A Comprehensive Study on DES Pretreatment Application to Microalgae for Enhanced Lipid Recovery Suitable for Biodiesel Production: Combined Experimental and Theoretical Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Solvent Preparation and Analysis
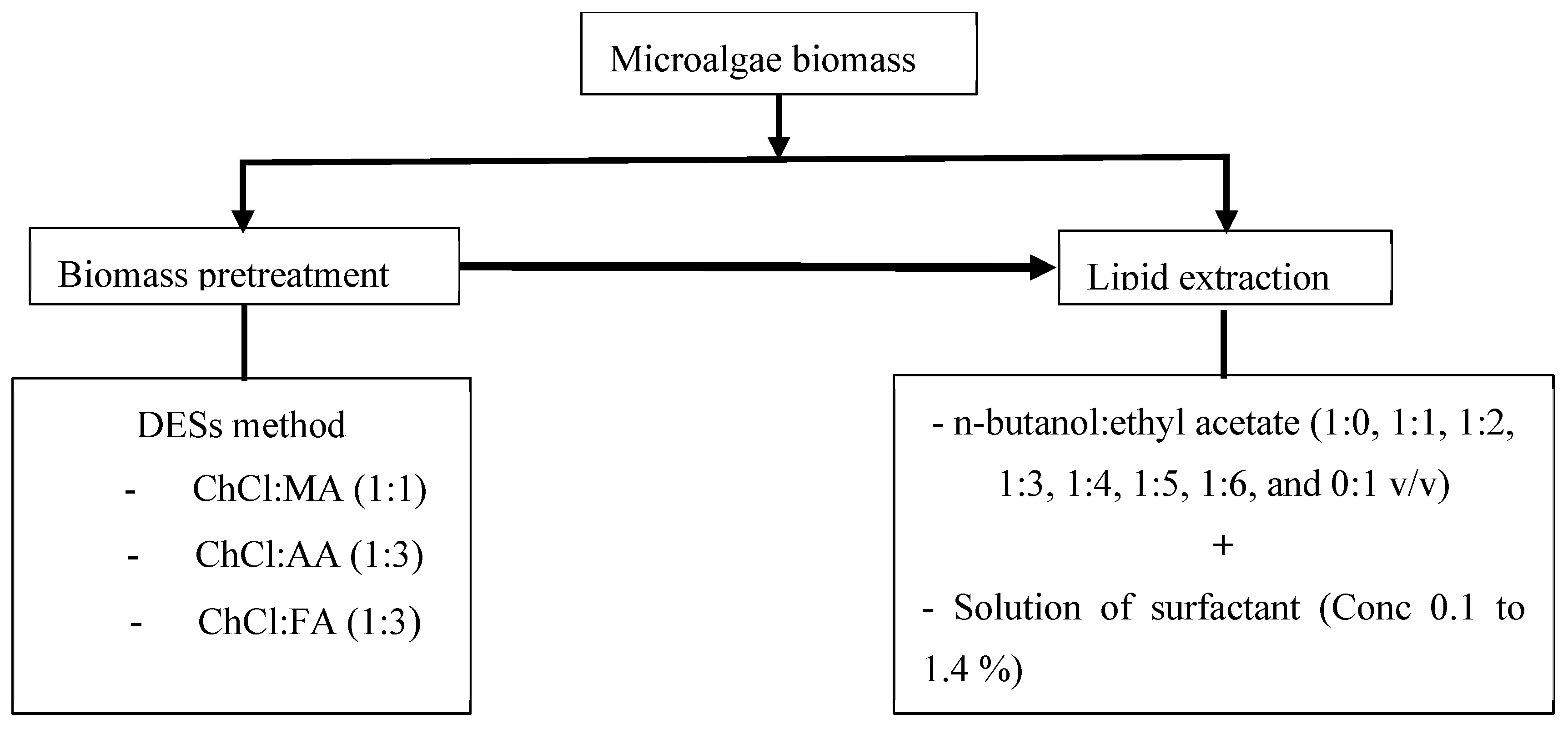
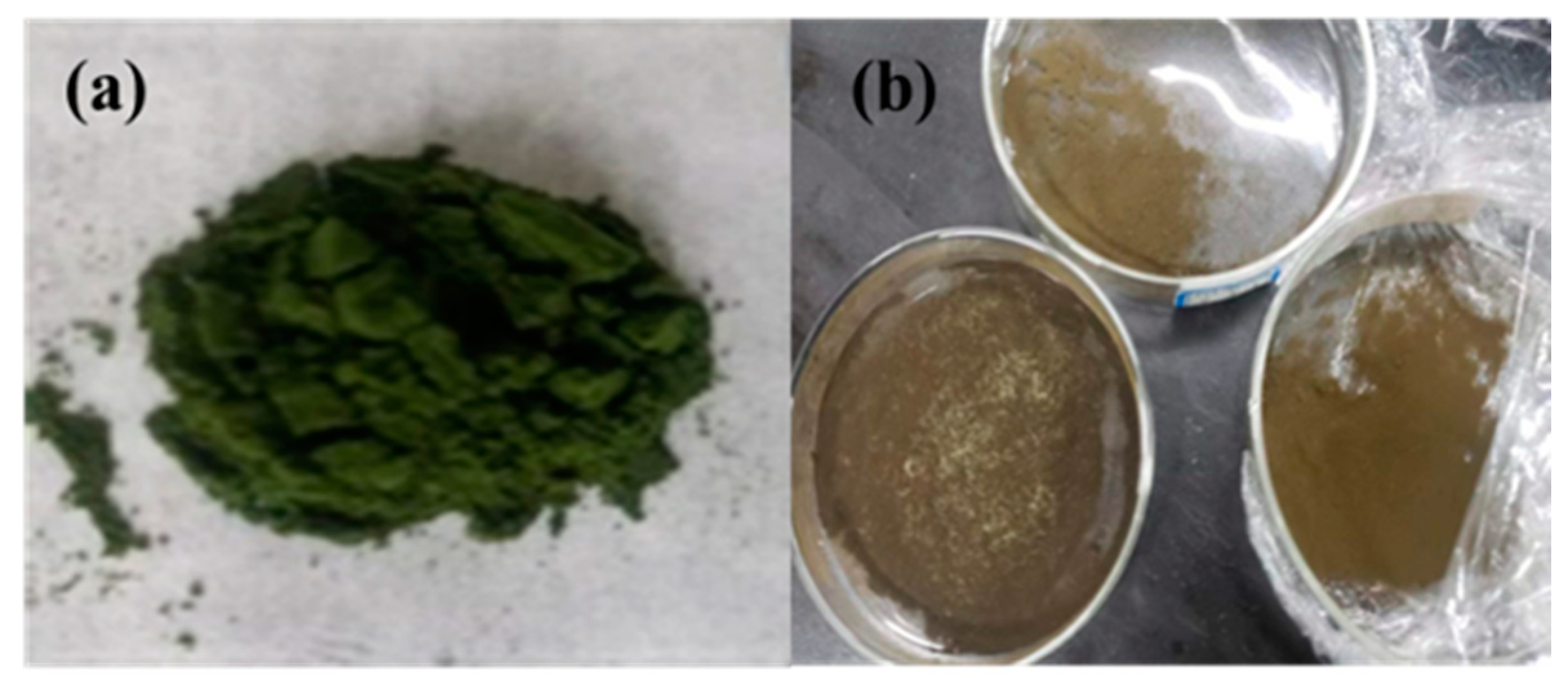
2.3. Pretreatment of Microalgae Biomass
2.4. Total Lipid Content and Optimization of the Lipid Extraction Process
2.5. Fatty Acid Methyl Ester Profile and Biodiesel Analysis
2.6. Analytical Examination
2.7. Statistical Examination
3. Results and Discussion
3.1. FTIR Analysis of Synthetized Deep Eutectic Solvents
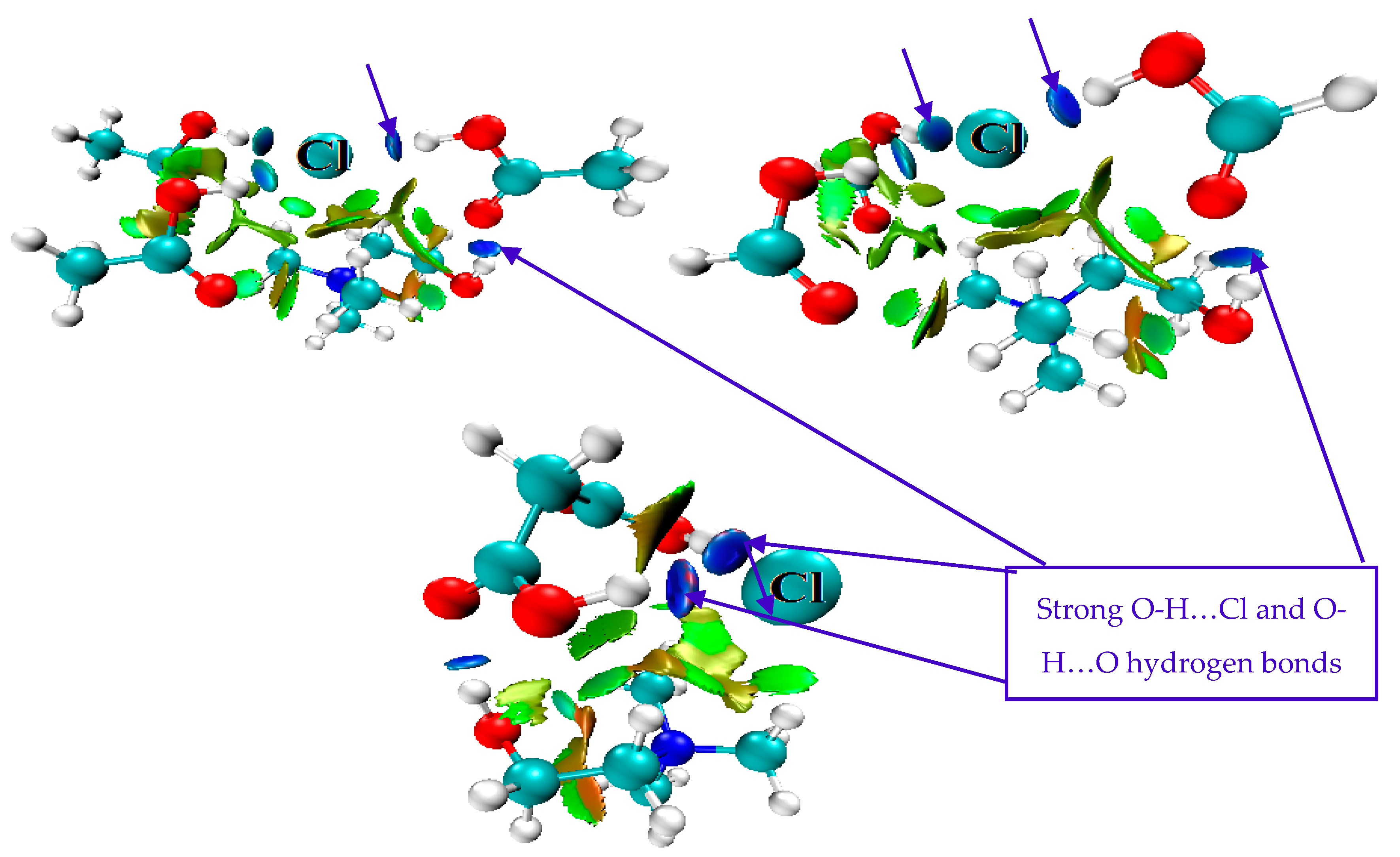
3.2. Geometric Optimization of DESs’ Structures
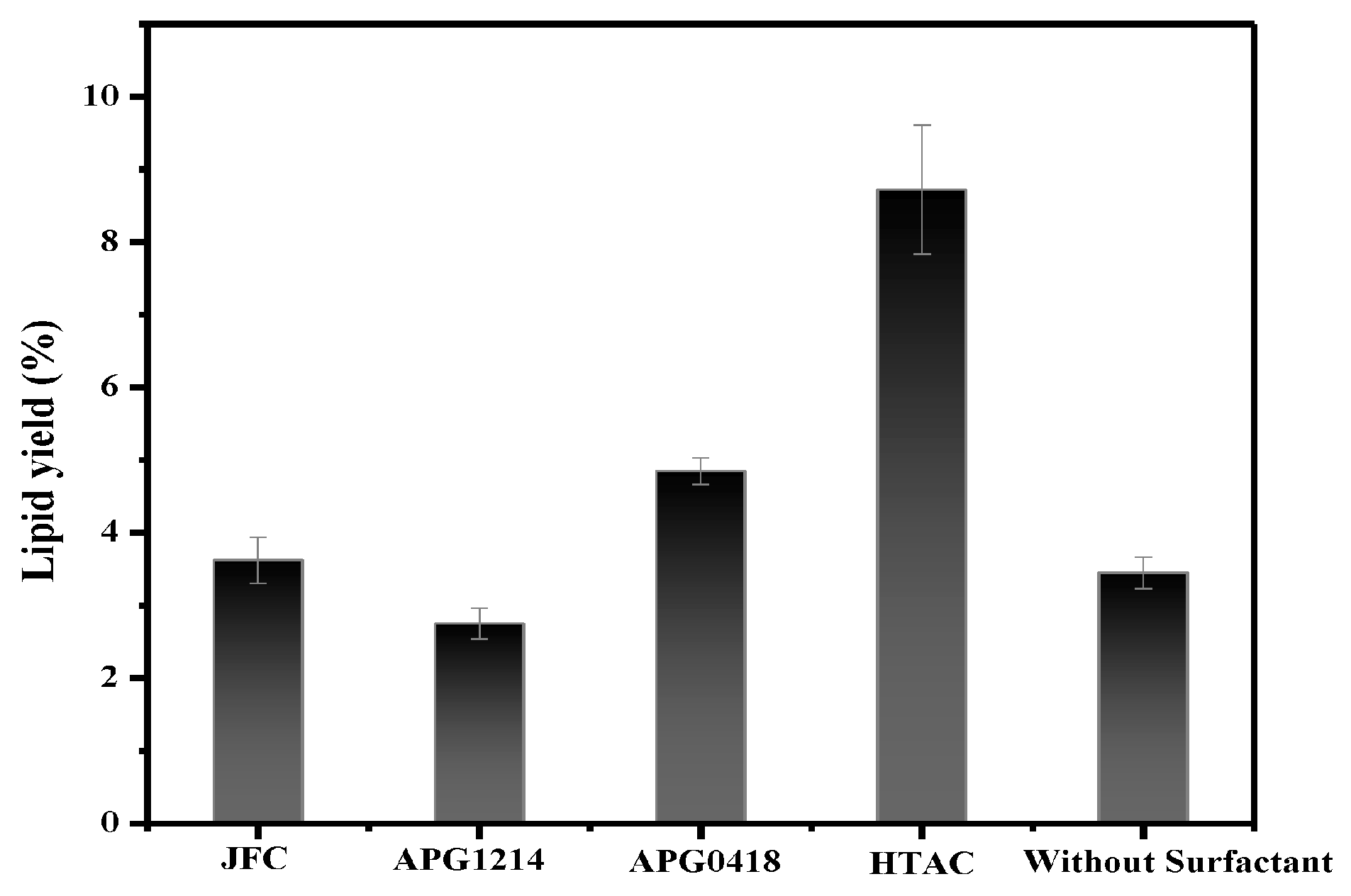
3.3. Surfactant Screening
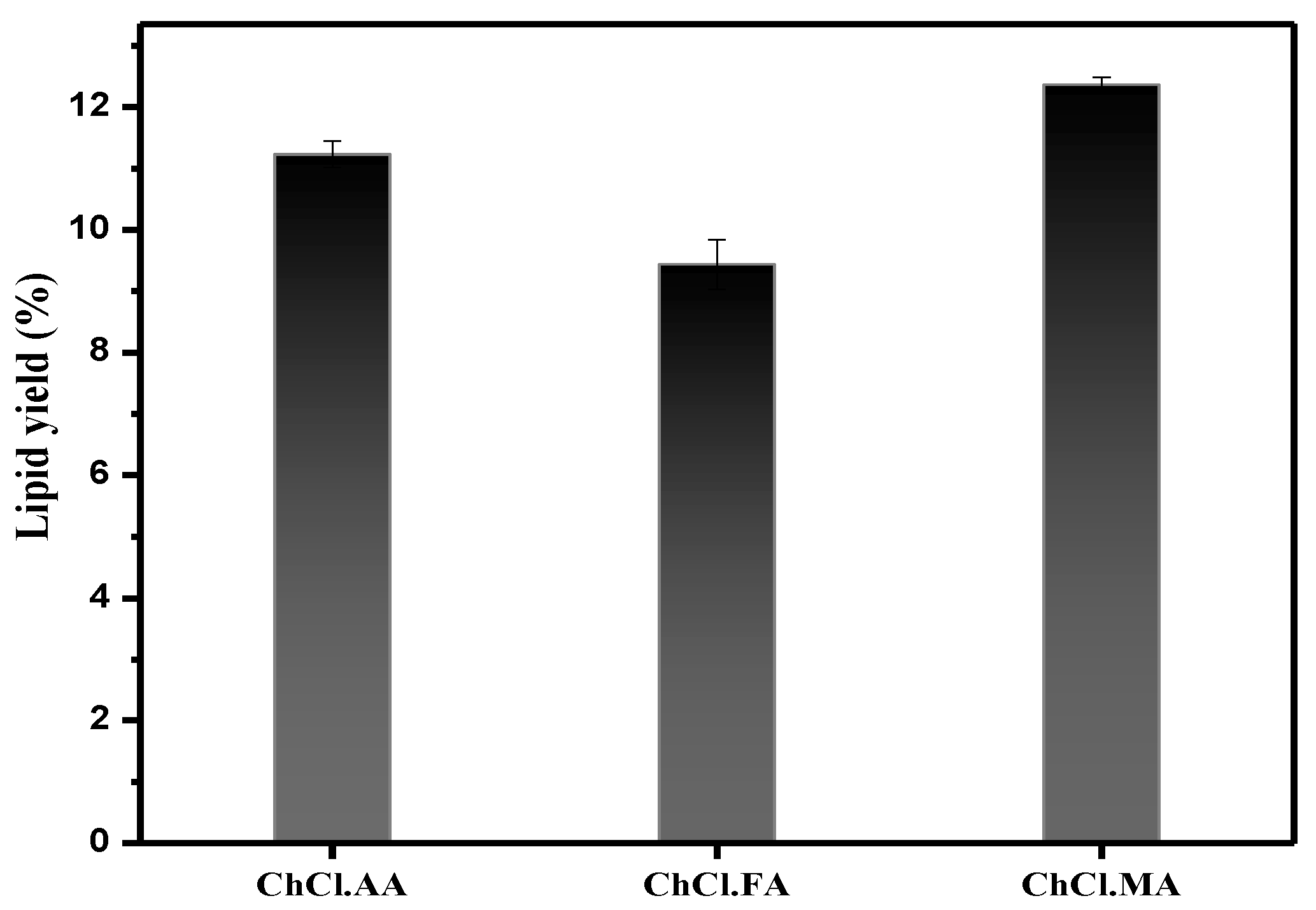
3.4. DESs Effect on Biomass Pretreatment
3.5. Characterization of Microalgae
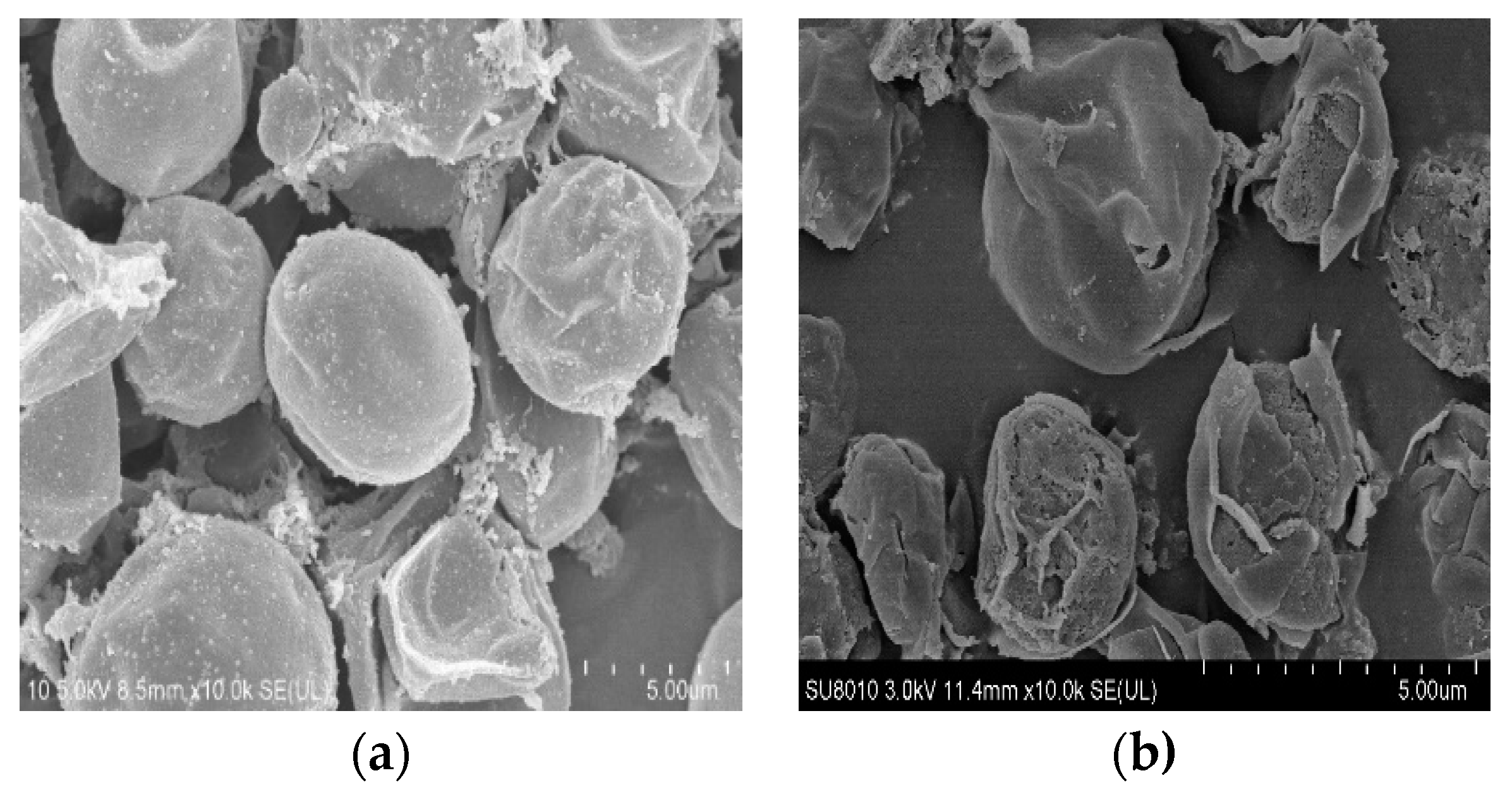
3.5.1. SEM Analysis of the Biomass
3.5.2. Thermogravimetric Analysis of the Biomass
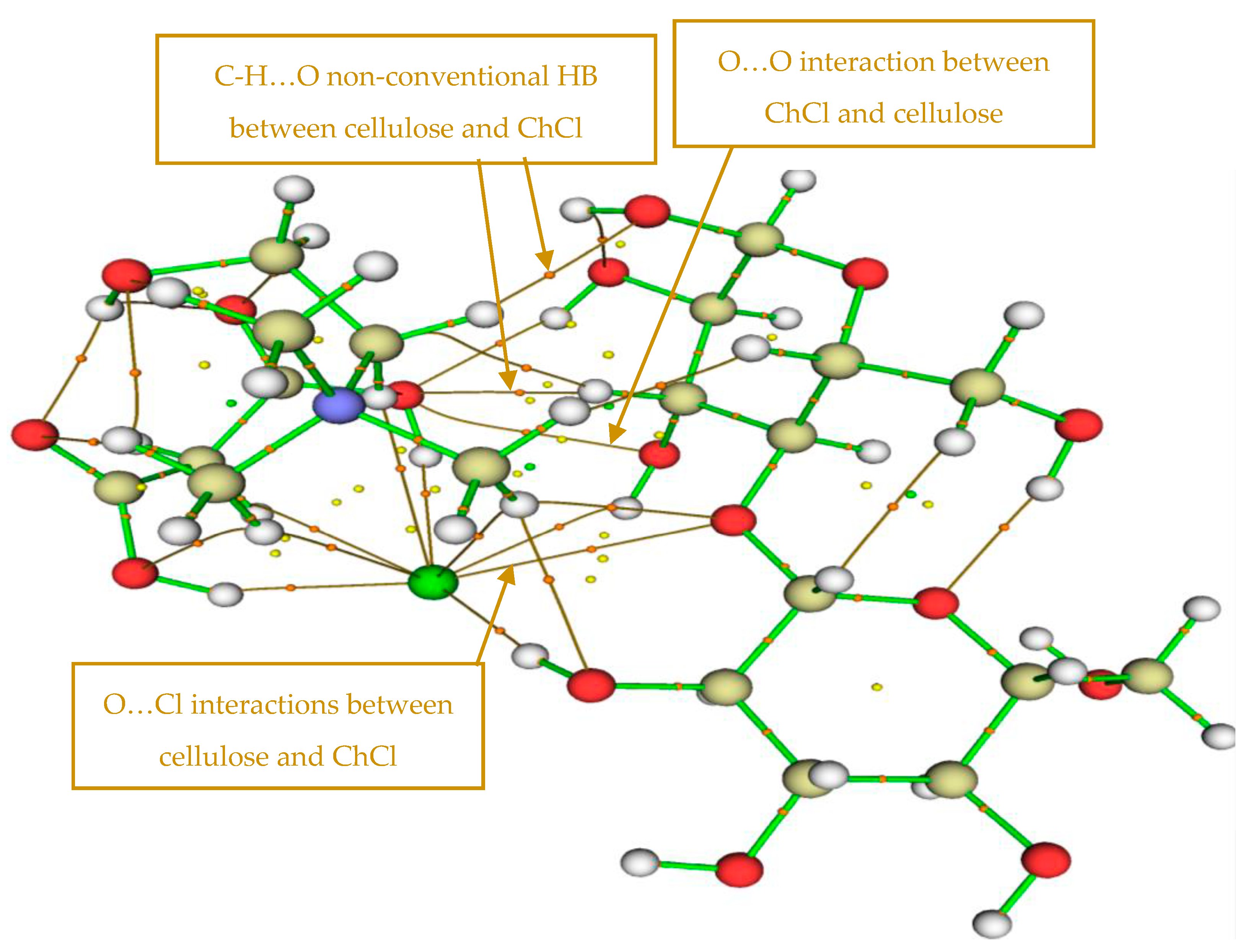
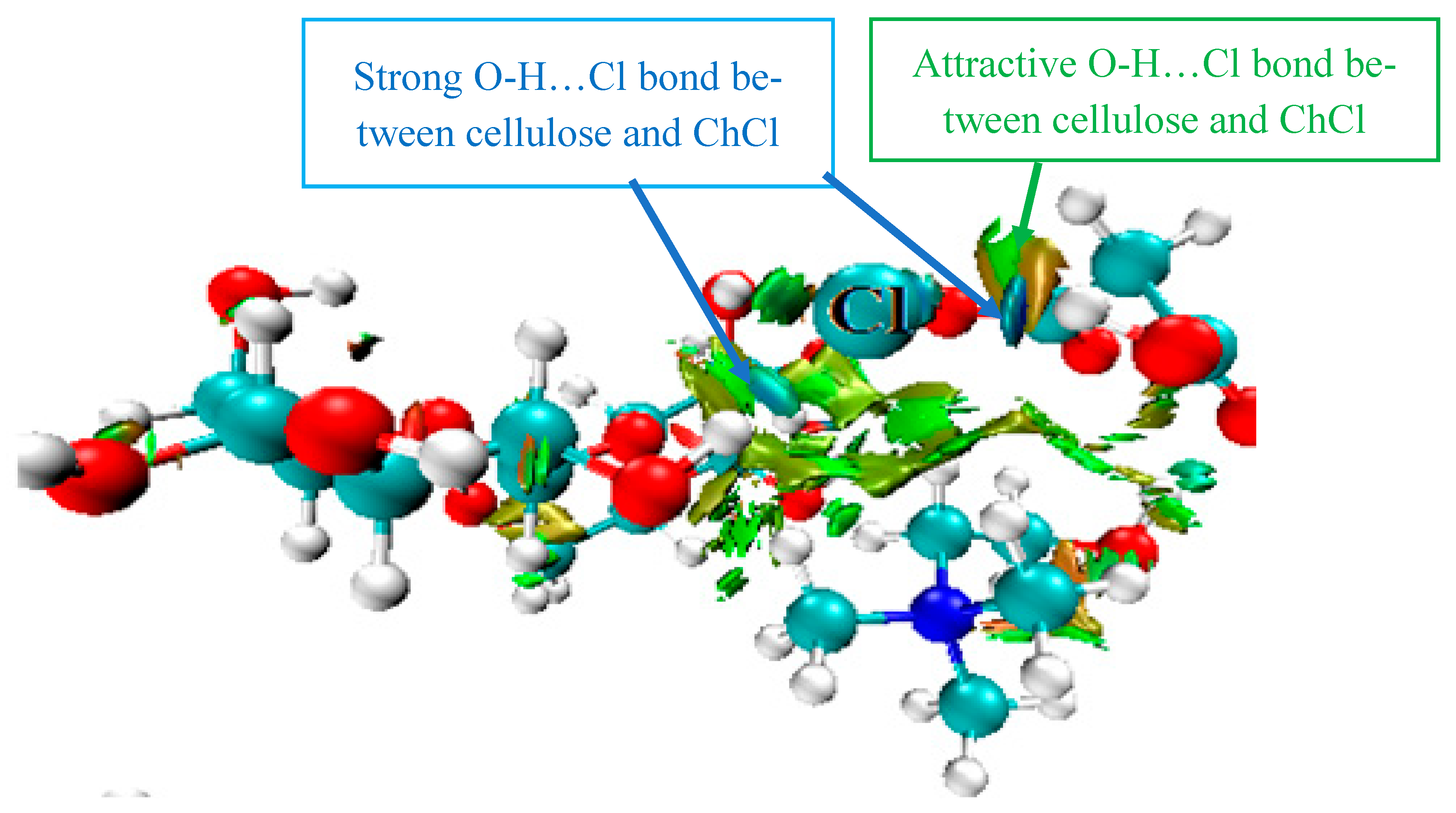
3.6. Mechanism of Cell Wall Disruption Inflicted by DES
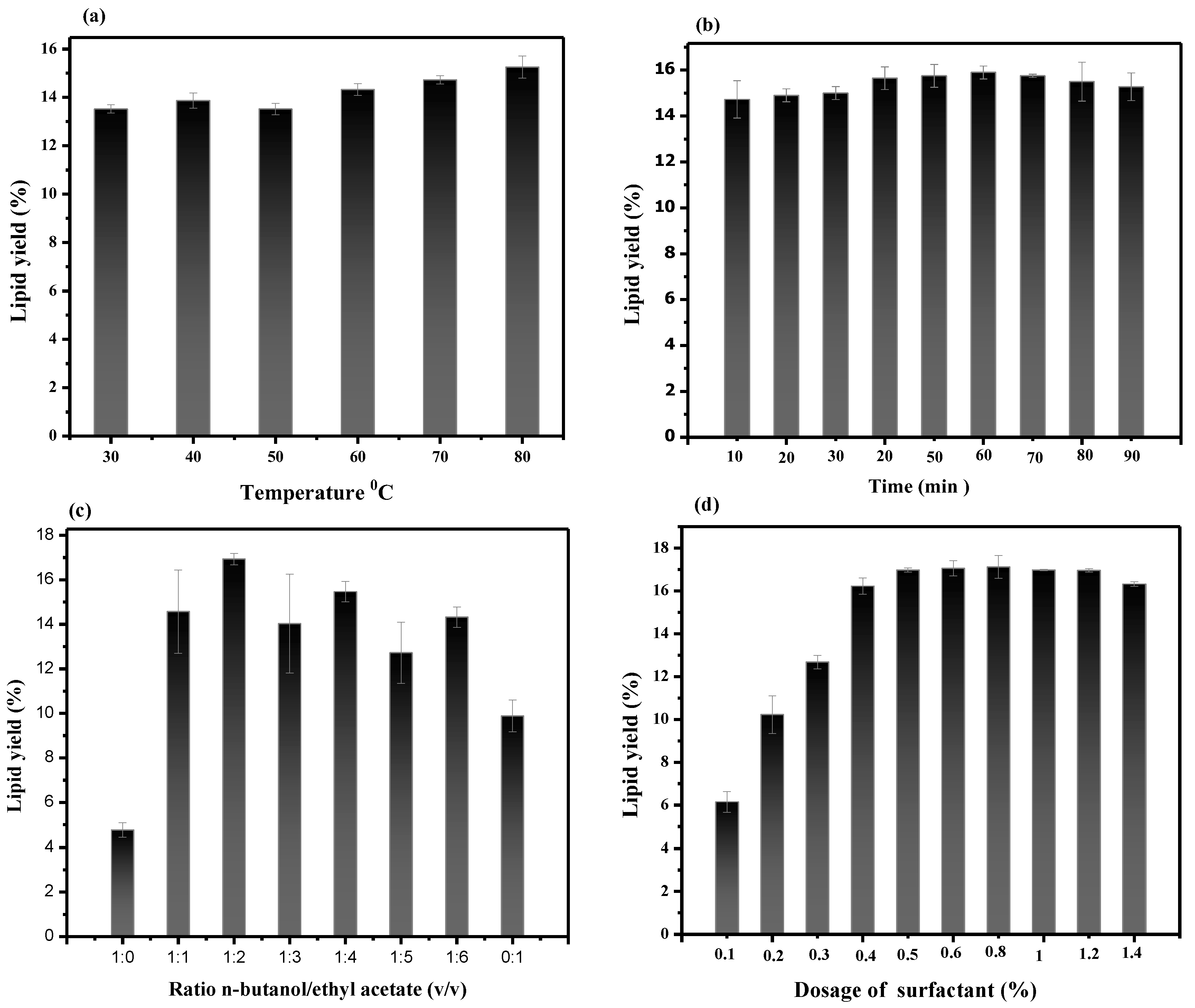
3.7. Optimization of Lipid Extraction Process Using Single-Factor Experiment
3.7.1. Effect Temperature on Lipid Extraction
3.7.2. Effect of Time on Lipid Extraction
3.7.3. Effect of n-Butanol-to-Ethyl-Acetate Ratio on Lipid Extraction Yield
3.7.4. Effect of Surfactant Concentration on Lipid Extraction
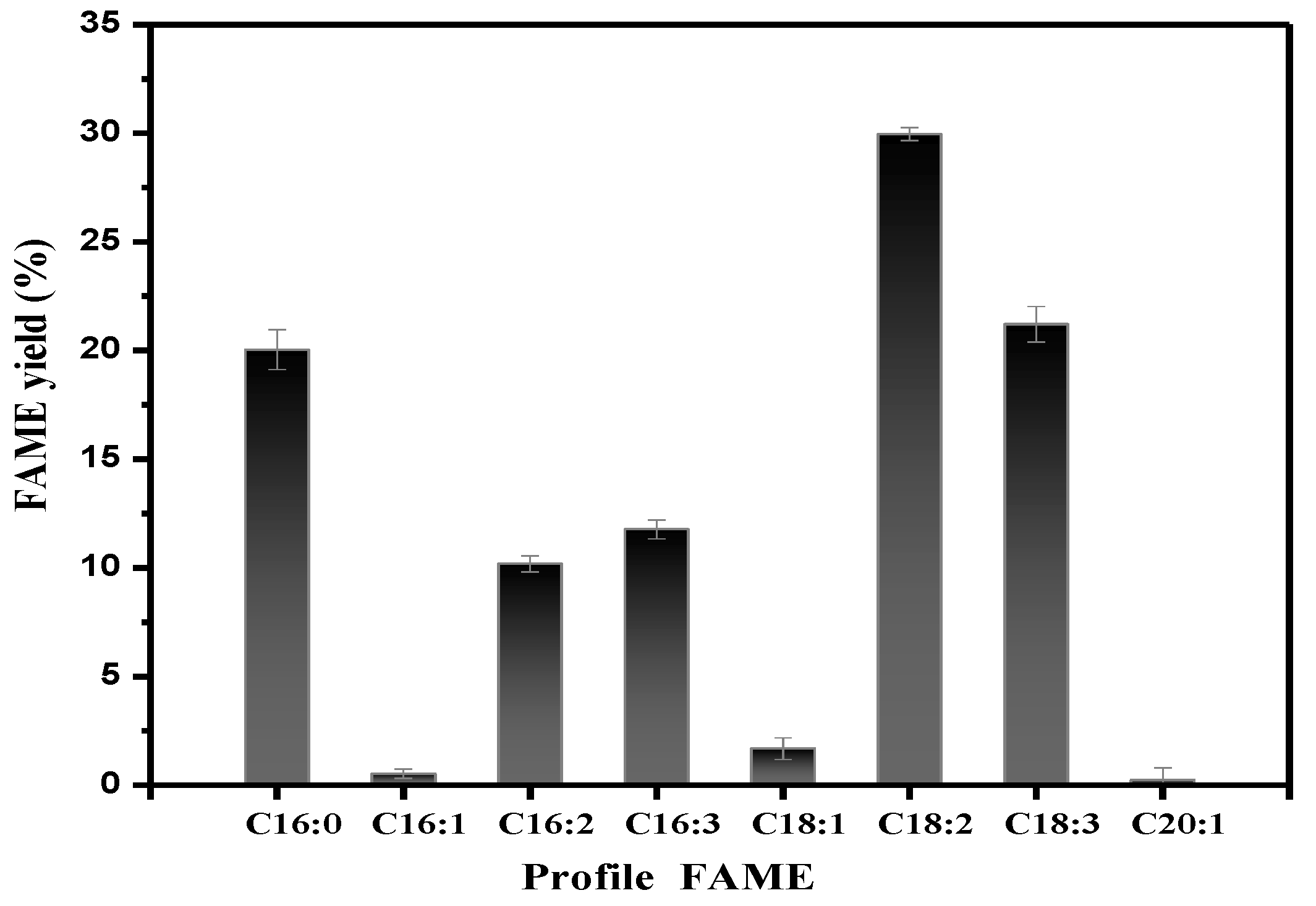
3.8. Fatty Acid Methyl Ester Profiles of Lipids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, A.; Gami, B.; Patel, P.; Patel, B. Biodiesel production from microalgae Dunaliella tertiolecta: A study on economic feasibility on large-scale cultivation systems. Biomass Convers. Biorefin. 2023, 13, 1071–1085. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.-S.; Bai, F.; Zhao, X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products—A review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Dinakaran, S.; Dahms, H.-U.; Ponnusamy, V.K. Passive cell disruption lipid extraction methods of microalgae for biofuel production—A review. Fuel 2019, 252, 699–709. [Google Scholar] [CrossRef]
- Chen, M.; Liu, T.; Chen, X.; Chen, L.; Zhang, W.; Wang, J.; Gao, L.; Chen, Y.; Peng, X. Subcritical co-solvents extraction of lipid from wet microalgae pastes of Nannochloropsis sp. Eur. J. Lipid Sci. Technol. 2012, 114, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, G.; Coutens, C.; Sánchez, M.; Sineiro, J.; Fábregas, J.; Deive, F.J.; Rodríguez, A.; Núñez, M.J. On the double role of surfactants as microalga cell lysis agents and antioxidants extractants. Green Chem. 2012, 14, 1044–1051. [Google Scholar] [CrossRef]
- Wu, C.; Xiao, Y.; Lin, W.; Zhu, J.; Siegler, H.D.L.H.; Zong, M.; Rong, J. Surfactants assist in lipid extraction from wet Nannochloropsis sp. Bioresour. Technol. 2017, 243, 793–799. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Gao, M.-Z.; Cui, Q.; Wang, L.-T.; Meng, Y.; Yu, L.; Li, Y.-Y.; Fu, Y.-J. A green and integrated strategy for enhanced phenolic compounds extraction from mulberry (Morus alba L.) leaves by deep eutectic solvent. Microchem. J. 2020, 154, 104598. [Google Scholar]
- Ngatcha, A.D.P.; Muhammad, G.; Lv, Y.; Xiong, W.; Zhao, A.; Xu, J.; Alam, M. Microalgae biomass pre-treatment with deep eutectic solvent to optimize lipid isolation in biodiesel production. Biomass Convers. Biorefin. 2022, 12 (Suppl. S1), 133–143. [Google Scholar] [CrossRef]
- Lu, W.; Alam, M.A.; Pan, Y.; Wu, J.; Wang, Z.; Yuan, Z. A new approach of microalgal biomass pretreatment using deep eutectic solvents for enhanced lipid recovery for biodiesel production. Bioresour. Technol. 2016, 218, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Naik, P.K.; Banerjee, T.; Goud, V.V.; Paul, S. Solubility of glucose in tetrabutylammonium bromide based deep eutectic solvents: Experimental and molecular dynamic simulations. Fluid Phase Equilibria 2017, 448, 168–177. [Google Scholar] [CrossRef]
- Tommasi, E.; Cravotto, G.; Galletti, P.; Grillo, G.; Mazzotti, M.; Sacchetti, G.; Samorì, C.; Tabasso, S.; Tacchini, M.; Tagliavini, E. Enhanced and Selective Lipid Extraction from the Microalga P. tricornutum by Dimethyl Carbonate and Supercritical CO2 Using Deep Eutectic Solvents and Microwaves as Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 8316–8322. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef]
- Feng, W.; Wang, S.; Duan, X.; Wang, W.; Yang, F.; Xiong, J.; Wang, T.; Wang, C. A novel approach for enhancing lipid recovery for biodiesel production from wet energy biomass using surfactants-assisted extraction. Renew. Energy 2021, 170, 462–470. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Shiwaku, I.A.; Maximo, G.J.; Caldas Batista, E.A. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M.A.; Wang, Z.; Huang, D.; Hu, K.; Chen, H.; Yuan, Z. One-step production of biodiesel from wet and unbroken microalgae biomass using deep eutectic solvent. Bioresour. Technol. 2017, 238, 157–163. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Citation; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Binkley, J.S.; Pople, J.A.; Hehre, W.J. Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J. Am. Chem. Soc. 1980, 102, 939–947. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Song, L.; Sommerfeld, M.; Hu, Q. Automated accelerated solvent extraction method for total lipid analysis of microalgae. Algal Res. 2020, 51, 102080. [Google Scholar] [CrossRef]
- Howlader, M.S.; Rai, N.; Todd French, W. Improving the lipid recovery from wet oleaginous microorganisms using different pretreatment techniques. Bioresour. Technol. 2018, 267, 743–755. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mousavi, S.M.; Shojaosadati, S.A. A novel surfactant-assisted ionic liquid pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Bioresour. Technol. 2014, 169, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Alam, A.; Pan, Y.; Nock, W.J.; Wang, Z.; Yuan, Z. Optimization of algal lipid extraction by mixture of ethyl acetate and ethanol via response surface methodology for biodiesel production. Korean J. Chem. Eng. 2016, 33, 2575–2581. [Google Scholar] [CrossRef]
- Loos, E.; Meindl, D. Composition of the cell wall of Chlorella fusca. Planta 1982, 156, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer: A user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J. 2014, 1, 55–57. [Google Scholar] [CrossRef]
- Suastes-Rivas, J.K.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Gómez, E.J.B.; Chairez, I. Biodiesel production, through intensification and profitable distribution of fatty acid methyl esters by a microalgae-yeast co-culture, isolated from wastewater as a function of the nutrients’ composition of the culture media. Fuel 2020, 280, 118633. [Google Scholar] [CrossRef]
- Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Tamafo Fouegue, A.D.; Nono, J.H.; Nkungli, N.K.; Ghogomu, J.N. A theoretical study of the structural and electronic properties of some titanocenes using DFT, TD-DFT, and QTAIM. Struct. Chem. 2021, 32, 353–366. [Google Scholar] [CrossRef]
- Nkungli, N.K.; Ghogomu, J.N. Theoretical analysis of the binding of iron (III) protoporphyrin IX to 4-methoxyacetophenone thiosemicarbazone via DFT-D3, MEP, QTAIM, NCI, ELF, and LOL studies. J. Mol. Model. 2017, 23, 200. [Google Scholar] [CrossRef]
- Mya, K.Y.; Sirivat, A.; Jamieson, A.M. Effect of Ionic Strength on the Structure of Polymer− Surfactant Complexes. J. Phys. Chem. B 2003, 107, 5460–5466. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Yang, X.; Zhang, J. Synergism and Phase Behavior of Alcohol Polyoxyethylene Ether Acetate and Cationic Surfactant-Mixed Systems. J. Surfactants Deterg. 2020, 23, 145–151. [Google Scholar] [CrossRef]
- Belsito, M.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Ronald, C. Decyl Glucoside and Other Alkyl Glucosides as Used in Cosmetics; Cosmetic Ingredient Review (CIR): Washington, DC, USA, 2011. [Google Scholar]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of decyl glucoside and other alkyl glucosides as used in cosmetics. Int. J. Toxicol. 2013, 32 (Suppl. S5), 22S–48S. [Google Scholar] [CrossRef]
- Lai, Y.S.; De Francesco, F.; Aguinaga, A.; Parameswaran, P.; Rittmann, B.E. Improving lipid recovery from Scenedesmus wet biomass by surfactant-assisted disruption. Green Chem. 2016, 18, 1319–1326. [Google Scholar] [CrossRef]
- Callejón, M.J.J.; Medina, A.R.; Sánchez, M.D.M.; Peña, E.H.; Cerdán, L.E.; Moreno, P.A.G.; Grima, E.M. Extraction of saponifiable lipids from wet microalgal biomass for biodiesel production. Bioresour. Technol. 2014, 169, 198–205. [Google Scholar] [CrossRef]
- Do, L.D.; Sabatini, D.A. Aqueous extended-surfactant based method for vegetable oil extraction: Proof of concept. J. Am. Oil Chem. Soc. 2010, 87, 1211–1220. [Google Scholar] [CrossRef]
- Huang, W.-C.; Kim, J.-D. Cationic surfactant-based method for simultaneous harvesting and cell disruption of a microalgal biomass. Bioresour. Technol. 2013, 149, 579–581. [Google Scholar] [CrossRef]
- Arif, M.; Li, Y.; El-Dalatony, M.M.; Zhang, C.; Li, X.; Salama, E.-S. A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: An approach for biofuel generation and nutrients removal. Renew. Energy 2021, 163, 1973–1982. [Google Scholar] [CrossRef]
- Pouliot, R.; Germain, L.; Auger, A.; Tremblay, N.; Juhasz, J. Physical characterization of the stratum corneum of an in vitro human skin equivalent produced by tissue engineering and its comparison with normal human skin by ATR-FTIR spectroscopy and thermal analysis (DSC). Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 1999, 1439, 341–352. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of innovative processing methods on microalgae cell wall: Prospects towards digestibility of protein-rich biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Popelier, P.L.A. The QTAIM perspective of chemical bonding. In The Chemical Bond: Fundamental Aspects of Chemical Bonding; Wiley-VCH: Weinheim, Germany, 2014; pp. 271–308. [Google Scholar]
- Boto, R.A.; Piquemal, J.-P.; Contreras-García, J. Revealing strong interactions with the reduced density gradient: A benchmark for covalent, ionic and charge-shift bonds. Theor. Chem. Acc. 2017, 136, 139. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Wu, J.; Alam, A.; Pan, Y.; Huang, D.; Wang, Z.; Wang, T. Enhanced extraction of lipids from microalgae with eco-friendly mixture of methanol and ethyl acetate for biodiesel production. J. Taiwan Inst. Chem. Eng. 2017, 71, 323–329. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Zheng, X.; Wang, Q.; Huang, J.; Ebaid, R. Enhancement of biodiesel yield and characteristics through in-situ solvo-thermal co-transesterification of wet microalgae with spent coffee grounds. Bioresour. Technol. 2021, 323, 124640. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energy Convers. Manag. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- Shahid, A.; Usman, M.; Atta, Z.; Musharraf, S.G.; Malik, S.; Elkamel, A.; Shahid, M.; Alkhattabi, N.A.; Gull, M.; Mehmood, M.A. Impact of wastewater cultivation on pollutant removal, biomass production, metabolite biosynthesis, and carbon dioxide fixation of newly isolated cyanobacteria in a multiproduct biorefinery paradigm. Bioresour. Technol. 2021, 333, 125194. [Google Scholar] [CrossRef]
- Pedro, G.A.; Amaral, M.S.; Pereira, F.M.; Flumignan, D.L.; Da Rós, P.C.M.; Reis, C.E.R.; Silva, M.B. Highly wet Chlorella minutissima biomass for in situ biodiesel production and residual biomass rich in labile carbohydrates. Bioenergy Res. 2022, 15, 154–165. [Google Scholar] [CrossRef]
- Munari, F.; Cavagnino, D. Determination of Total FAME and Linolenic Acid Methyl Ester in Pure Biodiesel (B100) by GC in Compliance with EN 14103; Thermo Fisher Scientific: Milan, Italy, 2007. [Google Scholar]
- Kumbhar, V.; Pandey, A.; Sonawane, C.R.; El-Shafay, A.; Panchal, H.; Chamkha, A.J. Statistical analysis on prediction of biodiesel properties from its fatty acid composition. Case Stud. Therm. Eng. 2022, 30, 101775. [Google Scholar] [CrossRef]



| Organic Salts | Hydrogen Bond Donor | DES Molar Ratio | Density ρ, (g·mL−1) |
|---|---|---|---|
| Choline chloride | Malonic acid | 1:1 | 1.08 |
| Choline chloride | Formic acid | 1:3 | 1.12 |
| Choline chloride | Acetic acid | 1:3 | 1.02 |
| Type | Surfactants | CMC (a) (mM) at 296.15 K–248.15 K |
|---|---|---|
| Cation | N-Hexadecyl trimethylammonium chloride (HTAC) | 0.94–1.19 [32] |
| Non-ionic | Fatty alcohol polyoxy-ethylene ether (JFC) | 0.019 [33] |
| Coconut glucoside (APG 0814) | 0.13 [34] | |
| Lauryl glucoside (APG 1214) | 0.13 [35] |
| Fuel Property | International Standards | Obtained Biodiesel | |
|---|---|---|---|
| EN14214 | ASTMD6751 | ||
| Degree of unsaturation (DU) (mg KOHg−1) | - | - | 104.73 |
| Saponification value (SV) (gI 2100g−-1 fat) | - | - | 151.09 |
| Iodine value (IV) | ≤120 | - | 114.50 |
| Cetane number (CN) | 51–120 | ˃47 | 56.65 |
| Long-chain saturated factor (LCSF) | - | - | 2.00 |
| Kinematic viscosity 40 °C (mm2 s−1) | 3.5 to 5.0 | 1.9 to 6 | 2.40 |
| Cold filter plugging point (CFPP) (°C) | −20 to 5 | - | −10.18 |
| Cloud point (°C) | ˃4 | −3 to −15 | 5.54 |
| Oxidative stability (OS) (h) | ˃6 | - | 4.89 |
| Density (kg·m−3) | - | 0.8 to 0.86 | 0.65 |
| Higher heating value HHV (MJ Kg−1) | - | - | 28.90 |
| Fatty acid methyl ester content (wt %) | ≥96.50 | - | 95.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matchim Kamdem, M.C.; Tamafo Fouegue, A.D.; Lai, N. A Comprehensive Study on DES Pretreatment Application to Microalgae for Enhanced Lipid Recovery Suitable for Biodiesel Production: Combined Experimental and Theoretical Investigations. Energies 2023, 16, 3806. https://doi.org/10.3390/en16093806
Matchim Kamdem MC, Tamafo Fouegue AD, Lai N. A Comprehensive Study on DES Pretreatment Application to Microalgae for Enhanced Lipid Recovery Suitable for Biodiesel Production: Combined Experimental and Theoretical Investigations. Energies. 2023; 16(9):3806. https://doi.org/10.3390/en16093806
Chicago/Turabian StyleMatchim Kamdem, Michele Corneille, Aymard Didier Tamafo Fouegue, and Nanjun Lai. 2023. "A Comprehensive Study on DES Pretreatment Application to Microalgae for Enhanced Lipid Recovery Suitable for Biodiesel Production: Combined Experimental and Theoretical Investigations" Energies 16, no. 9: 3806. https://doi.org/10.3390/en16093806
APA StyleMatchim Kamdem, M. C., Tamafo Fouegue, A. D., & Lai, N. (2023). A Comprehensive Study on DES Pretreatment Application to Microalgae for Enhanced Lipid Recovery Suitable for Biodiesel Production: Combined Experimental and Theoretical Investigations. Energies, 16(9), 3806. https://doi.org/10.3390/en16093806










