Investigation of the Heat Storage Capacity and Storage Dynamics of a Novel Polymeric Macro-Encapsulated Core-Shell Particle Using a Paraffinic Core
Abstract
1. Introduction
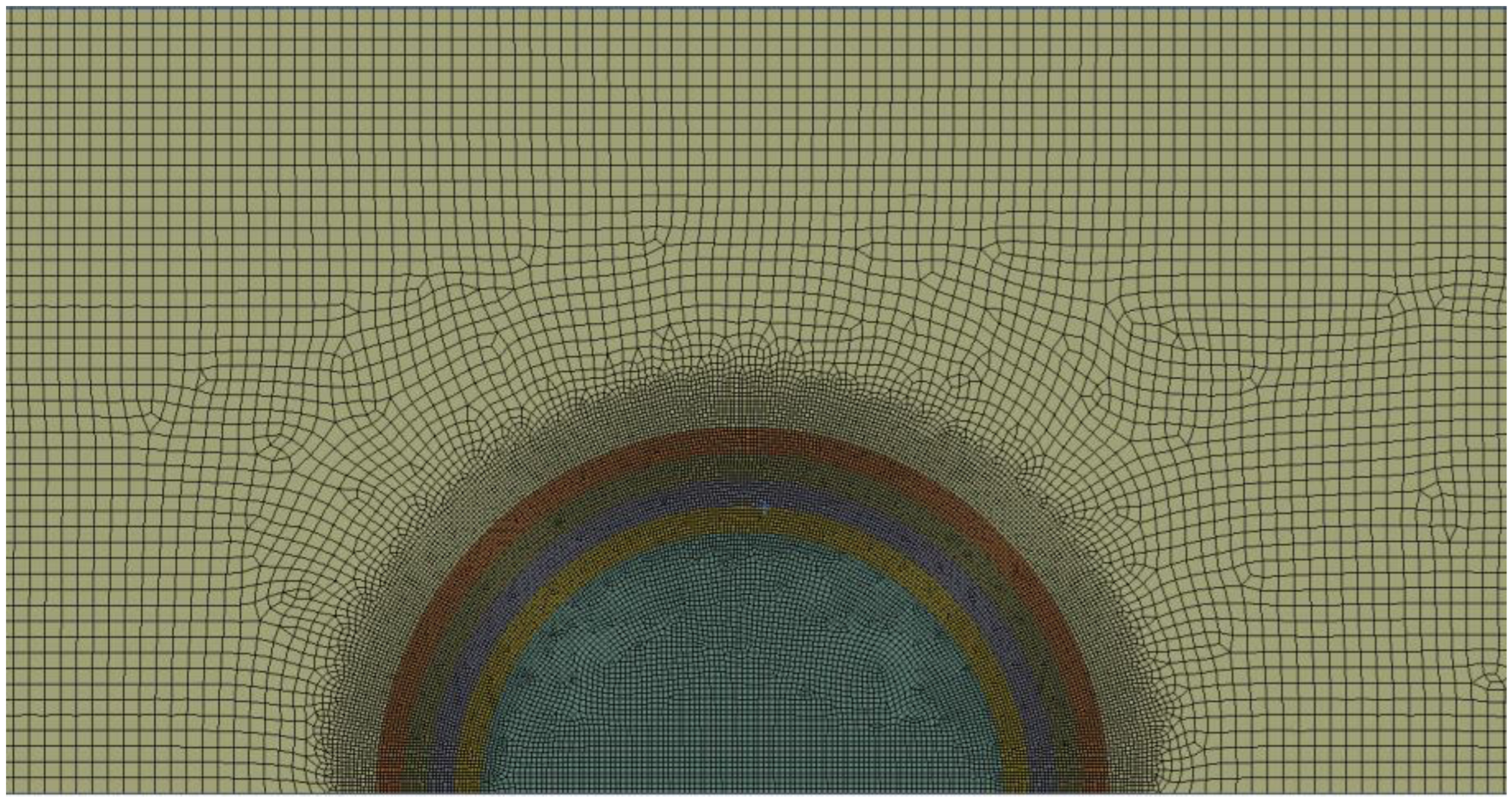
2. Methods—Computational Fluid Dynamics
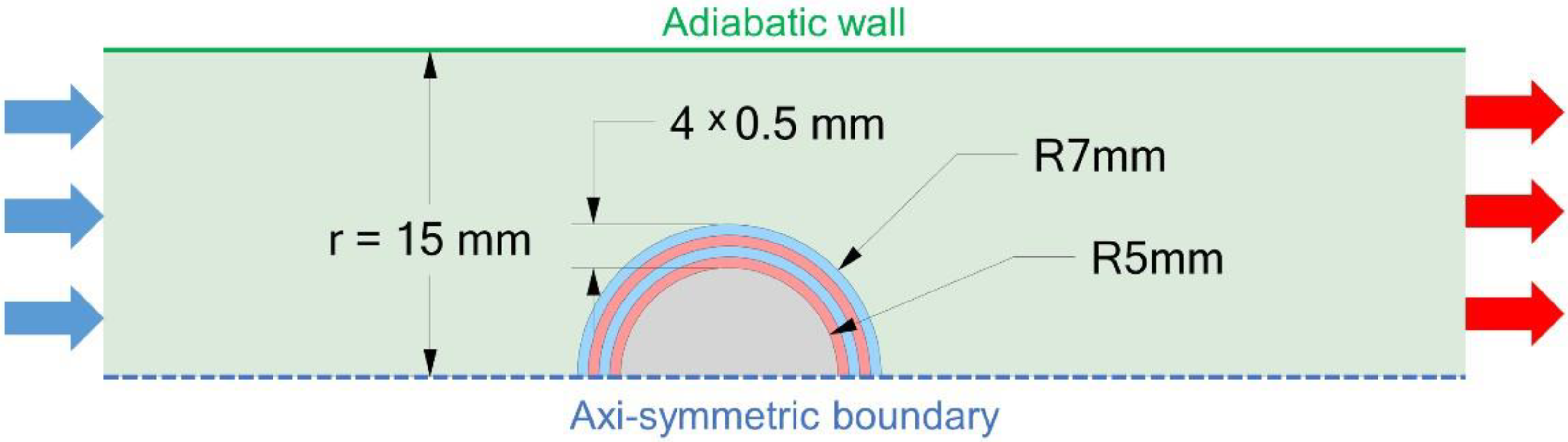
2.1. Simulation Domain and Boundary Conditions
2.2. Phase Transition Model Approach
3. Results
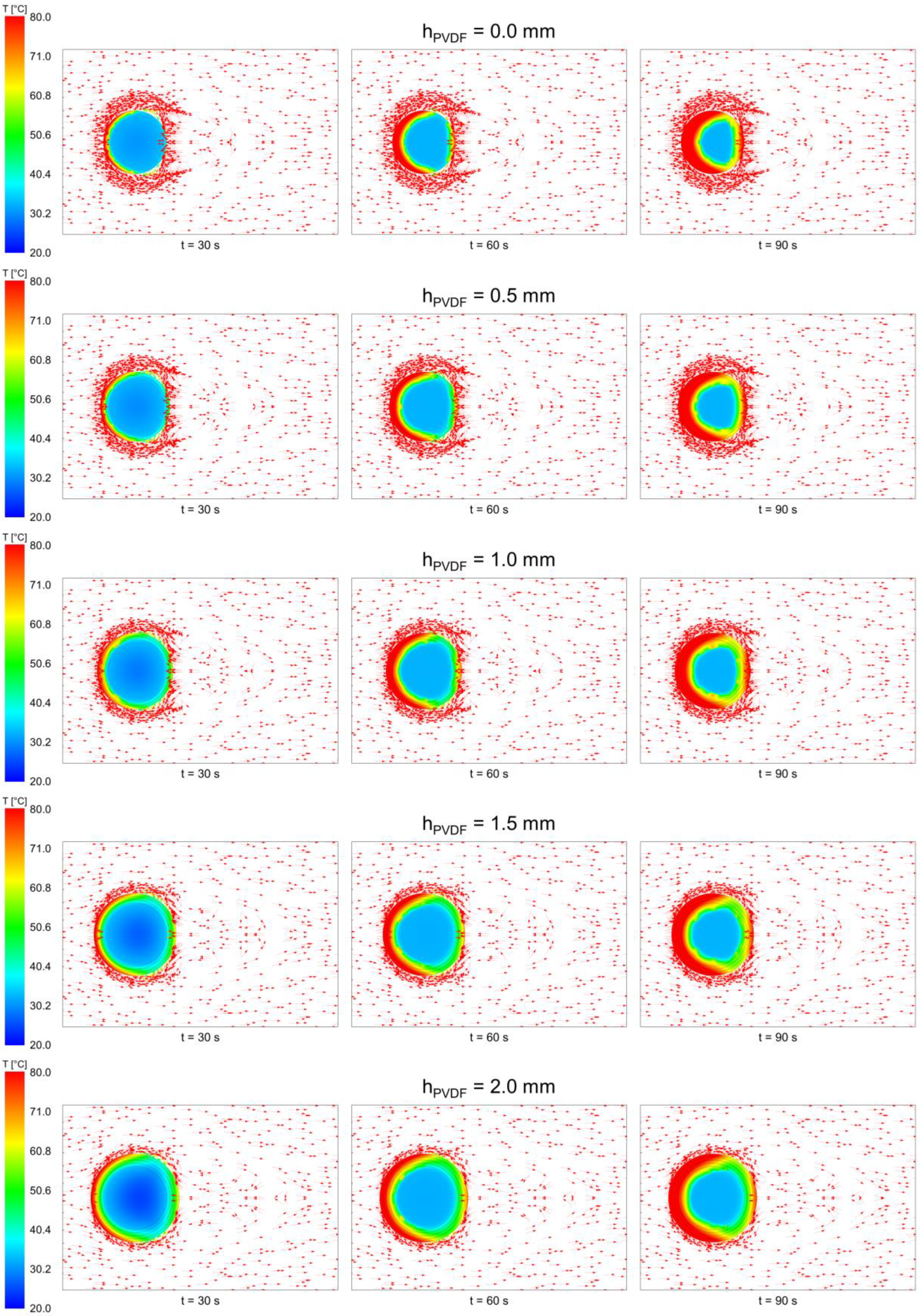
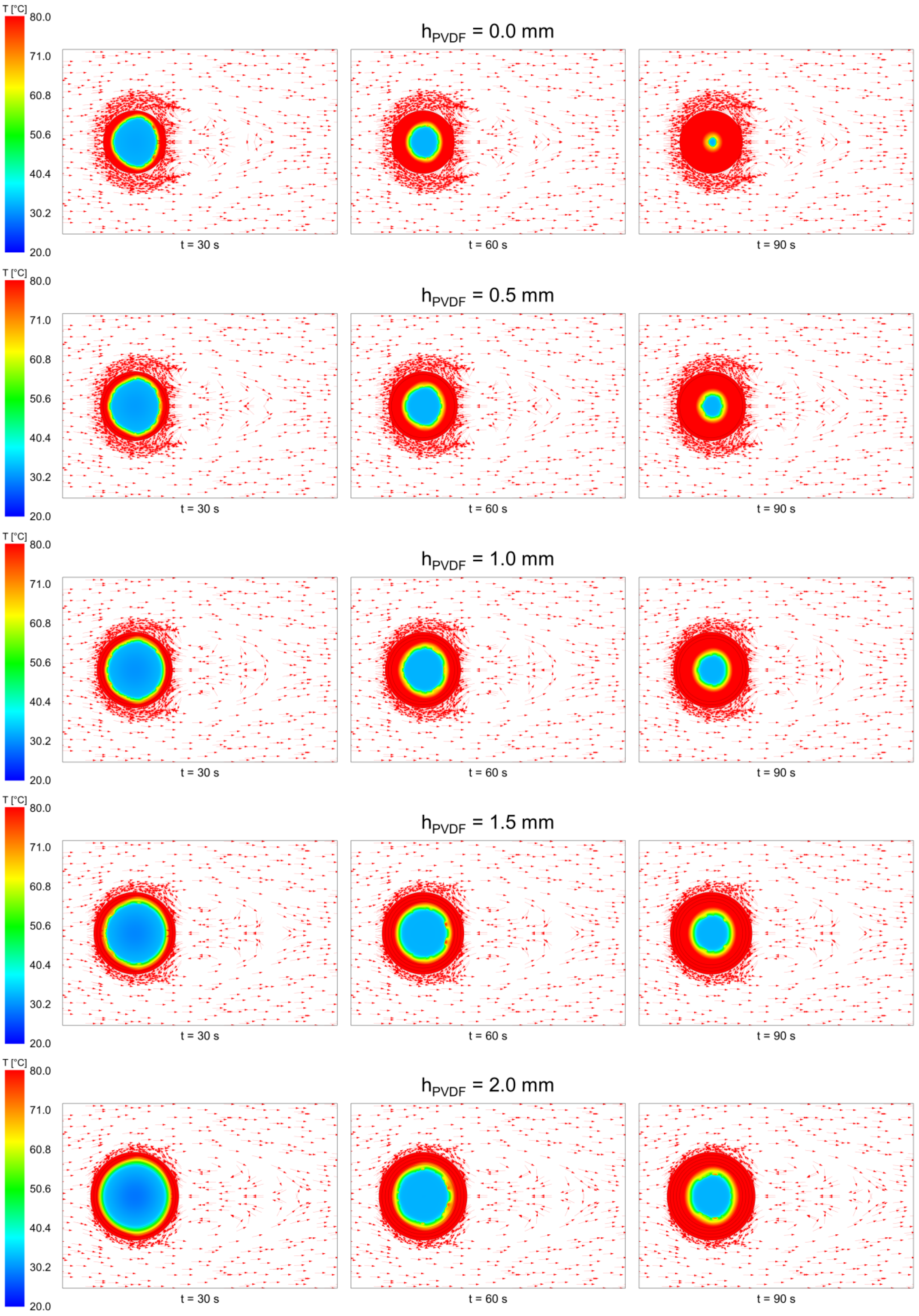
3.1. Temperature Field of the Core-Shell Particle during Heat Loading Process
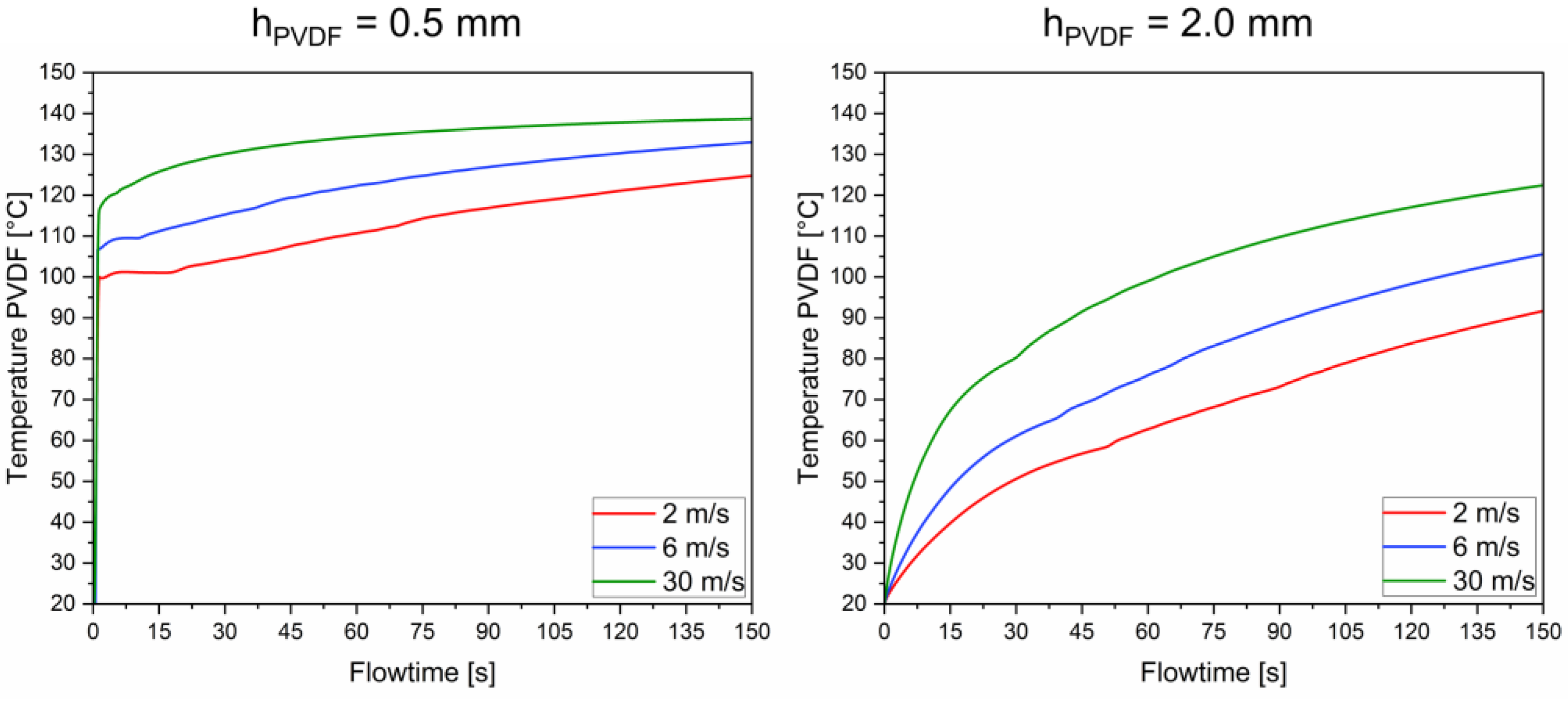
3.2. Velocity-Dependent Average Temperature Evolution in the PVDF Shell and the n-Eicosane Core
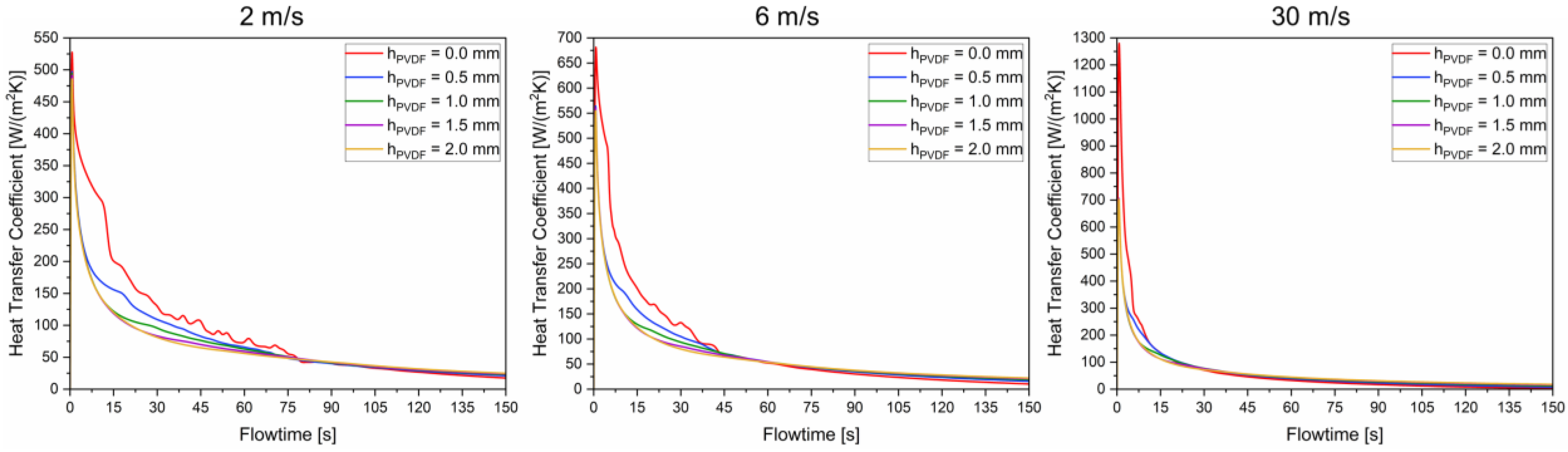
3.3. Influence of Shell Thickness and Working Fluid Velocity on the Heat Transfer Coefficient
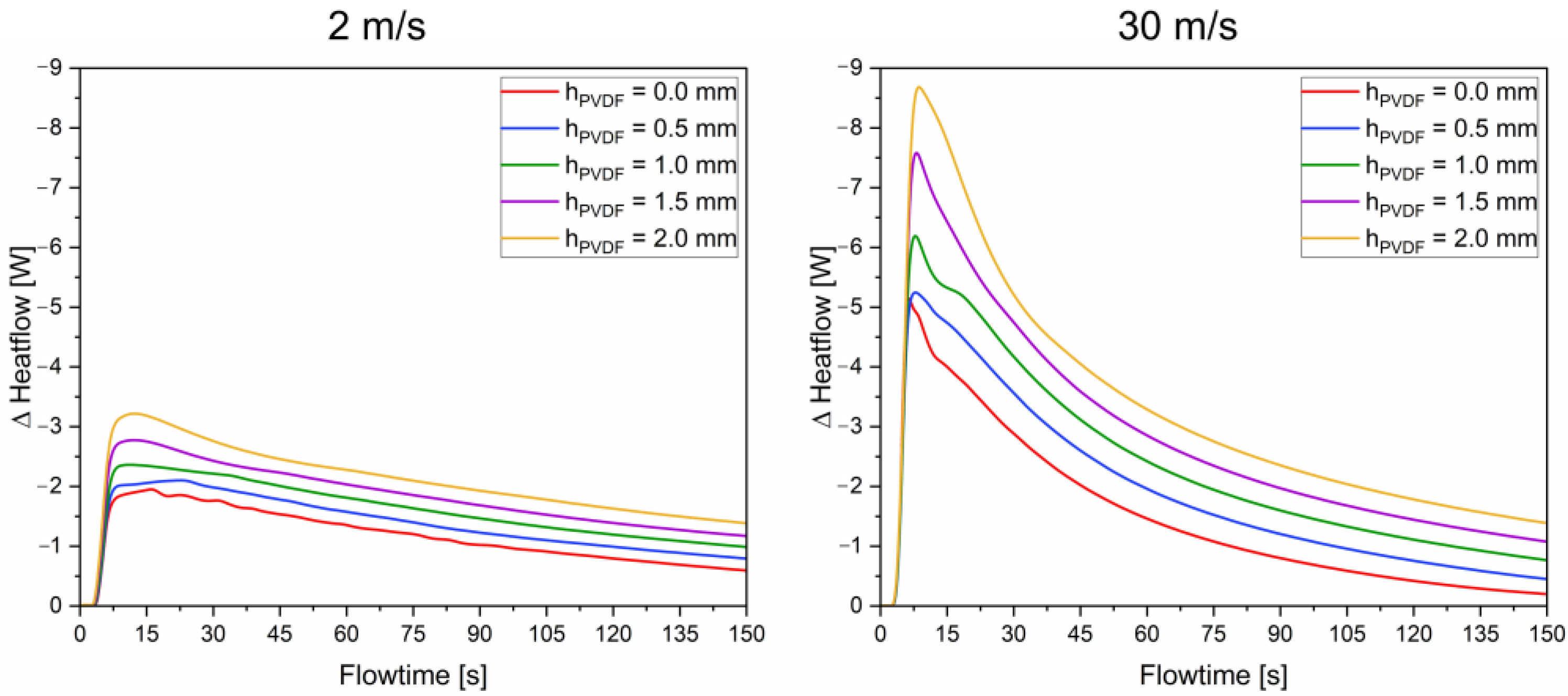
3.4. Impact of Shell Thickness and Working Fluid Velocity on the Heat Flow and Storage Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miró, L.; Gasia, J.; Cabeza, L.F. Thermal energy storage (TES) for industrial waste heat (IWH) recovery: A review. Appl. Energy 2016, 179, 284–301. [Google Scholar] [CrossRef]
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage 2021, 3, e135. [Google Scholar] [CrossRef]
- Preißinger, M.; Brüggemann, D. Thermoeconomic evaluation of modular organic Rankine cycles for waste heat recovery over a broad range of heat source temperatures and capacities. Energies 2017, 10, 269. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Thermal Energy Storage: Systems and Applications; John Wiley & Sons: New York, NY, USA, 2021. [Google Scholar]
- Benalcazar, P. Optimal sizing of thermal energy storage systems for CHP plants considering specific investment costs: A case study. Energy 2021, 234, 121323. [Google Scholar] [CrossRef]
- Ramos, A.; López, E.; del Cañizo, C.; Datas, A. Cost-effective ultra-high temperature latent heat thermal energy storage systems. J. Energy Storage 2022, 49, 104131. [Google Scholar] [CrossRef]
- Omara, A.A. Phase change materials for waste heat recovery in internal combustion engines: A review. J. Energy Storage 2021, 44, 103421. [Google Scholar] [CrossRef]
- Sharma, A.; Chen, C.R. Solar water heating system with phase change materials. Int. Rev. Chem. Eng. 2009, 1, 297–307. [Google Scholar]
- Mu, M.; Zhang, S.; Yang, S.; Wang, Y. Phase change materials applied in agricultural greenhouses. J. Energy Storage 2022, 49, 104100. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles-An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Zalba, B.; Marın, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Sharma, S.D.; Sagara, K. Latent heat storage materials and systems: A review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Hua, W. Review of preparation technologies of organic composite phase change materials in energy storage. J. Mol. Liq. 2021, 336, 115923. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Aramesh, M.; Shabani, B. Metal foams application to enhance the thermal performance of phase change materials: A review of experimental studies to understand the mechanisms. J. Energy Storage 2022, 50, 104650. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Ansu, A.K.; Goyal, R.; Sarı, A.; Tyagi, V.V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Li, J.; Guo, L.; Fang, M. Review of encapsulated salt hydrate core-shell phase change materials. KONA Powder Part. J. 2020, 37, 85–96. [Google Scholar] [CrossRef]
- Dutil, Y.; Rousse, D.R.; Salah, N.B.; Lassue, S.; Zalewski, L. A review on phase-change materials: Mathematical modeling and simulations. Renew. Sustain. Energy Rev. 2011, 15, 112–130. [Google Scholar] [CrossRef]
- Al-Abidi, A.A.; Mat, S.B.; Sopian, K.; Sulaiman, M.Y.; Mohammed, A.T. CFD applications for latent heat thermal energy storage: A review. Renew. Sustain. Energy Rev. 2013, 20, 353–363. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P. Numerical and experimental study of heat transfer characteristics of a shell-tube latent heat storage system: Part I–Charging process. Energy 2015, 79, 337–350. [Google Scholar] [CrossRef]
- Bouzennada, T.; Mechighel, F.; Ghachem, K.; Kolsi, L. Numerical Simulation of the Impact of the Heat Source Position on Melting of a Nano-Enhanced Phase Change Material. Nanomaterials 2021, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Maneengam, A.; Bouzennada, T.; Abderrahmane, A.; Guedri, K.; Weera, W.; Younis, O.; Bouallegue, B. Numerical Study of Lid-Driven Hybrid Nanofluid Flow in a Corrugated Porous Cavity in the Presence of Magnetic Field. Nanomaterials 2022, 12, 2390. [Google Scholar] [CrossRef]
- Maneengam, A.; Bouzennada, T.; Abderrahmane, A.; Ghachem, K.; Kolsi, L.; Younis, O.; Guedri, K.; Weera, W. Numerical Study of 3D MHD Mixed Convection and Entropy Generation in Trapezoidal Porous Enclosure Filled with a Hybrid Nanofluid: Effect of Zigzag Wall and Spinning Inner Cylinder. Nanomaterials 2022, 12, 1974. [Google Scholar] [CrossRef]
- Bouzennada, T.; Mechighel, F.; Ismail, T.; Kolsi, L.; Ghachem, K. Heat transfer and fluid flow in a PCM-filled enclosure: Effect of inclination angle and mid-separation fin. Int. Commun. Heat Mass Transf. 2021, 124, 105280. [Google Scholar] [CrossRef]
- Bouzennada, T.; Mechighel, F.; Filali, A.; Ghachem, K.; Kolsi, L. Numerical investigation of heat transfer and melting process in a PCM capsule: Effects of inner tube position and Stefan number. Case Stud. Therm. Eng. 2021, 27, 101306. [Google Scholar] [CrossRef]
- Bouzennada, T.; Mechighel, F.; Filali, A.; Kolsi, L. Study of the usability of sinusoidal function heat flux based on enthalpy-porosity technique for PCM-related applications. J. Therm. Anal. Calorim. 2020, 141, 1769–1784. [Google Scholar] [CrossRef]
- Sarani, I.; Xie, B.; Bao, Z.; Huo, W.; Li, X.; Xu, Y.; Wang, B.; Jiao, K. Analysis of phase change material thermal effects in large-scale proton-exchange membrane fuel cell based on open-source computational fluid dynamics. Appl. Therm. Eng. 2022, 216, 119143. [Google Scholar] [CrossRef]
- Jaguemont, J.; Omar, N.; Van den Bossche, P.; Mierlo, J. Phase-change materials (PCM) for automotive applications: A review. Appl. Therm. Eng. 2018, 132, 308–320. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Sasmito, A.P. Numerical investigation of heat transfer performance of a rotating latent heat thermal energy storage. Appl. Energy 2018, 227, 542–554. [Google Scholar] [CrossRef]
- Kumarasamy, K.; An, J.; Yang, J.; Yang, E.H. Novel CFD-based numerical schemes for conduction dominant encapsulated phase change materials (EPCM) with temperature hysteresis for thermal energy storage applications. Energy 2017, 132, 31–40. [Google Scholar] [CrossRef]
- Klimeš, L.; Charvát, P.; Joybari, M.M.; Zálešák, M.; Haghighat, F.; Panchabikesan, K.; Mankibi, M.E.; Yuan, Y. Computer modelling and experimental investigation of phase change hysteresis of PCMs: The state-of-the-art review. Appl. Energy 2020, 263, 114572. [Google Scholar] [CrossRef]
- Abdellatef, Y.; Kavgic, M. Thermal, microstructural and numerical analysis of hempcrete-microencapsulated phase change material composites. Appl. Therm. Eng. 2020, 178, 115520. [Google Scholar] [CrossRef]
- Menter, F.R. Two-equation eddy-viscosity turbulence models for engineering applications. AIAA J. 1994, 32, 1598–1605. [Google Scholar] [CrossRef]
- Dos Santos, W.N.; Iguchi, C.Y.; Gregorio, R., Jr. Thermal properties of poly (vinilidene fluoride) in the temperature range from 25 to 210 °C. Polym. Test. 2008, 27, 204–208. [Google Scholar] [CrossRef]
- Abdi, A.; Ignatowicz, M.; Gunasekara, S.N.; Chiu, J.N.; Martin, V. Experimental investigation of thermo-physical properties of n-octadecane and n-eicosane. Int. J. Heat Mass Transf. 2020, 161, 120285. [Google Scholar] [CrossRef]
- Wärmeatlas, V.D.I. Verein Deutscher Ingenieure VDI-Gesellschaft Verfahrenstechnik und Chemieingenieurwesen (GVC); Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]

| Material | Viscosity | Density | Thermal Conductivity | Isobaric Heat Capacity |
|---|---|---|---|---|
| Ammonia [40] (gas) | ||||
| PVDF [38] (solid) | - | |||
| n-eicosane [39] (solid/liquid) | - | |||
| hPVDF [mm] | Average Melting Time | ||
|---|---|---|---|
| 2 m/s | 6 m/s | 30 m/s | |
| 0.0 | 151.0 | 123.0 | 93.5 |
| 0.5 | 164.0 | 138.5 | 109.0 |
| 1.0 | 177.0 | 153.0 | 124.0 |
| 1.5 | 190.0 | 167.5 | 139.5 |
| 2.0 | 203.0 | 181.0 | 156.0 |
| hPVDF [mm] | Heat Accumulation | ||
|---|---|---|---|
| 2 m/s | 6 m/s | 30 m/s | |
| 0.0 | −175.57 | −200.86 | −220.82 |
| 0.5 | −205.59 | −245.53 | −285.83 |
| 1.0 | −238.48 | −289.83 | −352.03 |
| 1.5 | −272.28 | −337.60 | −421.34 |
| 2.0 | −311.31 | −386.05 | −491.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, M.; Fischlschweiger, M.; Zeiner, T. Investigation of the Heat Storage Capacity and Storage Dynamics of a Novel Polymeric Macro-Encapsulated Core-Shell Particle Using a Paraffinic Core. Energies 2023, 16, 957. https://doi.org/10.3390/en16020957
Singer M, Fischlschweiger M, Zeiner T. Investigation of the Heat Storage Capacity and Storage Dynamics of a Novel Polymeric Macro-Encapsulated Core-Shell Particle Using a Paraffinic Core. Energies. 2023; 16(2):957. https://doi.org/10.3390/en16020957
Chicago/Turabian StyleSinger, Matthias, Michael Fischlschweiger, and Tim Zeiner. 2023. "Investigation of the Heat Storage Capacity and Storage Dynamics of a Novel Polymeric Macro-Encapsulated Core-Shell Particle Using a Paraffinic Core" Energies 16, no. 2: 957. https://doi.org/10.3390/en16020957
APA StyleSinger, M., Fischlschweiger, M., & Zeiner, T. (2023). Investigation of the Heat Storage Capacity and Storage Dynamics of a Novel Polymeric Macro-Encapsulated Core-Shell Particle Using a Paraffinic Core. Energies, 16(2), 957. https://doi.org/10.3390/en16020957







