Research on the Influence of Ripple Voltage on the Performance of a Proton Exchange Membrane Electrolyzer
Abstract
:1. Introduction
- (1)
- The hydrogen production mechanism of the proton exchange membrane electrolyzer is described, and a dynamic model of the proton exchange membrane electrolyzer was established according to the principles of electrochemistry and material conservation.
- (2)
- A simulation model of a proton exchange membrane electrolyzer was established in PSCAD/EMTDC, and its correctness and effectiveness were verified via simulation. The influence of relevant performance parameters on the terminal voltage of the electrolyzer was analyzed.
- (3)
- Based on the established model, the relationship between the ripple voltage and the performance of proton exchange membrane electrolyzers was interpreted, and the hydrogen production and power consumption performance of electrolyzers were analyzed by using three common forms of ripple voltage.
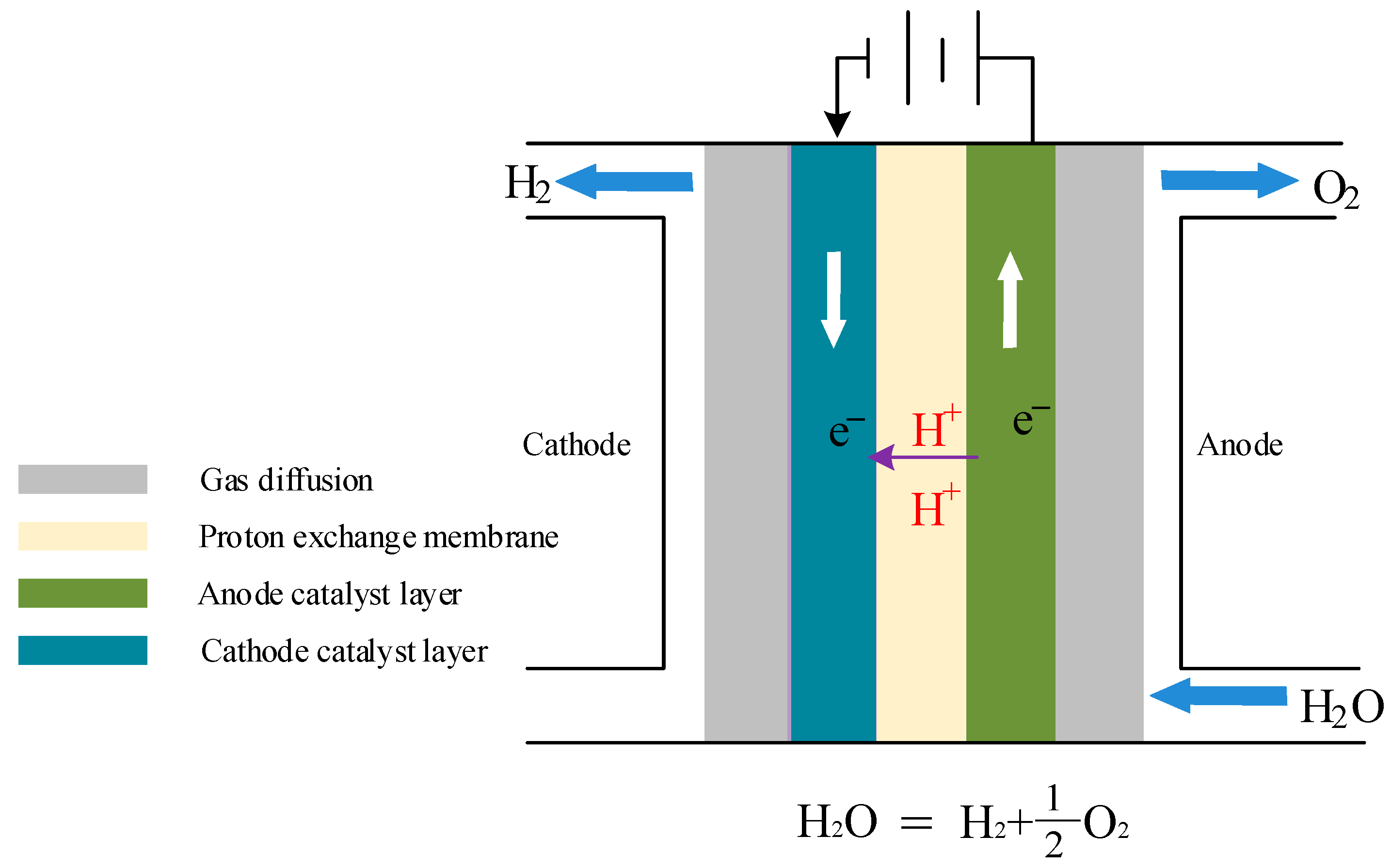
2. Introduction of Proton Exchange Membrane Electrolyzer
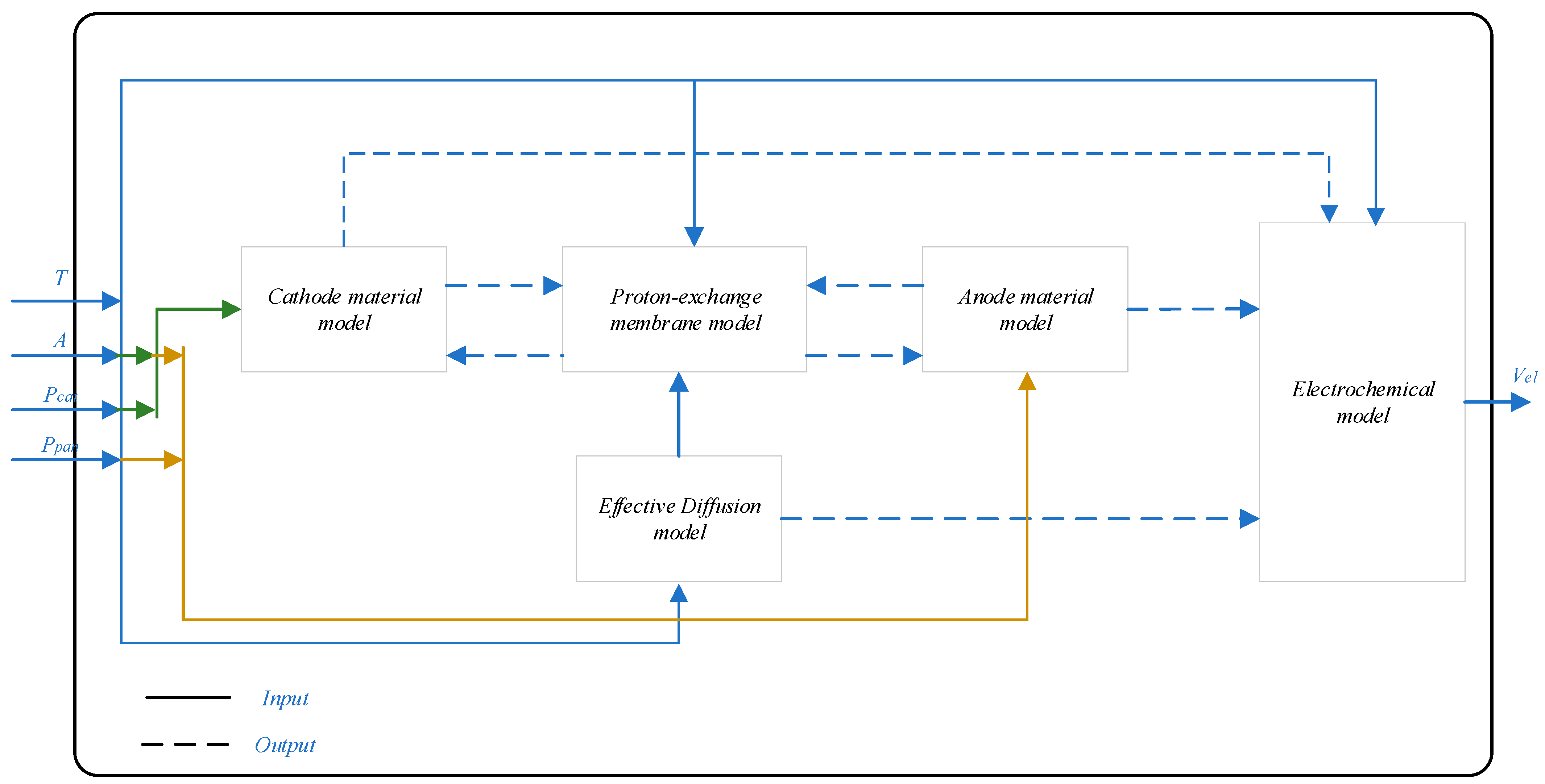
3. Mathematical Model of Proton Exchange Membrane Electrolyzer
- ▪
- The reaction inside the electrolytic cell is carried out under isothermal conditions;
- ▪
- The reaction inside the electrolytic cell is carried out under isobaric conditions;
- ▪
- The ohmic resistance and exchange membrane resistance of the electrolytic cell are fixed values;
- ▪
- The friction loss of water and gas in the pipeline is 0.
3.1. Voltage Model
3.2. Material Transport Model
4. Performance Analysis of Proton Exchange Membrane Electrolyzer
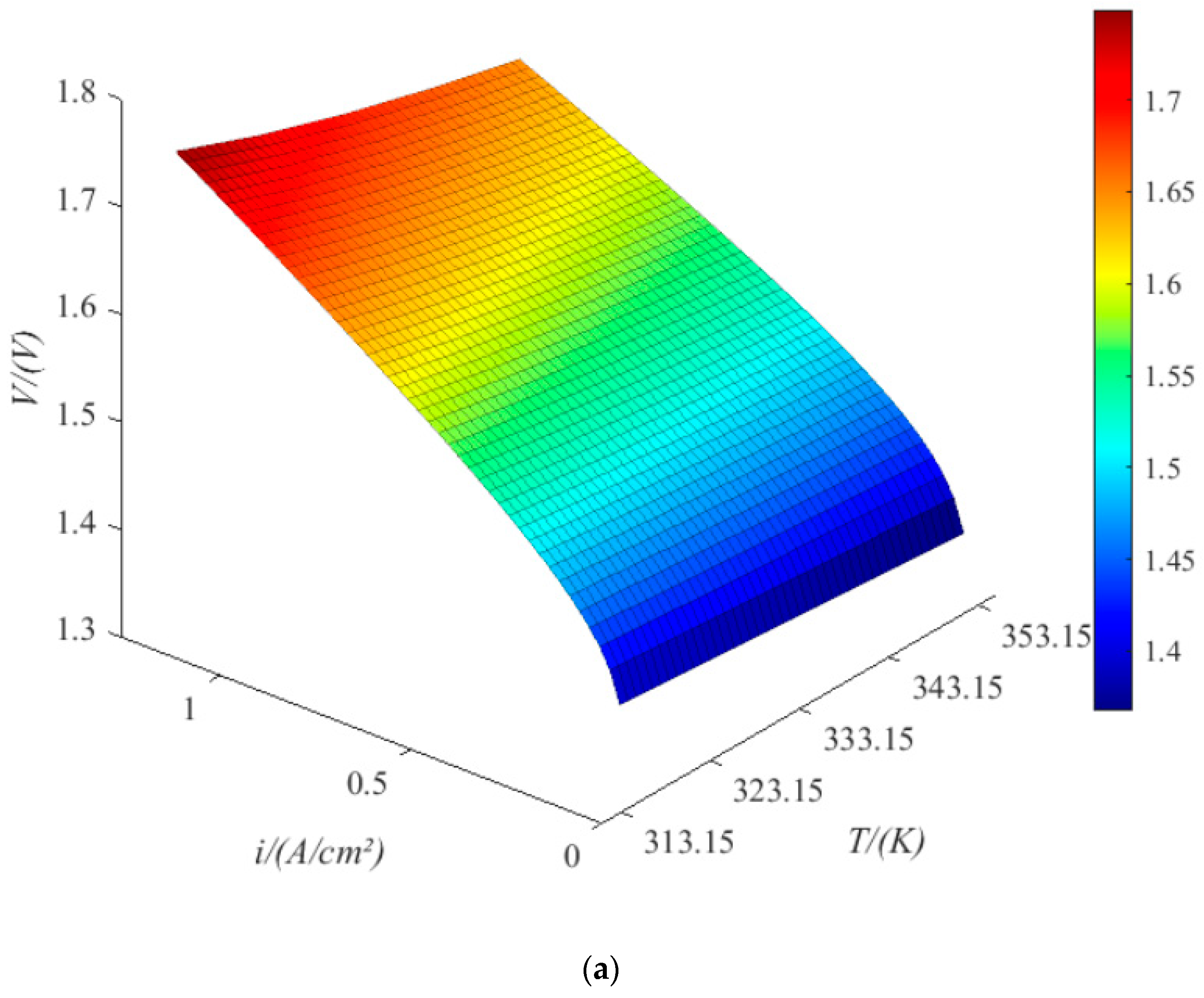
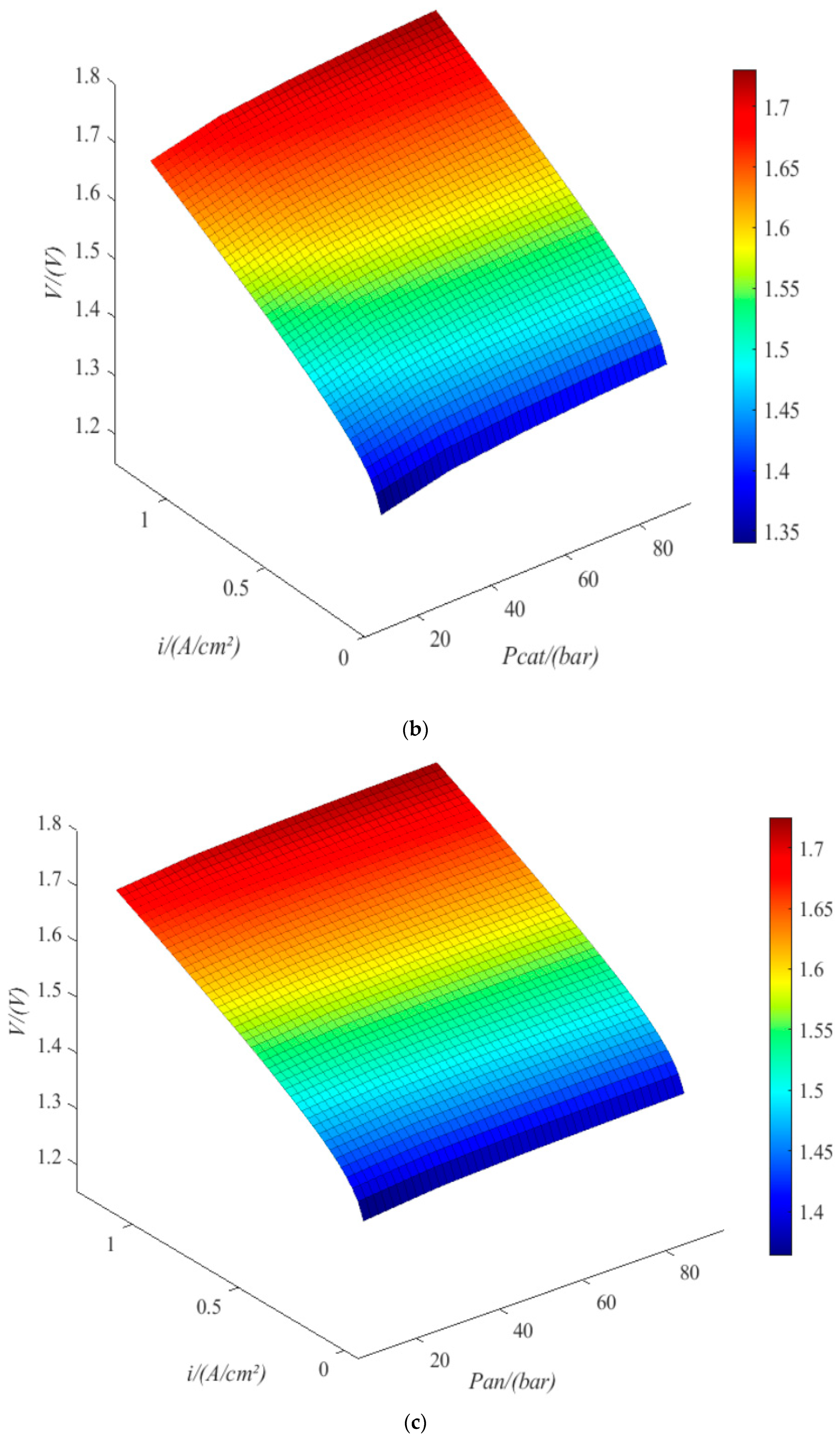
4.1. PEMEL Properties
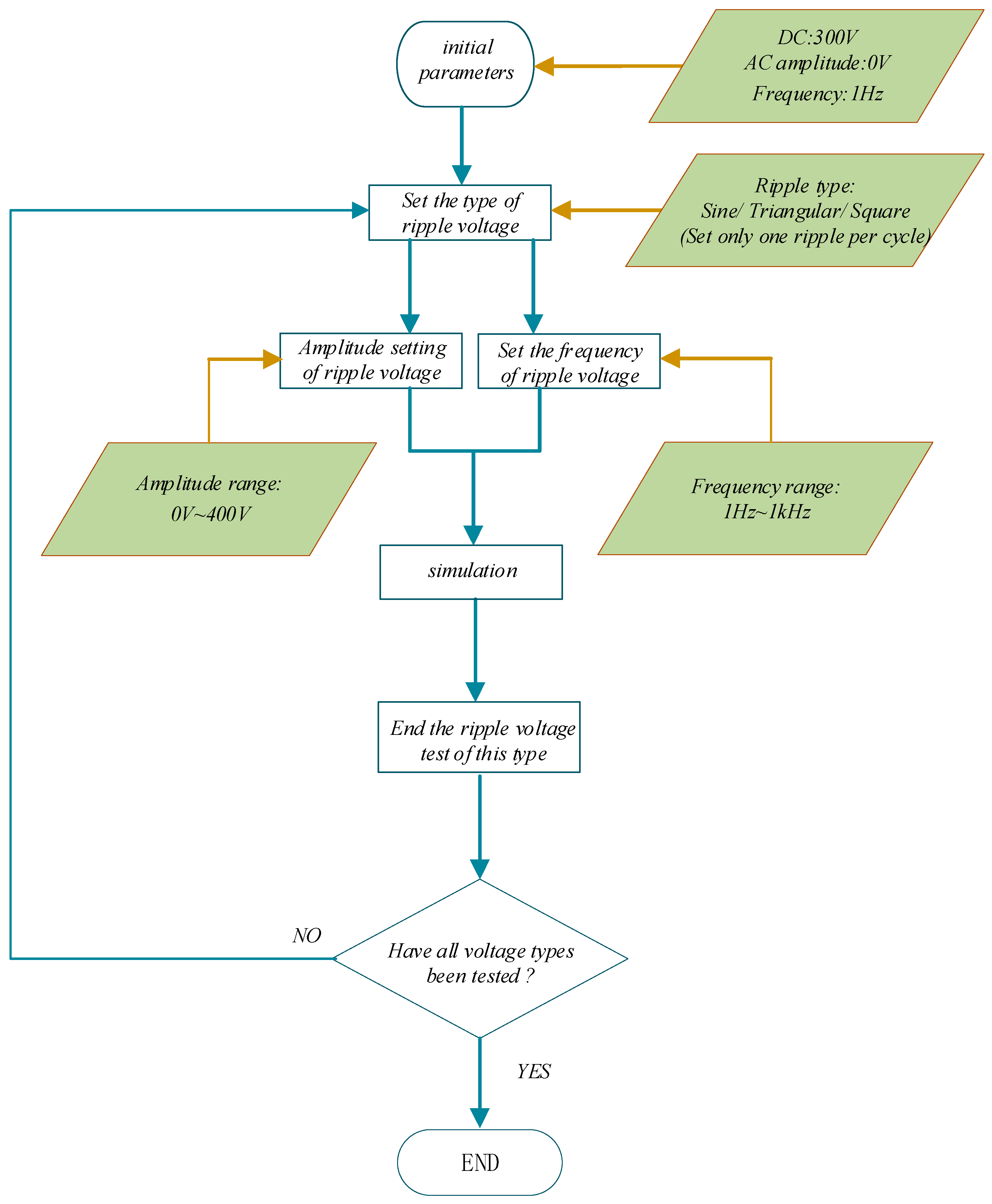
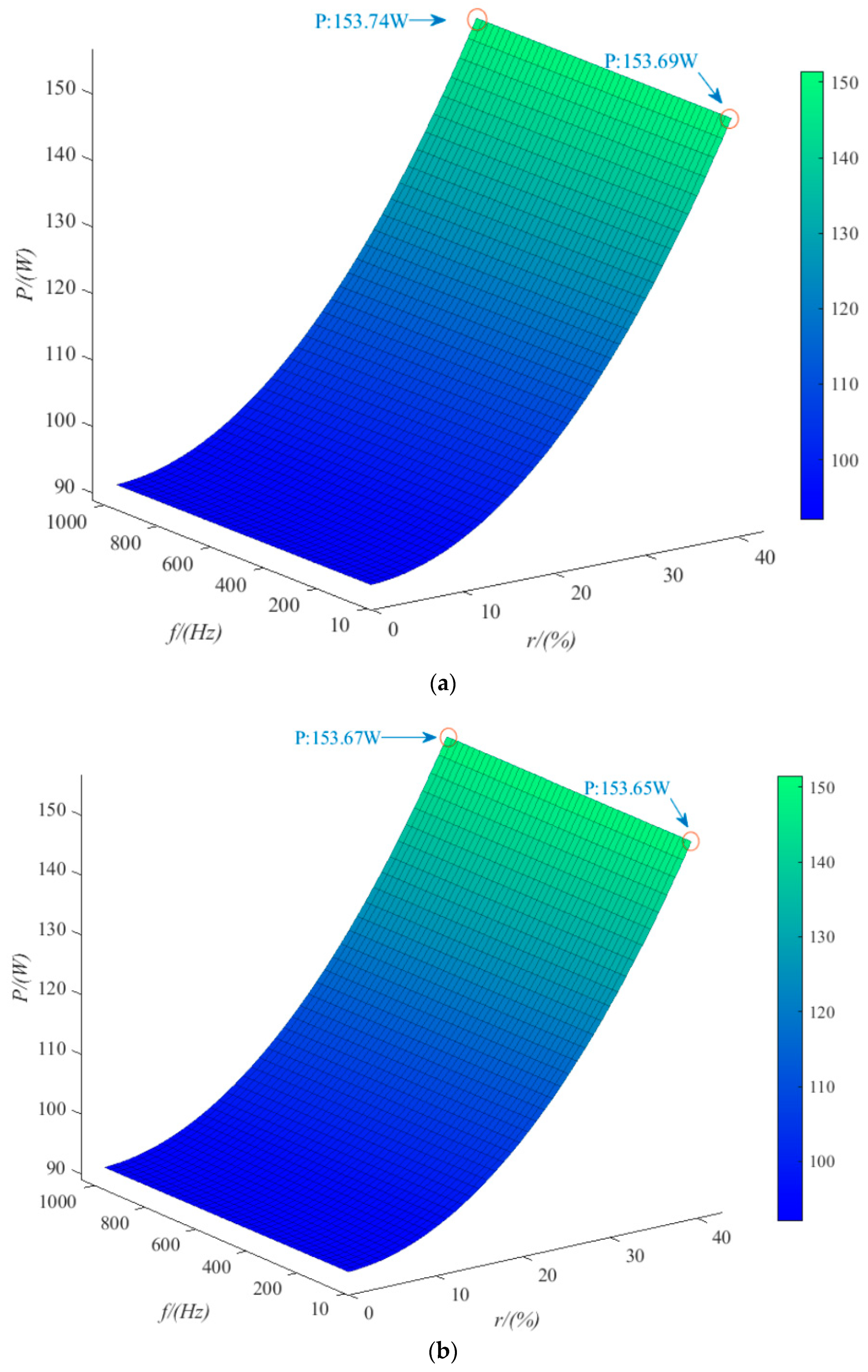
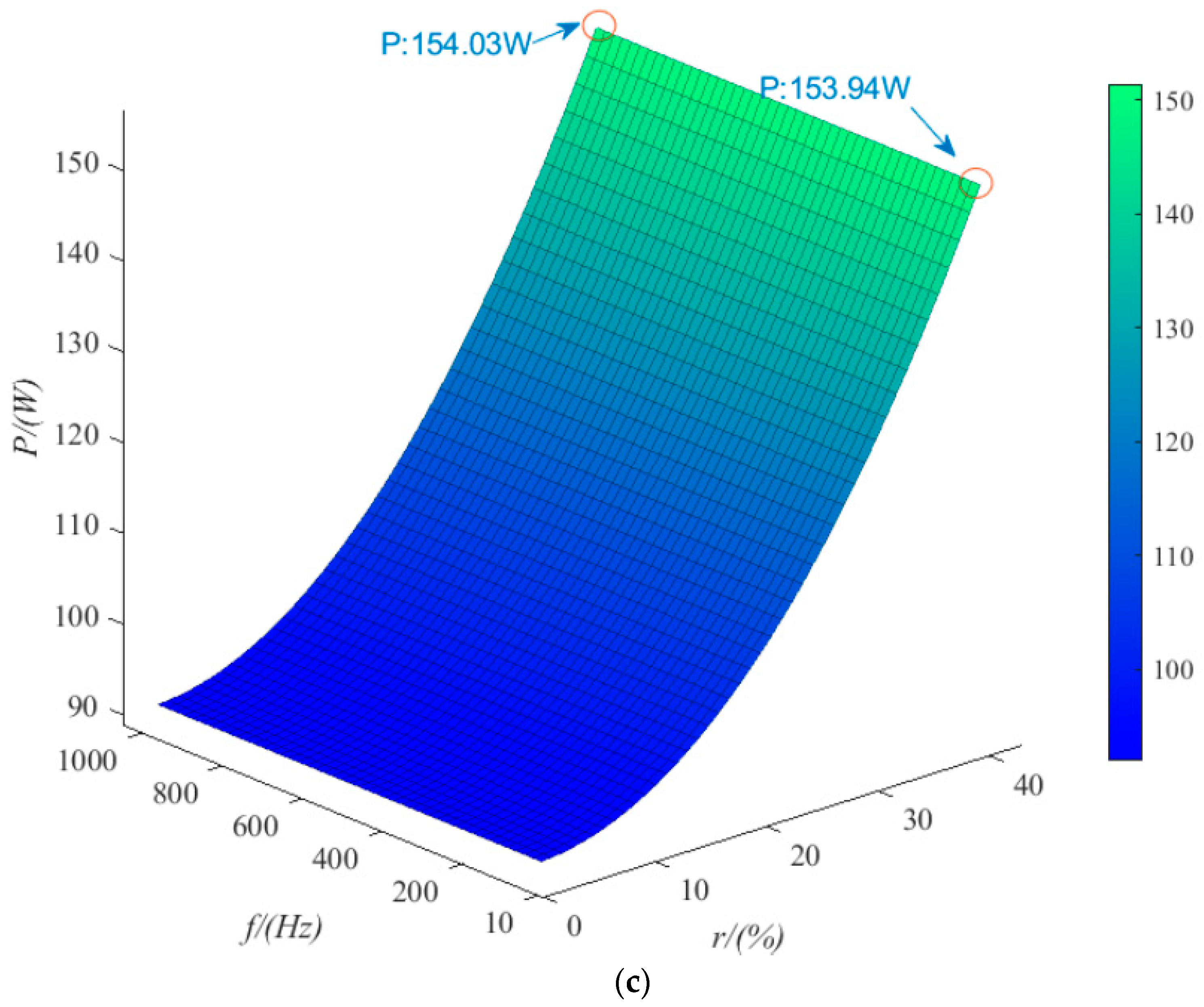
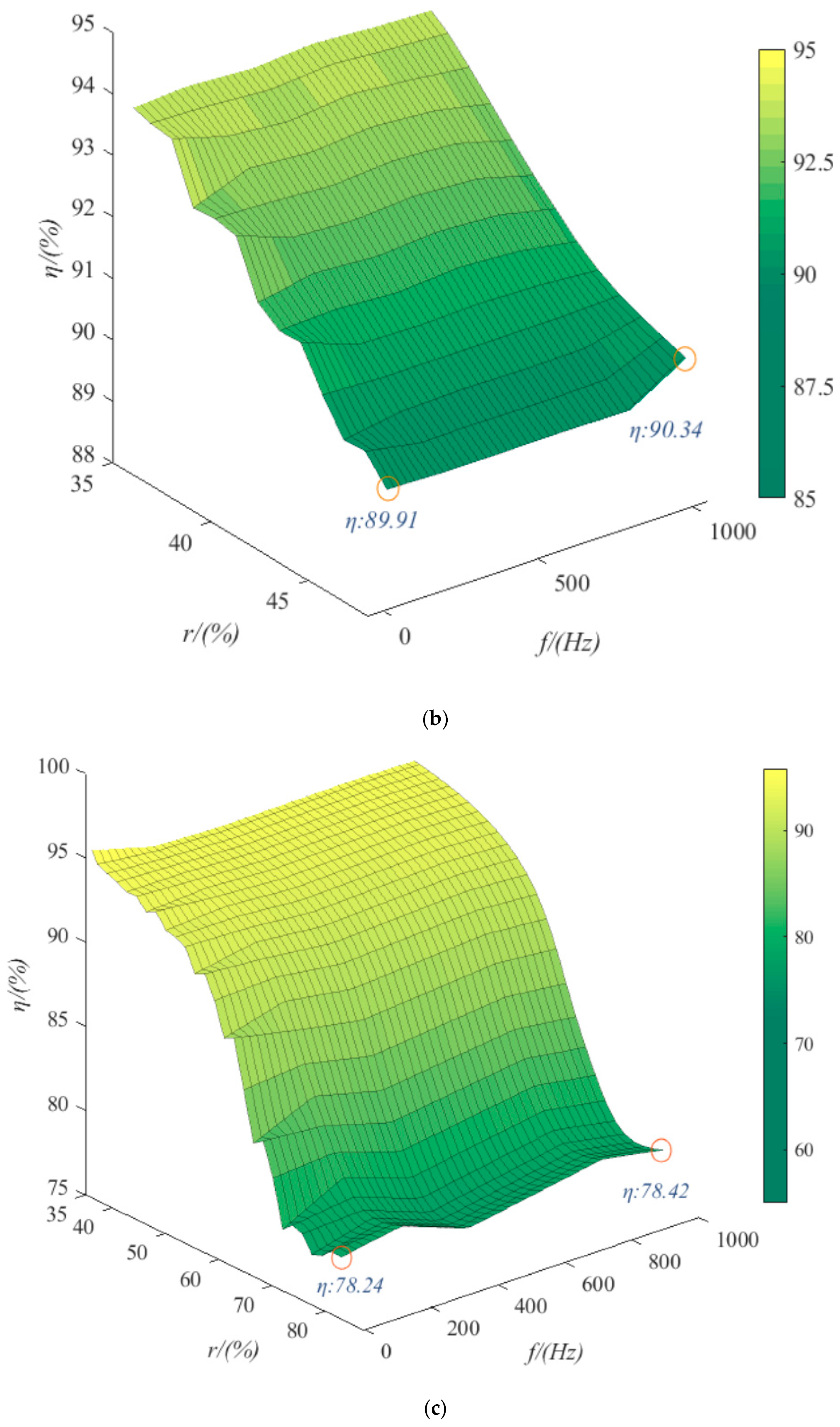
4.2. Ripple Voltage Effect
5. Conclusions
- (1)
- The established mathematical model and simulation model of the proton exchange membrane electrolyzer are correct and can be used for reference in subsequent research.
- (2)
- When the current density is 1.18 A/cm2, the terminal voltage is 1.75 V at a temperature of 314 K, 1.65 V at a temperature of 352 K, 1.4 V at an exchange current density of 10–1.34 A/cm2, 1.57 V at an exchange current density of 10−9.04 A/cm2, 1.67 V at a cathode pressure of 11.5 bar, 1.73 V at a cathode pressure of 88.5 bar, 1.69 V at an anode pressure of 11.5 bar, and 1.72 V at an anode pressure of 88.5 bar. The trend is the same under low current density. Therefore, increasing the pressure of anode and cathode is beneficial to improving the working current density; increasing the exchange current density and increasing the temperature of the electrolytic cell are conducive to reducing the power consumption of the electrolytic cell.
- (3)
- The trends of the effects of the three kinds of ripple are consistent. Taking sinusoidal ripple voltage as an example, when the ripple coefficient increases by 45%, the average power consumption increases by 61%. When the ripple coefficient is constant, the frequency increases by 1000%, and the average power consumption increases by only 0.033%. In the range of low ripple coefficients (0~35%), the hydrogen production rate can be reduced by 2% at most. When the ripple coefficient is in the range of 35~70%, the hydrogen production rate can be reduced by 12% at most. The ripple coefficient has a great influence on the power consumption and hydrogen production rate of the electrolytic cell, but the frequency is small. Among the three kinds of ripple, the triangular wave has the least influence on the power consumption and hydrogen production rate of the electrolytic cell. Therefore, the influence of the ripple coefficient and the overall smoothness of the waveform should be considered in practical application.
- (4)
- Although the test results are under specific parameters, because the working principle, internal chemical reaction and mechanical structure of the electrolytic cell are fixed, the conclusions are qualitatively consistent. It is universal for different types of equipment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Li, H.; Peng, Y.; Xiao-Yan, L.; Xin-Yu, Z. Modeling and grid connection control of wind hydrogen storage coupling system. China South. Power Grid Technol. 2022, 16, 141–150. (In Chinese) [Google Scholar]
- Zhang, W. Technical progress and economic analysis of hydrogen production. Contemp. Pet. Petrochem. 2022, 30, 31–36. (In Chinese) [Google Scholar]
- Grigoriev, S.; Porembsky, V.; Fateev, V. Pure hydrogen production by PEM electrolysis for hydrogen energy. Int. J. Hydrogen Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- Rashid, M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 2249–8958. [Google Scholar]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J. Few, Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Yodwong, B.; Guilbert, D.; Phattanasak, M.; Kaewmanee, W.; Hinaje, M.; Vitale, G. Proton exchange membrane electrolyzer modeling for power electronics control: A short review. C J. Carbon Res. 2020, 6, 29. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Li, G.; Niu, M. Research progress of proton exchange membrane electrolyzer modeling based on model hierarchy analysis. High Volt. Technol. 2023, 49, 1105–1117. (In Chinese) [Google Scholar]
- Rahim, A.H.; Tijani, A.S.; Kamarudin, S.K.; Hanapi, S. An overview of polymer electrolyte membrane electrolyzer for hydrogen production: Modeling and mass transport. J. Power Sources 2016, 309, 56–65. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Gong, M.; Zhao, H.; Wang, X.; Huang, H. Process simulation of factors affecting hydrogen production from water electrolysis with proton exchange membrane. Compr. Smart Energy 2022, 44, 88–94. (In Chinese) [Google Scholar]
- Zheng, J.; Mo, J. Three-dimensional modeling of the reaction characteristic parameters of PEM electrolysis cells. Chin. J. Power Sources 2021, 45, 1401–1404, 1504. (In Chinese) [Google Scholar]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Koponen, J.; Ruuskanen, V.; Hehemann, M.; Rauls, E.; Kosonen, A.; Ahola, J.; Stolten, D. Effect of power quality on the design of proton exchange membrane water electrolysis systems. Appl. Energy 2020, 279, 115791. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Energy and exergy analysis of hydrogen production by a proton exchange membrane (PEM) electro-lyzer plant. Energy Convers. Manag. 2008, 49, 2748–2756. [Google Scholar] [CrossRef]
- Ursu´a, A.; Marroyo, L.; Gubı´a, E.; Gandı´a, L.M.; Dieguez, P.M.; Sanchis, P. Influence of the power supply on the energy efficiency of an alkaline water electrolyser. Int. J. Hydrogen Energy 2009, 34, 3221e33. [Google Scholar] [CrossRef]
- Demir, N.; Kaya, M.F.; Albawabiji, M.S. Effect of pulse potential on alkaline water electrolysis performance. Int. J. Hydrogen Energy 2018, 43, 17013–17020. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de SáA, I.; Teixeira, A.P.S.; Ortiz-Martinez, V.M.; Ortiz, A.; Ortiz, I.; Rangel, C.M. New modified Nafion-bisphosphonic acid composite membranes for enhanced proton conductivity and PEMFC performance. Int. J. Hydrogen Energy 2021, 46, 17562–17571. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-fluorinated polymer composite proton exchange membranes for fuel cell applications-Areview. Chem. Phys. Chem. 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Hubert, M.A.; Capuano, C.; Manco, J.; Danilovic, N.; Valle, E.; Hellstern, T.R.; Ayers, K.; Jaramillo, T.F. A non-precious metalhydrogen catalyst in a commercial polymer electrolyte membrane electrolyser. Nat. Nanotechnol. 2019, 14, 1071–1074. [Google Scholar] [CrossRef]
- Bernt, M.; Hartig-weiss, A.; Tovini, M.F.; El-Sayed, H.A.; Schramm, C.; Schroter, J.; Gebauer, C.; Gasteiger, H.A. Current challenges incatalyst development for PEM water electrolyzers. Chem. Ing. Tech. 2020, 92, 31–39. [Google Scholar] [CrossRef]
- Guo, Y.; Cui, Z.; Xu, X.; Feng, Q. Preparation of alloy catalysts by electroplating method for hydrogen production from hydrolysis of sodium borohydride. Lab. Res. Explor. 2015, 34, 14–17. (In Chinese) [Google Scholar]
- Liu, C.; Zeng, G.; Liao, M.; Dong, X. Dynamic simulation modeling and fast frequency response analysis of large capacity electrolytic cell. Power Grid Technol. 2023, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Raúl, M.; Luis, M.; Fernández, R.; Garcia-Vazquez, C.A.; Jurado, F. Electrolyzer models for hydrogen production from wind energy systems. J. Hydrogen Energy 2015, 40, 2927–2938. [Google Scholar]
- Abdullah, A.; Ibrahim, D. Thermodynamic and electrochemical analyses of a solid oxide electrolyzer for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 21404–21413. [Google Scholar]
- Keller, R.; Rauls, E.; Hehemann, M.; Muller, M.; Carmo, M. An adaptive model-based feedforward temperature control of a 100 kW PEM electrolyzer. Control. Eng. Pract. 2022, 120, 104992. [Google Scholar] [CrossRef]
- Huang, P. Umidity standard of compressed hydrogen for fuel cell technology. ECS Trans. 2008, 12, 479–484. [Google Scholar] [CrossRef]
- Wang, Z.M. Simulation and Experimental Study on the Performance of Proton Exchange Membrane Cell. Ph.D. Thesis, North China Electric Power University, Beijing, China, 2021. (In Chinese). [Google Scholar]
- Abdin, Z.; Webb, C.; Gray, E. Modelling and simulation of a proton exchange membrane (PEM) electrolyser cell. Int. J. Hydrogen Energy 2015, 40, 13243–13257. [Google Scholar] [CrossRef]



| Subscript i | ai | bi |
|---|---|---|
| 0 | 0.0089 | 0.0093 |
| 1 | −1.468 × 10−6 | −8.012 × 10−6 |
| 2 | 4.101 × 10−5 | 4.213 × 10−6 |
| 3 | −3.703 × 10−7 | −3.782 × 10−6 |
| 4 | 9.8 × 10−9 | 1 × 10−8 |
| Parameter | Numerical Value | Parameter | Numerical Value |
|---|---|---|---|
| Membrane area A | 160 cm2 | Curvature ξ | 4 |
| Film thickness δmem | 0.0254 cm | Average pore radius r | 1 nm |
| Electro-osmosis coefficient nd | 7 | Water diffusion coefficient in membrane Dw | 1.28 × 10−6 |
| Membrane moisture content λ | 21% | Permeability of Membrane to water KDarcy | 1.58 × 10−14 |
| Porosity ε | 0.3 | Water density ρH2O | 1 (g/cm3) |
| Faraday constant F | 96,485 (C/mol) | Gas constant R | 8.314 (J/mol/K) |
| Oxygen pressure PO2 | 3 atm | Hydrogen pressure PH2 | 3 atm |
| AC current density i0 | 0.4 (A/cm2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, T.; Li, H.; Wang, J.; Jia, D. Research on the Influence of Ripple Voltage on the Performance of a Proton Exchange Membrane Electrolyzer. Energies 2023, 16, 6912. https://doi.org/10.3390/en16196912
Yuan T, Li H, Wang J, Jia D. Research on the Influence of Ripple Voltage on the Performance of a Proton Exchange Membrane Electrolyzer. Energies. 2023; 16(19):6912. https://doi.org/10.3390/en16196912
Chicago/Turabian StyleYuan, Tianze, Hua Li, Jikang Wang, and Dong Jia. 2023. "Research on the Influence of Ripple Voltage on the Performance of a Proton Exchange Membrane Electrolyzer" Energies 16, no. 19: 6912. https://doi.org/10.3390/en16196912
APA StyleYuan, T., Li, H., Wang, J., & Jia, D. (2023). Research on the Influence of Ripple Voltage on the Performance of a Proton Exchange Membrane Electrolyzer. Energies, 16(19), 6912. https://doi.org/10.3390/en16196912







