Abstract
The present study deals with the physicochemical and catalytic/kinetic investigation of Fe, Au, Fe-Au, and Mo-Au modified Ni/GDC electrocatalysts towards their performance for the DRM, RWGS, and CH4 decomposition reactions. For this purpose, Au-NiO/GDC (where Au = 1 or 3 wt.%), Fe-NiO/GDC (where Fe = 0.5 or 2 wt.%), 0.5Fe-3Au-NiO/GDC, and 0.4Mo-3Au-NiO/GDC were synthesized via deposition (co-) precipitation. There is discussion on the structural properties of the electrocatalysts on the oxidized and reduced state, as well as their use as electrolyte-supported (half) cells. A key remark after H2-reduction is the formation of binary or ternary solid solutions. Ni/GDC was the most active for the catalytic CO2 reforming of CH4 and the CH4 decomposition reactions and as a result the most prone to carbon deposition. On the other hand, the modified 3Au-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC exhibited the following properties: (i) the highest Ea,app for the non-desired RWGS reaction, (ii) high tolerance to carbon formation due to lower activity for the CH4 decomposition, and (iii) were also less active for H2 and CO production. Finally, 0.4Mo-3Au-Ni/GDC seems to perform the DRM reaction through a different mechanism when compared to Ni/GDC. Overall, the above three samples are proposed as potential fuel electrodes for further electrocatalytic measurements for the SOFC internal DRM process.
1. Introduction
Recycling CO2 and CH4 to produce syngas (H2 + CO) through Dry Reforming of Methane (DRM) has received considerable attention and prominent research interest. CO2 and CH4 are greenhouse gases and the main compounds of biogas (55–65% CH4 and 45–55% CO2), which is widely produced by anaerobic fermentation of biomass []. The DRM process offers a feasible solution to abate CO2 and CH4, responsible for global warming and major climate changes, via production of synthesis gas (or syngas) with a molar H2/CO ratio close to unity [,]. Furthermore, the syngas from DRM is suitable for further usage and synthesis of long-chain hydrocarbons in the Fischer-Tropsch industry [,].
Among the existing technologies for DRM energy applications, high temperature (750–1000 °C) Solid Oxide Fuel Cells (SOFCs) are the most efficient devices for electricity production [,,,]. A SOFC fed by biogas can operate either by using an external reformer, which converts the biogas to syngas, or without using an external reformer, inside the fuel cell, by utilizing advanced fuel electrodes [,]. The first approach represents the State of the Art (SoA) of the current technology, where the external reformer converts biogas to syngas and then this stream is fed to the fuel side of SOFC [,,]. The second approach which is also known as “internal dry reforming of methane (IDRM)” uses modified improved electrodes to convert biogas to syngas through a catalytic DRM reaction and then to electricity through charge transfer reactions inside the SOFC [,,,,]. The main advantages of this concept include simplified system design without external reformer and reduced air-flow/energy-losses because the heat released by the exothermic charge transfer reactions can also be used for the endothermic catalytic reactions (DRM, etc.) [,,,,].
In general, when SOFCs operate at temperatures of 750–900 °C under internal dry reforming of methane conditions, several (electro)catalytic reactions may occur simultaneously on the fuel electrode (Equations (1)–(6)) [,,,,,]. In this respect, in the fuel side CO2 and CH4 are converted into H2 and CO via the DRM reaction (Equation (1)). Due to the strongly endothermic character of DRM, high reaction temperatures (>750 °C) are required to achieve high H2 and CO yields [,].
During operation, the produced H2 from the DRM may be consumed through the Reverse Water Gas Shift (RWGS) reaction (Equation (2)), resulting in decreased H2/CO ratio values (<1) [].
Moreover, carbon deposition on the surface of the electrocatalyst may occur through cracking of CH4 (Equation (3)), and this is a very important issue since it is responsible for the progressive electrocatalyst deactivation [,]. The CH4 decomposition reaction (Equation (3)) is thermodynamically favored at the high temperature region of >600 °C, whereas at lower temperatures (<650 °C) carbon is mainly produced through the CO disproportionation (Boudouard) reaction (2CO → C + CO2) [,,].
During fuel cell operating conditions, the produced H2 and CO, as well as the supplied CH4 can be electrochemically oxidized in the triple phase boundary (TPB) region by oxygen ions (O2−) to produce power, according to Equations (4)–(6) [,,,,].
Although significant progress has been made on the development of advanced SOFC systems, the main issue that hinders the commercialization of this technology, particularly under CH4 reforming processes, is the performance degradation of the cells and especially of the fuel electrodes. The performance of cells/fuel electrodes is affected by the thermal stresses resulting from the endothermic (Equations (1)–(3)) and the exothermic (Equations (4)–(6)) (electro)catalytic reactions, as well as the chemical degradation due to coke formation [].
The State-Of-the-Art (SoA) electrocatalysts in SOFCs are Ni-ceramic-metal composites comprising Yttria Stabilized Zirconia (YSZ) and Gadolinia Doped Ceria (GDC), due to their excellent activity for electrochemical reactions and low cost [,]. In this respect, Ni/GDC is widely used as a fuel electrode, since it exhibits higher catalytic performance in CH4 reforming reactions and resistance to coke formation when compared to Ni/YSZ electrodes [,,]. The improved performance of Ni/GDC is attributed to the capacity of ceria to store and release oxygen. This property favours the oxidation and thus the removal of surface carbon species and the oxidation of CH4 instead of its decomposition [,]. An alternative solution to suppress the carbon deposition and sintering tendency of nickel can be achieved by dispersing small amounts of transition noble (Ru, Rh, Pd, Pt, Au) or non-noble (Fe, Co, Cu, Mo, W) metals [,,].
In the same direction, our research group has studied the effect of Au and/or Mo or Fe addition on commercial NiO/GDC powder in solid oxide applications [,,,,]. The modifications have so far resulted in electrocatalysts with enhanced electrochemical performance and tolerance against nickel oxidation and carbon or sulphur poisoning under steam (or steam + CO2) electrolysis [,,] and under internal steam reforming of methane (ISRM) operating conditions in the presence/absence of 10 ppm H2S [,]. In these studies, the modified cermets exhibited variations in their physicochemical and electrochemical properties, which were correlated with the formation of bimetallic Fe-Ni, Au-Ni, and ternary Mo–Au–Ni solid solutions during the H2-reduction process. According to the ISRM studies, 0.4Mo-3Au-Ni/GDC was found to be the most carbon tolerant electrode when compared to Ni/GDC, 0.4Mo-Ni/GDC, and 3Au-Ni/GDC under both helium diluted or non-diluted harsh H2O/CH4 reaction feed conditions []. The improved performance of 0.4Mo-3Au-Ni/GDC was ascribed to the inhibited complete dehydrogenation of methyl species (CHx) towards carbon on the active sites of the electrode []. Although Au utilization is not cost-effective compared to non-precious metals, its usage may be compensated if long-term operation and carbon tolerance is achieved.
In regards to the substitution of noble metals, iron is indicated as a promising transition metal dopant of Ni, due to its improved redox properties, its abundancy, and its lower price. The first research efforts of our group focused on the modification of Ni/GDC with various wt.% Fe with quite promising results for the solid oxide steam electrolysis reaction []. Notably, 0.5Fe-Ni/GDC displayed the highest performance compared to Ni/GDC and 2Fe-Ni/GDC, whereas the main problem was the poor stability that resulted in the decrease in the electrocatalytic activity. The CO2 reforming of CH4 has also been studied over Fe-Ni-based catalysts, focusing on the synergistic interaction of Fe-Ni towards the DRM activity and carbon formation propensity. According to some of the above research studies [,,,,], under DRM reaction conditions FeOx species are formed due to partial segregation of metallic Fe from the Fe-Ni alloyed particles, favouring the interaction of FeOx with the accumulated carbon towards its gasification. A recent study over Fe-Ni/MgO catalysts [] showed that Fe modification can inhibit carbon deposition under DRM reaction conditions, but at the same time may alter the nature of the deposited carbon towards a form that can be easily removed from the catalyst surface via gasification by CO2.
In the present work, the performance of the following electrocatalysts (Ni/GDC, 1 wt.% Au-Ni/GDC, 3 wt.% Au-Ni/GDC, 0.5 wt.% Fe-Ni/GDC, 2 wt.% Fe-Ni/GDC, 0.5 wt.% Fe-3 wt.% Au-Ni/GDC, and 0.4 wt.% Mo-3 wt.% Au-Ni/GDC) was evaluated at Open Circuit Potential (OCP) conditions under biogas fuel operation. The samples were tested in the form of Electrolyte Supported (half) Cells (ESCs) at 750–900 °C by applying a reaction fuel mixture of CH4/CO2 = 1 with inlet gas flows that varied in the range between 150 to 300 cm3/min. The latter approach provided a reference profile for the catalytic performance of the candidate electrocatalysts by applying the same CH4/CO2 fuel feed conditions as those under IDRM operational mode but without the effect of applied current/potential. In addition, selected samples were further studied through specific kinetic measurements under differential conditions at various CH4 and CO2 partial pressures in an attempt to clarify the effect of modification on the intrinsic catalytic activity of Ni/GDC for the DRM reaction. The research objective of this investigation focuses on the sustainable conversion of the greenhouse CH4 and CO2 gases towards syngas by means of Solid Oxide Fuel cells, which is a highly efficient and environmentally friendly electrochemical source of energy/power and useful chemicals.
2. Experimental
2.1. Preparation of Powders
The modified powders were prepared via the Deposition—Precipitation (D.P.) and Deposition—Co Precipitation (D.CP.) methods by using the commercial NiO/GDC cermet (65 wt.% NiO-35 wt.% GDC, Marion Technologies) as the support. The precursors for the 1 wt.% Au-Ni/GDC, 3 wt.% Au-Ni/GDC, 0.5 wt.% Fe-Ni/GDC, 2 wt.% Fe-Ni/GDC, 0.5 wt.% Fe-3 wt.% Au-Ni/GDC, and 0.4 wt.% Mo-3 wt.% Au-Ni/GDC samples were HAuCl4 (99.99% trace metals basis, 30 wt.% in dilute HCl), Fe(NO3)3x9H2O (ACS reagent ≥ 98%), and (NH4)6Mo7O24 (99.98% metals basis) purchased from Sigma-Aldrich (St. Louis, MO, USA). Full details about the synthesis of the electrocatalysts can be found elsewhere [,]. During the process, the temperature was fixed at 70 °C and pH was adjusted by using NH3 (1 M), at 7.0 for Au-NiO/GDC, 8.0 for Fe-NiO/GDC, and 6.0 for Fe-Au-NiO/GDC and Mo-Au-NiO/GDC samples. In the case of Au-modified electrocatalysts, after filtering the precipitate was mildly washed in order to eliminate any residual Cl−. Finally, in all cases, the precipitate was dried at 110 °C for 24 h and then each powder was calcined in air at 600 °C/90 min and a part of it at 1100 °C/75 min. The first batch was used for the preparation of a paste for electrode production and the last one for physicochemical characterization. Calcination at 1100 °C is considered necessary in order to examine the powders at similar calcination conditions, such as those where the cells are prepared. In the following sections, the examined samples will be reported as 1Au-NiO/GDC, 3Au-NiO/GDC, 0.5Fe-NiO/GDC, 2Fe-NiO/GDC, 0.5Fe-3Au-NiO/GDC, and 0.4Mo-3Au-NiO/GDC.
2.2. Preparation of Half Cells
The half cells were supported on a circular shaped planar 8YSZ electrolyte with 25 mm diameter and 300 μm thickness, purchased from Kerafol. As reported in previous studies [,], the deposition of the electrode was made by using the screen-printing method and a paste which consisted of a proper amount of calcined powder at 600 °C, terpineol as the dispersant, and PVB (polyvinylbutyral) as the binder, purchased from Sigma-Aldrich. After the paste-deposition, the cell was sintered at 1150 °C with a heating/cooling ramp rate of 2 °C/min. The loading of the examined fuel electrodes was ~6 mg cm−2 and the active surface area was 1.8 cm2.
2.3. Physicochemical Characterization
The powders, in their oxidized and H2-reduced form, were characterized with XRF (X-ray Fluorescence), BET (Brunauer-Emmett-Teller), XRD (X-ray Diffraction), and TGA (Thermogravimetric Analysis) in the presence of CH4. Specifically, the elemental wt.% concentration in each sample was determined by means of the non-destructive X-ray fluorescence (XRF) analysis using a portable Bruker Tracer III SD set with a beam diameter of 3 mm. The BET Specific Surface Area values (SSAs) were measured with a Micromeritics TriStar 3000 apparatus, employing nitrogen physisorption at the temperature of liquid nitrogen (77 K). In each measurement, the sample was pre-heated and outgased under dynamic vacuum at 250 °C for 2 h.
XRD measurements were performed by using a Bruker D8 Advance instrument equipped with a nickel-filtered Cu Kα (0.15418 nm) radiation source. The step size and the time per step were respectively fixed at 0.02° and 5 s, in the range of 20° 2θ 60°. The patterns were analyzed via the DiffracPlus-EVA software. The peaks were identified by using the standard reference crystallographic patterns of NiO: 044-1159, Ni: 004-0850, GDC: 046-0508, Au: 065-2870, and Fe2O3: 039-1346. The primary crystallite size of the main detected nanocrystals was estimated by means of Scherrer’s formula:
where is the X-ray wavelength corresponding to Cu Kα radiation (0.15418 nm), is the diffraction angle, and is the line broadening (in radians) at half maximum. For the estimation of the values, the diffraction peaks that were used are located at 2 equal to 43.4° for NiO (0 1 2), 44.4° for Ni (1 1 1), 28.6° for GDC (2 2 2), 38.1° for Au (1 1 1), and 35.6° for Fe2O3 (3 1 1).
TGA measurements were performed in a TA Q50 instrument isothermally at 800 °C under 10 vol.% CH4/Ar with a total flow rate of 100 cm3 min−1. The weight of each sample was ~25 mg and before the measurement the powder was reduced in-situ with 80 vol.% H2/Ar at 800 °C for 100 min. After the H2-reduction period, the reaction mixture was switched and CH4/Ar was added in the feed.
2.4. Catalytic/Kinetic Measurements
The electrolyte-supported half cells were attached on a YSZ tube-reactor and were sealed airtight by using a glass sealing material. The half cells were investigated catalytically at OCP conditions under biogas fuel operation. The experiments were performed in the presence of a Ni mesh at 750–900 °C under a fuel mixture with CH4/CO2 = 1 without dilution in a carrier gas. Specifically, four experimental cycles were performed per temperature. Each cycle consisted of a total inlet gas flow ranging between 150–300 cm3/min with a step of 50 cm3/min, while the operating temperature was fixed. By changing the cycle, the experimental procedure was the same, but the temperature varied from 750 to 900 °C with a step of 50 °C. The kinetic measurements were carried out under differential conditions at various CH4 and CO2 partial pressures. Reactants and products were detected by using an on-line gas chromatograph (Varian CP-3800) with a thermal conductivity detector. A Porapak Q column (80–100 mesh, 1.8 m × 1/8 in. × 2 mm) was used for the analysis of H2O at 150 °C while a Carbosieve S-11 column (80–100 mesh, 2 m × 1/8 in. × 2 mm) was used for the analysis of H2, CO, CH4, and CO2 (in parallel with the Porapak Q).
The catalytic rates of reactants and products were calculated through Equation (8) and the carbon formation rates were determined by using the measured production rates of H2, H2O, and CO in the mass balance equation of carbon (Equation (9)).
where is the consumption/production rate for H2O, H2, CO, CH4 and CO2, is the total volumetric flow, is the molecular volume of ideal gases (24,451 at 25 °C and 1 atm), and are the reactor inlet/outlet concentrations of each compound, respectively.
The corresponding % conversions of the reactants (CH4, CO2) were calculated according to Equations (10) and (11).
3. Results and Discussion
Physicochemical Characterization
The wt.% amount of each dopant (Mo, Fe, or Au) in the total mass of the oxidized NiO/GDC powder was investigated by means of XRF analysis and the results confirmed that the calculated wt.% loadings are close to the nominal values which are presented in Table 1.

Table 1.
XRF analysis of the Mo, Au, and Fe wt.% concentration on the examined oxidized powders after calcination at 1100 °C.
The Specific Surface Area (SSA) values of modified cermets, in their oxidized and reduced form, are presented in Table 2.

Table 2.
Specific Surface Area (SSA) values determined with the BET method of the calcined powders in their oxidized and reduced form. Error/accuracy = ±0.2 m2 g−1.
Concerning the effect of the dopants, and by considering the error/accuracy limit of BET measurements on these materials (±0.2 m2 g−1), the addition of 3 wt.% Au, with or without 0.4 wt.% Mo, caused a ~33% decrease in the SSA of the oxidized NiO/GDC, whereas in the H2-reduced form there was no difference in the SSA of Ni/GDC. Interestingly and opposite to the case of Mo/Au, the addition of Fe caused an increase in the SSA value. Specifically, this was ~24% and ~36% for 0.5Fe-Ni/GDC and 2Fe-Ni/GDC, respectively. The beneficial effect of Fe addition in inhibiting the decrease in SSANi/GDC upon H2-reduction was also reported in previous studies [,]. Another remark refers to the SSA of the oxidized ternary 0.5Fe-3Au-NiO/GDC sample, which exhibited higher value than that of 0.5Fe-NiO/GDC and 3Au-NiO/GDC. This can be realized via a synergistic interaction between the co-deposited Fe and Au dopants with NiO/GDC, which seems to retain the SSA of the specific sample. However, upon H2-reduction the SSA decreased to a similar value like that in the majority of the examined samples.
The effect of modifiers on the bulk phase of NiO/GDC powder was further studied by means of XRD analysis. Figure 1 and Figure S1A in the Supplementary Materials present the XRD patterns of cermets calcined at 1100 °C. Typical diffraction peaks of NiO (JCPDS 044-1159) and GDC (Gd0.6Ce0.4)2O3.2 (JCPDS 046-0508) phases were detected in all samples. It is worth mentioning that the diffraction peaks of NiO and GDC appeared at the same 2θ position for all samples and no displacement was observed, as it can be seen in the magnification of the main peaks for NiO (0 1 2) and GDC (2 2 2) in the regions (42.8° ≤ 2θ ≤ 43.6°) and (28° ≤ 2θ ≤ 28.8°), respectively (Figure S1B,C in the Supplementary Materials).
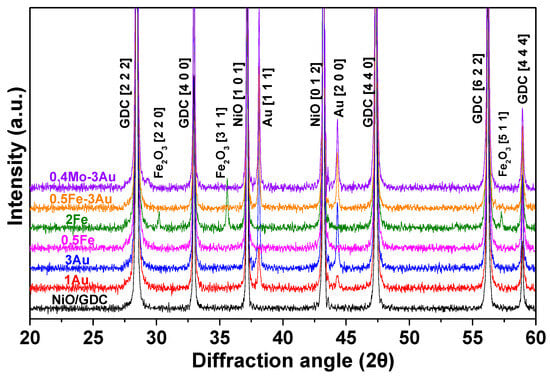
Figure 1.
XRD patterns of the oxidized Mo-Au-Fe-NiO/GDC powders after calcination in air at 1100 °C/75 min. Reflections indexed are shown in brackets.
Iron was detected in the form of maghemite-C, syn-Fe2O3 (JCPDS 039-1346), only for the 2Fe-NiO/GDC sample with three peaks at 2θ = 30.2° for the plane (2 2 0), 35.6° for (3 1 1), and 57.3° for (5 1 1). The absence of Fe2O3 diffraction peaks in the 0.5Fe-NiO/GDC and 0.5Fe-3Au-NiO/GDC samples can be attributed to the low wt.% content of iron which is below the detection limit of the XRD technique.
Moreover, gold was detected in the form of metallic Au (JCPDS 065-2870) with two peaks at 2θ = 38.1° for the plane (1 1 1) and 44.3° for (2 0 0). Concerning molybdenum, the XRD pattern of 0.4Mo-3Au-NiO/GDC highlighted its possible absence as MoOx bulk particles. Complementary results from previous XRD and “quasi” in-situ (H2 or H2O) XPS analysis on Au or/and Mo and Mo-Au-NiO/GDC samples [,,] confirm the above XRD analysis, but also highlight the presence of molybdate species on the surface of the oxidized 0.4Mo-3Au-NiO/GDC sample, which interact with Ni (or Ni-Au) upon H2-reduction towards the formation of a Mo-Au-Ni solid solution.
The corresponding XRD patterns of the H2-reduced powders at 900 °C for 2 h are presented in Figure 2A and Figure S1D in the Supplementary Materials. Two magnified areas of the main diffraction peaks of Ni (syn-Ni, JCPDS 004-0850) with the plane (1 1 1) and GDC (JCPDS 046-0508) with the plane (2 2 2) are also presented in Figure 2B and Figure 2C, respectively. It is observed that after H2-reduction at 900 °C, both iron and gold were not detected in the XRD patterns of the samples, suggesting their possible absence in the bulk phase of the samples, as well as a re-arrangement in the structure of the reduced materials.
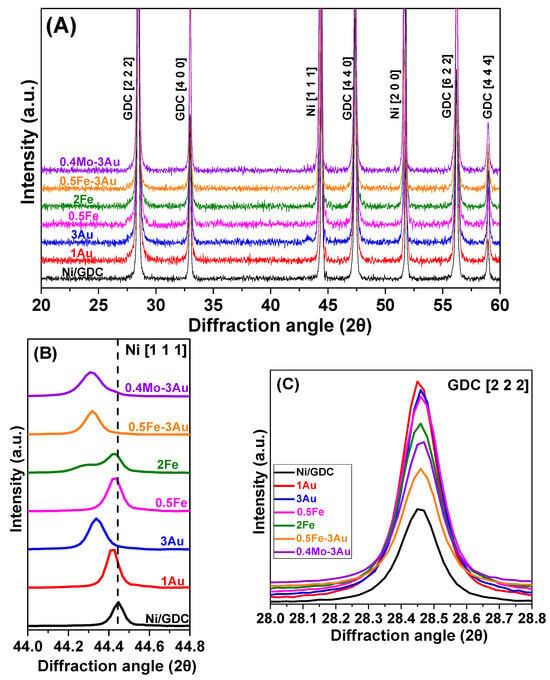
Figure 2.
XRD patterns of Mo-Au-Fe-Ni/GDC powders after H2-reduction at 900 °C. Specifically, there are depictions of the (A) whole pattern, (B) magnification of the Ni (1 1 1) peak in the range of 44.0° ≤ 2θ ≤ 44.8°, (C) magnification of the GDC (2 2 2) peak in the range of 28.0° ≤ 2θ ≤ 28.8°. Reflections indexed are shown in brackets.
More specifically, the absence of bulk iron particles in 2Fe-Ni/GDC is confirmed by a shift of the Ni diffraction peaks to lower 2θ values, indicating the formation of a Ni-Fe solid solution during H2-reduction, which is further confirmed by previous studies in the literature [,,,,] (Figure 2B). This shift in the present study can be realized through the detection of a shoulder at 44.3° of the main diffraction peak Ni (1 1 1), which at this point is ascribed to Ni-2Fe solid solution and is currently under further evaluation. The latter effect is also detectable in the XRD pattern of 0.5Fe-Ni/GDC, where Ni (1 1 1) was slightly shifted.
Similarly, an absence of bulk gold particles was observed in 1Au-Ni/GDC, 3Au-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC, which was further verified by a shift of the Ni diffraction peaks to lower 2θ values, indicating the formation of Ni-Au or Ni-Fe-Au and Ni-Mo-Au solid solutions during the H2-reduction process. Particularly, Ni-Au and Ni-Mo-Au solid solutions in Ni/YSZ and Ni/GDC cermets have been thoroughly reported in previous studies of our research group [,].
Concerning the Ni-Fe-Au solid solution, this is the first time that it is studied by means of XRD measurements. Specifically, the incorporation of the larger atomic radius ions of Fe (1.56 Å) or/and Au (1.74 Å) or/and Mo (1.90 Å) into the Ni (1.49 Å) [] lattice has been reported to cause an increase in the crystal lattice parameter value of the Ni-Fe, Ni-Au, Ni-Fe-Au, and Ni-Mo-Au phases [,,,]. The lattice parameters for the above solid solution phases were calculated by the (1 1 1) diffraction plane of Ni (Figure 2B) via the DiffracPlus-EVA software. In particular, the lattice parameter values were estimated as equal to 3.530 Å for Ni, 3.532 Å for 1Au-Ni, 3.539 Å for 3Au-Ni, 3.532 Å for 0.5Fe-Ni, and 3.540 Å for 2Fe-Ni, 0.5Fe-3Au-Ni, and 0.4Mo-3Au-Ni. On the other hand, it should be mentioned that no shift was detected for the GDC diffraction peaks, as it can be seen in the magnification of the main peak of GDC (2 2 2) (Figure 2C).
The mean primary size of the detected nanocrystals in the XRD patterns was estimated from the main peaks of NiO (0 1 2), Ni (1 1 1), GDC (2 2 2), Fe2O3 (3 1 1), and Au (1 1 1), according to the Scherrer equation (Equation (7)). The calculated values for the oxidized and reduced samples are presented in Table 3. More specifically, the crystal size of GDC did not change after calcination or H2-reduction or after Fe, Au, and Mo modification, with a mean diameter of ~65 nm. Moreover, the crystallite size of NiO showed minor deviation (~15% increase) upon modification with Fe or/and Au, whereas a ~37% increase was observed upon modification with 0.4 wt.% Mo and 3 wt.% Au in the oxidized samples. Finally, by increasing the loading of Au from 1 to 3 wt.%, the Au crystal size increased by 34% and this value did not change upon addition of 0.5 wt.% Fe or 0.4 wt.% Mo. On the other hand, a decrease in the Ni crystallite size was detected on the H2-reduced samples. Specifically, the primary size of Ni decreased by 12% and 31% in the cases of 1Au-Ni/GDC and 3Au-Ni/GDC, by 24% and 40% in 0.5Fe-Ni/GDC and 2Fe-Ni/GDC, and by 24% and 45% in 0.5Fe-3Au-Ni/GDC and 0.4Mo-3Au-Ni/GDC.

Table 3.
Primary, mean, crystallite size (nm) of NiO, Ni, GDC, Fe2O3, and Au, estimated from XRD line broadening and by using the Scherrer equation (Equation (7)), for Mo-Au-Fe-NiO/GDC electrocatalysts in their oxidized and reduced form.
Overall, concerning the physicochemical properties of the oxidized powders, XRF analysis revealed that the wt.% concentration of each dopant was close to the nominal, whereas XRD verified the detection of iron in the form of syn-Fe2O3 and gold as metallic Au. After H2-reduction, there was formation of Ni-Fe, Ni-Au, Ni-Fe-Au, and Ni-Mo-Au solid solutions.
4. Catalytic—Kinetic Measurements
4.1. Carbon Deposition from the Catalytic CH4 Dissociation Reaction—TGA Measurements
The carbon tolerance of the modified samples was studied isothermally at 800 °C by means of thermogravimetric (TG) analysis under 10 vol.% CH4/Ar flow. The TG profiles (Figure 3) depict the change in weight (Δwt.%), as a function of time due to carbon deposition.
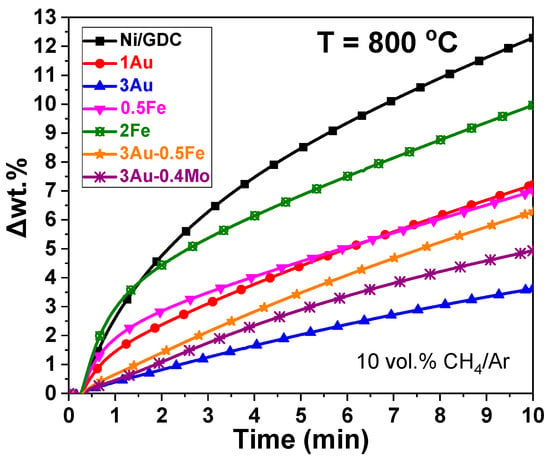
Figure 3.
TG isothermal analysis of Mo-Au-Fe-Ni/GDC powders under 10 vol.% CH4 in Ar at 800 °C. Ftot = 100 cm3 min−1.
Specifically, the presented TG measurements investigated the activity of the examined samples for the catalytic CH4 dissociation reaction and the concomitant carbon formation/deposition. In this reaction scheme, CH4 is adsorbed and dissociates on the Ni surface sites (*) towards adsorbed H* and CHx species, according to Equations (12)–(16) [,].
CH4 + 2* ↔ CH3* + H*
CH4 + * ↔ CH4*
CH3* + * ↔ CH2* + H*
CH2* + * ↔ CH* + H*
CH* + * ↔ C* + H*
The resulting adsorbed carbon (C*) species cover the surface of Ni, with subsequent diffusion and coverage of the bulk phase. The basic remark in this scheme is that the strongest CH4 interaction with the Ni sites results in a higher carbon formation/deposition rate.
The TG profiles (Figure 3) show that the modified samples exhibited lower activity for the catalytic CH4 decomposition, and as a result sufficient tolerance to carbon formation/deposition when compared to Ni/GDC. Concerning the iron-modified samples, 2Fe-Ni/GDC and to a lesser extent 0.5Fe-Ni/GDC exhibited immediate and rapid weight increase due to carbon deposition, whereas after the first minute the wt.% increase was less acute. After 10 min under 10 vol.% CH4/Ar exposure, the Fe-modified samples exhibited a slightly higher tolerance against coking than Ni/GDC. On the other hand, modification of Ni/GDC with 3 wt.% Au resulted in the highest carbon resistance when compared to the other samples. In regards to the ternary samples 0.5Fe-3Au-Ni/GDC and 0.4Mo-3Au-Ni/GDC, they exhibited adequate tolerance against carbon formation. Thus, the presence of 3 wt.% Au, with or without Fe/Mo, makes Ni/GDC less prone to carbon deposition.
The presented TGA results on Au and Mo-Au-Ni/GDC samples confirm previous findings from our research group []. Moreover, studies in the literature verify that Au can modify the catalytic activity of a Ni-based catalyst by inhibiting the dissociative adsorption of CH4 or at least the dehydrogenation reaction steps that lead to carbon formation (Equations (12)–(16)) [,]. In particular, Fan et al. [] employed DFT calculations to study the synergistic interaction between Ni and transition metal atoms (M = Cu, Ru, Rh, Pd, Ag, Pt, and Au) on M/Ni (1 1 1) surface alloys for CH4 dissociation. In accordance with our results, it was found that the binding strength of CHx was lower over Au-Ni (1 1 1) compared to Ni3 sites. Additionally, the adsorption energy of C on Ni-Au sites was found to be less negative than that on the Ni (1 1 1) surface, indicating the inhibition of coke formation over Au/Ni catalysts.
4.2. Catalytic—Kinetic Investigation for the Dry CH4 Reforming Reaction
Catalytic experiments at OCP mode on electrolyte-supported half cells were performed in order to have a reference on the performance and carbon tolerance of each electrocatalyst under CO2 reforming of CH4 reaction conditions, without the effect of the applied current (and consequently of the O2− flux). Regarding the homogenous catalytic reaction, no activity was observed. In addition, since the specific measurements are going to be used as a reference catalytic profile for further full cell electrocatalytic measurements, it was decided to examine the effect of the applied current collector for the fuel electrode. In this respect, comparative measurements of Ni/GDC with and without the presence of Ni mesh (Figure S2 in the Supplementary Materials) showed that there is no direct catalytic contribution of the Ni mesh on the electrocatalysts’ activity.
Figure 4 and Figure 5 show the catalytic performance of each sample for the CO2 reforming of CH4 at 750–900 °C (CH4/CO2 = 50/50, Ftot,in = 150 cm3 min−1). Specifically, Figure 4 depicts the consumption/production rates of CH4, CO2, H2, CO, H2O, and the formed carbon, whereas Figure 5 shows the % conversions of CH4 and CO2. The measurements were performed on electrolyte supported (half) cells that comprised only the fuel electrocatalyst and Ni mesh.
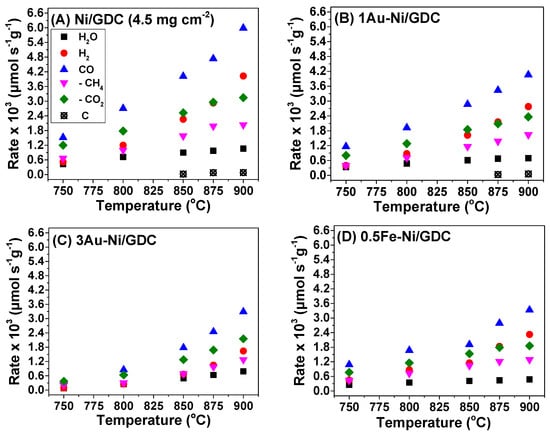
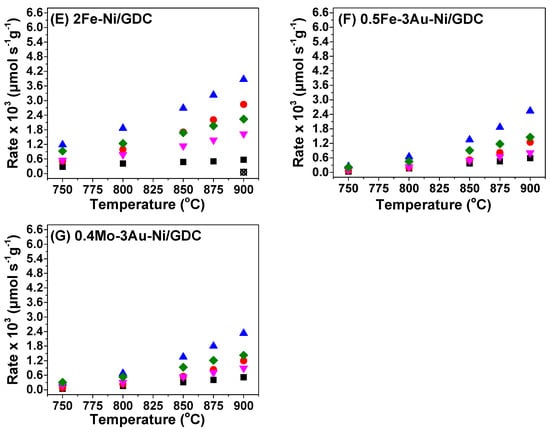
Figure 4.
Production and consumption rates (μmol s−1 g−1) of (A) Ni/GDC [], (B) 1Au-Ni/GDC, (C) 3Au-Ni/GDC, (D) 0.5Fe-Ni/GDC, (E) 2Fe-Ni/GDC, (F) 0.5Fe-3Au-Ni/GDC [], and (G) 0.4Mo-3Au-Ni/GDC [] half cells at 750–900 °C. The reaction mixture comprised 50 vol.% CH4 and 50 vol.% CO2. Ftotal = 150 cm3/min. Mass of each electrode ~6 mg/cm2. OCP conditions. Data from (A,F,G) are reprinted from Ref. []. 2023, © The Electrochemical Society. Reproduced by permission of IOP Publishing Ltd. All rights reserved.
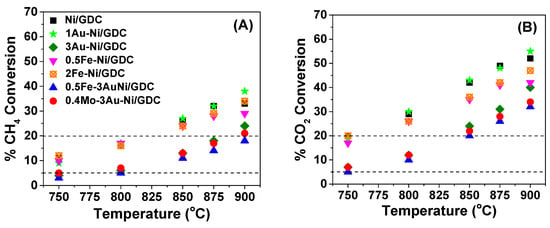
Figure 5.
% Conversion of (A) CH4 and (B) CO2 for Ni/GDC, 1Au-Ni/GDC, 3Au-Ni/GDC, 0.5Fe-Ni/GDC, 2Fe-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC half cells at 750–900 °C. Reaction mixture = 50 vol.% CH4−50 vol.% CO2. Ftotal = 150 cm3/min. Mass of each electrode = ~6 mg/cm2. OCP conditions. The dashed lines correspond to the differential conditions region, where the CH4 and CO2 conversions varied in the region 5–20%.
In respect to the catalytic activity of Ni/GDC, it was found to be the most active sample for the CO2 reforming of CH4, yielding the highest consumption/production rates (Figure 4) and % conversions (Figure 5). However, it also exhibited measurable carbon formation rates at high temperatures (≥850 °C). The modified electrocatalysts were less active in terms of H2 and CO production, but at the same time they were less prone to carbon formation (Figure 4). In particular, the majority of them did not show measurable carbon rates within the examined temperature region.
More specifically, 1Au-Ni/GDC and 2Fe-Ni/GDC were less active than Ni/GDC, but more active compared to the other modified samples (Figure 4). However, carbon formation was measured for both of these electrocatalysts at temperatures higher than 875 °C (Figure 4), which is a drawback for their use as potential fuel electrodes for the IDRM process. Although the above samples exhibited adequate tolerance to carbon formation under the catalytic decomposition of 10 vol.% CH4/Ar at 800 °C (Figure 3), this did not apply for the DRM catalytic measurements under 50 vol.% CH4−50 vol.% CO2 (Figure 4). Furthermore, the fact that 1Au-Ni/GDC was prone to carbon formation (Figure 4) suggests that the 1 wt.% content of Au is probably too low to inhibit the coke formation under DRM reaction conditions.
On the other hand, 3Au-Ni/GDC and 0.5Fe-Ni/GDC showed sufficient carbon tolerance, but slightly lower rates than 1Au-Ni/GDC and 2Fe-Ni/GDC (Figure 4). Thus, it seems that the modification of Ni/GDC with 3 wt.% Au or 0.5 wt.% Fe inhibits carbon formation under DRM conditions and this observation concurs with the results from the TG analysis (Figure 3). Finally, the ternary samples 0.5Fe-3Au-Ni/GDC and 0.4Mo-3Au-Ni/GDC exhibited the lowest catalytic activity (Figure 4), but at the same time zero carbon deposits, which is also in agreement with the TGA results (Figure 3).
Regarding the % conversions of CO2 and CH4 at 750–900 °C (Figure 5), a general observation is that these values were lower than the thermodynamic equilibrium values at the corresponding temperatures under a mixture of CH4/CO2 = 1 at 1 atm (Table 4).
In addition, the % conversion of CO2 at each temperature was always higher than the % conversion of CH4. This is ascribed to the contribution of the RWGS reaction, which consumes CO2 and H2, resulting in a H2/CO ratio less than unity [,,,,]. Finally, the % conversions (Figure 5) and the consumption/production rates (Figure 4) increased by increasing the reaction temperature, due to the endothermic character of the DRM reaction [,].

Table 4.
Equilibrium % conversions of CH4 and CO2 for stoichiometric carbon dioxide reforming of methane (at 1 atm) as a function of temperature [,].
Table 4.
Equilibrium % conversions of CH4 and CO2 for stoichiometric carbon dioxide reforming of methane (at 1 atm) as a function of temperature [,].
| Temperature (°C) | % CH4 | % CO2 |
|---|---|---|
| 750 | 89.5 | 93.5 |
| 800 | 95.0 | 97.3 |
| 850 | 96.7 | 98.3 |
| 875 | 97.5 | 98.8 |
| 900 | 98.3 | 99.2 |
The “used” electrocatalysts, after the applied DRM conditions, were examined by means of scanning electron microscopy (SEM) with a HR − SEM (Zeiss SUPRA 35VP) (Figure S4 in the Supplementary Materials). Further analysis of the SEM images with the Gwyddion 2.49 software enabled the calculation of the particle size (Table S2 in the Supplementary Materials). As a general remark, the observed particles consist of both Ni and GDC and the mean diameter of their size was calculated in the range of 200 nm (std. error ± 10 nm), indicating similar agglomeration after the DRM measurement. Moreover, 0.5Fe-3Au-Ni/GDC and 0.4Mo-3Au-Ni/GDC exhibited larger “void space” when compared to Ni/GDC which is an indication of larger macro-porosity/tortuosity [].
According to the literature [,,,,,,], Au-Ni surface alloys exhibit less activity for methane reforming, but stronger resistance to carbon formation, even with small amounts of Au doping. Indicatively, DFT studies from Besenbacher et al. [] on Au-Ni catalysts led to the suggestion that Au-Ni is less active in methane reforming, but more stable because the effect of Au on the atomic carbon adsorption was found to be stronger than on CH4 activation. The low catalytic activity of Au-Ni-based catalysts is in accordance with the d-band center theory that was proposed by Nørskov et al. [,] dealing with the dissociation of CH4 on the Ni surface doped with Au. According to these studies, the rate of CH4 dissociation and consequently the amount of carbon formation (Equations (12)–(16)) can be inhibited on Au-modified surfaces, due to the fact that the center of the d-band density of states is displaced to lower energies below the Fermi level of Ni. The same trend can be considered for the case where Au, Mo, and Ni coexist through the formation of the ternary solid solution []. The latter remark has been confirmed in previous studies [,,] for the catalytic and electrocatalytic internal H2O reforming of CH4 (also in the presence of 10 ppm H2S) where the 0.4Mo-3Au-Ni/GDC electrode was the least prone to carbon deposition (and sulfur poisoning) and the most active electrocatalytically, compared to both the non-doped and the Au-doped electrodes.
Other studies proposed that carbon formation in reforming processes depends on the size of metal particles [,]. Thus, smaller Ni particles have stronger ability to inhibit coke deposition, suggesting high stability of the catalytic activity []. In the present study, the particle size of Ni was smaller in all modified samples compared to Ni/GDC. Another remark especially for the case of the CO2 reforming of CH4 is that the addition of a small amount of a noble metal (i.e., Au in our case) favors CO2 dissociation, resulting in oxygen formation that can assist the coke removal from the catalyst surface [].
Furthermore, in regards to the Fe-Ni surface alloys/solid solutions, these have been proposed to partially promote carbon gasification [,,,,,]. Specifically, S.M. Kim et al. reported [] that Fe in Ni-Fe alloys is partially oxidized by CO2 to FeO (CO2 + Fe → CO + FeO), which in turn provides lattice oxygen to the deposited carbon on the surface, leading to its partial oxidation to CO (C + FeO → CO + Fe), and therefore results in materials with improved coke tolerance. In the present study, only the 0.5Fe-Ni/GDC sample was found to be tolerant towards coke formation, whereas 2Fe-Ni/GDC was prone to carbon deposition. Finally, the 0.5Fe-3Au-Ni/GDC is a new proposed electrocatalyst that combines the improved catalytic characteristics of Au and Fe towards elimination of carbon deposits, although it exhibited lower catalytic performance compared to Ni/GDC.
Therefore, in an attempt to further investigate the observed differences on the catalytic activity, kinetic measurements were performed by applying fuel reaction inlet flows in the range of 150–300 cm3/min. The objective of the applied flows was to keep the CH4 and CO2 conversions in the region between 5–20%, thus assuring operation under differential conditions. It should be noted that in the above fuel inlet range the reaction rates were found to remain practically constant, which indicates the absence of mass transfer limitations.
Specifically, Ni/GDC was the most catalytically active sample, yielding high % CH4 and CO2 conversions that were beyond the differential conditions region. In order to achieve this experimental parameter, it was necessary to decrease the mass loading of the sample from 5 mg/cm2 to 2.5 mg/cm2 and to perform a new series of kinetic measurements (Figure S3 in the Supplementary Materials). Similarly, in the case of the modified samples, the Ftotal was fixed in the range of 150–300 cm3/min with a mass loading of ~6 mg/cm2 (Table S1 in the Supplementary Materials). In addition, for reliable comparison reasons, a Ni/GDC electrode with a similar loading (5 mg/cm2) as the modified samples was studied kinetically in the range of 250–300 cm3/min (Table S1 in the Supplementary Materials).
Figure 6 presents the Arrhenius plots for the production rates of CO, H2, and H2O and the consumption rates of CH4 and CO2 and Table 5 presents the calculated apparent activation energies (Ea,app) for each electrocatalyst under differential conditions. The Ea, app for the production of H2O (Figure 6C) corresponds to the apparent activation energy for the RWGS reaction, which consumes the valuable H2 for the fuel cell and is considered as a non-desired side reaction for the SOFC IDRM process. Modification of Ni/GDC with iron seems to favor the RWGS reaction, since Ea,app values of 0.5Fe-Ni/GDC and 2Fe-Ni/GDC were quite low for H2O production. The same observation also applies for the 1Au-Ni/GDC electrode. Moreover, the above samples exhibited quite similar Ea,app values like Ni/GDC for CH4 and CO2 consumption. Concerning H2 and CO production, 0.5Fe-Ni/GDC, 1Au-Ni/GDC, and mainly 2Fe-Ni/GDC exhibited lower Ea,app values than that of Ni/GDC.
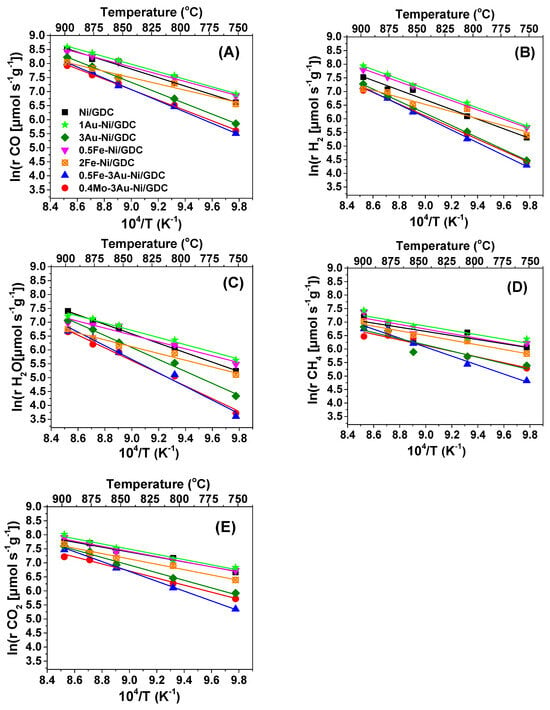
Figure 6.
Arrhenius plots of the inherent production of (A) CO, (B) H2, and (C) H2O and consumption of (D) CH4 and (E) CO2 as a function of temperature (750–900 °C). Differential conditions (Conversions: 5–20%) under the reaction mixture of 50 vol.% CH4−50 vol.% CO2. Ftotal (cm3 min−1) and mass (mg cm−2) of each half cell as a function of temperature (750–900 °C) is depicted in Table S1 in the Supplementary Materials.

Table 5.
Apparent Activation Energies (Ea,app, kJ mol−1) of modified electrodes under differential conditions for H2O, H2, and CO production and CH4, CO2 consumption derived from Figure 6 and calculated from the Arrhenius equation.
On the other hand, modification of Ni/GDC with 3 wt.% Au seems to hinder the activity for the RWGS reaction, since the Ea,app values of 3Au-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC were higher than those of the other examined samples. In addition, the specific electrocatalysts were less prone to carbon formation and less active for H2 and CO production with relatively high Ea,app values when compared to Ni/GDC and the Fe-modified electrodes. Finally, the fact that 2Fe-Ni/GDC and Ni/GDC were the samples where carbon formation was detected is a point that needs to be also taken into account.
According to the literature, CH4 decomposition (Equation (12)) is the rate-determining step at high temperatures for both DRM and decomposition reactions [,,]. Experimental kinetic studies [,] coincide with theoretical studies [,] for the reaction mechanism, which suggests that CH4 decomposition demands higher energy than further decomposition of CHx (x ≤ 3) (Equations (14)–(16)). Thus, the available research data focus on activation energy values for CH4 activation/consumption. Moreover, it is generally accepted that CH4 is decomposed on metal surface sites (e.g., Ni), whereas CO2 is mainly activated on support sites (e.g., GDC in the case of the examined electrocatalysts) on the vicinity of dispersed metal particles or/and on the metallic sites [,]. As reported in the literature, the calculated activation energies for Ni-based catalysts in the DRM process vary within a wide range between 29 and 360 kJ/mol, which depends on the nature of the support, the presence of additives, and the catalytic conditions [,,]. The most frequently reported Ea,app value for Ni-based catalysts is ~60 kJ/mol, which concurs with our calculated Ea,app for Ni/GDC and coincides with the Ea,app for CH4 dissociation on Ni (1 1 0) and Ni (1 1 1) []. It should be noted that very low Ea,app values may reflect mass transfer limitation phenomena [,].
The modification of Ni/GDC with 3 wt.% Au and 0.4 wt.% Mo-3 wt.% Au, as well as with 0.5 wt.% Fe-3 wt.% Au, increased the Ea,app for CH4 dissociation, suggesting that the catalytic activity of the surface Ni active sites has been inhibited for this reaction. Furthermore, the fact that Ni-Au modification increased the Ea,app for CH4 reforming reactions has been also observed in other studies [,]. Overall concerning the selection of the proper electrocatalyst for the IDRM reaction, it is suggested that this should exhibit (a) the lowest Ea,app for H2 and CO production, (b) the highest Ea,app for H2O production, and (c) negligible carbon formation rates.
The kinetic investigation dealt also with the dependency of the H2, CO, and H2O production rates on various CH4 and CO2 molar fractions (yCH4, yCO2). These measurements also took place under differential conditions, by varying the applied gas flows between 150 and 300 cm3/min. The examined electrocatalysts (in the form of half cells) were Ni/GDC and 0.4Mo-3Au-Ni/GDC with mass loadings of 2.5 and 7.5 mg/cm2, respectively. The effect of yCH4 and yCO2 on the production rates for Ni/GDC is shown in Figure 7 and Figure 8, whereas for 0.4Mo-3Au-Ni/GDC it is shown in Figure 9 and Figure 10.
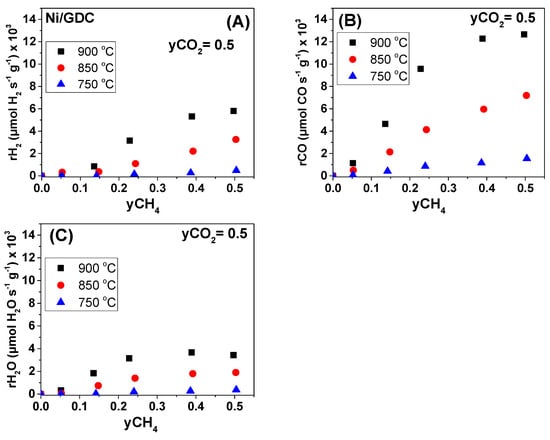
Figure 7.
Steady-state effect of CH4 molar fraction (yCH4) under fixed CO2 molar fraction (yCO2 = 0.5) on the (A) H2 production rate, (B) CO production rate, and (C) H2O production rate under OCP conditions. Half-cell with Ni/GDC (2.5 mg/cm2). Differential conditions (Conversions: 5–20%).
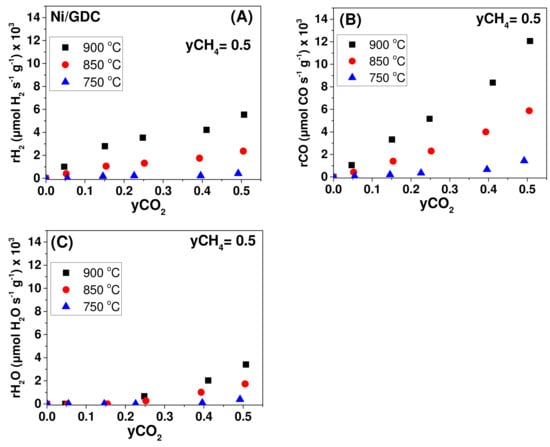
Figure 8.
Steady-state effect of CO2 molar fraction (yCO2) under fixed CH4 molar fraction (yCH4 = 0.5) on the (A) H2 production rate, (B) CO production rate, and (C) H2O production rate under OCP conditions. Half-cell with Ni/GDC (2.5 mg/cm2). Differential conditions (Conversions: 5–20%).
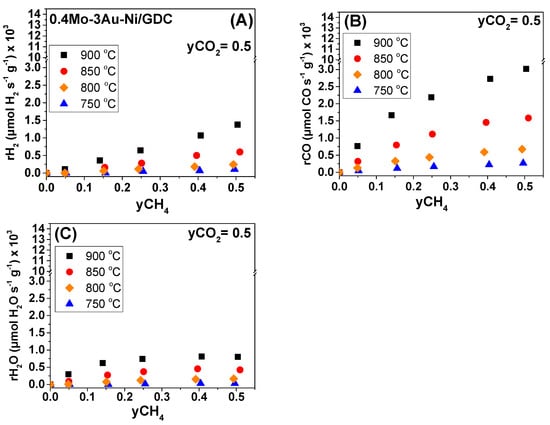
Figure 9.
Steady-state effect of CH4 molar fraction (yCH4) under fixed CO2 molar fraction (yCO2 = 0.5) on the (A) H2 production rate, (B) CO production rate, and (C) H2O production rate under OCP conditions. Half-cell with 0.4Mo-3Au-Ni/GDC (7.5 mg/cm2). Differential conditions (Conversions: 5–20%).
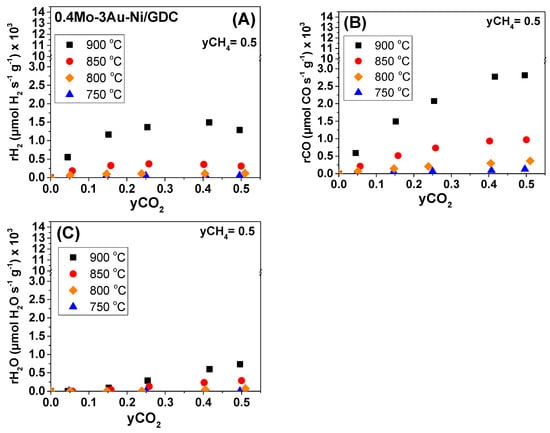
Figure 10.
Steady-state effect of CO2 molar fraction (yCO2) under fixed CH4 molar fraction (yCH4 = 0.5) on the (A) H2 production rate, (B) CO production rate, and (C) H2O production rate under OCP conditions. Half-cell with 0.4Mo-3Au-Ni/GDC (7.5 mg/cm2). Differential conditions (Conversions: 5–20%).
Concerning Ni/GDC, it is observed that the production rates of H2 and CO exhibited a positive order dependence either on yCH4 (Figure 7A,B) or on yCO2 (Figure 8A,B) at 850 and 900 °C. On the other hand, the production rate of H2O showed an initially positive and then zero order dependence on yCH4 (Figure 7C), whereas it was positive on yCO2 (Figure 8C) at the same temperatures (850 and 900 °C). At 750 °C the general remark is the inhibition of the catalytic activity towards the production of H2, CO, and H2O. Moreover, at each temperature and fuel feed, the CO production rates were higher than that of H2, due to the fact that CO is produced both through the DRM (Equation (1)) and the RWGS (Equation (2)) reactions.
Interestingly, at the high temperature of 900 °C the production rates of H2, CO, and H2O approached a maximum at yCH4 ≥ 0.4 by keeping constant yCO2 = 0.5 (Figure 7). On the contrary, at the same temperature, rH2, rCO, and rH2O increased within the whole yCO2 range, while yCH4 = 0.5 (Figure 8). The positive reaction order of the production rates versus yCH4 and yCO2 and the appearance of a maximum at yCH4 ≥ 0.4 at 900 °C indicate that most probably the dissociative adsorption of CH4 can be the limiting step of the DRM reaction on Ni/GDC, which is in agreement with other studies [,,,]. Finally, it should be noted that under low yCO2 (<0.25) (Figure 8C) the production of H2O, through the RWGS reaction, was negligible and as a result the produced CO is derived solely from the DRM reaction. In this case and in the region of yCO2 < 0.25, the produced rH2 should be identical with the produced rCO, which was confirmed (Figure 8A,B).
In regards to the 0.4Mo-3Au-Ni/GDC sample at T < 800 °C (i.e., 750 °C), the overall catalytic activity was inhibited, similar to Ni/GDC. At ≥ 800 °C, a positive order dependence was also observed for the production rates of H2 and CO by varying yCH4 (Figure 9A,B). However, by varying the yCO2 and at temperatures > 800 °C (Figure 10A,B), the trend of rH2 and rCO was different when compared to Ni/GDC. Specifically, in the non-modified sample the production rate of H2 and CO increased within the whole range of the applied yCO2, while yCH4 = 0.5 (Figure 8A,B). On the other hand, in the case of the Mo-Au-modified sample, at T ≥ 800 °C the rH2 and rCO reached to a maximum for yCO2 ≥ 0.25 (Figure 10A,B). This maximum indicates that the DRM reaction mechanism for 0.4Mo-3Au-Ni/GDC might be different when compared to Ni/GDC. In particular, it can be suggested that on the active sites of the Mo-Au-Ni-modified electrocatalyst there is a point where the dissociative adsorption of CO2 reached faster to equilibrium and to a stable surface coverage. As a result, the production of CO and H2 did not increase any further by increasing the yCO2 when compared to Ni/GDC.
Concerning the production rate of H2O on the 0.4Mo-3Au-Ni/GDC, it exhibited the same trend like on the non-modified sample. Specifically, rH2O initially showed a positive order dependence on yCH4 (Figure 9C), which altered to zero at yCH4 ≥ 0.25. Moreover, the dependence of rH2O on the applied yCO2 (Figure 10C) was positive.
Therefore, the observation that in the presented DRM reactions scheme H2O is produced only through the RWGS reaction, in combination with the similar dependence of rH2O on yCH4 and yCO2, suggest that both of the Ni/GDC and 0.4Mo-3Au-Ni/GDC electrocatalysts perform the RWGS reaction through the same mechanism. On the other hand, by considering that CO is produced both through DRM and RWGS, it can be assumed that the mechanism of the main DRM reaction may be different for 0.4Mo-3Au-Ni/GDC than that of Ni/GDC, which is a remark that needs to be further studied. Furthermore, the calculated Ea,app from the Arrhenius plots and the formation rates of carbon highlight that the modified 3Au-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC samples exhibited (i) the highest Ea,app for the non-desired RWGS reaction, (ii) high tolerance to carbon formation, and (iii) they were less active for H2 and CO production, as well as for the CH4 decomposition. Therefore, and for the purposes of a following separate manuscript, there will be selection among these three samples for electro-catalytic measurements and comparison with the SoA Ni/GDC in the form of full cells for the internal DRM process.
5. Conclusions
The present study dealt with the physicochemical and kinetic investigation of Fe, Au, Fe-Au, and Mo-Au modified Ni/GDC electrocatalysts towards their performance for the DRM, RWGS, and CH4 decomposition reactions, as well as their tolerance to carbon formation. The research objective of this investigation focuses on the sustainable conversion of the greenhouse CH4 and CO2 gases towards syngas by means of Solid Oxide Fuel cells, which is a highly efficient and environmentally friendly electrochemical source of energy/power and useful chemicals. In this respect, the catalytic-kinetic investigation, in combination with the detailed physicochemical characterization, of a variety of new candidate fuel electrodes is the first key step to understand the underlying catalytic profile and to elucidate the occurring reactions during the IDRM process.
Regarding the physicochemical properties of the oxidized powders, XRF analysis revealed that the wt.% concentration of each dopant was close to the nominal, whereas XRD verified the detection of iron in the form of syn-Fe2O3 and gold as metallic Au. After H2-reduction, there was formation of Ni-Fe, Ni-Au, Ni-Fe-Au, and Ni-Mo-Au solid solutions. TG analysis at 800 °C, under 10 vol.% CH4/Ar, showed that the modified samples exhibited lower activity for the catalytic CH4 decomposition and were less prone to carbon deposition when compared to Ni/GDC. Specifically, modification with 3 wt.% Au, with or without Fe/Mo, resulted in the highest carbon tolerance.
In respect to the catalytic performance of electrolyte-supported half cells, Ni/GDC was found to be the most active sample for the CO2 reforming of CH4. However, it exhibited carbon formation rates at high temperatures (≥850 °C). On the other hand, 3Au-Ni/GDC, 0.5Fe-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC were less active catalytically, but at the same time exhibited higher tolerance to carbon deposition. Concerning the selection of the proper electrocatalyst for the IDRM reaction, it is suggested that this should preferably exhibit (a) the lowest Ea,app for H2 and CO production, (b) the highest Ea,app for H2O production which results mainly from the RWGS and is considered as an undesired side reaction for the SOFC IDRM process, and (c) negligible carbon formation. These kinetic pre-conditions seem to be followed by the modified 3Au-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC electrocatalysts.
Further kinetic analysis on Ni/GDC and 0.4Mo-3Au-Ni/GDC showed similar dependence of rH2O on yCH4 and yCO2, suggesting that the RWGS side reaction may be performed through the same mechanism on both samples. On the other hand, it was observed that the mechanism of the main DRM reaction may be different for the 0.4Mo-3Au-Ni/GDC when compared to Ni/GDC. This is because the Mo-Au-modified sample (i) was less active for the catalytic CH4 decomposition reaction and thus less prone to carbon formation, and (ii) at high temperature (≥800 °C) and constant yCH4 the dissociative adsorption of CO2 seems to reach faster to a stable surface coverage when compared to Ni/GDC.
Overall, the present study is a thorough investigation on the development of modified Ni/GDC electrocatalysts with enhanced efficiency and carbon tolerance when compared to the SoA. The selected samples, namely 3Au-Ni/GDC, 0.5Fe-3Au-Ni/GDC, and 0.4Mo-3Au-Ni/GDC, meet the experimentally defined kinetic prerequisites for the SOFC IDRM reaction. Therefore, the reported findings are considered as a significant contribution for the practical application of this technology with a valuable impact in the research field of sustainable energy. These samples are currently under further investigation as electrodes for the purposes of a separate manuscript in full cell SOFC measurements and long-term stability operation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/en17010184/s1, Figure S1. XRD patterns of the oxidized [(A),(B),(C)] (calcined at 1100 °C) and [(D)] H2-reduced (900 °C) powders. Furthermore, there are magnifications of the main XRD peaks for the oxidized (1100 °C) powders: (B) NiO (0 1 2) peak in the range of 42.8° ≤ 2θ ≤ 43.6° and (C) GDC (2 2 2) peak in the range of 28.0° ≤ 2θ ≤ 28.8°. Figure S2. Catalytic investigation of a half cell with: (A), (C) Ni/GDC without Ni mesh and (B), (D) Ni/GDC with Ni mesh, at 750–900 °C, in terms of (A), (B) rate and (C), (D) % conversion. The reaction mixture comprised 50 vol.% CH4 and 50 vol.% CO2. OCP conditions. Ftotal = 150 cm3/min. Mass of Ni/GDC electrode ~5 mg/cm2. Figure S3. Catalytic investigation of Ni/GDC under differential conditions, in terms of (A) % conversion of CH4 and CO2, (B) production and consumption inherent rates (μmol s−1 g−1) and (C) Arrhenius plots of the inherent rates as a function of temperature (750–900 °C). Reaction mixture: 50 vol.% CH4−50 vol.% CO2. Ftotal was 150 cm3/min at 750–850 °C and 200 cm3/min at 875 and 900 °C. Mass: 3 mg/cm2. Figure S4. SEM of the surface side of the used Mo-Au-Fe-Ni/GDC half cells, after DRM catalytic study. Table S1: Ftotal,inlet (cm3 min−1) per half cell, under differential conditions (Conversions: 5–20%) as a function of temperature (750–900 °C). Mass (mg cm−2) of each cell is also depicted. Table S2: Mean diameter (nm) of 100 particles, per Mo-Au-Fe-Ni/GDC half cell, from Figure S4 after SEM analysis. Used cells after DRM study.
Author Contributions
Conceptualization, S.G.N. and D.K.N.; Methodology, E.I., S.G.N. and D.K.N.; Validation, E.I., S.G.N. and D.K.N.; Formal analysis, E.I.; Investigation, E.I.; Resources, D.K.N.; Data curation, E.I. and D.K.N.; Writing—original draft, E.I.; Writing—review & editing, E.I. and D.K.N.; Visualization, E.I. and D.K.N.; Supervision, S.G.N. and D.K.N.; Project administration, D.K.N.; Funding acquisition, S.G.N. and D.K.N. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the European Union and Greek national funds through the operational program ‘Regional Excellence’ and the operational program ‘Competitiveness, Entrepreneurship, and Innovation’, under the call “RESEARCH-CREATE-INNOVATE” (Project code: Eco-Bio-H2-FCs, T2EΔK-00955) and from the Fuel Cells and Hydrogen Two Joint Undertaking (now Clean Hydrogen Partnership) under the project 24_7 ZEN [Horizon Europe], Grant Agreement No. 101101418. This Joint Undertaking receives support from the European Union’s Horizon 2020 Research and Innovation program, Hydrogen Europe and Hydrogen Europe Research.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
The authors would like to thank Vayia Xanthopoulou, researcher at the Laboratory of Electron Microscopy and Microanalysis, School of Natural Sciences, University of Patras, for the XRF analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Escudero, M.J.; Maffiotte, C.A.; Serrano, J.L. Long-term operation of a solid oxide fuel cell with MoNi–CeO2 as anode directly fed by biogas containing simultaneously sulphur and siloxane. J. Power Sources 2021, 481, 229048. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Dong, F. Grand Challenges for Catalytic Remediation in Environmental and Energy Applications toward a Cleaner and Sustainable Future. Front. Environ. Chem. 2020, 1, 5. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Niu, J.; Guo, F.; Ran, J.; Qi, W.; Yang, Z. Methane dry (CO2) reforming to syngas (H2/CO) in catalytic process: From experimental study and DFT calculations. Int. J. Hydrogen Energy 2020, 45, 30267–30287. [Google Scholar] [CrossRef]
- Faro, M.L.; Vita, A.; Pino, L.; Aricò, A.S. Performance evaluation of a solid oxide fuel cell coupled to an external biogas tri-reforming process. Fuel Process. Technol. 2013, 115, 238–245. [Google Scholar] [CrossRef]
- Chiodo, V.; Galvagno, A.; Lanzini, A.; Papurello, D.; Urbani, F.; Santarelli, M.; Freni, S. Biogas reforming process investigation for SOFC application. Energy Convers. Manag. 2015, 98, 252–258. [Google Scholar] [CrossRef]
- Shiratori, Y.; Ijichi, T.; Oshima, T.; Sasaki, K. Internal reforming SOFC running on biogas. Int. J. Hydrogen Energy 2010, 35, 7905–7912. [Google Scholar] [CrossRef]
- Sarno, C.; Luisetto, I.; Zurlo, F.; Licoccia, S.; Di Bartolomeo, E. Lanthanum chromite based composite anodes for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 14742–14750. [Google Scholar] [CrossRef]
- Papadam, T.; Goula, G.; Yentekakis, I.V. Long-term operation stability tests of intermediate and high temperature Ni-based anodes’ SOFCs directly fueled with simulated biogas mixtures. Int. J. Hydrogen Energy 2012, 37, 16680–16685. [Google Scholar] [CrossRef]
- Santoro, M.; Di Bartolomeo, E.; Luisetto, I.; Aricò, A.; Squadrito, G.; Zignani, S.; Faro, M.L. Insights on the electrochemical performance of indirect internal reforming of biogas into a solid oxide fuel cell. Electrochim. Acta 2022, 409, 139940. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Thattai, A.T.; Fan, L.; Lindeboom, R.E.F.; Spanjers, H.; Aravind, P.V. Solid Oxide Fuel Cells fuelled with biogas: Potential and constraints. Renew. Energy 2019, 134, 194–214. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G. Biogas management: Advanced utilization for production of renewable energy and added-value chemicals. Front. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Barelli, L.; Ottaviano, A. Solid oxide fuel cell technology coupled with methane dry reforming: A viable option for high efficiency plant with reduced CO2 emissions. Energy 2014, 71, 118–129. [Google Scholar] [CrossRef]
- Qiu, P.; Sun, S.; Yang, X.; Chen, F.; Xiong, C.; Jia, L.; Li, J. A review on anode on-cell catalyst reforming layer for direct methane solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 25208–25224. [Google Scholar] [CrossRef]
- Gür, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Prog. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Wang, J.; Yang, J.; Chen, Y.; Guan, W.; Singhal, S.C. Power generation from a symmetric flat-tube solid oxide fuel cell using direct internal dry-reforming of methane. J. Power Sources 2021, 516, 230662. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Tanveer, W.H.; Sayed, E.T.; Assad, M.E.H.; Allagui, A.; Cha, S.W. On the technical challenges affecting the performance of direct internal reforming biogas solid oxide fuel cells. Renew. Sustain. Energy Rev. 2019, 101, 361–375. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Özkara-Aydnolu, Ş. Thermodynamic equilibrium analysis of combined carbon dioxide reforming with steam reforming of methane to synthesis gas. Int. J. Hydrogen Energy 2010, 35, 12821–12828. [Google Scholar] [CrossRef]
- Escudero, M.J.; Serrano, J.L. Individual impact of several impurities on the performance of direct internal reforming biogas solid oxide fuel cell using W-Ni-CeO2 as anode. Int. J. Hydrogen Energy 2019, 44, 20616–20631. [Google Scholar] [CrossRef]
- Chlipała, M.; Błaszczak, P.; Wang, S.F.; Jasiński, P.; Bochentyn, B. In Situ study of a composition of outlet gases from biogas fuelled Solid Oxide Fuel Cell performed by the Fourier Transform Infrared Spectroscopy. Int. J. Hydrogen Energy 2019, 44, 13864–13874. [Google Scholar] [CrossRef]
- Liu, M.; van der Kleij, A.; Verkooijen, A.H.M.; Aravind, P.V. An experimental study of the interaction between tar and SOFCs with Ni/GDC anodes. Appl. Energy 2013, 108, 149–157. [Google Scholar] [CrossRef]
- Ioannidou, E.; Chavani, M.; Neophytides, S.G.; Niakolas, D.K. Effect of the PH2O/PCO2 and PH2 on the intrinsic electro-catalytic interactions and the CO production pathway on Ni/GDC during solid oxide H2O/CO2 co-electrolysis. J. Catal. 2021, 404, 174–186. [Google Scholar] [CrossRef]
- Zhan, Z.; Lin, Y.; Pillai, M.; Kim, I.; Barnett, S.A. High-rate electrochemical partial oxidation of methane in solid oxide fuel cells. J. Power Sources 2006, 161, 460–465. [Google Scholar] [CrossRef]
- Sun, C.; Stimming, U. Recent anode advances in solid oxide fuel cells. J. Power Sources 2007, 171, 247–260. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.P.; Tok, A.I.Y.; Luo, L. GDC-impregnated Ni anodes for direct utilization of methane in solid oxide fuel cells. J. Power Sources 2006, 159, 68–72. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Wu, Y.; Ran, R.; Shao, Z. Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 2013, 113, 8104–8151. [Google Scholar] [CrossRef]
- Yue, W.; Li, Y.; Zheng, Y.; Wu, T.; Zhao, C.; Zhao, J.; Geng, G.; Zhang, W.; Chen, J.; Zhu, J.; et al. Enhancing coking resistance of Ni/YSZ electrodes: In Situ characterization, mechanism research, and surface engineering. Nano Energy 2019, 62, 64–78. [Google Scholar] [CrossRef]
- Niakolas, D.K.; Neofytidis, C.S.; Neophytides, S.G. Effect of Au and/or Mo doping on the development of carbon and sulfur tolerant anodes for SOFCs-A short review. Front. Environ. Sci. 2017, 5, 78. [Google Scholar] [CrossRef]
- Niakolas, D.K.; Athanasiou, M.; Dracopoulos, V.; Tsiaoussis, I.; Bebelis, S.; Neophytides, S.G. Study of the synergistic interaction between nickel, gold and molybdenum in novel modified NiO/GDC cermets, possible anode materials for CH4 fueled SOFCs. Appl. Catal. A Gen. 2013, 456, 223–232. [Google Scholar] [CrossRef]
- Neofytidis, C.; Dracopoulos, V.; Neophytides, S.G.; Niakolas, D.K. Electrocatalytic performance and carbon tolerance of ternary Au-Mo-Ni/GDC SOFC anodes under CH4-rich Internal Steam Reforming conditions. Catal. Today 2018, 310, 157–165. [Google Scholar] [CrossRef]
- Neofytidis, C.; Ioannidou, E.; Sygellou, L.; Kollia, M.; Niakolas, D.K. Affecting the H2O electrolysis process in SOECs through modification of NiO/GDC; experimental case of Au-Mo-Ni synergy. J. Catal. 2019, 373, 260–275. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neophytides, S.; Niakolas, D.K. Experimental clarification of the RWGS reaction effect in H2O/CO2 SOEC co-electrolysis conditions. Catalysts 2019, 9, 151. [Google Scholar] [CrossRef]
- Neofytidis, C.; Ioannidou, E.; Kollia, M.; Neophytides, S.; Niakolas, D. The promoting effect of Fe on Ni/GDC for the Solid Oxide H2O electrolysis. Int. J. Energy Res. 2020, 44, 10982–10995. [Google Scholar] [CrossRef]
- Kan, H.; Lee, H. Enhanced stability of Ni-Fe/GDC solid oxide fuel cell anodes for dry methane fuel. Catal. Commun. 2010, 12, 36–39. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Batchu, R.; Galvita, V.V.; Poelman, H.; Marin, G.B. Carbon gasification from Fe-Ni catalysts after methane dry reforming. Appl. Catal. B Environ. 2016, 185, 42–55. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, Q.; Guo, C.; Li, S.; Yan, W.; Jiao, W.; Qiu, L.; Yan, X.; Li, R. Improved effect of Fe on the stable NiFe/Al2O3 catalyst in low-temperature dry reforming of methane. Ind. Eng. Chem. Res. 2020, 59, 17250–17258. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.A.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264, 118497. [Google Scholar] [CrossRef]
- Zaravelis, F.; Sygellou, L.; Souvalioti, A.; Niakolas, D.K. Transition metals in Ni/GDC for the reversible solid oxide cell operation: Optimization of the Mo-Au-Ni synergy and further enhancement via substitution of Mo with Fe. Electrochim. Acta 2023, 453, 142343. [Google Scholar] [CrossRef]
- Wu, Y.; Su, C.; Wang, W.; Wang, H.; Shao, Z. Effect of fabrication method on properties and performance of bimetallic Ni0.75 Fe0.25 anode catalyst for solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 9287–9297. [Google Scholar] [CrossRef]
- HJeong; Kim, S.; Bae, Y.; Yoon, K.J.; Lee, J.; Hong, J. Effect of Fe infiltration to Ni/YSZ solid-oxide-cell fuel electrode on steam/CO2 co-electrolysis. Int. J. Energy Res. 2019, 43, 4949–4958. [Google Scholar]
- Simonsen, S.B.; Muhl, T.T.; Thydén, K.T.S.; Chatzichristodoulou, C.; Nielsen, J.; Sudireddy, B.R. Effect of Fe on high performing nanostructured Ni/Gd-doped ceria electrocatalysts. Solid State Ion. 2019, 340, 115019. [Google Scholar] [CrossRef]
- Fiuza, P.R.; da Silva, M.A.; Boaventura, J.S. Development of Fe–Ni/YSZ–GDC electrocatalysts for application as SOFC anodes: XRD and TPR characterization and evaluation in the ethanol steam reforming reaction. Int. J. Hydrogen Energy 2010, 35, 11216–11228. [Google Scholar] [CrossRef]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic screening constants from SCF functions. II. Atoms with 37 to 86 electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- Majewski, A.J.; Singh, S.K.; Labhasetwar, N.K.; Steinberger-Wilckens, R. Nickel–molybdenum catalysts for combined solid oxide fuel cell internal steam and dry reforming. Chem. Eng. Sci. 2021, 232, 116341. [Google Scholar] [CrossRef]
- Horváth, A.; Guczi, L.; Kocsonya, A.; Sáfrán, G.; La Parola, V.; Liotta, L.; Pantaleo, G.; Venezia, A. Sol-derived AuNi/MgAl2O4 catalysts: Formation, structure and activity in dry reforming of methane. Appl. Catal. A Gen. 2013, 468, 250–259. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Fan, C.; Zhu, Y.A.; Xu, Y.; Zhou, Y.; Zhou, X.G.; Chen, D. Origin of synergistic effect over Ni-based bimetallic surfaces: A density functional theory study. J. Chem. Phys. 2012, 137, 014703. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Neophytides, S.G.; Niakolas, D.K. Au-Mo-Fe-Ni/CeO2 (Gd2O3) as Potential Fuel Electrodes for Internal CO2 Reforming of CH4 in Single SOFCs. ECS Trans. 2023, 111, 2473–2485. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.C.; Green, M.L.H.; Claridge, J.B. Methane oxyforming for synthesis gas production. Catal. Rev. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Matralis, H.; Verykios, X. Utilization of biogas as a renewable carbon source: Dry reforming of methane. In Catalysis for Alternative Energy Generation; Springer: New York, NY, USA, 2012; pp. 57–127. [Google Scholar]
- Guilhaume, N.; Bianchi, D.; Wandawa, R.A.; Yin, W.; Schuurman, Y. Study of CO2 and H2O adsorption competition in the combined dry/steam reforming of biogas. Catal. Today 2021, 375, 282–289. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Fouskas, A.; Kollia, M.; Kambolis, A.; Papadopoulou, C.; Matralis, H. Boron-modified Ni/Al2O3 catalysts for reduced carbon deposition during dry reforming of methane. Appl. Catal. A Gen. 2014, 474, 125–134. [Google Scholar] [CrossRef]
- Cesario, M.R.; Souza, G.S.; Loureiro, F.J.; Araújo, A.J.; Grilo, J.P.; Aouad, S.; Tidahy, H.L.; Macedo, D.A.; Fagg, D.P.; Gennequin, C.; et al. Synthesis of Co–Ni and Cu–Ni based-catalysts for dry reforming of methane as potential components for SOFC anodes. Ceram. Int. 2021, 47, 33191–33201. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Ling, C.; Zhou, T.; Wang, S. Methane dehydrogenation on Au/Ni surface alloys-a first-principles study. Catal. Sci. Technol. 2013, 3, 1343–1354. [Google Scholar] [CrossRef]
- Besenbacher, F.; Chorkendorff, I.; Clausen, B.S.; Hammer, B.; Molenbroek, A.M.; Nørskov, J.K.; Stensgaard, I. Design of a surface alloy catalyst for steam reforming. Science 1998, 279, 1913–1915. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, P.; Hammer, B.; Nørskov, J.K. A theoretical study of CH4 dissociation on pure and gold-alloyed Ni(111) surfaces. J. Chem. Phys. 1996, 105, 5595–5604. [Google Scholar] [CrossRef]
- Li, L.; Anjum, D.H.; Zhu, H.; Saih, Y.; Laveille, P.V.; D’Souza, L.; Basset, J. Synergetic Effects Leading to Coke-Resistant NiCo Bimetallic Catalysts for Dry Reforming of Methane. ChemCatChem 2015, 7, 427–433. [Google Scholar] [CrossRef]
- Bengaard, H.; Nørskov, J.; Sehested, J.; Clausen, B.; Nielsen, L.; Molenbroek, A.; Rostrup-Nielsen, J. Steam reforming and graphite formation on Ni catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Ge, X.; Deng, S.; Chen, B.; Shen, J. A review of different catalytic systems for dry reforming of methane: Conventional catalysis-alone and plasma-catalytic system. J. CO2 Util. 2021, 46, 101462. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Jones, G.; Jakobsen, J.G.; Shim, S.S.; Kleis, J.; Andersson, M.P.; Rossmeisl, J.; Abildpedersen, F.; Bligaard, T.; Helveg, S.; Hinnemann, B.; et al. First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J. Catal. 2008, 259, 147–160. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Akpan, E.; Sun, Y.; Kumar, P.; Ibrahim, H.; Aboudheir, A.; Idem, R. Kinetics, experimental and reactor modeling studies of the carbon dioxide reforming of methane (CDRM) over a new Ni/CeO2-ZrO2 catalyst in a packed bed tubular reactor. Chem. Eng. Sci. 2007, 62, 4012–4024. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Bobrova, L.N.; Bobin, A.S.; Mezentseva, N.V.; Sadykov, V.A.; Thybaut, J.W.; Marin, G.B. Kinetic assessment of dry reforming of methane on Pt+Ni containing composite of fluorite-like structure. Appl. Catal. B Environ. 2016, 182, 513–524. [Google Scholar] [CrossRef]
- Gavrielatos, I.; Drakopoulos, V.; Neophytides, S.G. Carbon tolerant Ni–Au SOFC electrodes operating under internal steam reforming conditions. J. Catal. 2008, 259, 75–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).