Abstract
In thermal cracking and collision-induced dissociation (CID) processes, molecules/ions mainly undergo cleavage reactions. In theory, the cleavage reaction is preferred for weak bonds in both processes. The present study investigates the thermal cracking and CID behavior of polar compounds in vacuum residue. By controlling the thermal reaction temperature and collision energy, different degrees of fragmentation were achieved. The molecular composition before and after the cracking process was analyzed through electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry (ESI FT-ICR MS). There was a correlation between the reaction temperature and the collision energy for the average carbon number value. Both desulfurization and decarboxylation were also observed in two processes due to the low C-S bond energy and poor stability of carboxylic acid groups. Nevertheless, the two processes still had some differences in reaction selectivity. Polar species tended to be directly dealkylated down to the C1–C5 substituted aromatic core in the CID process, showing a discontinuity in the carbon number vs. double bond equivalence (DBE) distribution for the CID product. On the contrary, the carbon number distribution in the thermal reaction showed a continuously reduced trend. In summary, the CID process can qualitatively reflect the cracking behavior. However, the product structural distribution of the thermal cracking product cannot be fully predicted, especially for sidechain cracking reactions. In addition, the research results can provide a new method to realize the simulation of the thermal cracking without energy and time consumption, so as to guide the selection of the feedstock and optimization of the reaction condition.
1. Introduction
The increasing demand for energy and a reduction in light oil are driving the research for better utilization of heavy oil. The high viscosity of heavy oil inhibits its transportation and processing [1]. Thermal visbreaking is a popular heavy oil upgrading technology to reduce the viscosity through a heat-treatment process [2]. It has advantages in terms of a low equipment investment, low operating expenses, and few limitations regarding feedstock quality. The understanding of the visbreaking behavior and product composition prediction is an import research subject.
Visbreaking is a moderate thermal cracking process that mainly consists of bond breaking and a low degree of bimolecular condensation reactions. The latter leads to coke formation, shortening the process operating cycle [2]. The condensation reaction is inhibited in most practical applications. The cracking reaction is promoted and is the main reaction in the visbreaking process.
Mass spectrometry (MS) is an important technique to characterize petroleum molecular composition. Dissociation techniques combined with mass spectrometry have been widely used to study molecular structural characteristics [3,4]. Dissociation techniques such as collision-induced dissociation (CID) [5,6], surface-induced dissociation (SID) [7,8], photo-dissociation (PD) [9,10], blackbody infrared dissociation (BIRD) [11,12], and electron capture dissociation (ECD) [13,14] were reported in the past and have played an important role in molecular structure study. These fragmentation techniques have also been applied to analyze complex petroleum systems. A different level of structural information was revealed according to the resolving power of the mass spectrometer. The molecular composition and structure of petroleum determine its physical and chemical properties. The value and processing properties of isomers vary greatly, so it is necessary to analyze petroleum components at a molecular structure level.
Dissociation techniques coupled with a relatively low-resolution mass spectrometer were frequently used in the past to investigate the petroleum molecular structure. Because of the complex nature of petroleum composition, petroleum molecule structural characteristics were indirectly revealed through the overall molecular weight distribution change. Mullins et al. [15] analyzed the molecular weight of asphaltene by two-step laser desorption laser ionization mass spectrometry (L2-TOF MS), which minimized the possible plasma concentration effects in ordinary mass spectra. They proved that asphaltene molecular weight distribution was centered around 600 Da. Hassan et al. [16,17] also used the L2-TOF MS technique to study Middle East asphaltene and found that mass distribution variation before and after fragmentation was consistent with that of island-type model compounds. Borton et al. [18] analyzed the asphaltene fragment ions using the collision-induced dissociation (CID) technique and laser-induced acoustic desorption (LIAD) technology, and found that the side chains in the asphaltene molecules contained at least 2–13 carbons and that there were a large number of branched ethyl or alkyl chains on the α-carbon and methyl on the naphthalene.
The increasing resolution of the mass spectrum provides more accurate molecular weight and ion elemental composition information. The milestone in this field is the application of ultra-high magnetic field Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS). It provides the molecular composition information for most of the complex heavy oil systems [19,20,21,22,23]. Qian et al. [19,20] identified more than 3000 compound elemental constituents in heavy petroleum by 9.4T FT-ICR MS. FT-ICR MS revealed the chemical composition of petroleum at a molecular level and formed the concept and methodology of “petroleomics” [24,25]. Since then, fragmentation techniques have been introduced into FT-ICR MS to study the molecular structure of petroleum [26,27,28,29]. Qian et al. [30] investigated the changes of the double bond equivalent (DBE) of heavy oil molecules using the CID FT-ICR MS technique, verifying the existence of archipelago structures [31,32]. Rodgers et al. [33] and Nyadong et al. [34] also demonstrated the overwhelming contribution of archipelago motifs to the structure of petroleum. Recent work by Rodgers [35] showed that the mass spectrometric analysis of asphaltenes tended to preferential ionize island structural motifs, and this explained the overwhelming mass spectral support of the island model. Rudzinski et al. [28] applied CID technology to identify alkyl sulfonic acid homologues in Maya crude oil. Rodgers et al. [27] used infrared multiphoton dissociation (IRMPD) FT-ICR MS techniques to identify the acidic substance of crude oils and naphthenates and to explore their compositional differences. CID FT-ICR MS was performed by Rüger et al. [36] to characterize the structure of five heavy oils by observing their carbon number and DBE distribution change after CID process. Zhang et al. [29] used CID FT-ICR MS to study the fragmentation behavior of basic nitrogen compounds in the Sudan vacuum residue, indicating its preferential heterolytic bond cleavage due to the predominant odd-electron fragmentation ions. The DBE change before and after fragmentation was not obvious, revealing the possibility of an island-like structure. The fragmentation technique was recently applied to study the structure of asphaltenes. Qian et al. [37] directly traced asphaltenes structure through the single Dalton CID FT-ICR MS technique, indicating the island-type Z value distribution of asphaltenes, but it did not rule out the influence of other factors. Wang et al. [38,39,40,41,42] obtained more sulfur compound and hydrocarbon structure information from model compounds and petroleum samples through narrow window CID FT-ICR MS. The CID combined with hydrodesulfurization kinetics, by Deng et al., characterized the reactivity and molecules of sulfur compounds, revealing the reaction sequence and its influencing factors [43]. Rodgers et al. [33] employed IRMPD fragmentation techniques to research South American asphaltenes that were purified by different solvents, and found that C5 and C6 asphaltenes fragments were island-like, but C7 asphaltenes fragments showed an archipelago bimodal mass distribution. Dong et al. [44] employed CID to explore the structures of asphaltene molecular ions, and showed that the abundances of single-core and multicore compounds were not uniform within the entire asphaltene samples. Most of the current MS dissociation technology is limited to the discovery of the core structure, providing less information on the actual reaction and lacking a connection with the refining process, which still needs to be further explored.
Among all of the dissociation techniques, CID technology is widely used, in which the weakest bond will break first [4]. Previous work [45] has discussed the mass distribution similarity between thermal reaction products and CID products. Laskin et al. [46] reported that the internal energy distribution of the precursor ions in CID followed the Boltzmann distribution, which theoretically showed consistency between the CID process and the thermal reaction. However, systematic and comprehensive study of the CID process and thermal cracking was rarely conducted.
This paper compares the molecular compositional change of polar compounds in the CID process and thermal cracking process at different fragmentation degrees. We studied their similarity and difference to explore the possibility of simulating the heavy oil cracking process using CID technology, attempting to use CID MS as a “virtual thermal visbreaking reactor”, which will potentially provide valuable information regarding the heavy oil thermal reaction behavior, without conducting time-consuming and exhausting thermal experiments, in order to guide the selection of the feedstock and the optimization of the reaction condition.
2. Experimental
2.1. Feedstock and Thermal Visbreaking Reactions
The raw material studied in the present work was a Venezuela vacuum residue (VR, >425 °C). The thermal visbreaking reactions were conducted in a closed 60 mL volume batch reactor loaded with ~25 g feedstock. The reaction time was fixed at 1 h and the reaction temperature varied in the range of 25–430 °C to achieve different degrees of fragmentation. The specific temperature range and reaction time were selected in order to avoid coke formation. The experimental procedures were as follows: quickly heating to the reaction temperature and after reaching the prescribed time, the reactor was rapidly cooled to room temperature and liquid products were taken for analysis.
2.2. ESI FT-ICR MS and CID Fragmentation
About 10 mg of each sample was completely dissolved in 1 mL of toluene to a concentration of 10 mg/mL. For the FT-ICR MS measurement, the solution was further diluted with toluene/methanol (1:1, v/v) mixtures to 0.2 mg/mL. Then, 5 μL formic acid and 10 μL 28% ammonia were added as an ionization promoter for positive- and negative-ion ESI mode measurements.
The diluted solution was then injected by an Apollo electrospray ion source at a rate of 250 μL/h. The mass spectrometer used for the study was Bruker Apex ultra FT-ICR MS (9.4 T) and argon was used as the collision gas. The operating conditions for positive-ion mode were −4000 V spray shield e voltage, −4500 V capillary introduction voltage, 1.2 s TOF, and 2.0 s accumulation time. In the +ESI CID process, the value of the collision voltage (CV) varied from −1.5 eV to −150 eV. The conditions for negative-ion mode were 3800 V spray shield voltage, 4200 V capillary introduction voltage, 1.2 s TOF, and accumulation time 2.0 s. In the −ESI CID process, the CV value ranged from 1.5 eV to 60 eV.
2.3. Mass Calibration and Data Analysis
The data were calibrated internally according to a known and highly abundant homologous series of N-containing compounds using the Bruker Data Analysis software. Peak data with relative abundance greater than six times the standard deviation of the baseline noise were imported into custom software [47], where species with the same heteroatom class and its isotopes with different values for DBE and carbon number were searched within a set (0.001 Kendrick mass defect (KMD)) tolerance [48].
3. Results and Discussion
3.1. Molecular Weight Distribution
Cracking and condensation reactions are both involved in petroleum thermal reaction processes, which mainly undergo single-molecule and bi-molecule mechanisms, respectively. On the other hand, there is almost no bi-molecular reaction among analyte ions in the CID process due to the charge repulsion effect [45]. Bi-molecular condensation reactions in thermal processes lead to the formation of coke. Thus, it is more appropriate to compare CID MS with the visbreaking process, rather than the coking process. The main factors affecting thermal visbreaking are the reaction time and reaction temperature. In the present study, we fixed the reaction time at 1 h and different conversion depths were obtained by changing the reaction temperature. The thermal visbreaking reaction was controlled in a relatively mild temperature range (<430 °C) in order to minimize coke formation. The fragmentation behavior of the CID process is governed by the accumulation time and collision voltage in the collision cell with the same collision gas. Similarly, we fixed the accumulation time at 2.0 s and changed the collision voltage to control the degree of molecular fragmentation.
Positive- and negative-ion mode ESI selectively ionize basic and acidic compounds in petroleum. In order to fully understand the CID and thermal reaction behavior of polar species in heavy petroleum, positive- and negative-ion ESI FT-ICR MS were both used in the present research. Molecular weight reduction is an intuitive reflection of the fragmentation degree. Both the CID and thermal visbreaking processes’ products of polar species in heavy petroleum exhibited a clear molecular weight reduction, which is shown in Figure 1 and Figure 2.
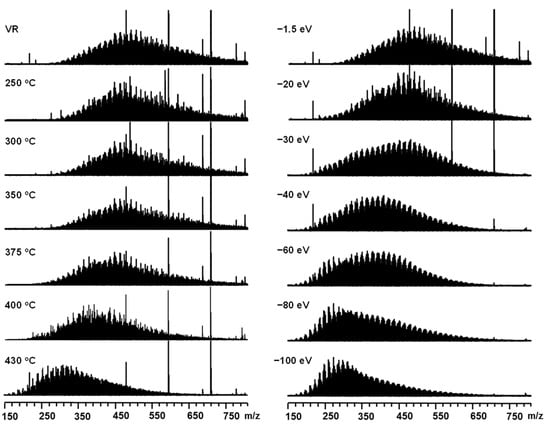
Figure 1.
Broadband +ESI FT-ICR mass spectra of Venezuela VR thermal reaction products under different temperatures (left). Broadband +ESI FT-ICR mass spectra of Venezuela VR under different collision voltages (right).
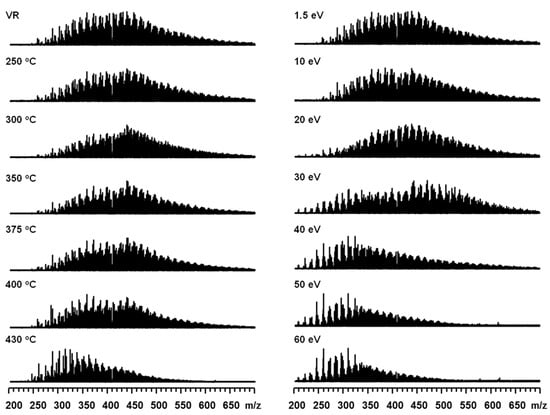
Figure 2.
Broadband −ESI FT-ICR mass spectra of Venezuela VR thermal reaction products under different temperatures (left). Broadband −ESI FT-ICR mass spectra of Venezuela VR under different collision voltages (right).
Figure 1 (left) shows the positive-ion ESI FT-ICR mass spectra of the thermal reaction products under different reaction temperatures. A threshold temperature (around 350 °C) for the thermal reaction process was observed, before which the molecular weight distribution remained unchanged. When the reaction temperature was higher than 350 °C, the molecular weight distribution began to shift to a lower value range. Figure 1 (right) shows the positive-ion ESI CID FT-ICR mass spectra of Venezuela VR at different collision voltages (CV). With the increasing CV, large ions produced small ions via cleavage reactions, resulting in a molecular weight reduction. From the perspective of the mass distribution range and peak shape, the overall distribution of the CID reaction products and thermal reaction products were very similar. The visbreaking product molecular weight distribution, which reached 400 °C and 430 °C, was quite similar to that of the CID product at −40 eV and −100 eV, respectively. This indicates that the thermal visbreaking and CID fragmentation degrees of basic species in Venezuela VR were similar at these conditions. For a clearer observation, overlapped mass spectrum and expanded mass spectrum at the two processes are listed in the supporting information (Figures S1 and S2). Negative-ion ESI FT-ICR MS revealed the molecular composition of non-basic nitrogen and acidic compounds [19,49,50,51] in Venezuelan VR. The visbreaking and CID fragmentation results are shown in Figure 2. Similar to the behavior of the basic compounds, the mass center shifted to the left when increasing the reaction temperature and CV. The mass shift pattern of visbreaking products was almost the same. In the CID process, the negative ions were likely to be less stable than the positive ion. Complete fragmentation was observed at 60 eV for the negative ions.
3.2. Dealkylation
The ultra-high resolving power and mass accuracy of FT-ICR MS provided the molecular composition in terms of the chemical formula. The mass spectra in Figure 1 and Figure 2 were further processed to identify the molecular identity of all of the peaks. In the thermal reaction and CID processes, the molecule/ion side-chain breaking reaction contributed significantly to the molecular weight reduction. Many works have reported the dealkylation reactions [30,34,45,52,53] of petroleum and model compounds. The nitrogen compounds in crude oil are mainly pyrrolic and pyridinic compounds; among them, the nitrogen atoms are located in an aromatic ring. Neither thermal visbreaking nor CID processes were capable of breaking an aromatic ring. Therefore, the carbon number change of the N1 class in ESI FT-ICR MS reveal edthe C-C bond cleavage behavior during thermal visbreaking and CID processes.
The basic polar species that contained only one nitrogen atom were most abundant in the positive-ion FT-ICR MS. The carbon number distribution of N1 class species in thermal reaction products and CID fragmentation product are shown in Figure 3. The carbon number distribution of N1 compounds showed a bell-shaped curve with a monomodal distribution for all of the results. Both the N1 class species in the thermal reaction and CID process products showed a decreasing trend in the overall carbon number. The peak in the carbon number distribution declined from ~40 to ~25. The carbon number range of CID products was somewhat narrower than that of the thermal reaction products.
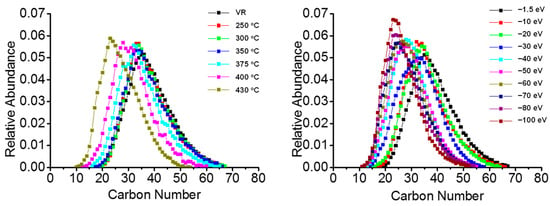
Figure 3.
Carbon number distribution of basic N1 class species in thermal visbreaking products at different temperatures (left). Carbon number distribution of N1 class species for Venezuela VR +ESI FT-ICR mass spectra under different collision voltages (right).
In order to give a clearer illustration of the carbon number change, we calculated the average carbon number for N1 class species for all visbreaking and CID conditions. The results are shown in Figure 4. When the reacting temperature was less than 350 °C, the average carbon number for N1 class species in the visbreaking product remained constant. The average carbon number began to decline drastically when the temperature was higher than 350 °C. This indicates that ~350 °C was the threshold temperature for C−C bond cleavage in the thermal process. This is consistent with the general experience in the petroleum refining industry, in which thermal cracking only occurs when the system temperature is above 350 °C [54]. Figure 4 (right) shows the average carbon number values of the N1 class species in CID products under different collision voltages. Different from the thermal visbreaking reactions, there were no threshold conditions. The average carbon number dramatically decreased when the CV value increased from 1.5 to 100 eV. It can be concluded that under the current CID operating conditions, the increase in the collision voltage immediately induced C−C bond cleavage, which was equivalent to the stage of the thermal reaction that was higher than 350 °C (The blue part in Figure 4). The average carbon number values (~30 and ~27) of the N1 class for the thermal visbreaking product at 400 and 430 °C were in agreement with that of the CID fragment ion at CV = 40 eV and 100 eV, respectively. The variation in non-basic nitrogen compound identified in the −ESI FT-ICR mass spectrum was not discussed in detail here, as their variance was analog to basic nitrogen compounds.
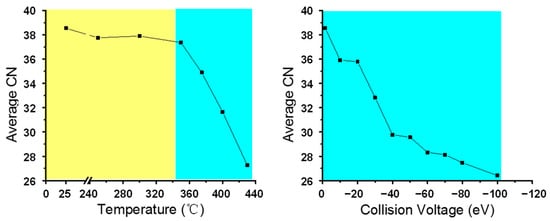
Figure 4.
Average carbon number of basic N1 class species in thermal visbreaking products at different temperatures (left). Average carbon number of N1 class species for Venezuela VR +ESI FT-ICR mass spectra under different collision voltages (right).
To reveal the detail structural change in the thermal visbreaking and CID processes, double bond equivalence (DBE) vs. carbon number plots for the N1 class are shown in Figure 5 and Figure 6. The overall trend of the two processes was similar. The carbon number was reduced with the increasing reaction temperature and CV, while the DBE value remained almost unchanged, but noticing an abnormal small-scale increase in DBE for the conditions of 400 °C, −60 eV, −80 eV, and 100 eV. We speculate that this could be because of the formation of new double bonds or rearrangement reactions during the dissociation process, and will be further explored in future work.
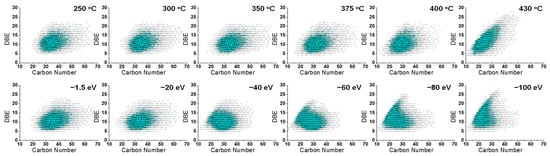
Figure 5.
DBE vs. carbon number distribution of N1 class species assigned from +ESI FT-ICR mass spectra of thermal reaction products (top) and feedstock (Venezuela VR) +ESI FT-ICR mass spectra at different collision voltages (bottom).
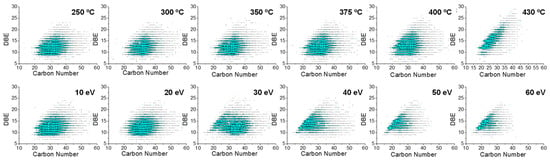
Figure 6.
DBE vs. carbon number distribution of N1 class species assigned from −ESI FT-ICR mass spectra of thermal reaction products (top) and feedstock (Venezuela VR) −ESI FT-ICR mass spectra at different collision voltages (bottom).
Among them, the DBE versus carbon number distribution of the 400 °C thermal reaction products roughly corresponded to that of the CID product at −40 eV. N1 species with a carbon number greater than 40 almost disappeared under these conditions. The carbon number distribution of the product was similar at 430 °C and −100 eV. The N1 species with a carbon number greater than 35 almost disappeared. These two sets of conditions had a similar impact on the structure of the N1 compound, which again confirmed the previous discussion regarding molecular weight.
The carbon number distribution of the N1 class compounds in the thermal cracking and CID process were statistically similar (as shown in the Supporting Information Figure S5). However, the C−C cracking path in the two processes still had some differences, which could be found from DBE versus carbon number distribution. Taking Figure 6 as an example, when the CV increased to 30 eV, the structural distribution of the N1 class ions was clearly divided into two regions: the molecular nuclear region close to the planar limit [55] and the long carbon chain region. As the CV increased (around 40 eV), the long carbon chain region gradually disappeared, producing more ions near the plane limit (at 60 eV). On the contrary, in the thermal process, the decrease in molecular carbon number was continuous, and no gapping region was observed. The basic N1 class species (in Figure 5) displayed a similar bimodal phenomenon after CID, but was less obvious. We assume the CID fragmentation had priority in breaking the weakest bond linked to aromatic rings, leaving the aromatic core attached with very short alkyl substitutes. The thermal visbreaking process did not show such a significant selectivity. The bond breaking position was not preferred during heavy petroleum thermal cracking. Moreover, bi-molecular reactions, such as hydrogen abstraction, may also have resulted in a wider product carbon number distribution. The bond cleavage that occurred in the ion during the CID process was different than that in the thermal reaction process, which completely followed a free radical reaction mechanism. Thus, we concluded that CID showed a higher bond cleavage than the thermal cracking. Both processes produced small molecules/ions. CID fragmentation technology could only qualitatively predict the carbon number reduction in the thermal visbreaking process. It should be noted that the high selectivity in the CID process potentially provided information about molecule/ion sidechain number in a petroleum molecule with fused aromatic rings. We are still working on this subject and will publish our results in the future.
3.3. Desulfurization
The bond energy of the C-S bond in petroleum molecules is usually smaller than that of the C-C bond in a similar chemical environment. Therefore, the cleavage of the C-C bond is usually accompanied with the cleavage of the C-S bond, resulting in sulfur atom removal. Because of the poor ionization efficiency of sulfur compounds, it is hard to directly study the C-S bond cleavage by the ESI ionization source. However, the cleavage information of the C-S bond can be indirectly obtained by the variation of the multi-heteroatom containing compound with both nitrogen and sulfur atoms. We chose to use high abundant N1S1 class species as a subject to study this problem.
To evaluate the desulfurization effect of the two processes, we summed up the total abundances of the N1S1 class compounds in all of the conditions. As the N1 compound is very stable, there was almost no change in abundance during the CID process, and the actual content change for other types of compounds was reflected on the N1 compound basis, so we renormalized the total abundance ratio of N1S1 to N1 as the relative abundance. The results are shown in Figure 7. The sulfur atom removal effect of the two processes can be clearly seen as the relative abundance of N1S1 was reduced with the increase in fragmentation degree. The thermal reaction behavior of N1S1 was similar to that of dealkylation. When the temperature was less than 350 °C, the relative abundance of N1S1 varied at a low degree. This decreased sharply when the temperature was higher than 350 °C. This means that 350 °C was also the threshold temperature for the N1S1 class species C-S bond cleavage. In CID, the relative abundance of N1S1 obviously decreased with the increase in CV. Again, no threshold CV value could be observed. The C-S bond and C-C bond cleavage showed a very similar behavior.
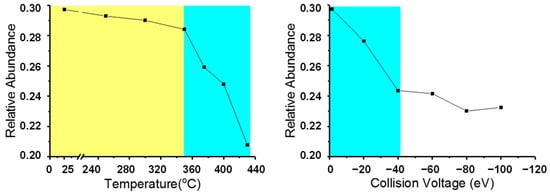
Figure 7.
Relative abundance of N1S1 class species of N1 class species assigned from +ESI FT-ICR mass spectra of thermal reaction products (left) and feedstock (Venezuela VR) +ESI FT-ICR mass spectra at different collision voltages (right).
To investigate the effects of the thermal reaction and CID on the structure of sulfur-containing compounds, the distributions of the DBE versus carbon number for the products at the highest temperature and collision energy were analyzed. Figure 8 shows the DBE versus carbon number distribution of the N1S1 class species of the Venezuela vacuum residue (left), 100 eV CID products (middle), and 430 °C thermal reaction products (right). Interestingly, the boundary slope of the CID product was greater than that of the thermal reaction product. For a given DBE value, the N1S1 class fragment ion contained a smaller carbon number in the CID process than that of the thermal reaction process. This was probably due to the rearrangement reaction during the ion dissociation. Qian reported that the alkyl chain could not be completely removed from the aromatic ring during the thermal reaction, but in CID, it was observed that the aromatics were alkylated into the C1 substituted structures [30].
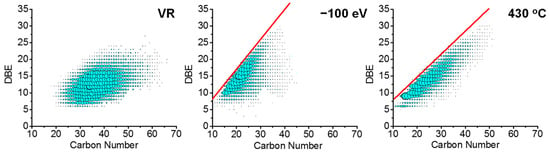
Figure 8.
DBE vs. carbon number distribution of N1S1 species in positive-ion ESI FT-ICR MS of VR (left), CID process at −100 eV (middle), and thermal reaction products at 430 °C (right).
3.4. Deoxygenation
The last functional group we considered in the present study was the carboxyl group (−COOH). The acidic oxygen compounds in crude oil are mainly carboxylic acids and phenolic compounds, among which carboxylic acid compounds are widely considered for their significant contribution to the corrosion problem. Therefore, it is of great significance to remove acid from the oil in various oil product upgrading processes. Here, the effect of acid removal in the thermal reaction and CID process was investigated by observing the change in O2 class species in −ESI FT-ICR MS.
To evaluate the de-carboxylation effect in two processes, we calculated the relative abundance of the O2 class species under different conditions and the results are shown in Figure 9. In general, both the thermal reaction and the CID process had an obvious de-carboxylation effect; the relative abundance of the O2 class species decreased with the increase in the thermal reaction temperature and CV value. At relatively high temperatures and CID collision voltages, all of the carboxylic group were completely removed and the relative abundance of the O2 class species almost reached to zero. The carboxylic group was significantly unstable compared with the C-C bond and C-S bond. During thermal visbreaking, their relative abundance decreased at a relatively low temperature (around 240 °C) and small CV, which was different from the C-C and C-S dissociation processes. Before reaching 350 °C, the changes in O2 classes were relatively moderate, and we observed that the relative abundance of the O2 class abnormally increased at 350 °C and then decreased sharply. We assumed that the deviation was caused by the air oxidation of the sample. In the CID process, the relative abundance of the O2 declined sharply throughout.
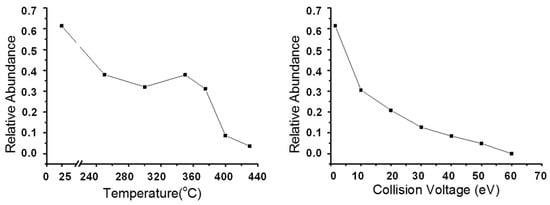
Figure 9.
Relative abundance of O2 class species assigned from +ESI FT-ICR mass spectra of thermal reaction products (left) and feedstock (Venezuela VR) +ESI FT-ICR mass spectra at different collision voltages (right).
To investigate the structural changes of O2 compounds during thermal reaction and CID processes, the DBE and carbon number distribution were analyzed (see Figure 10). Petroleum oxygen compounds with DBE = 1 were fatty acids, while those with DBE = 2~7 were naphthenic acids with 1–6 rings. The ion with DBE > 7 may be polycyclic naphthenic acids with 18–50 carbon atoms or aromatic acids and phenolic compounds with one or two hydroxyl groups. It is obvious that although the DBE versus carbon number distribution of the O2 class species did not change at 250 °C and 20 eV (Figure 10), their relative abundance decreased drastically (Figure 9). This is because carboxyl directly resulted from molecular/ion removal before the cleavage of the C-C bond.
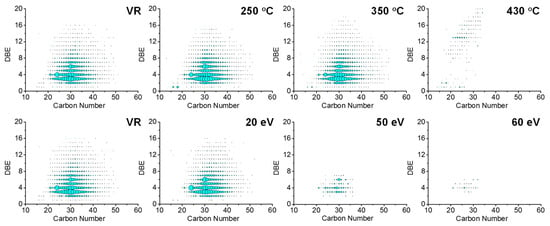
Figure 10.
DBE vs. carbon number distribution of O2 class species assigned from −ESI FT-ICR mass spectra of thermal reaction products (top) and feedstock (Venezuela VR) −ESI FT-ICR mass spectra at different collision voltages (bottom).
4. Conclusions
Through characterizing and comparing the polar species in the CID and thermal visbreaking processes, similarities and differences in the fragmentation behavior were observed. With the increase in CV and thermal reaction temperature, the molecular weight and distribution range for the carbon number were obviously decreased because of the side-chain cracking. The CID process was preferred to break the entire side chain, while the selectivity in the thermal reaction process was much lower. The sulfur atom removal was observed both in the thermal reaction and in the CID process, which was proven by the reduction in N1S1 class species relative abundance. The N1 and N1S1 compounds in the petroleum were stable, and the relative abundance began to decrease significantly when the reaction temperature reached 350 °C. The O2 class species were unstable. The carboxyl group was directly removed through molecular/ion removal before fracture of the C-C bond. After obtaining the polar species fragmentation behavior in CID and the thermal cracking of heavy oil, in the future, more effort should be made for the mechanistic study of cleavage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16083448/s1. Figure S1: Overlapped +ESI FT-ICR mass spectra of Venezuela VR thermal reaction products and CID products. Figure S2: Expanded +ESI FT-ICR mass spectra of Venezuela VR thermal reaction products and CID products. Figure S3: Overlapped −ESI FT-ICR mass spectra of Venezuela VR thermal reaction products and CID products. Figure S4: Expanded −ESI FT-ICR mass spectra of Venezuela VR thermal reaction products and CID products. Figure S5: Overlapped carbon number distribution in +ESI FT-ICR mass spectra of Venezuela VR thermal reaction products and CID products.
Author Contributions
Conceptualization, L.Z. and X.F.; methodology, X.F.; investigation, X.F.; data curation, X.F.; writing—original draft preparation, X.F., H.Y. and Y.Z.; writing—review and editing, L.Z.; visualization, X.F.; supervision, L.Z.; project administration, L.Z., H.Y., X.J. and L.W.; funding acquisition, L.Z., H.Y., and Y.Z.; H.Y. and X.F. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Speight, J.G. The Chemistry and Technology of Petroleum; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Joshi, J.B.; Pandit, A.B.; Kataria, K.L.; Kulkarni, R.P.; Sawarkar, A.N.; Tandon, D.; Ram, Y.; Kumar, M.M. Petroleum residue upgradation via visbreaking: A review. Ind. Eng. Chem. Res. 2008, 47, 8960–8988. [Google Scholar] [CrossRef]
- Marshall, A.G.; Hendrickson, C.L.; Jackson, G.S. Fourier transform ion cyclotron resonance mass spectrometry: A primer. Mass Spectrom. Rev. 1998, 17, 1–35. [Google Scholar] [CrossRef]
- Scigelova, M.; Hornshaw, M.; Giannakopulos, A.; Makarov, A. Fourier transform mass spectrometry. Mol. Cell. Proteom. 2011, 10, M111.009431. [Google Scholar] [CrossRef] [PubMed]
- McLuckey, S.A. Principles of collisional activation in analytical mass spectrometry. J. Am. Soc. Mass Spectrom. 1992, 3, 599–614. [Google Scholar] [CrossRef]
- Cody, R.; Burnier, R.; Freiser, B. Collision-induced dissociation with Fourier transform mass spectrometry. Anal. Chem. 1982, 54, 96–101. [Google Scholar] [CrossRef]
- Dongre, A.R.; Somogyi, Á.; Wysocki, V.H. Surface-induced dissociation: An effective tool to probe structure, energetics and fragmentation mechanisms of protonated peptides. J. Mass Spectrom. 1996, 31, 339–350. [Google Scholar] [CrossRef]
- Mabud, M.A.; Dekrey, M.J.; Cooks, R.G. Surface-induced dissociation of molecular ions. Int. J. Mass Spectrom. Ion Process. 1985, 67, 285–294. [Google Scholar] [CrossRef]
- Bowers, W.D.; Delbert, S.S.; Hunter, R.L.; McIver, R.T., Jr. Fragmentation of oligopeptide ions using ultraviolet laser radiation and Fourier transform mass spectrometry. J. Am. Chem. Soc. 1984, 106, 7288–7289. [Google Scholar] [CrossRef]
- Little, D.P.; Speir, J.P.; Senko, M.W.; O’Connor, P.B.; McLafferty, F.W. Infrared multiphoton dissociation of large multiply charged ions for biomolecule sequencing. Anal. Chem. 1994, 66, 2809–2815. [Google Scholar] [CrossRef]
- Dunbar, R.C. BIRD (blackbody infrared radiative dissociation): Evolution, principles, and applications. Mass Spectrom. Rev. 2004, 23, 127–158. [Google Scholar] [CrossRef]
- Price, W.D.; Schnier, P.D.; Williams, E.R. Tandem mass spectrometry of large biomolecule ions by blackbody infrared radiative dissociation. Anal. Chem. 1996, 68, 859–866. [Google Scholar] [CrossRef]
- Qi, Y.; Volmer, D.A. Electron-based fragmentation methods in mass spectrometry: An overview. Mass Spectrom. Rev. 2017, 36, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, R.A. Electron-capture dissociation tandem mass spectrometry. Curr. Opin. Biotechnol. 2004, 15, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, A.E.; Hammond, M.R.; Morrow, A.L.; Mullins, O.C.; Zare, R.N. Two-step laser mass spectrometry of asphaltenes. J. Am. Chem. Soc. 2008, 130, 7216–7217. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.; Morrow, A.L.; Pomerantz, A.E.; Zare, R.N. Evidence for island structures as the dominant architecture of asphaltenes. Energy Fuels 2011, 25, 1597–1604. [Google Scholar] [CrossRef]
- Sabbah, H.; Morrow, A.L.; Pomerantz, A.E.; Mullins, O.C.; Tan, X.; Gray, M.R.; Azyat, K.; Tykwinski, R.R.; Zare, R.N. Comparing laser desorption/laser ionization mass spectra of asphaltenes and model compounds. Energy Fuels 2010, 24, 3589–3594. [Google Scholar] [CrossRef]
- Borton, D.; Pinkston, D.S.; Hurt, M.R.; Tan, X.; Azyat, K.; Scherer, A.; Tykwinski, R.; Gray, M.; Qian, K.; Kenttämaa, H.I. Molecular structures of asphaltenes based on the dissociation reactions of their ions in mass spectrometry. Energy Fuels 2010, 24, 5548–5559. [Google Scholar] [CrossRef]
- Qian, K.; Robbins, W.K.; Hughey, C.A.; Cooper, H.J.; Rodgers, R.P.; Marshall, A.G. Resolution and Identification of Elemental Compositions for More than 3000 Crude Acids in Heavy Petroleum by Negative-Ion Microelectrospray High-Field Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2001, 15, 1505–1511. [Google Scholar] [CrossRef]
- Qian, K.; Rodgers, R.P.; Hendrickson, C.L.; Emmett, M.R.; Marshall, A.G. Reading chemical fine print: Resolution and identification of 3000 nitrogen-containing aromatic compounds from a single electrospray ionization Fourier transform ion cyclotron resonance mass spectrum of heavy petroleum crude oil. Energy Fuels 2001, 15, 492–498. [Google Scholar] [CrossRef]
- Kim, S.; Stanford, L.A.; Rodgers, R.P.; Marshall, A.G.; Walters, C.C.; Qian, K.; Wenger, L.M.; Mankiewicz, P. Microbial alteration of the acidic and neutral polar NSO compounds revealed by Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2005, 36, 1117–1134. [Google Scholar] [CrossRef]
- Cho, Y.; Witt, M.; Kim, Y.H.; Kim, S. Characterization of crude oils at the molecular level by use of laser desorption ionization Fourier-transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2012, 84, 8587–8594. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ahmed, A.; Kim, S. Application of Atmospheric Pressure Photo Ionization Hydrogen/Deuterium Exchange High-Resolution Mass Spectrometry for the Molecular Level Speciation of Nitrogen Compounds in Heavy Crude Oils. Anal. Chem. 2013, 85, 9758–9763. [Google Scholar] [CrossRef]
- Marshall, A.G.; Rodgers, R.P. Petroleomics: Chemistry of the underworld. Proc. Natl. Acad. Sci. USA 2008, 105, 18090–18095. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.G.; Rodgers, R.P. Petroleomics: The next grand challenge for chemical analysis. Acc. Chem. Res. 2004, 37, 53–59. [Google Scholar] [CrossRef]
- Wei, W.; Yingrong, L.; Zelong, L.; Huandi, H.; Songbai, T. Linkage of aromatic ring structures in saturates, aromatics, resins and asphaltenes fractions of vacuum residues determined by collision-induced dissociation technology. China Pet. Process. Petrochem. Technol. 2016, 18, 59–65. [Google Scholar]
- Mapolelo, M.M.; Rodgers, R.P.; Blakney, G.T.; Yen, A.T.; Asomaning, S.; Marshall, A.G. Characterization of naphthenic acids in crude oils and naphthenates by electrospray ionization FT-ICR mass spectrometry. Int. J. Mass Spectrom. 2011, 300, 149–157. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Oehlers, L.; Zhang, Y.; Najera, B. Tandem mass spectrometric characterization of commercial naphthenic acids and a Maya crude oil. Energy Fuels 2002, 16, 1178–1185. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhao, S.; Xu, C.; Chung, K.H.; Shi, Q. Characterization of heavy petroleum fraction by positive-ion electrospray ionization FT-ICR mass spectrometry and collision induced dissociation: Bond dissociation behavior and aromatic ring architecture of basic nitrogen compounds. Sci. China Chem. 2013, 56, 874–882. [Google Scholar] [CrossRef]
- Qian, K.; Edwards, K.E.; Mennito, A.S.; Freund, H.; Saeger, R.B.; Hickey, K.J.; Francisco, M.A.; Yung, C.; Chawla, B.; Wu, C. Determination of structural building blocks in heavy petroleum systems by collision-induced dissociation fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2012, 84, 4544–4551. [Google Scholar] [CrossRef]
- Strausz, O.P.; Safarik, I.; Lown, E.; Morales-Izquierdo, A. A critique of asphaltene fluorescence decay and depolarization-based claims about molecular weight and molecular architecture. Energy Fuels 2008, 22, 1156–1166. [Google Scholar] [CrossRef]
- Gray, M.R. Consistency of Asphaltene Chemical Structures with Pyrolysis and Coking Behavior. Energy Fuels 2003, 17, 1566–1569. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in Asphaltene Petroleomics. Part 1: Asphaltenes Are Composed of Abundant Island and Archipelago Structural Motifs. Energy Fuels 2017, 31, 13509–13518. [Google Scholar] [CrossRef]
- Nyadong, L.; Lai, J.; Thompsen, C.; LaFrancois, C.J.; Cai, X.-H.; Song, C.; Wang, J.; Wang, W. High-Field Orbitrap Mass Spectrometry and Tandem Mass Spectrometry for Molecular Characterization of Asphaltenes. Energy Fuel 2018, 32, 294–305. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in Asphaltene Petroleomics 2. A Selective Separation Method that Reveals Fractions Enriched in Island and Archipelago Structural Motifs by Mass Spectrometry. Energy Fuels 2018, 32, 314–328. [Google Scholar] [CrossRef]
- Rüger, C.P.; Neumann, A.; Sklorz, M.; Schwemer, T.; Zimmermann, R. Thermal Analysis Coupled to Ultrahigh Resolution Mass Spectrometry with Collision Induced Dissociation for Complex Petroleum Samples: Heavy Oil Composition and Asphaltene Precipitation Effects. Energy Fuels 2017, 31, 13144–13158. [Google Scholar] [CrossRef]
- Wittrig, A.M.; Fredriksen, T.R.; Qian, K.; Clingenpeel, A.C.; Harper, M.R. Single Dalton Collision-Induced Dissociation for Petroleum Structure Characterization. Energy Fuels 2017, 31, 13338–13344. [Google Scholar] [CrossRef]
- Liu, L.; Song, C.; Tian, S.; Zhang, Q.; Cai, X.; Liu, Y.; Liu, Z.; Wang, W. Structural characterization of sulfur-containing aromatic compounds in heavy oils by FT-ICR mass spectrometry with a narrow isolation window. Fuel 2019, 240, 40–48. [Google Scholar] [CrossRef]
- Wang, W.; Dong, M.; Song, C.; Cai, X.; Liu, Y.; Liu, Z.; Tian, S. Structural information of asphaltenes derived from petroleum vacuum residue and its hydrotreated product obtained by FT-ICR mass spectrometry with narrow ion isolation windows. Fuel 2018, 227, 111–117. [Google Scholar] [CrossRef]
- Cai, X.; Shi, R.; Wang, W.; Hou, H.; Peng, D.; Wang, N.; Deng, Z.; Liu, Z.; Zhang, Q. Molecular Structures of Refractory Sulfur Compounds in Heavy Oil Hydrodesulfurization Characterized by Collision-Induced Dissociation Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2022, 36, 1326–1337. [Google Scholar] [CrossRef]
- Liu, L.; Qundan, Z.; Huandi, H.; Songbai, T.; Wei, W. Structural Characterization of Petroleum Molecules by CID FT-ICR MS with Narrow Isolation Window. China Pet. Process. Petrochem. Technol. 2018, 20, 32–41. [Google Scholar]
- Zhao, J.; Dai, L.; Wang, W.; Liu, T.; Ren, L.; Zhang, L.; Han, W.; Li, D. Unraveling the molecular-level structures and distribution of refractory sulfur compounds during residue hydrotreating process. Fuel Process. Technol. 2021, 224, 107025. [Google Scholar] [CrossRef]
- Deng, Z.; Dai, L.; Han, W.; Cai, X.; Nie, X.; Fang, Q.; Nie, H. Towards a deep understanding of the evolution and molecular structures of refractory sulfur compounds during deep residue hydrotreating process. Fuel Process. Technol. 2022, 231, 107235. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.; Milton, J.; Yerabolu, R.; Easterling, L.; Kenttämaa, H.I. Investigation of the relative abundances of single-core and multicore compounds in asphaltenes by using high-resolution in-source collision-activated dissociation and medium-energy collision-activated dissociation mass spectrometry with statistical considerations. Fuel 2019, 246, 126–132. [Google Scholar]
- Qian, K.; Edwards, K.E.; Mennito, A.S.; Freund, H. Determination of Cores or Building Blocks and Reconstruction of Parent Molecules in Heavy Petroleums and Other Hydrocarbon Resources; U.S. Patent and Trademark Office: Washington, DC, USA, 2015.
- Laskin, J.; Byrd, M.; Futrell, J. Internal energy distributions resulting from sustained off-resonance excitation in FTMS. I. Fragmentation of the bromobenzene radical cation. Int. J. Mass Spectrom. 2000, 195, 285–302. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, C.; Zhao, S.; Chung, K.H.; Zhang, Y.; Gao, W. Characterization of basic nitrogen species in coker gas oils by positive-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2009, 24, 563–569. [Google Scholar] [CrossRef]
- Hughey, C.A.; Hendrickson, C.L.; Rodgers, R.P.; Marshall, A.G.; Qian, K. Kendrick mass defect spectrum: A compact visual analysis for ultrahigh-resolution broadband mass spectra. Anal. Chem. 2001, 73, 4676–4681. [Google Scholar] [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G.; Qian, K.; Robbins, W.K. Identification of acidic NSO compounds in crude oils of different geochemical origins by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2002, 33, 743–759. [Google Scholar] [CrossRef]
- Shi, Q.; Hou, D.; Chung, K.H.; Xu, C.; Zhao, S.; Zhang, Y. Characterization of heteroatom compounds in a crude oil and its saturates, aromatics, resins, and asphaltenes (SARA) and non-basic nitrogen fractions analyzed by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2010, 24, 2545–2553. [Google Scholar]
- Shi, Q.; Zhao, S.; Xu, Z.; Chung, K.H.; Zhang, Y.; Xu, C. Distribution of acids and neutral nitrogen compounds in a Chinese crude oil and its fractions: Characterized by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2010, 24, 4005–4011. [Google Scholar] [CrossRef]
- Podgorski, D.C.; Corilo, Y.E.; Nyadong, L.; Lobodin, V.V.; Bythell, B.J.; Robbins, W.K.; McKenna, A.M.; Marshall, A.G.; Rodgers, R.P. Heavy petroleum composition. 5. Compositional and structural continuum of petroleum revealed. Energy Fuels 2013, 27, 1268–1276. [Google Scholar] [CrossRef]
- Ruddy, B.M.; Huettel, M.; Kostka, J.E.; Lobodin, V.V.; Bythell, B.J.; McKenna, A.M.; Aeppli, C.; Reddy, C.M.; Nelson, R.K.; Marshall, A.G. Targeted petroleomics: Analytical investigation of Macondo well oil oxidation products from Pensacola Beach. Energy Fuels 2014, 28, 4043–4050. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Moghaddas, J.; Aghjeh, M.R. Study on thermal cracking behavior of petroleum residue. Fuel 2008, 87, 1623–1627. [Google Scholar] [CrossRef]
- Hsu, C.S.; Lobodin, V.V.; Rodgers, R.P.; McKenna, A.M.; Marshall, A.G. Compositional boundaries for fossil hydrocarbons. Energy Fuels 2011, 25, 2174–2178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).