiang, M.; Yan, J.; Jiang, K. Controllable electrolytic formation of Ti2O as an efficient sulfur host in lithium-sulfur (Li-S) batteries. J. Mater. Chem. A 2020, 8, 11224–11232. [Google Scholar] [CrossRef]
- Luo, Z.; Lei, W.; Wang, X.; Pan, J.; Pan, Y.; Xia, S. AlF3 coating as sulfur immobilizers in cathode material for high performance lithium-sulfur batteries. J. Alloys Compd. 2020, 812, 152132. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision synthesis versus bulk-scale fabrication of graphenes. Nat. Rev. Chem. 2018, 2, 100. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as electrode materials for energy storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, A.B.; Ferrero, G.A.; Diez, N.; Sevilla, M. A Green Route to High-Surface Area Carbons by Chemical Activation of Biomass-Based Products with Sodium Thiosulfate. ACS Sustain. Chem. Eng. 2018, 6, 16323–16331. [Google Scholar] [CrossRef]
- Knoop, J.E.; Ahn, S. Review recent advances in nanomaterials for high-performance Li–S batteries. J. Energy Chem. 2020, 47, 86–106. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.; Wang, X.; Shi, X.; Mulder, F.M. The electrochemical performance of super P carbon black in reversible Li/Na ion uptake. Sci. China Phys. Mech. Astron. 2017, 60, 064611. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, J.; Wang, Q.; Lin, D.; Li, K.; Zhou, L. Thermally etched porous carbon cloth catalyzed by metal organic frameworks as sulfur hosts for lithium–sulfur batteries. Carbon 2019, 150, 76–84. [Google Scholar] [CrossRef]
- Dong, C.; Gao, W.; Jin, B.; Jiang, Q. Advances in Cathode Materials for High-Performance Lithium-Sulfur Batteries. iScience 2018, 6, 151–198. [Google Scholar] [CrossRef]
- Song, M.K.; Zhang, Y.; Cairns, E.J. A long-life, high-rate lithium/sulfur cell: A multifaceted approach to enhancing cell performance. Nano Lett. 2013, 13, 5891–5899. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Z.; Huang, S.; Chen, L.; Liang, Y.; Mai, W.; Zhong, H.; Fu, R.; Wu, D. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres for enhanced adsorption and energy storage. Nat. Commun. 2015, 6, 7221. [Google Scholar] [CrossRef]
- Cho, I.; Choi, J.; Kim, K.; Ryou, M.H.; Lee, Y.M. A comparative investigation of carbon black (Super-P) and vapor-grown carbon fibers (VGCFs) as conductive additives for lithium-ion battery cathodes. RSC Adv. 2015, 5, 95073–95078. [Google Scholar] [CrossRef]
- Song, J.-Y.; Lee, H.-H.; Hong, W.G.; Huh, Y.S.; Lee, Y.S.; Kim, H.J.; Jun, Y.-S. A Polysulfide-Infiltrated Carbon Cloth Cathode for High-Performance Flexible Lithium–Sulfur Batteries. Nanomaterials 2018, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wu, J.; Ma, Z.; Li, B.; Zhang, X.; Zu, X.; Xiang, X.; Li, S. A melt diffusion strategy for tunable sulfur loading on CC@MoS2 for lithium–sulfur batteries. Energy Rep. 2020, 6, 172–180. [Google Scholar] [CrossRef]
- Ni, Y.; Liao, K.; Li, J. In situ template route for synthesis of porous Ni12P5 superstructures and their applications in environmental treatments. CrystEngComm 2010, 12, 1568–1575. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, X.; Zhou, G.; Zhang, W.; Luo, J.; Huang, H.; Gan, Y.; Liang, C.; Xia, Y.; Zhang, J.; et al. Efficient Activation of Li2S by Transition Metal Phosphides Nanoparticles for Highly Stable Lithium-Sulfur Batteries. ACS Energy Lett. 2017, 2, 1711–1719. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Tian, W.; Chen, W.; Xi, B.; Li, H.; Feng, J.; Xiong, S. Ni12P5 nanoparticles bound on graphene sheets for advanced lithium-sulfur batteries. Nanoscale 2020, 12, 10760–10770. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, C.; Chen, X.; Liang, P.; Wan, H.; Liu, X.; Tan, Q.; Wu, H.; Rao, H.; Wang, H.; et al. High conductivity Ni12P5 nanowires as high-rate electrode material for battery-supercapacitor hybrid devices. Chem. Eng. J. 2020, 392, 123661. [Google Scholar] [CrossRef]
- Mandal, S.; Bera, T.; Dubey, G.; Saha, J.; Laha, J.K. Uses of K2S2O8 in Metal-Catalyzed and Metal-Free Oxidative Transformations. ACS Catal. 2018, 8, 5085–5144. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X.; Zhu, Z.; Dai, J. A simple mild hydrothermal route for the synthesis of nickel phosphide powders. Ceram. Int. 2010, 36, 1155–1158. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Bai, A.; Li, P.; Li, J.; Zhang, X.; Yu, M.; Wang, J.; Sun, H. Preparation of a Nitrogen-Doped Reduced Graphene Oxide-Modified Graphite Felt Electrode for VO2+/VO2+ Reaction by Freeze-Drying and Pyrolysis Method. J. Chem. 2019, 2019, 8958946. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Zhang, F.; Liu, C.; Shaw, L.L. PVP-Assisted Synthesis of Uniform Carbon Coated Li2S/CB for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2015, 7, 25748–25756. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Z.; Zhang, H.; Tan, H.; Yu, J.; Wu, J. Mesoporous β-MnO2/sulfur composite as cathode material for Li–S batteries. Electrochim. Acta 2013, 106, 307–311. [Google Scholar] [CrossRef]
- Medenbach, L.; Escher, I.; Köwitsch, N.; Armbrüster, M.; Zedler, L.; Dietzek, B.; Adelhelm, P. Sulfur Spillover on Carbon Materials and Possible Impacts on Metal–Sulfur Batteries. Angew. Chem. Int. Ed. 2018, 57, 13666–13670. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzanowicz, A.M.; Abeywickrama, T.M.; Lin, H.; Alramahi, D.; Segre, C.U.; Mandal, B.K. Scalable Ni12P5-Coated Carbon Cloth Cathode for Lithium–Sulfur Batteries. Energies 2024, 17, 4356. https://doi.org/10.3390/en17174356
Suzanowicz AM, Abeywickrama TM, Lin H, Alramahi D, Segre CU, Mandal BK. Scalable Ni12P5-Coated Carbon Cloth Cathode for Lithium–Sulfur Batteries. Energies. 2024; 17(17):4356. https://doi.org/10.3390/en17174356
Chicago/Turabian StyleSuzanowicz, Artur M., Thulitha M. Abeywickrama, Hao Lin, Dana Alramahi, Carlo U. Segre, and Braja K. Mandal. 2024. "Scalable Ni12P5-Coated Carbon Cloth Cathode for Lithium–Sulfur Batteries" Energies 17, no. 17: 4356. https://doi.org/10.3390/en17174356
APA StyleSuzanowicz, A. M., Abeywickrama, T. M., Lin, H., Alramahi, D., Segre, C. U., & Mandal, B. K. (2024). Scalable Ni12P5-Coated Carbon Cloth Cathode for Lithium–Sulfur Batteries. Energies, 17(17), 4356. https://doi.org/10.3390/en17174356








