Ternary Fe- or Mo-Au-Ni/GDC as Candidate Fuel Electrodes for the Internal Dry Reforming of CH4: Physicochemical and Kinetic Investigation
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Powders
2.2. Preparation of Half Cells
2.3. Physicochemical Characterization
2.4. Catalytic/Kinetic Measurements
3. Results and Discussion
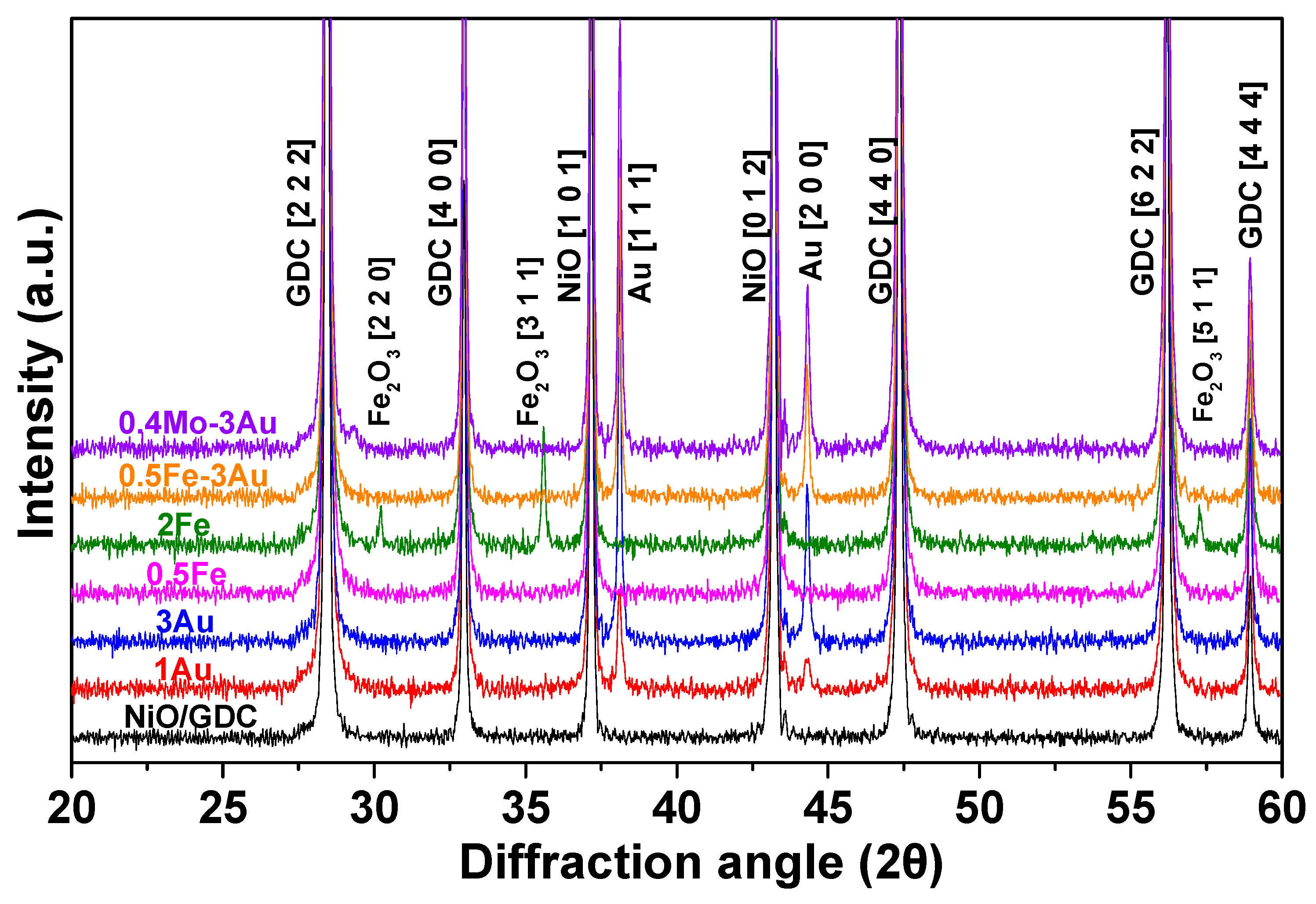
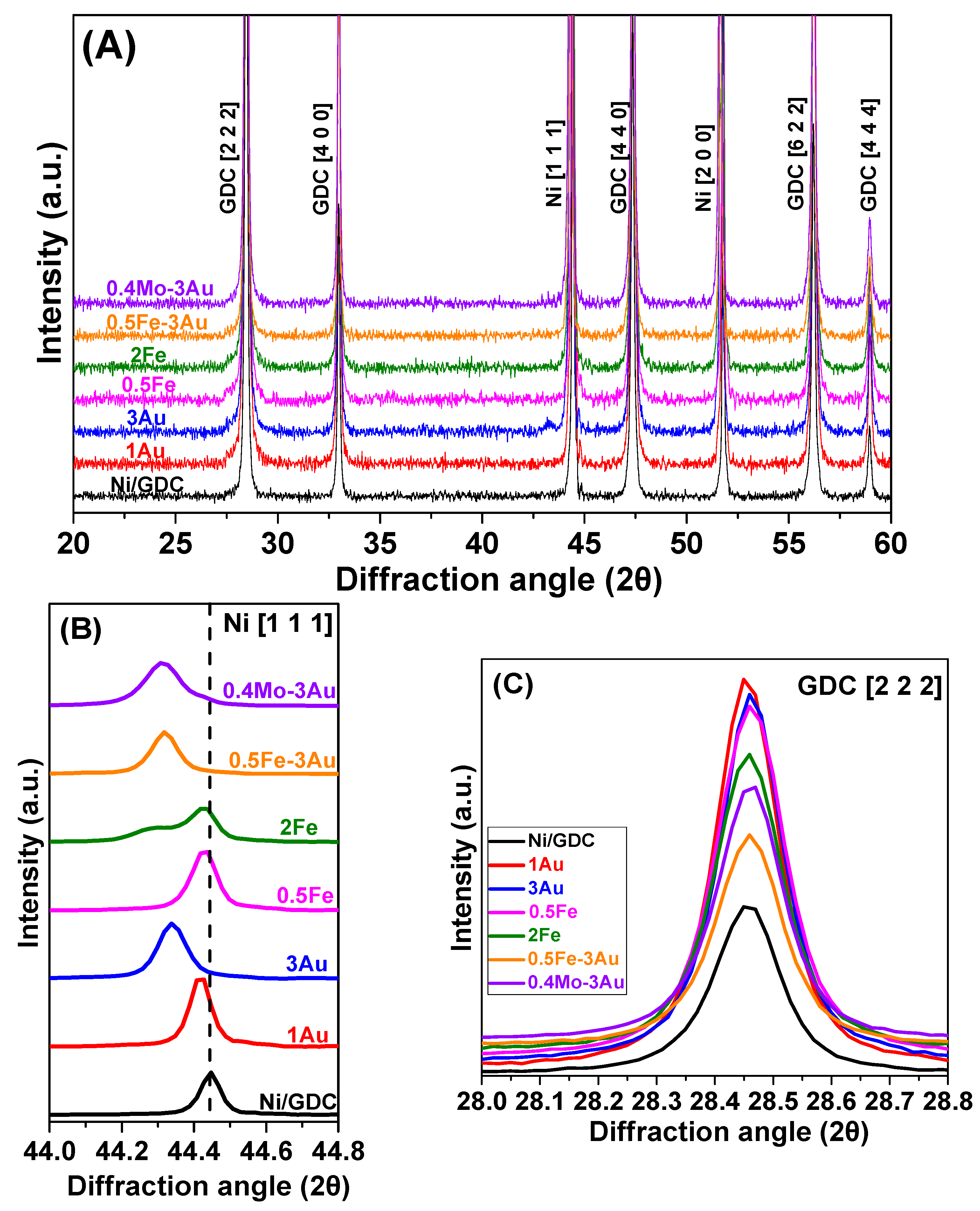
Physicochemical Characterization
4. Catalytic—Kinetic Measurements
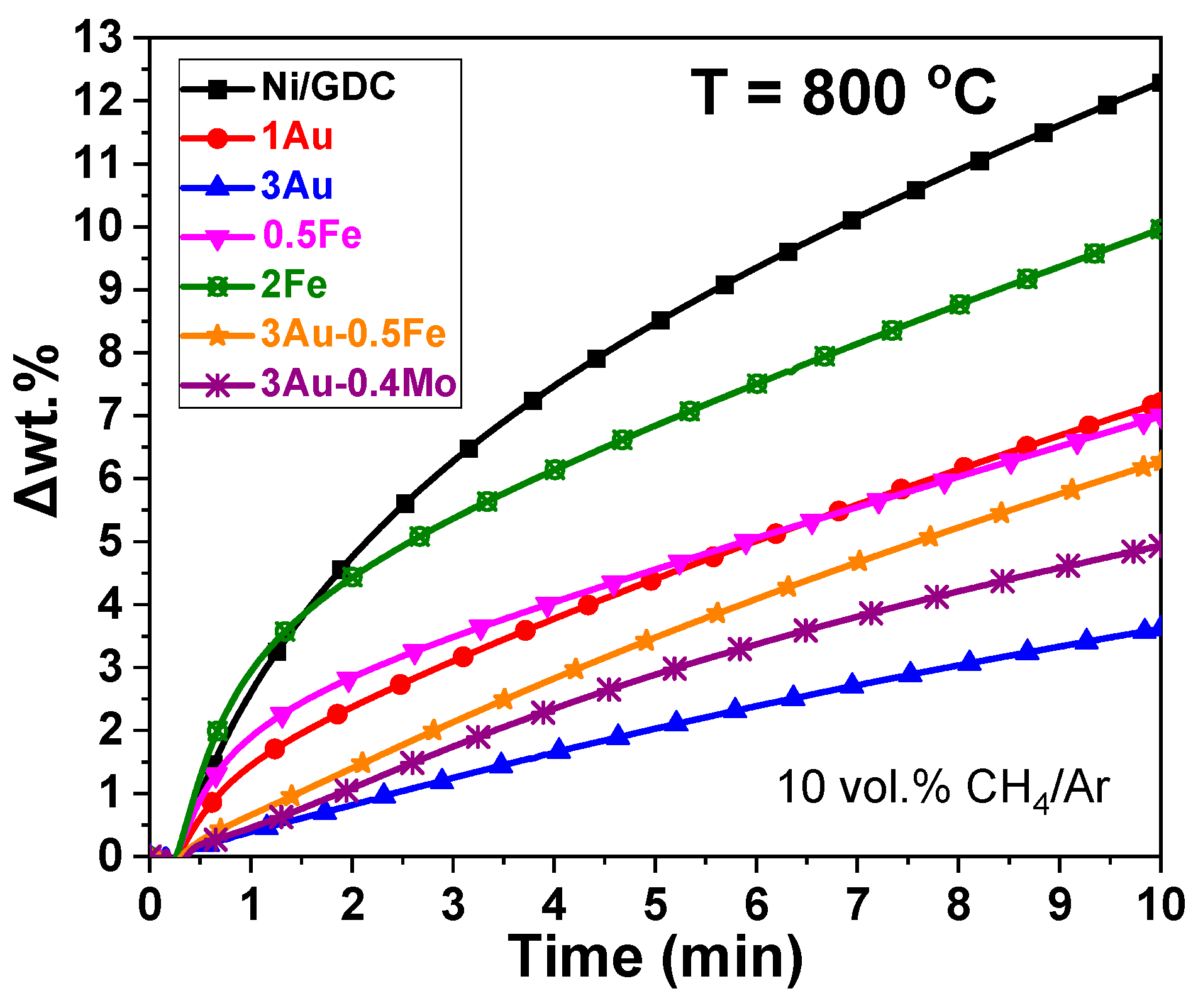
4.1. Carbon Deposition from the Catalytic CH4 Dissociation Reaction—TGA Measurements
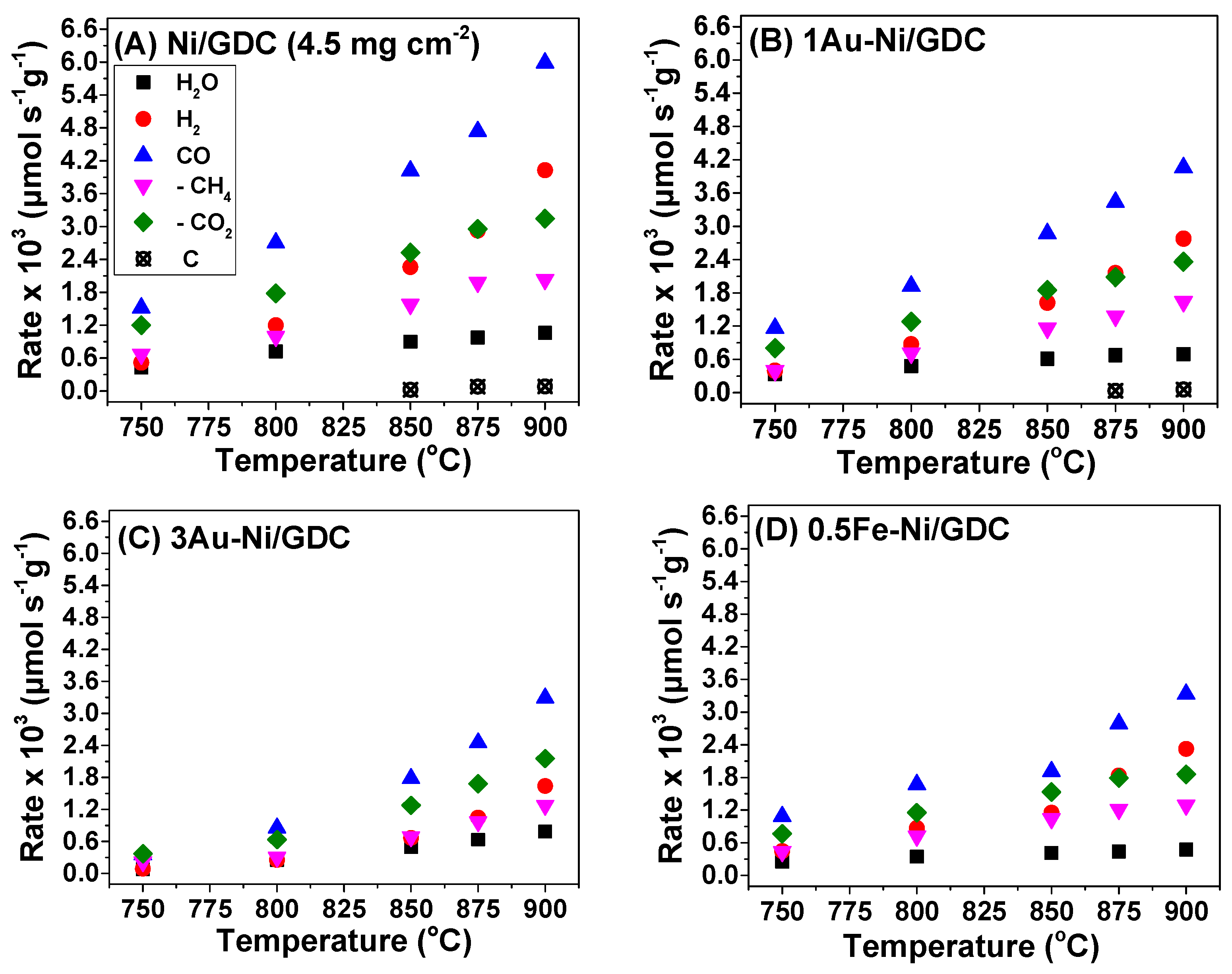
4.2. Catalytic—Kinetic Investigation for the Dry CH4 Reforming Reaction
| Temperature (°C) | % CH4 | % CO2 |
|---|---|---|
| 750 | 89.5 | 93.5 |
| 800 | 95.0 | 97.3 |
| 850 | 96.7 | 98.3 |
| 875 | 97.5 | 98.8 |
| 900 | 98.3 | 99.2 |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escudero, M.J.; Maffiotte, C.A.; Serrano, J.L. Long-term operation of a solid oxide fuel cell with MoNi–CeO2 as anode directly fed by biogas containing simultaneously sulphur and siloxane. J. Power Sources 2021, 481, 229048. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Dong, F. Grand Challenges for Catalytic Remediation in Environmental and Energy Applications toward a Cleaner and Sustainable Future. Front. Environ. Chem. 2020, 1, 5. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Niu, J.; Guo, F.; Ran, J.; Qi, W.; Yang, Z. Methane dry (CO2) reforming to syngas (H2/CO) in catalytic process: From experimental study and DFT calculations. Int. J. Hydrogen Energy 2020, 45, 30267–30287. [Google Scholar] [CrossRef]
- Faro, M.L.; Vita, A.; Pino, L.; Aricò, A.S. Performance evaluation of a solid oxide fuel cell coupled to an external biogas tri-reforming process. Fuel Process. Technol. 2013, 115, 238–245. [Google Scholar] [CrossRef]
- Chiodo, V.; Galvagno, A.; Lanzini, A.; Papurello, D.; Urbani, F.; Santarelli, M.; Freni, S. Biogas reforming process investigation for SOFC application. Energy Convers. Manag. 2015, 98, 252–258. [Google Scholar] [CrossRef]
- Shiratori, Y.; Ijichi, T.; Oshima, T.; Sasaki, K. Internal reforming SOFC running on biogas. Int. J. Hydrogen Energy 2010, 35, 7905–7912. [Google Scholar] [CrossRef]
- Sarno, C.; Luisetto, I.; Zurlo, F.; Licoccia, S.; Di Bartolomeo, E. Lanthanum chromite based composite anodes for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 14742–14750. [Google Scholar] [CrossRef]
- Papadam, T.; Goula, G.; Yentekakis, I.V. Long-term operation stability tests of intermediate and high temperature Ni-based anodes’ SOFCs directly fueled with simulated biogas mixtures. Int. J. Hydrogen Energy 2012, 37, 16680–16685. [Google Scholar] [CrossRef]
- Santoro, M.; Di Bartolomeo, E.; Luisetto, I.; Aricò, A.; Squadrito, G.; Zignani, S.; Faro, M.L. Insights on the electrochemical performance of indirect internal reforming of biogas into a solid oxide fuel cell. Electrochim. Acta 2022, 409, 139940. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Thattai, A.T.; Fan, L.; Lindeboom, R.E.F.; Spanjers, H.; Aravind, P.V. Solid Oxide Fuel Cells fuelled with biogas: Potential and constraints. Renew. Energy 2019, 134, 194–214. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G. Biogas management: Advanced utilization for production of renewable energy and added-value chemicals. Front. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Barelli, L.; Ottaviano, A. Solid oxide fuel cell technology coupled with methane dry reforming: A viable option for high efficiency plant with reduced CO2 emissions. Energy 2014, 71, 118–129. [Google Scholar] [CrossRef]
- Qiu, P.; Sun, S.; Yang, X.; Chen, F.; Xiong, C.; Jia, L.; Li, J. A review on anode on-cell catalyst reforming layer for direct methane solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 25208–25224. [Google Scholar] [CrossRef]
- Gür, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Prog. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Wang, J.; Yang, J.; Chen, Y.; Guan, W.; Singhal, S.C. Power generation from a symmetric flat-tube solid oxide fuel cell using direct internal dry-reforming of methane. J. Power Sources 2021, 516, 230662. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Tanveer, W.H.; Sayed, E.T.; Assad, M.E.H.; Allagui, A.; Cha, S.W. On the technical challenges affecting the performance of direct internal reforming biogas solid oxide fuel cells. Renew. Sustain. Energy Rev. 2019, 101, 361–375. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Özkara-Aydnolu, Ş. Thermodynamic equilibrium analysis of combined carbon dioxide reforming with steam reforming of methane to synthesis gas. Int. J. Hydrogen Energy 2010, 35, 12821–12828. [Google Scholar] [CrossRef]
- Escudero, M.J.; Serrano, J.L. Individual impact of several impurities on the performance of direct internal reforming biogas solid oxide fuel cell using W-Ni-CeO2 as anode. Int. J. Hydrogen Energy 2019, 44, 20616–20631. [Google Scholar] [CrossRef]
- Chlipała, M.; Błaszczak, P.; Wang, S.F.; Jasiński, P.; Bochentyn, B. In Situ study of a composition of outlet gases from biogas fuelled Solid Oxide Fuel Cell performed by the Fourier Transform Infrared Spectroscopy. Int. J. Hydrogen Energy 2019, 44, 13864–13874. [Google Scholar] [CrossRef]
- Liu, M.; van der Kleij, A.; Verkooijen, A.H.M.; Aravind, P.V. An experimental study of the interaction between tar and SOFCs with Ni/GDC anodes. Appl. Energy 2013, 108, 149–157. [Google Scholar] [CrossRef]
- Ioannidou, E.; Chavani, M.; Neophytides, S.G.; Niakolas, D.K. Effect of the PH2O/PCO2 and PH2 on the intrinsic electro-catalytic interactions and the CO production pathway on Ni/GDC during solid oxide H2O/CO2 co-electrolysis. J. Catal. 2021, 404, 174–186. [Google Scholar] [CrossRef]
- Zhan, Z.; Lin, Y.; Pillai, M.; Kim, I.; Barnett, S.A. High-rate electrochemical partial oxidation of methane in solid oxide fuel cells. J. Power Sources 2006, 161, 460–465. [Google Scholar] [CrossRef]
- Sun, C.; Stimming, U. Recent anode advances in solid oxide fuel cells. J. Power Sources 2007, 171, 247–260. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.P.; Tok, A.I.Y.; Luo, L. GDC-impregnated Ni anodes for direct utilization of methane in solid oxide fuel cells. J. Power Sources 2006, 159, 68–72. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Wu, Y.; Ran, R.; Shao, Z. Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 2013, 113, 8104–8151. [Google Scholar] [CrossRef]
- Yue, W.; Li, Y.; Zheng, Y.; Wu, T.; Zhao, C.; Zhao, J.; Geng, G.; Zhang, W.; Chen, J.; Zhu, J.; et al. Enhancing coking resistance of Ni/YSZ electrodes: In Situ characterization, mechanism research, and surface engineering. Nano Energy 2019, 62, 64–78. [Google Scholar] [CrossRef]
- Niakolas, D.K.; Neofytidis, C.S.; Neophytides, S.G. Effect of Au and/or Mo doping on the development of carbon and sulfur tolerant anodes for SOFCs-A short review. Front. Environ. Sci. 2017, 5, 78. [Google Scholar] [CrossRef]
- Niakolas, D.K.; Athanasiou, M.; Dracopoulos, V.; Tsiaoussis, I.; Bebelis, S.; Neophytides, S.G. Study of the synergistic interaction between nickel, gold and molybdenum in novel modified NiO/GDC cermets, possible anode materials for CH4 fueled SOFCs. Appl. Catal. A Gen. 2013, 456, 223–232. [Google Scholar] [CrossRef]
- Neofytidis, C.; Dracopoulos, V.; Neophytides, S.G.; Niakolas, D.K. Electrocatalytic performance and carbon tolerance of ternary Au-Mo-Ni/GDC SOFC anodes under CH4-rich Internal Steam Reforming conditions. Catal. Today 2018, 310, 157–165. [Google Scholar] [CrossRef]
- Neofytidis, C.; Ioannidou, E.; Sygellou, L.; Kollia, M.; Niakolas, D.K. Affecting the H2O electrolysis process in SOECs through modification of NiO/GDC; experimental case of Au-Mo-Ni synergy. J. Catal. 2019, 373, 260–275. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neophytides, S.; Niakolas, D.K. Experimental clarification of the RWGS reaction effect in H2O/CO2 SOEC co-electrolysis conditions. Catalysts 2019, 9, 151. [Google Scholar] [CrossRef]
- Neofytidis, C.; Ioannidou, E.; Kollia, M.; Neophytides, S.; Niakolas, D. The promoting effect of Fe on Ni/GDC for the Solid Oxide H2O electrolysis. Int. J. Energy Res. 2020, 44, 10982–10995. [Google Scholar] [CrossRef]
- Kan, H.; Lee, H. Enhanced stability of Ni-Fe/GDC solid oxide fuel cell anodes for dry methane fuel. Catal. Commun. 2010, 12, 36–39. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Batchu, R.; Galvita, V.V.; Poelman, H.; Marin, G.B. Carbon gasification from Fe-Ni catalysts after methane dry reforming. Appl. Catal. B Environ. 2016, 185, 42–55. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, Q.; Guo, C.; Li, S.; Yan, W.; Jiao, W.; Qiu, L.; Yan, X.; Li, R. Improved effect of Fe on the stable NiFe/Al2O3 catalyst in low-temperature dry reforming of methane. Ind. Eng. Chem. Res. 2020, 59, 17250–17258. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.A.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264, 118497. [Google Scholar] [CrossRef]
- Zaravelis, F.; Sygellou, L.; Souvalioti, A.; Niakolas, D.K. Transition metals in Ni/GDC for the reversible solid oxide cell operation: Optimization of the Mo-Au-Ni synergy and further enhancement via substitution of Mo with Fe. Electrochim. Acta 2023, 453, 142343. [Google Scholar] [CrossRef]
- Wu, Y.; Su, C.; Wang, W.; Wang, H.; Shao, Z. Effect of fabrication method on properties and performance of bimetallic Ni0.75 Fe0.25 anode catalyst for solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 9287–9297. [Google Scholar] [CrossRef]
- HJeong; Kim, S.; Bae, Y.; Yoon, K.J.; Lee, J.; Hong, J. Effect of Fe infiltration to Ni/YSZ solid-oxide-cell fuel electrode on steam/CO2 co-electrolysis. Int. J. Energy Res. 2019, 43, 4949–4958. [Google Scholar]
- Simonsen, S.B.; Muhl, T.T.; Thydén, K.T.S.; Chatzichristodoulou, C.; Nielsen, J.; Sudireddy, B.R. Effect of Fe on high performing nanostructured Ni/Gd-doped ceria electrocatalysts. Solid State Ion. 2019, 340, 115019. [Google Scholar] [CrossRef]
- Fiuza, P.R.; da Silva, M.A.; Boaventura, J.S. Development of Fe–Ni/YSZ–GDC electrocatalysts for application as SOFC anodes: XRD and TPR characterization and evaluation in the ethanol steam reforming reaction. Int. J. Hydrogen Energy 2010, 35, 11216–11228. [Google Scholar] [CrossRef]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic screening constants from SCF functions. II. Atoms with 37 to 86 electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- Majewski, A.J.; Singh, S.K.; Labhasetwar, N.K.; Steinberger-Wilckens, R. Nickel–molybdenum catalysts for combined solid oxide fuel cell internal steam and dry reforming. Chem. Eng. Sci. 2021, 232, 116341. [Google Scholar] [CrossRef]
- Horváth, A.; Guczi, L.; Kocsonya, A.; Sáfrán, G.; La Parola, V.; Liotta, L.; Pantaleo, G.; Venezia, A. Sol-derived AuNi/MgAl2O4 catalysts: Formation, structure and activity in dry reforming of methane. Appl. Catal. A Gen. 2013, 468, 250–259. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Fan, C.; Zhu, Y.A.; Xu, Y.; Zhou, Y.; Zhou, X.G.; Chen, D. Origin of synergistic effect over Ni-based bimetallic surfaces: A density functional theory study. J. Chem. Phys. 2012, 137, 014703. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Neophytides, S.G.; Niakolas, D.K. Au-Mo-Fe-Ni/CeO2 (Gd2O3) as Potential Fuel Electrodes for Internal CO2 Reforming of CH4 in Single SOFCs. ECS Trans. 2023, 111, 2473–2485. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.C.; Green, M.L.H.; Claridge, J.B. Methane oxyforming for synthesis gas production. Catal. Rev. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Matralis, H.; Verykios, X. Utilization of biogas as a renewable carbon source: Dry reforming of methane. In Catalysis for Alternative Energy Generation; Springer: New York, NY, USA, 2012; pp. 57–127. [Google Scholar]
- Guilhaume, N.; Bianchi, D.; Wandawa, R.A.; Yin, W.; Schuurman, Y. Study of CO2 and H2O adsorption competition in the combined dry/steam reforming of biogas. Catal. Today 2021, 375, 282–289. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Fouskas, A.; Kollia, M.; Kambolis, A.; Papadopoulou, C.; Matralis, H. Boron-modified Ni/Al2O3 catalysts for reduced carbon deposition during dry reforming of methane. Appl. Catal. A Gen. 2014, 474, 125–134. [Google Scholar] [CrossRef]
- Cesario, M.R.; Souza, G.S.; Loureiro, F.J.; Araújo, A.J.; Grilo, J.P.; Aouad, S.; Tidahy, H.L.; Macedo, D.A.; Fagg, D.P.; Gennequin, C.; et al. Synthesis of Co–Ni and Cu–Ni based-catalysts for dry reforming of methane as potential components for SOFC anodes. Ceram. Int. 2021, 47, 33191–33201. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Ling, C.; Zhou, T.; Wang, S. Methane dehydrogenation on Au/Ni surface alloys-a first-principles study. Catal. Sci. Technol. 2013, 3, 1343–1354. [Google Scholar] [CrossRef]
- Besenbacher, F.; Chorkendorff, I.; Clausen, B.S.; Hammer, B.; Molenbroek, A.M.; Nørskov, J.K.; Stensgaard, I. Design of a surface alloy catalyst for steam reforming. Science 1998, 279, 1913–1915. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, P.; Hammer, B.; Nørskov, J.K. A theoretical study of CH4 dissociation on pure and gold-alloyed Ni(111) surfaces. J. Chem. Phys. 1996, 105, 5595–5604. [Google Scholar] [CrossRef]
- Li, L.; Anjum, D.H.; Zhu, H.; Saih, Y.; Laveille, P.V.; D’Souza, L.; Basset, J. Synergetic Effects Leading to Coke-Resistant NiCo Bimetallic Catalysts for Dry Reforming of Methane. ChemCatChem 2015, 7, 427–433. [Google Scholar] [CrossRef]
- Bengaard, H.; Nørskov, J.; Sehested, J.; Clausen, B.; Nielsen, L.; Molenbroek, A.; Rostrup-Nielsen, J. Steam reforming and graphite formation on Ni catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Ge, X.; Deng, S.; Chen, B.; Shen, J. A review of different catalytic systems for dry reforming of methane: Conventional catalysis-alone and plasma-catalytic system. J. CO2 Util. 2021, 46, 101462. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Jones, G.; Jakobsen, J.G.; Shim, S.S.; Kleis, J.; Andersson, M.P.; Rossmeisl, J.; Abildpedersen, F.; Bligaard, T.; Helveg, S.; Hinnemann, B.; et al. First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J. Catal. 2008, 259, 147–160. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Akpan, E.; Sun, Y.; Kumar, P.; Ibrahim, H.; Aboudheir, A.; Idem, R. Kinetics, experimental and reactor modeling studies of the carbon dioxide reforming of methane (CDRM) over a new Ni/CeO2-ZrO2 catalyst in a packed bed tubular reactor. Chem. Eng. Sci. 2007, 62, 4012–4024. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Bobrova, L.N.; Bobin, A.S.; Mezentseva, N.V.; Sadykov, V.A.; Thybaut, J.W.; Marin, G.B. Kinetic assessment of dry reforming of methane on Pt+Ni containing composite of fluorite-like structure. Appl. Catal. B Environ. 2016, 182, 513–524. [Google Scholar] [CrossRef]
- Gavrielatos, I.; Drakopoulos, V.; Neophytides, S.G. Carbon tolerant Ni–Au SOFC electrodes operating under internal steam reforming conditions. J. Catal. 2008, 259, 75–84. [Google Scholar] [CrossRef]
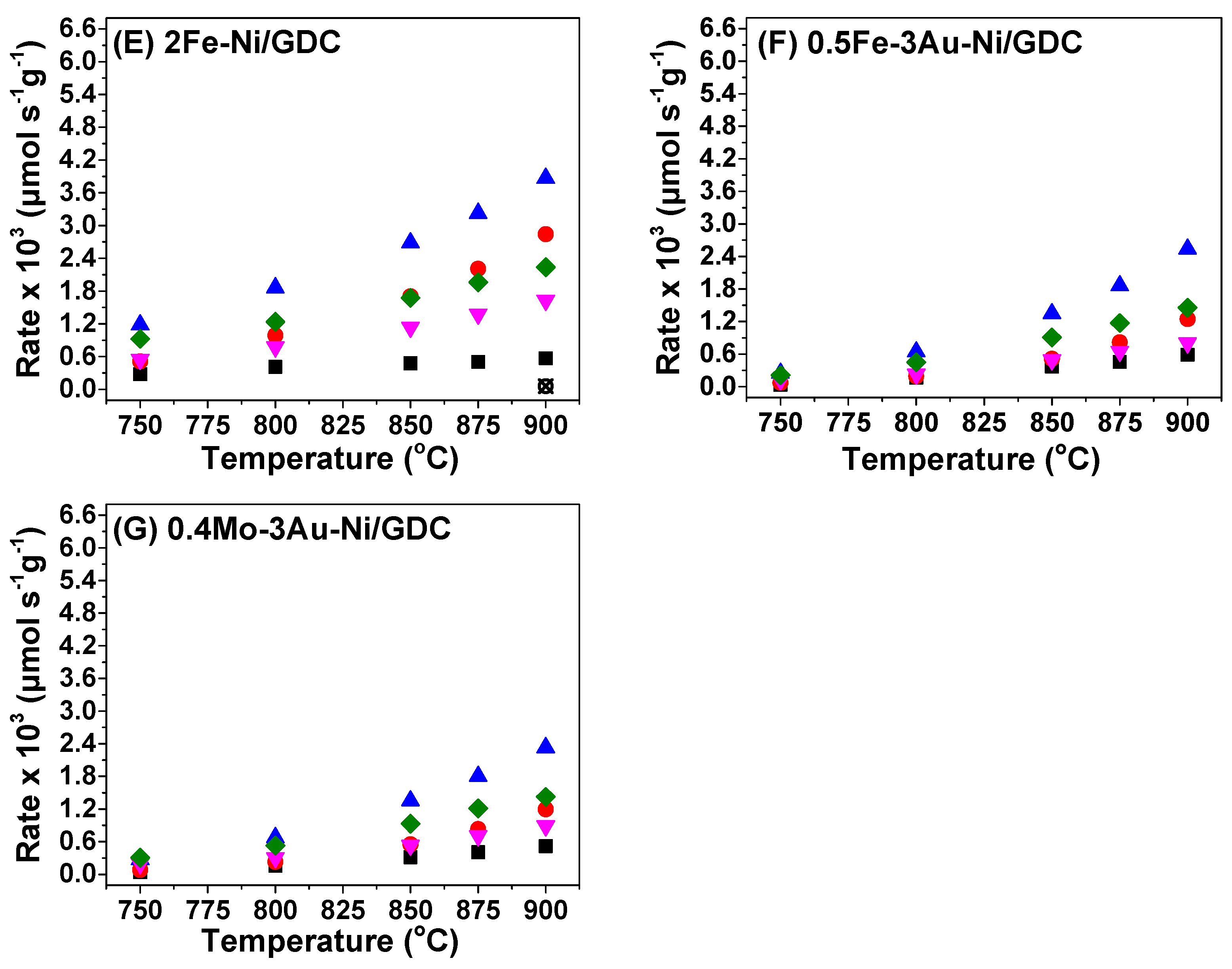

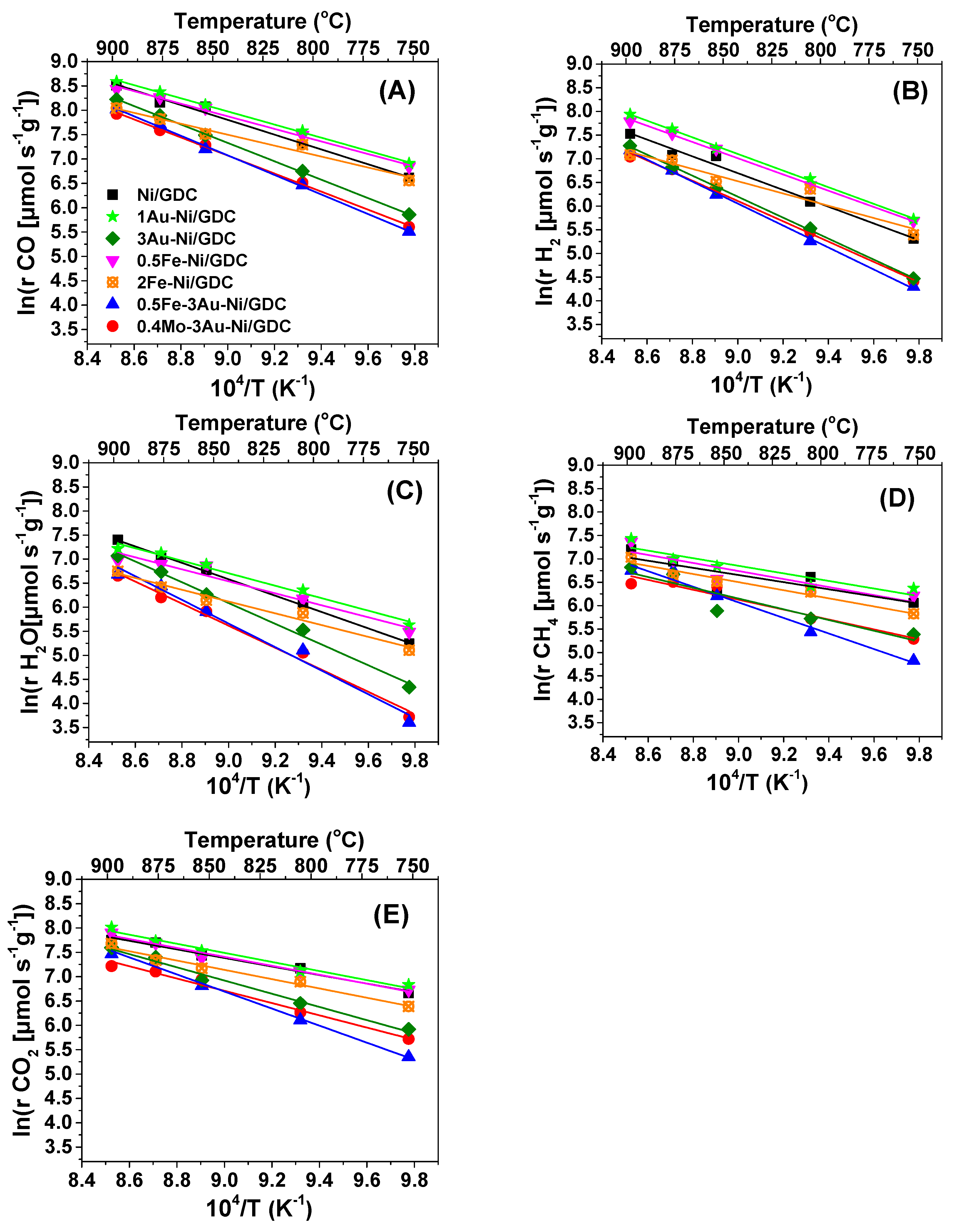
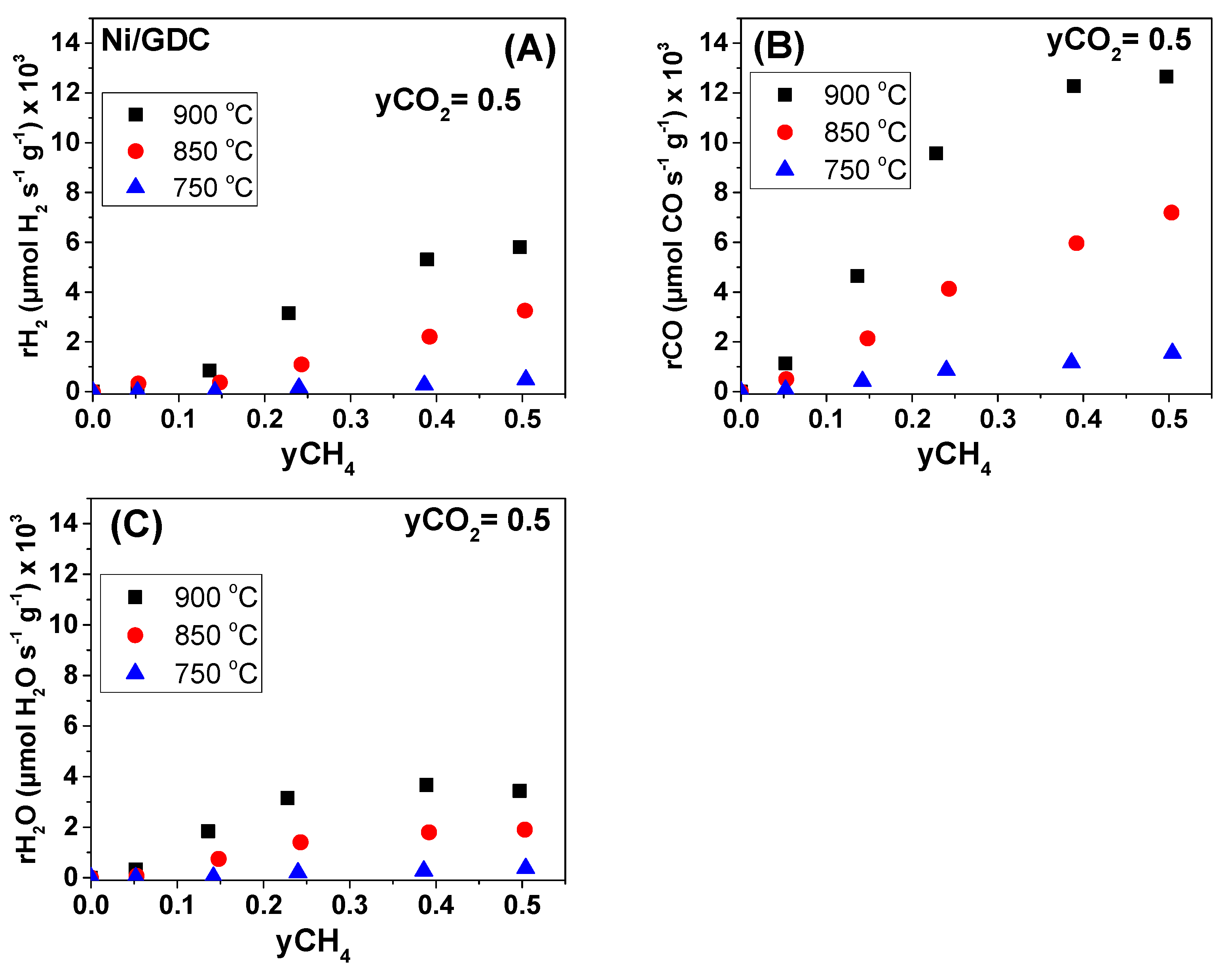
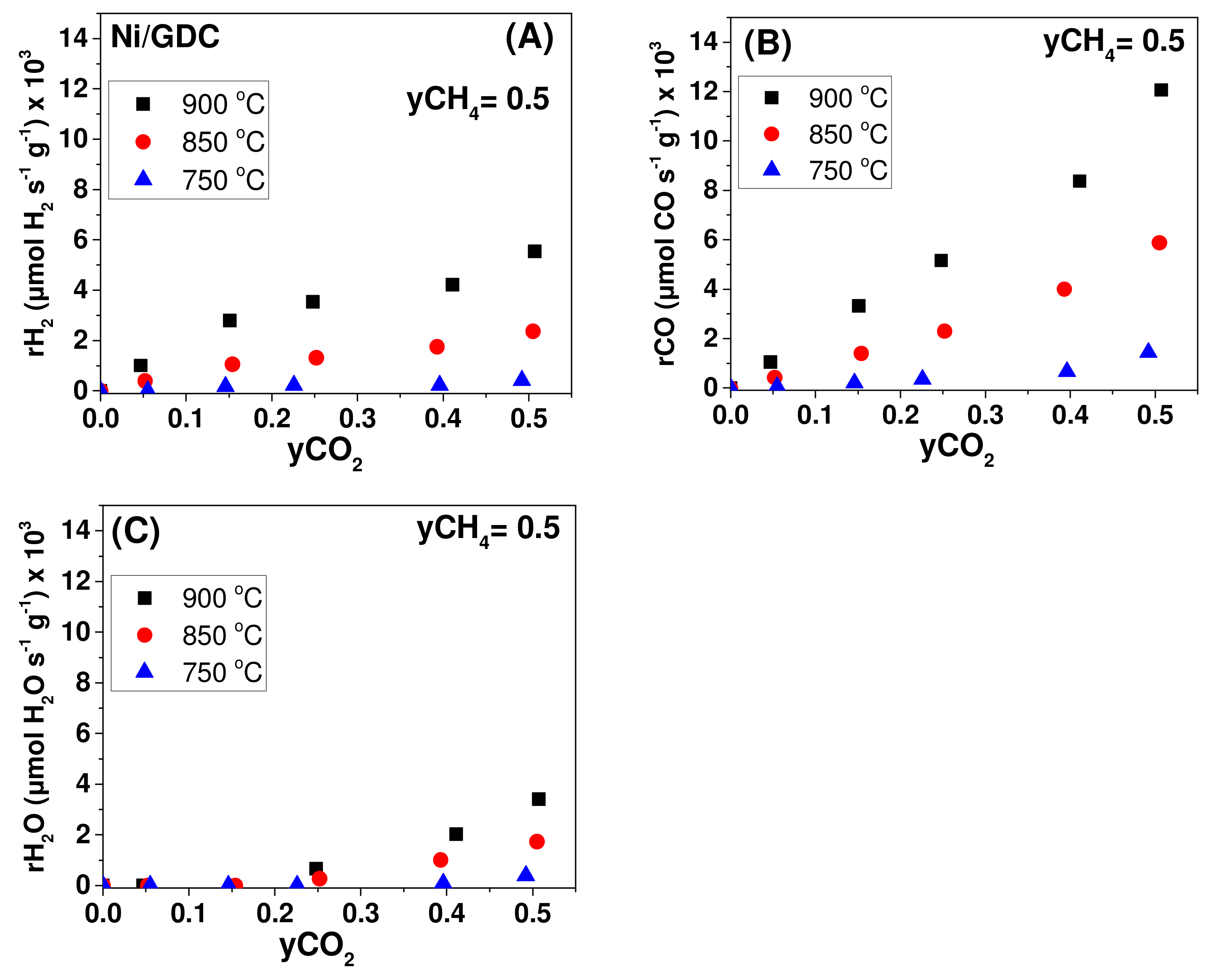


| Sample | wt.% Concentration | ||
|---|---|---|---|
| Mo | Au | Fe | |
| 1Au-NiO/GDC | − | 0.8 | − |
| 3Au-NiO/GDC | − | 2.7 | − |
| 0.5Fe-NiO/GDC | − | − | 0.5 |
| 2Fe-NiO/GDC | − | − | 2.1 |
| 0.5Fe-3Au-NiO/GDC | − | 2.5 | 0.6 |
| 0.4Mo-3Au-NiO/GDC | 0.7 | 2.6 | − |
| Sample | SSA (m2 g−1) | |
|---|---|---|
| T = 1100 °C, (Oxidized) | After H2-Reduction at T = 900 °C/2 h | |
| NiO/GDC | 4.2 | 2.5 |
| 1Au-NiO/GDC | 4.3 | 2.2 |
| 3Au-NiO/GDC | 2.7 | 2.1 |
| 0.5Fe-NiO/GDC | 3.4 | 3.1 |
| 2Fe-NiO/GDC | 4.0 | 3.4 |
| 0.5Fe-3Au-NiO/GDC | 3.9 | 2.1 |
| 0.4Mo-3Au-NiO/GDC | 2.8 | 2.3 |
| Primary, Mean, Crystallite Size, d, (nm) | |||||||
|---|---|---|---|---|---|---|---|
| NiO/GDC | 1Au | 3Au | 0.5Fe | 2Fe | 0.5Fe-3Au | 0.4Mo-3Au | |
| Oxidized, calcined at T = 1100 °C | |||||||
| NiO | 98 | 116 | 119 | 113 | 106 | 111 | 134 |
| GDC | 67 | 67 | 70 | 69 | 65 | 71 | 69 |
| Au | – | 56 | 75 | – | – | 83 | 72 |
| Fe2O3 | – | – | – | – | 67 | – | – |
| After H2-reduction at T = 900 °C | |||||||
| Ni | 132 | 116 | 91 | 100 | 79 | 100 | 73 |
| GDC | 61 | 63 | 64 | 58 | 59 | 60 | 61 |
| Sample | Ea,app * (kJ mol−1) per Product or Reactant | ||||
|---|---|---|---|---|---|
| H2O | H2 | CO | CH4 | CO2 | |
| Ni/GDC_1* | 139 | 167 | 125 | 64 | 85 |
| Ni/GDC_2* | 142 | 147 | 126 | 63 | 73 |
| 1Au | 108 | 146 | 112 | 67 | 78 |
| 3Au | 179 | 184 | 157 | 95 | 113 |
| 0.5Fe | 104 | 143 | 107 | 71 | 76 |
| 2Fe | 102 | 108 | 93 | 72 | 79 |
| 0.5Fe-3Au | 204 | 193 | 167 | 138 | 146 |
| 0.4Mo-3Au | 191 | 178 | 154 | 88 | 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidou, E.; Neophytides, S.G.; Niakolas, D.K. Ternary Fe- or Mo-Au-Ni/GDC as Candidate Fuel Electrodes for the Internal Dry Reforming of CH4: Physicochemical and Kinetic Investigation. Energies 2024, 17, 184. https://doi.org/10.3390/en17010184
Ioannidou E, Neophytides SG, Niakolas DK. Ternary Fe- or Mo-Au-Ni/GDC as Candidate Fuel Electrodes for the Internal Dry Reforming of CH4: Physicochemical and Kinetic Investigation. Energies. 2024; 17(1):184. https://doi.org/10.3390/en17010184
Chicago/Turabian StyleIoannidou, Evangelia, Stylianos G. Neophytides, and Dimitrios K. Niakolas. 2024. "Ternary Fe- or Mo-Au-Ni/GDC as Candidate Fuel Electrodes for the Internal Dry Reforming of CH4: Physicochemical and Kinetic Investigation" Energies 17, no. 1: 184. https://doi.org/10.3390/en17010184
APA StyleIoannidou, E., Neophytides, S. G., & Niakolas, D. K. (2024). Ternary Fe- or Mo-Au-Ni/GDC as Candidate Fuel Electrodes for the Internal Dry Reforming of CH4: Physicochemical and Kinetic Investigation. Energies, 17(1), 184. https://doi.org/10.3390/en17010184






