Common Reed and Maize Silage Co-Digestion as a Pathway towards Sustainable Biogas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Experimental Setup
2.3. Chemical Analyses
2.4. Calculations and Statistical Analyses
3. Results
3.1. Feedstock and Inoculum Characteristics
3.2. Methane Yield from Mono- and Co-Digestion of Maize and Reed Silages
3.3. Concentration of Hydrogen Sulphide and Ammonia in Biogas from Mono- and Co-Digestion of Maize and Reed Silages
3.4. Energy Balance and GHG Emissions
4. Discussion
4.1. Feedstock Characteristics
4.2. Methane Yield from Mono- and Co-Digestion of Maize and Reed Silages
4.3. Concentration of Hydrogen Sulfide and Ammonia in Biogas from Mono- and Co-Digestion of Maize and Reed Silages
4.4. Energy Balance and GHG Emissions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Bioenergy Association. Global Bioenergy Statistics 2021. Available online: https://www.worldbioenergy.org/uploads/211214%20WBA%20GBS%202021.pdf (accessed on 2 November 2022).
- International Energy Agency. Biogas Production by Region and by Feedstock Type. 2018. Available online: https://www.iea.org/data-and-statistics/charts/biogas-production-by-region-and-by-feedstock-type-2018 (accessed on 2 November 2022).
- Green Planet Energy. Paludicultur Und Biomethan—Nachhaltige Landnutzung, 1st ed.; Green Planet Energy: Hamburg, Germany, 2022. [Google Scholar]
- Fachagentur Nachwachsende Rohstoffe e.V., 2020. Bioenergy in Germany Facts and Figures 2020. Available online: https://www.fnr.de/fileadmin/allgemein/pdf/broschueren/broschuere_basisdaten_bioenergie_2020_engl_web.pdf (accessed on 3 November 2022).
- García-Lara, S.; Serna-Saldivar, S.O. Chapter 1—Corn History and Culture. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 1–18. ISBN 978-0-12-811971-6. [Google Scholar]
- FAOSTAT Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 December 2022).
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops That Feed the World 6. Past Successes and Future Challenges to the Role Played by Maize in Global Food Security. Food Sec. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Ni, T. Feedstock for Bioethanol Production from a Technological Paradigm Perspective. BioResources 2015, 10, 6285–6304. [Google Scholar] [CrossRef]
- Mumm, R.H.; Goldsmith, P.D.; Rausch, K.D.; Stein, H.H. Land Usage Attributed to Corn Ethanol Production in the United States: Sensitivity to Technological Advances in Corn Grain Yield, Ethanol Conversion, and Co-Product Utilization. Biotechnol. Biofuels 2014, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Dobers, G.M. Acceptance of Biogas Plants Taking into Account Space and Place. Energy Policy 2019, 135, 110987. [Google Scholar] [CrossRef]
- Wicki, L.; Naglis-Liepa, K.; Filipiak, T.; Parzonko, A.; Wicka, A. Is the Production of Agricultural Biogas Environmentally Friendly? Does the Structure of Consumption of First- and Second-Generation Raw Materials in Latvia and Poland Matter? Energies 2022, 15, 5623. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, E. Biogas Production from Different Lignocellulosic Biomass Sources: Advances and Perspectives. 3 Biotech 2018, 8, 233. [Google Scholar] [CrossRef]
- Kumar, R.; Bhardwaj, A.; Singh, L.P.; Singh, G. Environmental Impact Assessment of Maize Production in Northern India. IOP Conf. Ser. Earth Environ. Sci. 2022, 1084, 012042. [Google Scholar] [CrossRef]
- Rode, M.; Schneider, C.; Ketelhake, G.; Reißhauer, D. Naturschutzverträgliche Erzeugung Und Nutzung von Biomasse Zur Wärme–Und Stromgewinnung, 1st ed.; BfN–Skripten: Bonn, Germany, 2015. (In German) [Google Scholar]
- Vergara, F.; Lakes, T.M. Maizification of the Landscape for Biogas Production? Identifying the Likelihood of Silage Maize for Biogas in Brandenburg from 2008–2018; FORLand-Working Paper; Humboldt-Universität zu Berlin: Berlin, Germany, 2019. [Google Scholar]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.-L. Functional Landscape Heterogeneity and Animal Biodiversity in Agricultural Landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does Conservation on Farmland Contribute to Halting the Biodiversity Decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef]
- Banaszuk, P.; Wysocka-Czubaszek, A.; Czubaszek, R.; Roj-Rojewski, S. Skutki energetycznego wykorzystania biomasy. Wieś I Rol. 2015, 4, 139–152. (In Polish) [Google Scholar]
- Agostini, A.; Battini, F.; Padella, M.; Giuntoli, J.; Baxter, D.; Marelli, L.; Amaducci, S. Economics of GHG Emissions Mitigation via Biogas Production from Sorghum, Maize and Dairy Farm Manure Digestion in the Po Valley. Biomass Bioenergy 2016, 89, 58–66. [Google Scholar] [CrossRef]
- Lüker-Jans, N.; Simmering, D.; Otte, A. The Impact of Biogas Plants on Regional Dynamics of Permanent Grassland and Maize Area—The Example of Hesse, Germany (2005–2010). Agric. Ecosys. Environ. 2017, 241, 24–38. [Google Scholar] [CrossRef]
- Markou, G.; Brulé, M.; Balafoutis, A.; Kornaros, M.; Georgakakis, D.; Papadakis, G. Biogas Production from Energy Crops in Northern Greece: Economics of Electricity Generation Associated with Heat Recovery in a Greenhouse. Clean. Techn. Environ. Policy 2017, 19, 1147–1167. [Google Scholar] [CrossRef]
- Couwenberg, J. Biomass Energy Crops on Peatlands: On Emissions and Perversions. Int. Mire Conserv. Group (IMCG) Newsl. Issue 2007, 3, 12–14. [Google Scholar]
- Bacenetti, J.; Sala, C.; Fusi, A.; Fiala, M. Agricultural Anaerobic Digestion Plants: What LCA Studies Pointed out and What Can Be Done to Make Them More Environmentally Sustainable. Appl. Energy 2016, 179, 669–686. [Google Scholar] [CrossRef]
- Balcioglu, G.; Jeswani, H.K.; Azapagic, A. Evaluating the Environmental and Economic Sustainability of Energy from Anaerobic Digestion of Different Feedstocks in Turkey. Sustain. Prod. Consum. 2022, 32, 924–941. [Google Scholar] [CrossRef]
- Mu, H.; Zhao, C.; Zhao, Y.; Li, Y.; Hua, D.; Zhang, X.; Xu, H. Enhanced Methane Production by Semi-Continuous Mesophilic Co-Digestion of Potato Waste and Cabbage Waste: Performance and Microbial Characteristics Analysis. Bioresour. Technol. 2017, 236, 68–76. [Google Scholar] [CrossRef]
- Chuanchai, A.; Ramaraj, R. Sustainability Assessment of Biogas Production from Buffalo Grass and Dung: Biogas Purification and Bio-Fertilizer. 3 Biotech 2018, 8, 151. [Google Scholar] [CrossRef]
- Vats, N.; Khan, A.A.; Ahmad, K. Options for Enhanced Anaerobic Digestion of Waste and Biomass—A Review. J. Biosyst. Eng. 2020, 45, 1–15. [Google Scholar] [CrossRef]
- Miramontes-Martínez, L.R.; Rivas-García, P.; Albalate-Ramírez, A.; Botello-Álvarez, J.E.; Escamilla-Alvarado, C.; Gomez-Gonzalez, R.; Alcalá-Rodríguez, M.M.; Valencia-Vázquez, R.; Santos-López, I.A. Anaerobic Co-Digestion of Fruit and Vegetable Waste: Synergy and Process Stability Analysis. J. Air Waste Manag. Assoc. 2021, 71, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Riau, V.; Burgos, L.; Camps, F.; Domingo, F.; Torrellas, M.; Antón, A.; Bonmatí, A. Closing Nutrient Loops in a Maize Rotation. Catch Crops to Reduce Nutrient Leaching and Increase Biogas Production by Anaerobic Co-Digestion with Dairy Manure. Waste Manag. 2021, 126, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Wilinska-Lisowska, A.; Ossowska, M.; Czerwionka, K. The Influence of Co-Fermentation of Agri-Food Waste with Primary Sludge on Biogas Production and Composition of the Liquid Fraction of Digestate. Energies 2021, 14, 1907. [Google Scholar] [CrossRef]
- Vijin Prabhu, A.; Sivaram, A.R.; Prabhu, N.; Sundaramahalingam, A. A Study of Enhancing the Biogas Production in Anaerobic Digestion. Mater. Today Proc. 2021, 45, 7994–7999. [Google Scholar] [CrossRef]
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass. Valori. 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Aklilu, E.G.; Waday, Y.A. Optimizing the Process Parameters to Maximize Biogas Yield from Anaerobic Co-Digestion of Alkali-Treated Corn Stover and Poultry Manure Using Artificial Neural Network and Response Surface Methodology. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Chakraborty, D.; Palani, S.G.; Ghangrekar, M.M.; Anand, N.; Pathak, P. Dual Role of Grass Clippings as Buffering Agent and Biomass during Anaerobic Co-Digestion with Food Waste. Clean Techn. Environ. Policy 2022, 24, 2787–2799. [Google Scholar] [CrossRef]
- Velásquez Piñas, J.A.; Venturini, O.J.; Silva Lora, E.E.; Calle Roalcaba, O.D. Technical Assessment of Mono-Digestion and Co-Digestion Systems for the Production of Biogas from Anaerobic Digestion in Brazil. Renew. Energy 2018, 117, 447–458. [Google Scholar] [CrossRef]
- Böjti, T.; Kovács, K.L.; Kakuk, B.; Wirth, R.; Rákhely, G.; Bagi, Z. Pretreatment of Poultry Manure for Efficient Biogas Production as Monosubstrate or Co-Fermentation with Maize Silage and Corn Stover. Anaerobe 2017, 46, 138–145. [Google Scholar] [CrossRef]
- Kalamaras, S.D.; Kotsopoulos, T.A. Anaerobic Co-Digestion of Cattle Manure and Alternative Crops for the Substitution of Maize in South Europe. Bioresour. Technol. 2014, 172, 68–75. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gómez, X.; Martínez, E.J.; Fierro, J.; Otero, M. Feasibility of Anaerobic Co-Digestion of Poultry Blood with Maize Residues. Bioresour. Technol. 2013, 144, 513–520. [Google Scholar] [CrossRef]
- Gruber-Brunhumer, M.R.; Montgomery, L.F.R.; Nussbaumer, M.; Schoepp, T.; Zohar, E.; Muccio, M.; Ludwig, I.; Bochmann, G.; Fuchs, W.; Drosg, B. Effects of Partial Maize Silage Substitution with Microalgae on Viscosity and Biogas Yields in Continuous AD Trials. J. Biotechnol. 2019, 295, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Battini, F.; Giuntoli, J.; Tabaglio, V.; Padella, M.; Baxter, D.; Marelli, L.; Amaducci, S. Environmentally Sustainable Biogas? The Key Role of Manure Co-Digestion with Energy Crops. Energies 2015, 8, 5234–5265. [Google Scholar] [CrossRef]
- Lask, J.; Martínez Guajardo, A.; Weik, J.; von Cossel, M.; Lewandowski, I.; Wagner, M. Comparative Environmental and Economic Life Cycle Assessment of Biogas Production from Perennial Wild Plant Mixtures and Maize (Zea mays L.) in Southwest Germany. Glob. Change Biol. Bioenergy 2020, 12, 571–585. [Google Scholar] [CrossRef]
- De Vries, J.W.; Vinken, T.M.W.J.; Hamelin, L.; De Boer, I.J.M. Comparing Environmental Consequences of Anaerobic Mono- and Co-Digestion of Pig Manure to Produce Bio-Energy—A Life Cycle Perspective. Bioresour. Technol. 2012, 125, 239–248. [Google Scholar] [CrossRef]
- Tanneberger, F.; Wichtmann, W. Land Use Options for Rewetted Peatlands. In Carbon Credits from Peatland Rewetting. Climate-Biodiversity-Land Use, 1st ed.; Tanneberger, F., Wichtmann, W., Eds.; Schweizerbart Science Publishers: Stuttgart, Germany, 2011; pp. 107–132. [Google Scholar]
- Granéli, W. Reed Phragmites australis (Cav.) Trin. Ex Steudel as an Energy Source in Sweden. Biomass 1984, 4, 183–208. [Google Scholar] [CrossRef]
- Brix, H.; Sorrell, B.K.; Lorenzen, B. Are Phragmites-Dominated Wetlands a Net Source or Net Sink of Greenhouse Gases? Aquat. Bot. 2001, 69, 313–324. [Google Scholar] [CrossRef]
- Hallam, A.; Anderson, I.C.; Buxton, D.R. Comparative Economic Analysis of Perennial, Annual, and Intercrops for Biomass Production. Biomass Bioenergy. 2001, 21, 407–424. [Google Scholar] [CrossRef]
- Jasinskas, A.; Zaltauskas, A.; Kryzeviciene, A. The Investigation of Growing and Using of Tall Perennial Grasses as Energy Crops. Biomass Bioenergy 2008, 32, 981–987. [Google Scholar] [CrossRef]
- Massé, D.; Gilbert, Y.; Savoie, P.; Bélanger, G.; Parent, G.; Babineau, D. Methane Yield from Switchgrass and Reed Canarygrass Grown in Eastern Canada. Bioresour. Technol. 2011, 102, 10286–10292. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The Utilisation of Reed (Phragmites australis): A Review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Pocienė, L.; Šarūnaitė, L.; Tilvikienė, V.; Šlepetys, J.; Kadžiulienė, Ž. The Yield and Composition of Reed Canary Grass Biomass as Raw Material for Combustion. Biologija 2013, 59, 195–200. [Google Scholar] [CrossRef]
- Hartung, C.; Andrade, D.; Dandikas, V.; Eickenscheidt, T.; Drösler, M.; Zollfrank, C.; Heuwinkel, H. Suitability of Paludiculture Biomass as Biogas Substrate—Biogas Yield and Long-Term Effects on Anaerobic Digestion. Renew. Energy 2020, 159, 64–71. [Google Scholar] [CrossRef]
- Geurts, J.J.M.; van Duinen, G.-J.A.; van Belle, J.; Wichmann, S.; Wichtmann, W.; Fritz, C. Recognize the High Potential of Paludiculture on Rewetted Peat Soils to Mitigate Climate Change. Landbauforsch. J. Sustain. Org. Agric. Syst. 2019, 69, 5–8. [Google Scholar] [CrossRef]
- United Nations Environment Programme Global Peatlands Assessment: The State of the World’s Peatlands. Available online: http://www.unep.org/resources/global-peatlands-assessment-2022 (accessed on 27 November 2022).
- Humpenöder, F.; Karstens, K.; Lotze-Campen, H.; Leifeld, J.; Menichetti, L.; Barthelmes, A.; Popp, A. Peatland Protection and Restoration Are Key for Climate Change Mitigation. Environ. Res. Lett. 2020, 15, 104093. [Google Scholar] [CrossRef]
- Joosten, H.; Tanneberger, F.; Moen, A. (Eds.) Mires and Peatlans of Europe. Status. Distribution and Conservation, 1st ed.; Schweizerbart Science Publishers: Stuttgart, Germany, 2017. [Google Scholar]
- Tanneberger, F.; Appulo, L.; Ewert, S.; Lakner, S.; Ó Brolcháin, N.; Peters, J.; Wichtmann, W. The Power of Nature-Based Solutions: How Peatlands Can Help Us to Achieve Key EU Sustainability Objectives. Adv. Sustain. Syst. 2021, 5, 2000146. [Google Scholar] [CrossRef]
- Raichel, D.L.; Able, K.W.; Hartman, J.M. The Influence of Phragmites (Common Reed) on the Distribution, Abundance, and Potential Prey of a Resident Marsh Fish in the Hackensack Meadowlands, New Jersey. Estuaries 2003, 26, 511–521. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Pisello, A.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable Ethanol Production from Common Reed (Phragmites australis) through Simultaneuos Saccharification and Fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef]
- Banaszuk, P.; Kamocki, A.K.; Wysocka-Czubaszek, A.; Czubaszek, R.; Roj-Rojewski, S. Closing the Loop—Recovery of Nutrients and Energy from Wetland Biomass. Ecol. Eng. 2020, 143, 105643. [Google Scholar] [CrossRef]
- Gómez-Sánchez, M.D.; Sánchez, R.; Espinosa, E.; Rosal, A.; Rodríguez, A. Production of Cellulosic Pulp from Reed (Phragmites Australis) to Produce Paper and Paperboard. Bioprocess Eng. 2017, 1, 65. [Google Scholar] [CrossRef]
- Dragoni, F.; Giannini, V.; Ragaglini, G.; Bonari, E.; Silvestri, N. Effect of Harvest Time and Frequency on Biomass Quality and Biomethane Potential of Common Reed (Phragmites australis) Under Paludiculture Conditions. Bioenergy Res. 2017, 10, 1066–1078. [Google Scholar] [CrossRef]
- Roj-Rojewski, S.; Wysocka-Czubaszek, A.; Czubaszek, R.; Kamocki, A.; Banaszuk, P. Anaerobic Digestion of Wetland Biomass from Conservation Management for Biogas Production. Biomass Bioenergy 2019, 122, 126–132. [Google Scholar] [CrossRef]
- Jagadabhi, P.S.; Kaparaju, P.; Rintala, J. Two-Stage Anaerobic Digestion of Tomato, Cucumber, Common Reed and Grass Silage in Leach-Bed Reactors and Upflow Anaerobic Sludge Blanket Reactors. Bioresour. Technol. 2011, 102, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Wichtmann, W.; Banaszuk, P. Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies. Energies 2021, 14, 8373. [Google Scholar] [CrossRef]
- Müller, J.; Jantzen, C.; Wiedow, D. The Energy Potential of Soft Rush (Juncus effusus L.) in Different Conversion Routes. Energy Sustain. Soc. 2020, 10, 26. [Google Scholar] [CrossRef]
- Baute, K.; Van Eerd, L.; Robinson, D.; Sikkema, P.; Mushtaq, M.; Gilroyed, B. Comparing the Biomass Yield and Biogas Potential of Phragmites Australis with Miscanthus x Giganteus and Panicum virgatum Grown in Canada. Energies 2018, 11, 2198. [Google Scholar] [CrossRef]
- Ohlsson, L.-O.; Karlsson, S.; Rupar-Gadd, K.; Albers, E.; Welander, U. Evaluation of Laminaria digitata and Phragmites australis for Biogas Production and Nutrient Recycling. Biomass Bioenergy 2020, 140, 105670. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Xi, B.; Sun, W.; Xia, X.; Zhu, C.; He, X.; Li, M.; Yang, T.; Wang, P.; et al. Biogas Production Improvement and C/N Control by Natural Clinoptilolite Addition into Anaerobic Co-Digestion of Phragmites australis, Feces and Kitchen Waste. Bioresour. Technol. 2015, 180, 192–199. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and Ternary Trace Elements to Enhance Anaerobic Digestion of Cattle Manure: Focusing on Kinetic Models for Biogas Production and Digestate Utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef]
- APHA. Standard Methods: For the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Pollution Control Federation: Washington, DC, USA, 1998; ISBN 978-0-87553-235-6.
- Statistics Poland. Production of Agricultural and Horticultural Crops in 2021, 1st ed.; Zakład Wydawnictw Statystycznych: Warsaw, Poland, 2022. [Google Scholar]
- European Biomass Association. Statistical Report 2017; European Biomass Association: Brussels, Belgium, 2017. [Google Scholar]
- The National Centre for Emissions Management. Emission Factors for CO2, SO2, NOx and Total Particulate Matter for Electrical Energy on the Basis of Information in National Database on Emissions of Greenhouse Gases and Other Substances for 2020; The National Centre for Emissions Management: Warsaw, Poland, 2020. (In Polish) [Google Scholar]
- Hryniewicz, M.; Grzybek, A. Estimation of Greenhouse Gases Emission from Maize for Silage Crop by LCA Method. Probl. Agric. Eng. 2016, 1, 63–73. (in Polish). [Google Scholar]
- Schittenhelm, S. Chemical Composition and Methane Yield of Maize Hybrids with Contrasting Maturity. Eur. J. Agron. 2008, 29, 72–79. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, X.; Zhu, W.; Wang, X.; Chen, S.; Cheng, X.; Cui, Z. Methane Yield through Anaerobic Digestion for Various Maize Varieties in China. Bioresour. Technol. 2012, 118, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorowska, B.; Płaza, A.; Rzążewska, E.; Waranica, M. Effect of Weather Conditions on the Content of Sugars in Plants of European Maize Cultivars Grown for Silage. Acta Agroph. 2020, 26, 57–64. [Google Scholar] [CrossRef]
- Nenova, L.; Benkova, M.; Simeonova, T.; Atanassova, I. Nitrogen, Phosphorus and Potassium Content in Maize Dry Biomass under the Effect of Different Levels of Mineral Fertilization. Agric. Sci. Technol. 2019, 11, 311–316. [Google Scholar] [CrossRef]
- Bojtor, C.; Mousavi, S.M.N.; Illés, Á.; Golzardi, F.; Széles, A.; Szabó, A.; Nagy, J.; Marton, C.L. Nutrient Composition Analysis of Maize Hybrids Affected by Different Nitrogen Fertilisation Systems. Plants 2022, 11, 1593. [Google Scholar] [CrossRef]
- Lin, S.; Pi, Y.; Long, D.; Duan, J.; Zhu, X.; Wang, X.; He, J.; Zhu, Y. Impact of Organic and Chemical Nitrogen Fertilizers on the Crop Yield and Fertilizer Use Efficiency of Soybean–Maize Intercropping Systems. Agriculture 2022, 12, 1428. [Google Scholar] [CrossRef]
- Seppälä, M.; Pyykkönen, V.; Väisänen, A.; Rintala, J. Biomethane Production from Maize and Liquid Cow Manure—Effect of Share of Maize, Post-Methanation Potential and Digestate Characteristics. Fuel 2013, 107, 209–216. [Google Scholar] [CrossRef]
- Skowrońska, M.; Filipek, T. Accumulation of Nitrogen and Phosphorus by Maize as a Result of a Reduction in the Potassium Fertilization Rate. Ecol. Chem. Eng. S 2010, 17, 83–88. [Google Scholar]
- Zhao, X.; Liu, J.; Liu, J.; Yang, F.; Zhu, W.; Yuan, X.; Hu, Y.; Cui, Z.; Wang, X. Effect of Ensiling and Silage Additives on Biogas Production and Microbial Community Dynamics during Anaerobic Digestion of Switchgrass. Bioresour. Technol. 2017, 241, 349–359. [Google Scholar] [CrossRef]
- Herrmann, C.; Prochnow, A.; Heiermann, M.; Idler, C. Biomass from Landscape Management of Grassland Used for Biogas Production: Effects of Harvest Date and Silage Additives on Feedstock Quality and Methane Yield. Grass Forage Sci. 2014, 69, 549–566. [Google Scholar] [CrossRef]
- Mosier, N. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Wenzel, M.; Kabengele, G.; Dahms, T.; Barz, M.; Wichtmann, W. Bioenergie Aus Nassen Mooren. Thermische Verwertung von Halmgutartiger Biomasse Aus Paludikultur, 1st ed.; Institut für Botanik und Landschaftsökologie, Universität Greifswald, Greifswald Moor Centrum: Greifswald, Germany, 2022. (In German) [Google Scholar]
- Lizasoain, J.; Rincón, M.; Theuretzbacher, F.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; Zweckmair, T.; Gronauer, A.; Bauer, A. Biogas Production from Reed Biomass: Effect of Pretreatment Using Different Steam Explosion Conditions. Biomass Bioenergy 2016, 95, 84–91. [Google Scholar] [CrossRef]
- Borin, M.; Florio, G.; Barbera, A.; Cirelli, G.L.; Albergo, R.; Palazzo, S. Preliminary Evaluation of Macrophyte Wetland Biomasses to Obtain Second Generation Ethanol. In Proceedings of the 19th European Biomass Conference and Exhibition, Berlin, Germany, 6–10 June 2011. [Google Scholar]
- Van Tran, G.; Unpaprom, Y.; Ramaraj, R. Methane Productivity Evaluation of an Invasive Wetland Plant, Common Reed. Biomass Conv. Bioref. 2020, 10, 689–695. [Google Scholar] [CrossRef]
- Wöhler-Geske, A.; Moschner, C.R.; Gellerich, A. Provenances and Properties of Thatching Reed (Phragmites australis). Appl. Agric. For. Res. 2016, 86, 1–10. [Google Scholar] [CrossRef]
- López-González, D.; Avalos-Ramirez, A.; Giroir-Fendler, A.; Godbout, S.; Fernandez-Lopez, M.; Sanchez-Silva, L.; Valverde, J.L. Combustion Kinetic Study of Woody and Herbaceous Crops by Thermal Analysis Coupled to Mass Spectrometry. Energy 2015, 90, 1626–1635. [Google Scholar] [CrossRef]
- Baran, M.; Váradyová, Z.; Kráčmár, S.; Hedvábný, J. The Common Reed (Phragmites australis) as a Source of Roughage in Ruminant Nutrition. Acta Vet. Brno 2002, 71, 445–449. [Google Scholar] [CrossRef]
- Kwietniewska, E.; Tys, J. Process Characteristics, Inhibition Factors and Methane Yields of Anaerobic Digestion Process, with Particular Focus on Microalgal Biomass Fermentation. Renew. Sust. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Herrmann, A.; Rath, J. Biogas Production from Maize: Current State, Challenges, and Prospects. 1. Methane Yield Potential. Bioenergy Res. 2012, 5, 1027–1042. [Google Scholar] [CrossRef]
- Kreuger, E.; Nges, I.; Björnsson, L. Ensiling of Crops for Biogas Production: Effects on Methane Yield and Total Solids Determin. Biotechnol. Biofuels 2011, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Idler, C.; Heiermann, M. Improving Aerobic Stability and Biogas Production of Maize Silage Using Silage Additives. Bioresour. Technol. 2015, 197, 393–403. [Google Scholar] [CrossRef]
- Gross, F.; Riebe, K. Gärfutter: Betriebswirtschaft, Erzeugung, Verfütterung, 1st ed.; UlmeVerlag Eugen: Stuttgart, Germany, 1974; ISBN 978-3-8001-4321-4. (In German) [Google Scholar]
- Eller, F.; Ehde, P.M.; Oehmke, C.; Ren, L.; Brix, H.; Sorrell, B.K.; Weisner, S.E.B. Biomethane Yield from Different European Phragmites australis genotypes, compared with other herbaceous wetland species grown at different fertilization regimes. Resources 2020, 9, 57. [Google Scholar] [CrossRef]
- Bareha, Y.; Faucher, J.-P.; Michel, M.; Houdon, M.; Vaneeckhaute, C. Evaluating the Impact of Substrate Addition for Anaerobic Co-Digestion on Biogas Production and Digestate Quality: The Case of Deinking Sludge. J. Environ. Manag. 2022, 319, 115657. [Google Scholar] [CrossRef] [PubMed]
- Wickham, R.; Galway, B.; Bustamante, H.; Nghiem, L.D. Biomethane Potential Evaluation of Co-Digestion of Sewage Sludge and Organic Wastes. Int. Biodeterior. Biodegrad. 2016, 113, 3–8. [Google Scholar] [CrossRef]
- Feng, L.; Wahid, R.; Ward, A.J.; Møller, H.B. Anaerobic Co-Digestion of Cattle Manure and Meadow Grass: Effect of Serial Configurations of Continuous Stirred Tank Reactors (CSTRs). Biosyst. Eng. 2017, 160, 1–11. [Google Scholar] [CrossRef]
- Pelegrin, J.; Holzem, R.M. Evaluating the Impacts of Phragmites australis Pretreatment Methods on Biogas and Methane. MJUR 2017, 7, 244–259. [Google Scholar]
- Motte, J.-C.; Escudié, R.; Hamelin, J.; Steyer, J.-P.; Bernet, N.; Delgenes, J.-P.; Dumas, C. Substrate Milling Pretreatment as a Key Parameter for Solid-State Anaerobic Digestion Optimization. Bioresour. Technol. 2014, 173, 185–192. [Google Scholar] [CrossRef]
- Wahid, R.; Hjorth, M.; Kristensen, S.; Møller, H.B. Extrusion as Pretreatment for Boosting Methane Production: Effect of Screw Configurations. Energy Fuels 2015, 29, 4030–4037. [Google Scholar] [CrossRef]
- Shah, T.A.; Tabassum, R. Enhancing Biogas Production from Lime Soaked Corn Cob Residue. Int. J. Renew. Energy Res. 2018, 8, 761–766. [Google Scholar]
- Amnuaycheewa, P.; Hengaroonprasan, R.; Rattanaporn, K.; Kirdponpattara, S.; Cheenkachorn, K.; Sriariyanun, M. Enhancing Enzymatic Hydrolysis and Biogas Production from Rice Straw by Pretreatment with Organic Acids. Ind. Crops Prod. 2016, 87, 247–254. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the Methane Fermentation Process with Particular Emphasis on the Microbiological Aspect: A Review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Fortuny, M.; Baeza, J.A.; Gamisans, X.; Casas, C.; Lafuente, J.; Deshusses, M.A.; Gabriel, D. Biological Sweetening of Energy Gases Mimics in Biotrickling Filters. Chemosphere 2008, 71, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.R.A.; Torii, S. Removal of Hydrogen Sulfide (H₂S) from Biogas Using Zero-Valent Iron. J. Clean Energy Technol. 2015, 3, 428–432. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas Composition from Agricultural Sources and Organic Fraction of Municipal Solid Waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Żarczyński, A.; Rosiak, K.; Anielak, P.; Wolf, W. Practical Methods of Cleaning Biogas from Hydrogen Sulphide. Part 1. Application of Solid Sorbents. Acta Innov. 2014, 12, 24–34. [Google Scholar]
- Aita, B.C.; Mayer, F.D.; Muratt, D.T.; Brondani, M.; Pujol, S.B.; Denardi, L.B.; Hoffmann, R.; da Silveira, D.D. Biofiltration of H2S-Rich Biogas Using Acidithiobacillus thiooxidans. Clean. Techn. Environ. Policy 2016, 18, 689–703. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.; Choi, H.; Lee, C. A Review of Technologies for In-Situ Sulfide Control in Anaerobic Digestion. Renew. Sust. Energy Rev. 2022, 157, 112068. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Moreno, G.; Quijano, G. Theoretical Framework for the Estimation of H2S Concentration in Biogas Produced from Complex Sulfur-Rich Substrates. Environ. Sci. Pollut. Res. 2020, 27, 15959–15966. [Google Scholar] [CrossRef]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamamoto, Y.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. Prospects for Biogas Production and H2S Control from the Anaerobic Digestion of Cattle Manure: The Influence of Microscale Waste Iron Powder and Iron Oxide Nanoparticles. Waste Manag. 2020, 101, 141–149. [Google Scholar] [CrossRef]
- Arias, D.E.; Veluchamy, C.; Habash, M.B.; Gilroyed, B.H. Biogas Production, Waste Stabilization Efficiency, and Hygienization Potential of a Mesophilic Anaerobic Plug Flow Reactor Processing Swine Manure and Corn Stover. J. Environ. Manag. 2021, 284, 112027. [Google Scholar] [CrossRef]
- Tahir, M.S.; Shahzad, K.; Shahid, Z.; Sagir, M.; Rehan, M.; Nizami, A. Producing Methane Enriched Biogas Using Solvent Absorption Method. Chem. Eng. Trans. 2015, 45, 1309–1314. [Google Scholar] [CrossRef]
- Herout, M.; Malaťák, J.; Kučera, L.; Dlabaja, T. Biogas Composition Depending on the Type of Plant Biomass Used. Res. Agric. Eng. 2011, 57, 137–143. [Google Scholar] [CrossRef]
- Hutňan, M. Maize Silage as Substrate for Biogas Production. In Advances in Silage Production and Utilization; da Silva, T., Santos, E.M., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Rajagopal, R.; Massé, D.I.; Singh, G. A Critical Review on Inhibition of Anaerobic Digestion Process by Excess Ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process Disturbances in Agricultural Biogas Production—Causes, Mechanisms and Effects on the Biogas Microbiome: A Review. Energies 2019, 12, 365. [Google Scholar] [CrossRef]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of Ammonia Removal in Anaerobic Digestion: A Review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.V.; Keesman, K.J.; Zeeman, G.; van Lier, J.B. Effect of Ammonia on the Anaerobic Hydrolysis of Cellulose and Tributyrin. Biomass Bioenergy 2012, 47, 316–323. [Google Scholar] [CrossRef]
- Statistics Poland. Energy Consumption in Households in 2018; Zakład Wydawnictw Statystycznych: Warsaw, Poland, 2019.
- Demko, J.; Machava, J.; Saniga, M. Energy Production Analysis of Common Reed—Phragmites australis (Cav.) Trin. Folia Oecologica 2017, 44, 107–113. [Google Scholar] [CrossRef]
- Dahms, T.; Oehmke, C.; Kowatsch, A.; Abel, S.; Wichmann, S.; Wichtmann, W.; Schröder, C. Halmgutartige Festbrennstoffe Aus Nassen Mooren, 1st ed.; Universität Greifswald, Greifswald Moor Centrum: Greifswald, Germany, 2017. (In German) [Google Scholar]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Banaszuk, P. GHG Emissions and Efficiency of Energy Generation through Anaerobic Fermentation of Wetland Biomass. Energies 2020, 13, 6497. [Google Scholar] [CrossRef]
- Tanneberger, F.; Birr, F.; Couwenberg, J.; Kaiser, M.; Luthardt, V.; Nerger, M.; Pfister, S.; Oppermann, R.; Zeitz, J.; Beyer, C.; et al. Saving Soil Carbon, Greenhouse Gas Emissions, Biodiversity and the Economy: Paludiculture as Sustainable Land Use Option in German Fen Peatlands. Reg. Environ. Change 2022, 22, 69. [Google Scholar] [CrossRef]
- Hijazi, O.; Munro, S.; Zerhusen, B.; Effenberger, M. Review of Life Cycle Assessment for Biogas Production in Europe. Renew. Sust. Energy Rev. 2016, 54, 1291–1300. [Google Scholar] [CrossRef]
- Tonini, D.; Hamelin, L.; Alvarado-Morales, M.; Astrup, T.F. GHG Emission Factors for Bioelectricity, Biomethane, and Bioethanol Quantified for 24 Biomass Substrates with Consequential Life-Cycle Assessment. Bioresour. Technol. 2016, 208, 123–133. [Google Scholar] [CrossRef] [PubMed]
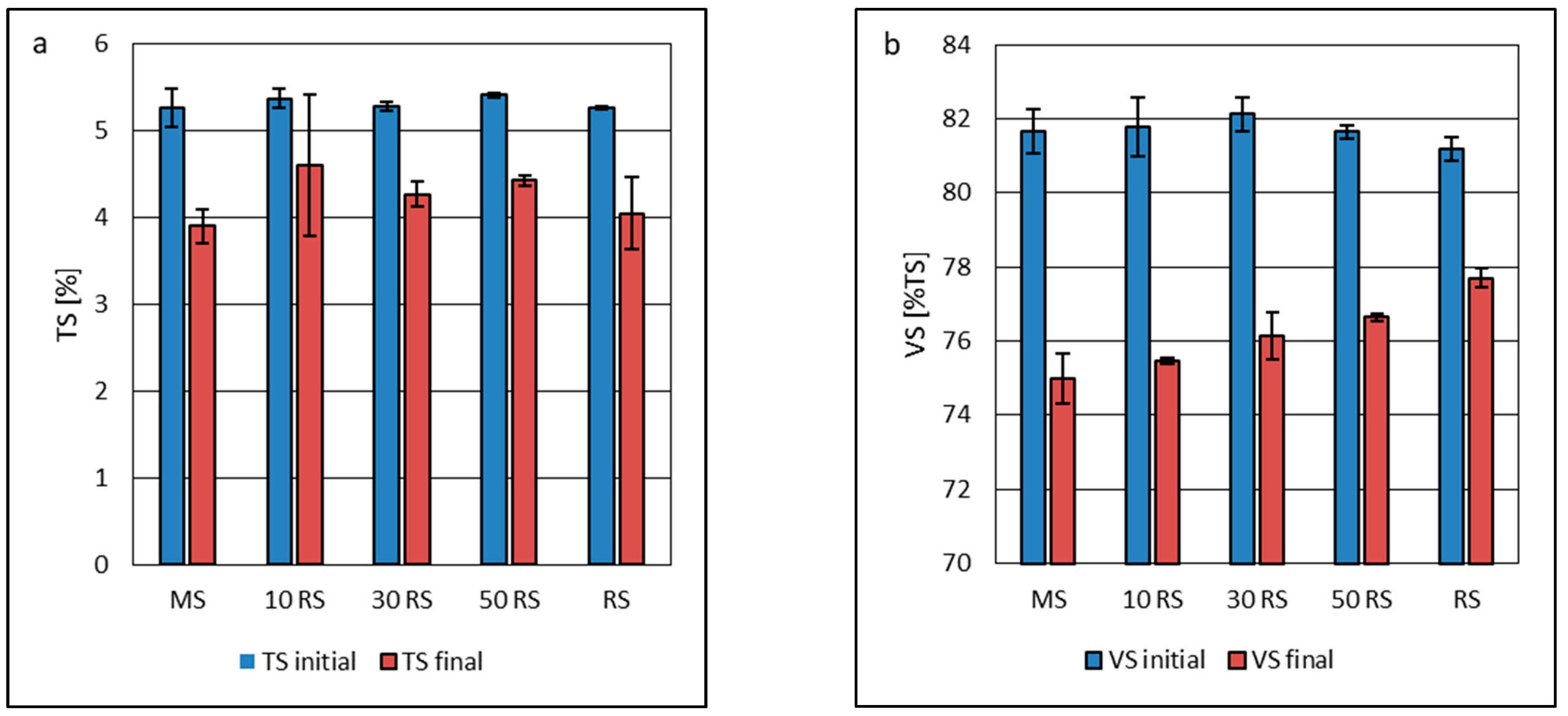
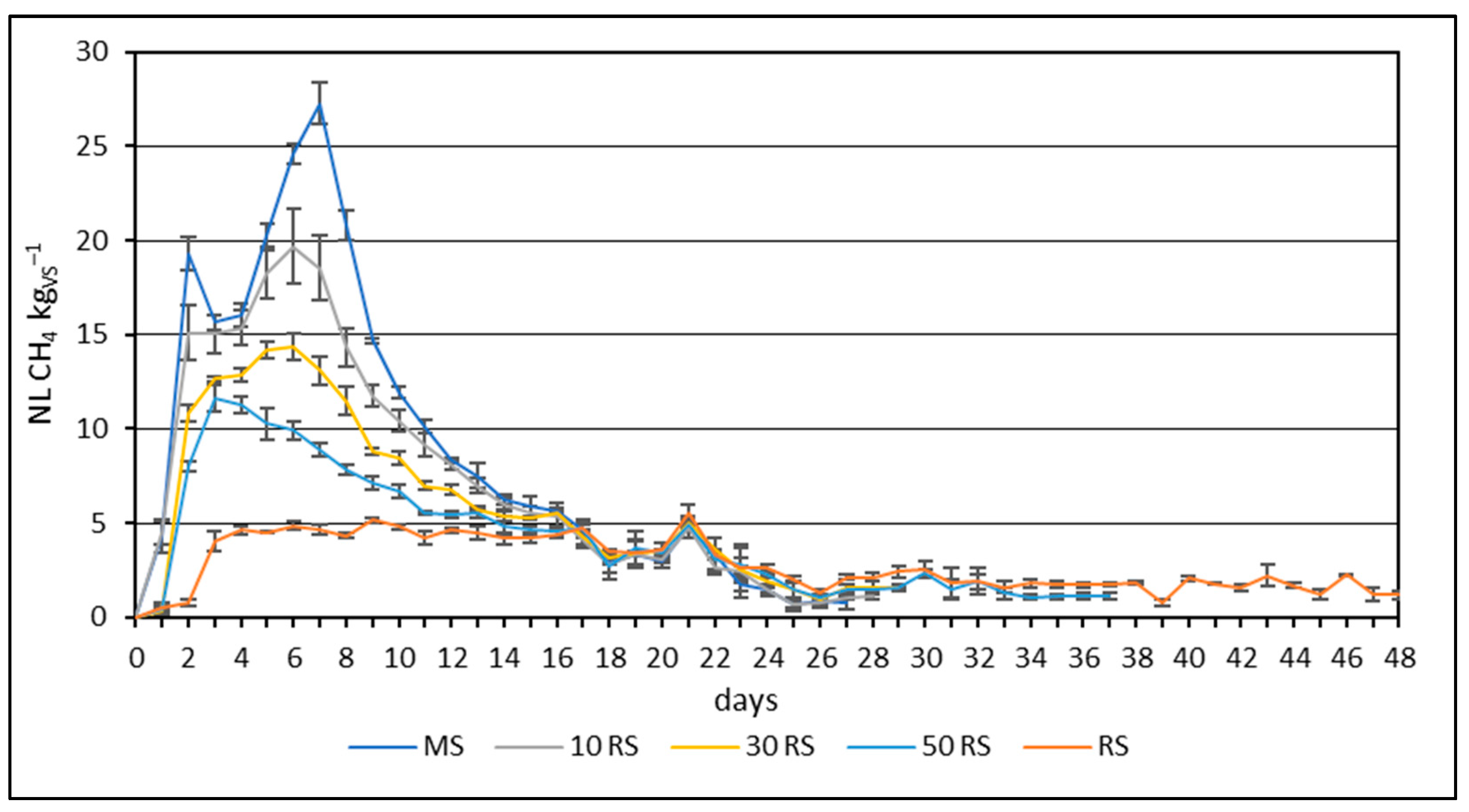
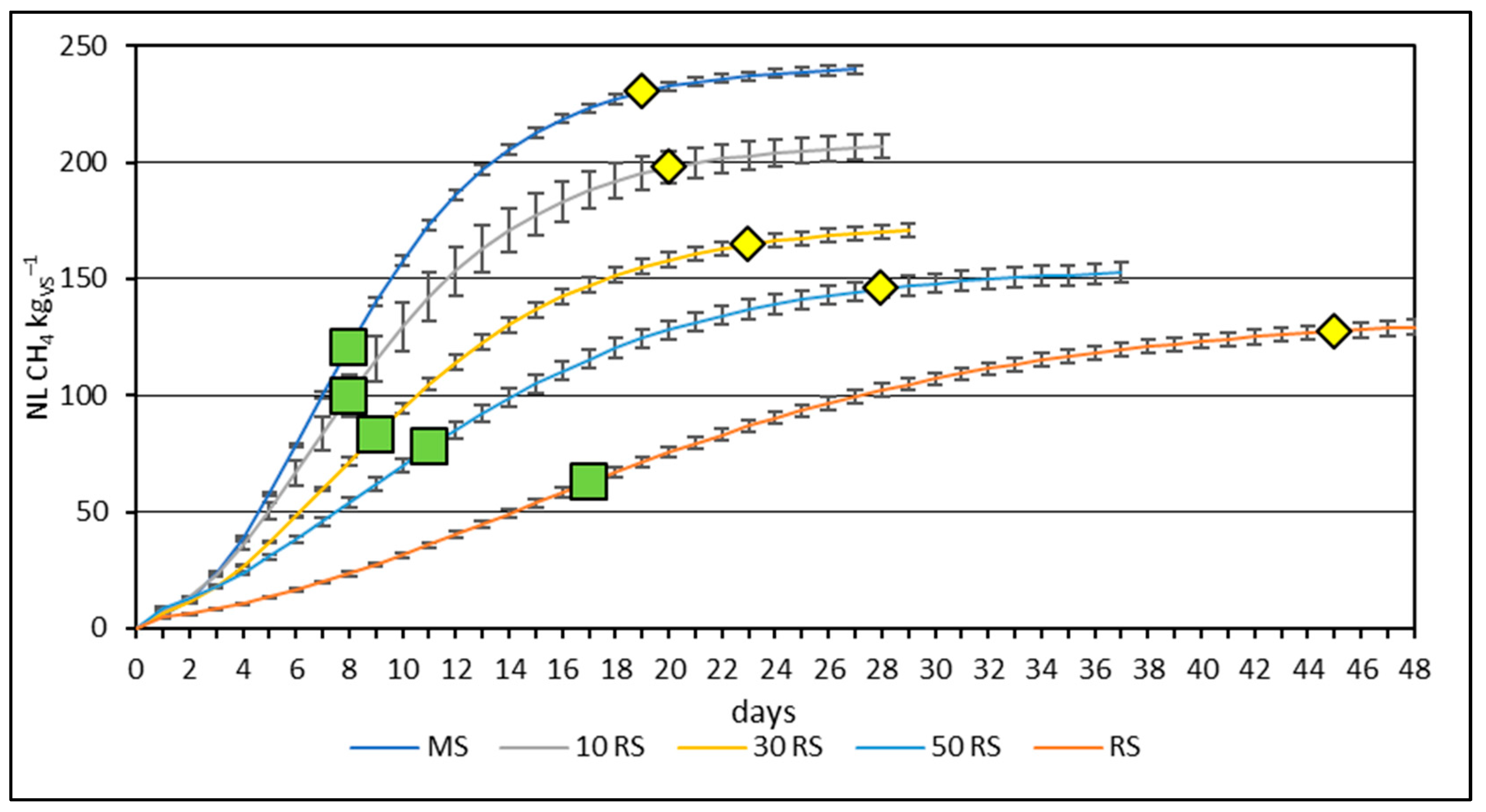
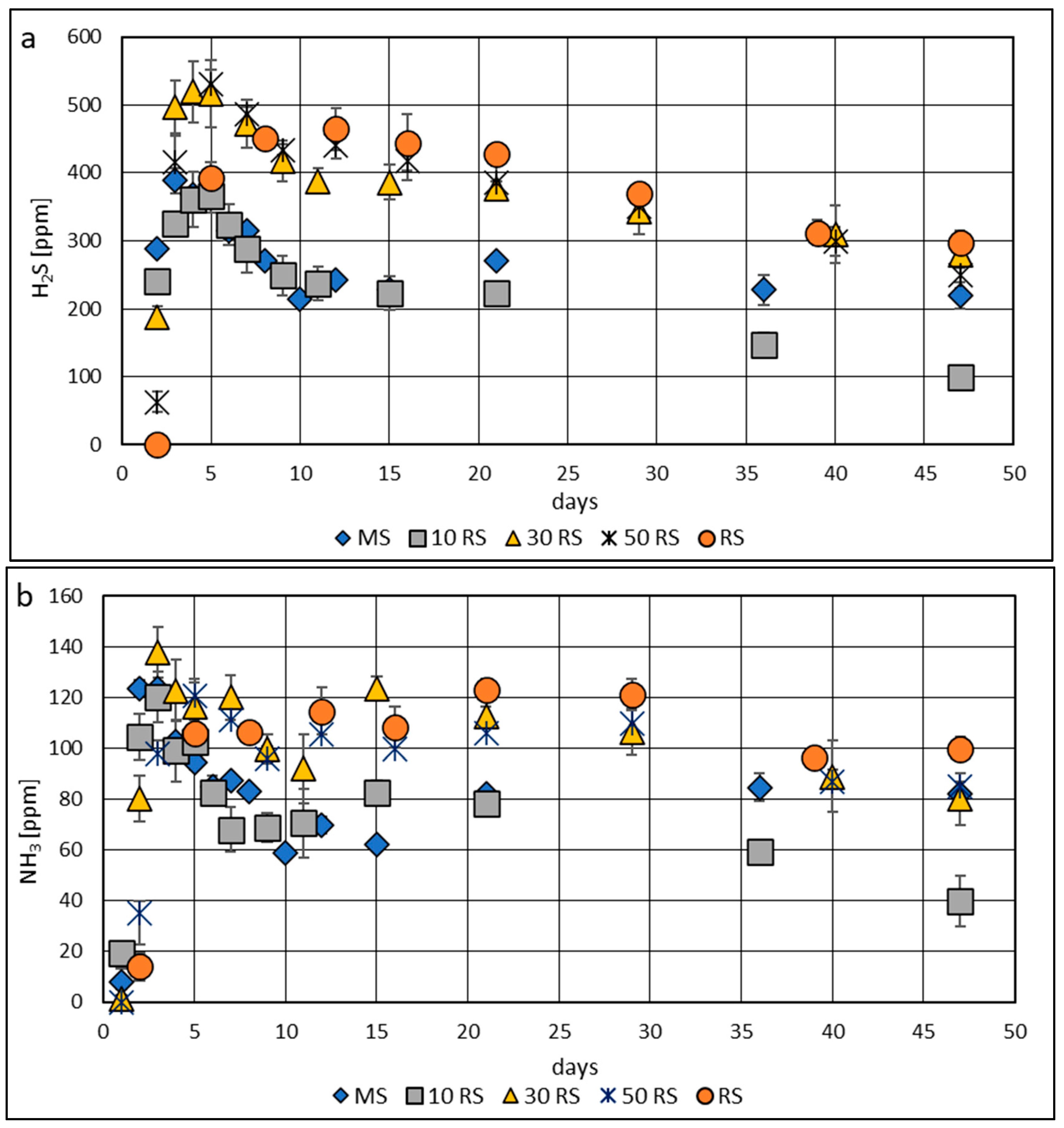

| Parameter | Maize Silage | Reed Silage | Inoculum |
|---|---|---|---|
| Total solids (TS), % | 31.66 ± 0.32 a * | 62.85 ± 0.99 b | 5.28 ± 0.03 c |
| Volatile solids (VS), %TS | 95.51 ± 0.53 a | 91.16 ± 0.27 b | 75.62 ± 0.02 c |
| Total Kjeldahl Nitrogen (TKN), g kgDM−1 | 13.88 ± 0.16 a | 14.60 ± 0.53 a | 94.50 ± 0.18 b |
| Total phosphorus (TP), g kgDM−1 | 1.98 ± 0.02 a | 1.26 ± 0.07 b | 8.29 ± 0.18 c |
| Total potassium (K), g kgDM−1 | 9.56 ± 0.20 a | 2.68 ± 0.16 b | 62.59 ± 1.15 c |
| Total organic carbon (TOC) g kgDM−1 | 396.04 ± 5.45 a | 379.11 ± 7.81 b | 368.03 ± 5.61 b |
| C:N | 29:1 | 26:1 | 4:1 |
| Feedstock | Methane Production | Maximum Daily Methane Production | Lag Time |
|---|---|---|---|
| NL kgVS−1 | NL kgVS−1 | Days | |
| MS | 241.42 ± 1.97 a * | 27.26 ± 1.09 a | 1.36 ± 0.06 a |
| 10 RS | 208.92 ± 4.91 b | 19.69 ± 2.02 b | 1.03 ± 0.06 b |
| 30 RS | 173.87 ± 2.26 c | 14.39 ± 0.72 c | 0.99 ± 0.15 b |
| 50 RS | 155.18 ± 4.59 d | 11.24 ± 0.43 d | 0.38 ± 0.07 c |
| RS | 135.22 ± 3.42 e | 5.54 ± 0.40 e | 2.23 ± 0.10 d |
| Feedstock | Electricity Generation | Heat Generation | ||
|---|---|---|---|---|
| kWh tDM−1 | kWh tFM−1 | GJ tDM−1 | GJ tFM−1 | |
| MS | 731 | 231 | 2.29 | 0.73 |
| 10 RS | 630 | 219 | 1.97 | 0.69 |
| 30 RS | 519 | 213 | 1.63 | 0.67 |
| 50 RS | 459 | 217 | 1.44 | 0.68 |
| RS | 391 | 246 | 1.22 | 0.77 |
| Combustion Heat HHV | Calorific Value LHV | Calorific Value * LHV | Calorific Value ** LHV | Ash |
|---|---|---|---|---|
| GJ tDM−1 | GJ tDM−1 | GJ tFM−1 | GJ tOM−1 | % |
| 18.250 ± 0.079 | 16.903 ± 0.079 | 9.716 ± 0.05 | 13.034 ± 0.050 | 7.38 ± 0.08 |
| Share of Reed Silage in Co-Digested Combinations of Reed and Maize Silages | Electric Power Installed in a Biogas Plant (kWel) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 500 | 1000 | ||||||
| B | BP | B | BP | B | BP | B | BP | B | BP | |
| GJ | ||||||||||
| 10% | 1677 | 23 | 3354 | 45 | 6709 | 90 | 16,772 | 226 | 33,544 | 452 |
| 30% | 5174 | 172 | 10,348 | 345 | 20,695 | 689 | 51,738 | 1723 | 103,476 | 3446 |
| 50% | 8464 | 415 | 16,928 | 831 | 33,857 | 1662 | 84,641 | 4154 | 169,283 | 8308 |
| Feedstock | Electric Power Installed in a Biogas Plant (kWel) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 500 | 1000 | ||||||
| Area Required for Maize and Reed Cultivation (ha) | ||||||||||
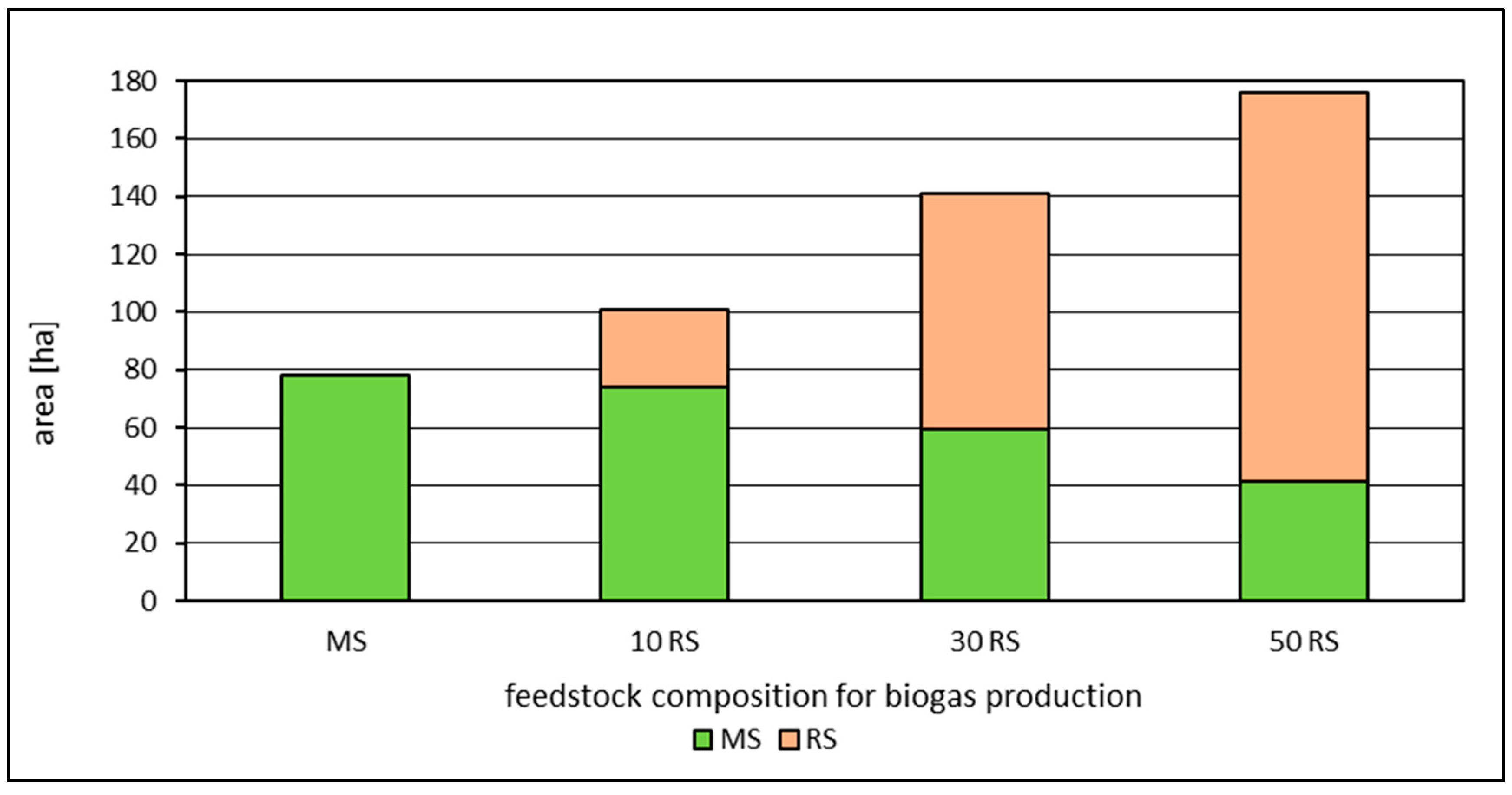
| Maize | Reed | Maize | Reed | Maize | Reed | Maize | Reed | Maize | Reed | |
| MS | 39 | 79 | 157 | 394 | 787 | |||||
| 10 RS | 37 | 13 | 75 | 27 | 150 | 53 | 374 | 133 | 749 | 267 |
| 30 RS | 30 | 41 | 60 | 82 | 120 | 164 | 299 | 411 | 599 | 822 |
| 50 RS | 21 | 67 | 42 | 135 | 84 | 269 | 210 | 673 | 420 | 1345 |
| Feedstock | Electric Power Installed in a Biogas Plant (kWel) | ||||
|---|---|---|---|---|---|
| 50 | 100 | 200 | 500 | 1000 | |
| t CO2 eq. | |||||
| 10 RS | 6.45 | 12.89 | 25.78 | 64.46 | 128.92 |
| 30 RS | 31.52 | 63.04 | 126.07 | 315.19 | 630.37 |
| 50 RS | 61.45 | 122.90 | 245.80 | 614.49 | 1228.98 |
| Share of Reed Silage in Co-Digested Combinations of Reed and Maize Silages | Electric Power Installed in a Biogas Plant (kWel) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 500 | 1000 | ||||||
| E | H | E | H | E | H | E | H | E | H | |
| t CO2 | ||||||||||
| 10% | 27.8 | 11.7 | 55.6 | 23.4 | 111.3 | 46.7 | 278.2 | 116.8 | 556.4 | 233.5 |
| 30% | 83.5 | 35.0 | 166.9 | 70.1 | 333.9 | 140.1 | 1391.0 | 350.3 | 1669.3 | 700.6 |
| 50% | 139.1 | 58.4 | 278.2 | 116.8 | 556.4 | 233.5 | 1391.0 | 583.8 | 2782.1 | 1167.7 |
| Electric Power Installed in a Biogas Plant | Electricity Generation | Heat Generation |
|---|---|---|
| kWel | Number of residences with an area of 100 m2 | |
| 50 | 146 | 16 |
| 100 | 292 | 32 |
| 200 | 584 | 65 |
| 500 | 1459 | 162 |
| 1000 | 2918 | 324 |
| Share of Reed Silage in Co-Digested Combinations of Reed and Maize Silages | Electric Power Installed in a Biogas Plant (kWel) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 500 | 1000 | ||||||
| E | H | E | H | E | H | E | H | E | H | |
| Number of Residences with an Area of 100 m2 | ||||||||||
| 10% | 1 | 0 | 3 | 1 | 6 | 1 | 15 | 3 | 29 | 6 |
| 30% | 13 | 2 | 26 | 4 | 53 | 9 | 131 | 22 | 263 | 45 |
| 50% | 36 | 5 | 73 | 11 | 146 | 22 | 365 | 54 | 729 | 108 |
| Share of Reed Silage in Co-Digested Combinations of Reed and Maize Silages | Electric Power Installed in a Biogas Plant (kWel) | ||||
|---|---|---|---|---|---|
| 50 | 100 | 200 | 500 | 1000 | |
| 10% | 22 | 44 | 87 | 218 | 436 |
| 30% | 67 | 134 | 269 | 672 | 1344 |
| 50% | 110 | 220 | 440 | 1099 | 2198 |
| 100% | 194 | 388 | 777 | 1942 | 3885 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubaszek, R.; Wysocka-Czubaszek, A.; Wichtmann, W.; Zając, G.; Banaszuk, P. Common Reed and Maize Silage Co-Digestion as a Pathway towards Sustainable Biogas Production. Energies 2023, 16, 695. https://doi.org/10.3390/en16020695
Czubaszek R, Wysocka-Czubaszek A, Wichtmann W, Zając G, Banaszuk P. Common Reed and Maize Silage Co-Digestion as a Pathway towards Sustainable Biogas Production. Energies. 2023; 16(2):695. https://doi.org/10.3390/en16020695
Chicago/Turabian StyleCzubaszek, Robert, Agnieszka Wysocka-Czubaszek, Wendelin Wichtmann, Grzegorz Zając, and Piotr Banaszuk. 2023. "Common Reed and Maize Silage Co-Digestion as a Pathway towards Sustainable Biogas Production" Energies 16, no. 2: 695. https://doi.org/10.3390/en16020695
APA StyleCzubaszek, R., Wysocka-Czubaszek, A., Wichtmann, W., Zając, G., & Banaszuk, P. (2023). Common Reed and Maize Silage Co-Digestion as a Pathway towards Sustainable Biogas Production. Energies, 16(2), 695. https://doi.org/10.3390/en16020695






