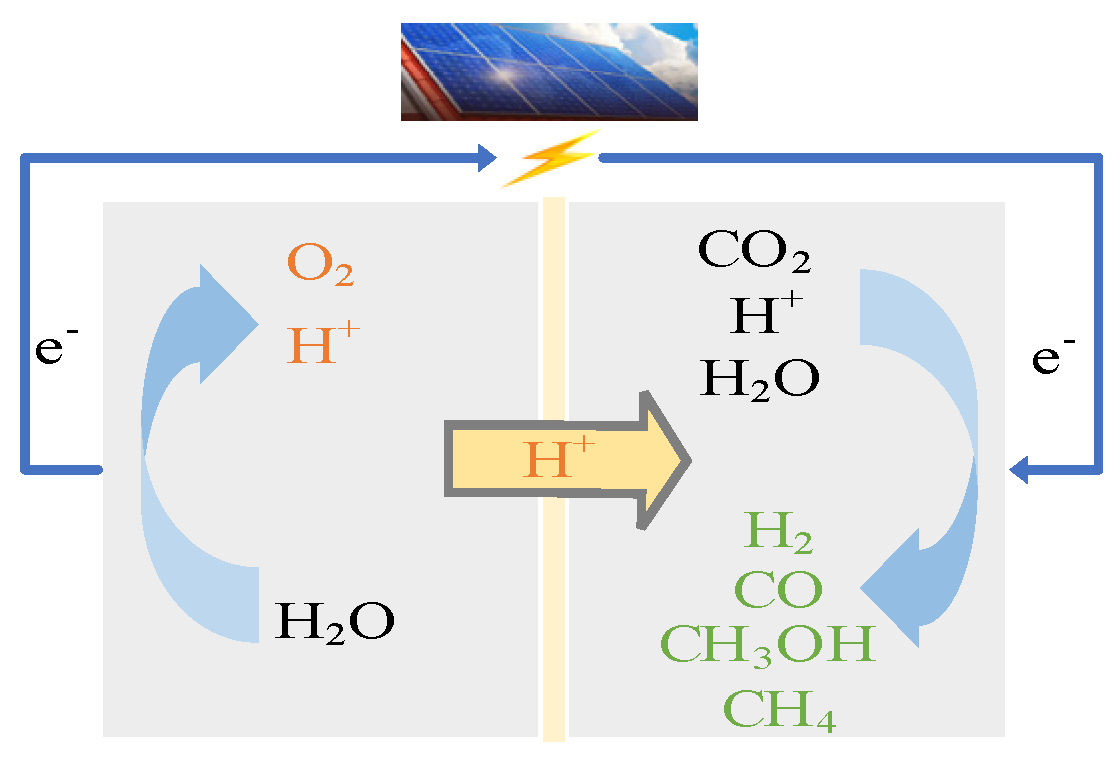
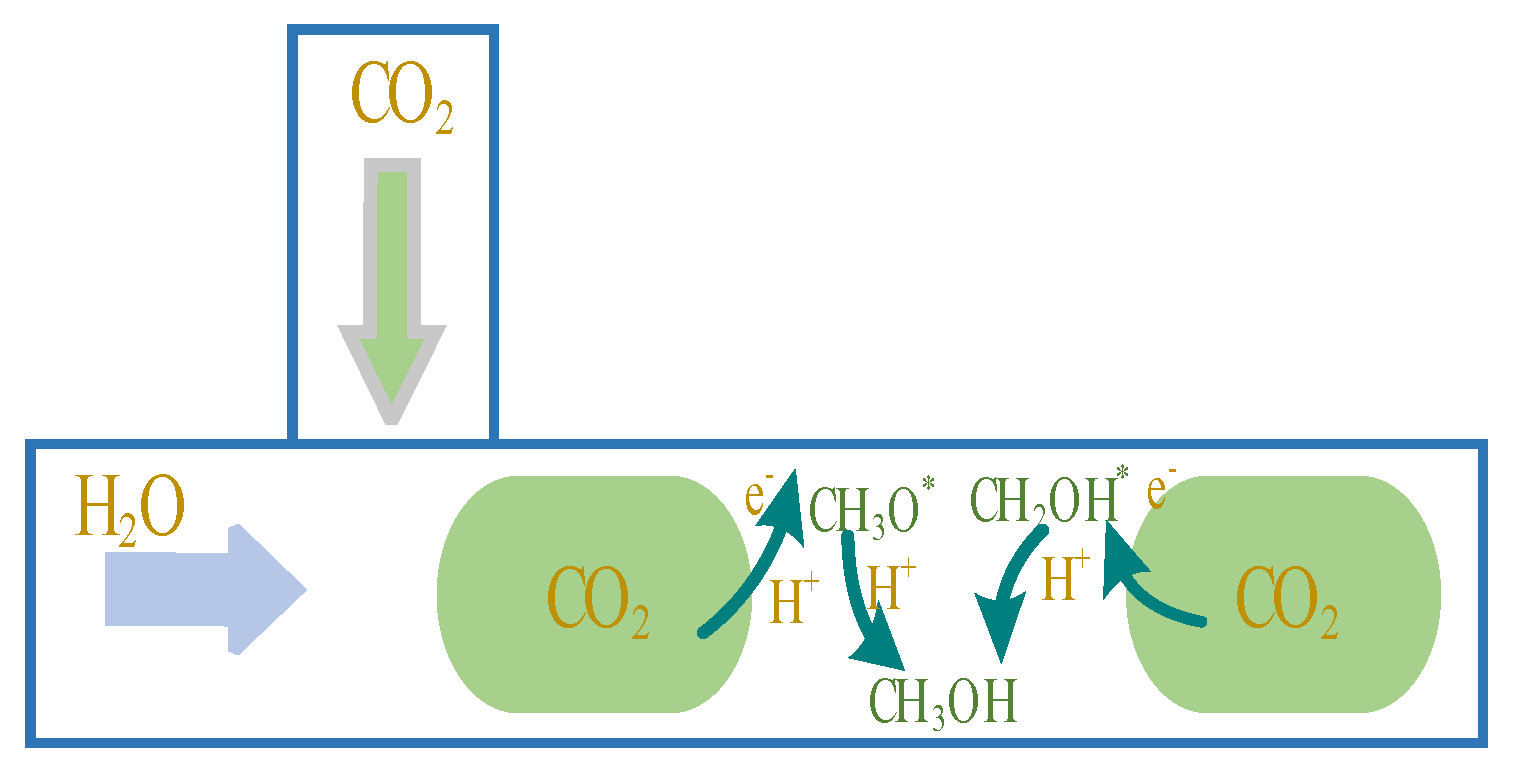
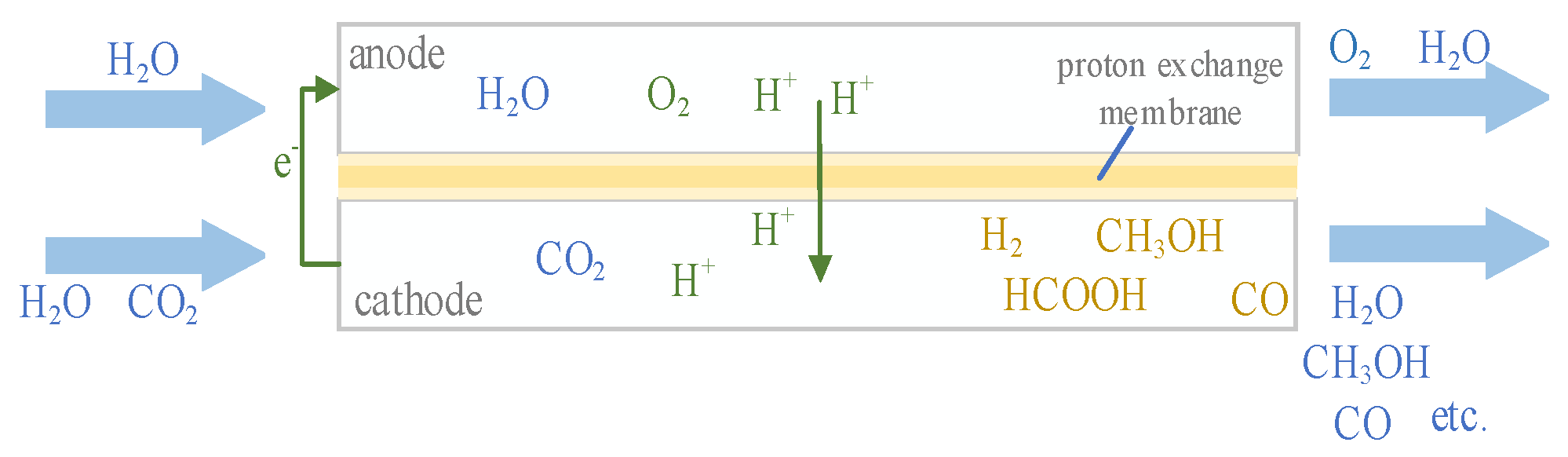
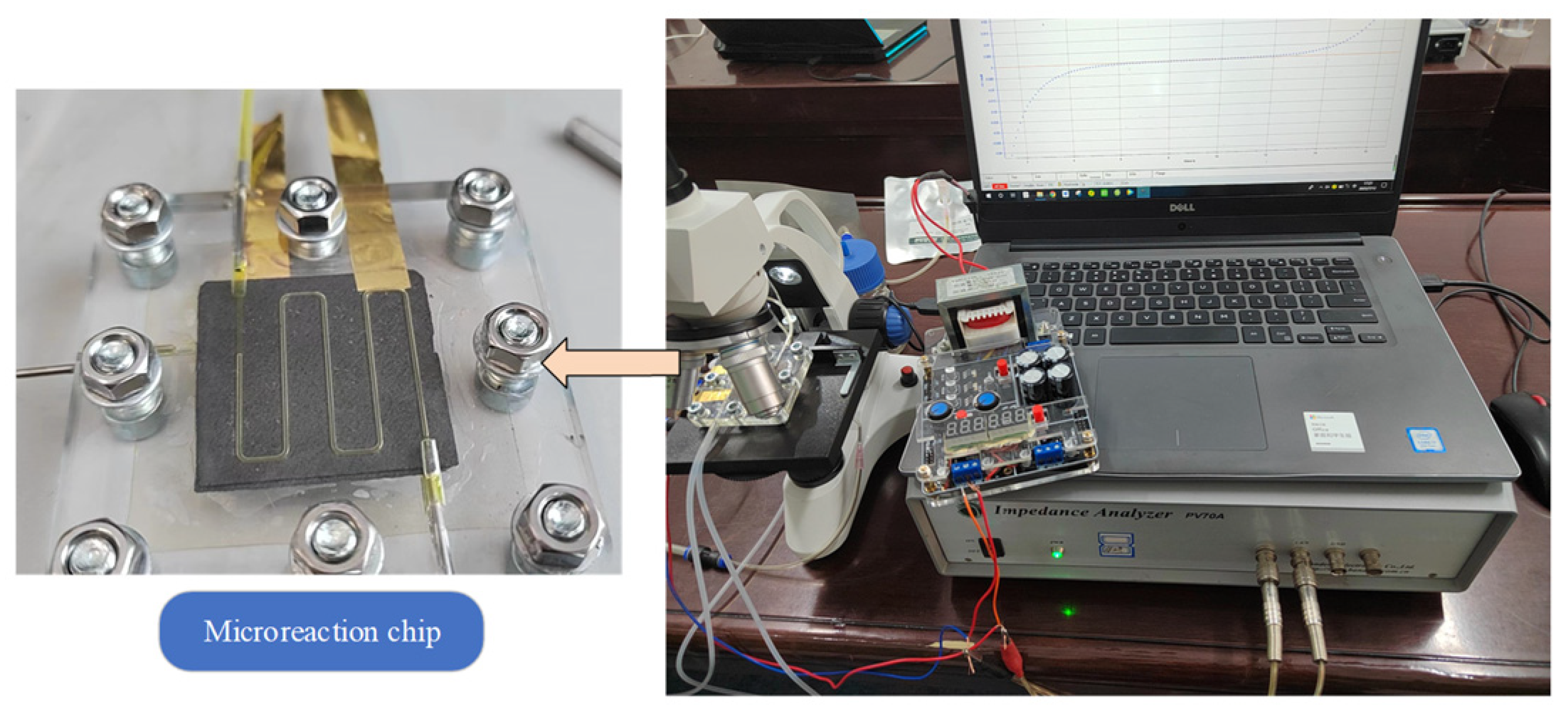
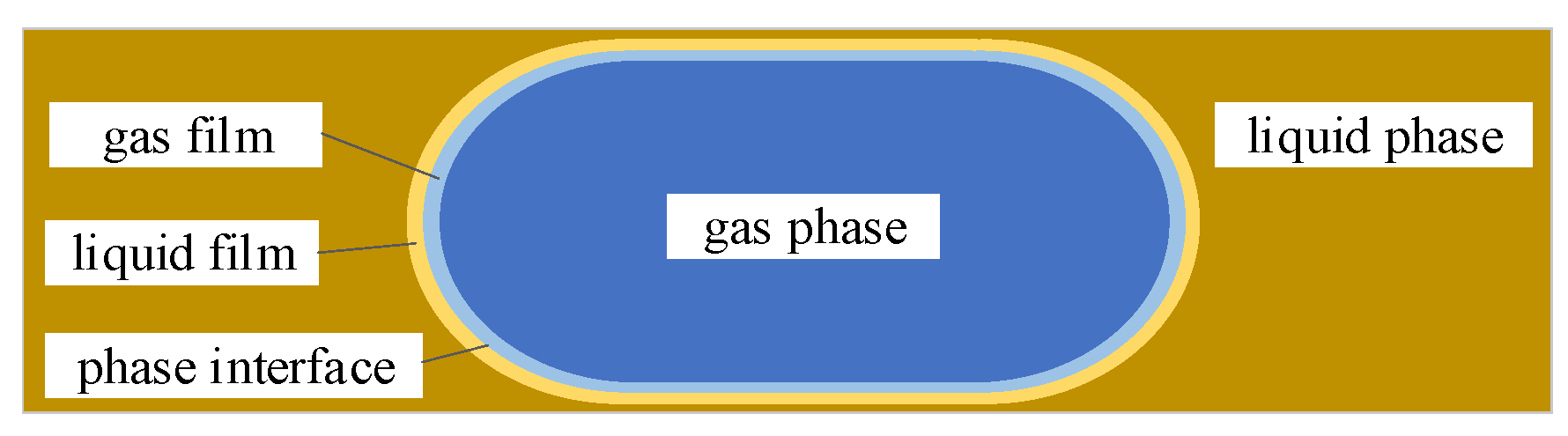
1. Introduction
The sustainable development of space exploration requires sufficient energy and material. Due to the limitation of the weight that aircraft can carry, further development of deep space exploration is greatly restricted. In situ resource utilization technology (ISRU) proposed in recent years to use raw materials from outer space for material conversion and energy extraction, in order to achieve energy and material supply [
1,
2]. Taking Mars exploration as an example, there is abundant CO
2 on Mars, and using CO
2 as raw material for fuel and oxygen conversion can provide sufficient material for Mars development [
3,
4].
In the 1970s, the in situ production of propellants on Mars was put forward. Ash et al. proposed the use of raw materials on Mars for the production of vehicle ascent propellants, and conducted a study on in situ propellant production (ISPP) [
2]. With the progress of research, the utilization of resources on Mars has been more widely used, and ISPP has been gradually replaced by ISRU. Since 1990s, ISRU has gained rapid development. The most commonly used ISRU technology for CO
2 conversion and oxygen production is the Sabatier conversion method [
5,
6,
7]. The reaction principle of the Sabatier reduction method is as follows:
Many researchers have developed many transformation systems based on the Sabatier method [
8,
9]. In 2004, NASA proposed a manned Mars exploration system based on the Sabatier method to convert H
2 and CO
2 into CH
4 and H
2O [
10]. The device includes two reaction modules of CO
2 reduction and H
2O electrolysis, and a series of experiments have been carried out on the space station [
11,
12]. In 2007, researchers designed a reactor based on the Sabatier principle, which can achieve 70% to 80% CO
2 conversion at 400 °C and produce 0.0125 kg/h CH
4 [
13]. In 2013, Pioneer Astronautics in the United States developed the Integrated Mars In-situ Propellant Production System (IMISPPS) [
7], which combines the Sabatier reaction and the RWGS reaction. The overall reaction is:
The reactor simplifies the system, reduces the power consumption of the reaction, and improves the quality of the apparatus.
In the last ten years, Sabatier transformation has been developed rapidly, and some experiments have been carried out to verify it. Sabatier reaction technology is mature and has a high conversion rate, but the high temperature and pressure limit the efficiency of energy conversion; it is also not conducive to accurate control of the product.
In 2021, Nanjing University and the Chinese Academy of Space Technology proposed the use of artificial photosynthesis technology for ISRU [
14], which can reduce CO
2 and generate O
2 at normal temperature and pressure. The characteristics of less energy consumption and easy control of reaction is very important for space technology. There are three main ways to reduce CO
2 by artificial photosynthesis: photocatalysis, electrocatalysis, and photoelectrocatalysis [
15,
16,
17]. Photocatalysis has the characteristic of high energy utilization, but the light source is unstable in an outer space environment and the reaction is not easy to continue. Electrocatalysis is the use of electrical energy generated by solar energy for reactions, which is stable and sustainable. Moreover, due to the simple and compact structure of the reaction device, the modular production can be realized. However, if we only rely on electricity to carry out chemical reactions, we will need to carry a large amount of energy, resulting in an increase in costs. Photoelectrocatalysis is a photoelectrochemical reaction carried out by a semiconductor photoelectrode material changing the surface state of an electrode [
18]. In photoelectrocatalysis, electrical energy can be obtained by solar energy conversion. Photoelectrocatalysis has strong sustainability and a high energy utilization rate. Therefore, photoelectrocatalysis was chosen for the reduction of CO
2.
The chemical reaction in the microreactor has the following advantages: the reaction is safe, easy to control, and has high mass transfer efficiency [
19,
20]. At the same time, due to the influence of surface tension in the channel being far greater than that of gravity, gravity can be ignored when chemical reactions are carried out in outer space [
21]. Therefore, artificial photosynthesis in microchannels is an important way to reduce CO
2 in situ.
Researchers have conducted numerous studies on the reduction of CO
2 in microchannels. Rongwei Guo [
22] studied CO
2 absorption by AMP and EG; a mass transfer prediction model was proposed, and the results showed that the microchannel had obvious advantages in the mass transfer. Wen-Ling Li [
23] used a numerical simulation method to study the absorption of CO
2 by MEA, and analyzed the flow and mass transfer law. Yaran Yin [
24] studied the CO
2 absorbed by MEA/[Bmim] [BF4], the variation of bubbles was analyzed, and the prediction formula of mass transfer coefficient considering the Re number and Damköhler number was proposed. Daofan Ma [
25] studied CO
2 absorption by MEA and DEEA through visualization. It was found that the mass transfer coefficient of the microreactor was higher than that of the conventional reactor. Mengmeng Huang [
26] studied the CO
2 absorption by silica nanostream; the mass transfer coefficient, CO
2 absorption, and pressure drop were analyzed. Physical mass transfer, chemical mass transfer, and CO
2 absorption have been studied extensively by researchers [
27,
28,
29]. However, there are few studies on the photoelectrocatalysis reduction of CO
2 in microchannels.
In this study, the reduction of CO2 under electrocatalysis in microchannels was studied, including the bubble and liquid slug changes, pressure, porosity, and mass transfer coefficient. We also proposed a prediction model for mass transfer coefficient in electrochemical reactions.
3. Mass Transfer of Physical Absorption in Microreactor
3.1. Two-Phase Flow Mixing
CO2 and electrolytes are introduced into the cathode. The solubility of CO2 in the electrolyte is low, and CO2 exists in the micro-channel in the form of bubbles. Taylor flow formed by two-phase flow mixing is often studied as the main flow pattern because of its stable structure and obvious mass transfer. Parameters such as the frequency of bubble generation, the bubble velocity, and the relevant parameters of the Taylor cell are analyzed.
As shown in
Figure 7, when the flow rate of CO
2 increases, the concentration of the gas phase in the microchannel increases, thus increasing the proportion of the gas phase in the microchannel; therefore, the bubble volume becomes larger. When the liquid flow rate is low, the proportion of the gas phase in the two-phase flow increases rapidly with the increase of gas flow rate, increasing the bubble volume greatly. When the liquid phase flow rate is high, the proportion of the liquid phase in the microchannel is high, and when the gas phase flow rate is increased, the proportion of the gas phase in the microchannel increases slightly, increasing the bubble volume slightly. When the flow rate of the gas phase is constant, the proportion of the gas phase decreases with the increase of the flow rate of the liquid phase, decreasing the bubble volume. When the flow rate of the liquid phase is low, increasing the flow rate of the liquid phase can increase the squeezing effect of bubbles, and the increase of solvent can make more gas phase enter the liquid phase, so the length of bubbles decreases significantly. When the liquid flow rate is higher, the two-phase flow velocity is gradually accelerated, and the gas-liquid mass exchange reaches saturation in unit time. At the same time, the bubble pressure increases, and the bubble volume change caused by the pressure decreases, decreasing the bubble length slightly.
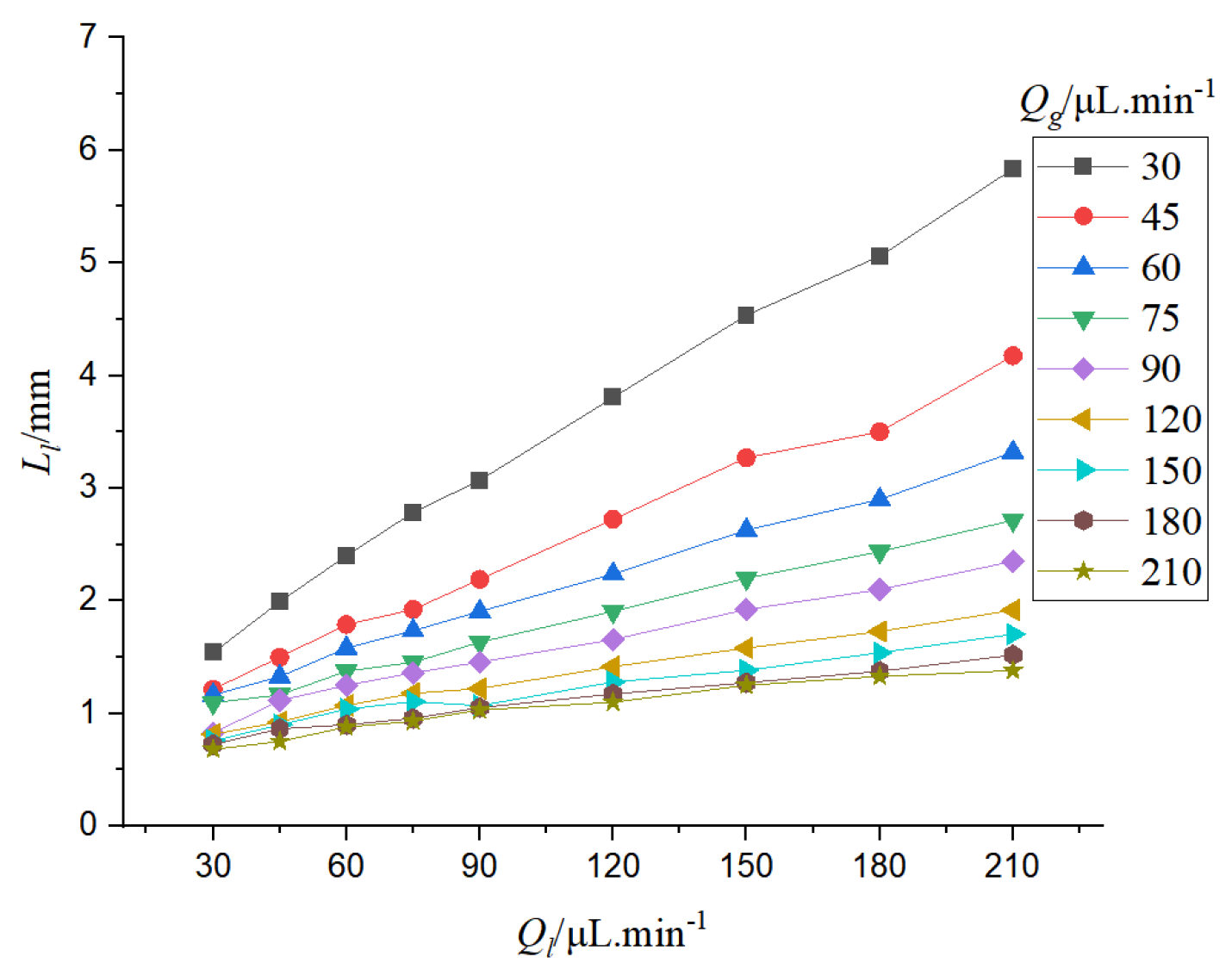
As shown in
Figure 8, when the liquid flow rate is low, increasing the liquid flow rate can increase the liquid content in the microchannel, enhance the aggregation of the liquid, and lengthen the liquid slug. If the gas flow rate is low, the liquid phase is dominant in the microchannel, and the shear effect of the bubble on the liquid phase is weakened. With the increase of the liquid flow rate, the length of the liquid slug increases rapidly. When the gas flow rate is higher, the shear effect of the bubble is stronger, so the liquid slug length increases relatively slowly with the increase of the liquid flow rate.
As shown in
Figure 9, the Taylor cell is composed of bubbles and liquid slug. When the difference between the liquid velocity and the gas velocity is too large, the length of liquid slug or bubbles increases sharply, which makes the length of the Taylor cell increase. When the gas velocity and the liquid velocity are close to each other, the lengths of bubbles and liquid slug are not too large, and the length of the Taylor cell is short. The shorter the length of the Taylor unit, the more Taylor units exist in the microchannel at the same time, which is beneficial to the chemical reaction.
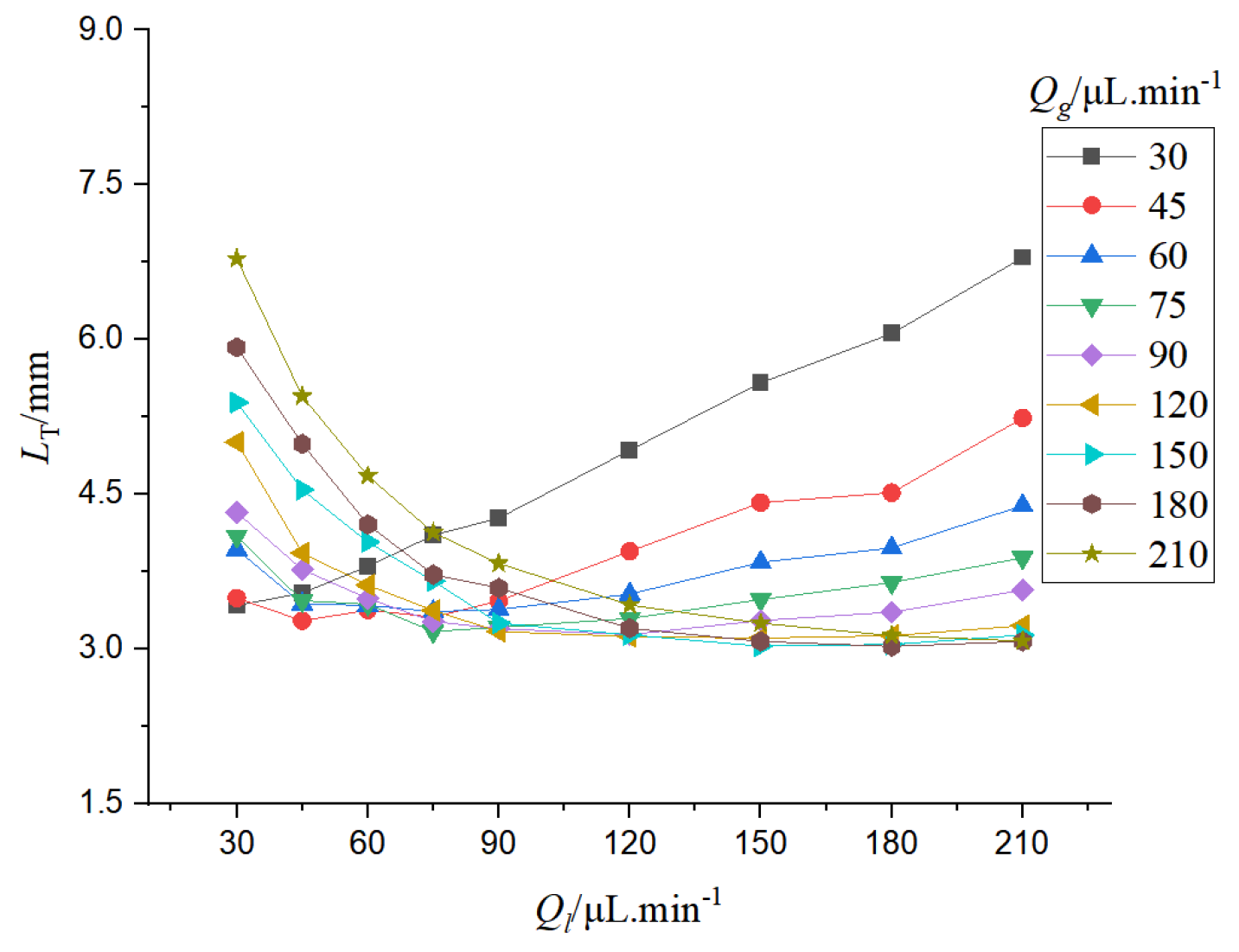
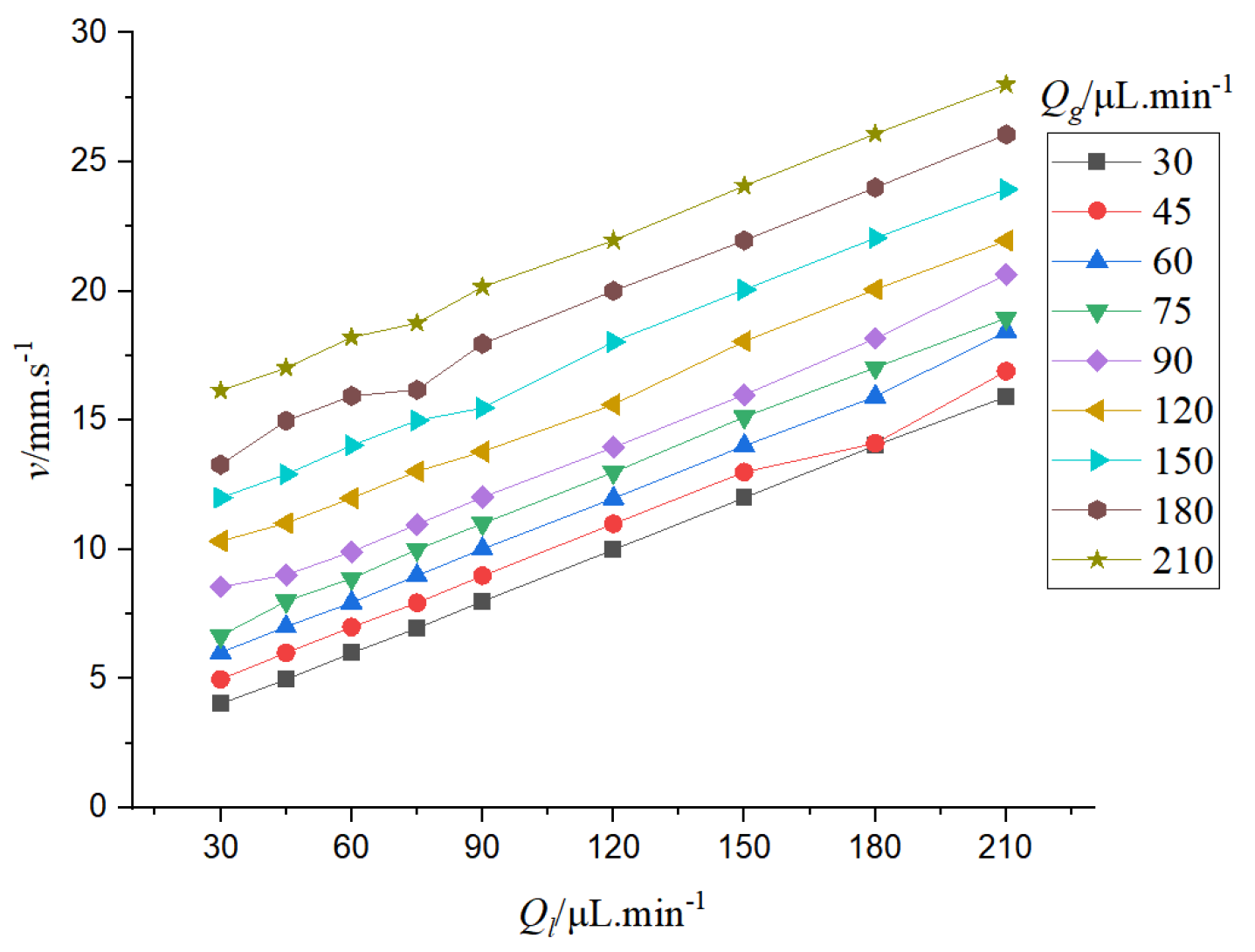
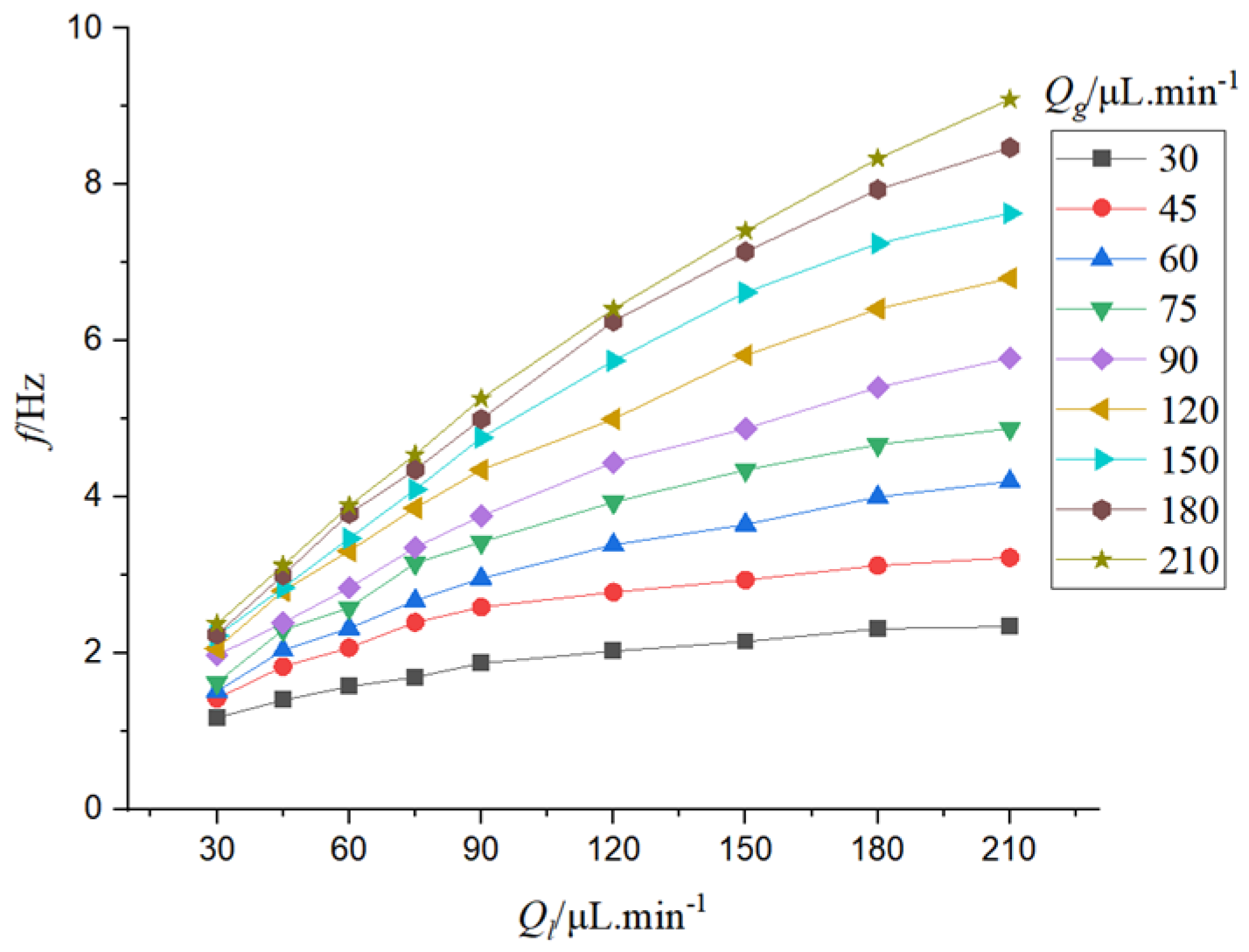
It can be seen from
Figure 10 that the bubble velocity increases as the gas-liquid phase flow rate increases. When the gas-liquid flow rate increases, the bubble velocity is accelerated, and the gas-liquid flow rate varies linearly with the bubble velocity.
It can be seen from
Figure 11, When the flow rate of the liquid phase is constant, the frequency of bubble formation increases as the flow rate of the gas phase increases. When the gas flow rate is low, the increase of the gas flow rate will lead to a larger increase in bubble frequency. When the gas flow rate is high, the bubble frequency increases slightly with the increase of gas flow rate. When the gas flow rate is constant, the bubble frequency increases with the increase of the liquid flow rate. When the liquid flow rate is low, the bubble frequency increases greatly with the increase of liquid flow rate. When the flow rate of the gas-liquid phase increases, the speed of bubbles is accelerated, and the shear force at the two-phase interface is strengthened, which makes the bubble fall off more easily at the two-phase mixing interface, and the frequency of bubble formation increases.
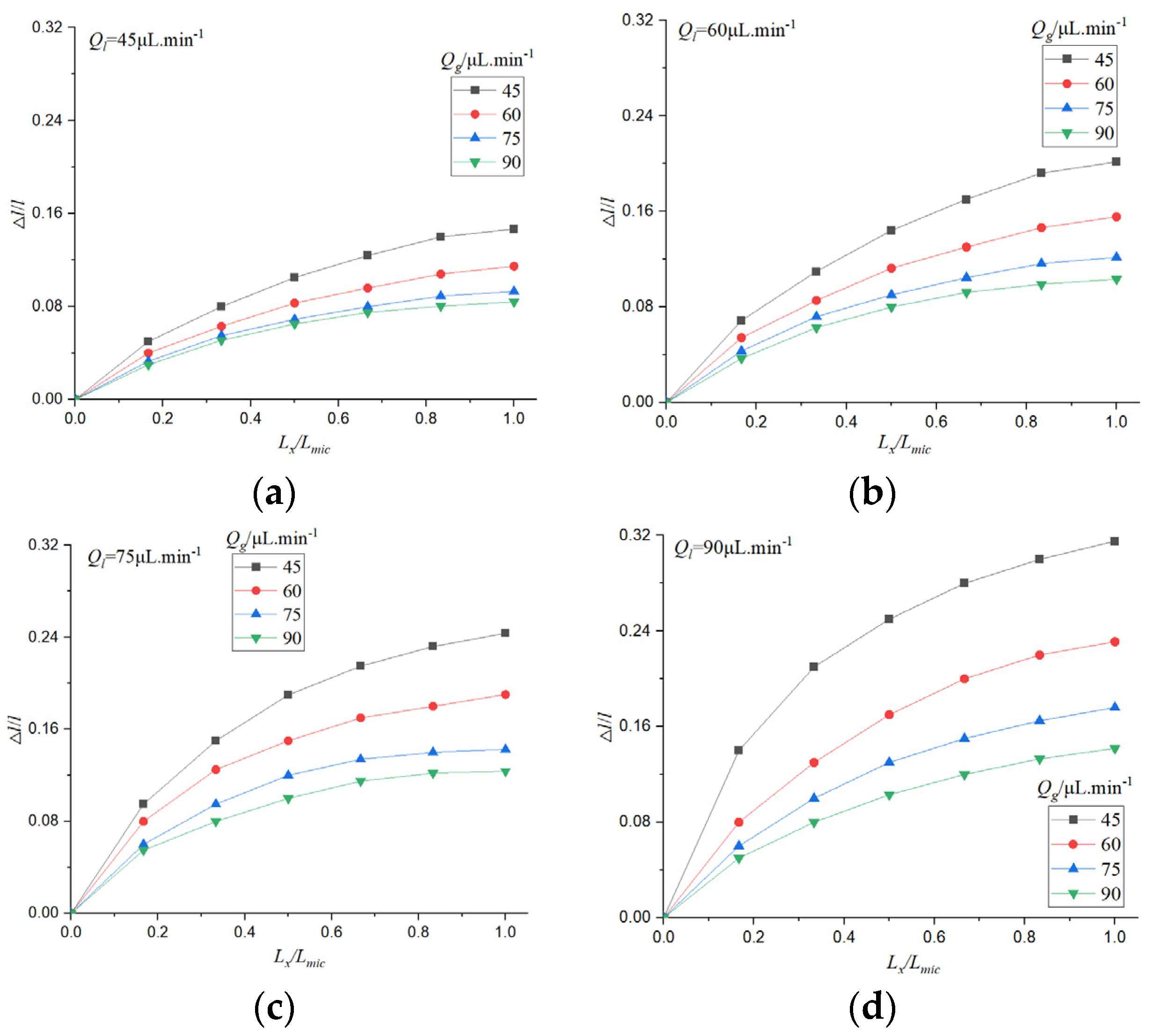
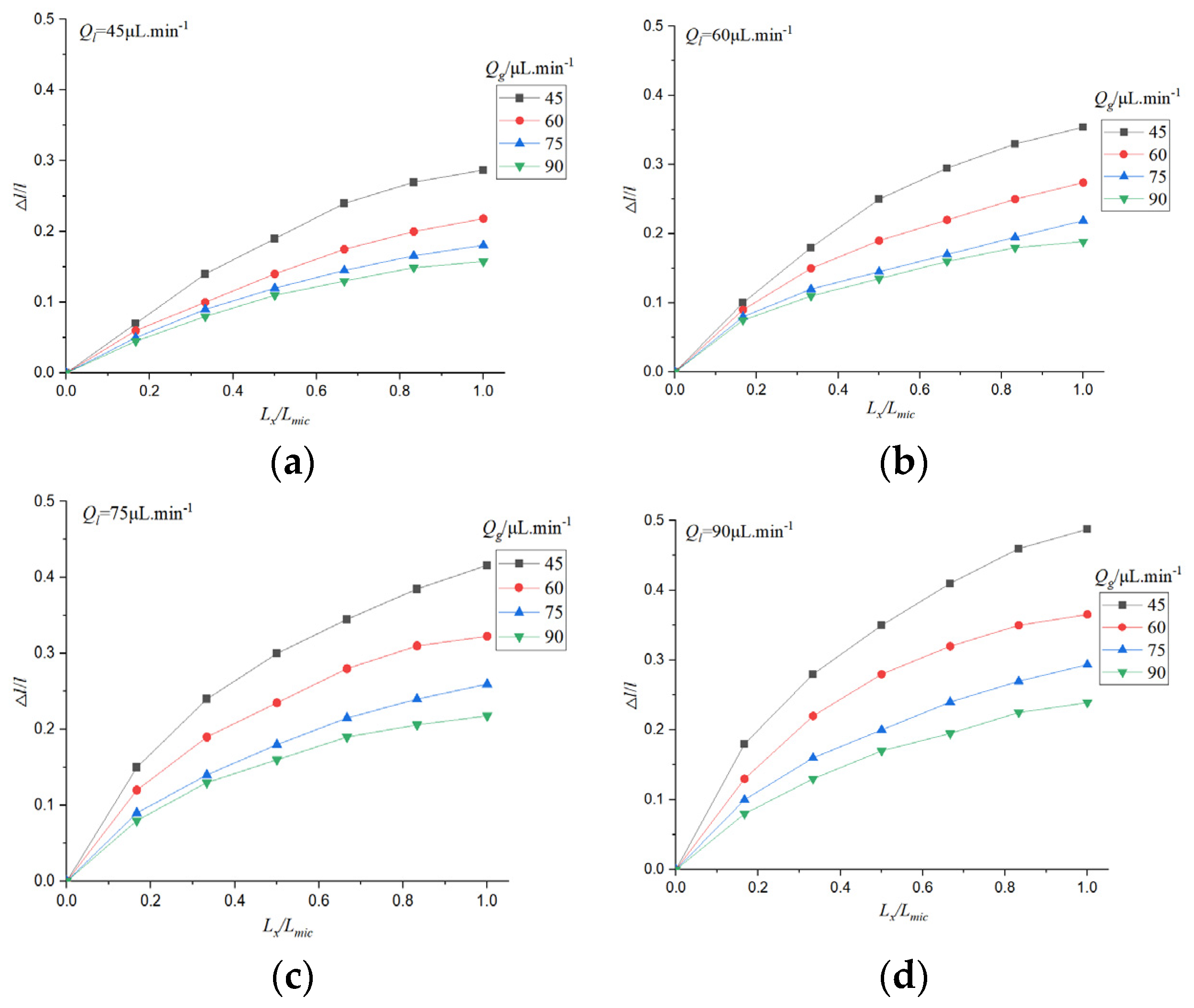
Due to the existence of dissolution and diffusion, the bubble length is continuously shortening. The evolution of bubble length in the process of CO2 absorption by electrolyte was studied.
The initial length of the bubble is l0, and the difference between the instantaneous length and the initial length is △l. The relative loss length of the bubble △l/ l0 can reflect the rate of gas-liquid circulation and mass transfer, and it is affected by temperature, microchannel structure, electrolyte type, and concentration. The length of the microchannel is Lmic, the position of the bubble is Lx, and the influence curve of gas-liquid flow rate on the relative loss length of bubbles under physical absorption is drawn.
△
l/
l0 can reflect the change of bubble position with time. It can be seen from
Figure 12 that the relative loss length of bubbles increases with the increase of time, and the bubble length changes significantly when the gas flow rate is low. In the initial stage of bubble formation, the change of bubble is more obvious, the relative loss length decreases gradually with the increase of time, and the outlet of microchannel is basically unchanged.
This is due to the fact that the gas phase in the microchannel is continuously absorbed, and the concentration of CO2 in the liquid phase is low initially, so the absorption rate is fast. The concentration of CO2 in the liquid phase increased gradually, and the absorption rate decreased. When the gas velocity is small, the bubble size is small, and the change of relative absorption length is more obvious. When the gas flow rate is large, the effect of gas flow rate on the relative absorption length is not obvious. The increase of liquid phase flow rate can increase the absorption of gas phase and increase the relative loss length.
3.2. Mass Transfer of CO2 Absorption in Electrolyte
CO2 and electrolytes were introduced into the cathode of the microchip, and the two-phase flow was mixed. The mass transfer coefficient of CO2 under physical absorption was studied.
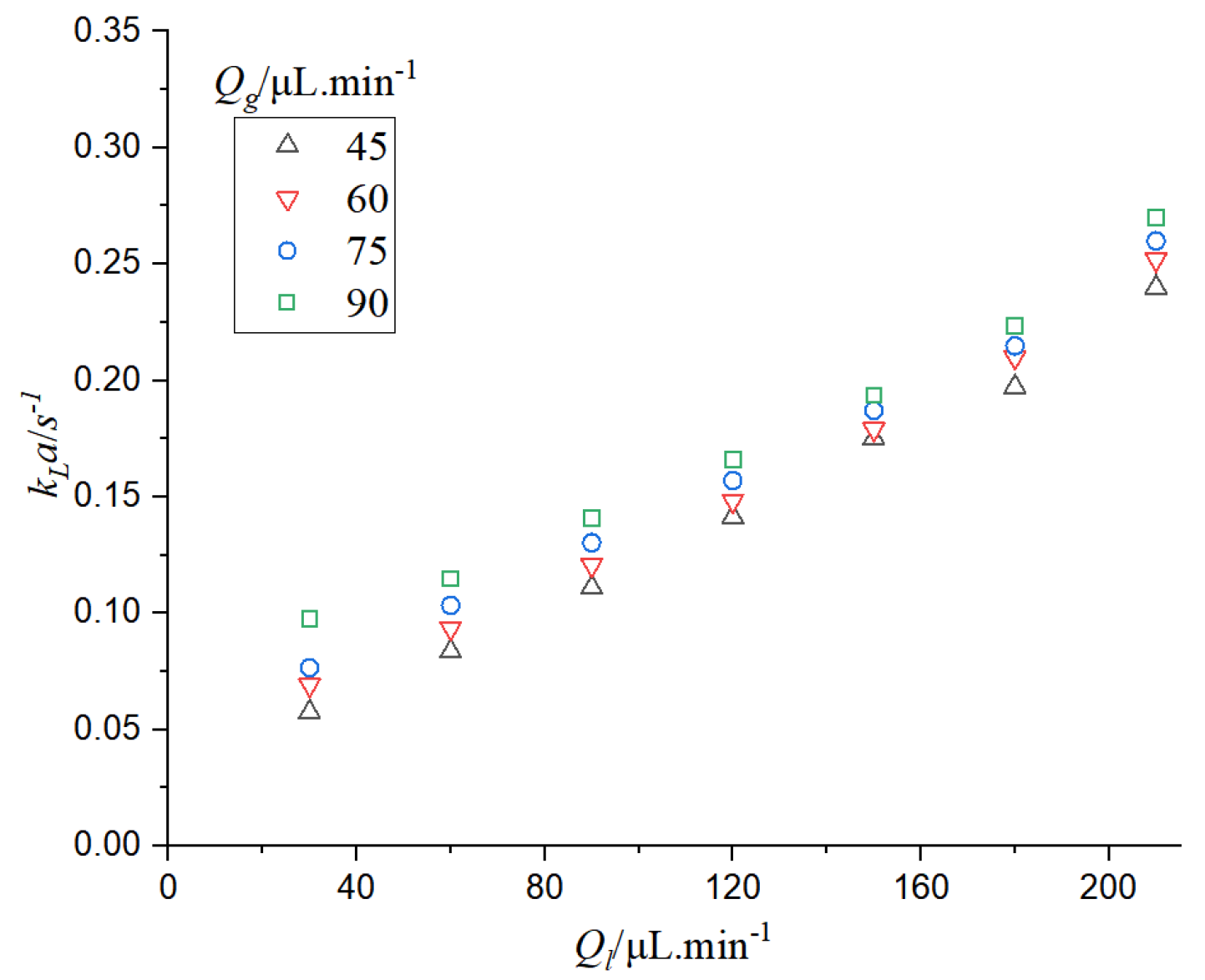
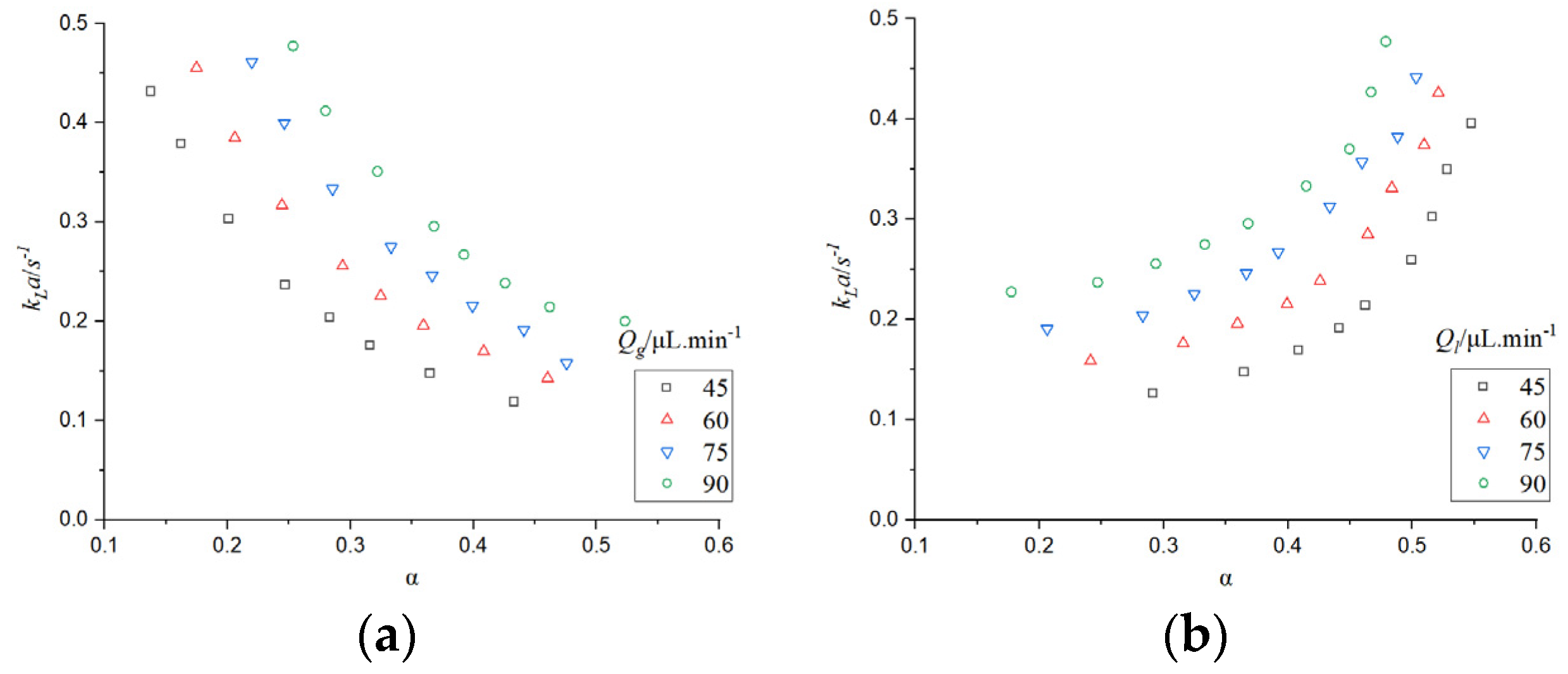
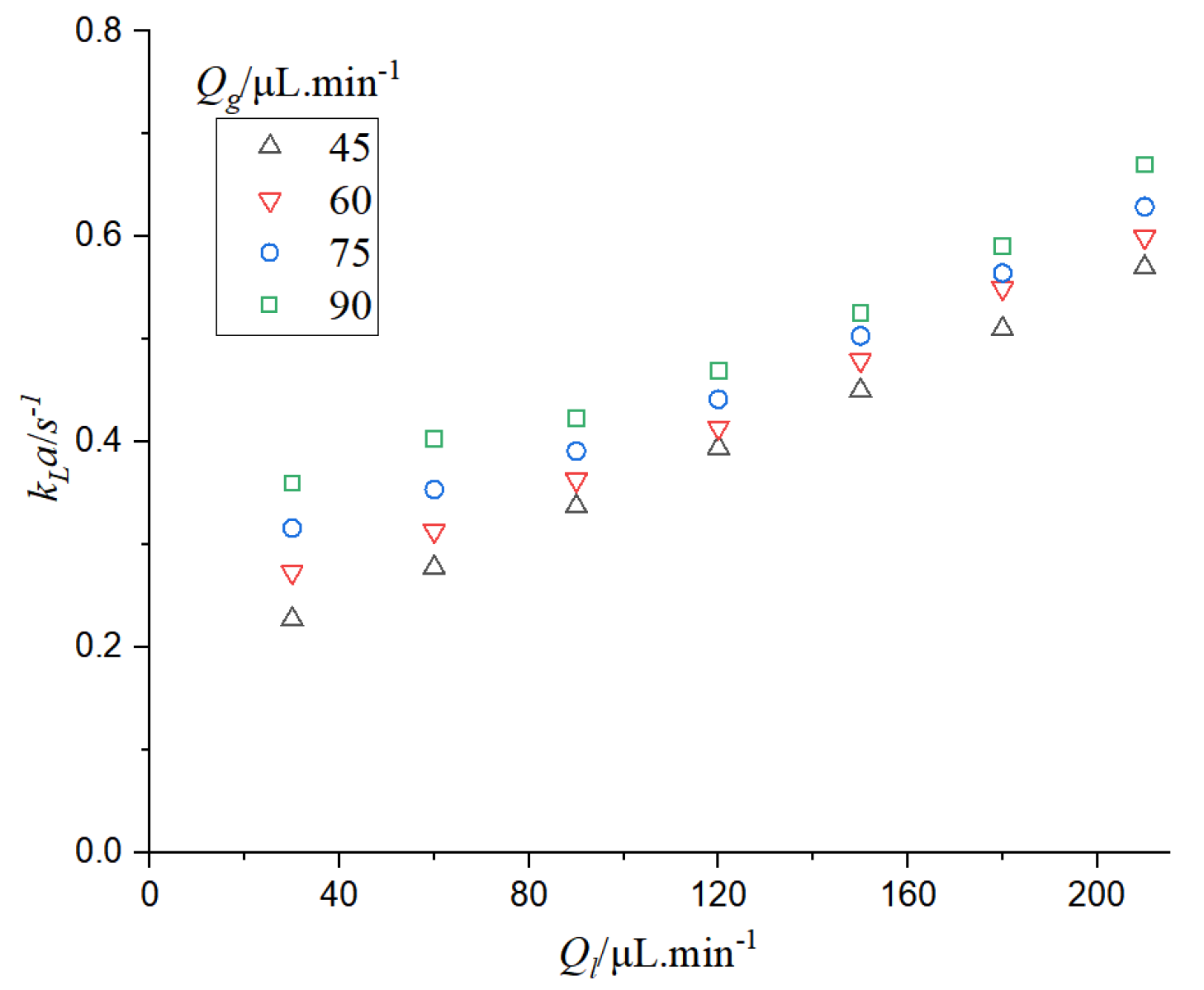
It can be seen from
Figure 13 that when the gas phase flow rate is constant, the mass transfer coefficient increases with the increase of the gas-liquid phase flow rate. When the gas-liquid flow rate increases, the mass circulation intensifies and the mass transfer effect enhances. When the gas flow rate increases, the specific surface area of bubbles increases, while the specific surface area decreases with the increase of liquid flow rate. The residence time of bubbles in the microchannel also decreases with the increase of gas-liquid flow rate. According to the calculation formula of the mass transfer coefficient, the decrease of residence time can increase the mass transfer coefficient, so the increase of
kLa is mainly caused by the increase of the specific surface area of bubbles and the decrease of the residence time of bubbles in the microchannel.
The change in gas-liquid flow rate will lead to the change of bubble and liquid slug length, thus affecting the absorption of CO2. The CO2 mass transfer coefficient under the influence of bubble and liquid slug was studied.
It can be seen from
Figure 14 that when the bubble length is constant and the liquid slug length increases, or when the liquid slug length is constant and the bubble length increases, the mass transfer coefficient increases, which verifies that the increase of gas-liquid phase flow rate can lead to the intensification of mass transfer.
The gas-liquid two-phase flow is introduced into the microchannel to carry out the chemical reaction, and the gas is dissolved into the liquid phase at the initial stage. Taylor flow mass transfer studies on physical absorption have shown [
43] that the pressure of the gas is the main factor in the initial stage of gas dissolution. Fluid transport in microchannels is affected by surface friction, resulting in a pressure drop. Pressure drop is an important parameter of gas-liquid two-phase flow, which affects the chemical reaction rate, gas-liquid flow rate, and the stability of the reaction.
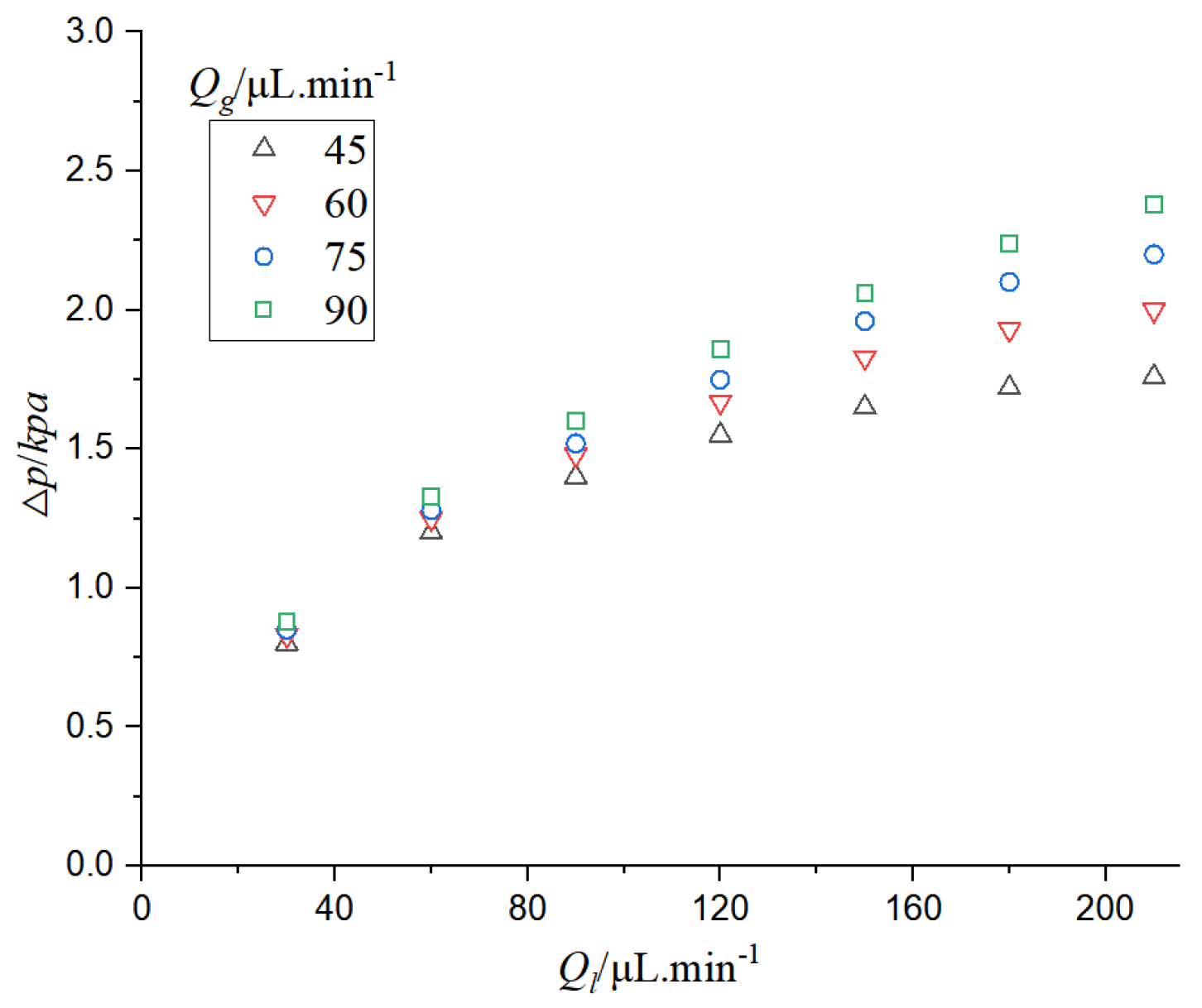
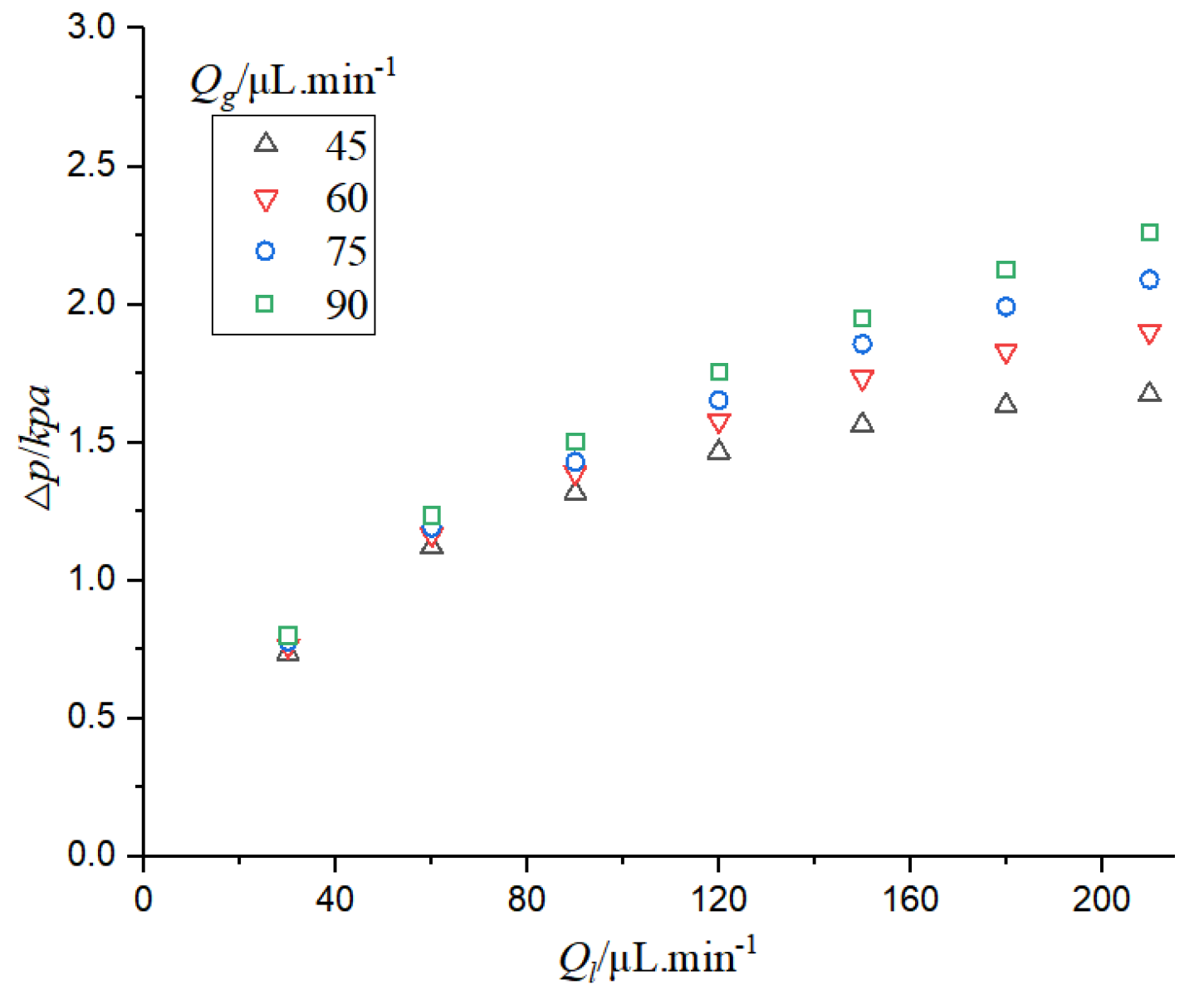
It can be seen from
Figure 15 that when the gas phase flow rate is constant, the pressure drop in the CO
2-H
2O system increases with the increase of the liquid phase flow rate. When the liquid velocity is constant, the pressure drop increases with the increase of gas velocity. When the flow velocity of the gas-liquid two-phase flow increases, the microfluidics need great pumping pressure, and thus its pressure drop increases. With the increase of gas-liquid flow rate, the growth trend of the pressure drop gradually slows down, especially when the gas-liquid flow rate difference is large; the velocity of the phase with larger flow rate continues to increase, and the increase of pressure drop is very small. This is because the increase of liquid phase flow and gas phase flow can lead to the increase of pressure drop, and the increase of pressure drop is also affected by the bubble absorption of the liquid phase. When the difference of gas-liquid flow is large, the change of bubble length is reduced, so the influence of gas phase absorption on pressure drop is reduced.
It can be seen from
Figure 16 that when the pressure drop increases, the mass transfer coefficient increases. The pressure drop of two-phase flow can promote mass transfer. The increase of pressure drop is mainly affected by the increase of liquid flow rate, and the increase of gas-liquid flow rate can accelerate the mass transfer. The increase of pressure can increase the solubility of CO
2 in the electrolyte, thus increasing the concentration of CO
2 on the surface of the catalyst and thereby increasing the rate of chemical reaction.
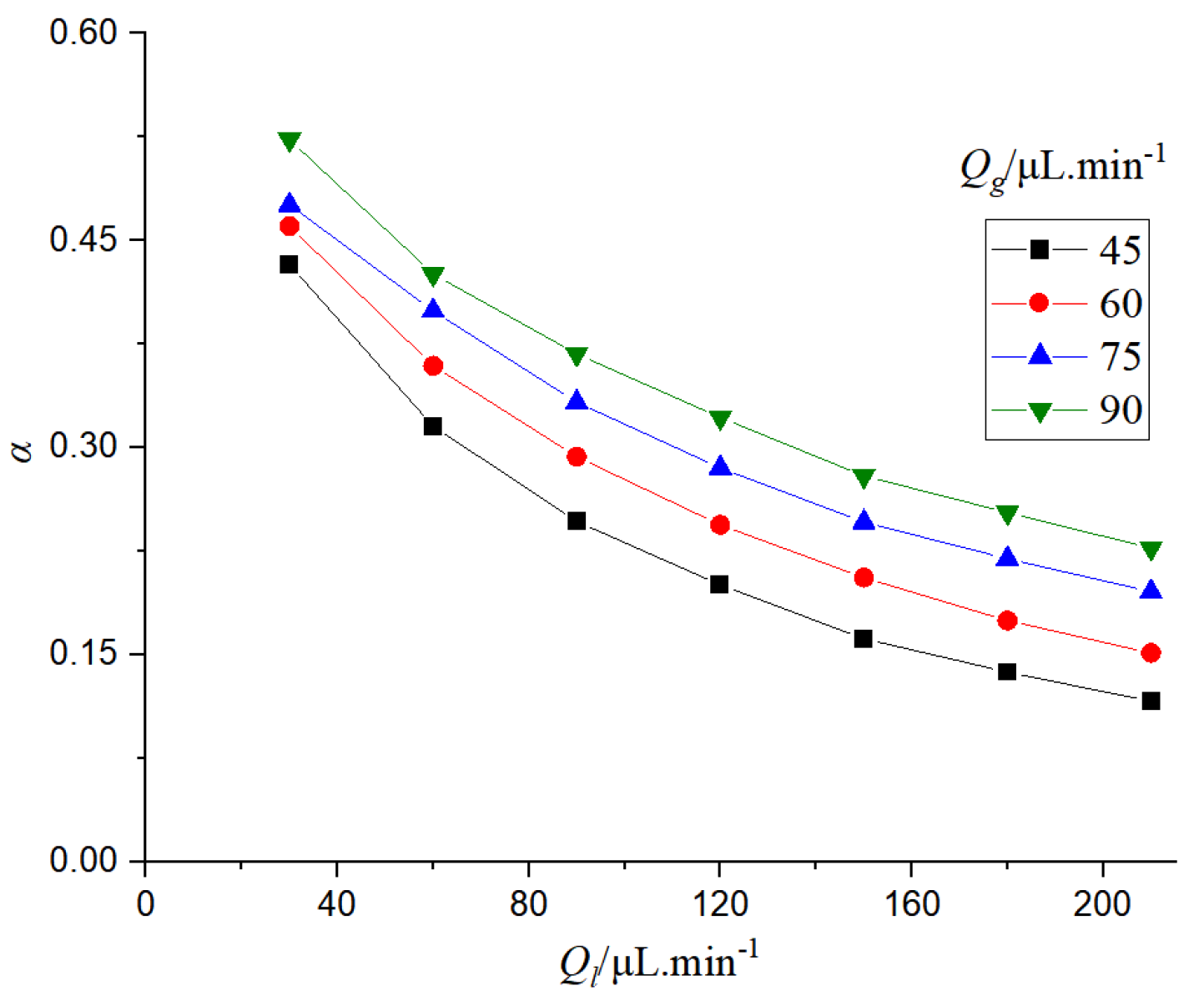
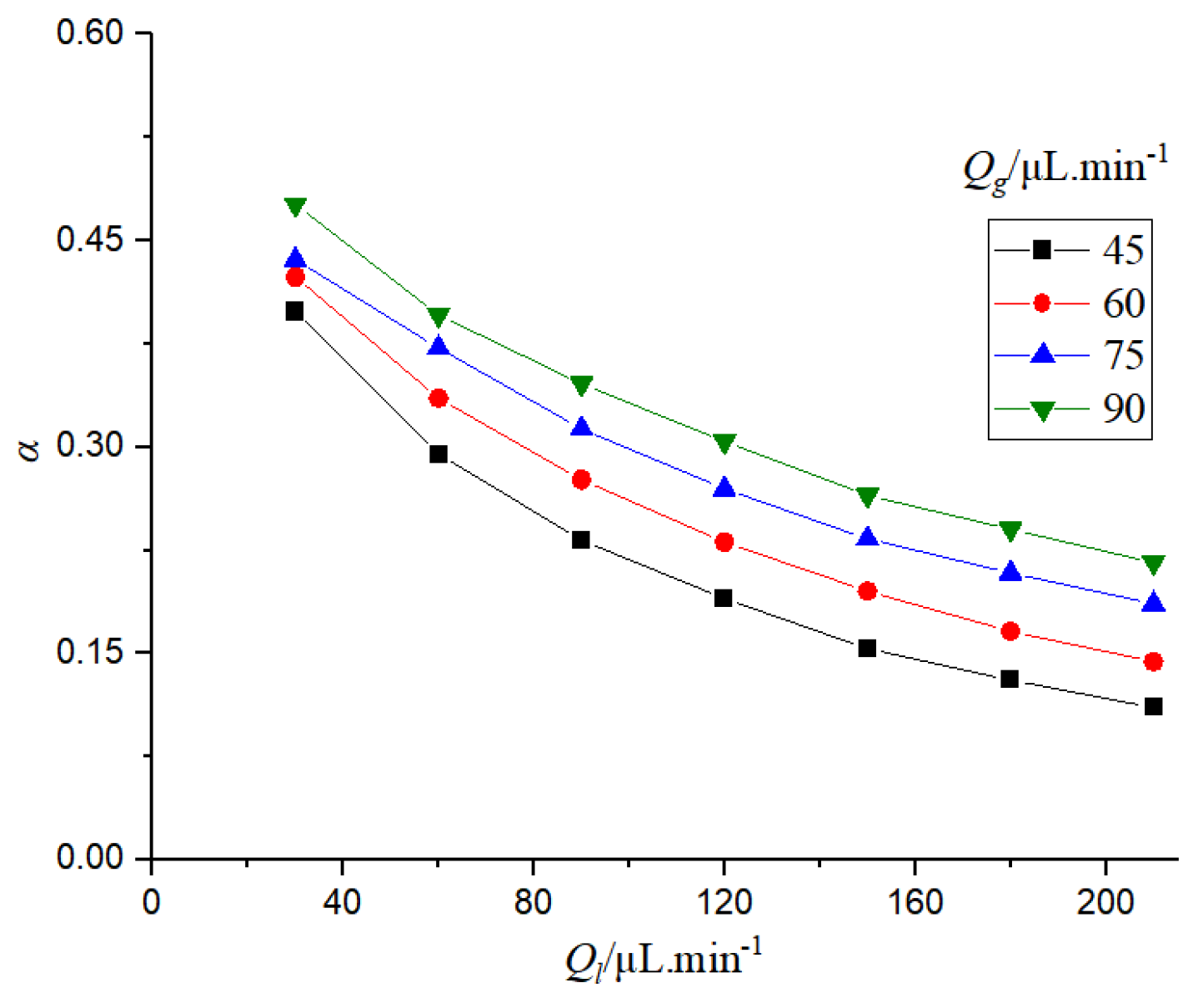
It can be seen from
Figure 17 that the porosity in the microchannel is determined by the distribution of bubbles and liquid slugs. When the gas flow rate is constant, the bubble length becomes shorter and the porosity decreases with the increase of the liquid flow rate. When the liquid flow rate is fixed, the porosity increases with the increase of the gas flow rate.
Porosity can reflect the flow relationship between gas and liquid, thus affecting the absorption and mass transfer performance of CO2. The effect of porosity on the mass transfer coefficient was studied.
It can be seen from
Figure 18 that when the gas phase flow rate is constant, if the porosity increases, the mass transfer efficiency will decrease. This is because the porosity reflects the ratio of gas-liquid flow rate in the microchannel. When the gas flow rate is constant, the increase of porosity is due to the decrease of liquid flow rate, and the decrease of liquid flow rate will reduce the mass transfer efficiency. When the liquid flow rate is constant, the increase of porosity will lead to the increase of the mass transfer coefficient, which is mainly due to the increase of gas flow rate.
3.3. Prediction of Mass Transfer Coefficients for Physical Absorption of CO2
Many scholars have studied the mass transfer of gas-liquid two-phase flow, and proposed a relevant prediction formula of the mass transfer coefficient.
Yue et al. [
44] predicted the mass transfer coefficient of CO
2 absorption by deionized water in a microchannel, and proposed a prediction formula for the correlation between the mass transfer coefficient, Reynolds number, and Schmidt number, which is as follows:
where
ShLadH is the Sherwood number,
ShLadH =
kLdH/
D, and
ScL is the liquid phase Schmidt number,
ScL =
μL/(
ρLD).
Ji et al. [
45] studied the mass transfer process of CO
2 absorption by deionized water, ethanol, and n-propanol in the microchannel, and proposed the mass transfer prediction method. The prediction formula of mass transfer coefficient is as follows:
where
CaL is the liquid phase Capillary number, and
CaL = U
Lμ
L/σ.
Yin et al. [
24,
42] studied the mass transfer of CO
2 absorbed by monoethanolamine and other solutions, and proposed a prediction method of mass transfer coefficient by using the Reynolds number and Damkohler number, and obtained the prediction formula of mass transfer coefficient as follows:
where
Da is the Damkohler number,
where
kov is the overall reaction rate constant.
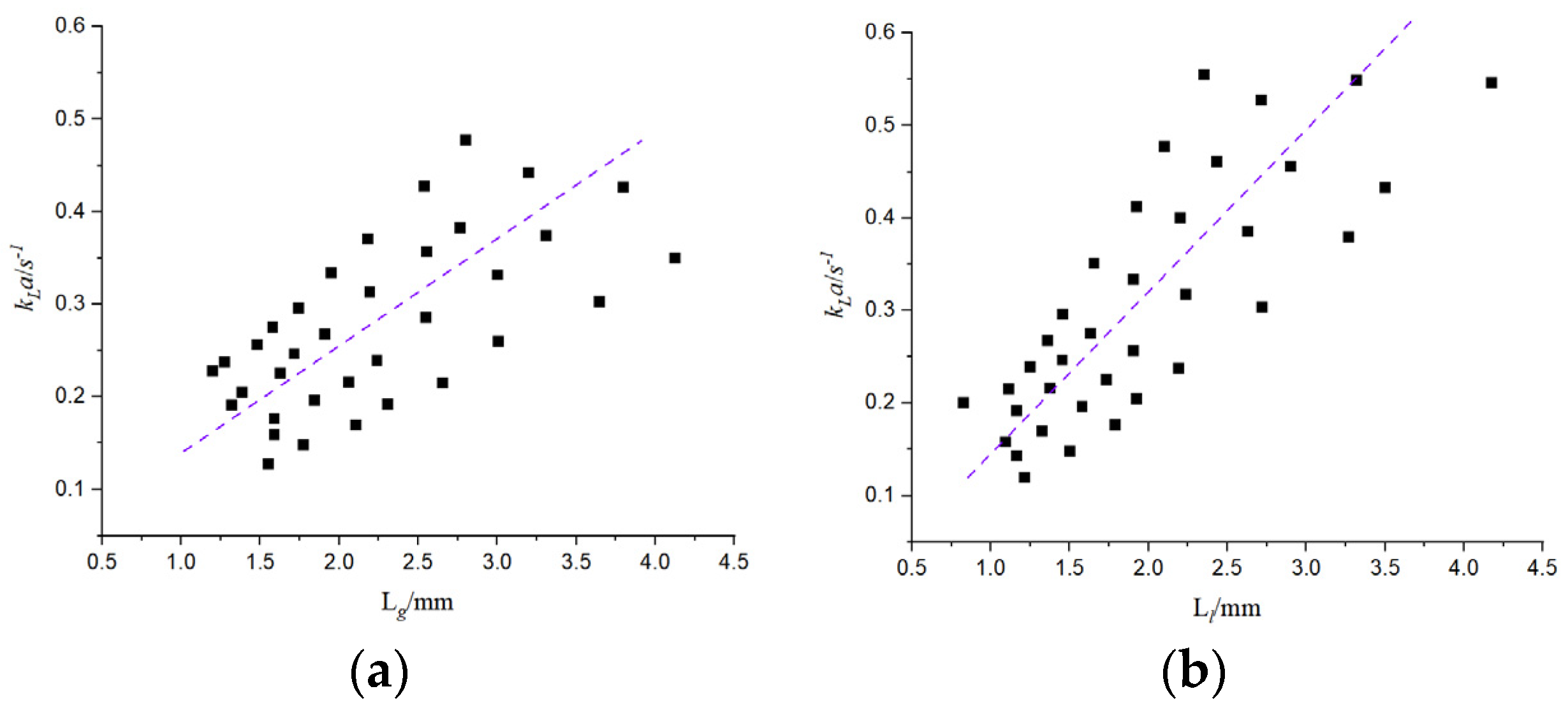
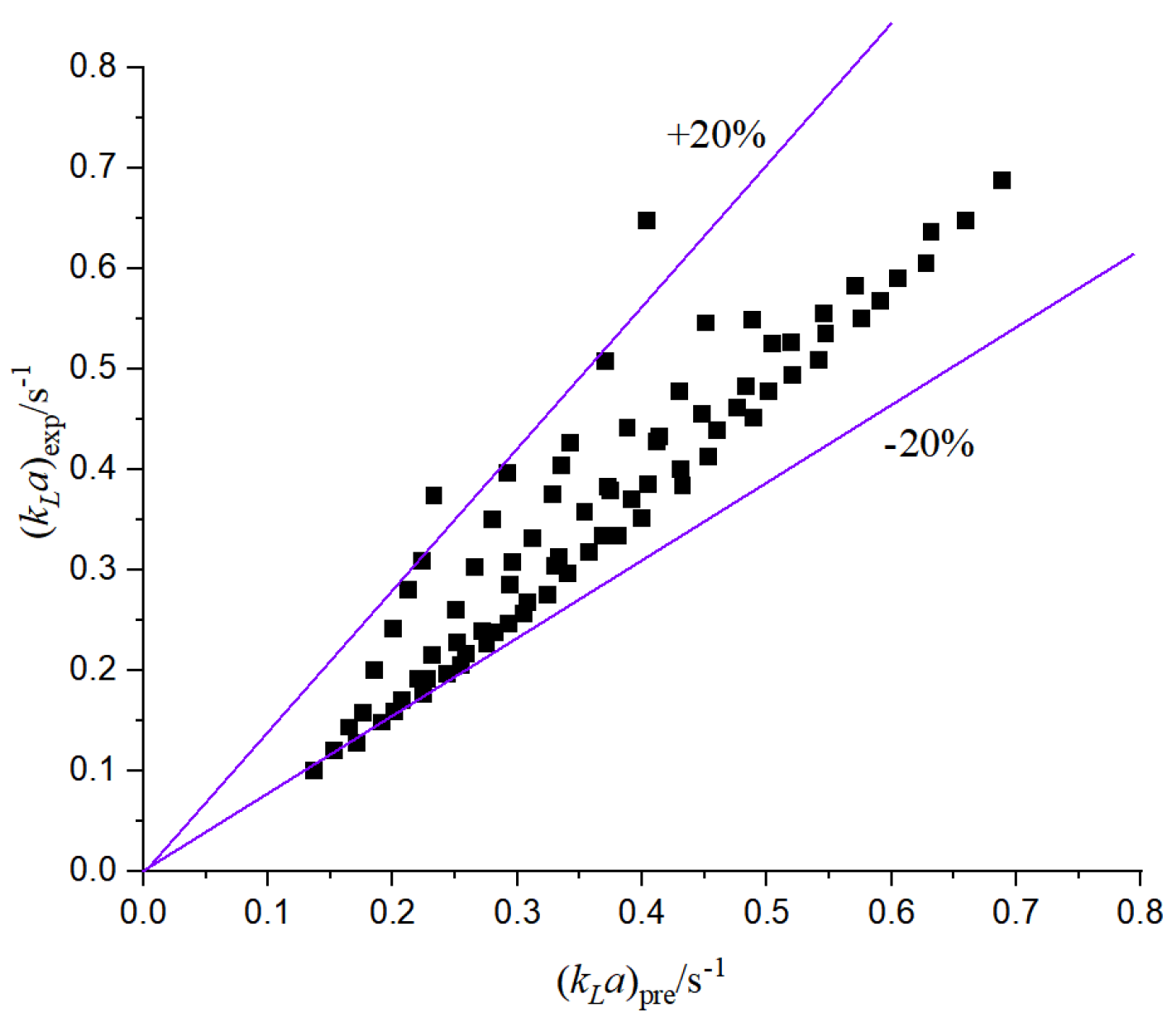
The above different prediction models are compared with this experiment, and there are some errors with the results of this paper. On the one hand, the reason lies in the influence of the size of the microchannel structure and the operating conditions. On the other hand, the micro-reaction channel has a cathode and an anode; the cathode and the anode are separated by a proton exchange membrane, and water molecules exchange substances between the cathode and the anode. Moreover, the gas-liquid two-phase flow rate in other literature is large, and the applicability is poor for the working conditions with a small flow rate. Therefore, based on the microchannel designed in this paper, the relevant mass transfer prediction formula is improved, as shown in
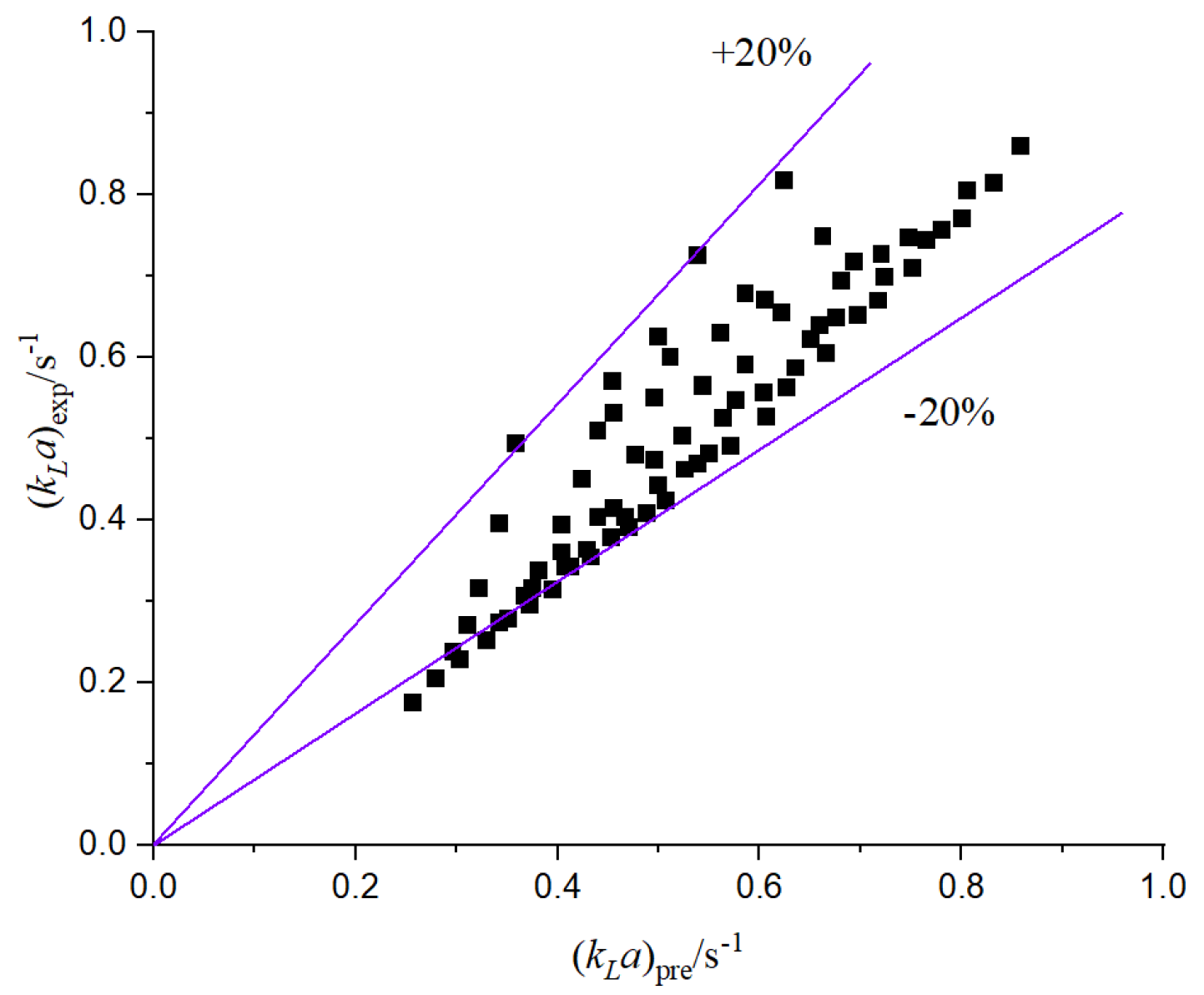
Figure 19, the mass transfer coefficient is fitted, and the following correlation is obtained:
According to the comparison between the experimental mass transfer coefficient and the predicted value, the prediction of the two-phase flow mass transfer coefficient is accurate, the correlation is better, and the relative error is basically within 20%.
4. Mass Transfer of Chemical Absorption in Microreactor
4.1. Mass Transfer in Electrochemical Reaction
The reduction reaction of CO2 is carried out at the cathode in the microreactor, and the reduction reaction is affected by many factors. The analysis of physical absorption shows that the mass transfer in the micro-channel is affected by the hydrodynamic characteristics of Taylor flow. For the CO2 absorption process with the participation of chemical reaction, the chemical reaction and fluid flow interact with each other. On the one hand, Taylor flow affects the mass transfer and product yield of chemical reactions. On the other hand, the chemical reaction affects the characteristics of Taylor bubble change, porosity, and pressure drop.
A NaHCO3 solution with a volume fraction of 5% was introduced into the liquid phase inlet, CO2 was introduced into the gas phase inlet, and the experiment was carried out by changing the flow rate of gas and liquid phases. The flow rate of the liquid phase in the negative and positive stages remain the same. The TiO2-Cu2O electrode was used for catalysis, the catalyst loading was 0. 01 g/cm2, and the applied voltage was applied on the cathode and anode. The length of bubbles and liquid slugs were observed and recorded at intervals during the reaction, and the yield of products was measured after a period of catalytic reaction.
The formation process of Taylor bubbles and liquid slug under chemical reaction was studied, and the changes in bubble length, porosity, pressure drop, and mass transfer coefficient under the chemical reaction were analyzed.
It can be seen from
Figure 20 that the bubble length gradually decreases with the chemical reaction and gas diffusion. At the initial moment, the length of the bubble decreases rapidly, and after a period of time, the change in length gradually decreases; that is, the slope of the curve in the figure decreases. Compared to the relative loss length of bubbles in the physical absorption process, it can be found that the relative loss length of bubbles in the chemical reaction is larger, and the change of bubbles is more obvious.
It can be seen from
Figure 21 that the electrochemical absorption is similar to the physical absorption. In the electrochemical reaction, when the liquid phase flow rate increases, the porosity decreases; when the gas phase flow rate increases, the porosity of the microchannel increases. When the gas flow rate increases, the gas content in the microchannel increases, so the porosity increases. When the liquid flow rate increases, the liquid content in the microchannel increases and the porosity decreases. By comparing the changes of porosity in physical absorption and chemical absorption, it can be found that the porosity in electrochemical absorption is smaller. This is because the rate of chemical reaction increases and the consumption of CO
2 increases, resulting in a decrease in porosity.
It can be seen from
Figure 22 that when the liquid phase flow rate is constant, the pressure drop increases with the increase of the gas phase flow rate. By comparing the pressure drop in the electrochemical reaction with that of physical absorption, it can be found that the pressure drop in electrochemical reaction is low. This is because the porosity of the electrochemical reaction is increased, the length of the bubble is reduced, and the friction resistance to the fluid movement is reduced, so that the pressure drop in the channel is reduced.
Next, the mass transfer coefficient of the electrochemical reduction of CO2 in the microchannel was studied.
It can be seen from
Figure 23 that the mass transfer coefficient increases when the gas-liquid phase flow rate increases. When the gas-liquid flow rate increases, the mass exchange of the two-phase flow in the microchannel is more sufficient, and the mass transfer coefficient increases. Moreover, the increase of gas-liquid phase flow rate also increases the flux of reaction substances, accelerates the rate of the chemical reaction, and is beneficial to mass transfer. By comparing the mass transfer coefficient in physical absorption, it can be found that the mass transfer coefficient in electrochemical absorption is larger than that in physical absorption. Chemical absorption includes the diffusion of substances and the absorption of chemical reactions.
Mass transfer in electrochemical reaction is affected by many factors, such as gas-liquid flow rate, applied voltage, catalyst type and loading, and micro-channel structure. It is difficult to predict the mass transfer coefficient in electrochemical reactions.
The mass transfer coefficient in the electrochemical reaction in this chapter is fitted and predicted. The applied voltage is 4 V, the catalyst is TiO
2-Cu
2O, the catalyst loading is 0.01 g/cm
2, and the fitting formula of the mass transfer coefficient is as follows:
According to the comparison between the experimental mass transfer coefficient and the predicted value in the electrochemical reaction, as shown in
Figure 24, the correlation between them is good, and the relative error is basically within 20%.
4.2. Enhancement Factor of CO2 Absorption
Chemical reactions are limited by mass transfer, but chemical reactions affect mass transfer. By comparing the mass transfer coefficients of the physical absorption of CO
2 and electrochemical reduction of CO
2, it can be found that the electrochemical reaction has a promoting effect on the absorption of CO
2. The enhancement factor can be used to reflect the relationship between chemical absorption and physical absorption, and its calculation formula is as follows:
Then, the effect of gas-liquid flow rate on enhancement factor was analyzed.
It can be seen from
Figure 25 that when the liquid phase flow rate is the same, if the gas phase flow rate increases, the enhancement factor will increase. When the gas flow rate increases, the initial disturbance force of the two-phase flow increases, the CO
2 reduction reaction accelerates, the gas consumption intensifies, and the mass transfer is intense. Moreover, the increase of gas phase flow rate also makes the bubble length larger, and the initial driving force of the gas phase operation is larger, so the mass transfer coefficient increases faster and the enhancement factor increases. When the gas flow rate is the same, the enhancement factor decreases with the increase of the liquid flow rate. This is contrary to the rule of CO
2 absorption by alkanolamine solution in the literature [
46]. On one hand, it is due to the different structural dimensions of the microchannel, but on the other hand, there is a water electrolysis reaction in this study, and the H
+ generated will affect the absorption of CO
2 by aqueous solution. When the liquid flow rate increases, on the one hand, the material circulation intensifies and the mass transfer coefficient increases. On the other hand, the increase of H + will hinder the absorption rate of CO
2 in the liquid phase.
4.3. Effect of Mass Transfer on Chemical Reaction
Gas-liquid circulation affects the substance formation in chemical reactions. The catalytic reaction was carried out for 3 h, and the yield of the product was measured. In this study, the main target product of artificial photosynthesis is CH3OH, in addition to H2, CO, HCOOH, and other by-products, and the yield was analyzed by gas chromatography. At present, the product evaluation of catalytic reactions usually uses the yield of unit mass catalyst for a period of time as the evaluation standard; that is, μmol/g is the yield unit. In this study, the catalyst was in good contact with the substrate, and the generation of the product was affected by many factors, such as the structural parameters of the microchip and the size of the electrode membrane. In order to evaluate the device more intuitively and optimize the microchip, the yield per unit area in a period of time was used as the evaluation standard; that is, μmol/cm2 was used as the yield unit.
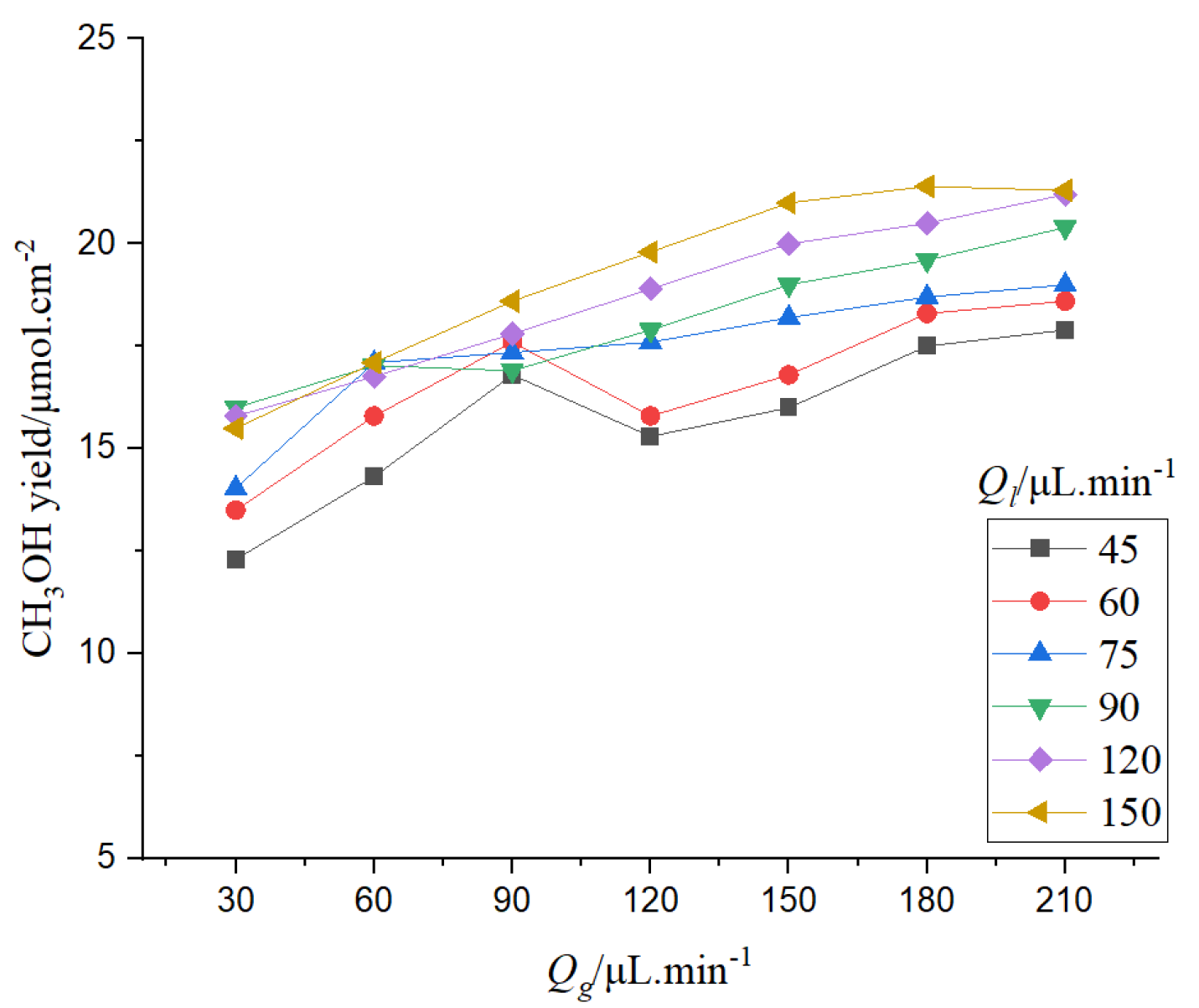
It can be seen from
Figure 26 that when the gas phase flow rate is constant, the chemical reaction rate generally shows an increasing trend with the increase of the liquid phase flow rate. However, when the liquid flow rate is 60 μL/min–90 μL/min, the chemical reaction rate does not increase with the increase of the liquid flow rate, and even has a decreasing trend. The mass transfer of two-phase flow is affected by the velocity of the gas-liquid phase. When the velocity of the liquid phase is low, the pumping pressure of the liquid phase is low, and the bubbles exist in a stable form. Moreover, from the analysis above, it can be seen that the relative flow velocity of the gas-liquid interface is slow at low velocity, which is not conducive to the rapid occurrence of mass transfer. When the electrolyte flow rate increases, the mass transfer at the gas-liquid interface is enhanced and the reaction is sufficient, so the reaction rate increases. When the liquid flow rate increases, the mass transfer increases, and the side reactions of hydrogenation and CO generation are intensified. The generated gas is adsorbed on the surface of the catalyst layer, which limits the increase of the reaction rate. If the liquid flow rate continues to increase, the bubbles adsorbed on the surface of the catalyst layer can be brought out of the channel in time, so the chemical reaction rate can continue to increase.
When the liquid flow rate is fixed, with the increase of the gas flow rate, the production of CH3OH increases first, then decreases, and finally increases. When the gas flow rate increases, the concentration of CO2 in the microchannel increases, the mass transfer at the gas-liquid interface is enhanced due to the higher flow rate, and the reaction rate is accelerated. When the gas flow rate reaches a certain value, the generated bubbles will aggregate in the microchannel, which hinders the contact between the reactant and catalyst, and affects the continued increase of the reaction rate. When the gas flow rate continues to increase, part of the bubbles adsorbed in the microchannel will be taken out of the channel; the reaction rate shows an increasing trend, but the increasing rate slows down.
From the analysis of CH3OH production by gas-liquid flow rate, it can be found that when the gas-liquid flow rate is small, the chemical reaction increases faster with the increase of gas-liquid flow rate. When the gas-liquid flow rate is high, the chemical reaction rate will not increase or even decrease slightly with the increase of gas-liquid flow rate. This is because the excessive flow rate reduces the reaction time in the microchannel and reduces the yield of the reaction. Therefore, too large or too small gas-liquid flow rate is not conducive to the chemical reaction.
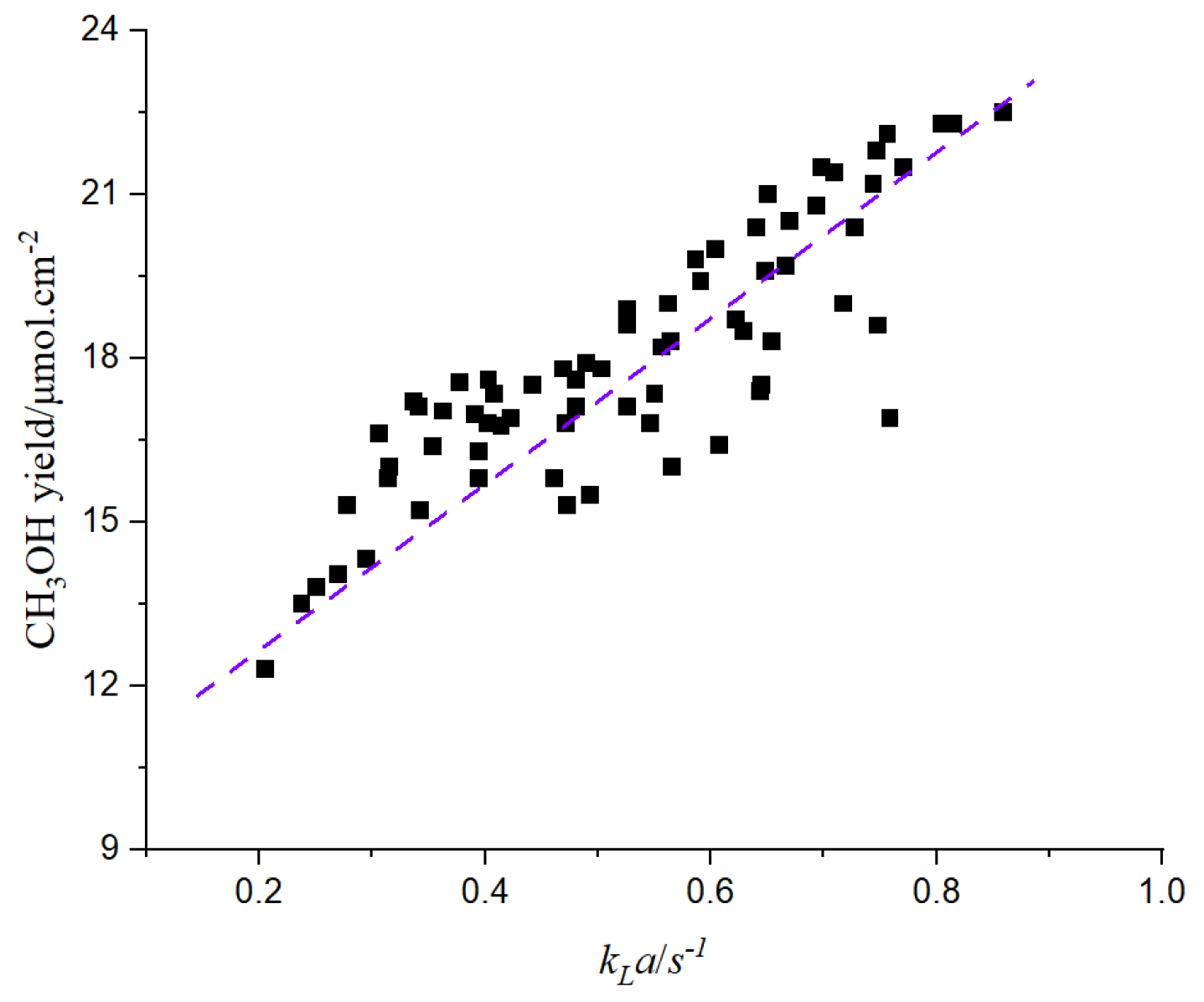
The chemical reaction is affected by the mass transfer rate. The following figure shows the relationship between the mass transfer coefficient and the yield of CH3OH.
It can be seen from
Figure 27 that the yield of CH
3OH increases with the increase of the mass transfer coefficient. The increase of the mass transfer coefficient leads to the intensification of material circulation, which accelerates the chemical reaction and increases the yield of products.