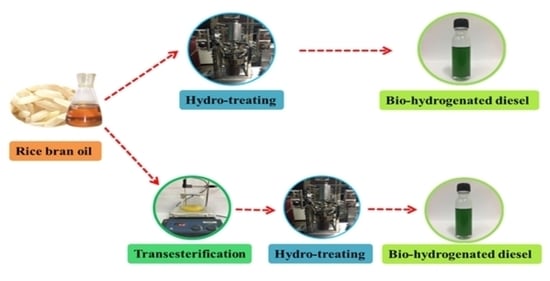
Transesterification and Hydrotreating Reactions of Rice Bran Oil for Bio-Hydrogenated Diesel Production
Abstract
1. Introduction
2. Results
2.1. Characterisation of Rice Bran Oil (RBO)
2.2. Bio-Hydrogenated (BHD) Production from RBO
2.2.1. Effect of Catalyst Loading
2.2.2. Effect of Reaction Temperature
2.2.3. Effect of Initial Hydrogen Pressure
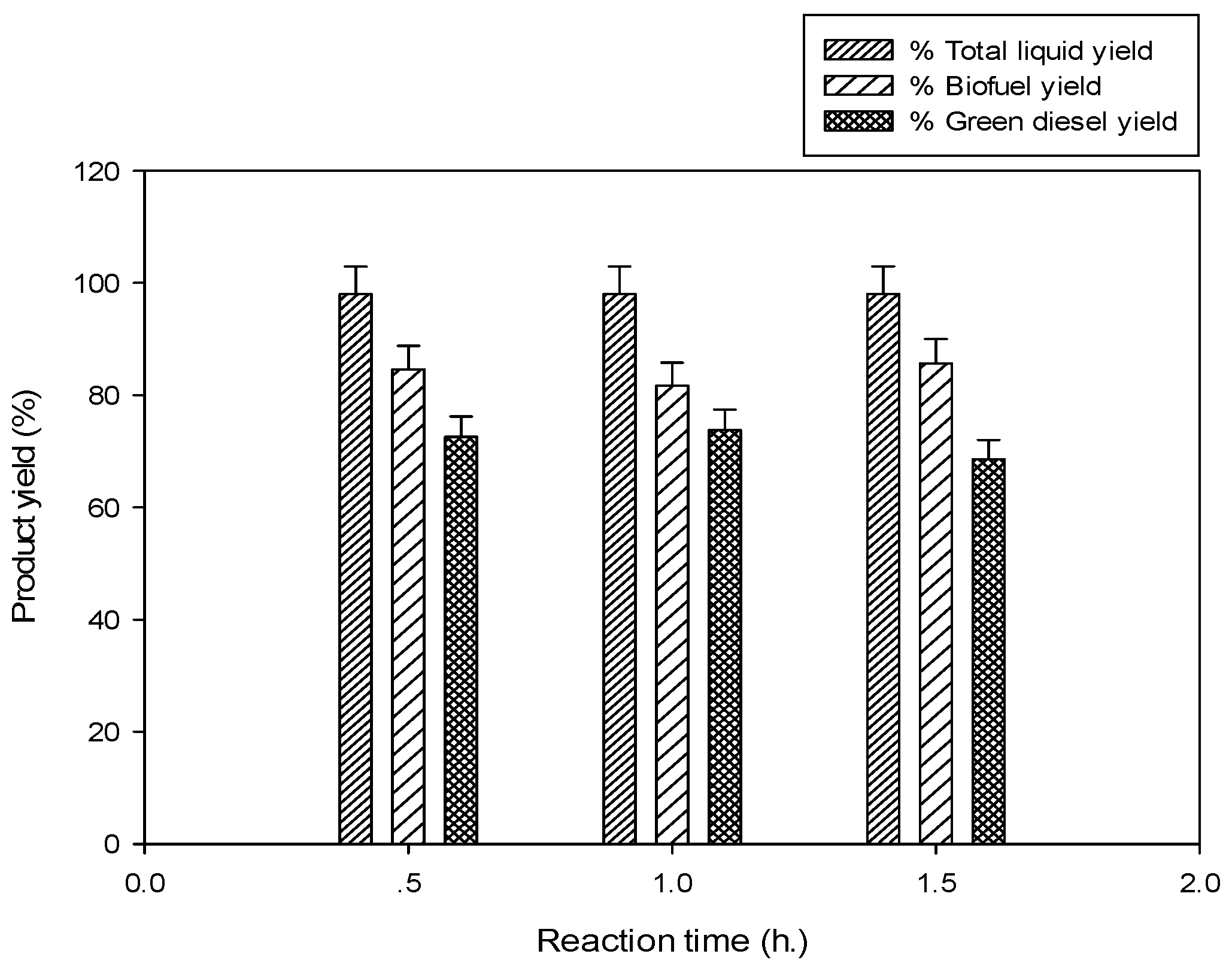
2.2.4. Effect of Reaction Time
2.3. Transesterification of RBO and Characterisation of RBME
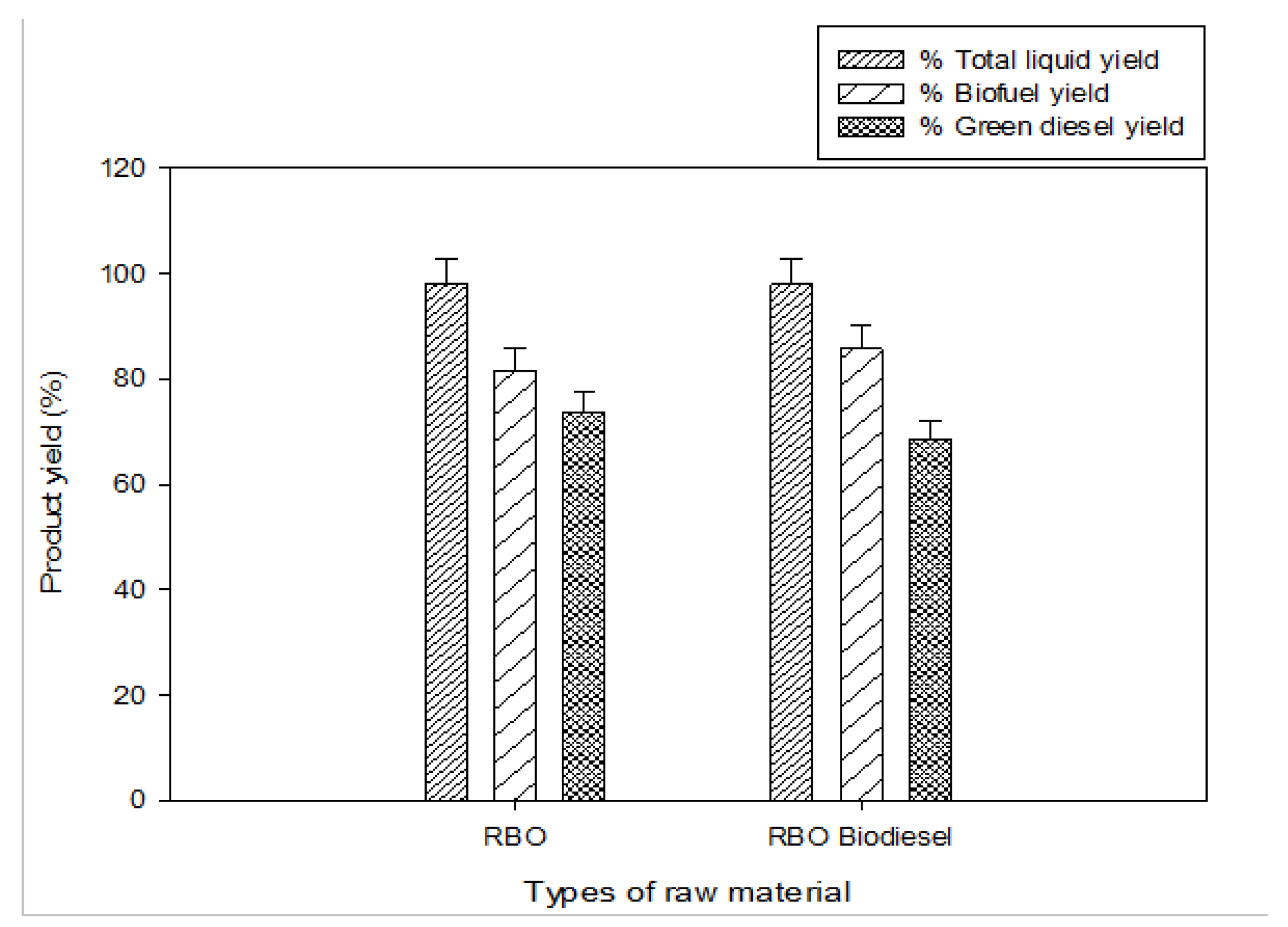
2.4. Production of Bio-Hydrogenated Diesel (BHD) from RBME
2.5. Properties of Biodiesel (RBME) and RBO/RBME Green Diesel
2.6. Potential of RBO/RBME for BHD Production with Other Feedstocks
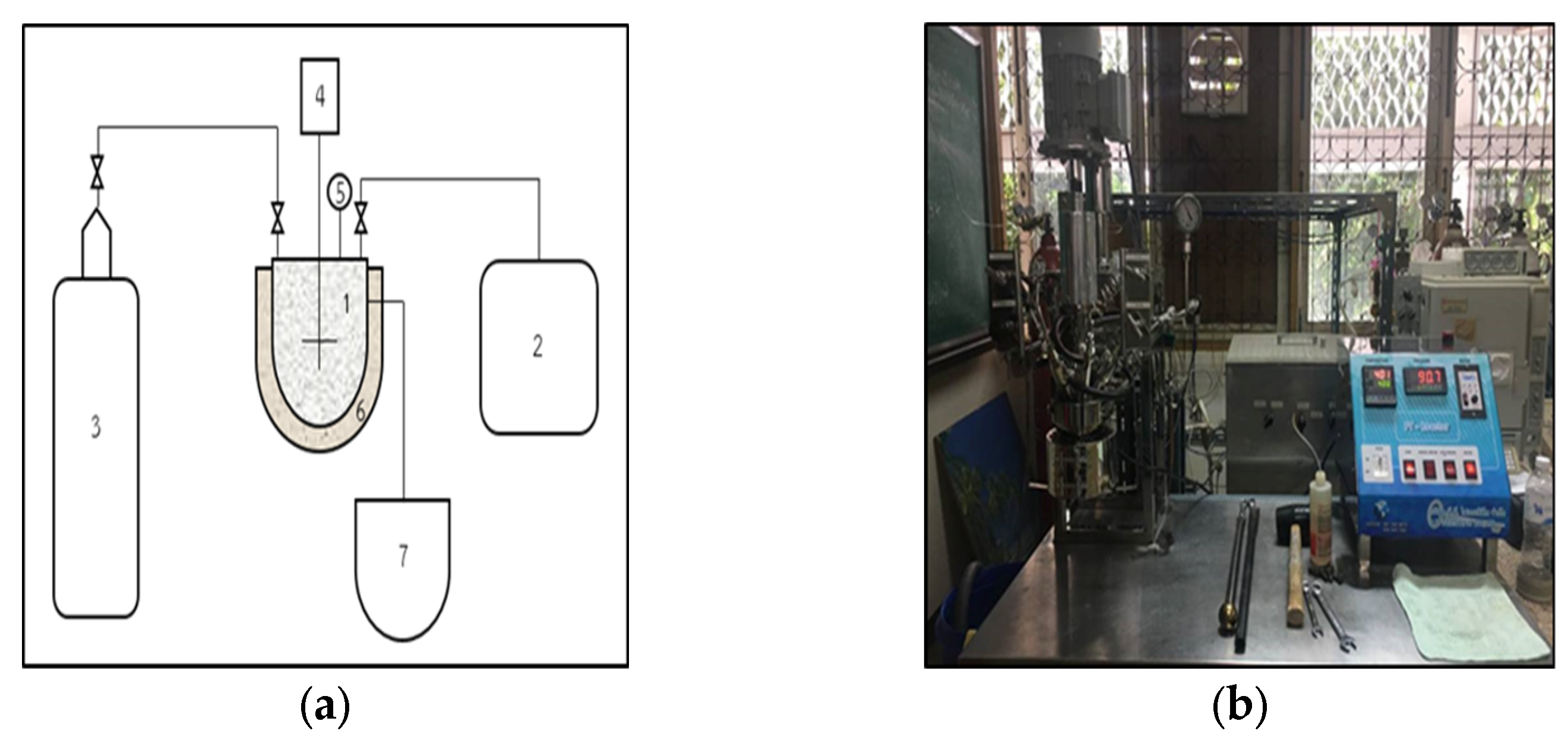
3. Materials and Methods
3.1. Raw Materials
3.2. Characterisation of RBO
3.3. Catalyst Characterisation
3.4. Bio-Hydrogenated Diesel (BHD) Production from RBO
3.4.1. Effect of Catalyst Loading
3.4.2. Effect of Reaction Temperature
3.4.3. Effect of Initial Hydrogen Pressure
3.4.4. Effect of Reaction Time
3.5. Transesterification of RB
3.6. Production of Bio-Hydrogenated Diesel from RBME
3.7. Analytical Techniques
3.7.1. Characterisation of RBO
3.7.2. Distillation following the Standard Method of ASTM D86
3.7.3. Thermogravimetric Analysis (TGA)
3.7.4. Biodiesel Yield
3.7.5. Characterisation of RBME
3.7.6. Properties of Biodiesel/Green Diesel Characterisation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Alternative Energy Development and Efficiency, Ministry of Energy, Thailand. Energy Situation of Thailand in January–December (2017); Energy Situation of Thailand; Department of Alternative Energy Development and Efficiency, Ministry of Energy: Bangkok, Thailand, 2018; pp. 1–5.
- Thai Rice Exporters Association, Thailand. Rice Exports by Destination 2018–2020. Available online: http://www.thairiceexporters.or.th (accessed on 1 January 2019).
- Wongwaiwech, D.; Weerawatanakorn, M.; Tharatha, S.; Ho, C.T. Comparative study on amount of nutraceuticals in by-products from solvent and cold pressing methods of rice bran oil processing. J. Food Drug Anal. 2019, 27, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ihoeghian, N.A.; Usman, M.A. Exergetic evaluation of biodiesel production from rice bran oil using heterogeneous catalyst. J. King Saud Univ.–Sci. 2020, 32, 101–107. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Masjuki, H.; Amini, Z.; Harrison, M.; Wang, C.; Kusumo, F.; Alwi, A. Rice bran oil based biodiesel production using calcium H.H. oxide catalyst derived from Chicoreus brunneus shell. Energy 2018, 144, 10–19. [Google Scholar] [CrossRef]
- Khatib, E.L.; Hanafi, S.A.; Barakat, A.S.; Al-Amrousi, E.F. Hydrotreating rice bran oil for biofuel production. Egypt J. Pet. 2018, 27, 1325–1331. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo-Boyás, R.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of vegetable oils to produce bio-hydrogenated diesel and liquefied petroleum gas fuel over catalysts containing sulfided Ni-Mo and solid acids. Energy Fuels 2011, 25, 4675–4685. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Morgan, T.; Lacny, J.; Mohapatra, S.; Crocker, M. Catalytic deoxygenation of triglycerides and fatty acids to hydrocarbons over carbon-supported nickel. Fuel 2013, 103, 1010–1017. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Gusmão, J.; Brodzki, D.; Djéga-Mariadassou, G.; Frety, R. Utilization of vegetable oils as an alternative source for diesel-type fuel: Hydrocracking on reduced Ni/SiO2 and sulphided Ni-Mo/γ-Al2O3. Catal. Today 1989, 5, 533–544. [Google Scholar] [CrossRef]
- Hongloi, N.; Prapainainar, P.; Seubsai, A.; Sudsakorn, K.; Prapainainar, C. Nickel catalyst with different supports for green diesel production. Energy 2019, 182, 306–320. [Google Scholar] [CrossRef]
- Boonrod, B.; Prapainainar, C.; Narataruksa, P.; Kantama, A.; Saibautrong, W.; Sudsakorn, K.; Mungcharoen, T.; Prapainainar, P. Evaluating the environmental impacts of bio-hydrogenated diesel production from palm oil and fatty acid methyl ester through life cycle assessment. J. Clean. Prod. 2017, 142, 1210–1221. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Phillips, C.B. Renewable fuels via catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2011, 397, 1–12. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. Optimization of bio-hydrogenated kerosene from refined palm oil by catalytic hydrocracking. Energies 2019, 12, 3196. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Kaewkannetra, P. Production of bio-hydrogenated kerosene by catalytic hydrocracking from refined bleached deodorised palm/palm kernel oils. Renew. Energy 2020, 147, 464–472. [Google Scholar] [CrossRef]
- El Boulifi, N.; Bouaid, A.; Martinez, M.; Aracil, J. Optimization and oxidative stability of biodiesel production from rice bran oil. Renew. Energy 2013, 53, 141–147. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energy Convers. Manag. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Fu, J.; Miao, C.; Lv, P.; Yuan, L.Z. Catalytic hydro-processing of fatty acid methyl esters to renewable alkane fuels over Ni/HZSM-5 catalyst. Catal. Today 2016, 259, 266–276. [Google Scholar] [CrossRef]
- Yang, L.; Carreon, M.A. Effect of reaction parameters on the decarboxylation of oleic acid over Pt/ZIF-67membrane/zeolite 5A bead catalysts. J. Chem. Technol. Biotechnol. 2017, 92, 52–58. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Viriya-empikul, N.; Charinpanitkul, T.; Assabumrungrat, S. Production of bio-hydrogenated diesel by catalytic hydrotreating of palm oil over NiMoS2/γ-Al2O3 catalyst. Bioresour. Technol. 2014, 158, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.J.; Vaidya, P.D. On the production of bio-hydrogenated diesel over hydrotalcite-like supported palladium and ruthenium catalysts. Fuel Process. Technol. 2018, 169, 142–149. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Phimsen, S.; Kiatkittipong, K.; Wongsakulphasatch, S.; Laosiripojana, N.; Assabumrungrat, S. Diesel-like hydrocarbon production from hydro-processing of relevant refining palm oil. Fuel Process. Technol. 2013, 116, 16–26. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Oil extracted from spent coffee grounds for bio-hydrotreated diesel production. Energy Convers. Manag. 2016, 126, 1028–1036. [Google Scholar] [CrossRef]
- Department of Energy Business, Thailand. Details Attached to the Announcement of the Department of Energy Business; Determining the Characteristics and Quality of Diesel; Department of Energy Business: Bangkok, Thailand, 2013; pp. 73–74.
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Lee, S.P.; Ramli, A. Methyl oleate deoxygenation for production of diesel fuel aliphatic hydrocarbons over Pd/SBA-15 catalysts. Chem. Cent. J. 2013, 7, 149. [Google Scholar] [CrossRef]
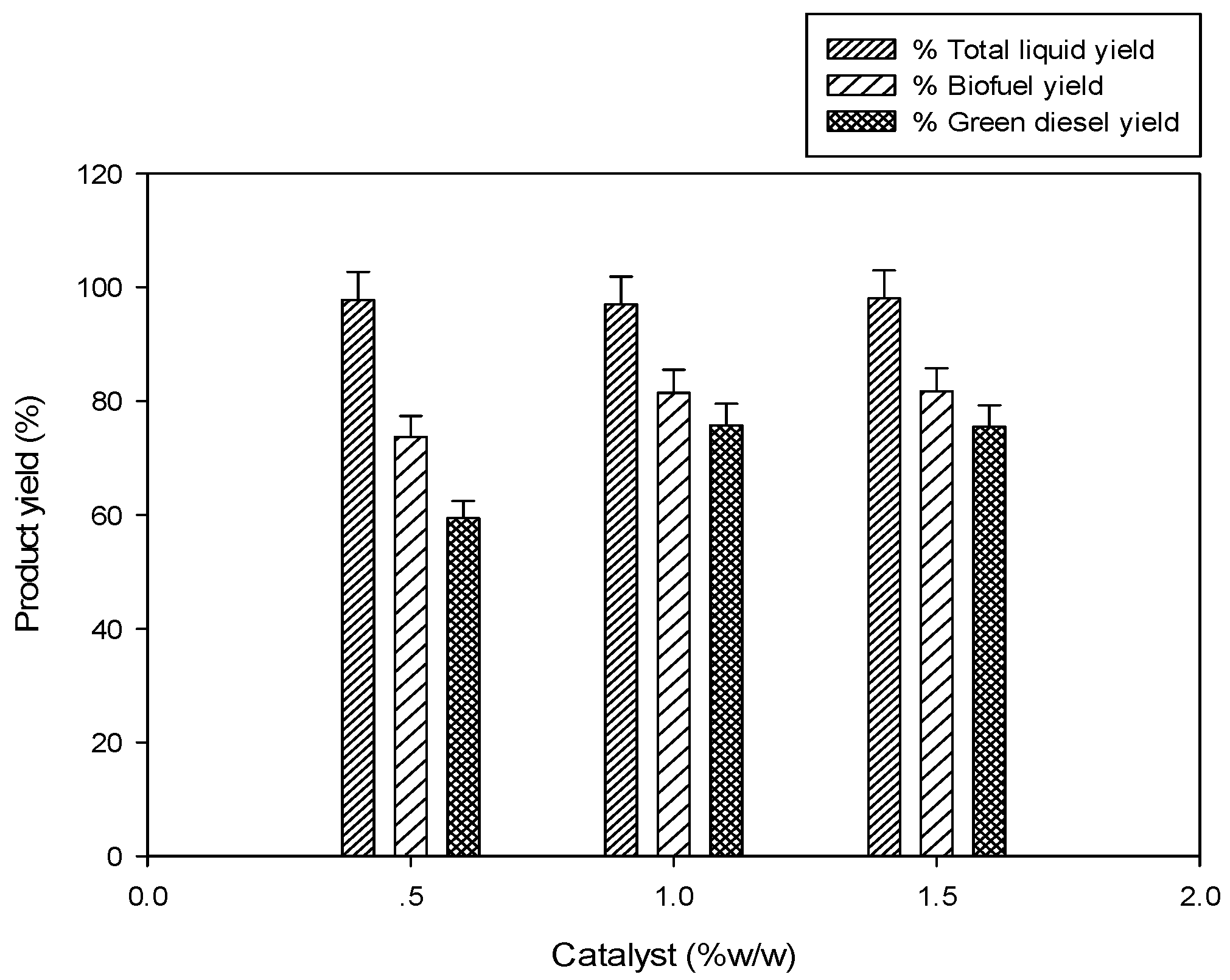
 0.5% catalyst,
0.5% catalyst,  1.0% catalyst,
1.0% catalyst,  1.5% catalyst).
1.5% catalyst).
 0.5% catalyst,
0.5% catalyst,  1.0% catalyst,
1.0% catalyst,  1.5% catalyst).
1.5% catalyst).
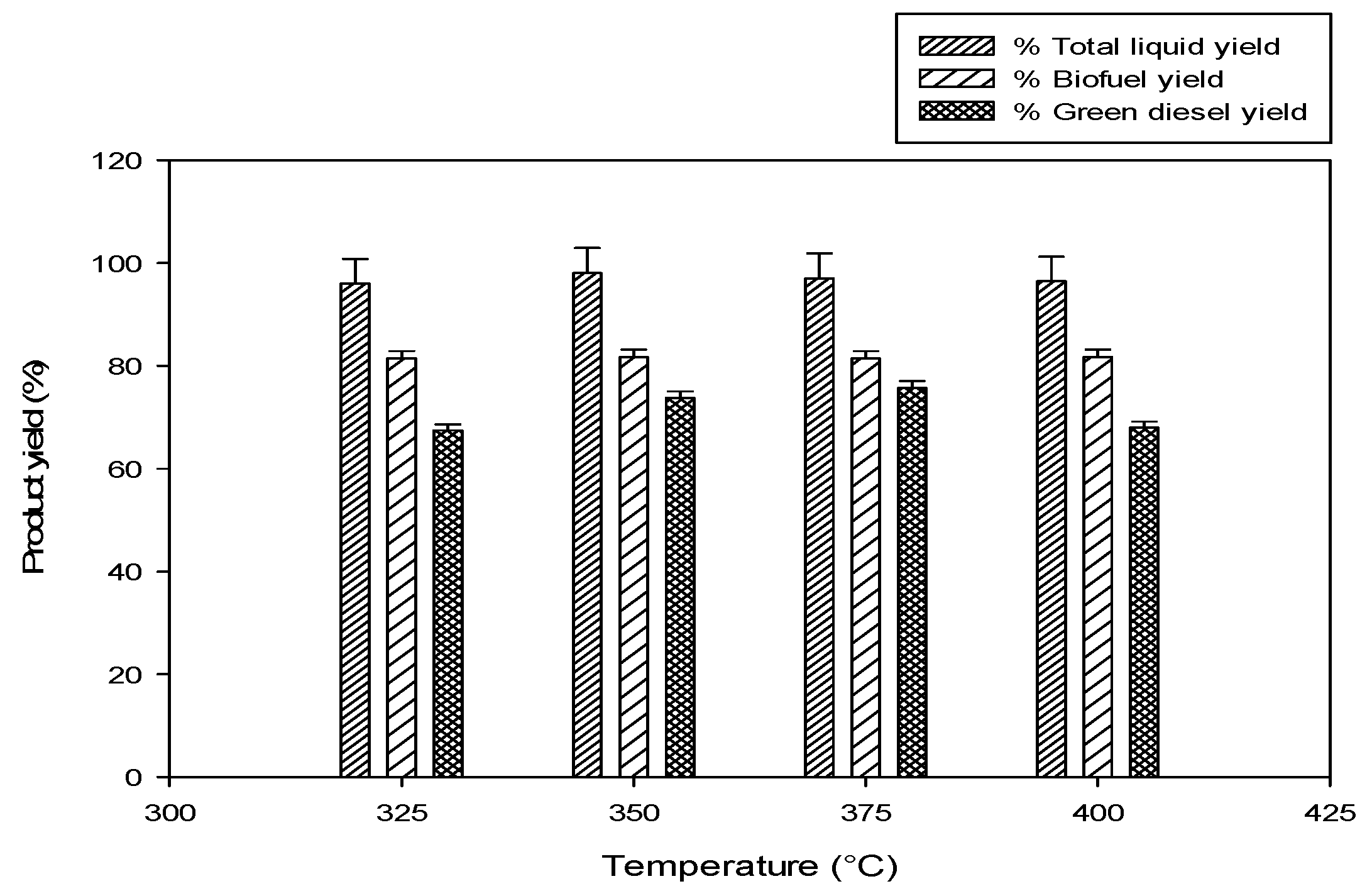
 325 °C,
325 °C,  350 °C,
350 °C,  375 °C,
375 °C,  400 °C).
400 °C).
 325 °C,
325 °C,  350 °C,
350 °C,  375 °C,
375 °C,  400 °C).
400 °C).
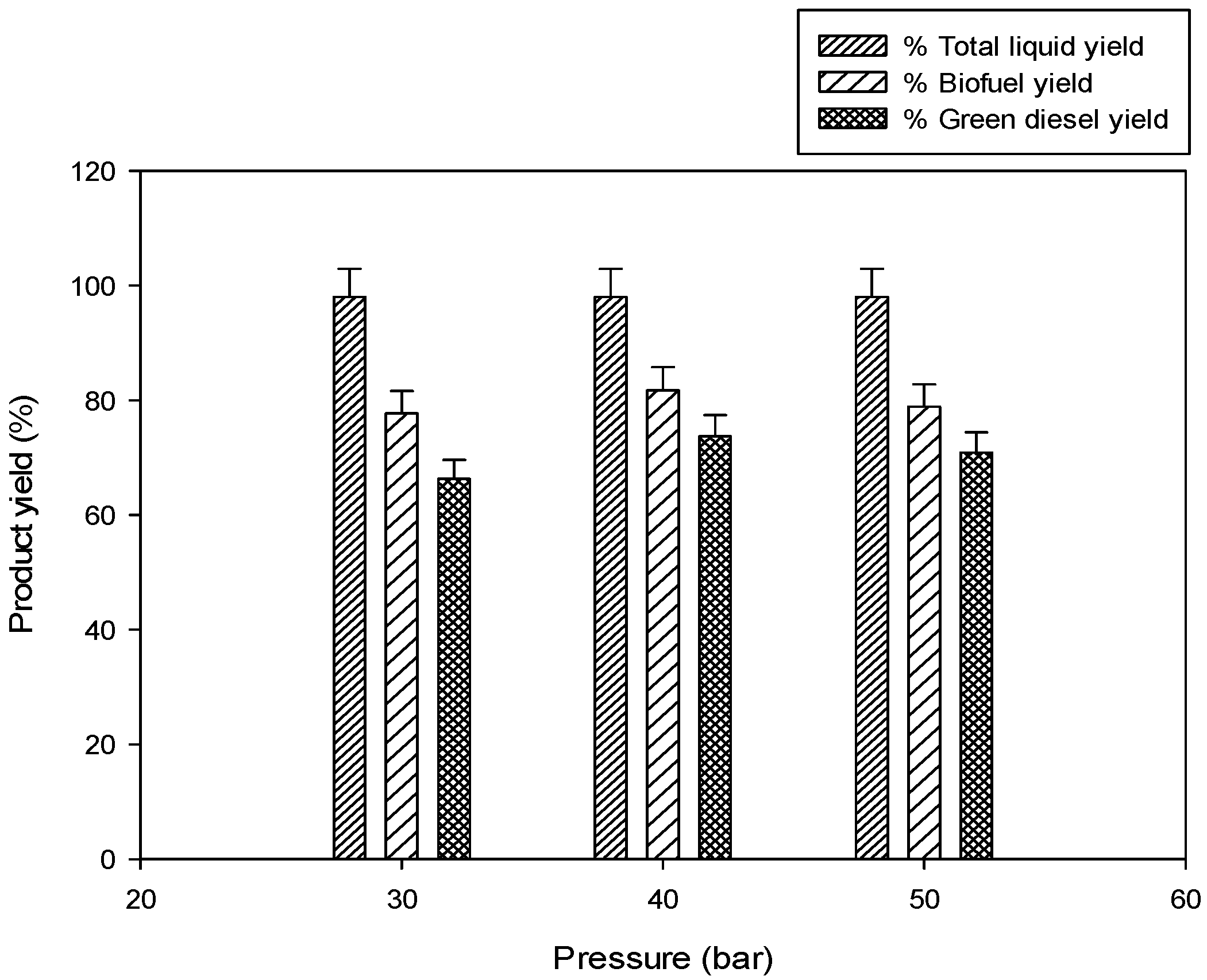
 30 bar,
30 bar, 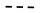 40 bar,
40 bar, 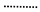 50 bar ).
50 bar ).
 30 bar,
30 bar, 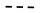 40 bar,
40 bar, 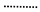 50 bar ).
50 bar ).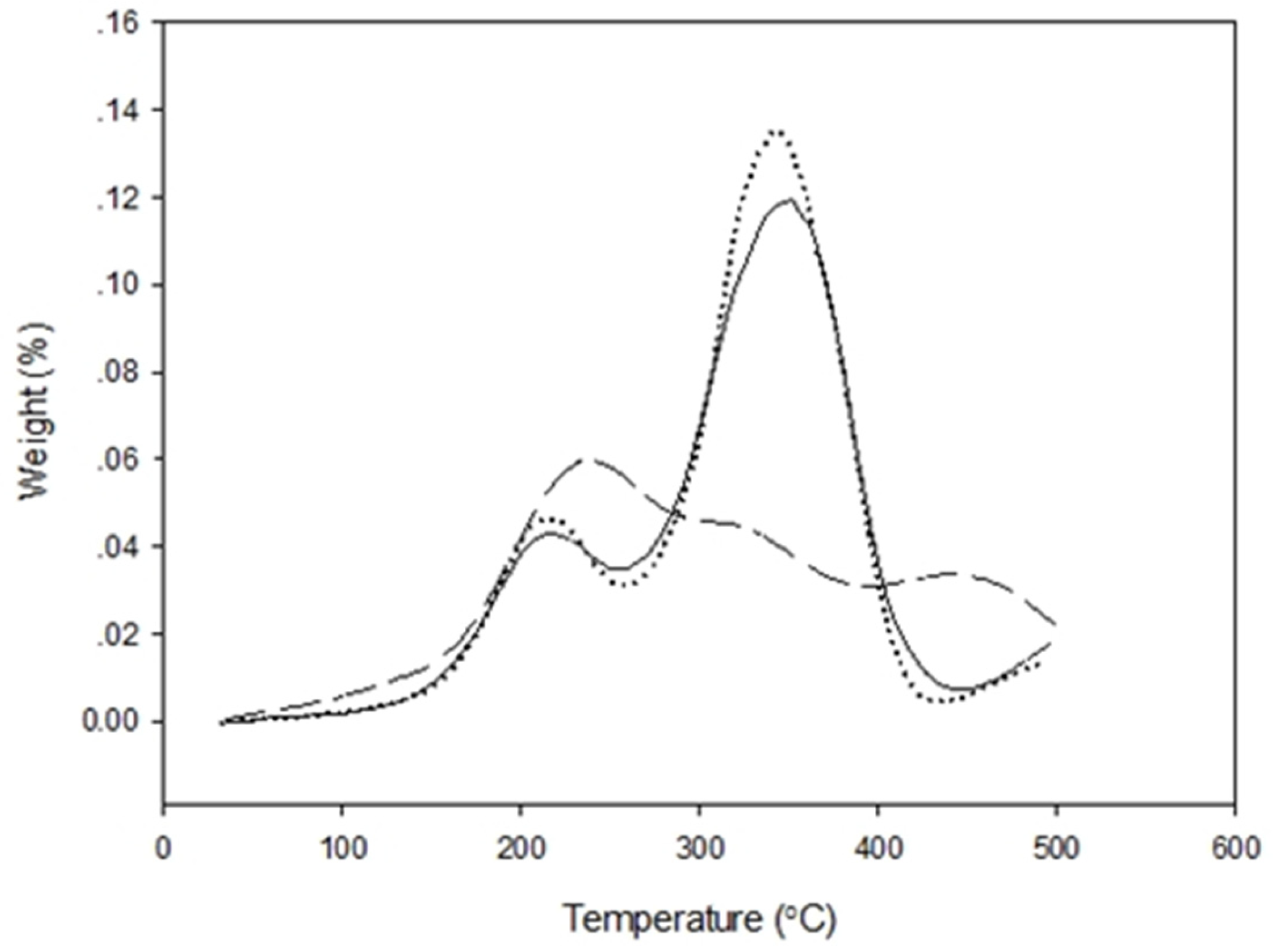
 30 min,
30 min,  60 min,
60 min,  90 min).
90 min).
 30 min,
30 min,  60 min,
60 min,  90 min).
90 min).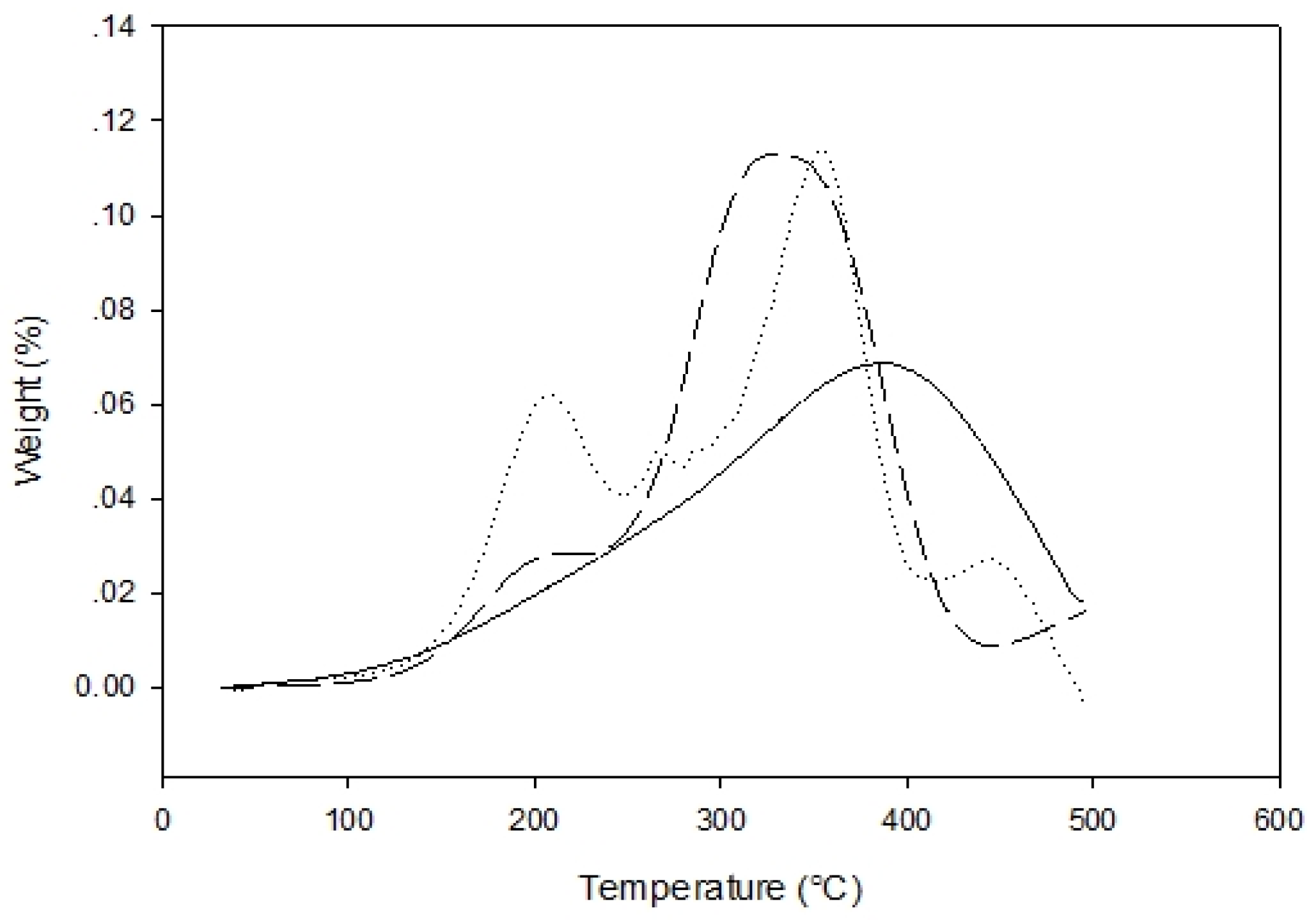

| Parameters | Methods | Value | Commercial Vegetable Oil |
|---|---|---|---|
| Acid value (mg KOH/g of oil) | AOAC 1990 | 0.4 | ≥0.6 |
| Specific gravity (ρ substance/ρ water) at 15.6 °C | ASTM D1298 | 0.9 | 0.9 |
| Viscosity (cSt: mm2/s) at 40 °C | ASTM D445 | 44.10 | 30–60 |
| Composition | Quantity (% wt) |
|---|---|
| Capric acid (C10:0) | 3.16 |
| Myristic acid (C14:0) | 8.02 |
| Palmitic acid (C16:0) | 2.95 |
| Palmitoleic acid (C16:1) | 5.37 |
| Stearic acid (C18:0) | 1.34 |
| Oleic acid (C18:1) | 56.48 |
| Linoleic acid (C18:2) | 1.14 |
| Linolenic acid (C18:3) | 0.67 |
| Arachidic acid (C20:0) | 2.45 |
| Gadoleic acid (C20:1) | 0.02 |
| Behenic acid (C22:0) | 7.01 |
| Erucic acid (C22:1) | 0.41 |
| Lignoceric acid (C24:0) | 10.98 |
| Total | 100 |
| Parameters | Methods | Diesel * | RBME | BHD from RBO | BHD from RBME | |
|---|---|---|---|---|---|---|
| High Speed | Low Speed | |||||
| Specific gravity at 15.6 °C | ASTM D1289 | 0.81–0.87 | Max. 0.92 | 0.89 | 0.95 | 0.88 |
| Viscosity (cSt) at 40 °C | ASTM D445 | 1.80–4.10 | Max. 8.0 | 5.55 | 7.53 | 4.40 |
| Distillation | ASTM D86 | 90% | - | 90% | 89.6% | 92% |
| Feedstocks | Catalysts | Conditions | %BHD Yield | References |
|---|---|---|---|---|
| Rice bran oil (RBO) | 0.3% Pd/Al2O3 | 350 °C, 40 bar H2, 1 h | 73.71 | This work |
| Soybean oil | 5% Pd/C | 350 °C, 10 bar N2, 5 h | 38 | [13] |
| Crude palm oil | 5% Pd/C | 400 °C, 40 bar H2, 3 h | 51% | [22] |
| Degummed palm oil | 5% Pd/C | 400 °C, 40 bar H2, 1 h | 70% | [22] |
| Palm fatty acid distillate | 5% Pd/C | 375 °C, 40 bar H2, 0.5 h | 81% | [22] |
| Coffee ground oil | 5% Pd/C | 400 °C, 40 bar H2, 2 h | 22.3 | [23] |
| Rice bran methyl ester (RBME) | 0.3% Pd/Al2O3 | 350 °C, 40 bar H2, 1 h | 68.51 | This work |
| Fatty acid methyl esters (FAMEs) | 10% Ni/HZSM-5 | 280 °C, 8 bar H2, 80 h, LHSV of 4 h−1 | 47.7 | [18] |
| Methyl oleate | 3.8% Pd/SBA-15 | 270 °C, 60 bar H2, 6 h | 70 | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujjanutat, P.; Srihanun, N.; Muanruksa, P.; Winterburn, J.; Kaewkannetra, P. Transesterification and Hydrotreating Reactions of Rice Bran Oil for Bio-Hydrogenated Diesel Production. Energies 2023, 16, 1347. https://doi.org/10.3390/en16031347
Dujjanutat P, Srihanun N, Muanruksa P, Winterburn J, Kaewkannetra P. Transesterification and Hydrotreating Reactions of Rice Bran Oil for Bio-Hydrogenated Diesel Production. Energies. 2023; 16(3):1347. https://doi.org/10.3390/en16031347
Chicago/Turabian StyleDujjanutat, Praepilas, Nithinun Srihanun, Papasanee Muanruksa, James Winterburn, and Pakawadee Kaewkannetra. 2023. "Transesterification and Hydrotreating Reactions of Rice Bran Oil for Bio-Hydrogenated Diesel Production" Energies 16, no. 3: 1347. https://doi.org/10.3390/en16031347
APA StyleDujjanutat, P., Srihanun, N., Muanruksa, P., Winterburn, J., & Kaewkannetra, P. (2023). Transesterification and Hydrotreating Reactions of Rice Bran Oil for Bio-Hydrogenated Diesel Production. Energies, 16(3), 1347. https://doi.org/10.3390/en16031347