Using Algae for Biofuel Production: A Review
Abstract
1. Introduction
2. Methodology
3. The Problem of Eutrophication in the Aquatic Environment
4. Algae in Eutrophicated Reservoirs
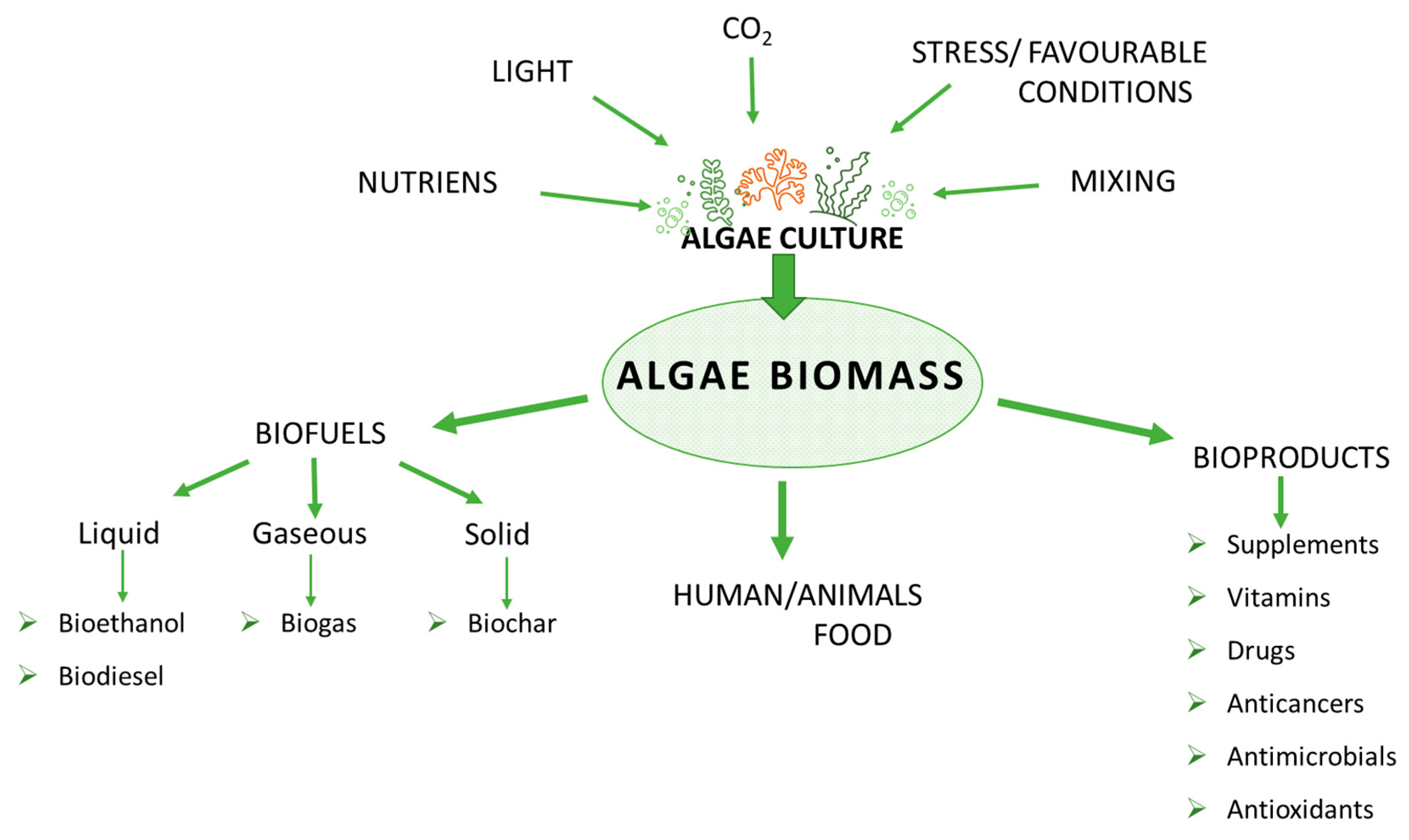
5. Algae Cultivation
6. Pretreatment of Algae Biomass
7. Biofuels
8. Algae-Derived Biofuels
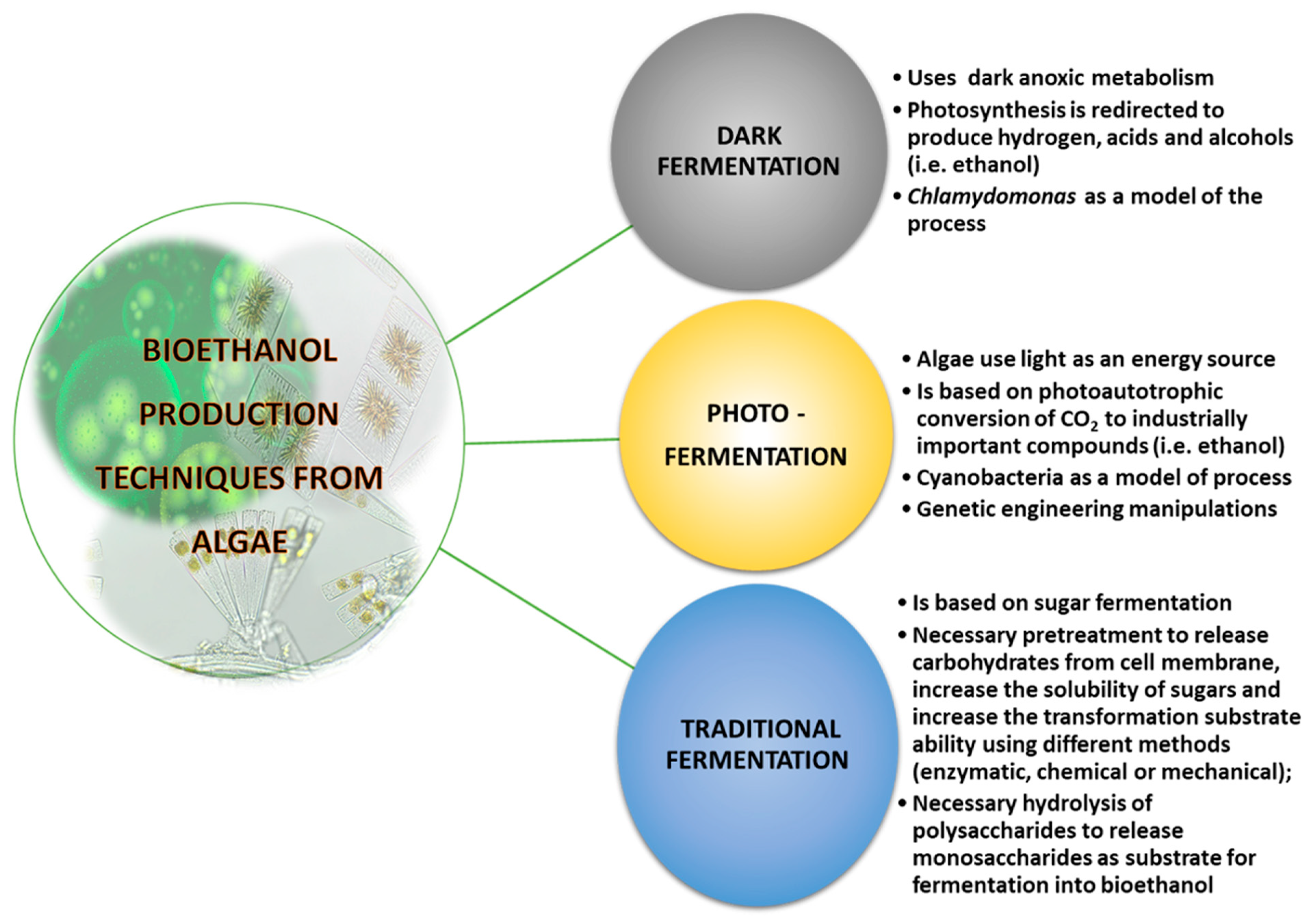
8.1. Bioethanol
8.2. Biogas
8.3. Biodiesel
| Microalgae Species | Biodiesel 1 Content (Productivity) | Comments | References |
|---|---|---|---|
| Chlorella vulgaris | 46% dw 2 | growth under nitrogen limitation | [153] |
| Chlorella vulgaris | 57% dw | growth under nitrogen limitation | [152] |
| Auxenochlorella protothecoides | 1.8–30.9% | Coculturing with E. coli | [156] |
| Tetraselmis striata | 18–23% dw | Coculturing with P. bermudensis | [157] |
| Chlorella sp. MTF-7 | Up to 39.3% dw | harvest with ferrofluids | [160] |
| Graesiella emersonii | 3.18 mg/L/d | cultivation with vermicompost extract | [161] |
| Scenedesmus quadricauda | 0.3 g/L | cultivation in the presence of sewage sludge, glucose or flue gases | [151] |
9. Conclusions and Future Research Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nymark, M.; Sharma, A.K.; Sparstad, T.; Bones, A.M.; Winge, P. A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci. Rep. 2016, 6, 24951. [Google Scholar] [CrossRef]
- Hopes, A.; Nekrasov, V.; Kamoun, S.; Mock, T. Editing of the urease gene by CRISPR-Cas in the diatom Thalassiosira pseudonana. Plant Methods 2016, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Brodie, J.; Ball, S.G.; Bouget, F.Y.; Chan, C.X.; De Clerck, O.; Cock, J.M.; Gachon, C.; Grossman, A.R.; Mock, T.; Raven, J.A.; et al. Biotic interactions as drivers of algal origin and evolution. New Phytol. 2017, 216, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Price, D.C.; Chan, C.X.; Yoon, H.S.; Yang, E.C.; Qiu, H.; Weber, A.P.; Schwacke, R.; Gross, J.; Blouin, N.A.; Lane, C.; et al. Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. Science 2012, 335, 843–847. [Google Scholar] [CrossRef]
- Cenci, U.; Bhattacharya, D.; Weber, A.P.; Colleoni, C.; Subtil, A.; Ball, S.G. Biotic host–pathogen interactions as major drivers of plastid endosymbiosis. Trends Plant Sci. 2017, 22, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, V.; Barsanti, L.; Frassanito, A.M.; Passarelli, V.; Gualieri, P. Algal toxins: Nature, occurrence, effect and detection. In Proceedings of the NATO Advanced Study Institute on Sensor Systems for Biological Threats; The Algal Toxins Case: Pisa, Italy, 2008; ISBN 978-1-4020-8479-9. [Google Scholar]
- Graham, L.E.; Graham, J.E.; Wilcox, L.W. Algae, 2nd ed.; Benjamin-Cummings Publishing: Menlo Park, CA, USA, 2008; ISBN 0321559657. [Google Scholar]
- Zhang, J.; Chen, W.-T.; Zhang, P.; Luo, Z.; Zhang, Y. Hydrothermal Liquefaction of Chlorella Pyrenoidosa in Sub- and Supercritical Ethanol with Heterogeneous Catalysts. Bioresour. Technol. 2013, 133, 389–397. [Google Scholar] [CrossRef]
- Almomani, F.; Hosseinzadeh-Bandbafha, H.; Aghbashlo, M.; Omar, A.; Joo, S.-W.; Vasseghian, Y.; Karimi-Maleh, H.; Shiung Lam, S.; Tabatabaei, M.; Rezania, S. Comprehensive Insights into Conversion of Microalgae to Feed, Food, and Biofuels: Current Status and Key Challenges towards Implementation of Sustainable Biorefineries. Chem. Eng. J. 2022, 455, 140588. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Pate, R.C. Resource Requirements for the Large-Scale Production of Algal Biofuels. Biofuels 2013, 4, 409–435. [Google Scholar] [CrossRef]
- Posten, C.; Shaub, G. Microalgae and terrestrial biomass as source for fuel—A process review. J. Biotechnol. 2009, 142, 64–69. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from Algae: Challenges and Potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Shams Esfandabadi, Z.; Ranjbari, M.; Scagnelli, S. The imbalance of food and biofuel markets amid Ukraine-Russia crisis: A systems thinking perspective. Biofuel Res. J. 2022, 9, 1640–1647. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, Harmful Algae and Biodiversity—Challenging Paradigms in a World of Complex Nutrient Changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-E.; Wu, X.; Hao, H.-L.; He, Z.-L. Mechanisms and Assessment of Water Eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. Recent Advances in the Understanding and Management of Eutrophication. Limnol. Oceanogr. 2006, 51 Pt 2, 356–363. [Google Scholar] [CrossRef]
- Carpenter, S.R. Submersed Vegetation: An Internal Factor in Lake Ecosystem Succession. Am. Nat. 1981, 118, 372–383. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Kluwer: Norwell, MA, USA, 1998. [Google Scholar]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. Freshwaters: Analysis of Potential Economic Damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Engström-Öst, J.; Viitasalo, M. Turbidity Decreases Anti-Predator Behaviour in Pike Larvae, Esox Lucius. Environ. Biol. Fishes 2005, 73, 1–8. [Google Scholar] [CrossRef]
- Turner, A.M.; Chislock, M.F. Blinded by the Stink: Nutrient Enrichment Impairs the Perception of Predation Risk by Freshwater Snails. Ecol. Appl. 2010, 20, 2089–2095. [Google Scholar] [CrossRef]
- Arend, K.K.; Beletsky, D.; DePINTO, J.V.; Ludsin, S.A.; Roberts, J.J.; Rucinski, D.K.; Scavia, D.; Schwab, D.J.; Höök, T.O. Seasonal and Interannual Effects of Hypoxia on Fish Habitat Quality in Central Lake Erie: Hypoxia Effects on Fish Habitat. Freshw. Biol. 2011, 56, 366–383. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine Macroalgae in a Circular Economy Context: A Comprehensive Analysis Focused on Residual Biomass. Biotechnol. Adv. 2022, 60, 107987. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Burkholder, J.M. Harmful Algal Blooms: A Compendium Desk Reference; Burkholder, J.M., Morton, S.L., Eds.; Wiley Blackwell: Singapore, 2018. [Google Scholar]
- Glibert, P.; University of Maryland Center for Environmental Science; Burford, M. Globally Changing Nutrient Loads and Harmful Algal Blooms: Recent Advances, New Paradigms, and Continuing Challenges. Oceanography 2017, 30, 58–69. [Google Scholar] [CrossRef]
- Adams, J.M.; Gallagher, J.A.; Donnison, I.S. Fermentation Study on Saccharina Latissima for Bioethanol Production Considering Variable Pre-Treatments. J. Appl. Phycol. 2009, 21, 569–574. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Gouveia, L.; Marques, A.E.; da Silva, T.L.; Reis, A. Neochloris Oleabundans UTEX #1185: A Suitable Renewable Lipid Source for Biofuel Production. J. Ind. Microbiol. Biotechnol. 2009, 36, 821–826. [Google Scholar] [CrossRef]
- Shen, Y.; Pei, Z.; Yuan, W.; Mao, E. Effect of nitrogen and extraction method on algae lipid yield. Int. J. Agric. Biol. Eng. 2009, 2, 51–57. [Google Scholar] [CrossRef]
- Coppola, F.; Simonciniand, E.; Pulselli, R.M. Bioethanol potentials from marine residual biomass: An energy evaluation. Energy Environ. 2009, 122, 379–387. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.Y.; Gan, K.; Yang, J. Growth and nutrient removal properties of a freshwater microalga Scenedesmus sp. LX1 under different kinds of nitrogen sources. Ecol. Eng. 2010, 36, 379–381. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Sommerfeld, M.; Hu, Q. Photosynthetic Carbon Partitioning and Lipid Production in the Oleaginous Microalga Pseudochlorococcum Sp. (Chlorophyceae) under Nitrogen-Limited Conditions. Bioresour. Technol. 2011, 102, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.L.; Wolfrum, E.J. Feasibility of Spectroscopic Characterization of Algal Lipids: Chemometric Correlation of NIR and FTIR Spectra with Exogenous Lipids in Algal Biomass. Bioenergy Res. 2011, 4, 22–35. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella Strains Calorific Values When Grown in Low Nitrogen Medium. Enzym. Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, G.-C.; Zhou, B.-C. Effect of Iron on Growth and Lipid Accumulation in Chlorella Vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Q. Biodiesel Production from Heterotrophic Microalgal Oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Yusoff, F.M.; Shariff, M.; Abas, F.; Mariana, N.S. Screening of Malaysian Indigenous Microalgae for Antioxidant Properties and Nutritional Value. J. Appl. Phycol. 2007, 19, 711–718. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae—Review. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Tornabene, T.G.; Holzer, G.; Lien, S.; Burris, N. Lipid Composition of the Nitrogen Starved Green Alga Neochloris Oleoabundans. Enzym. Microb. Technol. 1983, 5, 435–440. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.; Xiang, J.; Wu, Q. High-Density Fermentation of Microalga Chlorella Protothecoides in Bioreactor for Microbio-Diesel Production. Appl. Microbiol. Biotechnol. 2008, 78, 29–36. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Rasoul-Amini, S.; Naseri, A.T.; Montazeri-Najafabady, N.; Mobasher, M.A.; Dabbagh, F. Microalgae Biofuel Potentials (Review). Prikl. Biokhim. Mikrobiol. 2012, 48, 150–168. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel Production from Oleaginous Microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Carbohydrate metabolism and respiration in algae. In Photosynthesis in Algae; Springer: Berlin/Heidelberg, Germany, 2003; pp. 205–224. [Google Scholar] [CrossRef]
- Kadouche, D.; Ducatez, M.; Cenci, U.; Tirtiaux, C.; Suzuki, E.; Nakamura, Y.; Putaux, J.-L.; Terrasson, A.D.; Diaz-Troya, S.; Florencio, F.J.; et al. Characterization of Function of the GlgA2 Glycogen/Starch Synthase in Cyanobacterium Sp. Clg1 Highlights Convergent Evolution of Glycogen Metabolism into Starch Granule Aggregation. Plant Physiol. 2016, 171, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- De Porcellinis, A.; Frigaard, N.-U.; Sakuragi, Y. Determination of the Glycogen Content in Cyanobacteria. J. Vis. Exp. 2017, 125, e56068. [Google Scholar] [CrossRef]
- Wang, H.; Ji, C.; Bi, S.; Zhou, P.; Chen, L.; Liu, T. Joint Production of Biodiesel and Bioethanol from Filamentous Oleaginous Microalgae Tribonema sp. Bioresour. Technol. 2014, 172, 169–173. [Google Scholar] [CrossRef]
- Lehr, F.; Posten, C. Closed Photo-Bioreactors as Tools for Biofuel Production. Curr. Opin. Biotechnol. 2009, 20, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Busi, M.V.; Barchiesi, J.; Martín, M.; Gomez-Casati, D.F. Starch Metabolism in Green Algae. Starke 2014, 66, 28–40. [Google Scholar] [CrossRef]
- Andersen, T.; Andersen, F.Ø. Effects of CO2 Concentration on Growth of Filamentous Algae and Littorella Uniflora in a Danish Softwater Lake. Aquat. Bot. 2006, 84, 267–271. [Google Scholar] [CrossRef]
- Hanagata, N.; Takeuchi, T.; Fukuju, Y.; Barnes, D.J.; Karube, I. Tolerance of Microalgae to High CO2 and High Temperature. Phytochemistry 1992, 31, 3345–3348. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S. Assessing the Potential of Algal Biomass Opportunities for Bioenergy Industry: A Review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Jayamuthunagai, J.; Chakravarthy, M.; Kumar, R.R.; Yogendran, D.; Praveenkumar, R. Algae: Promising Future Feedstock for Biofuels. In Algae and Environmental Sustainability; Springer: New Delhi, India, 2015; pp. 1–8. [Google Scholar]
- Dahmani, S.; Zerrouki, D.; Ramanna, L.; Rawat, I.; Bux, F. Cultivation of Chlorella Pyrenoidosa in Outdoor Open Raceway Pond Using Domestic Wastewater as Medium in Arid Desert Region. Bioresour. Technol. 2016, 219, 749–752. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rahimpour, M.; Ghasemi, Y.; Raeissi, S. The Biodiesel of Microalgae as a Solution for Diesel Demand in Iran. Energies 2018, 11, 950. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, A.; Malcata, F.X. Microalgal Reactors: A Review of Enclosed System Designs and Performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Michel, E.; Fabrice, F. Photosynthesis of Scenedesmus obliquus in outdoor open thin-layer cascade system in high and low CO2 in Belgium. J. Biotechnol. 2015, 215, 2–12. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as Green Energy Reserve: Technological Outlook on Biofuel Production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef] [PubMed]
- Qiang, H.; Richmond, A. Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, algal density and rate of mixing in a flat plate photobioreactor. J. Appl. Phycol. 1996, 8, 139–145. [Google Scholar] [CrossRef]
- Dogaris, I.; Welch, M.; Meiser, A.; Walmsley, L.; Philippidis, G. A novel horizontal photobioreactor for high-density cultivation of microalgae. Bioresour. Technol 2015, 198, 316–324. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of Suitable Photobioreactor for Algae Production—A Review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Ermis, H.; Güven-Gülhan, Ü.; Çakır, T.; Altınbaş, M. Microalgae growth and diversity in anaerobic digestate compared to synthetic media. Biofuel Res. J. 2022, 9, 1551–1561. [Google Scholar] [CrossRef]
- Lage, S.; Gojkovic, Z.; Funk, C.; Gentili, F.G. Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies 2018, 11, 664. [Google Scholar] [CrossRef]
- Zheng, S.; Zou, S.; Wang, H.; Feng, T.; Sun, S.; Chen, H.; Wang, Q. Reducing Culture Medium Nitrogen Supply Coupled with Replenishing Carbon Nutrient Simultaneously Enhances the Biomass and Lipid Production of Chlamydomonas Reinhardtii. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, J.; Tian, G. Growth Characteristics of Botryococcus Braunii 765 under High CO2 Concentration in Photobioreactor. Bioresour. Technol. 2011, 102, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Cervantes, A.; Morales, M.; Novelo, E.; Revah, S. Carbon Dioxide Fixation and Lipid Storage by Scenedesmus Obtusiusculus. Bioresour. Technol. 2013, 130, 652–658. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Craggs, R.; Farid, M.M. Biodiesel Production Potential of Wastewater Treatment High Rate Algal Pond Biomass. Bioresour. Technol. 2016, 221, 222–233. [Google Scholar] [CrossRef]
- Barua, V.B.; Munir, M. A Review on Synchronous Microalgal Lipid Enhancement and Wastewater Treatment. Energies 2021, 14, 7687. [Google Scholar] [CrossRef]
- Chávez-Fuentes, P.; Ruiz-Marin, A.; Canedo-López, Y. Biodiesel Synthesis from Chlorella Vulgaris under Effect of Nitrogen Limitation, Intensity and Quality Light: Estimation on the Based Fatty Acids Profiles. Mol. Biol. Rep. 2018, 45, 1145–1154. [Google Scholar] [CrossRef]
- Khalili, A.; Najafpour, G.D.; Amini, G.; Samkhaniyani, F. Influence of Nutrients and LED Light Intensities on Biomass Production of Microalgae Chlorella Vulgaris. Biotechnol. Bioprocess Eng. 2015, 20, 284–290. [Google Scholar] [CrossRef]
- Markou, G. Effect of Various Colors of Light-Emitting Diodes (LEDs) on the Biomass Composition of Arthrospira Platensis Cultivated in Semi-Continuous Mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef]
- McGee, D.; Archer, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Influence of Spectral Intensity and Quality of LED Lighting on Photoacclimation, Carbon Allocation and High-Value Pigments in Microalgae. Photosynth. Res. 2020, 143, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing Lutein Productivity of an Indigenous Microalga Scenedesmus Obliquus FSP-3 Using Light-Related Strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Gim, G.H.; Ryu, J.; Kim, M.J.; Kim, P.I.; Kim, S.W. Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J. Ind. Microbiol. Biotechnol. 2016, 43, 605–616. [Google Scholar] [CrossRef]
- Sousa, A.I.; Martins, I.; Lillebø, A.I.; Flindt, M.R.; Pardal, M.A. Influence of Salinity, Nutrients and Light on the Germination and Growth of Enteromorpha Sp. Spores. J. Exp. Mar. Bio. Ecol. 2007, 341, 142–150. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Effect of Nitrogen, Salt, and Iron Content in the Growth Medium and Light Intensity on Lipid Production by Microalgae Isolated from Freshwater Sources in Thailand. Bioresour. Technol. 2011, 102, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Okada, S.; Honda, M. Culture of the Hydrocarbon Producing Microalga Botryococcus Braunii Strain Showa: Optimal CO2, Salinity, Temperature, and Irradiance Conditions. Bioresour. Technol. 2013, 133, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, H.-Y.; Zhang, Y.-P. Growth and Lipid Accumulation Properties of a Freshwater Microalga Scenedesmus Sp. under Different Cultivation Temperature. Bioresour. Technol. 2011, 102, 3098–3102. [Google Scholar] [CrossRef]
- Janeeshma, E.; Johnson, R.; Amritha, M.S.; Noble, L.; Aswathi, K.P.R.; Telesiński, A.; Kalaji, H.M.; Auriga, A.; Puthur, J.T. Modulations in Chlorophyll a Fluorescence Based on Intensity and Spectral Variations of Light. Int. J. Mol. Sci. 2022, 23, 5599. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, X.; Xi, Y.; Wang, J.; Kong, F.; Zhao, Y.; Chi, Z. Progress on the development of floating photobioreactor for microalgae cultivation and its application potential. World J. Microbiol. Biotechnol. 2019, 35, 190. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Hernández-Mariné, M.; García, J.; Ferrer, I. Long-Term Anaerobic Digestion of Microalgae Grown in HRAP for Wastewater Treatment. Effect of Microwave Pretreatment. Water Res. 2014, 49, 351–359. [Google Scholar] [CrossRef]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed Electric Field Assisted Extraction of Intracellular Valuables from Microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment Technologies for an Efficient Bioethanol Production Process Based on Enzymatic Hydrolysis: A Review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Nakamura, K.; Ariga, O.; Nakasaki, K. Production of High Concentrations of Bioethanol from Seaweeds That Contain Easily Hydrolyzable Polysaccharides. Process Biochem. 2011, 46, 2111–2116. [Google Scholar] [CrossRef]
- Moore, E.K.; Hoare, M.; Dunnill, P. Disruption of Baker’s Yeast in a High-Pressure Homogenizer: New Evidence on Mechanism. Enzym. Microb. Technol. 1990, 12, 764–770. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of Several Methods for Effective Lipid Extraction from Microalgae. Bioresour. Technol. 2010, 101 (Suppl. S1), S75–S77. [Google Scholar] [CrossRef]
- Singh, S.; Meena, P.; Saharan, V.K.; Bhoi, R.; George, S. Enhanced Lipid Recovery from Chlorella Sp. Biomass by Green Approach: A Combination of Ultrasonication and Homogenization Pre-Treatment Techniques (Hybrid Method) Using Aqueous Deep Eutectic Solvents. Mater. Today 2022, 57, 179–186. [Google Scholar] [CrossRef]
- Balasubramanian, R.K.; Yen Doan, T.T.; Obbard, J.P. Factors Affecting Cellular Lipid Extraction from Marine Microalgae. Chem. Eng. J. 2013, 215–216, 929–936. [Google Scholar] [CrossRef]
- Bierau, H.; Zhang, Z.; Lyddiatt, A. Direct Process Integration of Cell Disruption and Fluidised Bed Adsorption for the Recovery of Intracellular Proteins. J. Chem. Technol. Biotechnol. 1999, 74, 208–212. [Google Scholar] [CrossRef]
- Alzate, M.E.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biochemical Methane Potential of Microalgae: Influence of Substrate to Inoculum Ratio, Biomass Concentration and Pretreatment. Bioresour. Technol. 2012, 123, 488–494. [Google Scholar] [CrossRef]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella Vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef]
- Iqbal, J.; Theegala, C. Microwave Assisted Lipid Extraction from Microalgae Using Biodiesel as Co-Solvent. Algal Res. 2013, 2, 34–42. [Google Scholar] [CrossRef]
- Lorente, E.; Farriol, X.; Salvadó, J. Steam Explosion as a Fractionation Step in Biofuel Production from Microalgae. Fuel Process. Technol. 2015, 131, 93–98. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.-P.; Del Pino, V.; Uronen, P.; Legrand, C. Combined Effects of Nitrogen Concentration and Seasonal Changes on the Production of Lipids in Nannochloropsis Oculate. Mar. Drugs 2014, 12, 1891–1910. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Saccharification of Carbohydrates in Microalgal Biomass by Physical, Chemical and Enzymatic Pre-Treatments as a Previous Step for Bioethanol Production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Mercer, P.; Armenta, R.E. Developments in Oil Extraction from Microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Hosseinzadeh-Bandbafha, H.; Shahbeik, H.; Tabatabaei, M. The role of sustainability assessment tools in realizing bioenergy and bioproduct systems. Biofuel Res. J. 2022, 9, 1697–1706. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Legacy of Fossil Fuels. Chem. Asian J. 2011, 6, 768–784. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction, 2nd ed.; Wiley-VCH Verlag: Weinheim, Germany, 2011; p. 443. [Google Scholar]
- Nehring, R. Traversing the Mountaintop: World Fossil Fuel Production to 2050. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 3067–3079. [Google Scholar] [CrossRef]
- Mol, A.P.J. Boundless Biofuels? Between Environmental Sustainability and Vulnerability. Sociol. Rural. 2007, 47, 297–315. [Google Scholar] [CrossRef]
- Gamborg, C.; Millar, K.; Shortall, O.; Sandøe, P. Bioenergy and Land Use: Framing the Ethical Debate. J. Agric. Environ. Ethics 2012, 25, 909–925. [Google Scholar] [CrossRef]
- Kraan, S. Mass-Cultivation of Carbohydrate Rich Macroalgae, a Possible Solution for Sustainable Biofuel Production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Sinitsyn, A.P.; Sinitsyna, O.A. Bioconversion of Renewable Plant Biomass. Second-Generation Biofuels: Raw Materials, Biomass Pretreatment, Enzymes, Processes, and Cost Analysis. Biochemistry 2021, 86 (Suppl. 1), S166–S195. [Google Scholar] [CrossRef]
- Islam, Z.U.; Zhisheng, Y.; Hassan, E.B.; Dongdong, C.; Hongxun, Z. Microbial Conversion of Pyrolytic Products to Biofuels: A Novel and Sustainable Approach toward Second-Generation Biofuels. J. Ind. Microbiol. Biotechnol. 2015, 42, 1557–1579. [Google Scholar] [CrossRef]
- Harfouche, A.; Grant, K.; Selig, M.; Tsai, D.; Meilan, R. Protecting Innovation: Genomics-Based Intellectual Property for the Development of Feedstock for Second-Generation Biofuels. Recent Pat. DNA Gene Seq. 2010, 4, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ying, K.; Chen, G.; Zhou, C.; Zhang, W.; Zhang, X.; Cai, Z.; Holmes, T.; Tao, Y. Growth of Chlorella Vulgaris and Nutrient Removal in the Wastewater in Response to Intermittent Carbon Dioxide. Chemosphere 2017, 186, 977–985. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second Generation Biofuels: High-Efficiency Microalgae for Biodiesel Production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Abdullah, B.; Syed Muhammad, S.A.F.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth Generation Biofuel: A Review on Risks and Mitigation Strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Khan, S.; Fu, P. Biotechnological Perspectives on Algae: A Viable Option for next Generation Biofuels. Curr. Opin. Biotechnol. 2020, 62, 146–152. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae Beats Bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef]
- Borowiak, D.; Krzywonos, M. Bioenergy, Biofuels, Lipids and Pigments—Research Trends in the Use of Microalgae Grown in Photobioreactors. Energies 2022, 15, 5357. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Silva Benavides, A.M.; Torzillo, G. Variables Governing Photosynthesis and Growth in Microalgae Mass Cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Singh, N.K.; Dhar, D.W. Microalgae as Second Generation Biofuel. A Review. Agron. Sustain. Dev. 2011, 31, 605–629. [Google Scholar] [CrossRef]
- Heredia, V.; Gonçalves, O.; Marchal, L.; Pruvost, J. Producing Energy-Rich Microalgae Biomass for Liquid Biofuels: Influence of Strain Selection and Culture Conditions. Energies 2021, 14, 1246. [Google Scholar] [CrossRef]
- Bajpai, D.; Tyagi, V.K. Biodiesel: Source, Production, Composition, Properties and Its Benefits. J. Oleo Sci. 2006, 55, 487–502. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic Phototrophs: Efficient Alternatives to Land-Based Crops for Biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Sandefur, H.N.; Matlock, M.D.; Costello, T.A. Seasonal Productivity of a Periphytic Algal Community for Biofuel Feedstock Generation and Nutrient Treatment. Ecol. Eng. 2011, 37, 1476–1480. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Bertucco, A. Bioethanol from Microalgae and Cyanobacteria: A Review and Technological Outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and Macroalgal Biomass: A Renewable Source for Bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef]
- Zhu, L.D.; Hiltunen, E.; Antila, E.; Zhong, J.J.; Yuan, Z.H.; Wang, Z.M. Microalgal Biofuels: Flexible Bioenergies for Sustainable Development. Renew. Sustain. Energy Rev. 2014, 30, 1035–1046. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Silva Benavides, A.M.; Ranglová, K.; Malapascua, J.R.; Masojídek, J.; Torzillo, G. Diurnal Changes of Photosynthesis and Growth of Arthrospira Platensis Cultured in a Thin-Layer Cascade and an Open Pond. Algal Res. 2017, 28, 48–56. [Google Scholar] [CrossRef]
- Malibari, R.; Sayegh, F.; Elazzazy, A.M.; Baeshen, M.N.; Dourou, M.; Aggelis, G. Reuse of Shrimp Farm Wastewater as Growth Medium for Marine Microalgae Isolated from Red Sea—Jeddah. J. Clean. Prod. 2018, 198, 160–169. [Google Scholar] [CrossRef]
- Velazquez-Lucio, J.; Rodríguez-Jasso, R.M.; Colla, L.M.; Sáenz-Galindo, A.; Cervantes-Cisneros, D.E.; Aguilar, C.N.; Fernandes, B.D.; Ruiz, H.A. Microalgal Biomass Pretreatment for Bioethanol Production: A Review. Biofuel Res. J. 2018, 5, 780–791. [Google Scholar] [CrossRef]
- Magneschi, L.; Catalanotti, C.; Subramanian, V.; Dubini, A.; Yang, W.; Mus, F.; Posewitz, M.C.; Seibert, M.; Perata, P.; Grossman, A.R. A Mutant in the ADH1 Gene of Chlamydomonas Reinhardtii Elicits Metabolic Restructuring during Anaerobiosis. Plant Physiol. 2012, 158, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Dexter, J.; Armshaw, P.; Sheahan, C.; Pembroke, J.T. The State of Autotrophic Ethanol Production in Cyanobacteria. J. Appl. Microbiol. 2015, 119, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Dexter, J.; Fu, P. Metabolic Engineering of Cyanobacteria for Ethanol Production. Energy Environ. Sci. 2009, 2, 857. [Google Scholar] [CrossRef]
- Deng, M.D.; Coleman, J.R. Ethanol Synthesis by Genetic Engineering in Cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Badger, P.C. Ethanol from cellulose: A general review. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 17–21. [Google Scholar]
- Algenol Biofuels. 2011. Available online: http://www.algenolbiofuels.com (accessed on 20 December 2021).
- Górka, J.; Cimochowicz-Rybicka, M. Algae biomass as a co-substrate in methane digestion of sewage sludge. Tech. Transactions. 2015, 112, 25–37. [Google Scholar]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas Production from Algal Biomass: A Review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of Microalgae to Improve Biogas Production: A Review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Wiley, P.E.; Campbell, J.E.; McKuin, B. Production of Biodiesel and Biogas from Algae: A Review of Process Train Options. Water Environ. Res. 2011, 83, 326–338. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, J.; Zhao, Q.; Laurens, L.; Jarvis, E.; Chen, S.; Frear, C. Efficient Anaerobic Digestion of Whole Microalgae and Lipid-Extracted Microalgae Residues for Methane Energy Production. Bioresour. Technol. 2014, 161, 423–430. [Google Scholar] [CrossRef]
- Demirbas, A. Potential Applications of Renewable Energy Sources, Biomass Combustion Problems in Boiler Power Systems and Combustion Related Environmental Issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Submariner-Network.eu. Available online: https://www.submariner-network.eu/images/projects/SubmarinerCompendium2013_PL_compressed.pdf (accessed on 5 December 2022).
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.-P. Impact of Microalgae Characteristics on Their Conversion to Biofuel. Part II: Focus on Biomethane Production. Biofuel. Bioprod. Biorefin. 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Veerabadhran, M.; Gnanasekaran, D.; Wei, J.; Yang, F. Anaerobic Digestion of Microalgal Biomass for Bioenergy Production, Removal of Nutrients and Microcystin: Current Status. J. Appl. Microbiol. 2021, 131, 1639–1651. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Keller, T.A.; Phan, D.; Parris, M.L.; Li, M.; Richardson, L.; Kopachevsky, A.M. Co-Digestion of Wastewater-Grown Filamentous Algae with Sewage Sludge Improves Biomethane Production and Energy Balance Compared to Thermal, Chemical, or Thermochemical Pretreatments. Front. Energy Res. 2019, 7, 47. [Google Scholar] [CrossRef]
- Cho, D.H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.H.; Oh, H.M.; Kim, H.S. Enhancing microalgal biomass productivity by engineering a microalgal-bacterial community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef]
- Liu, P.-R.; Yang, Z.-Y.; Hong, Y.; Hou, Y.-L. An in situ Method for Synthesis of Magnetic Nanomaterials and Efficient Harvesting for Oleaginous Microalgae in Algal Culture. Algal Res. 2018, 31, 173–182. [Google Scholar] [CrossRef]
- Scott, S.A.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.G. Biodiesel from Algae: Challenges and Prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef]
- Kandimalla, P.; Desi, S.; Vurimindi, H. Mixotrophic Cultivation of Microalgae Using Industrial Flue Gases for Biodiesel Production. Environ. Sci. Pollut. Res. Int. 2016, 23, 9345–9354. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Lipid Productivity, Settling Potential and Fatty Acid Profile of 11 Microalgal Species Grown under Nitrogen Replete and Limited Conditions. J. Appl. Phycol. 2012, 24, 989–1001. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Influence of Nitrogen-Limitation Regime on the Production by Chlorella Vulgaris of Lipids for Biodiesel Feedstocks. Biofuels 2010, 1, 47–58. [Google Scholar] [CrossRef]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae-Bacteria Symbiosis in Microalgal Growth and Biofuel Production: A Review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef]
- Guo, Z.; Tong, Y.W. The Interactions between Chlorella Vulgaris and Algal Symbiotic Bacteria under Photoautotrophic and Photoheterotrophic Conditions. J. Appl. Phycol. 2014, 26, 1483–1492. [Google Scholar] [CrossRef]
- Higgins, B.T.; Gennity, I.; Samra, S.; Kind, T.; Fiehn, O.; VanderGheynst, J.S. Cofactor Symbiosis for Enhanced Algal Growth, Biofuel Production, and Wastewater Treatment. Algal Res. 2016, 17, 308–315. [Google Scholar] [CrossRef]
- Park, J.; Park, B.S.; Wang, P.; Patidar, S.K.; Kim, J.H.; Kim, S.-H.; Han, M.-S. Phycospheric Native Bacteria Pelagibaca Bermudensis and Stappia Sp. Ameliorate Biomass Productivity of Tetraselmis Striata (KCTC1432BP) in Co-Cultivation System through Mutualistic Interaction. Front. Plant Sci. 2017, 8, 289. [Google Scholar] [CrossRef]
- Han, S.-F.; Jin, W.; Tu, R.; Gao, S.-H.; Zhou, X. Microalgae Harvesting by Magnetic Flocculation for Biodiesel Production: Current Status and Potential. World J. Microbiol. Biotechnol. 2020, 36, 105. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, K.; Praveenkumar, R.; Kim, B.; Lee, S.Y.; Oh, Y.-K.; Park, S.B. Tri-Functionality of Fe3O4-Embedded Carbon Microparticles in Microalgae Harvesting. Chem. Eng. J. 2015, 280, 206–214. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chiu, S.-Y.; Kao, C.-Y.; Chen, T.-Y.; Chang, Y.-B.; Chang, J.-S.; Lin, C.-S. Ferrofluid-Assisted Rapid and Directional Harvesting of Marine Microalgal Chlorella Sp. Used for Biodiesel Production. Bioresour. Technol. 2017, 244 Pt 2, 1337–1340. [Google Scholar] [CrossRef]
- Santhana Kumar, V.; Das Sarkar, S.; Das, B.K.; Sarkar, D.J.; Gogoi, P.; Maurye, P.; Mitra, T.; Talukder, A.K.; Ganguly, S.; Nag, S.K.; et al. Sustainable Biodiesel Production from Microalgae Graesiella Emersonii through Valorization of Garden Wastes-Based Vermicompost. Sci. Total Environ. 2022, 807 Pt 3, 150995. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Microalgae as Feedstock for Biodiesel Production under Ultrasound Treatment—A Review. Bioresour. Technol. 2018, 250, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Upadhyay, R.; Shankar, R.; Pandey, S.P. Performance and Emission Characteristics of Micro-Algae Biodiesel with Butanol and TiO2 Nano-Additive over Diesel Engine. Sustain. Energy Technol. Assess. 2023, 55, 102975. [Google Scholar] [CrossRef]
- Castiglia, D.; Landi, S.; Esposito, S. Advanced Applications for Protein and Compounds from Microalgae. Plants 2021, 10, 1686. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.-F.; Li, R.-Y.; Yang, W.-D.; Liu, J.-S.; Lin, C.S.K.; Balamurugan, S.; Li, H.-Y. TAG Pathway Engineering via GPAT2 Concurrently Potentiates Abiotic Stress Tolerance and Oleaginicity in Phaeodactylum Tricornutum. Biotechnol. Biofuels 2020, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Chungjatupornchai, W.; Fa-Aroonsawat, S. Enhanced Triacylglycerol Production in Oleaginous Microalga Neochloris Oleoabundans by Co-Overexpression of Lipogenic Genes: Plastidial LPAAT1 and ER-Located DGAT2. J. Biosci. Bioeng. 2021, 131, 124–130. [Google Scholar] [CrossRef]


| Algae Species | Lipids (% dw) |
|---|---|
| Scenedesmus obliquus | 11–22/35–55 |
| Scenedesmus dimorphus | 6–7/16–40 |
| Botryococcus brauni | 25–75 |
| Chlorella sp. | 28–32 |
| Chlorella vulgaris | 14–40/56 |
| Chlorella protothecoides | 23/55 |
| Chlorella emersonii | 63 |
| Chlorella minutissima | 57 |
| Chlorella sorokiana | 22 |
| Spirulina maxima | 4–9 |
| Neochloris oleoabundans | 35–65 |
| Dunaliella bioculata | 8 |
| Dunaliella primolecta | 23 |
| Dunaliella salina | 14–20 |
| Crypthecodinium cohnii | 20 |
| Cylindrotheca sp. | 16–37 |
| Isochrysis sp. | 25–33 |
| Tetraselmis sueica | 15–23 |
| Phaeodactylum tricornutum | 20–30 |
| Neochloris oleoabundans | 35–54 |
| Nitzschia sp. | 45–47 |
| Schizochytrium sp. | 50–77 |
| Factor | Cultivation Conditions | Algae Species | References |
|---|---|---|---|
| Culture medium | BG-11 and Chu 13 media with CO2 supplementation | Botryococcus braunii, Scenedesmus obtusiusculus | [67,68] |
| synthetic media with organic carbon sources | Pediastrm sp., Micractinium sp., Ankistrodesmus falcatus, Monoraphodium sp., Desmodesmus sp., Coleastrum sp. Mucidosphaerium sp. | [66,69] | |
| nitrogen-rich media or nitrogen limiting media | Chlorella vulgaris ESP-31 | [70] | |
| Lighting | 50–200 μmol·m/s | Chlorella vulgaris | [71,72] |
| red light | Arthrospira (Spirulina) platensis | [73] | |
| High- and low-intensity green LEDs | Brachiomonas submarina, Scenedesmus obliquus | [74,75,76] | |
| High-intensity blue and white LED | Rhodella sp., Stauroneis sp. Phaeothamnion sp. | [74,76] | |
| Temperature | 20 °C | Enteromorpha sp. | [77] |
| 25 ± 1 °C | Botryococcus strain SK | [78] | |
| 25 °C | B. braunii 765 | [79] | |
| 10–30 °C | Scenedesmus sp. | [80] |
| Type of Pretreatment | Technology | Comments | References |
|---|---|---|---|
| Mechanical Pretreatment (reduce cell wall particle size, prevent the cells from being contaminated, increase the cell surface area, produce more disruption efficiency) | high-pressure homogenisation | recover lipids during cell rupture | [87,88] |
| high-speed homogenisation, | simple but aggressive cell disruption technique, achieves effective results, short operating time, generate lipids and other compounds | [89,90] | |
| bead milling | good disruption efficiency, easy operating procedures, easily available equipment | [91] | |
| Physical Pretreatment (cost effectiveness, ease of commercialization, and time saving) | Ultrasound Pretreatment | efficient increase in algae biomass | [92] |
| Microwave Techniques | increase lipid efficiency and in cell disruption efficiency | [93,94] | |
| Thermal Pretreatment (high biomass yields and low energy requirements) | Steam Explosion | efficiently extract lipids | [95] |
| Autoclaving | good biomass yield | [96] | |
| Chemical Pretreatments | alkaline and acidic reagents | corrosive, toxic, produce inhibitory components | [97] |
| Enzymatic Pretreatment | cellulases and amylases | low energy requirement, effective lipid production, low investment requirements, mild operating conditions, and less energy consumption and represent the best alternative to the aggressive mechanical techniques | [97,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska-Trypuć, A.; Wołejko, E.; Ernazarovna, M.D.; Głowacka, A.; Sokołowska, G.; Wydro, U. Using Algae for Biofuel Production: A Review. Energies 2023, 16, 1758. https://doi.org/10.3390/en16041758
Jabłońska-Trypuć A, Wołejko E, Ernazarovna MD, Głowacka A, Sokołowska G, Wydro U. Using Algae for Biofuel Production: A Review. Energies. 2023; 16(4):1758. https://doi.org/10.3390/en16041758
Chicago/Turabian StyleJabłońska-Trypuć, Agata, Elżbieta Wołejko, Mahmudova Dildora Ernazarovna, Aleksandra Głowacka, Gabriela Sokołowska, and Urszula Wydro. 2023. "Using Algae for Biofuel Production: A Review" Energies 16, no. 4: 1758. https://doi.org/10.3390/en16041758
APA StyleJabłońska-Trypuć, A., Wołejko, E., Ernazarovna, M. D., Głowacka, A., Sokołowska, G., & Wydro, U. (2023). Using Algae for Biofuel Production: A Review. Energies, 16(4), 1758. https://doi.org/10.3390/en16041758











