Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum
Abstract
1. Introduction
2. Experimental
2.1. Micro-Organism and Culture Media
2.2. Chemicals
2.3. Electrodes
2.4. Toxicity of VC
2.5. MFC
2.6. Carbon Cloth Characterization
3. Results and Discussion
3.1. Stability of VC
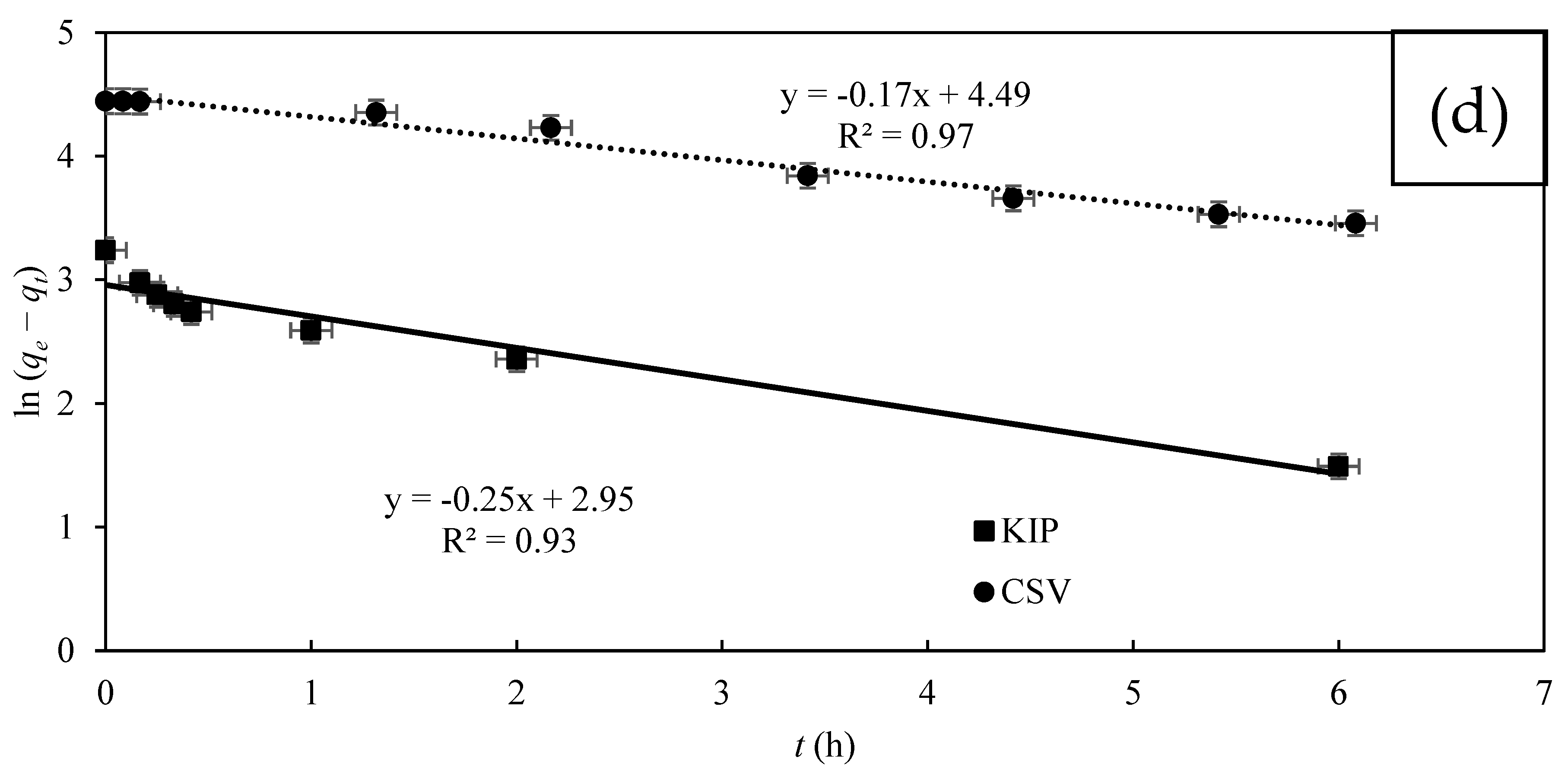
3.2. CV Removal by MFC
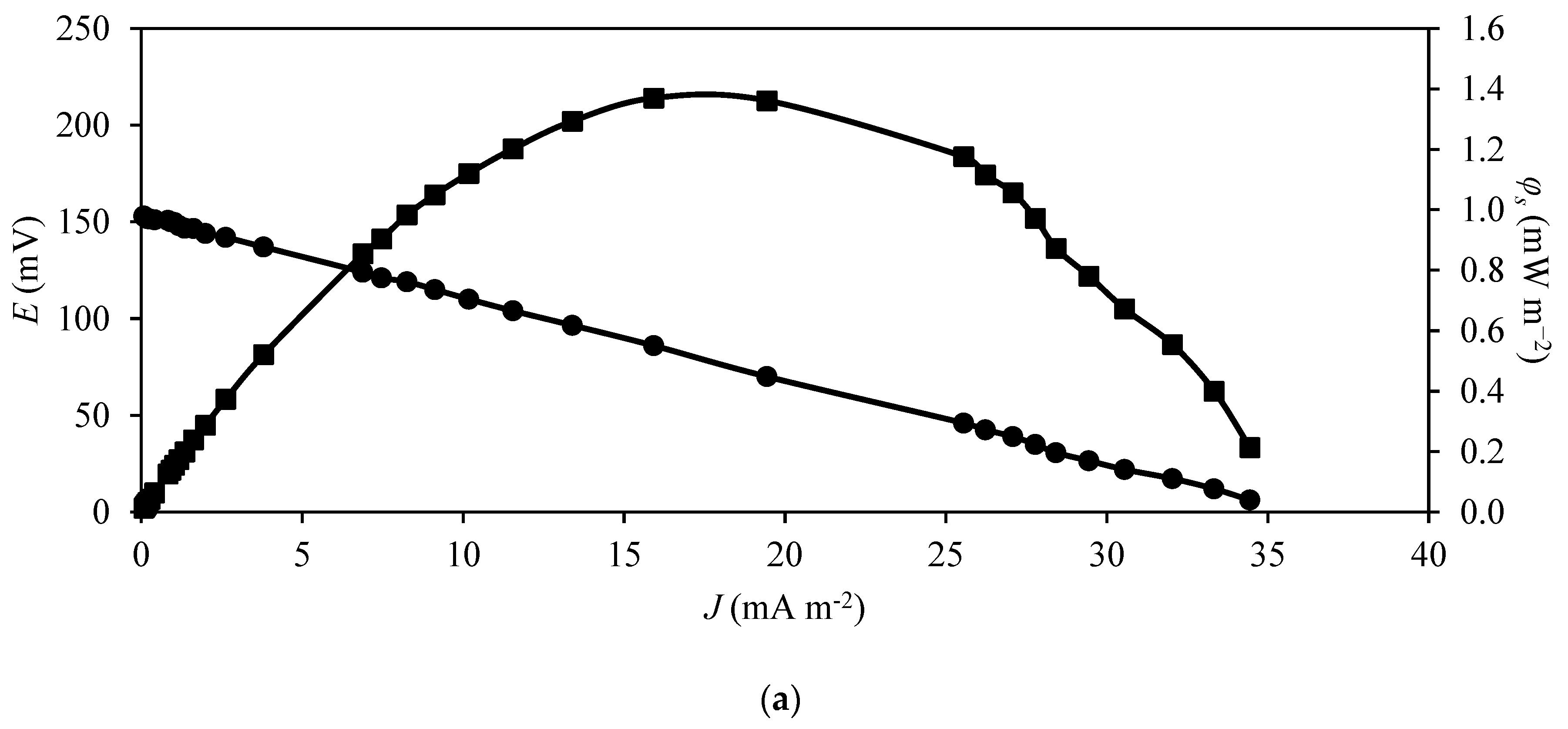
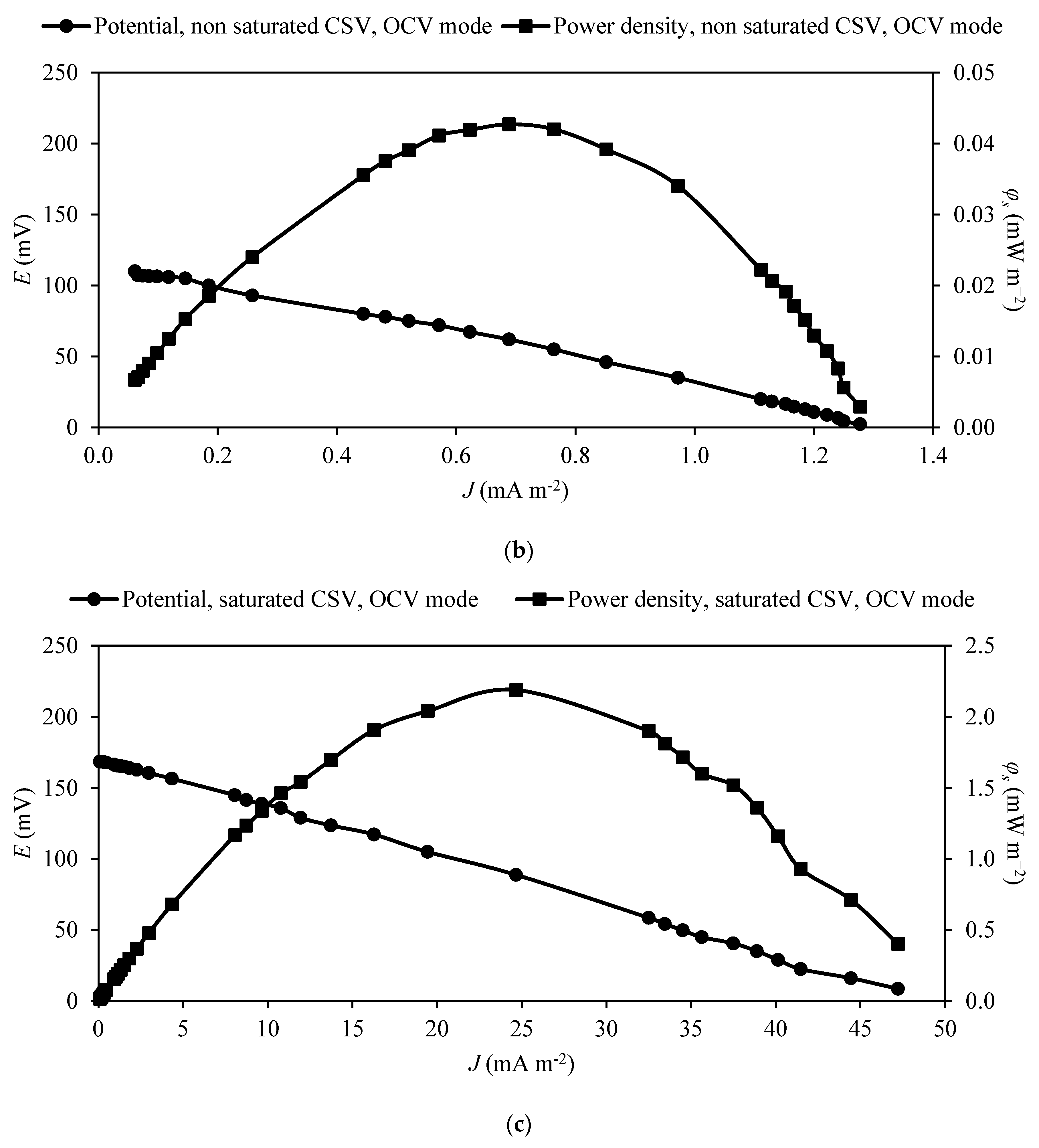
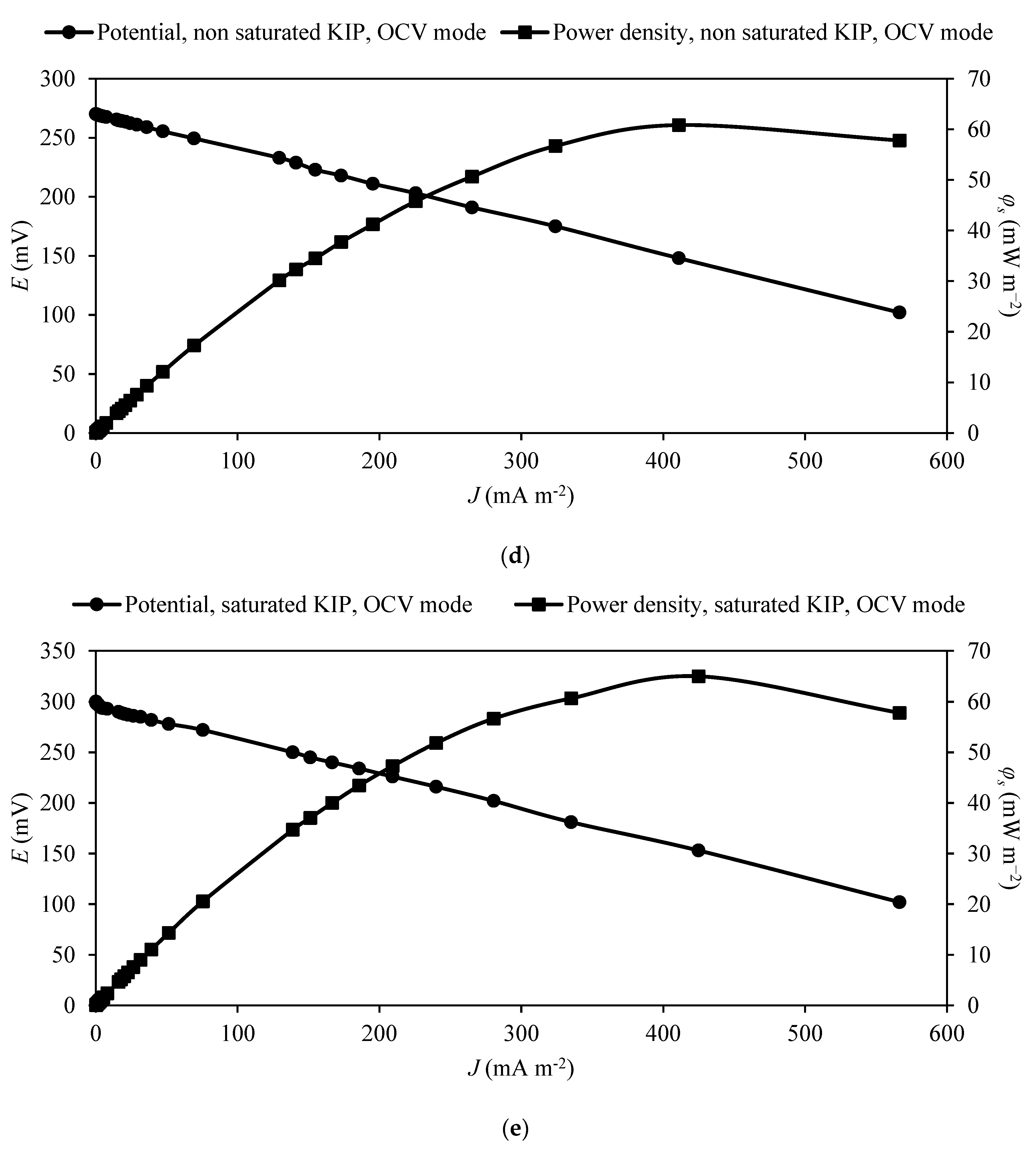
3.3. Power Production by MFC
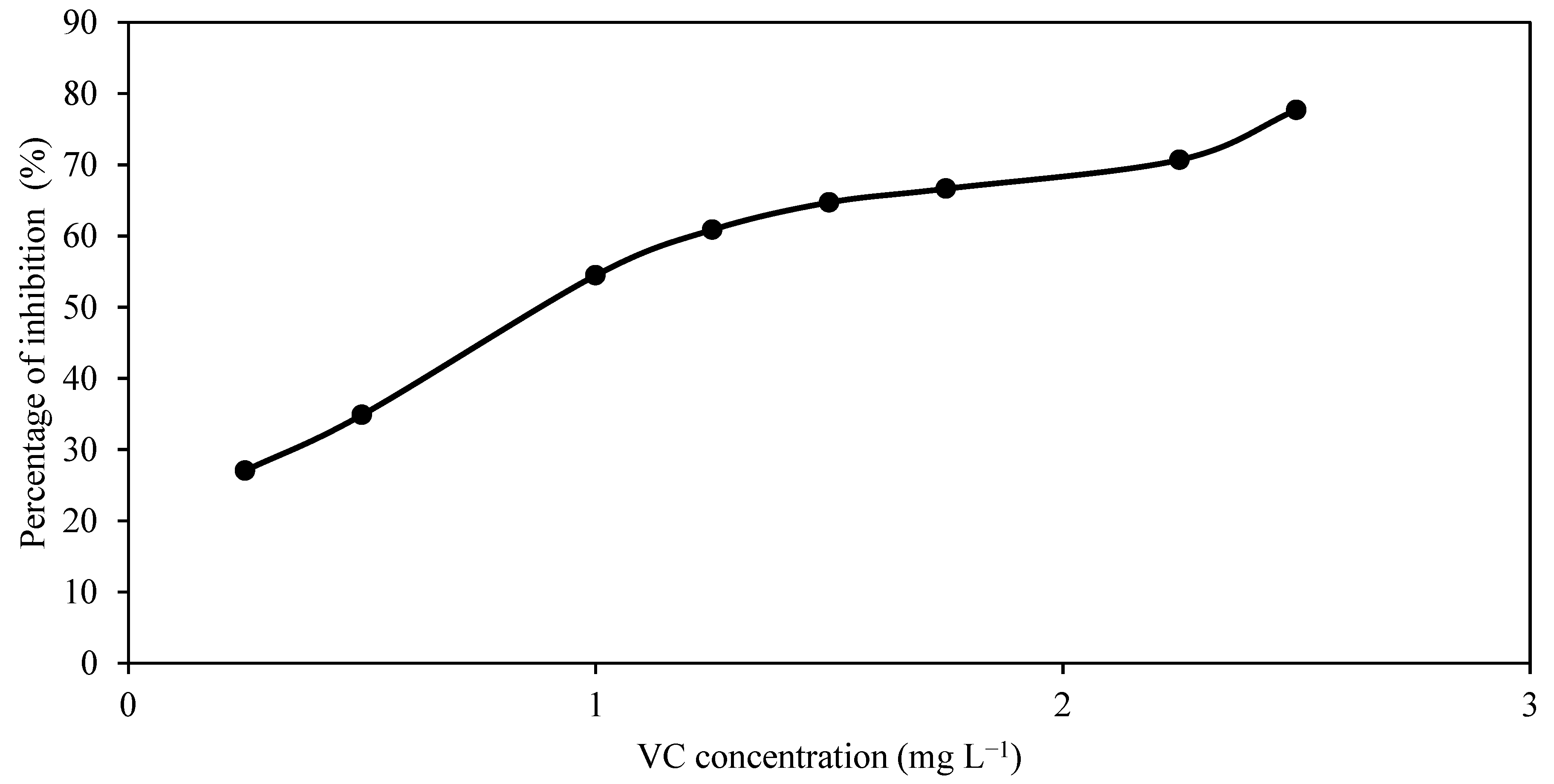
3.4. IC50 of CV for T. harzianum
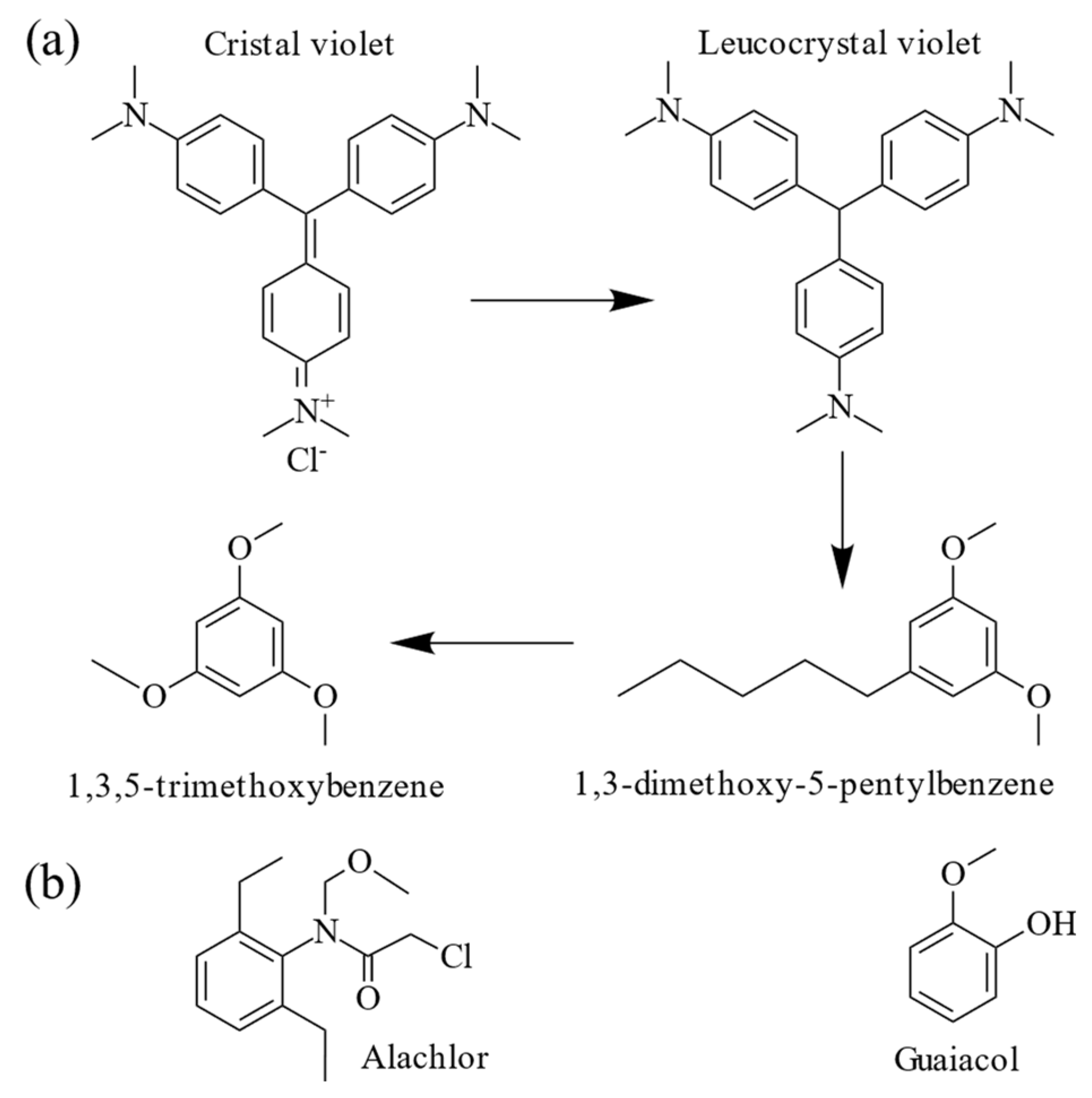
3.5. Hypothesis for the Mechanism of CV Degradation by T. harzianum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Bumpus, J.A.; Brock, B.J. Biodegradation of Crystal Violet by the White Rot Fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1988, 54, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Idaka, E.; Ogawa, T.; Yatome, C.; Horitsu, H. Behavior of activated sludge with dyes. Bull. Environ. Contam. Toxicol. 1985, 35, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Au, W.; Pathak, S.; Collie, C.J.; Hsu, T.C. Cytogenetic Toxicity of Gentian Violet and Crystal Violet on Mammalian Cells in Vitro. Mutat. Res. Genet. Toxicol. 1978, 58, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, S.A.; Zinatizadeh, A.A.; Zangeneh, H. Photocatalytic Decolorization of Direct Red16 from an Aqueous Solution Using B-ZnO/TiO2 Nano Photocatalyst: Synthesis, Characterization, Process Modeling, and Optimization. Water 2023, 15, 1203. [Google Scholar] [CrossRef]
- Karami, A.; Shomal, R.; Sabouni, R.; Al-Sayah, M.H.; Aidan, A. Parametric Study of Methyl Orange Removal Using Metal–Organic Frameworks Based on Factorial Experimental Design Analysis. Energies 2022, 15, 4642. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Walczak, P. The Use of Aminated Wheat Straw for Reactive Black 5 Dye Removal from Aqueous Solutions as a Potential Method of Biomass Valorization. Energies 2022, 15, 6257. [Google Scholar] [CrossRef]
- Claus, H.; Faber, G.; König, H. Redox-Mediated Decolorization of Synthetic Dyes by Fungal Laccases. Appl. Microbiol. Biotechnol. 2002, 59, 672–678. [Google Scholar] [CrossRef]
- Wesenberg, D.; Kyriakides, I.; Agathos, S.N. White-Rot Fungi and Their Enzymes for the Treatment of Industrial Dye Effluents. Biotechnol. Adv. 2003, 22, 161–187. [Google Scholar] [CrossRef]
- Forootanfar, H.; Moezzi, A.; Aghaie-Khozani, M.; Mahmoudjanlou, Y.; Ameri, A.; Niknejad, F.; Ali Faramarzi, M. Synthetic Dye Decolorization by Three Sources of Fungal Laccase. Iranian J. Environ. Health Sci. Eng. 2012, 9, 27. [Google Scholar] [CrossRef]
- Ostadhadi-Dehkordi, S.; Tabatabaei-Sameni, M.; Forootanfar, H.; Kolahdouz, S.; Ghazi-Khansari, M.; Faramarzi, M.A. Degradation of Some Benzodiazepines by a Laccase-Mediated System in Aqueous Solution. Bioresour. Technol. 2012, 125, 344–347. [Google Scholar] [CrossRef]
- Sadighi, A.; Faramarzi, M.A. Congo Red Decolorization by Immobilized Laccase through Chitosan Nanoparticles on the Glass Beads. J. Taiwan Inst. Chem. Eng. 2013, 44, 156–162. [Google Scholar] [CrossRef]
- D’Souza, D.T.; Tiwari, R.; Sah, A.K.; Raghukumar, C. Enhanced Production of Laccase by a Marine Fungus during Treatment of Colored Effluents and Synthetic Dyes. Enzyme Microb. Technol. 2006, 38, 504–511. [Google Scholar] [CrossRef]
- Moturi, B.; Charya Singara, M. Decolourisation of Crystal Violet and Malachite Green by Fungi. Sci. World J. 2009, 4. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ulaganathan, P.; Sivasubramanian, S.; Esakkimuthu, S.; Krishnaswamy, S.; Subramaniam, S. Trichoderma Asperellum Laccase Mediated Crystal Violet Degradation-Optimization of Experimental Conditions and Characterization. J. Environ. Chem. Eng. 2017, 5, 222–231. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical Effects Accompanying the Decomposition of Organic Compounds. R. Soc. Publ. 1911, 84, 260–276. [Google Scholar]
- Cohen, B. The Bacterial Culture as an Electrical Half-Cell. J. Bacteriol. 1931, 21, 18–19. [Google Scholar]
- Jayashree, C.; Sweta, S.; Arulazhagan, P.; Yeom, I.T.; Iqbal, M.I.I.; Rajesh Banu, J. Electricity Generation from Retting Wastewater Consisting of Recalcitrant Compounds Using Continuous Upflow Microbial Fuel Cell. Biotechnol. Bioprocess Eng. 2015, 20, 753–759. [Google Scholar] [CrossRef]
- Kebaili, H.; Kameche, M.; Innocent, C.; Ziane, F.Z.; Sabeur, S.A.; Sahraoui, T.; Ouis, M.; Zerrouki, A.; Charef, M.A. Treatment of Fruit Waste Leachate Using Microbial Fuel Cell: Preservation of Agricultural Environment. Acta Ecol. Sin. 2021, 41, 97–105. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Kakarla, R.; Min, B. Algae Cathode Microbial Fuel Cells for Electricity Generation and Nutrient Removal from Landfill Leachate Wastewater. Int. J. Hydrogen Energy 2017, 42, 29433–29442. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Agro-Food Industry Wastewater Treatment with Microbial Fuel Cells: Energetic Recovery Issues. Int. J. Hydrogen Energy 2018, 43, 500–511. [Google Scholar] [CrossRef]
- Çetinkaya, A.Y.; Köroğlu, E.O.; Demir, N.M.; Baysoy, D.Y.; Özkaya, B.; Çakmakçi, M. Electricity Production by a Microbial Fuel Cell Fueled by Brewery Wastewater and the Factors in Its Membrane Deterioration. Cuihua Xuebao/Chin. J. Catal. 2015, 36, 1068–1076. [Google Scholar] [CrossRef]
- Chen, F.; Zeng, S.; Luo, Z.; Ma, J.; Zhu, Q.; Zhang, S. A Novel MBBR–MFC Integrated System for High-Strength Pulp/Paper Wastewater Treatment and Bioelectricity Generation. Sep. Sci. Technol. 2020, 55, 2490–2499. [Google Scholar] [CrossRef]
- Naina Mohamed, S.; Ajit Hiraman, P.; Muthukumar, K.; Jayabalan, T. Bioelectricity Production from Kitchen Wastewater Using Microbial Fuel Cell with Photosynthetic Algal Cathode. Bioresour. Technol. 2020, 295, 122226. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Jiang, Z.H.; Lay, C.H.; Zhou, D. Electricity Generation from Swine Wastewater in Microbial Fuel Cell: Hydraulic Reaction Time Effect. Int. J. Hydrogen Energy 2016, 41, 21820–21826. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, X.; Wang, H.; Zhang, Y.; Gao, Y.; Bian, C.; Wang, X.; Xu, P. Power Generation and Microbial Community Analysis in Microbial Fuel Cells: A Promising System to Treat Organic Acid Fermentation Wastewater. Bioresour. Technol. 2019, 284, 72–79. [Google Scholar] [CrossRef]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse Metabolic Capacities of Fungi for Bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A. Yeast as a Biocatalyst in Microbial Fuel Cell. In Old Yeasts—New Questions; InTech: Nappanee, IN, USA, 2017. [Google Scholar]
- Pontié, M.; Jaspard, E.; Friant, C.; Kilani, J.; Fix-Tailler, A.; Innocent, C.; Chery, D.; Mbokou, S.F.; Somrani, A.; Cagnon, B.; et al. A Sustainable Fungal Microbial Fuel Cell (FMFC) for the Bioremediation of Acetaminophen (APAP) and Its Main by-Product (PAP) and Energy Production from Biomass. Biocatal. Agric. Biotechnol. 2019, 22, 101376. [Google Scholar] [CrossRef]
- Simões, M.F.; Maiorano, A.E.; dos Santos, J.G.; Peixoto, L.; de Souza, R.F.B.; Neto, A.O.; Brito, A.G.; Ottoni, C.A. Microbial Fuel Cell-Induced Production of Fungal Laccase to Degrade the Anthraquinone Dye Remazol Brilliant Blue R. Environ. Chem. Lett. 2019, 17, 1413–1420. [Google Scholar] [CrossRef]
- Osman, M.E.; Khattab, O.-K.H.; Abo-Elnasr, A.A.; Abdel Basset, S. Acid Black 172 Dye Decolorization and Bioelectricity Generation by Microbial Fuel Cell with Filamentous Fungi on Anode. Biosci. Biotechnol. Res. Asia 2018, 15, 981–986. [Google Scholar] [CrossRef]
- Gineys, M.; Benoit, R.; Cohaut, N.; Béguin, F.; Delpeux-Ouldriane, S. Behavior of Activated Carbon Cloths Used as Electrode in Electrochemical Processes. Chem. Eng. J. 2017, 310, 1–12. [Google Scholar] [CrossRef]
- Masson, S. Étude de L’adsorption de Micropolluants Émergents Sur Des Tissus de Carbone Activé. Ph.D. Thesis, Université de Grenoble, Grenoble, France, 2017. [Google Scholar]
- Kosimaningrum, W. Modification of Carbon Felt for Construction of Air-Breathing Cathode and Its Application in Microbial Fuel Cell. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2018. [Google Scholar]
- Palma-Goyes, R.E.; Guzmán-Duque, F.L.; Peñuela, G.; González, I.; Nava, J.L.; Torres-Palma, R.A. Electrochemical Degradation of Crystal Violet with BDD Electrodes: Effect of Electrochemical Parameters and Identification of Organic by-Products. Chemosphere 2010, 81, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Wang, Z.; Zhang, D. Electrochemical Oxidation of Vrystal Ciolet in the Presence of Hydrogen Peroxide. J. Chem. Technol. Biotechnol. 2010, 85, 1436–1444. [Google Scholar] [CrossRef]
- He, H.; Yang, S.; Yu, K.; Ju, Y.; Sun, C.; Wang, L. Microwave Induced Catalytic Degradation of Crystal Violet in Nano-Nickel Dioxide Suspensions. J. Hazard Mater. 2010, 173, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Mei, J.; Huang, P.; Tian, Y.; Liang, Y.; Jiang, X.; Li, M. Gα3 Subunit Thga3 Positively Regulates Conidiation, Mycoparasitism, Chitinase Activity, and Hydrophobicity of Trichoderma Harzianum. AMB Express 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.F.; Lebrun, L. Mercury Removal from Wastewater Using a Poly(Vinylalcohol)/Poly(Vinylimidazole) Complexing Membrane. Chem. Eng. J. 2010, 164, 37–48. [Google Scholar] [CrossRef]
- Fu, B.R.; Shen, C.; Ren, J.; Chen, J.Y.; Zhao, L. Advanced Oxidation of Biorefractory Organics in Aqueous Solution Together with Bioelectricity Generation by Microbial Fuel Cells with Composite FO/GPEs. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Toronto, ON, Canada, 1–3 November 2017; Institute of Physics Publishing: Bristol, UK, 2018; Volume 127. [Google Scholar]
- Danish Khan, M.; Abdulateif, H.; Ismail, I.M.; Sabir, S.; Zain Khan, M. Bioelectricity Generation and Bioremediation of an Azo-Dye in a Microbial Fuel Cell Coupled Activated Sludge Process. PLoS ONE 2015, 10, e0138448. [Google Scholar] [CrossRef]
- Annadurai, G.; Rajesh Babu, S.; Nagarajan, G.; Ragu, K. Use of Box Behnken Design of Experiments in the Production of Manganese Peroxidase by Phanerochaete Chrysosporium (MTCC 767) and Decolorization of Crystal Violet. Bioprocess Eng. 2000, 23, 715–719. [Google Scholar] [CrossRef]
- Yatome, C.; Yamada, S.; Ogawa, T.; Matsui, M. Degradation of Crystal Violet by Nocardia Corallina. Appl. Microbiol. Biotechnol. 1993, 38, 565–569. [Google Scholar] [CrossRef]
- Roy, D.C.; Biswas, S.K.; Saha, A.K.; Sikdar, B.; Rahman, M.; Roy, A.K.; Prodhan, Z.H.; Tang, S.S. Biodegradation of Crystal Violet Dye by Bacteria Isolated from Textile Industry Effluents. PeerJ 2018, 2018, e5015. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Liang, F.Y.; Chung, Y.C. Electricity Generation from Crystal Violet Using a Single-Chambered Microbial Fuel Cell Inoculated Aeromonas Hydrophila YC 57. Adv. Mater. Res. 2014, 860–863, 466–471. [Google Scholar] [CrossRef]
- Babanova, S.; Hubenova, Y.; Mitov, M. Influence of Artificial Mediators on Yeast-Based Fuel Cell Performance. J. Biosci. Bioeng. 2011, 112, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, A.; Fernando, S.; To, F. Performance of a Yeast-Mediated Biological Fuel Cell. Int. J. Mol. Sci. 2008, 9, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Mbokou, S.F.; Tonle, I.K.; Pontié, M. Development of a Novel Hybrid Biofuel Cell Type APAP/O2 Based on a Fungal Bioanode with a Scedosporium Dehoogii Biofilm. J. Appl. Electrochem. 2017, 47, 273–280. [Google Scholar] [CrossRef]
- Volpe, D.A.; Hamed, S.S.; Zhang, L.K. Use of Different Parameters and Equations for Calculation of IC 50 Values in Efflux Assays: Potential Sources of Variability in IC50 Determination. AAPS J. 2014, 16, 172–180. [Google Scholar] [CrossRef]
- JGI Database MycoCosm. Available online: https://mycocosm.jgi.doe.gov/mycocosm/home (accessed on 18 December 2023).
- Venice, F.; Davolos, D.; Spina, F.; Poli, A.; Prigione, V.P.; Varese, G.C.; Ghignone, S. Genome Sequence of Trichoderma Lixii Mut3171, a Promising Strain for Mycoremediation of Pah-Contaminated Sites. Microorganisms 2020, 8, 1258. [Google Scholar] [CrossRef]
- He, X.-L.; Song, C.; Li, Y.Y.; Wang, N.; Xu, L.; Han, X.; Wei, D. sheng Efficient Degradation of Azo Dyes by a Newly Isolated Fungus Trichoderma Tomentosum under Non-Sterile Conditions. Ecotoxicol. Environ. Saf. 2018, 150, 232–239. [Google Scholar] [CrossRef]



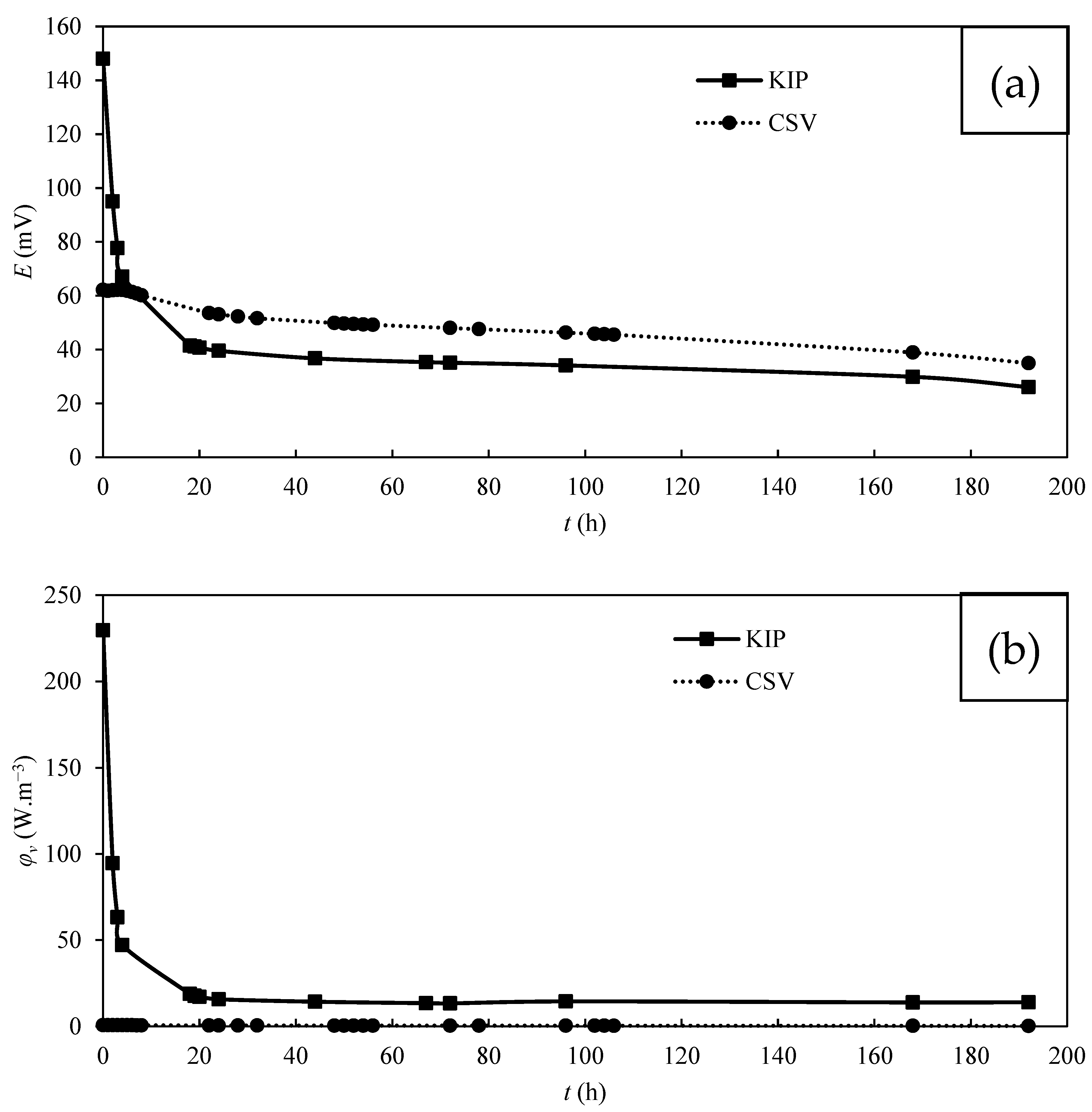
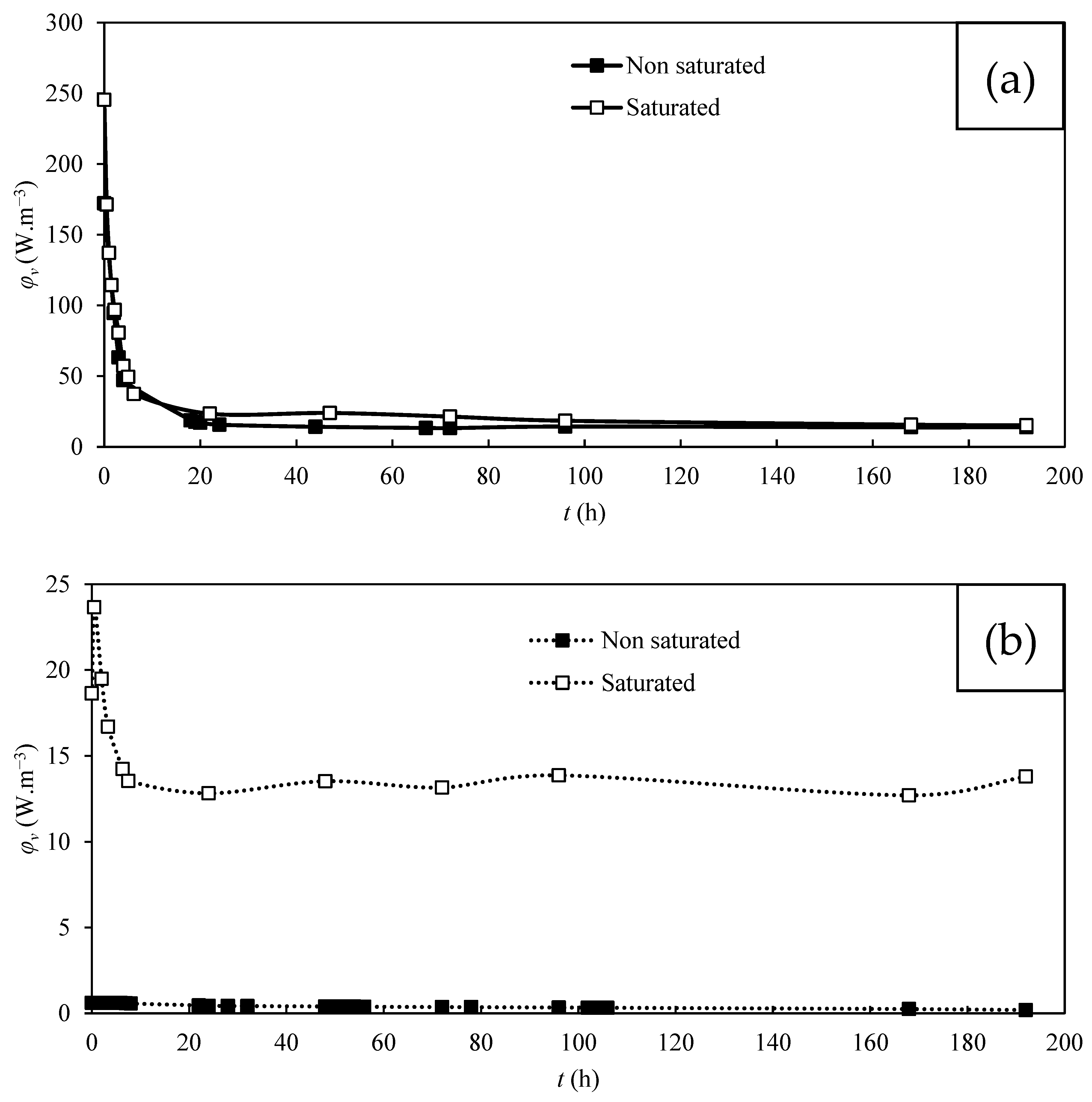
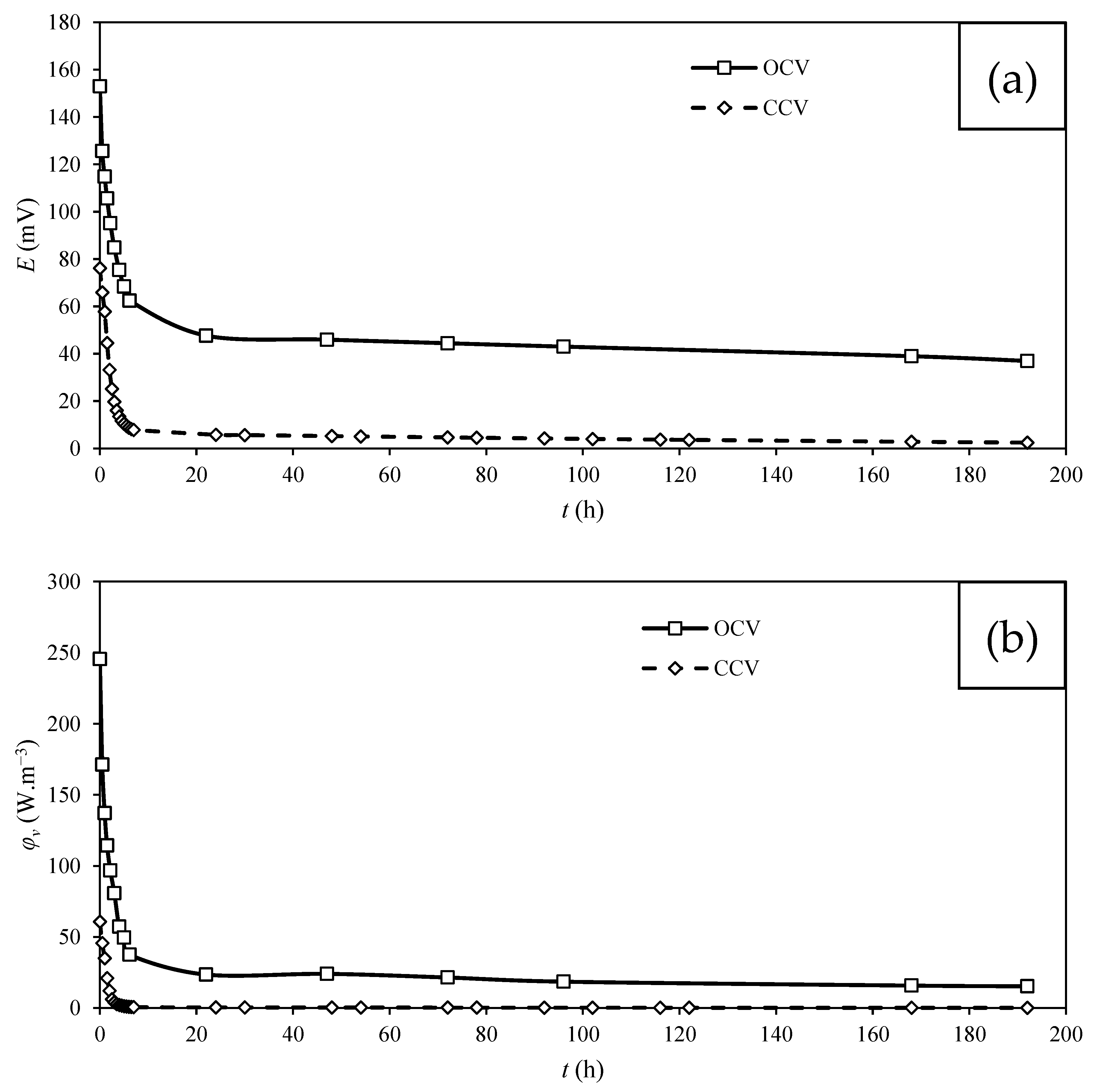
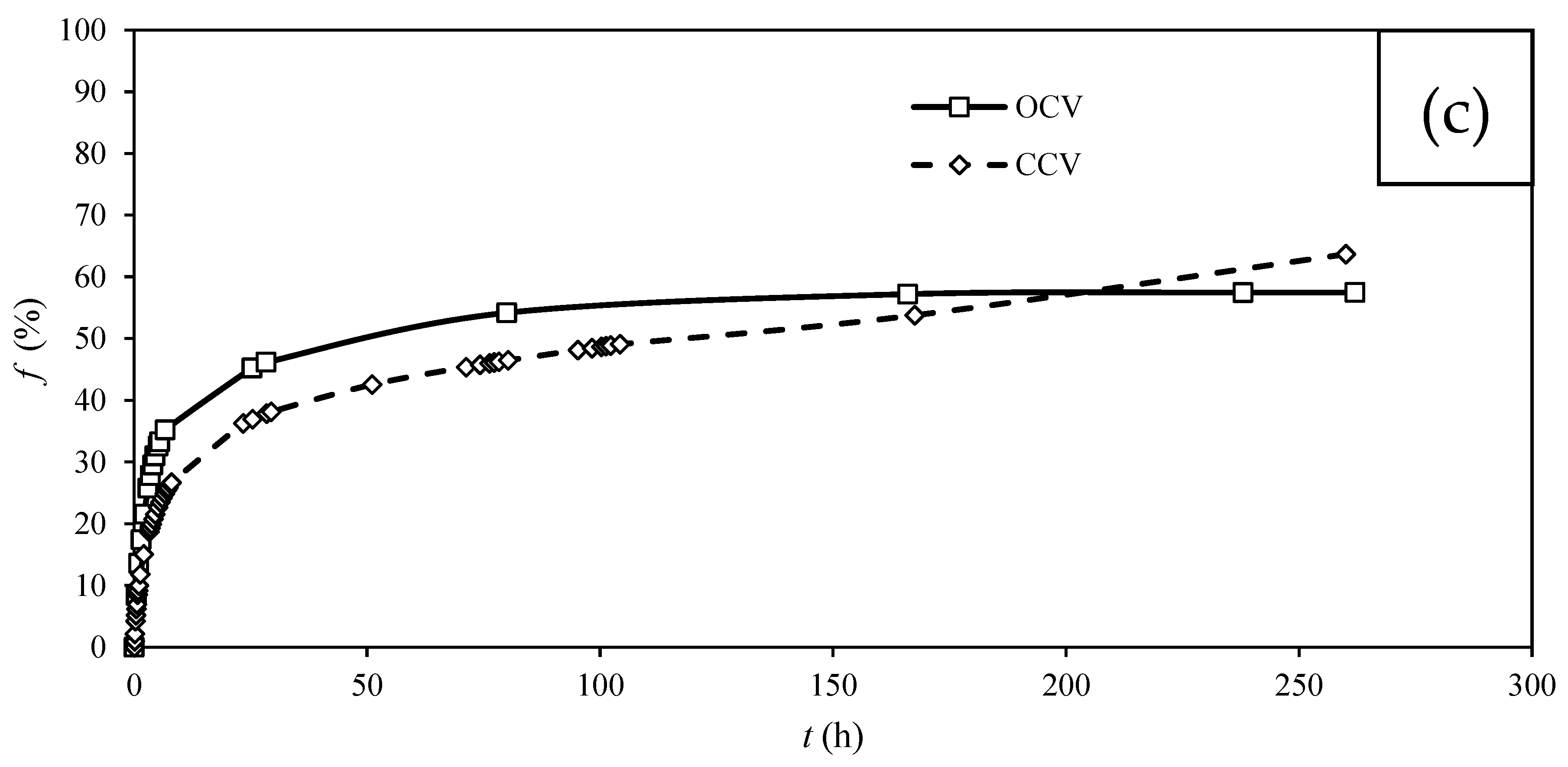

| Carbon Cloth | T. harzianum | Saturation | Operating Mode | emf (mV) | Maximum φs (mW.m−2) | Maximum φv’ (mW.m−3) | Maximum φv (W.m−3) |
|---|---|---|---|---|---|---|---|
| CSV | Yes | Non-saturated | OCV * | 111.6 | 0.8 | 14.1 | 0.6 |
| CSV | Yes | Saturated | OCV * | 178.5 | 2.7 | 49.9 | 23.6 |
| KIP | Yes | Non-saturated | OCV * | 270.1 | 58.8 | 1095.8 | 229.6 |
| KIP | No | Non-saturated | OCV * | 153.0 | 1.4 | 162.7 | 5.5 |
| KIP | Yes | Saturated | OCV * | 300.1 | 62.8 | 1170.3 | 245.4 |
| KIP | Yes | Saturated | CCV ** | 143.7 | 15.5 | 288.8 | 60.7 |
| T. harzianum’s Strains | Number of Genes |
|---|---|
| T. harzianum T22 v1.0 | 12,762 |
| T. harzianum M10 v1.0 | 12,842 |
| T. harzianum TR274 v1.0 | 13,932 |
| T. harzianum CBS 226.95 v1.0 | 14,095 |
| Enzyme families | Enzymes |
| Multicopper oxidases | 6 laccases, 2 tyrosinases |
| Monooxygenases | From 41 to 71 P450 mono-oxygenases, 1 benzoate-specific mono-oxygenase, 2 phenol 2-mono-oxygenases |
| Flavin-dependent monooxygenases | From 9 to 10 flavoprotein monooxygenases, from 0 to 2 meta-dichlorophenol 6-mono-oxygenases, from 17 to 26 flavin-binding monooxygenases-like, 2 nitroreductases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Votat, S.; Pontié, M.; Jaspard, E.; Lebrun, L. Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum. Energies 2024, 17, 247. https://doi.org/10.3390/en17010247
Votat S, Pontié M, Jaspard E, Lebrun L. Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum. Energies. 2024; 17(1):247. https://doi.org/10.3390/en17010247
Chicago/Turabian StyleVotat, Sébastien, Maxime Pontié, Emmanuel Jaspard, and Laurent Lebrun. 2024. "Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum" Energies 17, no. 1: 247. https://doi.org/10.3390/en17010247
APA StyleVotat, S., Pontié, M., Jaspard, E., & Lebrun, L. (2024). Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum. Energies, 17(1), 247. https://doi.org/10.3390/en17010247









