Green Energy Revolution and Substitution of Hydrocarbons with Hydrogen: Distribution Network Infrastructure Materials
Abstract
1. Introduction
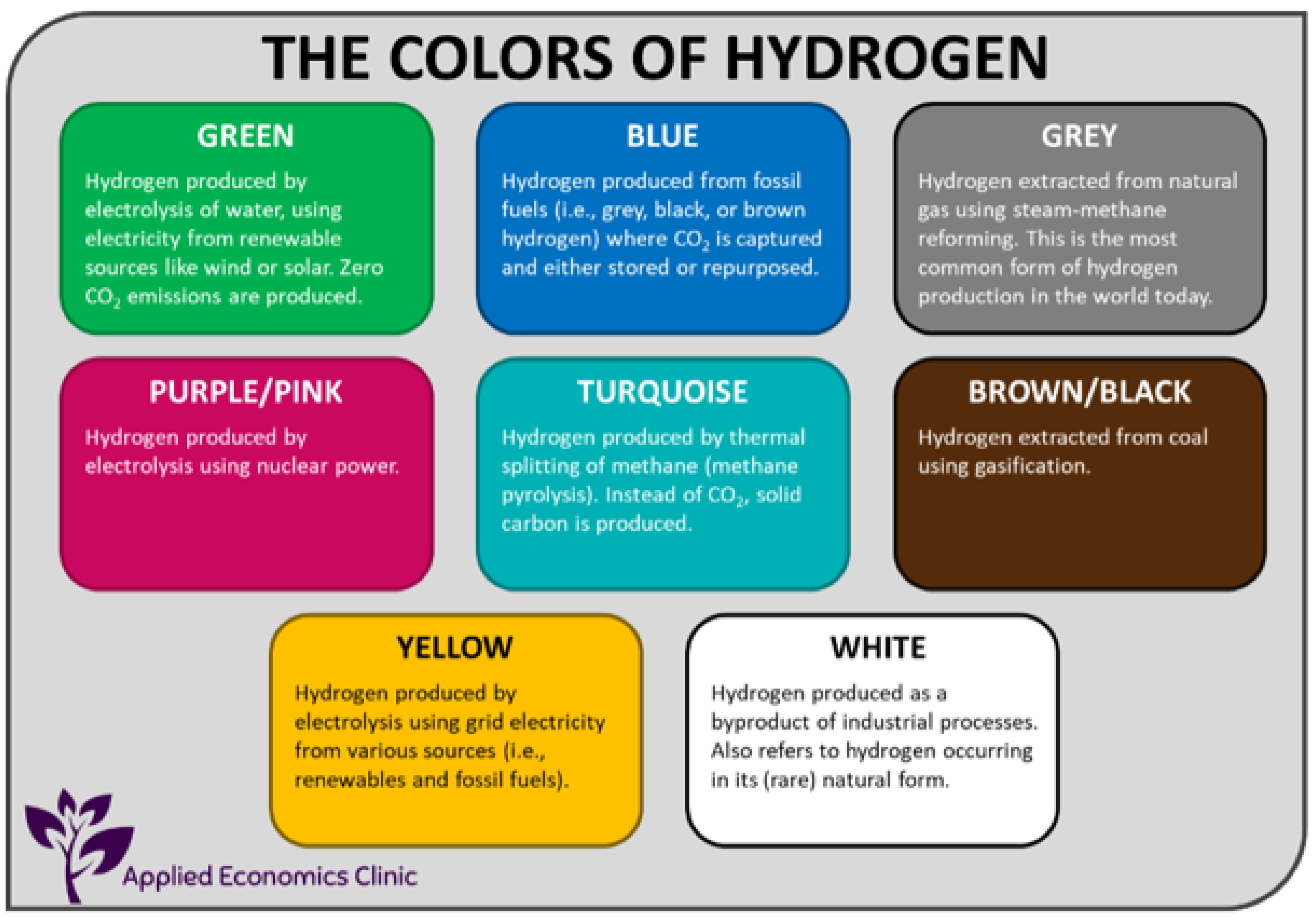
- Black/brown H2: produced from coal/lignite (brown coal) by gasification to generate hydrogen and other gases like CO, CO2, CH4, and H2O.
- Gray H2: produced from natural gas, and the resultant CO2 is released into the atmosphere.
- Blue H2: produced from natural gas, but the resultant CO2 is stored and not released into the atmosphere.
- Green H2: produced via electrolysis of water and is environment-friendly, since the power used for electrolysis is also provided by non-fossil fuel sources, like solar.
- Partial mixing of hydrogen in hydrocarbon gas pipelines with gradual increase to eventually achieving full replacement of hydrocarbon-based gases;
- Total replacement of hydrocarbons with hydrogen.
2. Hydrogen Facts
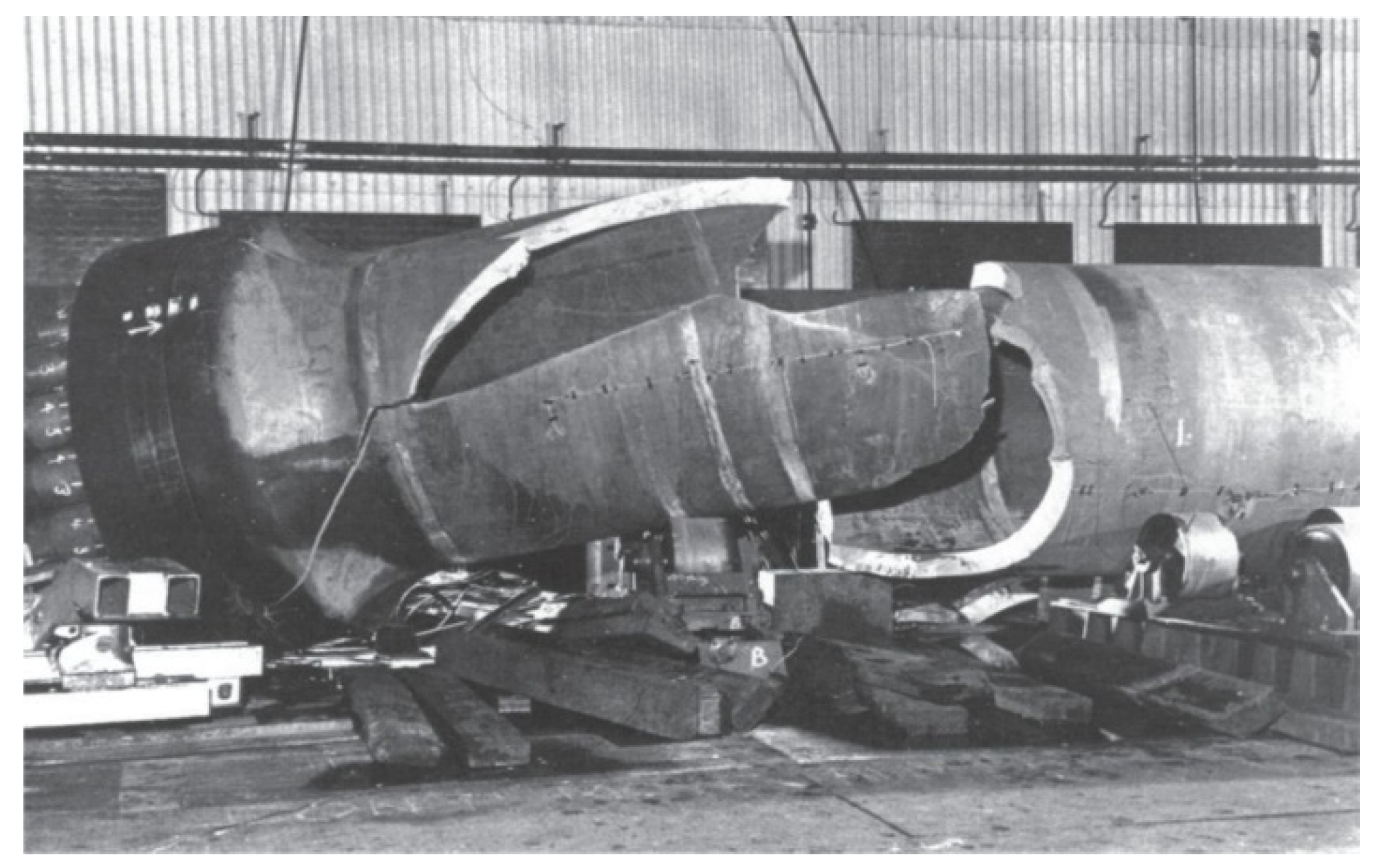
3. Materials Failure
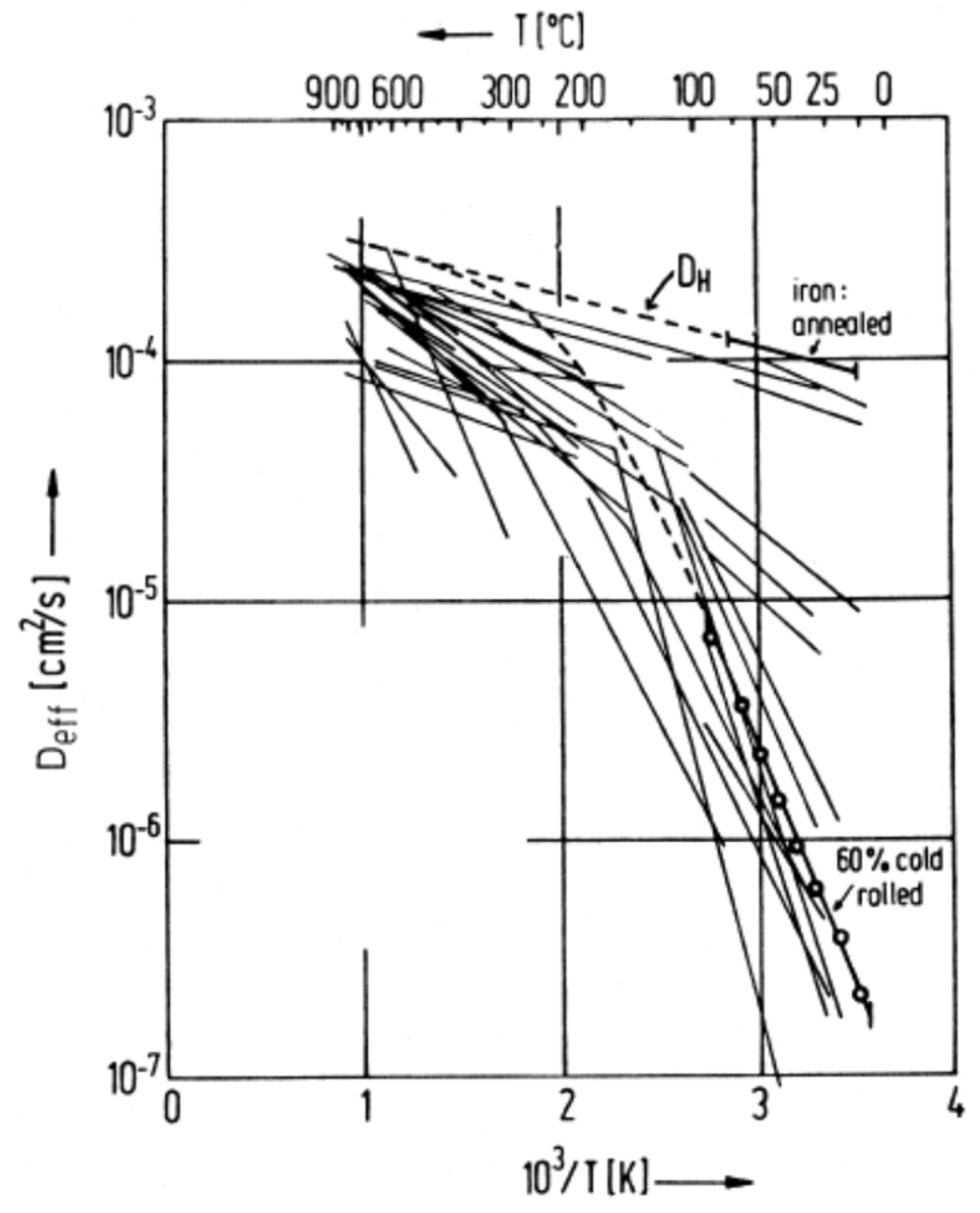
4. Hydrogen in Steels
- Electrochemical discharge, where the steel is immersed in a basic (e.g., NaOH) or acidic (e.g., H2SO4) solution as the cathode;
- The steel is placed in a hydrogen-gas-rich environment at specific pressure. The latter is the case encountered during the storage/transportation of hydrogen as the inner wall of the vessel/pipeline is exposed to hydrogen at pressure.
4.1. Hydrogen Embrittlement
4.2. Hydrogen Embrittlement Mechanisms
- Hydrogen-Enhanced Decohesion (HEDE) mechanism
- Adsorption-Induced Dislocation Emission (AIDE) Mechanism
- Hydrogen Enhanced Localized Plasticity (HELP) Mechanism
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, G.; Saikawa, E. Effectiveness of state climate and energy policies in reducing power-sector CO2 emissions. Nat. Clim. Chang. 2017, 7, 912–919. [Google Scholar] [CrossRef]
- Yusaf, T.; Goh, S.; Borserio, J.A. Potential of renewable energy alternatives in Australia. Renew. Sustain. Energy Rev. 2011, 15, 2214–2221. [Google Scholar] [CrossRef]
- Australian Renewable Energy Agency. Renewable Energy Options for Industrial Process Heat; Australian Renewable Energy Agency: Canberra, Australia, 2019. [Google Scholar]
- Available online: https://flowcharts.llnl.gov/ (accessed on 12 November 2023).
- Available online: https://ember-climate.org/insights/research/coal-power-emissions-per-capita-2020/ (accessed on 13 September 2023).
- Available online: https://ourworldindata.org/co2-emissions (accessed on 19 September 2023).
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. (Eds.) IPCC SR 1.5. IPCC Special Report on 1.5: Global Warming of 1.5 °C. In IPCC, 2018: Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2019; Volume 85. [Google Scholar]
- Available online: https://ember-climate.org/countries-and-regions/countries/australia/ (accessed on 13 September 2023).
- Available online: https://www.originenergy.com.au/about/who-we-are/what-we-do/generation/eraring-power-station/ (accessed on 19 September 2023).
- Stasio, T.; Castigliego, J. Colour of Hydrogen. Available online: https://aeclinic.org/aec-blog/2021/6/24/the-colors-of-hydrogen (accessed on 13 September 2023).
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Palmer, G.; Roberts, A.; Hoadley, A.; Dargaville, R.; Honnery, D. Life-cycle greenhouse gas emissions and net energy assessment of large-scale hydrogen production via electrolysis and solar PV. Energy Environ. Sci. 2021, 14, 5113–5131. [Google Scholar] [CrossRef]
- Neville, A. Lessons Learned from a Hydrogen Explosion. Power, 153 (5). Available online: https://www.powermag.com/lessons-learned-from-a-hydrogen-explosion/ (accessed on 9 September 2023).
- Available online: https://www.csb.gov/silver-eagle-refinery-flash-fire-and-explosion-and-catastrophic-pipe-explosion/ (accessed on 9 September 2023).
- Available online: https://reneweconomy.com.au/hydrogen-re-fuelling-station-explodes-in-norway-hyundai-and-toyota-suspend-fuel-cell-sales-99377/#:~:text=Great%20Solar%20Business-,Hydrogen%20re%2Dfuelling%20station%20explodes%20in%20Norway%2C%20Hyundai%20and,Toyota%20suspend%20fuel%20cell%20sales&text=A%20hydrogen%20refuelling%20station%20has,the%20re%2Dfuelling%20equipment%20itself (accessed on 9 September 2023).
- Available online: https://www.aiche.org/chs/conferences/international-center-hydrogen-safety-conference/2019/proceeding/paper/review-hydrogen-tank-explosion-gangneung-south-korea#:~:text=A%20devastating%20hydrogen%20tank%20explosion,into%20the%20hydrogen%20storage%20tank (accessed on 9 September 2023).
- Abdelgawad, A.; Salah, B.; Lu, Q.; Abdullah, A.M.; Chitt, M.; Ghanem, A.; Al-Hajri, R.S.; Eid, K. Template-free synthesis of M/g-C3N4 (M = Cu, Mn, and Fe) porous one-dimensional nanostructures for green hydrogen production. J. Electroanal. Chem. 2023, 938, 117426. [Google Scholar] [CrossRef]
- Salah, B.; Abdelgawad, A.; Lu, Q.; Ipadeola, A.K.; Luque, R.; Eid, K. Synergistically interactive MnFeM (M = Cu, Ti, and Co) sites doped porous g-C3N4 fiber-like nanostructures for an enhanced green hydrogen production. Green Chem. 2023, 25, 6032–6040. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps Between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2022, 6, e12441. [Google Scholar] [CrossRef]
- Shih, A.J.; Monteiro, M.C.; Dattila, F.; Pavesi, D.; Philips, M.; da Silva, A.H.; Vos, R.E.; Ojha, K.; Park, S.; van der Heijden, O.; et al. Water electrolysis. Nat. Rev. Methods Primers 2022, 2, 84. [Google Scholar] [CrossRef]
- Eid, K.; Lu, Q.; Abdel-Azeim, S.; Soliman, A.; Abdullah, A.M.; Abdelgwad, A.M.; Forbes, R.P.; Ozoemena, K.I.; Varma, R.S.; Shibl, M.F. Highly exfoliated Ti3C2Tx MXene nanosheets atomically doped with Cu for efficient electrochemical CO2 reduction: An experimental and theoretical study. J. Mater. Chem. A 2022, 10, 1965–1975. [Google Scholar] [CrossRef]
- Available online: https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_FullVolume.pdf (accessed on 12 September 2023).
- US Department of Energy. Available online: https://www.energy.gov/eere/fuelcells/articles/safety-codes-and-standards-fact-sheet (accessed on 13 May 2023).
- Opheim, D. Webinar Presentation, The ABCs of Hydrogen Safety: Always Be Cautious. 2023. Available online: https://www.globalspec.com/events/eventdetails?eventId=4024 (accessed on 20 September 2023).
- Linstrom, P. NIST Chemistry WebBook; NIST Standard Reference Database Number 69; NIST Office of Data and Informatics: Gaithersburg, MD, USA, 2021. [CrossRef]
- Knott, J. Brittle fracture in structural steels: Perspectives at different size-scales. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grabke, H.J.; Riecke, E. Absorption and diffusion of hydrogen in Steels. Mater. Tehnol. 2000, 34, 331. [Google Scholar]
- Alipooramirabad, H.; Paradowska, A.; Reid, M.; Ghomashchi, R. Effect of holding time on strain relaxation in high-strength low-alloy steel welds: An in-situ neutron diffraction approach. J. Manuf. Process. 2021, 73, 326–339. [Google Scholar] [CrossRef]
- Roccisano, A.; Nafisi, S.; Ghomashchi, R. Stress corrosion cracking observed in ex-service gas pipelines: A comprehensive study. Met. Mater. Trans. A 2019, 51, 167–188. [Google Scholar] [CrossRef]
- Alipooramirabad, H.; Paradowska, A.; Ghomashchi, R.; Reid, M. Investigating the effects of welding process on residual stresses, microstructure and mechanical properties in HSLA steel welds. J. Manuf. Process. 2017, 28, 70–81. [Google Scholar] [CrossRef]
- Kurji, R.; Coniglio, N.; Griggs, J.; Ghomashchi, R. Modified WIC test: An efficient and effective tool for evaluating pipeline girth weldability. Sci. Technol. Weld. Join. 2016, 22, 287–299. [Google Scholar] [CrossRef]
- Alipooramirabad, H.; Ghomashchi, R.; Paradowska, A.; Reid, M. Residual stress- microstructure- mechanical property interrelationships in multipass HSLA steel welds. J. Mater. Process. Technol. 2016, 231, 456–467. [Google Scholar] [CrossRef]
- Holappa, L. Secondary Steel Making, Ch. 1.6, Treatise on Process Metallurgy, Volume 3: Industrial Processes; Elsevier Ltd.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. STEELS, Microstructure and Properties, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- ASME 831.12:2019; Hydrogen Piping and Pipelines. ASME: New York, NY, USA, 2019.
- Steiner, M.; Marewski, U.; Silcher, H. DVGW Project SyWeSt H2: “Investigation of Steel Materials for Gas Pipelines and Plants for Assessment of their Suitability with Hydrogen”; DVGW Research Project G 202006; DVGW: Bonn, Germany, 2023. [Google Scholar]
- Parmelia Gas Pipeline. In Hydrogen Conversion Technical Feasibility Study, Public Knowledge Sharing Report; APA GROUP: Sydney, Australia, 2023.
- Kurji, R.N. Thermomechanical Factors Influencing Weld Metal Hydrogen Assisted Cold Cracking. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2016. [Google Scholar]
- Costin, W.L.; Lavigne, O.; Kotousov, A.; Ghomashchi, R. Valerie Linton Investigation of hydrogen assisted cracking in acicular ferrite using site-specific micro-fracture tests. Mater. Sci. Eng. 2016, A651, 859–868. [Google Scholar] [CrossRef]
- Venezuela, J.; Tapia-Bastidas, C.; Zhou, Q.; Depover, T.; Verbeken, K.; Gray, E.; Liu, Q.; Liu, Q.; Zhang, M.; Atrens, A. Determination of the equivalent hydrogen fugacity during electrochemical charging of 3.5NiCrMoV steel. Corros. Sci. 2018, 132, 90–106. [Google Scholar] [CrossRef]
- NACE Standard TM0284-2003 (Item No. 21215); Evaluation of Pipeline and Pressure Vessel Steels for Resistance to Hydrogen-Induced Cracking. NACE: Houston, TX, USA, 2003.
- Fetisov, V.; Davardoost, H.; Mogylevets, V. Technological Aspects of Methane–Hydrogen Mixture Transportation through Operating Gas Pipelines Considering Industrial and Fire Safety. Fire 2023, 6, 409. [Google Scholar] [CrossRef]
- Johnson, W.H. On Some Remarkable Changes Produced in Iron and Steel by the Action of Hydrogen and Acids. Nature 1875, 11, 393. [Google Scholar] [CrossRef]
- Frohmberg, R.P.; Barnett, W.J.; Troiano, A.R. Delayed Failure and Hydrogen Embrittlement in Steel; Technical Report; Wright-Patterson Air Force Base: Dayton, OH, USA, 1954; pp. 54–320. [Google Scholar]
- Dieter, G.E. Mechanical Metallurgy, 2nd ed.; McGraw Hill: Tokyo, Japan, 1976. [Google Scholar] [CrossRef]
- Watson, J.W.; Meshii, M. Treatise of Materials Science and Technology; Elsevier: Amsterdam, The Netherlands, 1989; p. 501. [Google Scholar]
- Ghomashchi, R.; Costin, W.; Kurji, R. Evolution of weld metal microstructure in shielded metal arc welding of X70 HSLA steel with cellulosic electrodes: A case study. Mater. Charact. 2015, 107, 317–326. [Google Scholar] [CrossRef]
- Thewlis, G. Classification and quantification of microstructures in steels. Mater. Sci. Technol. 2004, 20, 143–160. [Google Scholar] [CrossRef]
- Michler, T.; Lindner, M.; Eberle, U.; Meusinger, J. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies, Volume. 1: The Problem, Its Characterisation and Effects on Particular Alloy Classes; Gangloff, R.P., Somerday, B.P., Eds.; Woodhead Publishing: Sawston, UK, 2012; Volume 1, p. 94. [Google Scholar]
- Robertson, I.M.; Fenske, J.; Martin, M.; Briceno, M.; Dadfarnia, M.; Novak, P.; Ahn, D.C.; Sofronis, P.; Liu, J.B.; Johnson, D.D. Understanding How Hydrogen Influences the Mechanical Properties of Iron and Steel. In Proceedings of the 2nd International Symposium of Steel Science, Kyoto, Japan, 21–24 October 2009; Higashida, K., Tsuji, N., Eds.; The Iron and Steel Institute of Japan: Kyoto, Japan, 2009; p. 63. [Google Scholar]
- Shi, K.; Meng, X.; Xiao, S.; Chen, G.; Wu, H.; Zhou, C.; Jiang, S.; Chu, P.K. MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels. Nanomaterials 2021, 11, 2737. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, K.Y. An Overview on Hydrogen Uptake, Diffusion and Transport Behavior of Ferritic Steel, and Its Susceptibility to Hydrogen Degradation. Corros. Sci. Technol. 2017, 16, 209–225. [Google Scholar]
- Wang, J.; Li, Q.; Xiang, Q.-Y.; Cao, J.-L. Performances of AlN coatings as hydrogen isotopes permeation barriers. Fusion Eng. Des. 2016, 102, 94–98. [Google Scholar] [CrossRef]
- Yamabe, J.; Matsuoka, S.; Murakami, Y. Surface coating with a high resistance to hydrogen entry under high-pressure hydrogen-gas environment. Int. J. Hydrogen Energy 2013, 38, 10141–10154. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Y.; Cui, B.; Zhou, Q. Graphene coating on nickel as effective barriers against hydrogen embrittlement. Surf. Coat. Technol. 2019, 374, 610–616. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Oelrich, R.L.; Gates, R.O. Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review. Molecules 2022, 27, 6528. [Google Scholar] [CrossRef]
- Shi, K.; Xiao, S.; Ruan, Q.; Wu, H.; Chen, G.; Zhou, C.; Jiang, S.; Xi, K.; He, M.; Chu, P.K. Hydrogen permeation behavior and mechanism of multi-layered graphene coatings and mitigation of hydrogen embrittle-ment of pipe steel. Appl. Surf. Sci. 2021, 573, 151529. [Google Scholar] [CrossRef]
- Tazhibaeva, I.; Klepikov, A.; Romanenko, O.; Shestakov, V. Hydrogen permeation through steels and alloys with different protective coatings. Fusion Eng. Des. 2000, 51–52, 199–205. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, Y.; Yang, C.; Zhang, Y.; Cong, C.; Yuan, Y.; Lin, D.; Pei, L.; Zhu, Y.; Wang, H. A novel dual-functional epoxy-based composite coating with exceptional anti-corrosion and enhanced hydrogen gas barrier properties. Chem. Eng. J. 2022, 449. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, Y.; Cong, C.; Liu, Y.; Lin, D.; Pei, L.; Zhu, Y.; Wang, H. A bi-layer orientated and functionalized graphene-based composite coating with unique hydrogen gas barrier and long-term anti-corrosion performance. Carbon 2023, 205, 54–68. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Costin, W.L. On the Relationship between Microstructure Mechanical Properties and Weld Metal Hydrogen Assisted Cold Cracking. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2016. [Google Scholar]
- Zapffe, C.A. Hydrogen Embrittlement, Internal Stress and Defects in Steel; American Institute of Mining and Metallurgical Engineers: New York, NY, USA, 1941. [Google Scholar]
- Garofalo, F.; Chou, Y.; Ambegaokar, V. Effect of hydrogen on stability of micro cracks in iron and steel. Acta Met. 1960, 8, 504–512. [Google Scholar] [CrossRef]
- Bilby, B.A.; Hewitt, J. Hydrogen in steel–the stability of micro-cracks. Acta Metall. 1962, 10, 587–600. [Google Scholar] [CrossRef]
- Tetelman, A.; Robertson, W. Direct observation and analysis of crack propagation in iron-3% silicon single crystals. Acta Met. 1963, 11, 415–426. [Google Scholar] [CrossRef]
- Westlake, D.G. A generalized model for hydrogen embrittlement. Trans. ASM 1969, 62, 1000–1006. [Google Scholar]
- Gahr, S.; Grossbeck, M.; Birnbaum, H. Hydrogen embrittlement of Nb I—Macroscopic behavior at low temperatures. Acta Met. 1977, 25, 125–134. [Google Scholar] [CrossRef]
- Lufrano, J.; Sofronis, P.; Birnbaum, H. Modeling of hydrogen transport and elastically accommodated hydride formation near a crack tip. J. Mech. Phys. Solids 1996, 44, 179–205. [Google Scholar] [CrossRef]
- Hancock, G.G.; Johnson, H.H. Hydrogen oxygen and subcritical crack growth in a high strength steel. Trans. AIME 1965, 236, 513–516. [Google Scholar]
- Oriani, R.; Josephic, P. Equilibrium and kinetic studies of the hydrogen-assisted cracking of steel. Acta Met. 1977, 25, 979–988. [Google Scholar] [CrossRef]
- Boellinghaus, T. Numerical Modelling of Hydrogen Assisted Cracking. In Corrosion 2001; NACE International: Houston, TX, USA, 2001. [Google Scholar]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Met. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Johnson, H.H.; Troiano, A.R. Crack Initiation in Hydrogenated Steel. Nature 1957, 179, 777. [Google Scholar] [CrossRef]
- Oriani, R.; Josephic, P. Testing of the decohesion theory of hydrogen-induced crack propagation. Scr. Met. 1972, 6, 681–688. [Google Scholar] [CrossRef]
- Oriani, R.A.; Hirth, P.J.; Smialowski, M. Hydrogen Degradation of Ferrous Alloys; William Andrew Publishing/Noyes: Norwich, NY, USA, 1985; pp. 271–288, 641–685. [Google Scholar]
- Troiano, A.R. The role of hydrogen and other interstitials in the mechanical behaviour of metals. Trans. ASM 1960, 52, 54–80. [Google Scholar]
- Lynch, S.P. Mechanisms of hydrogen-assisted cracking. Met. Forum 1979, 2, 164, 189–200. [Google Scholar]
- Lynch, S. Metallographic contributions to understanding mechanisms of environmentally assisted cracking. Metallography 1989, 23, 147–171. [Google Scholar] [CrossRef]
- Birnbaum, H.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Ferreira, P.; Robertson, I.; Birnbaum, H. Hydrogen effects on the interaction between dislocations. Acta Mater. 1998, 46, 1749–1757. [Google Scholar] [CrossRef]
- Gerberich, W.W.; Oriani, R.A.; Lji, M.-J.; Chen, X.; Foecke, T. The necessity of both plasticity and brittleness in the fracture thresholds of iron. Philos. Mag. A 1991, 63, 363–376. [Google Scholar] [CrossRef]
- Vehoff, H.; Rothe, W. Gaseous hydrogen embrittlement in FeSi- and Ni-single crystals. Acta Met. 1983, 31, 1781–1793. [Google Scholar] [CrossRef]
- Li, J.C.M.; Oriani, R.A.; Darken, L.S. The Thermodynamics of Stressed Solids. Z. Für Phys. Chem. 1966, 49, 271–290. [Google Scholar] [CrossRef]
- Pressouyre, G. Trap theory of Hydrogen embrittlement. Acta Met. 1980, 28, 895–911. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall. Trans. 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Robertson, I.M.; Birnbaum, H.K.; Sofronis, P. Chapter 91 Hydrogen Effects on Plasticity; Hirth, J.P., Kubin, L., Eds.; Dislocations in Solids; Elsevier: Amsterdam, The Netherlands, 2009; Volume 15, pp. 249–293. [Google Scholar]
- Bhatnagar, P.; Zaferani, S.H.; Rafiefard, N.; Baraeinejad, B.; Vazifeh, A.R.; Mohammadpour, R.; Ghomashchi, R.; Dillersberger, H.; Tham, D.; Vashaee, D. Advancing personalized healthcare and entertainment: Progress in energy harvesting materials and techniques of self-powered wearable devices. Prog. Mater. Sci. 2023, 139, 101184. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Sams, M.W.; Shi, X.-L.; Mehrabian, N.; Ghomashchi, R.; Chen, Z.-G. Applications of thermoelectric generators to improve catalytic-assisted hydrogen production efficiency: Future directions. Energy Fuels 2022, 36, 8096–8106. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Jafarian, M.; Vashaee, D.; Ghomashchi, R. Thermal management systems and waste heat recycling by thermoelectric generators—An overview. Energies 2021, 14, 5646. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Ghomashchi, R.; Vashaee, D. Assessment of thermoelectric, mechanical, and microstructural reinforcement properties of graphene-mixed heterostructures. ACS Appl. Energy Mater. 2021, 4, 3573–3583. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Ghomashchi, R.; Vashaee, D. Thermoelectric, magnetic, and mechanical characteristics of antiferromagnetic manganese telluride reinforced with graphene nanoplates. Adv. Eng. Mater. 2020, 23, 2000816. [Google Scholar] [CrossRef]
- Lwin, M.L.; Dharmaiah, P.; Yoon, S.-M.; Song, S.H.; Kim, H.S.; Lee, J.-H.; Ghomashchi, R.; Hong, S.-J. Correlation with the composition of the different parts of p-type Bi0.5Sb1.5Te3 sintered bulks and their thermoelectric char-acteristics. J. Alloys Compd. 2020, 845, 156114. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Ghomashchi, R.; Vashaee, D. Strategies for engineering phonon transport in Heusler thermoelectric compounds. Renew. Sustain. Energy Rev. 2019, 112, 158–169. [Google Scholar] [CrossRef]



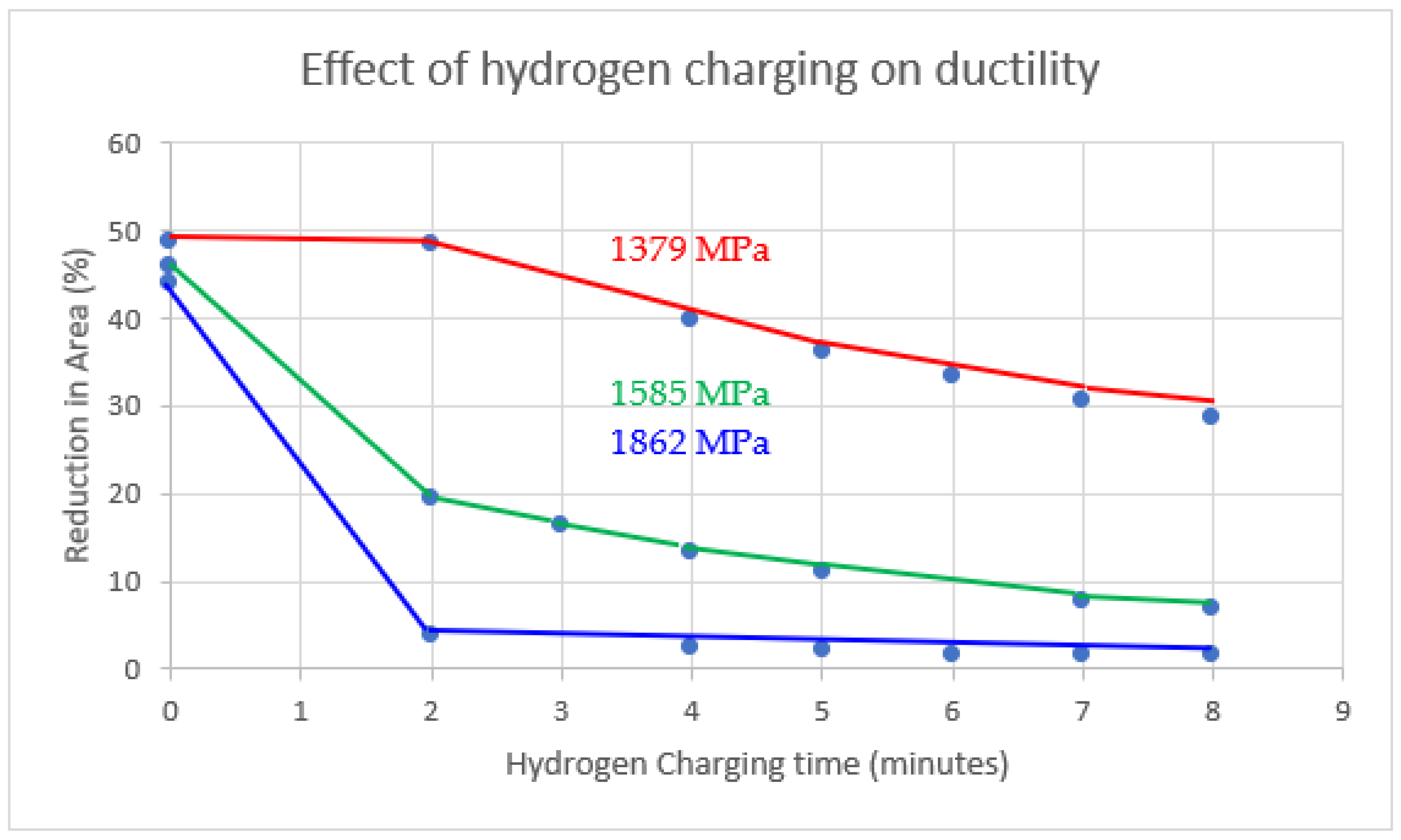
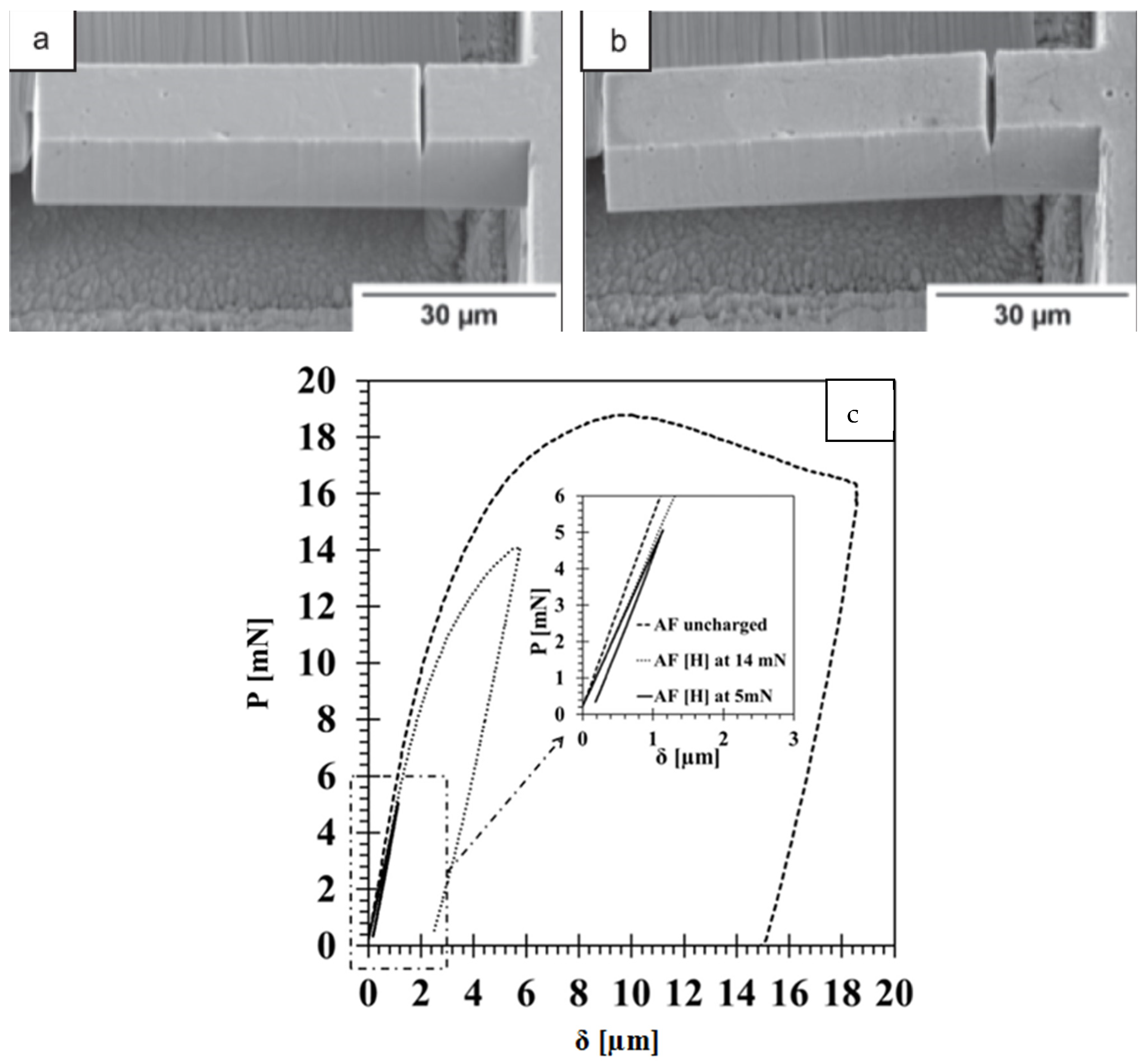
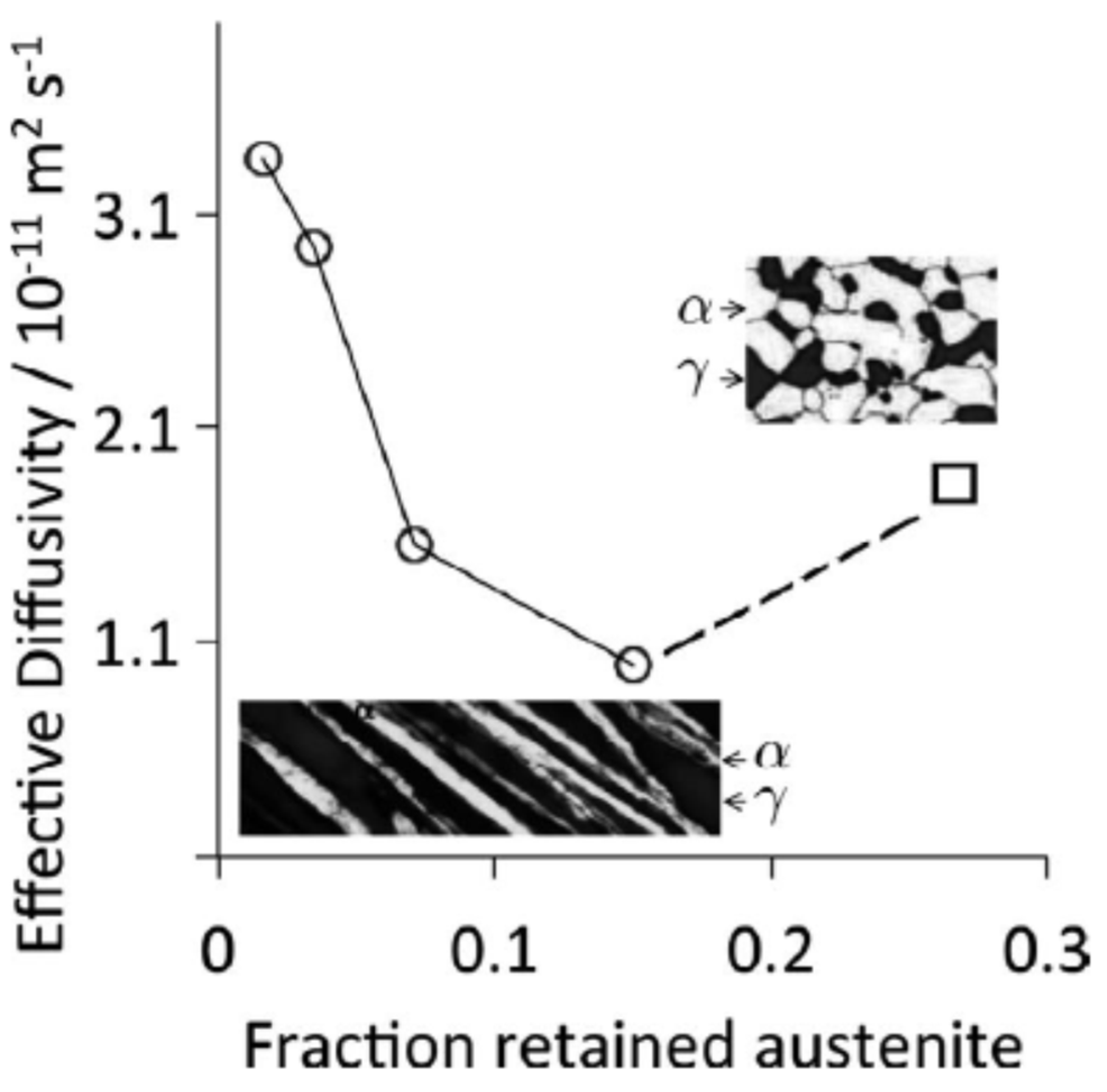
| Countries of Persian Gulf | CO2—Tonnes per Capita per Year (2021) |
|---|---|
| Bahrain | 26.7 |
| Iran | 8.5 |
| Iraq | 4.3 |
| Kingdom of Saudi Arabia | 18.7 |
| Kuwait | 25.0 |
| Oman | 17.9 |
| Qatar | 35.6 |
| United Arab Emirates | 21.8 |
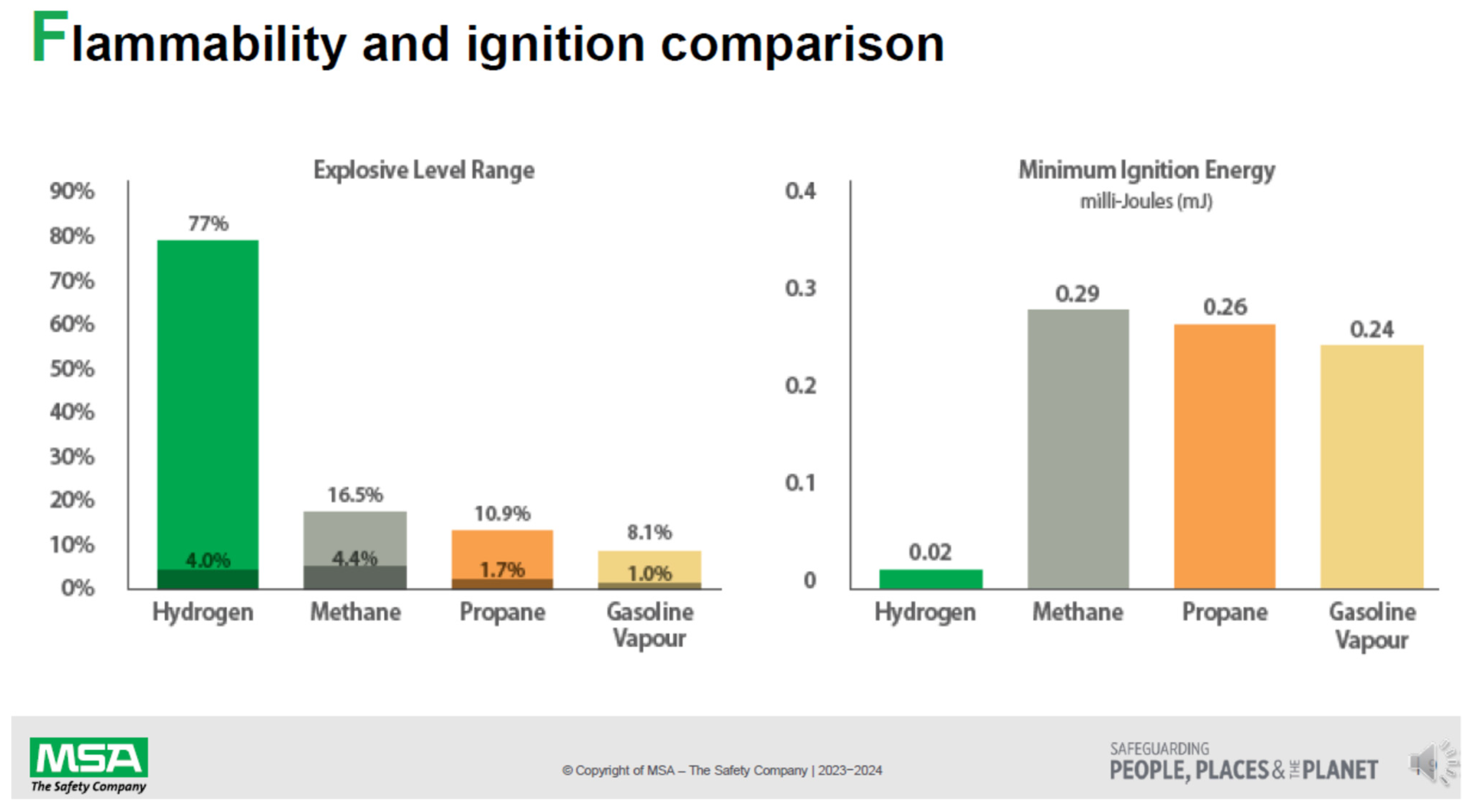
| Fuel | Thermal Energy of Combustion | Auto Ignition Temperature | |
|---|---|---|---|
| MJ/kg | BTU/lb | (°C) | |
| Hydrogen | 141.80 | 61,000 | 560 |
| Methane | 55.50 | 23,900 | 580 |
| Ethane | 51.90 | 22,400 | 515 |
| Propane | 50.35 | 21,700 | 455 |
| Butane | 49.50 | 20,900 | 405 |
| Pentane | 48.60 | 21,876 | 260 |
| Paraffin wax | 46.00 | 19,900 | 200–240 |
| Kerosene | 46.20 | 19,862 | 210 |
| Diesel | 44.80 | 19,300 | 210 |
| Coal (anthracite) | 32.50 | 14,000 | 930 |
| Gasoline (Petrol) | 46.00 | 19,800 | 246–280 |
| Wood fuel | 21.20 | 9142 | 180–230 |
| Mechanisms | Description | Reference |
|---|---|---|
| Hydrogen-enhanced decohesion (HEDE) | Reduction in interatomic cohesive bond strength due to interstitial segregation of dissolve hydrogen resulting in reduced local plasticity. | [74,75,76,77] |
| Adsorption-induced dislocation emission (AIDE) | Ease of dislocation generation and increased activity at the crack tip due to weakened interatomic bond strength initiated by hydrogen adsorption. | [78,79] |
| Hydrogen-enhanced localized plasticity (HELP) | Hydrogen dissolution and formation of hydrogen atmosphere at dislocation core reduces the barrier towards dislocation movement, promoting localized plasticity. | [80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghomashchi, R. Green Energy Revolution and Substitution of Hydrocarbons with Hydrogen: Distribution Network Infrastructure Materials. Energies 2023, 16, 8020. https://doi.org/10.3390/en16248020
Ghomashchi R. Green Energy Revolution and Substitution of Hydrocarbons with Hydrogen: Distribution Network Infrastructure Materials. Energies. 2023; 16(24):8020. https://doi.org/10.3390/en16248020
Chicago/Turabian StyleGhomashchi, Reza. 2023. "Green Energy Revolution and Substitution of Hydrocarbons with Hydrogen: Distribution Network Infrastructure Materials" Energies 16, no. 24: 8020. https://doi.org/10.3390/en16248020
APA StyleGhomashchi, R. (2023). Green Energy Revolution and Substitution of Hydrocarbons with Hydrogen: Distribution Network Infrastructure Materials. Energies, 16(24), 8020. https://doi.org/10.3390/en16248020





