Influence of Phosphorus Speciation on Its Chemical Removal from Reject Water from Dewatering of Municipal Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
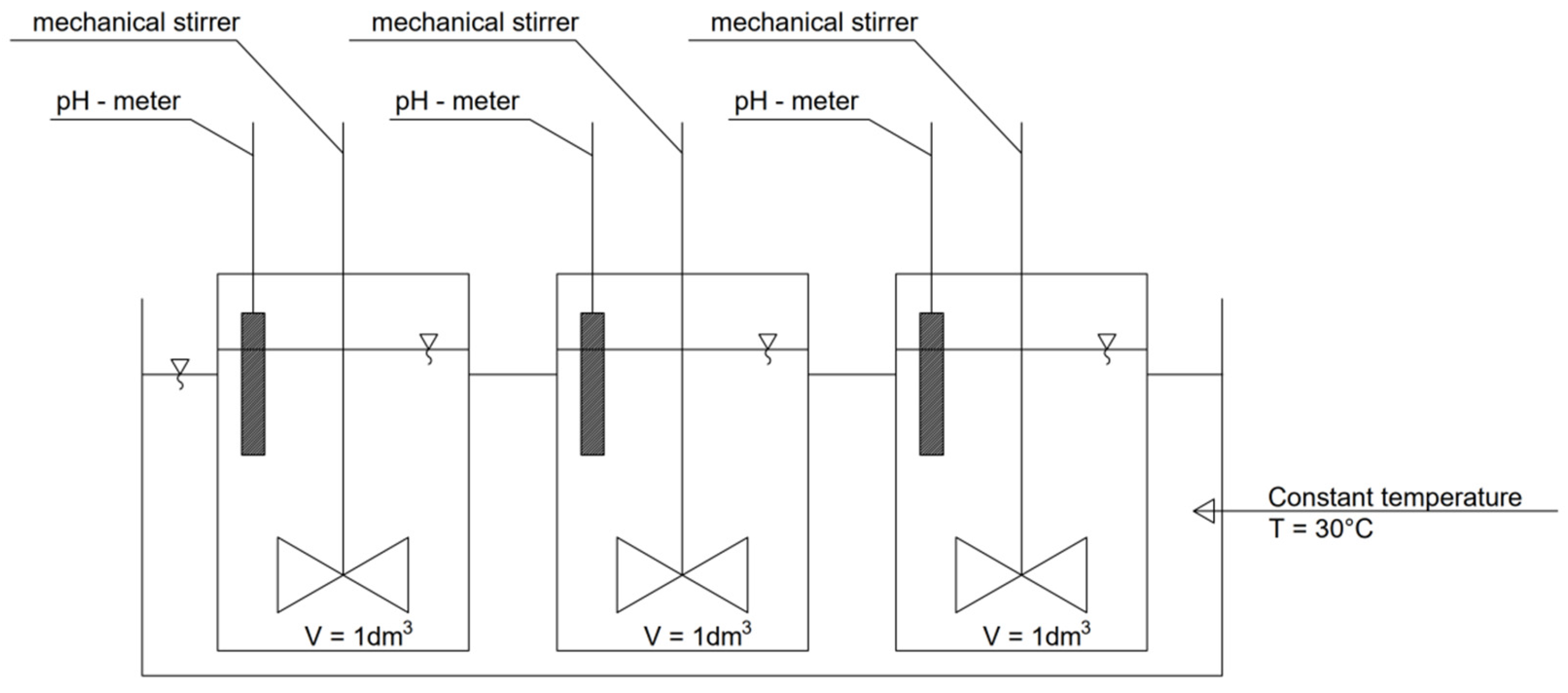
2.2. Research Methodology
2.3. Analytical Methods
2.4. Procedure for the Determination of Phosphorus Speciation
3. Results and Discussion
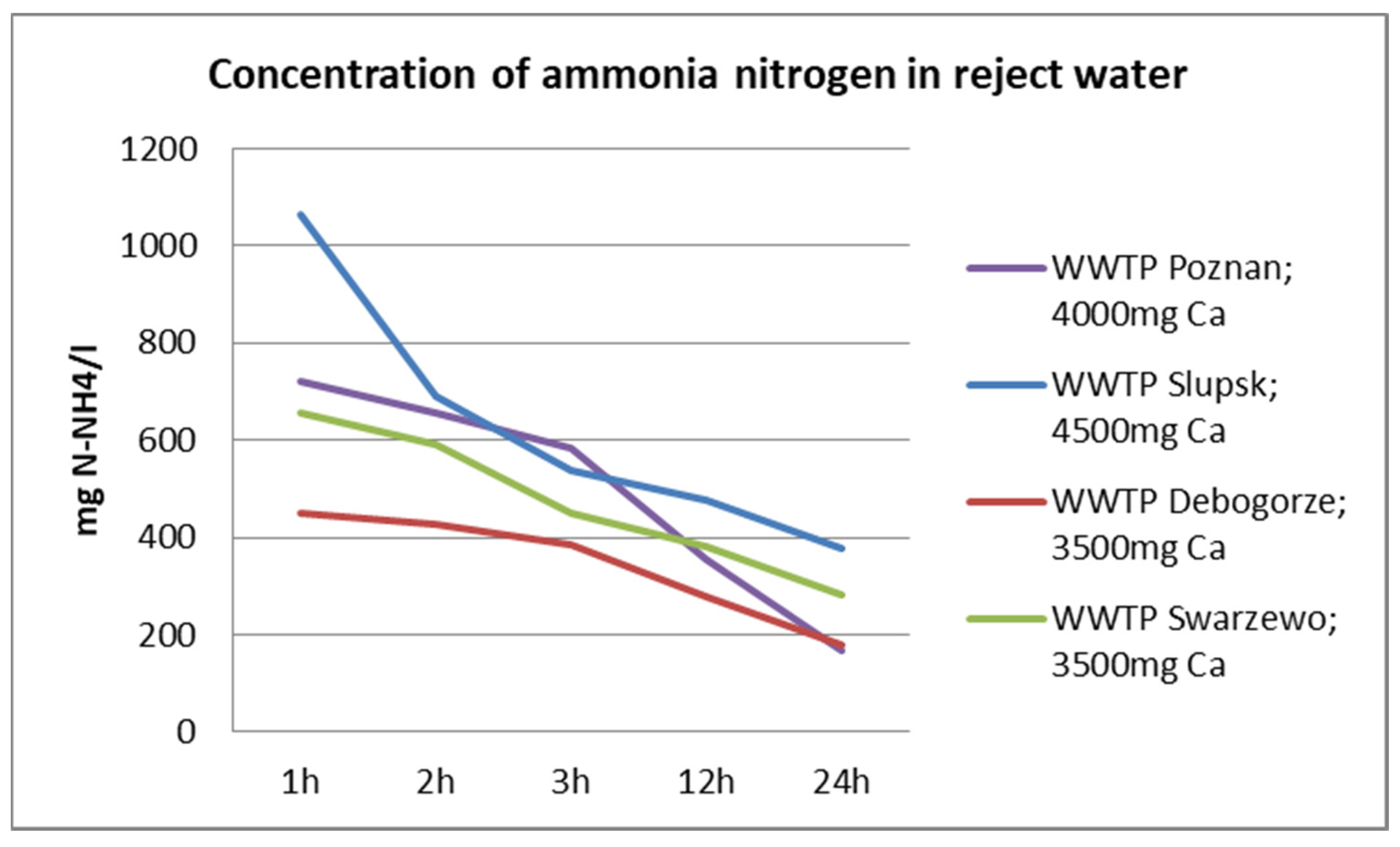
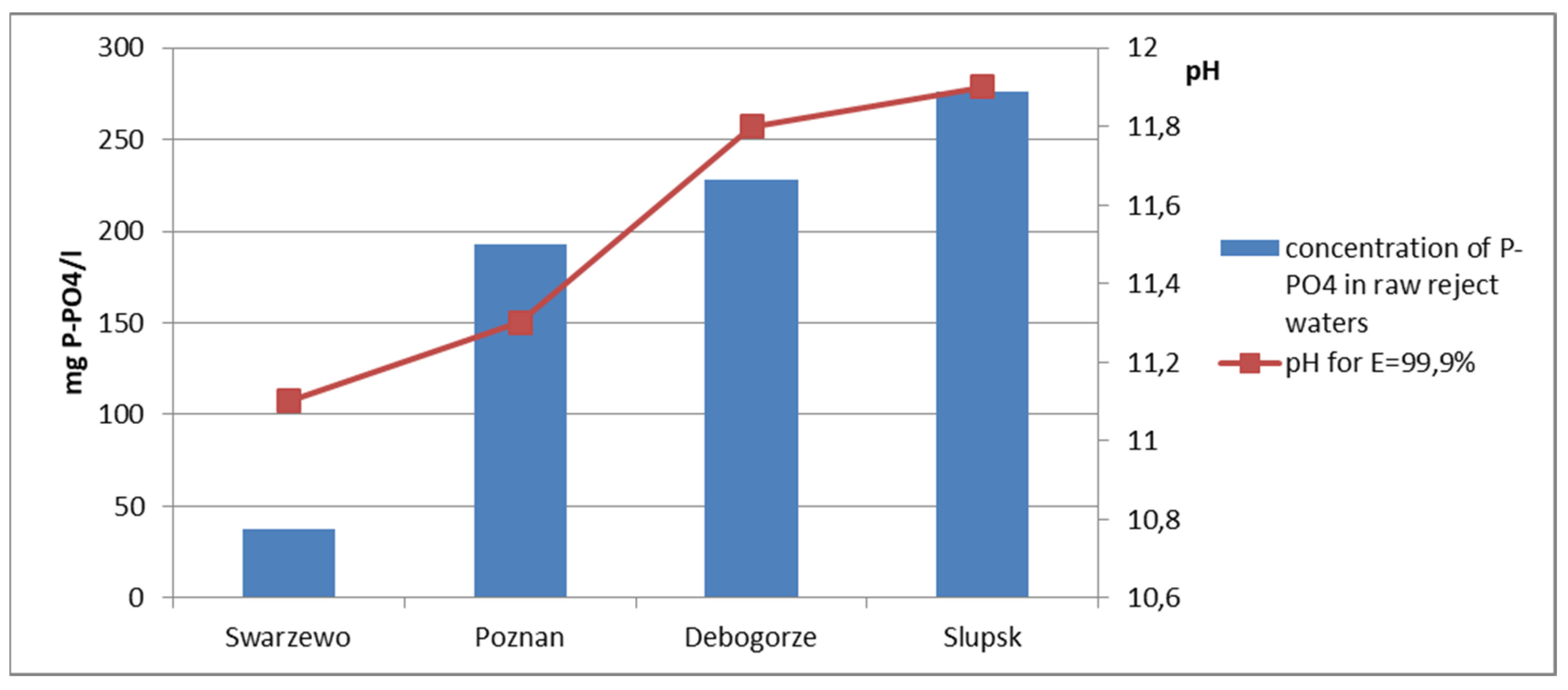
3.1. Characteristics of Reject Water
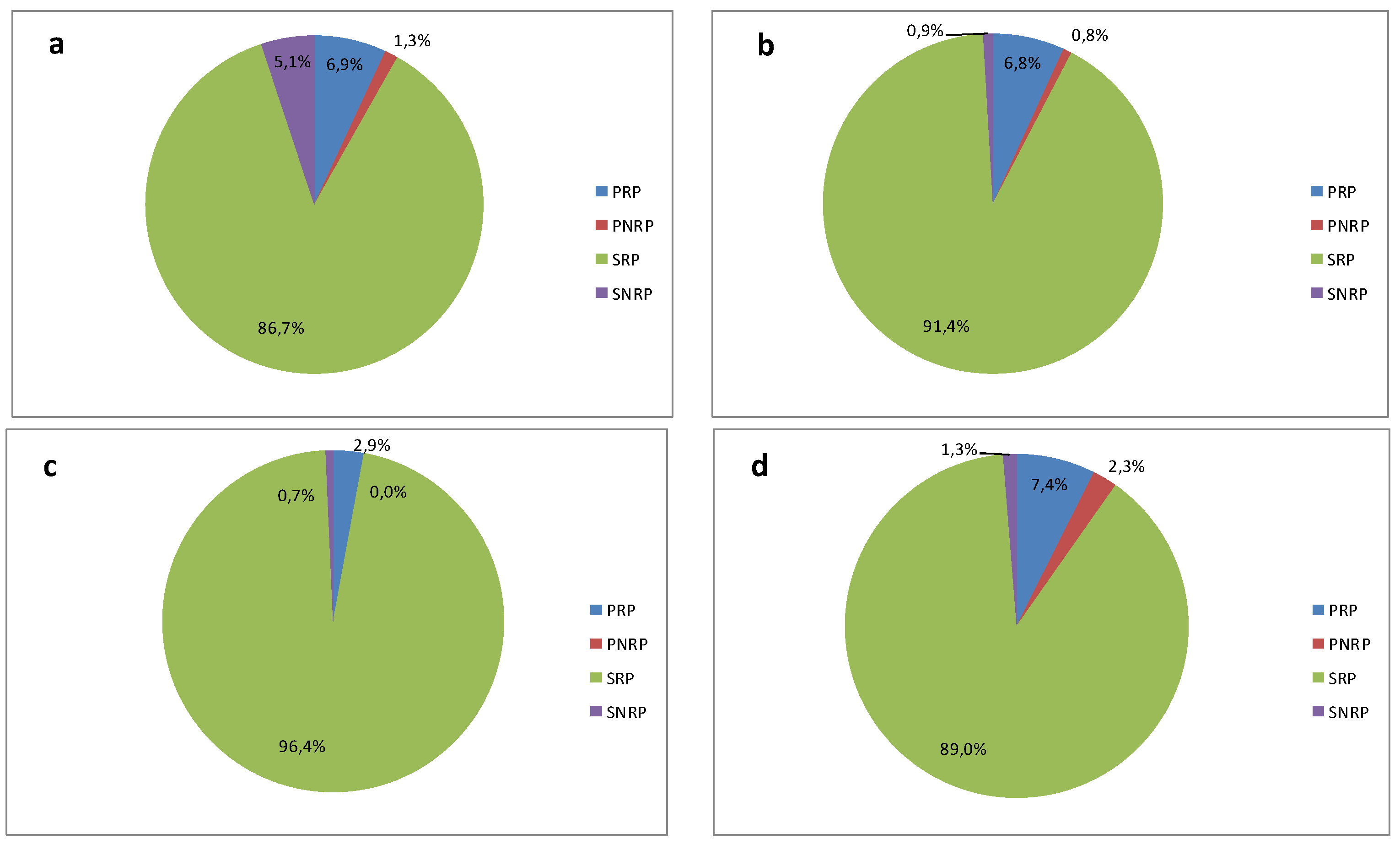
3.2. Phosphorus Speciation
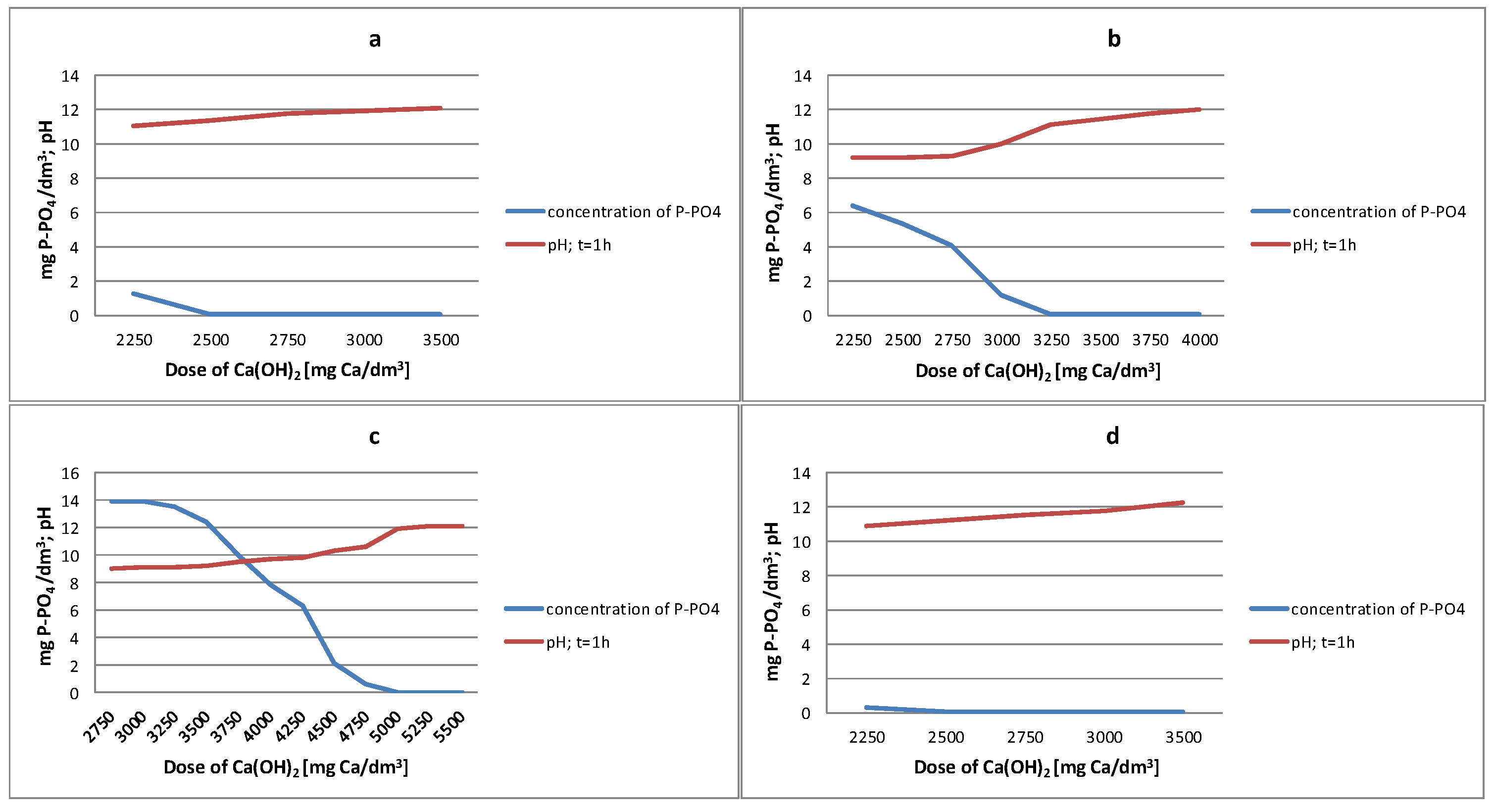
3.3. Precipitation of Phosphorus
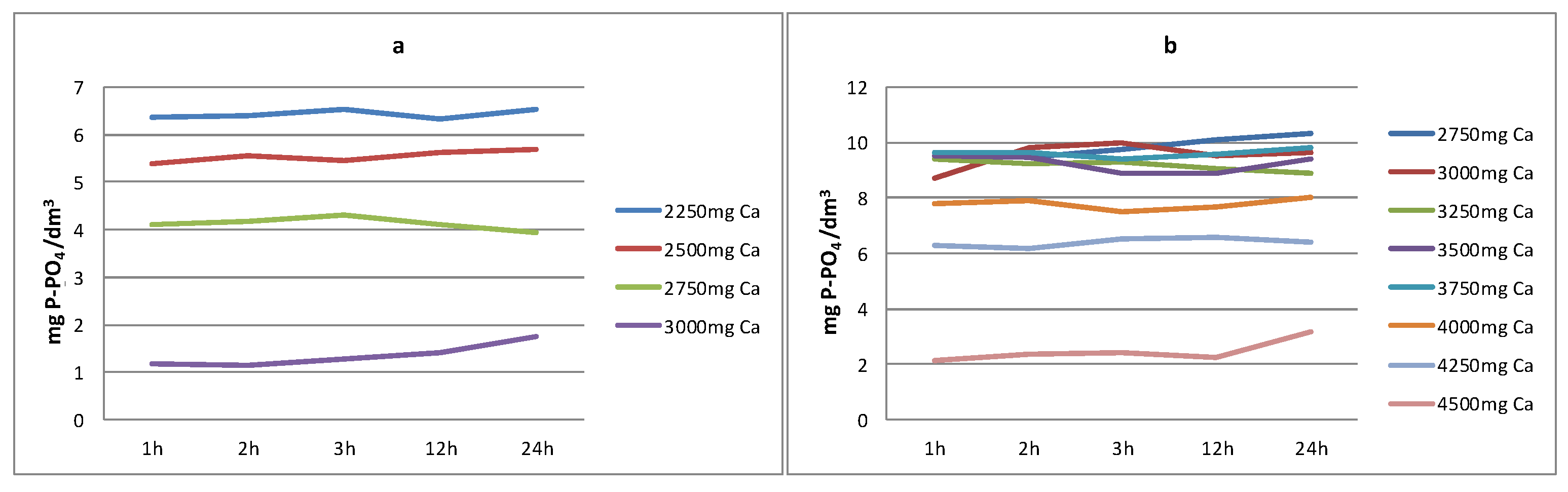
3.3.1. Reject Water from Debogorze WWTP
3.3.2. Reject Water from Poznan WWTP
3.3.3. Reject Water from Slupsk WWTP
3.3.4. Reject Water from Swarzewo WWTP
- WWTP Debogorze: 2500 mg Ca/dm3 and pH = 11.8
- WWTP Poznan 3250 mg Ca/dm3 and pH = 11.3
- WWTP Slupsk 5000 mg Ca/dm3 and pH = 11.9
- WWTP Swarzewo 2500 mg Ca/dm3 and pH = 11.1
4. Conclusions
- The concentration of phosphate phosphorus and ammonium nitrogen in reject waters from the dewatering of digested sewage sludge (with or without co-substrates) varies over a relatively wide range and depends on the wastewater treatment technology adopted at the plant, the co-substrate used for the digestion process and the efficiency of sludge dewatering.
- In all analysed reject waters, very high (exceeding 99.9%) phosphate phosphorus removal efficiencies were obtained using Ca(OH)2 for short reaction times (t = 1 h).
- The efficiency of phosphate phosphorus removal depends on the pH value of the reaction obtained during the precipitation process.
- The percentage of phosphorus forms determined in the raw reject water was similar for the four analysed treatment plants. Phosphorus in reject water was mainly bound in the SPR (soluble reactive phosphorus) fraction. However, slight differences were observed due to the composition of the feedstock.
- The forms of phosphorus in the analysed reject water did not affect the efficiency of the phosphorus precipitation process with calcium hydroxide. This means that the phosphorus precipitation method used in this work is very effective for both mono- and co-digestion processes, regardless of the composition of the feedstock.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statistic Poland. Environment 2022. Available online: https://stat.gov.pl/en/topics/environment-energy/environment/environment-2022,1,14.html (accessed on 5 December 2022).
- Piaskowski, K.; Cwikałowska, M. Profile of changes of orthophosphate concentrations during sewage and sewage sludge treatment. Rocz. Ochr. Sr. 2007, 9, 183–197. [Google Scholar]
- Wang, R.; Li, Y.; Wang, W.; Chen, Y.; Vanrolleghem, P.A. Effect of high orthophosphate concentration on mesophilic anaerobic sludge digestion and its modeling. Chem. Eng. J. 2015, 260, 791–800. [Google Scholar] [CrossRef]
- Dąbrowski, W. A study of the digestion process of sewage sludge from dairy WWTP to determine the composition and load ofreject water. Water Pract. Technol. 2014, 9, 71–78. [Google Scholar] [CrossRef]
- Morales, N.; Val del Río, A.; Vázquez-Padín, J.R.; Méndez, R.; Mosquera-Corral, A.; Campos, J.L. Integration of the Anammoxprocess to the rejection water and mainstream lines of WWTPs. Chemosphere 2015, 140, 99–105. [Google Scholar] [CrossRef]
- Di Costanzo, N.; Cesaro, A.; Di Capua, F.; Esposito, G. Exploiting the Nutrient Potential of Anaerobically Digested Sewage Sludge: A Review. Energies 2021, 14, 8149. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Gerbens-Leenes, W. The Water Footprint of Global Food Production. Water 2020, 12, 2696. [Google Scholar] [CrossRef]
- The World Bank. Water in Agriculture. Available online: https://www.worldbank.org/en/topic/water-in-agriculture (accessed on 15 July 2022).
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor MM, B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Zhang, W.; Villarini, G. Heavy precipitation is highly sensitive to the magnitude of future warming. Clim. Change 2017, 145, 249–257. [Google Scholar] [CrossRef]
- Myhre, G.; Alterskjær, K.; Stjern, C.W.; Hodnebrog, Ø.; Marelle, L.; Samset, B.H.; Sillmann, J.; Schaller, N.; Fischer, E.; Schulz, M.; et al. Frequency of extreme precipitation increases extensively with event rareness under global warming. Sci. Rep. 2019, 9, 16063. [Google Scholar] [CrossRef]
- Van der Bom, F.J.T.; Kopittke, P.M.; Raymond, N.S.; Sekine, R.; Lombi, E.; Mueller, C.W.; Doolette, C.L. Methods for assessing laterally-resolved distribution, speciation and bioavailability of phosphorus in soils. Rev. Environ. Sci. Biotechnol. 2022, 21, 53–74. [Google Scholar] [CrossRef]
- Booker, N.A.; Priestley, A.J.; Fraser, I.H. Struvite Formation in Wastewater Treatment Plants: Opportunities for Nutrient Recovery. Environ. Technol. 1999, 20, 777–782. [Google Scholar] [CrossRef]
- Ye, Z.; Shen, Y.; Xin Ye, X.; Zhang, Z.; Chen, S.; Shi, J. Phosphorus recovery from wastewater by struvite crystallization: Property of aggregates. J. Environ. Sci. 2014, 26, 991–1000. [Google Scholar] [CrossRef]
- Sena, M.; Hicks, A. Life cycle assessment review of struvite precipitation in wastewater treatment. Resour. Conserv. Recycl. 2018, 139, 194–204. [Google Scholar] [CrossRef]
- Hosni, K.; Ben Moussa, S.; Chachi, A.; Ben Amor, M. The removal of PO43− by calcium hydroxide from synthetic wastewater: Optimisation of the operating conditions. Desalination 2008, 223, 337–343. [Google Scholar] [CrossRef]
- González-Morales, C.; Fernández, B.; Molina, F.J.; Naranjo-Fernández, D.; Matamoros-Veloza, A.; Camargo-Valero, M.A. Influence of pH and Temperature on Struvite Purity and Recovery from Anaerobic Digestate. Sustainability 2021, 13, 10730. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Jefferson, B.; Parsons, S.A. Struvite crystallisation and recovery using a stainless steel structure as a seed material. Water Res. 2007, 41, 2449–2456. [Google Scholar] [CrossRef]
- Wilinska-Lisowska, A.; Ossowska, M.; Czerwionka, K. The Influence of Co-Fermentation of Agri-Food Waste with Primary Sludge on Biogas Production and Composition of the Liquid Fraction of Digestate. Energies 2021, 14, 1907. [Google Scholar] [CrossRef]
- Rasmeni, Z.Z.; Madyira, D.M.; Matheri, A.N. Optimum loading ratio for co-digested wastewater sludge and brewery spent yeast. Energy Rep. 2022, 8, 1141–1149. [Google Scholar] [CrossRef]
- Christensen, M.L.; Hjorth, M.; Keiding, K. Characterization of pig slurry with reference to flocculation and separation. Water Res. 2009, 43, 773–783. [Google Scholar] [CrossRef]
- Oliveira, V.; Labrincha, J.; Dias-Ferreira, C. Extraction of phosphorus and struvite production from the anaerobically digested organic fraction of municipal solid waste. J. Environ. Chem. Eng. 2018, 6, 2837–2845. [Google Scholar] [CrossRef]
- Tuszynska, A.; Czerwionka, K.; Obarska-Pempkowiak, H. Phosphorus concentration and availability in raw organic waste and post fermentation products. J. Environ. Manag. 2021, 278, 111468. [Google Scholar] [CrossRef]
- Li, B.; Dinkler, K.; Zhao, N.; Ran, X.; Sobhi, M.; Dong, R.; Müller, J.; Xiong, W.; Huang, G.; Guo, J.; et al. Response of phosphorus speciation to organic loading rates and temperatures during anaerobic co-digestion of animal manures and wheat straw. Sci. Total Environ. 2022, 838, 155921. [Google Scholar] [CrossRef] [PubMed]
- Tuszynska, A.; Wilinska, A.; Czerwionka, K. Phosphorus and nitrogen forms in liquid fraction of digestates from agricultural biogas plants. Environ. Technol. 2020, 42, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, H.; Liu, J.; Luo, J.; Qian, G.; Wang, A. pH dependent phosphorus release from waste activated sludge: Contributions of phosphorus speciation. Chem. Eng. J. 2015, 267, 260–265. [Google Scholar] [CrossRef]
- Li, B.; Brett, M.T. The influence of dissolved phosphorus molecular form on recalcitrance and bioavailability. Environ. Pollut. 2013, 182, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Brett, M.T. Characterization of the dissolved phosphorus uptake kinetics for the effluents from advanced nutrient removal processes. Water Res. 2015, 84, 181–189. [Google Scholar] [CrossRef]
- Xie, C.; Zhao, J.; Tang, J.; Xu, J.; Lin, X.; Xu, X. The phosphorus fractions and alkaline phosphatase activities in sludge. Bioresour. Technol. 2011, 102, 2455–2461. [Google Scholar] [CrossRef]
- Xie, C.; Tang, J.; Zhao, J.; Wu, D.; Xu, X. Comparison of phosphorus fractions and alkaline phosphatase activity in sludge, soils and sediments. J. Soils Sediments 2011, 11, 1432–1439. [Google Scholar] [CrossRef]
- He, Z.-W.; Liu, W.-Z.; Wang, L.; Tang, C.-C.; Guo, Z.-C.; Yang, C.-Y.; Wang, A.-J. Clarification of phosphorus fractions and phosphorus release enhancement mechanism related to pH during waste activated sludge treatment. Bioresour. Technol. 2016, 222, 217–225. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Bień, B.; Bień, J.D. Analysis of Reject Water Formed in the Mechanical Dewatering Process of Digested Sludge Conditioned by Physical and Chemical Methods. Energies 2022, 15, 1678. [Google Scholar] [CrossRef]
- Sperczynska, E. Phosphates removal from reject water from digestion of sludge. Ecol. Eng. 2016, 48, 196–201. (In Polish) [Google Scholar]
- Ju, L.-K.; Shah, H.K.; Porteous, J. Phosphorus Release in Aerobic Sludge Digestion. Water Environ. Res. 2005, 77, 553–559. [Google Scholar] [CrossRef]
- Guo, C.H.; Stabnikov, V.; Ivanova, V. The removal of nitrogen and phosphorus from reject water of municipal wastewater treatment plant using ferric and nitrate bioreductions. Bioresour. Technol. 2010, 101, 3992–3999. [Google Scholar] [CrossRef]
- Malinowski, P.; Dąbrowski, W.; Karolinczak, B. Application of SS-VF bed for the treatment of high concentrated reject water from autothermal thermophilic aerobic sewage sludge digestion. J. Ecol. Eng. 2018, 19, 103–110. [Google Scholar] [CrossRef]
- Wu, X.; Modin, O. Ammonium recovery from reject water combined with hydrogen production in a bioelectrochemical reactor. Bioresour. Technol. 2013, 146, 530–536. [Google Scholar] [CrossRef]
- Harris, W.G.; Wilkie, A.C.; Cao, X.; Sirengo, R. Bench-scale recovery of phosphorus from flushed dairy manure wastewater. Bioresour. Technol. 2008, 99, 3036–3043. [Google Scholar] [CrossRef]
- Song, Y.; Hahn, H.H.; Hoffmann, E. The effect of carbonate on the precipitation of calcium phosphate. Environ. Technol. 2002, 23, 207–215. [Google Scholar] [CrossRef]
- Moutin, T.; Gal, J.Y.; El Halouani, H.; Picot, B.; Bontoux, J. Decrease of phosphate concentration in a high rate pond by precipitation of calcium phosphate: Theoretical and experimental results. Water Res. 1992, 26, 1445–1450. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Capsoni, D.; Callegari, A.; Capodaglio, A.G. Impact of pH and Ionic Molar Ratios on Phosphorous Forms Precipitation and Recovery from Different Wastewater Sludges. Resources 2018, 7, 71. [Google Scholar] [CrossRef]
- Lu, N.C.; Liu, J.C. Removal of phosphate and fluoride from wastewater by a hybrid precipitation-microfiltration process. Sep. Purif. Technol. 2010, 74, 329–335. [Google Scholar] [CrossRef]
- Çelen, I.; Türker, M. Recovery of Ammonia as Struvite from Anaerobic Digester Effluents. Environ. Technol. 2001, 22, 1263–1272. [Google Scholar] [CrossRef]
- Stratful, I.; Scrimshaw, M.D.; Lester, J.N. Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res. 2001, 35, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Remiszewska-Skwarek, A.; Wierzchnicki, R.; Roubinek, O.K.; Kasinath, A.; Jeżewska, A.; Jasinska, M.; Byliński, H.; Chmielewski, A.G.; Czerwionka, K. The influence of low-temperature disintegration on the co-fermentation process of distillation residue and waste-activated sludge. Energies 2022, 15, 482. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]

| WWTP Size | Flow Rate | Configuration of Bioreactor | Sludge Handling |
|---|---|---|---|
| PE | m3/d | - | - |
| Debogorze 515,500 | 56,000 | A2/O with final simultanic denitrification | AnD * of primary and secondary sludge without the use of co-substrates |
| Poznan 1,200,000 | 102,200 | JHB | AnD of primary and secondary sludge with use of co-substrates (waste from the agri-food industry and external waste activated sludge) |
| Slupsk 200,000 | 20,000 | JHB modified to 5-stage reactor | AnD of primary and secondary sludge with use of co-substrates (fats from industrial plants) |
| Swarzewo 150,000 | 14,000 | SBR with final sedimentation in settling tanks | AnD of primary and secondary sludge with use of co-substrates (waste from the agri-food industry and external waste activated sludge) |
| WWTP | Dose of Ca(OH)2 [mg/dm3] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2250 | 2500 | 2750 | 3000 | 3250 | 3500 | 3750 | 4000 | 5500 | |
| Debogorze | + | + | + | + | + | + | − | − | − |
| Poznan | + | + | + | + | + | + | + | + | − |
| Slupsk | + | − | + | + | + | + | + | + * | + |
| Swarzewo | + | + | + | + | + | + | − | − | − |
| WWTP | pH | TP | P-PO4 | N-NH4 | COD | Ca | Mg |
|---|---|---|---|---|---|---|---|
| - | mg/dm3 | mg/dm3 | mg/dm3 | mg O2/dm3 | mg/dm3 | mg/dm3 | |
| Debogorze | 7.24 | 232 | 228 | 504 | 378 | 52.1 | 26.7 |
| Poznan | 7.48 | 196.2 | 192.8 | 926 | 519 | 55.3 | 23.4 |
| Slupsk | 7.88 | 278 | 276 | 1524 | 494 | 64.5 | 32.8 |
| Swarzewo | 7.80 | 39 | 37.6 | 724 | 437 | 72.1 | 58.4 |
| WWTP | TP | PRP | PNRP | SRP | SNRP | SP |
|---|---|---|---|---|---|---|
| Debogorze | 232 | 16 | 2.9 | 201.3 | 11.8 | 213 |
| Poznan | 196.2 | 13.4 | 1.6 | 179.4 | 1.8 | 181.2 |
| Slupsk | 278 | 8 | 0 | 268 | 2 | 270 |
| Swarzewo | 39 | 2.9 | 0.9 | 34.7 | 0.5 | 35.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulbat, E.; Czerwionka, K. Influence of Phosphorus Speciation on Its Chemical Removal from Reject Water from Dewatering of Municipal Sewage Sludge. Energies 2023, 16, 1260. https://doi.org/10.3390/en16031260
Kulbat E, Czerwionka K. Influence of Phosphorus Speciation on Its Chemical Removal from Reject Water from Dewatering of Municipal Sewage Sludge. Energies. 2023; 16(3):1260. https://doi.org/10.3390/en16031260
Chicago/Turabian StyleKulbat, Eliza, and Krzysztof Czerwionka. 2023. "Influence of Phosphorus Speciation on Its Chemical Removal from Reject Water from Dewatering of Municipal Sewage Sludge" Energies 16, no. 3: 1260. https://doi.org/10.3390/en16031260
APA StyleKulbat, E., & Czerwionka, K. (2023). Influence of Phosphorus Speciation on Its Chemical Removal from Reject Water from Dewatering of Municipal Sewage Sludge. Energies, 16(3), 1260. https://doi.org/10.3390/en16031260







