Dual Effect of Hydrothermal Fluid on Shale Oil Reservoir in Gulong Sag, Songliao Basin: Constrained by C-O Isotope and Geochemistry
Abstract
1. Introduction
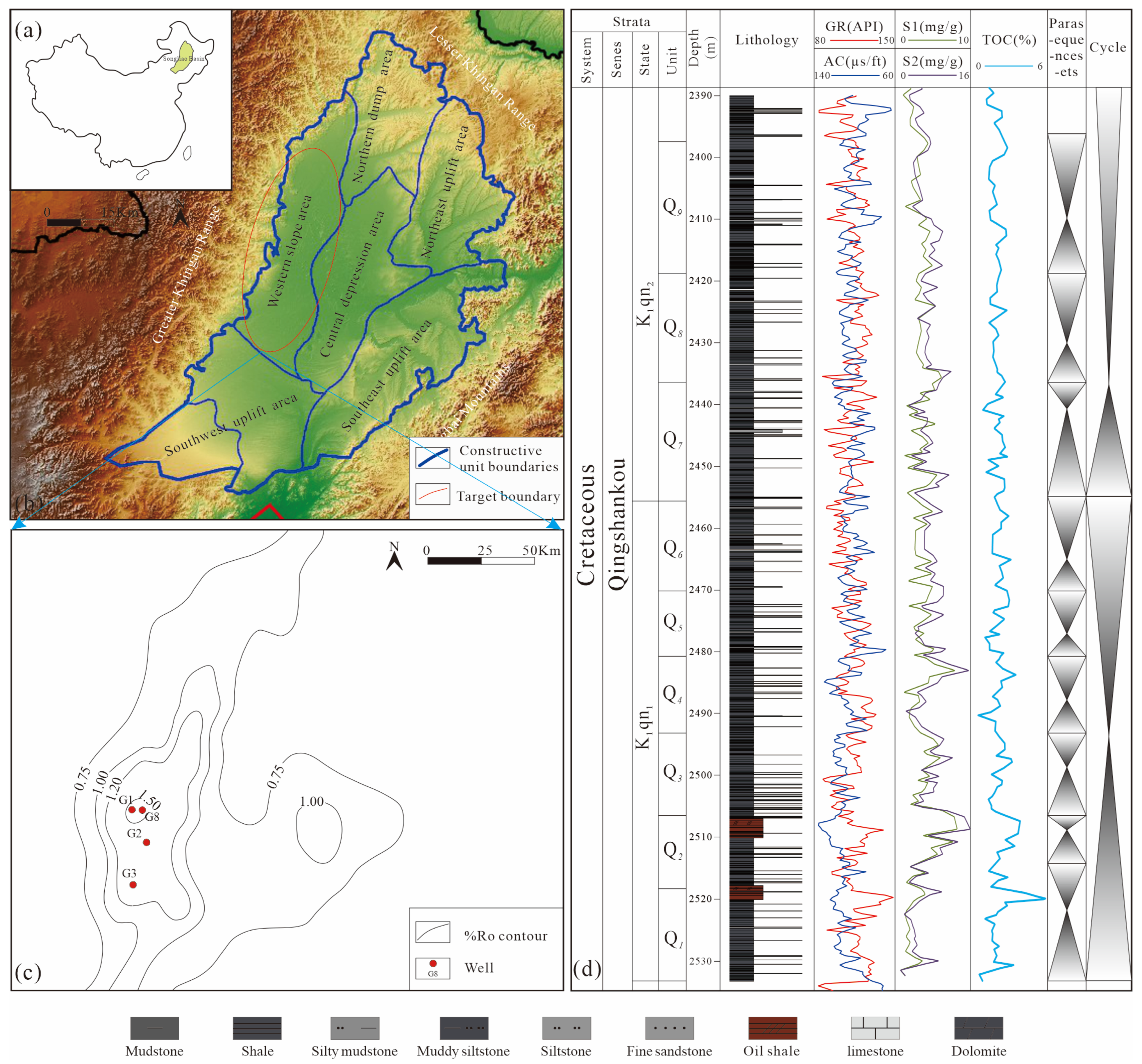
2. Geological Setting
3. Materials and Methods
3.1. Samples
3.2. Experiments
- FE-SEM and EDS
- 2.
- C-O isotope
- 3.
- XRD
4. Results
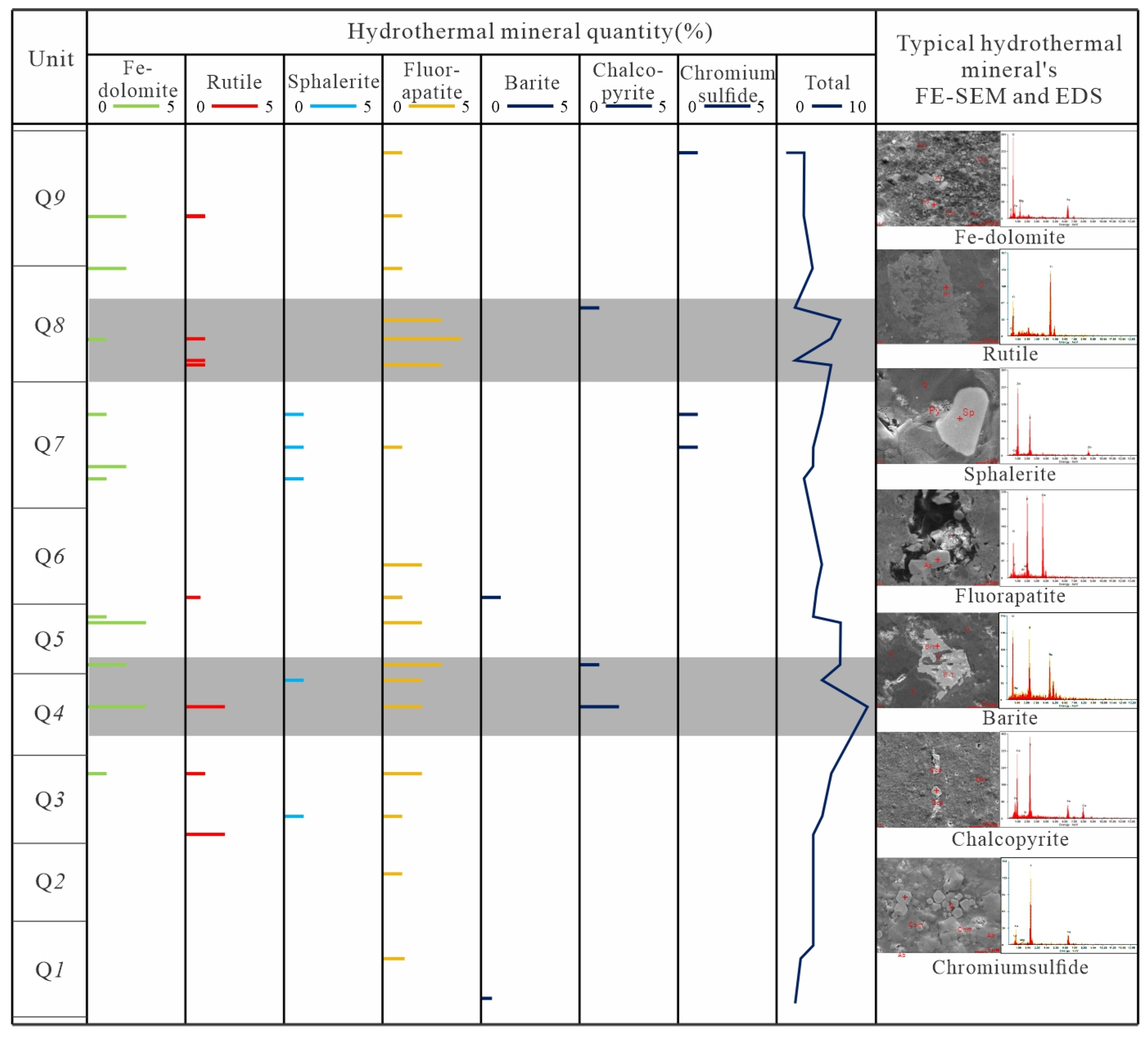
4.1. Petrological and Mineral Characteristics
4.2. Geochemical Characteristics
4.3. Brittleness Characteristics
5. Discussions
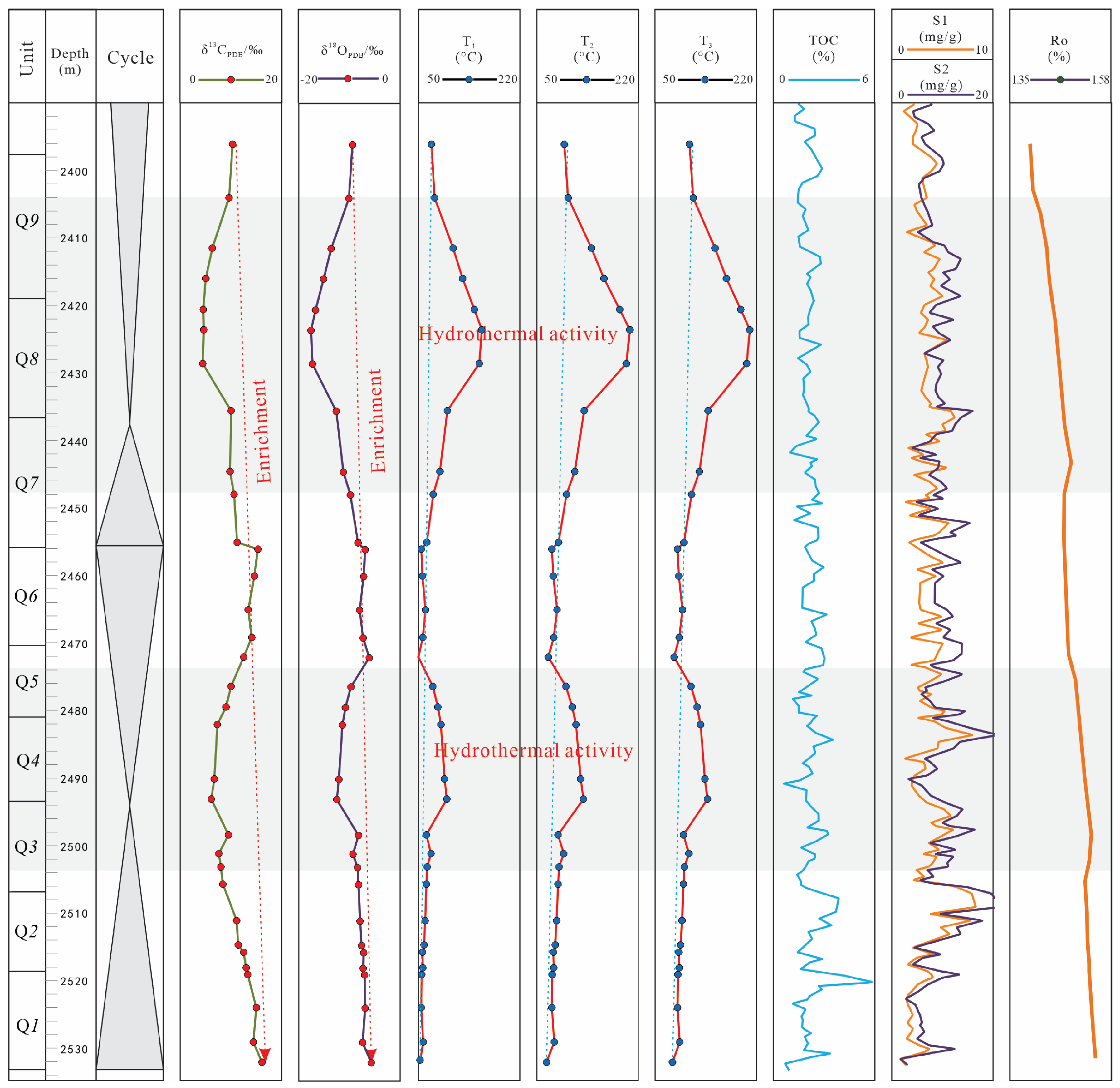
5.1. The Range Affected by Hydrothermal Fluids
5.1.1. Minerals Character
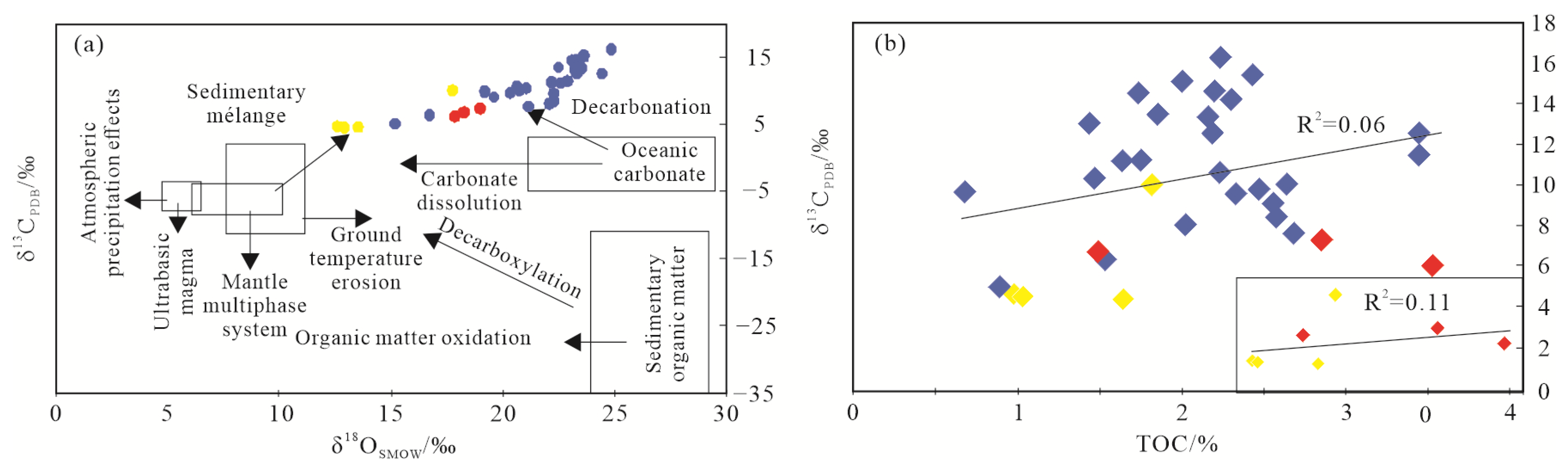
5.1.2. C-O Isotope Character
5.2. The Effect of Hydrothermal Fluid on Organic Matter Enrichment
5.3. The Dual Effect of Hydrothermal Fluid on Brittleness
6. Conclusions
- (1)
- Typical hydrothermal mineral identification (such as rutile, sphalerite, fluorapatite, barite, chalcopyrite, chromium sulfide) and shift to negative of C-O isotope character from Q1 to Q9 implied Q4 and Q8 unit are the main hydrothermal fluid influence area, and its effect become weaker in other units.
- (2)
- The Ro, TOC and S1 + S2 evolution character, accompany with the volcanic ash strata distribution character, implied that the hydrothermal activity cannot proliferation of algae blooms by nutrient elements input, but its high temperature (around 208 °C), calculated by δ18O temperature calculation formulas, promote the organic matter mature process around Q4 and Q8.
- (3)
- The high temperature and element from hydrothermal fluid changed the minerals composition and decreased the brittleness index based on XRD, though the overpressure caused by this process has a protection for the shale reservoir, but it also has a certain consistent effect on the formation of brittle minerals.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Bai, L.; Chi, Y.; Jia, R.; Fu, X.; Yang, L. Geochemical characterization and quantitative evaluation of shale oil reservoir by two-dimensional nuclear magnetic resonance and quantitative grain fluorescence on extract: A case study from the Qingshankou Formation in Southern Songliao Basin, northeast China. Mar. Pet. Geol. 2019, 109, 561–573. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Fu, X.; Bai, Y.; Bai, L.; Jia, M.; He, B. Lithofacies and depositional setting of a highly prospective lacustrine shale oil succession from the Upper Cretaceous Qingshankou Formation in the Gulong sag, northern Songliao Basin, northeast China. AAPG Bull. 2019, 103, 405–432. [Google Scholar] [CrossRef]
- Zhao, Z.; Littke, R.; Zieger, L.; Hou, D.; Froidl, F. Depositional environment, thermal maturity and shale oil potential of the Cretaceous Qingshankou Formation in the eastern Changling Sag, Songliao Basin, China: An integrated organic and inorganic geochemistry approach. Int. J. Coal Geol. 2020, 232, 103621. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, H.; Ren, L.; Deng, S.; Liu, Z. Carbon isotope fractionation characteristics during the oil shale water extraction process and its implications. Energy Fuels 2022, 36, 2993–3002. [Google Scholar] [CrossRef]
- He, W.; Zhu, R.; Cui, B.; Zhang, S.; Meng, Q.; Bai, B.; Feng, Z.; Lei, Z.; Wu, S.; He, K.; et al. The Geoscience Frontier of Gulong Shale Oil: Revealing the Role of Continental Shale from Oil Generation to Production. Engineering 2023, 28, 79–92. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, R.; Cai, Y.; Wang, H.; Lv, D.; Zou, H.; Fu, X.; Liu, C.; Cui, K.; Zhang, S.; et al. Distribution of organic matter in the Qingshankou Formation Shale, Gulong Sag, Songliao Basin observed within an isochronous sequence stratigraphic framework. Oil Gas Geol. 2023, 44, 869–886. (In Chinese) [Google Scholar]
- Fu, X.; Shi, H.; Meng, Q.; Liu, B.; Liang, J.; He, J.; Ran, Q.; Gong, L.; Jia, R. Controlling effects of the structure and deposition on the shale oil enrichment: Taking Formation qn1 in the Central Depression of Songliao Basin as an instance. Pet. Geol. Oilfield Dev. Daqing 2020, 39, 56–71. (In Chinese) [Google Scholar]
- Tao, H.; Qiu, Z.; Qu, Y.; Liu, J.; Qin, Z.; Xie, Z.; Qiu, J.; Liu, B. Geochemistry of Middle Permian lacustrine shales in the Jimusar Sag, Junggar Basin, NW China: Implications for hydrothermal activity and organic matter enrichment. J. Asian Earth Sci. 2022, 232, 505267. [Google Scholar] [CrossRef]
- You, J.; Liu, Y.; Li, Y.; Zhou, D.; Zheng, Q.; Yang, Y.; Shi, J.; Gao, H. Influencing factor of Chang 7 oil shale of Triassic Yanchang Formation in Ordos Basin: Constraint from hydrothermal fluid. J. Pet. Sci. Eng. 2021, 201, 108532. [Google Scholar] [CrossRef]
- Cao, J.; Luo, J.; Fan, C.; Li, S.; Wu, S.; Fu, Y.; Shi, X.; Dai, L.; Hou, J. Deep thermal fluid activity and its influence on the diagenesis and pore evolution of reservoirs: A case study from the Miocene Huangliu Formation reservoir in the LDX area, Yinggehai Basin, northern South China Sea. Earth Sci. Front. 2022, 29, 412–429. (In Chinese) [Google Scholar]
- Zhang, L.; Zhang, F.; Zhao, Z.; Gao, J.; Li, C.; Wang, Q.; Zhu, Z. Research progress on identification methods and geological significance for oil and gas of hydrothermal fluid activity. Mar. Pet. Geol. 2023, 28, 225–238. (In Chinese) [Google Scholar]
- Liu, J.; Liu, W.; Wang, C. Hydrothermal Fluids Flow Types in Sedimentary Basins and Its Significance of Petroleum Geology. Offshore Oil 2004, 24, 8–13. (In Chinese) [Google Scholar]
- Chen, Z.; Meng, X.; Song, X.; Long, K. Analysis of sequences and sources of diagenetic fluid in Leikoupo Formation, Western Sichuan Basin. Pet. Geol. Recovery Effic. 2021, 28, 33–40. (In Chinese) [Google Scholar]
- Xie, X.; Cheng, J.; Meng, Y. Basin fluid flow and associated diagenetic processes. Acta Sedmentological Sin. 2009, 27, 863–871. (In Chinese) [Google Scholar]
- Crerar, D.; Namson, J.; Chyi, M.; Williams, L.; Feigenson, M. Manganiferous cherts of the Franciscan assemblage. 1. General geology, ancient and modern analogs, and implications for hydrothermal convection at oceanic spreading centers. Econ. Geol. 1982, 77, 519–540. [Google Scholar] [CrossRef]
- Tiercelin, J.; Pflumio, C.; Castrec, M.; Boulégue, J.; Gente, P.; Rolet, J.; Coussement, C.; Stetter, K.; Huber, R.; Buku, S.; et al. Hydrothermal vents in Lake Tanganyika, East African Rift system. Geology 1993, 21, 499–502. [Google Scholar] [CrossRef]
- Zhong, D.; Jiang, Z.; Guo, Q.; Sun, H. A review about research history, situation and prospects of hydrothermal sedimentation. J. Palaeogeogr. 2015, 17, 285–296. [Google Scholar]
- He, C.; Ji, L.; Su, A.; Liu, Y.; Li, J.; Wu, Y.; Zhang, M. Relationship between hydrothermal sedimentation process and source rock development in the Yanchang Formation in southern Ordos Basin. Earth Sci. Front. 2017, 24, 277–285. [Google Scholar]
- Karl, D.; Wirsen, C.; Jannasch, H. Deep-sea primary production at the Galapagos hydrothermal vents. Science 1980, 2017, 1345–1347. [Google Scholar] [CrossRef]
- You, J.; Liu, Y.; Zhou, D.; Zheng, Q.; Vasichenko, K.; Chen, Z. Activity of hydrothermal fluid at the bottom of a lake and its influence on the development of high-quality source rocks: Triassic Yanchang Formation, southern Ordos Basin, China. Aust. J. Earth Sci. 2020, 67, 115–128. [Google Scholar] [CrossRef]
- Sun, B.; Yang, W. Folded calcite cracks in noncalcareous shales: A window into shale diagenesis and hydrothermal influence. J. Sediment. Res. 2023, 93, 875–894. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, Y.; Liang, C. Characteristics and origin of crystalline dolomite: A case from Paleogene lacustrine fine-grained rocks in Jiyang depression, Bohai Bay Basin, China. Mar. Pet. Geol. 2024, 162, 106694. [Google Scholar] [CrossRef]
- Li, D.; Huang, Y.; Cheng, H.; Wang, E.; Huang, Q.; Wang, H.; Wang, W.; Duan, C. Brittleness Estimation from Late Permian Mud Shale Well-Log Data at Songliao Basin Applying Rock Physical Simulation. Pure Appl. Geophys. 2023, 180, 3919–3945. [Google Scholar] [CrossRef]
- Liu, Y.; Duo, Y.; Zhang, M.; Meng, S.; Wang, S.; Su, Y.; Xu, L. Application of nano-scratch technology to identify continental shale mineral composition and distribution length of bedding interfacial transition zone—A case study of Cretaceous Qingshankou formation in Gulong Depression, Songliao Basin, NE China. Geoenergy Sci. Eng. 2024, 234, 212674. [Google Scholar]
- Liu, C.; Wang, Z.; Guo, Z.; Hong, W.; Dun, C.; Zhang, X.; Li, B.; Wu, L. Enrichment and distribution of shale oil in the Cretaceous Qingshankou Formation, Songliao Basin, Northeast China. Mar. Pet. Geol. 2017, 86, 751–770. [Google Scholar] [CrossRef]
- Chen, R.; Shang, F.; Cao, Y.; Song, L.; Li, Z. A comparative study of oil shale deposition in the Upper Cretaceous Nenjiang Formation, NE China: Evidence from petrographic and geochemical analyses. J. Pet. Sci. Eng. 2022, 219, 111130. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Zhang, J.; Chen, S.; Bai, J.; Liu, W.; Wang, Q. Hybrid Sedimentary Conditions of Organic-Rich Shales in Faulted Lacustrine Basin during Volcanic Eruption Episode: A Case Study of Shahezi Formation (K1sh Fm.), Lishu Faulted Depression, South Songliao Basin. J. China Univ. Geosci. 2022, 47, 1728–1747. [Google Scholar]
- Yuan, J.; Zhou, T.; Zhao, G. Identification marks of deep thermal fluid activity and its effect on reservoir transformation. Acta Geol. Sin. 2023, 97, 2067–2083. (In Chinese) [Google Scholar]
- Niu, P.; Han, J.; Zeng, Z.; Hou, H.; Liu, L.; Ma, G.; Guan, Y. Deep controlling factor of the geothermal field in the Songliao basin derived from magnetotelluric survey. Chin. J. Geophys. 2021, 64, 4060–4074. (In Chinese) [Google Scholar]
- Gazdewich, S.; Hauck, T.; Husson, J. Authigenic Carbonate Burial within the Late Devonian Western Canada Sedimentary Basin and Its Impact on the Global Carbon Cycle. Geochem. Geophys. Geosyst. 2024, 25, e2023GC011376. [Google Scholar] [CrossRef]
- Zhao, R.; Shan, X.; Yi, J.; Du, X.; Liang, Y.; Zhang, Y. Geochemistry of HCO3-Na thermal water from the Gudian slope: Insights into fluid origin, formation mechanism and circulation in the Yitong Basin, Northeast. Appl. Geochem. 2018, 91, 185–196. [Google Scholar] [CrossRef]
- Lawa, L.; Mahmoud, M.; Adebayo, A.; Sultan, A. Brittleness and microcracks: A new approach of brittleness characterization for shale fracking. J. Nat. Gas Sci. Eng. 2021, 87, 103793. [Google Scholar]
- Wang, K. Exploration and development prospects of unconventional oil and gas resources in China. Pet. Petrochem. Co. 2009, 17, 24–27. (In Chinese) [Google Scholar]
- Yang, X.; Yan, D.; Zhang, B.; Zhang, L.; Wei, X.; Li, T.; Zhang, J.; She, X. The impact of volcanic activity on the deposition of organic-rich shales: Evidence from carbon isotope and geochemical compositions. Mar. Pet. Geol. 2021, 128, 105010. [Google Scholar] [CrossRef]
- Zhao, R.; Shan, X.; Wu, C.; Yi, J.; Hao, G.; Wang, P. Formation and evolution of the Changbaishan volcanic geothermal system in a convergent plate boundary back-arc region constrained by boron isotope and gas data. J. Hydrol. 2019, 569, 188–202. [Google Scholar] [CrossRef]
- Lan, C.; Long, X. Redox-stratified seawater during the GOE: Evidences from rare earth elemental and C-O isotopic compositions of Paleoproterozoic BIF and carbonate rocks from the Taihua Group, North China Craton. Ore Geol. Rev. 2023, 157, 105424. [Google Scholar] [CrossRef]
- Makhnacha, A.; Pokrovskyb, B.; Murashkoa, O.; Petrov, O. Carbon and Oxygen Isotopes and Formation Conditions of the Upper Famennian Shale-Bearing Rocks in the Pripyat Trough, Southern Belarus. Lithol. Miner. Resour. 2020, 55, 24–35. [Google Scholar] [CrossRef]
- Hu, X.; Müller, I.; Zhao, A.; Ziegler, M.; Chen, Q.; Han, L.; Shi, Z. Clumped isotope thermometry reveals diagenetic origin of the dolomite layer within late Ordovician black shale of the Guanyinqiao Bed (SW China). Chem. Geol. 2022, 588, 120641. [Google Scholar] [CrossRef]
- Johnston, D.; Macdonald, F.; Gill, B.; Hoffman, P.; Schrag, D. Uncovering the Neoproterozoic carbon cycle. Nature 2012, 483, 320–323. [Google Scholar] [CrossRef]
- Kump, L.; Garrels, R. Modeling atmospheric O2 in the global sedimentary redox cycle. Am. J. Sci. 1986, 286, 337–360. [Google Scholar] [CrossRef]
- Derry, L. Causes and consequences of mid-Proterozoic anoxia. Geophys. Res. Lett. 2015, 42, 8538–8546. [Google Scholar] [CrossRef]
- Higgins, J.; Fischer, W.; Schrag, D. Oxygenation of the ocean and sediments: Consequences for the seafloor carbonate factory. Earth Planet. Sci. Lett. 2009, 284, 25–33. [Google Scholar] [CrossRef]
- Luan, G.; Azmy, K.; Meyer, R.; Olanipekun, B.; Dong, C. Origin of carbonate cements in Ben Nevis sandstones of Jeanne d’Arc Basin: Insights from geochemistry. Mar. Pet. Geol. 2023, 148, 106033. [Google Scholar] [CrossRef]
- Melezhik, V.; Fallick, A.; Pokrovsky, B. Enigmatic nature of thick sedimentary carbonates depleted in 13C beyond the canonicalmantle value: The challenges to our understanding of the terrestrial carbon cycle. Precambr. Res. 2005, 137, 131–165. [Google Scholar] [CrossRef]
- Feng, W.; Zheng, Y.; Zhou, J. Carbon and oxygen isotope Geochemistry of marbles from the Dabie-Sulu orogenic belt. Acta Petrol. Sin. 2003, 19, 468–478. (In Chinese) [Google Scholar]
- Aharon, P. Redox stratification and anoxia of the early Precambrian oceans: Implications for carbon isotope excursions and oxidation events. Precambr. Res. 2005, 137, 207–222. [Google Scholar] [CrossRef]
- Watson, A.; Bakker, D.; Ridgwell, A.; Boyd, P.; Law, C. Effect of iron supply on Southern Ocean CO2 glacial atmospheric CO2 uptake and implications for. Nature 2000, 407, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Bowie, A.; Boyd, P.; Buck, K.; Johnson, K.; Saito, M. The integral role of iron in ocean biogeochemistry. Nature 2017, 543, 51–59. [Google Scholar] [CrossRef]
- Garzanti, E. Petrographic classification of sand and sandstone. Earth Sci. Rev. 2018, 192, 545–563. [Google Scholar] [CrossRef]
- Isson, T.; Rauzi, S. Oxygen isotope ensemble reveals Earth’s seawater, temperature, and carbon cycle history. Science 2024, 383, 666–670. [Google Scholar] [CrossRef]
- Clark, I.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis: New York, NY, USA, 1997. [Google Scholar]
- Qiang, Z. Carbonate Reservoir Geology; University of Petroleum Press: Beijing, China, 2007. [Google Scholar]
- Shangola, S.; Al-Jaleel, H.; Tobia, F. Stable isotopes and rare-earth elements of carbonate rocks, Paleocene–Eocene Kolosh Formation, High Folded Zone, NE Iraq: Implication for depositional environment and Diagenesis. Carbonates Evaporites 2023, 38, 77. [Google Scholar] [CrossRef]
- Kang, S.; Sun, Y.; Qiao, M.; Li, S.; Deng, S.; Guo, W.; Li, J.; He, W. The enhancement on oil shale extraction of FeCl3 catalyst in subcritical water. Energy 2022, 238, 121763. [Google Scholar] [CrossRef]
- Friedman, I.; O’Neil, J. Compilation of stable isotopic fractionation factors of geochemical interest. In Data of Geochemistry; Fleischer, M., Ed.; U.S. Geological Survey Professional Paper 440-KK; U.S. Department of the Interior: Washington, DC, USA, 1977. [Google Scholar]
- Narthrop, D.; Clayton, R. Oxygen isotope fractionation in systems containing dolomite. J. Geol. 1966, 74, 174–196. [Google Scholar] [CrossRef]
- Fisher, S.; Land, L. Diagenetic history of the Eocene Wilcox sandstones and associated formation waters, south-Central Texas. Geochim. Cosmochim. Acta 1986, 60, 551–562. [Google Scholar] [CrossRef]
- Yang, T.; Cao, Y.; Friis, H.; Wang, Y.; Zhou, L. Diagenetic evolution and chemical changes of deep-water mudstones of Shahejie Formation in the Dongying Sag, Jiyang Depression, Eastern China. Mar. Petrol. Geol. 2018, 93, 14–32. [Google Scholar] [CrossRef]
- Shao, X.; Pang, X.; Li, M.; Qian, M.; Hu, T.; Li, Z.; Zhang, H.; Xu, Y. Hydrocarbon retention in lacustrine shales during thermal maturation: Insights from semi-open system pyrolysis. J. Pet. Sci. Eng. 2020, 184, 106480. [Google Scholar] [CrossRef]
- Zhao, R.; Ren, Q.; Deng, S.; Sun, Y.; Chang, Z. Constrain on Oil recovery stage during oil shale subcritical water extraction process based on carbon isotope fractionation character. Energies 2021, 14, 7839. [Google Scholar] [CrossRef]
- Lin, C.; Wang, W.; Dong, C.; Zhang, X.; Ren, L.; Lin, J. Status Quo of Sandstone Compaction Research and Its Advancement. Acta Sedimentol. Sin. 2020, 38, 538–553. (In Chinese) [Google Scholar]
- Zhao, R.; Shan, X.; Yi, J.; Liang, Y.; Li, C.; Qiu, C. Understanding fluid behavior through ion and isotope data from the Yitong Basin, Northeast China. Can. J. Earth Sci. 2018, 55, 308–320. [Google Scholar] [CrossRef]
- Shen, Y.; Neuweiler, F.; Immenhauser, A. Ordovician carbonate factory turnover tracked by its depositional, diagenetic, and carbon and oxygen isotope record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 621, 111570. [Google Scholar] [CrossRef]
- Hart, B.; Schieber, J.; Kalinec, J. Clay diagenesis and overpressure development in Upper Cretaceous and Tertiary shales of South Texas. Mar. Pet. Geol. 2023, 147, 105978. [Google Scholar] [CrossRef]
- Dunn, S. Stable isotopes in contact metamorphism of carbonate rocks. In Proceedings of the Teaching Petrology Workshop, Bozeman, MT, USA, 9–15 July 2003. [Google Scholar]
- Valley, J. Stable isotope geochemistry of metamorphic rocks. Rev. Mineral. Geochem. 1986, 16, 443–481. [Google Scholar]





| NO. | Sample NO. | Unit | Depth/m | δ13C/PDB/‰ | δ18O/PDB/‰ | Lithology Description |
|---|---|---|---|---|---|---|
| 1 | G8-9-1 | Q9 | 2396.1 | 10.4 | −9.5 | laminated shale |
| 2 | G8-9-2 | Q9 | 2404.0 | 9.7 | −10.2 | laminated shale |
| 3 | G8-9-3 | Q9 | 2411.5 | 6.4 | −13.7 | laminated shale |
| 4 | G8-9-5 | Q9 | 2416.0 | 5.1 | −15.2 | laminated shale |
| 5 | G8-8-1 | Q8 | 2420.6 | 4.6 | −16.8 | laminated shale |
| 6 | G8-8-2 | Q8 | 2423.6 | 4.7 | −17.7 | laminated shale |
| 7 | G8-8-3 | Q8 | 2428.6 | 4.5 | −17.4 | laminated shale |
| 8 | G8-8-5 | Q8 | 2435.6 | 10.1 | −12.7 | laminated shale |
| 9 | G8-7-2 | Q7 | 2444.6 | 9.9 | −11.3 | Dolomite-bearing banded lamellar shale |
| 10 | G8-7-3 | Q7 | 2448.0 | 10.7 | −9.9 | laminated shale |
| 11 | G8-7-5 | Q7 | 2455.1 | 11.3 | −8.4 | laminated shale |
| 12 | G8-6-1 | Q6 | 2456.1 | 15.4 | −7.0 | laminated shale |
| 13 | G8-6-2 | Q6 | 2460.1 | 14.7 | −7.3 | laminated shale |
| 14 | G8-6-3 | Q6 | 2465.1 | 13.5 | −8.1 | laminated shale |
| 15 | G8-6-5 | Q6 | 2469.2 | 14.2 | −7.4 | Dolomite-bearing banded lamellar shale |
| 16 | G8-5-2 | Q5 | 2472.1 | 12.6 | −6.2 | Dolomite-bearing banded lamellar shale |
| 17 | G8-5-3 | Q5 | 2476.5 | 10.1 | −9.8 | Dolomite-bearing banded lamellar shale |
| 18 | G8-5-4 | Q5 | 2479.5 | 9.1 | −10.9 | laminated shale |
| 19 | G8-4-1 | Q4 | 2482.1 | 7.4 | −11.5 | laminated shale |
| 20 | G8-4-3 | Q4 | 2490.1 | 6.8 | −12.2 | laminated shale |
| 21 | G8-4-4 | Q4 | 2493.1 | 6.2 | −12.6 | Dolomite-bearing banded lamellar shale |
| 22 | G8-3-2 | Q3 | 2498.4 | 9.6 | −8.3 | laminated shale |
| 23 | G8-3-3 | Q3 | 2501.2 | 7.7 | −9.4 | laminated shale |
| 24 | G8-3-4 | Q3 | 2503.1 | 8.1 | −8.5 | Dolomite-bearing banded lamellar shale |
| 25 | G8-3-5 | Q3 | 2505.7 | 8.5 | −8.3 | laminated shale |
| 26 | G8-2-2 | Q2 | 2511.1 | 11.2 | −8.0 | laminated shale |
| 27 | G8-2-3 | Q2 | 2514.7 | 11.5 | −7.7 | Dolomite-bearing banded lamellar shale |
| 28 | G8-2-4 | Q2 | 2515.8 | 12.6 | −7.3 | Dolomite-bearing banded lamellar shale |
| 29 | G8-2-5 | Q2 | 2518.1 | 13.1 | −7.4 | Dolomite-bearing banded lamellar shale |
| 30 | G8-1-1 | Q1 | 2519.1 | 13.4 | −7.1 | laminated shale |
| 31 | G8-1-3 | Q1 | 2524.0 | 15.1 | −7.0 | laminated shale |
| 32 | G8-1-5 | Q1 | 2529.1 | 14.5 | −7.5 | laminated shale |
| 33 | G8-1-6 | Q1 | 2532.1 | 16.2 | −5.8 | laminated shale |
| NO. | Sample NO. | Unit | Quartz /% | K-Feldspar /% | Plagioclase /% | Calcite /% | Fe-Dolomite /% | Siderite /% | Pyrite /% | Clay Mineral/% | Illite /% | Kaolinite /% | Chlorites /% | I/S /% | Brittleness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | G8-9-1 | Q9 | 36.6 | 1.7 | 23.8 | 1.6 | 0.0 | 0.0 | 3.5 | 32.8 | 76.0 | 0.0 | 9.0 | 15.0 | 0.50 |
| 2 | G8-9-2 | Q9 | 35.8 | 0.0 | 34.8 | 4.9 | 1.6 | 0.0 | 2.0 | 20.8 | 76.0 | 0.0 | 2.0 | 22.0 | 0.59 |
| 3 | G8-9-3 | Q9 | 30.9 | 0.0 | 25.4 | 3.3 | 1.8 | 1.34 | 3.4 | 33.8 | 73.0 | 0.0 | 10.0 | 17.0 | 0.46 |
| 4 | G8-9-5 | Q9 | 20.8 | 1.9 | 28.9 | 10.2 | 2.6 | 0.0 | 6.0 | 28.2 | 65.6 | 2.4 | 7.5 | 24.5 | 0.37 |
| 5 | G8-8-1 | Q8 | 32.6 | 0.0 | 21.4 | 3.7 | 1.0 | 1.1 | 7.2 | 33.0 | 63.0 | 0.0 | 10.0 | 26.0 | 0.47 |
| 6 | G8-8-2 | Q8 | 29.7 | 1.8 | 18.8 | 1.0 | 14.6 | 0.6 | 5.8 | 27.7 | 58.0 | 0.0 | 8.0 | 34.0 | 0.605 |
| 7 | G8-8-3 | Q8 | 32.6 | 1.56 | 19.3 | 0.8 | 2.5 | 0.0 | 6.2 | 37.1 | 56.0 | 0.0 | 8.0 | 36.0 | 0.47 |
| 8 | G8-8-5 | Q8 | 29.6 | 1.5 | 18.9 | 1.0 | 14.6 | 0.6 | 4.8 | 29.0 | 63.7 | 1.3 | 4.0 | 31.0 | 0.58 |
| 9 | G8-7-2 | Q7 | 21.4 | 0.0 | 10.9 | 0.0 | 37.1 | 0.0 | 2.9 | 27.7 | 65.4 | 0.7 | 5.0 | 28.9 | 0.66 |
| 10 | G8-7-3 | Q7 | 17.3 | 0.0 | 5.4 | 23.2 | 35.9 | 0.0 | 3.8 | 14.4 | 58.4 | 4.3 | 9.6 | 27.7 | 0.57 |
| 11 | G8-7-5 | Q7 | 2.0 | 0.0 | 2.6 | 3.0 | (88.8) | 0.0 | 0.0 | 3.6 | 45.0 | 0.0 | 28.0 | 20.0 | 0.92 |
| 12 | G8-6-1 | Q6 | 30.3 | 0.24 | 15.0 | 9.0 | 1.1 | 0.5 | 5.2 | 38.7 | 81.0 | 0.0 | 5.0 | 13.0 | 0.39 |
| 13 | G8-6-2 | Q6 | 30.4 | 0.96 | 16.9 | 2.9 | 1.1 | 0.6 | 4.6 | 42.5 | 68.0 | 0.0 | 10.0 | 20.0 | 0.40 |
| 14 | G8-6-3 | Q6 | 34.9 | 0.0 | 16.3 | 0.8 | 3.5 | 0.0 | 4.1 | 40.3 | 65.0 | 0.0 | 9.0 | 24.0 | 0.47 |
| 15 | G8-6-5 | Q6 | 31.5 | 3.36 | 25.8 | 20.8 | 5.8 | 0.0 | 2.2 | 10.9 | 63.8 | 2.2 | 10.0 | 24.0 | 0.52 |
| 16 | G8-5-2 | Q5 | 32.9 | 0.84 | 21.5 | 1.1 | 1.4 | 0.6 | 4.1 | 37.6 | 77.0 | 0.0 | 6.0 | 17.0 | 0.46 |
| 17 | G8-5-3 | Q5 | 28.5 | 0.0 | 24.7 | 0.8 | 10.4 | 0.0 | 3.1 | 32.6 | 61.0 | 0.0 | 7.0 | 31.0 | 0.52 |
| 18 | G8-5-4 | Q5 | 27.8 | 1.2 | 21.9 | 7.1 | 0.0 | 0.0 | 2.8 | 39.2 | 45.8 | 3.8 | 12.9 | 37.5 | 0.36 |
| 19 | G8-4-1 | Q4 | 1.5 | 0.0 | 1.8 | 0.0 | 93.0 | 1.4 | 0.0 | 2.3 | 74.0 | 0.0 | 7.0 | 19.0 | 0.95 |
| 20 | G8-4-3 | Q4 | 36.0 | 0.0 | 8.1 | 19.8 | 8.7 | 0.0 | 3.6 | 23.8 | 64.0 | 0.0 | 11.7 | 24.3 | 0.50 |
| 21 | G8-4-4 | Q4 | 37.9 | 0.0 | 15.4 | 0.0 | 1.7 | 0.0 | 1.9 | 43.1 | 53.0 | 0.0 | 6.0 | 41.0 | 0.46 |
| 22 | G8-3-2 | Q3 | 36.3 | 0.0 | 14.3 | 6.3 | 1.1 | 0.0 | 2.0 | 39.9 | 68.0 | 0.0 | 16.0 | 15.0 | 0.44 |
| 23 | G8-3-3 | Q3 | 30.1 | 0.0 | 10.2 | 2.9 | 0.0 | 0.0 | 0.7 | 56.1 | 55.2 | 4.6 | 9.4 | 30.8 | 0.33 |
| 24 | G8-3-4 | Q3 | 40.3 | 0.0 | 12.1 | 1.3 | 0.0 | 0.0 | 0.0 | 46.3 | 57.0 | 0.0 | 10 | 33 | 0.45 |
| 25 | G8-3-5 | Q3 | 35.6 | 0.0 | 15.0 | 0.0 | 7.0 | 0.7 | 2.2 | 39.5 | 53.0 | 0.0 | 18 | 27 | 0.50 |
| 26 | G8-2-2 | Q2 | 36.6 | 0.0 | 13.8 | 4.6 | 0.0 | 0.9 | 1.7 | 42.3 | 61.0 | 2.0 | 9.0 | 28 | 0.43 |
| 27 | G8-2-3 | Q2 | 32.58 | 0.0 | 16.9 | 1.6 | 1.1 | 0.8 | 3.3 | 43.7 | 57.0 | 0.0 | 18 | 25 | 0.41 |
| 28 | G8-2-4 | Q2 | 35 | 0.0 | 9.7 | 0.0 | 17.8 | 0.0 | 2.5 | 35 | 53.0 | 0.0 | 12 | 35 | 0.58 |
| 29 | G8-2-5 | Q2 | 30.2 | 0.0 | 9.6 | 0.0 | 18.6 | 0.0 | 0.0 | 41.5 | 55.0 | 0.0 | 27 | 18 | 0.53 |
| 30 | G8-1-1 | Q1 | 33.8 | 0.0 | 10.2 | 9.4 | 1.7 | 0.6 | 4.0 | 40.3 | 66.0 | 0.0 | 19 | 15 | 0.41 |
| 31 | G8-1-3 | Q1 | 3.0 | 0.0 | 0.5 | 92.7 | 0.0 | 0.0 | 0.6 | 3.2 | 72.0 | 0.0 | 13 | 15 | 0.03 |
| 32 | G8-1-5 | Q1 | 38 | 0.6 | 5.5 | 19.3 | 0.0 | 0.4 | 5.6 | 30.6 | 73.0 | 0.0 | 11 | 16 | 0.42 |
| 33 | G8-1-6 | Q1 | 36.8 | 0.0 | 9.6 | 12.3 | 0.0 | 0.4 | 7.0 | 33.9 | 62.0 | 0.0 | 10 | 28 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Fu, X.; Bai, Y.; Zhang, H.; Liu, Z.; Zhao, R. Dual Effect of Hydrothermal Fluid on Shale Oil Reservoir in Gulong Sag, Songliao Basin: Constrained by C-O Isotope and Geochemistry. Energies 2024, 17, 4159. https://doi.org/10.3390/en17164159
Li J, Fu X, Bai Y, Zhang H, Liu Z, Zhao R. Dual Effect of Hydrothermal Fluid on Shale Oil Reservoir in Gulong Sag, Songliao Basin: Constrained by C-O Isotope and Geochemistry. Energies. 2024; 17(16):4159. https://doi.org/10.3390/en17164159
Chicago/Turabian StyleLi, Junhui, Xiuli Fu, Yue Bai, Haixin Zhang, Zongbao Liu, and Rongsheng Zhao. 2024. "Dual Effect of Hydrothermal Fluid on Shale Oil Reservoir in Gulong Sag, Songliao Basin: Constrained by C-O Isotope and Geochemistry" Energies 17, no. 16: 4159. https://doi.org/10.3390/en17164159
APA StyleLi, J., Fu, X., Bai, Y., Zhang, H., Liu, Z., & Zhao, R. (2024). Dual Effect of Hydrothermal Fluid on Shale Oil Reservoir in Gulong Sag, Songliao Basin: Constrained by C-O Isotope and Geochemistry. Energies, 17(16), 4159. https://doi.org/10.3390/en17164159





