Abstract
The monoclinic BiVO4 in a powder state was prepared via a hydrothermal method, with the addition of KCl as a structure-directing agent. The as-prepared sample was calcined at different temperatures (400–600 °C), either in the air or in Ar gas. It is found that, though the morphology and crystal structure mostly remain unchanged, the bandgap properties are modified during calcination. A detailed analysis of the surface chemical states and optical absorption properties reveals the involvement of tetravalent vanadium ions and oxygen vacancies as the cause of the band modification. The bandgap properties are to be found tunable via changing the calcination condition, as well as the KCl concentration in the precursor. The photocatalytic properties of BiVO4 samples are greatly enhanced with the addition of KCl in the precursor, but degraded by post-annealing, where the residual Cl in the calcined sample may act as an inhibitor. The enhanced photoactivity is explained in terms of favorable faceting, bandgap modification, and heterojunction of BiVO4/BiOCl.
1. Introduction
Photocatalytic material is fascinating in that it can harness light energy to promote various chemical reactions for applications such as water spitting [1], pollutant degradation [2], hydrocarbon production [3], and self-cleaning effect [4]. A commonly used photocatalyst is TiO2, with a bandgap of around 3.2 eV. This wide-bandgap semiconductor material functions, however, only under ultraviolet light conditions, which occupy about 5% of sunlight at the surface of the Earth [5,6]. Therefore, numerous studies have been performed to seek alternative photocatalysts with narrower bandgaps, which can harness visible light energy, such as Fe2O3 [7], BiVO4 [1], SnS2 [8], and CdS [9].
In particular, the BiVO4 with monoclinic structure has attracted significant interest among researchers due to its narrower bandgap of around 2.5 eV [10] and other advantages, such as low cost of production, non-toxicity, abundance, and stability [11]. Though it can harness visible light with a wavelength shorter than ~500 nm, there is still a significant amount of sunlight energy that cannot be utilized, especially in the range with a wavelength of 500–600 nm. Further bandgap narrowing could then improve its light absorption efficiency and photocatalytic performance, as demonstrated by previous studies [12,13,14]. Hence, the ability to tune the bandgap of BiVO4 photocatalyst is of interest for achieving optimal performance for niche application. Various studies have been conducted to modify the bandgap via techniques such as doping [13,15], controlling oxygen vacancies [14,16], and incorporating with other photocatalysts [17].
Our previous study [18] has revealed that the addition of KCl in the BiVO4 precursor influences the morphology and bandgap of BiVO4, leading to an enhanced photocatalytic performance. Although the bandgap narrowing was suggested to be responsible for promotion in photocatalysis, the underlying cause had not been identified. Elucidation of the mechanism could lead to a better understanding of the BiVO4 system, which is beneficial for controlling bandgap properties of BiVO4 photocatalysis.
The likely responsible defects produced during BiVO4 synthesis are oxygen vacancies, common defects in metal oxides [19,20]. Oxygen vacancies are reported to reduce a bandgap via introducing defect levels inside the electronic bandgap [20,21]. Thermal treatment can be an effective method to study oxygen vacancies in metal oxides. Calcination in air (oxygen-rich atmosphere) may reduce the number of oxygen vacancies [22,23]. Conversely, in an oxygen-deficient atmosphere including nitrogen (N2) [13,23], hydrogen (H2) [24], or argon (Ar) [14,25], the number could remain the same or even increase. Among the latter calcination, the H2 gas may cause an undesirable reduction to the metal oxides [26], N2 gas may introduce an N-doping effect [13,23], and the Ar gas may induce a mild effect on oxygen vacancies in BiVO4, the doping effect of which is not well reported [14,25,27].
This study thus aims to elucidate possible cause of variation in the bandgap properties of BiVO4, synthesized via a hydrothermal method, where KCl is added to the BiVO4 precursor as a structure-directing agent. The change in oxygen vacancy level is explored via heating the powder sample in different atmospheres. The detailed analyses on surface chemical states and optical properties have revealed the involvement of oxygen vacancies as the cause of bandgap variation. The correlation of the KCl concentration with oxygen vacancies is discussed, in conjunction with the performance of detailed analyses on the morphology and the crystal structure.
2. Experimental
2.1. Materials
All the chemicals used in this study had analytical grades without further purification. Bismuth (III) nitrate pentahydrate (Bi(NO3)3·5H2O, 99.5%), ammonium vanadate (NH4VO3, 99.0%), and ethanolamine (2-aminoethanol, C2H7NO, ≥97.0%) were supplied by Nacalai Tesque. Potassium chloride (KCl, 99.5%) was purchased from FUJIFILM Wako Pure Chemical Corporation. Ultrapure water (18.2 MΩ·cm at 25 °C), obtained from the Direct-Q water purification system (Millipore), was used for all syntheses.
2.2. Preparation of BiVO4 Samples
The BiVO4 powder was prepared via a typical hydrothermal synthesis with KCl addition. First, 0.001 mol of Bi(NO3)3·5H2O was put in 30 mL of ultrapure water. After 5 min of stirring, 0.003 mol of KCl was added into the solution to form barely soluble BiOCl. Then, 0.001 mol of NH4VO3 was put into the suspension. The pH of the solution was adjusted to 1.8 using 0.6 mL of 1 M ethanolamine. After 1 h of constant stirring, ultrasonication (45 Hz) was utilized to agitate the suspension for another 1 h. Then, it was transferred to a 50 mL stainless-steel autoclave with a Teflon liner, which was sealed and placed in a pre-heated oven at 160 °C for 12 h. When the autoclave was cooled down to room temperature, the as-synthesized BiVO4 was taken out, washed several times with ultrapure water and ethyl alcohol, and dried at 90 °C overnight. This sample was denoted as KCl-BiVO4. For comparison, a pristine BiVO4 sample was prepared via the same procedure, albeit without KCl addition in the precursor. Finally, the KCl-BiVO4 sample was calcined in a tube furnace, either under ambient air or Ar atmosphere (pressure of 0.04 MPa at room temperature) for 1 h, at different temperatures of 400 °C, 500 °C, and 600 °C. The samples were denoted as “KCl-BiVO4 T-air” and “KCl-BiVO4 T-Ar”, where T represents the calcined temperature. A graphical flow chart of the whole span of BiVO4 sample preparation processes, including the hydrothermal powder synthesis and the post-annealing, is shown in Figure S1 (Supplementary Information).
2.3. Characterization
The X-ray diffractometry (XRD) was performed using a Rigaku RINT2100 (Rigaku, Tokyo, Japan) with Cu Kα radiation (λ = 0.15418 nm) operated at 40 kV and 30 mA. The microstructure and morphology of each sample was observed using an FE-SEM (Hitachi SU6600 Scanning Electron Microscope, Hitachi, Tokyo, Japan), where all samples were sputter-coated with thin Au film to prevent electronic charge-up. The X-ray photoelectron spectroscopy (XPS, JPS-9030 X-ray photoelectron spectrometer, JEOL, Tokyo, Japan) was conducted with Mg Kα radiation using O 1s peak at 530 eV as reference. The UV-vis diffuse reflectance spectra (DRS) were obtained using a Lambda 750S UV/Vis/NIR Spectrophotometer (Perkin Elmer, Waltham, MA, USA) equipped with a 60 mm integrating sphere.
2.4. Photocatalysis Evaluation
Photocatalytic activity was evaluated via the photodegradation of rhodamine B dye (RhB), purchased from Tokyo Chemical Industry (Tokyo, Japan), under visible light irradiation conditions. A xenon lamp with 500 W (Ushio, UXL-500D-O) equipped with a cutoff filter (λ > 420 nm) was used to illuminate RhB solution at room temperature (25 °C). The light intensity of the Xe lamp was calibrated with a spectroradiometer (S-2440 model II) to achieve 100 mW/cm2, but the light intensity that reached the sample solution was around 40 mW/cm2 due to cutoff and water filters. For each photodegradation measurement, 40 mL of RhB solution (0.01 mmol/L) containing 30 mg of sample powder was put in a beaker (100 mL). To achieve adsorption–desorption equilibrium between the sample powder and RhB solution, the solution was agitated under ultrasonication and magnetically stirred in the dark for 10 min and 50 min, respectively. After the start of light irradiation, about 3 mL of RhB solution was sampled every 60 min and filtered with a syringe filter (0.22 μm, PTFE) prior to the concentration analysis using Lambda 750S UV/Vis/NIR Spectrophotometer.
3. Results and Discussion
3.1. Morphology
Morphologies of the samples are shown in SEM micrographs in Figure 1. The pristine BiVO4 sample (Figure 1a) exhibits the intensive agglomeration of many tiny rods, with each rod length being around a few microns and rod diameters around a few submicrons, while the KCl-BiVO4 sample (Figure 1b) exhibits a “shuriken”-like structure with a size of ten microns. The morphology of shuriken-like BiVO4, caused by the addition of KCl, has been discussed in our previous study [18]. The KCl-BiVO4 samples are calcined at different temperatures, either under ambient air (Figure 1c,e,g) or under Ar atmosphere (Figure 1d,f,h). Based on Figure 1c–f, there is no significant change in morphology when KCl-BiVO4 is heated either at 400 °C or 500 °C for both in air and Ar gas atmospheres. However, the morphology is noticeably altered when the temperature becomes 600 °C, whether under air or Ar gas conditions (Figure 1g,h). The surface of KCl-BiVO4 shuriken-like samples becomes much smoother at 600 °C. Earlier studies have also found that the BiVO4 particles become more spherical with a smoother surface at a temperature above 500 °C due to the BiVO4 crystallization process [28,29].
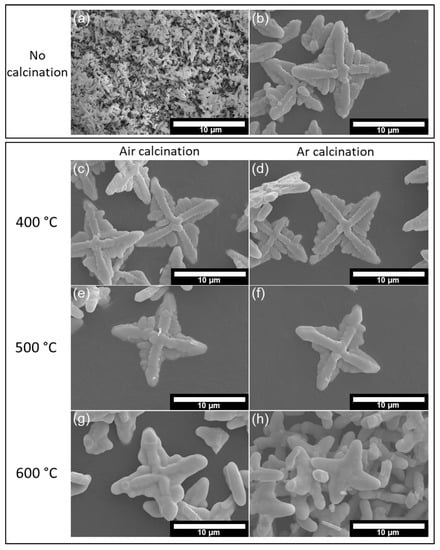
Figure 1.
SEM images of (a) pristine BiVO4, (b) KCl-BiVO4, (c) KCl-BiVO4 400-air, (d) KCl-BiVO4 400-Ar, (e) KCl-BiVO4 500-air, (f) KCl-BiVO4 500-Ar, (g) KCl-BiVO4 600-air, (h) KCl-BiVO4 600-Ar.
3.2. Crystal Structure
Figure 2 shows XRD patterns of BiVO4, with and without the addition of KCl. Both samples exhibit the monoclinic structure (ICDD PDF No. 00-014-0688), while the KCl-BiVO4 sample exhibits a strong intensity at 30.5°, corresponding to a (040) plane, which is ascribed to the effect of Clˉ on the crystal growth of BiVO4 [30]. Furthermore, the XRD diffractogram of the KCl-BiVO4 sample displays small peaks of a tetragonal BiOCl at 12°, 24.1°, and 25.9°, assigned to (001), (002), and (101) planes (ICDD PDF No.00-006-0249), respectively.
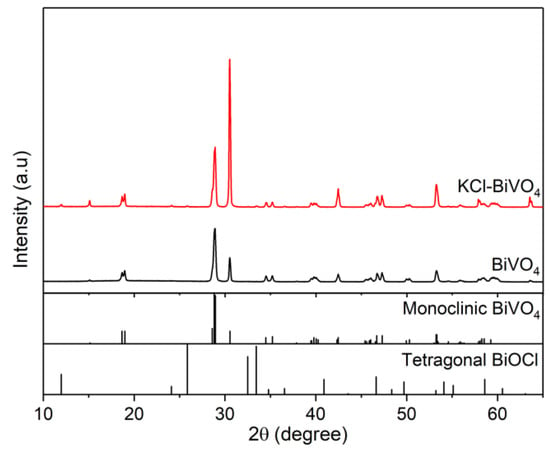
Figure 2.
XRD patterns of pristine BiVO4 and KCl-BiVO4.
After calcination at different temperatures either in the air, as shown in Figure 3a, or in Ar gas, as shown in Figure 3b, the KCl-BiVO4 sample retains the monoclinic structure of BiVO4. Here, “no calcination” signifies the KCl-BiVO4 sample, “T °C air” signifies the KCl-BiVO4 T-air, and “T °C Ar” indicates the KCl-BiVO4 T-Ar. The peak intensity of the retained BiOCl phase decreases with increasing the calcination temperature in either atmospheric condition, as BiOCl is reported to decompose and convert into Bi24O31Cl10 at temperatures above 400 °C [31,32,33]. However, there is no Bi24O31Cl10 phase observed in this study, as shown in Figure 3. Instead, another crystal phase appears in Figure 3b when KCl-BiVO4 is heated at 600 °C in Ar atmosphere conditions. The diffraction peaks of the mentioned crystal phase at 11.5°, 23.6°, and 32.6° can be assigned to (002), (013), and (200) planes of Bi4V2O10 (ICDD PDF No. 01-086-1181) [34], respectively.
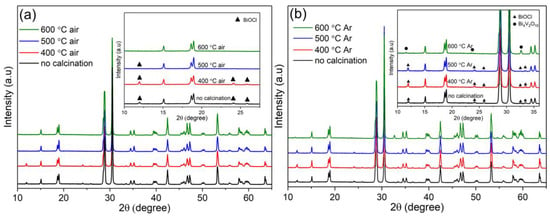
Figure 3.
XRD patterns of KCl-BiVO4 samples, calcined at different temperatures in (a) air and (b) Ar, both with magnified inset charts, where the peaks without ▲ or ● symbols correspond to BiVO4.
The formation of Bi4V2O10 in the present study can be explained as follows. The Bi4V2O10 belongs to Bi2O3–VO2 system, with V4+ instead of V5+ [34,35]. According to previous studies [34,36], Bi4V2O10 can be synthesized via heating the mixture of Bi2O3 and VO2 at temperatures higher than 550 °C in a vacuum. To date, however, there is scarce research on Bi4V2O10, and no report has indicated formation of Bi4V2O10 via thermal treatments of BiVO4 at a temperature range of 300–700 °C under Ar atmosphere conditions, as demonstrated by an earlier study [14]. The plausible explanation of Bi4V2O10 appearance in the KCl-BiVO4 600-Ar sample is that the pre-formed BiOCl decomposes and loses Cl at the high temperature, and further reacts with V4+ to form the Bi4V2O10 phase. It is worth mentioning that V4+ ions in BiVO4 are coupled with oxygen vacancies to maintain charge neutrality [37,38]. It is thus indicated that a considerable number of oxygen vacancies are already present in KCl-BiVO4, even prior to the oxygen-deficient calcination in Ar atmosphere.
The lattice parameters were determined via the Rietvelt analysis on all the BiVO4 powder samples (8 samples) in Figure 2 and Figure 3, and the results are shown in Table S1 (Supplementary Information). The crystallite size was also estimated for all the samples using the standard Scherrer equation, where the Scherrer constant was taken as 0.89 and the BiVO4 (040) plane at 30.5° was utilized for the estimation. The determined crystallite size was in the range from 45 to 51 nm, strongly suggesting that there is no significant difference in crystallinity among the samples, even after calcinations.
3.3. X-ray Photoelectron Spectroscope (XPS) Analysis
The XPS analysis was utilized to study the near-surface chemical state, including vacancies and compositions of the samples. The XPS survey spectra in Figure 4a demonstrate the existence of Bi, V, and O elements in all samples, i.e., BiVO4, KCl-BiVO4 and KCl-BiVO4 samples, calcined at various temperatures (400, 500, 600 °C) in the air. The high-resolution XPS spectra of Bi 4f orbitals, corresponding to Bi3+ of BiVO4, are shown in Figure 4b. The binding energy peak positions, appearing at 164.6 and 159.3 eV, correspond to Bi 4f5/2 and Bi 4f7/2, respectively [39]. There is no significant change in the two core-level excitation peaks when the KCl-BiVO4 sample is calcined up to 600 °C in the air. On the other hand, for V 2p orbitals, each asymmetric spectrum, assigned to the V 2p3/2 binding energy of BiVO4, can be deconvoluted into two spectra with peak positions at around 516.9 eV and 515.3 eV, as shown in Figure 4c. These correspond to V5+ and V4+, respectively [2,14]. The existence of V4+ has been reported to indicate the presence of oxygen vacancies in BiVO4 [37,38]. The spectral peak areas (relative percent, compared in cps) of V5+ and V4+ are listed in Table 1. In Figure 4d, it is displayed that, unlike the pristine BiVO4 sample, the KCl-BiVO4 sample exhibits a small spectrum with the peak position at 198.6 eV, which is assigned to Cl 2p of BiOCl [40]. The peak disappears when the calcination temperature becomes 600 °C, a result which is in agreement with XRD patterns seen in Figure 3a. Lastly, the binding energy peak position of the O 1s orbital (Figure 4e), appearing at 530 eV, corresponds to the Bi-O bonding of BiVO4 [40,41].
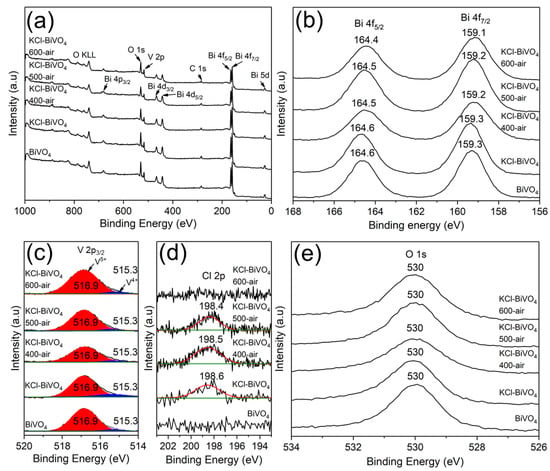
Figure 4.
XPS spectra of BiVO4 and KCl-BiVO4 samples calcined in the air at various temperatures: (a) survey spectra, and high-resolution spectra of (b) Bi 4f, (c) V 2p, (d) Cl 2p, and (e) O 1s orbitals.

Table 1.
XPS peak areas (relative %) of V5+ and V4+ from V 2p3/2, estimated % oxygen vacancy (Vo in %) and bandgaps (Eg in eV) of pristine BiVO4, KCl-BiVO4 and various KCl-BiVO4 samples calcined at different temperatures, either in Ar gas or in the air.
When the KCl-BiVO4 samples are calcined in Ar gas, instead of in the air, the Bi, V and O elements are observed as well in XPS survey spectra, as shown in Figure 5a, and there is no apparent change in the core-level excitation peaks of Bi 4f (Figure 5b) and O 1s (Figure 5e) orbitals when calcined up to 600 °C. The V 2p3/2 spectra (Figure 5c) are also similar, except for the relative peak area of V5+ and V4+, as shown in Table 1, which will be discussed later. As shown in Figure 5d, unlike the KCl-BiVO4 600-air sample, a small spectral peak (198.6 eV), derived from the Cl 2p orbital of BiOCl [40], is detected even in the KCl-BiVO4 600-Ar sample. However, the BiOCl phase disappears according to the XRD analysis (Figure 3b). This suggests that the residual Cl either exists in the form of amorphous or nano-sized grains possibly containing Bi, O, V, Cl (H, C), which cannot be detected via XRD analyses. It is also noted that the binding energy peak of the O 1s orbital (Figure 5e) appearing at 530 eV may exhibit a slight shoulder or tailing at the higher energy side, especially for samples of KCl-BiVO4 400-Ar and KCl-BiVO4 500-Ar. Although a well-defined deconvolution of the spectra was not successful, there might exist broad peaks, possibly corresponding to the unknown near-surface phases mentioned above, or the adsorbed oxygen [42].
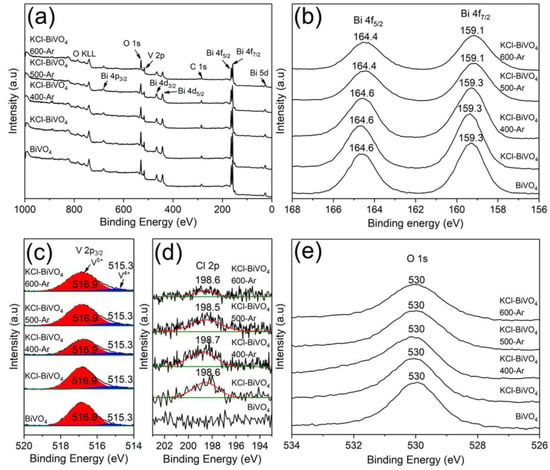
Figure 5.
XPS survey spectra (a) of BiVO4, KCl-BiVO4 and KCl-BiVO4 calcined in Ar at different temperatures, and high-resolution XPS spectra of (b) Bi 4f, (c) V 2p, (d) Cl 2p, and (e) O 1s orbitals.
The % oxygen vacancy (Vo) in Table 1 can be estimated using the peak areas of V5+ and V4+ in the V 2p3/2 XPS spectra, assuming that each oxygen vacancy site generates two equivalents of V4+. Thus, the % oxygen vacancies (Vo) is calculated using the following equation, Equation (1) [38].
3.4. Optical Properties and Bandgap Analysis
The optical properties were investigated using UV-vis diffuse reflectance spectra. Figure 6 demonstrates the absorption spectra and bandgap determination (Tauc plot) of various BiVO4 samples. The absorption spectra are obtained via conversion from the reflectance spectra using the Kubelka–Munk function, F(R) = (1 − R)2/2R, where R is diffuse reflectance. The absorption spectra of various samples, including pristine BiVO4 and KCl-BiVO4, calcined in the air (oxygen-rich condition) and in Ar (oxygen-deficient condition) are shown in Figure 6a,c, respectively. Since BiVO4 is a direct transition semiconductor, the Tauc plot, (F(R)hν)2 vs. hν, can be appropriately used to estimate the bandgap (Eg) [10,42,43]. Various Eg values, determined via extrapolating the linear part of the curve in Figure 6b,d, are listed in Table 1. The pristine BiVO4 sample exhibits the Eg value of 2.48 eV, similar to the earlier report [10], while the KCl-BiVO4 exhibits a smaller Eg of 2.34 eV, indicating that the KCl addition (in the precursor) would narrow the Eg value of BiVO4.
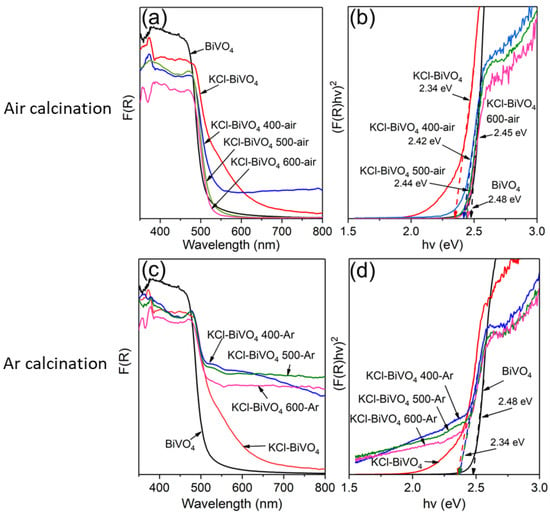
Figure 6.
UV-vis absorption spectra (left) and Tauc plots [(F(R)hv)2 vs. hv] (right) of pristine BiVO4, KCl-BiVO4 and KCl-BiVO4 calcined at different temperatures in (a,b) air and (c,d) Ar gas.
As shown in Figure 6a,b, the absorption edge of the KCl-BiVO4 sample (Eg ~2.34 eV) exhibits a blueshift after calcination in the air, and the Eg value of KCl-BiVO4 600-air almost reaches that of the pristine BiVO4 (Eg ~2.48 eV). On the other hand, the Eg value of the KCl-BiVO4 sample calcined in Ar remains 2.34 eV, as shown in Figure 6c,d. The bandgap modification is unlikely to have originated from the residual BiOCl phase (XRD results: Figure 3) since the BiOCl bandgap of ~3.3 eV [44] is substantially wider than that (2.34–2.48 eV) of BiVO4. Besides, an earlier study [44] reports no significant change in bandgap of BiVO4 in the BiOCl/BiVO4 composite, even with the interface at a nano-sized level. Further, the small amount of Bi4V2O10 would not influence the bandgap of BiVO4, as it is only observed in the KCl-BiVO4 600-Ar sample (Figure 3b), the Eg value (2.34 eV) of which is equal to other samples of KCl-BiVO4, KCl-BiVO4 400-Ar and 500-Ar. The most likely cause for the change in the bandgap would be the oxygen vacancies, which have been found to influence the light absorption and bandgap properties of BiVO4 [37,38]. This is also consistent with the XPS analyses, quantifying the presence of oxygen vacancies (Table 1). The oxygen vacancies in metal oxides generally introduce defect levels inside the electronic bandgap, either just below the bottom end of the conduction band or just above the upper end of the valence band. This would result in the bandgap narrowing due to the overlapping of the defect states with either the conduction band minimum or the valence band maximum [20,21].
The present study shows that the bandgaps of KCl-BiVO4 samples before and after Ar-calcination (at 400, 500, or 600 °C) are unchanged. This result differs from that of the earlier report [14], where the bandgap values of Ar-calcinated BiVO4 were slightly reduced by 0.02 and 0.05 eV at temperatures of 500 and 700 °C, respectively, compared with untreated BiVO4. This was attributed to the increased concentration in the oxygen vacancy. The different outcomes between the said report and our study might be due to a large number of oxygen vacancies already being present in the untreated KCl-BiVO4 sample (from our XPS analysis), the calcination of which in Ar may not effectively induce more oxygen vacancies in the sample. To confirm this hypothesis, the KCl-BiVO4 sample was first calcined at 600 °C for 1 h in the air, followed by 600 °C for 1 h in Ar, where the sample was allowed to cool to room temperature in between both calcinations. The bandgap value, determined from the Tauc plot (Figure S2, Supplementary Information), was found to increase from 2.34 to 2.45 eV when calcined in the air (600 °C for 1 h), and then decrease to 2.40 eV when calcined in Ar (600 °C for 1 h). This strongly suggests that, if the amount of oxygen vacancies in KCl-BiVO4 is relatively low, the Ar calcination could induce more oxygen vacancies to reduce the bandgap. This is in agreement with previous study [25], where BiVO4 was annealed in the air at 450 °C for 2 h followed by in Ar at 300–400 °C for 2 h.
It is worth mentioning that, while the onset of band gap absorption remains unchanged for Ar-calcination samples (Figure 6c,d), the spectral baselines visibly rise, enhancing light absorption in the entire wavelength range, which could correspond to the observed change in color (to dark green) (in Figure S3, Supplementary Information). A similar phenomenon has also been reported by Qin et al. [45], where the BiVO4 film was treated via electrochemical and chemical (NaBH4) reductions, generating a considerable amount of V4+ in BiVO4. In the case of the current study, however, a likely cause of the color change would be different, as the large amount of V4+ was present even prior to the calcination process (KCl-BiVO4 sample in Table 1). It is hypothesized that, due to calcination, a thin scale with an amorphous state or nanograined structure, composed of Bi–V–Cl–O(–H-C), none of which is detectable by XRD, could be formed on the powder, causing color alterations [46]. Diffuse light scattering or reflection of powders with high emissivity would cause effective light absorption due to the surface films with small band gaps and/or oxygen deficiencies [46]. It is further observed that, although the KCl-BiVO4 400-air sample exhibits a greenish-yellow color (Figure S3), both KCl-BiVO4 500-air and 600-air samples display a bright yellow color, similar to that of pristine BiVO4. It suggests that the light brown (or orange) color of KCl-BiVO4 (Figure S3) is caused by the (high) presence of V4+ and corresponding oxygen vacancies, and calcination at 400 °C would reduce V4+ to a moderate amount. However, at 500 and 600 °C, this would be reduced to a very low amount, as the oxygen in the air effectively fill the vacancies of the BiVO4 phase during calcinations.
The correlation between bandgap and oxygen vacancies in the present study can be examined from Vo and Eg values in Table 1, and a linear relationship is indicated, as shown in Figure 7. The Eg value decreases with an increase in the Vo value, strongly suggesting that an increase in oxygen vacancies (concentration) narrows the bandgap of the sample, which is consistent with earlier research [37,38,47]. A similar linear relationship between bandgap and oxygen vacancies has been reported in ZnO thin films, produced via varying the oxygen partial pressure [16]. It should be noted that the data of KCl-BiVO4 600-Ar are not used to construct Figure 7 because the sample contains Bi4V2O10 phase (XRD patterns in Figure 3b), possessing V4+ states that are not responsible for oxygen vacancies in BiVO4.
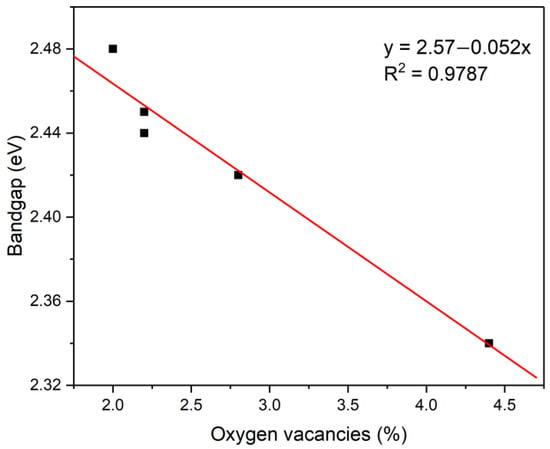
Figure 7.
Correlation between bandgap and oxygen vacancies of the samples.
Based on above results and analyses, it can be inferred that a large number of oxygen vacancies was produced during the sample preparation of BiVO4 with the addition of KCl, which in turn narrows the bandgap of BiVO4. The bandgap value of KCl-BiVO4 can be restored to that of pristine BiVO4 by heating the sample in the air, as the oxygen from the air can effectively fill the oxygen vacancies [22,23] of BiVO4. Conversely, when the KCl-BiVO4 sample is calcined in Ar gas (oxygen-deficient atmosphere), the oxygen vacancies remain in the system, resulting in no change in its Eg value. The underlying cause behind this effect will be discussed in the next section. Additionally, the bandgap value of KCl-BiVO4 can be tunable by heating the sample in different atmospheric conditions, as shown in Figure S2 (Supplementary Information).
3.5. Addition of KCl and Oxygen Vacancies
The concentration of added KCl to the BiVO4 precursor during sample preparation is known to influence the BiVO4 bandgap [18]. Additionally, in the present study, the bandgap value is found to change almost linearly with oxygen vacancy concentration of the KCl-BiVO4 sample. It is then suggested that there be a connection between the KCl concentration and the formation of oxygen vacancies. In order to confirm this point, the KCl-BiVO4 samples were prepared with different concentrations of KCl (0, 1, 2, 3, 4, and 5 mmol). The Eg and Voa (% oxygen vacancies) values, determined from Tauc plots (Figure S4) and XPS spectra (Figure S5), respectively, are listed in Table S2. The values (%) of oxygen vacancies plotted as a function of the KCl concentration are shown in Figure 8. There are two curved lines from two series of data. One set of data (Vob) is estimated from the Eg values (Figure S4 in Supplementary Information) and the linear correlation in Figure 7, and the other (Voa) is from the XPS V 2p3/2 spectra (Figure S5) and Equation (1). Both curves are relatively in good agreement, exhibiting a similar trend, where oxygen vacancies (in %) increase with an increase in KCl concentration up to 3 mmol and almost remain constant afterwards up to 5 mmol of KCl.
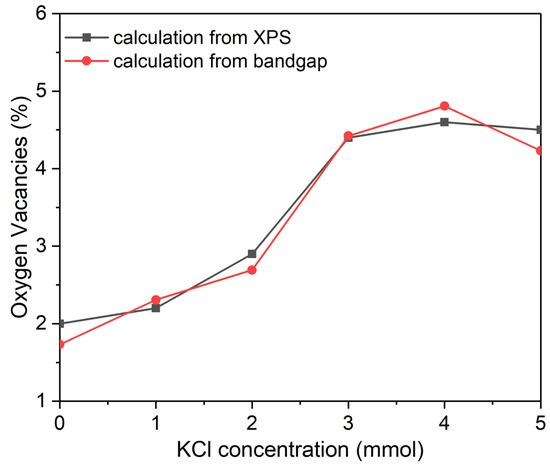
Figure 8.
Relationship between oxygen vacancies (%) and KCl concentration, where the data of vacancies are estimated from XPS spectra and bandgaps of KCl-BiVO4 samples.
The increasing trend of oxygen vacancies, seen with an increase in KCl concentration in Figure 8, can be explained as follows. The formation of oxygen vacancies in metal oxides is often involved with oxygen-deficient environment, chemical reduction, dynamics of ionic defects, plasma treatment, and hydrogen treatment [14,20,48]. In the current study, the reduction reaction of V5+ to V4+ through the action of Clˉ would have occurred during synthesis of BiVO4, generating excess oxygen vacancies to compensate for charge neutrality in the BiVO4 sample [38,49]. Upon considering the reaction (2) below, the formation of VO2+ (aq) with V4+ ions is favored instead of VO3ˉ (aq) with V5+ ions, as the change in Gibbs free energy is negative (ΔG0 = −11.66 kJ mol−1) at the standard condition of 298.15 K and 1 bar (see the calculation in Supplementary Information).
2VO3ˉ (aq) + 2Clˉ (aq) + 8H+ (aq) ⇌ 2VO2+ (aq) + Cl2 (g) + 4H2O (l)
An earlier study has also reported the formation of a small amount of V4+ in a solution of V5+ and chlorine [50]. Thus, adding more Clˉ would result in generating more VO2+ with V4+, which could contribute to the formation of excess oxygen vacancies in BiVO4. Although this does not accurately represent the actual condition of synthesis, which involves other chemicals and hydrothermal conditions, the phenomenological analysis may provide an insight into the correlation between concentrations of Clˉ and V4+, and then oxygen vacancies in the KCl-BiVO4 sample. Apparent saturation in concentration of oxygen vacancies above 3~4 mmol of KCl in Figure 8 may be attributed to the dependence of the process on acidity.
3.6. Photocatalytic Properties
Figure 9 represents photocatalytic properties of various BiVO4 samples in the current study under visible light irradiation (λ > 420 nm), where Figure 9a shows the photodegradation of 0.01 mmol/L RhB solution (C0: initial concentration, Ct: concentration after irradiation time t [in minutes]) by 30 mg of various BiVO4 powders, and Figure 9b shows the pseudo-first-order kinetic degradation rate (k) for each sample. Photolysis of the blank RhB solution was first conducted, as the control test, to confirm the solution stability, where, as shown in Figure 9a, the RhB concentration exhibited no significant change over 240 min of irradiation. On the other hand, the KCl-BiVO4 sample was able to degrade almost all of the RhB (about 94.7% removal) after irradiation for 240 min, followed by pristine the production of BiVO4, KCl-BiVO4 600-air, and KCl-BiVO4 600-Ar samples. According to the RhB-dye photodegradation kinetics analysis in Figure 9b, the degradation rate (k) of KCl-BiVO4 is more than twice that of pristine BiVO4. One of the reasons for great enhancement of the photocatalytic property is the favorable “shuriken-shape” morphology of KCl-BiVO4 powder with (040) faceting [18], and another reason would be the narrower bandgap, as shown in the current study, allowing for extended light absorption due to the high content of tetravalent vanadium ions and oxygen vacancies. Reusability of the sample is very high [18], whereby the RhB photodegradation efficiency only decreases from 94.7% to 90.8% after four cycles.
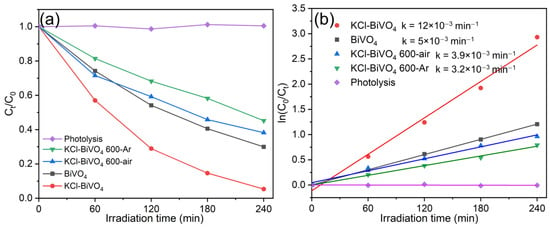
Figure 9.
(a) Photodegradation of RhB solution with various samples under visible light (λ > 420 nm) and (b) Pseudo-first-order kinetic degradation rate (k) of RhB for each sample.
The greatly enhanced photocatalytic property of the pristine KCl-BiVO4 sample is, however, much degraded after calcination at 600 °C in either air or Ar gas, even for the case with favorable morphology and narrower bandgaps. The likely cause may be the residual Cl retained in the sample, which possibly originated from BiOCl decomposition at 600 °C. As explained in Section 3.4, due to calcination, a thin scale with amorphous state or nanograined structure composed of Bi-V-Cl-O (-H-C) might have formed on the powder surface, possibly degrading the photocatalytic property. As for the crystallinity, no significant difference is exhibited among the samples even after calcinations, as explained in Section 3.2.
There would be, however, another reason for the photoactivity enhancement of pristine KCl-BiVO4 powder. This is, the heterojunction of BiVO4 with residual BiOCl retained in the KCl-BiVO4 sample. According to He et al. [51], though BiOCl (bandgap of 3.4~3.5 eV) cannot be active under visible light, the BiOCl/BiVO4 composite can promote photocatalytic performance, if BiOCl and BiVO4 form a p-n heterojunction, with BiVO4 acting as a sensitizer. When n-type BiVO4 is excited by visible light, the valence band becomes vacant. This would be filled by electrons from that of p-type BiOCl via the internal electric field at the interface. This process would promote the separation of photogenerated charge carriers, improving BiVO4 photocatalysis. The KCl-BiVO4 600-air sample in the current work does not contain residual Cl (from XPS analysis), but its photocatalytic activity is much lower than that of pristine KCl-BiVO4 and closer to pristine BiVO4. The KCl-BiVO4 600-Ar sample, on the other hand, does contain residual Cl, exhibiting a further lower photoactivity that is clearly lower than that of pristine BiVO4. Both samples do not possess tetragonal BiOCl, and the absence of tetragonal BiOCl may have affected the photocatalytic performance. The junction of BiVO4/BiOCl could be thus one of the factors improving photocatalytic activity of KCl-BiVO4 besides favorable faceting and bandgap narrowing.
Further investigation is required to deepen our knowledge on this system and elucidate the mechanism, as it is even reasonable that multiple contributions simultaneously coexist.
4. Conclusions
The KCl-BiVO4 sample was synthesized via the hydrothermal method with the addition of KCl in the BiVO4 precursor. The KCl-BiVO4 sample exhibits a narrower bandgap compared with the pristine BiVO4 sample. The bandgap modification is ascribed to the oxygen vacancies, which can be tunable via varying KCl concentrations in the precursor and/or post-annealing in different atmospheric conditions. A linear correlation is found between the concentration of oxygen vacancies in the KCl-BiVO4 sample and the bandgap value, in which an increase in the former reduces the latter value. In addition to the calcination conditions, altering the KCl concentration in the BiVO4 precursor could also influence the level of oxygen vacancies in BiVO4. It is thus possible that the bandgap of BiVO4 can be tunable via controlling these two factors. Photocatalytic properties are greatly enhanced for the KCl-BiVO4 sample, but degraded by post-annealing, where the residual Cl in the sample, when calcined, may act as an inhibitor for the photocatalytic performance. The enhanced photoactivity of KCl-BiVO4 is explained, in a consistent manner, in terms of favorable faceting, bandgap modification, and the heterojunction of BiVO4/BiOCl. We believe these findings are beneficial, not only in photocatalysis but also in other potential applications in bandgap engineering.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16020629/s1, Figure S1: A graphical flow chart of BiVO4 sample preparation; Figure S2: (a) UV-vis absorption spectra and (b) Tauc plots [(F(R)hv)2 vs hv] of KCl-BiVO4, KCl-BiVO4 600-air, and KCl-BiVO4 600-air-Ar (KCl-BiVO4 was calcined at 600 °C in air, and then in Ar gas); Figure S3: Photographs of pristine BiVO4, KCl-BiVO4, and calcined KCl-BiVO4 at various temperatures in air and Ar gas; Figure S4: Tauc plots [(F(R)hv)2 vs hv] and average bandgap values of KCl-BiVO4 with KCl concentrations of (a) 0 mmol, (b) 1 mmol, (c) 2 mmol, (d) 3 mmol, (e) 4 mmol, and (f) 5 mmol. Each sample was measured three times, and the bandgap value of each sample was determined using the average value; Figure S5: High-resolution XPS spectra of V 2p3/2 of KCl-BiVO4 samples with KCl concentration of (a) 0 mmol, (b) 1 mmol, (c) 2 mmol, (d) 3 mmol, (e) 4 mmol, and (f) 5 mmol; Table S1: The lattice parameters determined via the Rietvelt analysis on all the BiVO4 powder samples in Figure 2 and Figure 3; Table S2: XPS peak areas of V5+ and V4+ from V 2p3/2, estimated % oxygen vacancies (Vo), and bandgaps (Eg) of the KCl-BiVO4 with various concentrations of KCl; Table S3: Standard Gibbs free energies of formation (ΔGfo) for various species. Reference [52] is cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.M.; methodology, S.M. and K.N.I.; formal analysis, S.M. and H.O.; investigation, S.M.; writing—original draft preparation, S.M.; writing—review and editing, S.M., T.O. and H.O.; visualization, S.M.; supervision, K.N.I. and H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data that support the findings of this study are available from the first author upon reasonable request.
Acknowledgments
The authors thank to Japan International Cooperation Agency (JICA) for AUN/SEED-Net scholarship program.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Xi, G.; Ye, J. Synthesis of bismuth vanadate nanoplates with exposed {001} facets and enhanced visible-light photocatalytic properties. Chem. Commun. 2010, 46, 1893–1895. [Google Scholar] [CrossRef]
- Senasu, T.; Youngme, S.; Hemavibool, K.; Nanan, S. Sunlight-driven photodegradation of oxytetracycline antibiotic by BiVO4 photocatalyst. J. Solid State Chem. 2021, 297, 122088. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor. Nature 1979, 277, 637. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhang, D.; Zhao, D.; Wang, L.; Zheng, K. Near-infrared photocatalysis based on YF3:Yb3+, Tm3+/TiO2 core/shell nanoparticles. Chem. Commun. 2010, 46, 2304–2306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mao, X.; Chen, P.; Xiao, M.; Monny, S.A.; Wang, S.; Konarova, M.; Du, A.; Wang, L. Understanding the roles of oxygen vacancies in hematite-based photoelectrochemical processes. Angew. Chem. Int. Ed. 2019, 58, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Li, X.; Sun, Y.; Zhang, M.; Mu, J.; Zhang, P.; Guo, Z.; Liu, Y. Hierarchical assembly of ultrathin hexagonal SnS2 nanosheets onto electrospun TiO2 nanofibers: Enhanced photocatalytic activity based on photoinduced interfacial charge transfer. Nanoscale 2013, 5, 606–618. [Google Scholar] [CrossRef]
- Hao, X.; Hu, Y.; Cui, Z.; Zhou, J.; Wang, Y.; Zou, Z. Self-constructed facet junctions on hexagonal CdS single crystals with high photoactivity and photostability for water splitting. Appl. Catal. B Environ. 2019, 244, 694–703. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, W.; Hao, Y.; Zhang, Z.; Li, Q.; Zang, S. Nanostructured shuriken-like BiVO4 with preferentially exposed {010} facets: Preparation, formation mechanism, and enhanced photocatalytic performance. Dalton Trans. 2018, 47, 1325–1336. [Google Scholar] [CrossRef]
- Tan, H.L.; Amal, R.; Ng, Y.H. Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4: A review. J. Mater. Chem. A 2017, 5, 16498–16521. [Google Scholar] [CrossRef]
- Cooper, J.K.; Scott, S.B.; Ling, Y.; Yang, J.; Hao, S.; Li, Y.; Toma, F.M.; Stutzmann, M.; Lakshmi, K.V.; Sharp, I.D. Role of hydrogen in defining the n-type character of BiVO4 photoanodes. Chem. Mater. 2016, 28, 5761–5771. [Google Scholar] [CrossRef]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.-S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015, 6, 8769. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Suyanto, A.; De Denko, A.T.; Saputera, W.H.; Amal, R.; Osterloh, F.E.; Ng, Y.H. Enhancing the photoactivity of faceted BiVO4 via annealing in oxygen-deficient condition. Part. Part. Syst. Charact. 2017, 34, 1600290. [Google Scholar] [CrossRef]
- Qin, C.; Liao, H.; Rao, F.; Zhong, J.; Li, J. One-pot hydrothermal preparation of Br-doped BiVO4 with enhanced visible-light photocatalytic activity. Solid State Sci. 2020, 105, 106285. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, F.; Lin, Y.; Wang, G.; Pan, F. Correlation of oxygen vacancy variations to band gap changes in epitaxial ZnO thin films. Appl. Phys. Lett. 2013, 102, 181908. [Google Scholar] [CrossRef]
- Biswas, R.U.D.; Oh, W.-C. Synthesis of BiVO4-GO-PVDF nanocomposite: An excellent, newly designed material for high photocatalytic activity towards organic dye degradation by tuning band gap energies. Solid State Sci. 2018, 80, 22–30. [Google Scholar] [CrossRef]
- Meng, S.; Ogawa, T.; Okumura, H.; Ishihara, K.N. The effect of potassium chloride on BiVO4 morphology and photocatalysis. J. Solid State Chem. 2021, 302, 122291. [Google Scholar] [CrossRef]
- Künneth, C.; Batra, R.; Rossetti, G.A.; Ramprasad, R.; Kersch, A. Thermodynamics of phase stability and ferroelectricity from first principles. In Ferroelectricity in Doped Hafnium Oxide: Materials, Properties and Devices; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar] [CrossRef]
- Gunkel, F.; Christensen, D.V.; Chen, Y.Z.; Pryds, N. Oxygen vacancies: The (in)visible friend of oxide electronics. Appl. Phys. Lett. 2020, 116, 120505. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Chen, P.; Du, A.; Ostrikov, K.; Huang, W.; Wang, L. In situ formation of oxygen vacancies achieving near-complete charge separation in planar BiVO4 photoanodes. Adv. Mater. 2020, 32, 2001385. [Google Scholar] [CrossRef]
- Yu, W.; Chen, F.; Wang, Y.; Zhao, L. Rapid evaluation of oxygen vacancies-enhanced photogeneration of the superoxide radical in nano-TiO2 suspensions. RSC Adv. 2020, 10, 29082–29089. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yamane, H.; Matsushima, S.; Nakamura, H.; Ohira, K.; Kouya, M.; Kumada, K. Effect of thermal treatment on photocatalytic activity of N-doped TiO2 particles under visible light. Thin Solid Films 2008, 516, 7482–7487. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Lu, X.; Qian, F.; Tong, Y.; Zhang, J.Z.; Lordi, V.; Leao, C.R.; Li, Y. Computational and photoelectrochemical study of hydrogenated bismuth vanadate. J. Phys. Chem. C 2013, 117, 10957–10964. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Liu, B. Synthesis of BiVO4 nanoparticles with tunable oxygen vacancy level: The phenomena and mechanism for their enhanced photocatalytic performance. Ceram. Int. 2021, 47, 9849–9855. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Mtangi, W.; Auret, F.D.; Meyer, W.E.; Legodi, M.J.; Janse Van Rensburg, P.J.; Coelho, S.M.M.; Diale, M.; Nel, J.M. Effects of hydrogen, oxygen, and argon annealing on the electrical properties of ZnO and ZnO devices studied by current-voltage, deep level transient spectroscopy, and Laplace DLTS. J. Appl. Phys. 2012, 111, 094504. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Jiang, S.; Li, B.; Wang, J.; Shao, X.; Wang, D.; Wang, K.; Yan, Z. Bacitracin-assisted synthesis of spherical BiVO4 nanoparticles with C doping for remarkable photocatalytic performance under visible light. CrystEngComm 2020, 22, 1812–1821. [Google Scholar] [CrossRef]
- Ravidhas, C.; Josephine, A.J.; Sudhagar, P.; Devadoss, A.; Terashima, C.; Nakata, K.; Fujishima, A.; Raj, A.M.E.; Sanjeeviraja, C. Facile synthesis of nanostructured monoclinic bismuth vanadate by a co-precipitation method: Structural, optical and photocatalytic properties. Mater. Sci. Semicond. Process. 2015, 30, 343–351. [Google Scholar] [CrossRef]
- Xie, S.; Shen, Z.; Zhang, H.; Cheng, J.; Zhang, Q.; Wang, Y. Photocatalytic coupling of formaldehyde to ethylene glycol and glycolaldehyde over bismuth vanadate with controllable facets and cocatalysts. Catal. Sci. Technol. 2017, 7, 923–933. [Google Scholar] [CrossRef]
- Liu, X.; Su, Y.; Zhao, Q.; Du, C.; Liu, Z. Constructing Bi24O31Cl10/BiOCl heterojunction via a simple thermal annealing route for achieving enhanced photocatalytic activity and selectivity. Sci. Rep. 2016, 6, 28689. [Google Scholar] [CrossRef]
- Eggenweiler, U.; Keller, E.; Krämer, V. Redetermination of the crystal structures of the “arppe compound” Bi24O31Cl10 and the isomorphous Bi24O31Br10. Acta Crystallogr. Sect. B Struct. Sci. 2000, 56, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Wang, J.; Wang, Z.; Chen, J.; Xing, X.; Wang, L.; Yu, R. Bismuth oxychloride hollow microspheres with high visible light photocatalytic activity. Nano Res. 2016, 9, 593–601. [Google Scholar] [CrossRef]
- Sorokina, S.; Enjalbert, R.; Baulès, P.; Castro, A.; Galy, J. Continuous structural evolution of (Bi2O2)2V2yO4y+2(1≤y≤4) aurivillius phases in the Bi2O3–VO2 system. J. Solid State Chem. 1996, 125, 54–62. [Google Scholar] [CrossRef]
- Galy, J.; Enjalbert, R.; Millan, P.; Castro, A. New Aurivillius phases in the bismuth-vanadium-oxygen system crystal structure of Bi4V2O10. Comptes Rendus l’Academie Des Sci. Ser. 2 1993, 317, 43–48. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:24068739 (accessed on 14 June 2022). [CrossRef]
- Satto, C.; Millet, P.; Sciau, P.; Roucau, C.; Galy, J. α-Bi4V2O10 crystal structure and oxidation mechanism. X-ray and electron diffraction analysis. Mater. Res. Bull. 1999, 34, 655–664. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, Y.; Ma, F.; Zhang, Z.; Wei, X. Effects of oxygen vacancy on the mechanical, electronic and optical properties of monoclinic BiVO4. J. Mater. Sci. 2017, 52, 8546–8555. [Google Scholar] [CrossRef]
- Selim, S.; Pastor, E.; García-Tecedor, M.; Morris, M.R.; Francàs, L.; Sachs, M.; Moss, B.; Corby, S.; Mesa, C.A.; Gimenez, S.; et al. Impact of oxygen vacancy occupancy on charge carrier dynamics in BiVO4 photoanodes. J. Am. Chem. Soc. 2019, 141, 18791–18798. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-K.; Guan, M.-L.; Liu, S.-S.; Zhang, Y.-Q.; Zhang, C.-W.; He, Y.-X.; Huang, S.-M. Controlled synthesis of olive-shaped Bi2S3/BiVO4 microspheres through a limited chemical conversion route and enhanced visible-light-responding photocatalytic activity. Dalton Trans. 2012, 41, 5581–5586. [Google Scholar] [CrossRef]
- Feng, C.; Wang, D.; Jin, B.; Jiao, Z. The enhanced photocatalytic properties of BiOCl/BiVO4 p-n heterojunctions via plasmon resonance of metal Bi. RSC Adv. 2015, 5, 75947–75952. [Google Scholar] [CrossRef]
- Jaihindh, D.P.; Thirumalraj, B.; Chen, S.-M.; Balasubramanian, P.; Fu, Y.-P. Facile synthesis of hierarchically nanostructured bismuth vanadate: An efficient photocatalyst for degradation and detection of hexavalent chromium. J. Hazard. Mater. 2019, 367, 647–657. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, H.; Meng, X.; Ji, K.; Zhang, L.; Deng, J. Porous olive-like BiVO4: Alcoho-hydrothermal preparation and excellent visible-light-driven photocatalytic performance for the degradation of phenol. Appl. Catal. B 2011, 105, 326–334. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhou, L.; Xu, H. Efficient methylene blue removal over hydrothermally synthesized starlike BiVO4. Ind. Eng. Chem. Res. 2009, 48, 1735–1739. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, C.; Lin, H.; Xu, B.; Chen, S. Surface modification of m-BiVO4 with wide band-gap semiconductor BiOCl to largely improve the visible light induced photocatalytic activity. Appl. Surf. Sci. 2013, 284, 263–269. [Google Scholar] [CrossRef]
- Qin, D.-D.; Wang, T.; Song, Y.-M.; Tao, C.-L. Reduced monoclinic BiVO4 for improved photoelectrochemical oxidation of water under visible light. Dalton Trans. 2014, 43, 7691–7694. [Google Scholar] [CrossRef] [PubMed]
- Okumura, H.; Adachi, K.; Yamasue, E.; Ishihara, K.N. New LnOCl (Ln = Sm, Nd) photocatalyst and novel cocatalytic effect on BiOCl in humid environment. Chem. Commun. 2017, 53, 8854–8857. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, Q.; Fernandez-Alberti, S.; Long, R. Controlling charge carrier trapping and recombination in BiVO4 with the oxygen vacancy oxidation state. J. Phys. Chem. Lett. 2021, 12, 3514–3521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, S.; He, H.; Xie, C.; Tang, Y.; He, C.; Shao, M.; Wang, H. Oxygen vacancy engineering in titanium dioxide for sodium storage. Chem. Asian J. 2021, 16, 3–19. [Google Scholar] [CrossRef]
- Byun, S.; Jung, G.; Shi, Y.; Lanza, M.; Shin, B. Aging of a vanadium precursor solution: Influencing material properties and photoelectrochemical water oxidation performance of solution-processed BiVO4 photoanodes. Adv. Funct. Mater. 2020, 30, 1806662. [Google Scholar] [CrossRef]
- Kim, S.; Vijayakumar, M.; Wang, W.; Zhang, J.; Chen, B.; Nie, Z.; Chen, F.; Hu, J.; Li, L.; Yang, Z. Chloride supporting electrolytes for all-vanadium redox flow batteries. Phys. Chem. Chem. Phys. 2011, 13, 18186–18193. [Google Scholar] [CrossRef]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. BiOCl/BiVO4 p–n heterojunction with enhanced photocatalytic activity under visible-light irradiation. J. Phys. Chem. C 2014, 118, 389–398. [Google Scholar] [CrossRef]
- Wagman, D.; Evans, W.; Parker, V.; Schumm, R.; Halow, I.; Bailey, S.; Churney, K.; Nuttall, R. Erratum: The NBS tables of chemical thermodynamic properties. Selected values for inorganic and C1 and C2 organic substances in SI units. Phys. Chem. Ref. Data 11, Suppl. 2 (1982)]. J. Phys. Chem. Ref. Data 1989, 18, 1807–1812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).