Experimental and Theoretical Evaluation of a Commercial Luminescent Dye for PVT Systems
Abstract
:1. Introduction
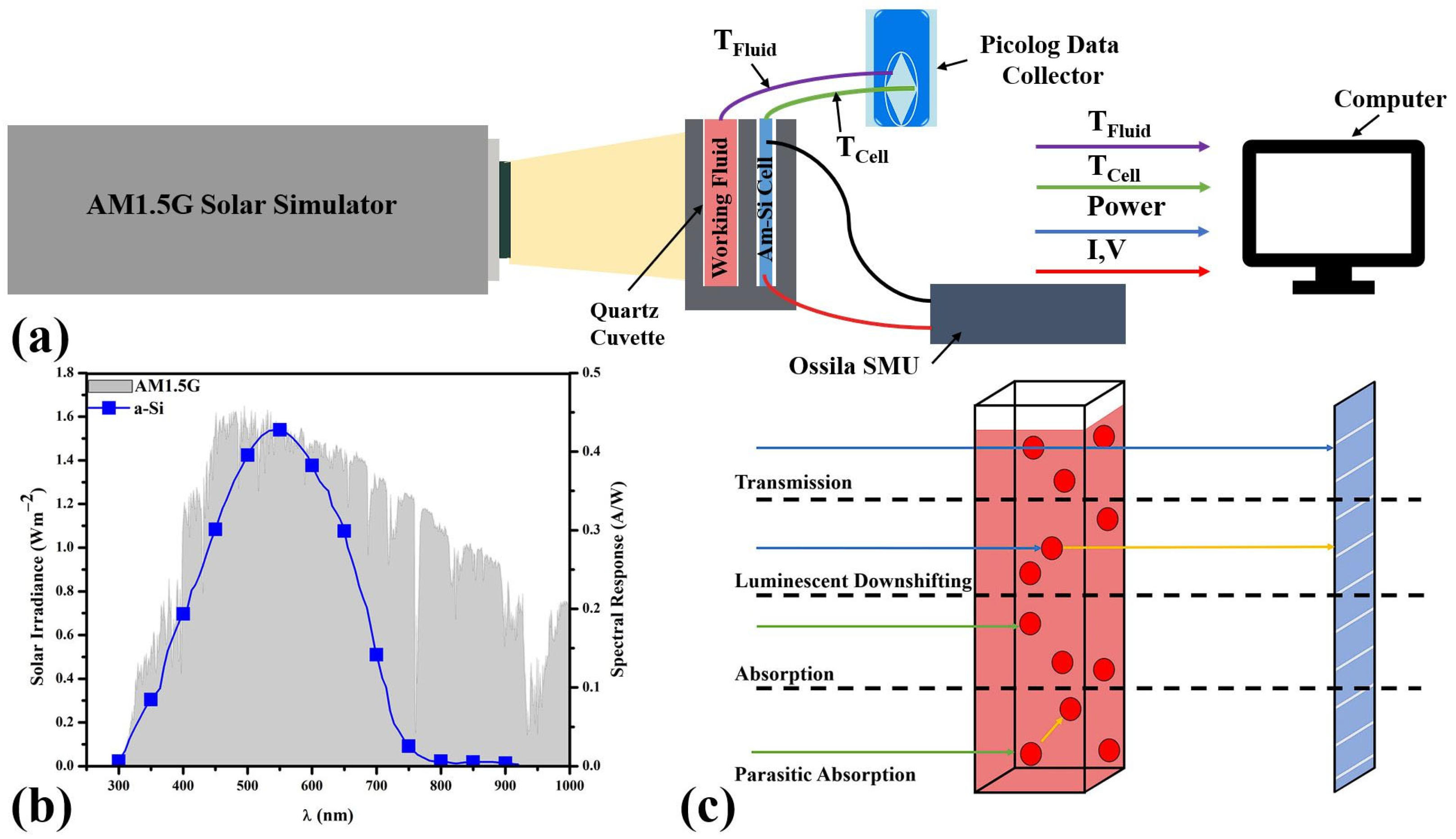
2. Materials and Methods
2.1. Preparation of Working Fluids
2.2. Photovoltaic-Thermal System Performance
2.3. Monte Carlo Raytrace Model
2.4. PVT Energy Conversion Calculation
3. Results
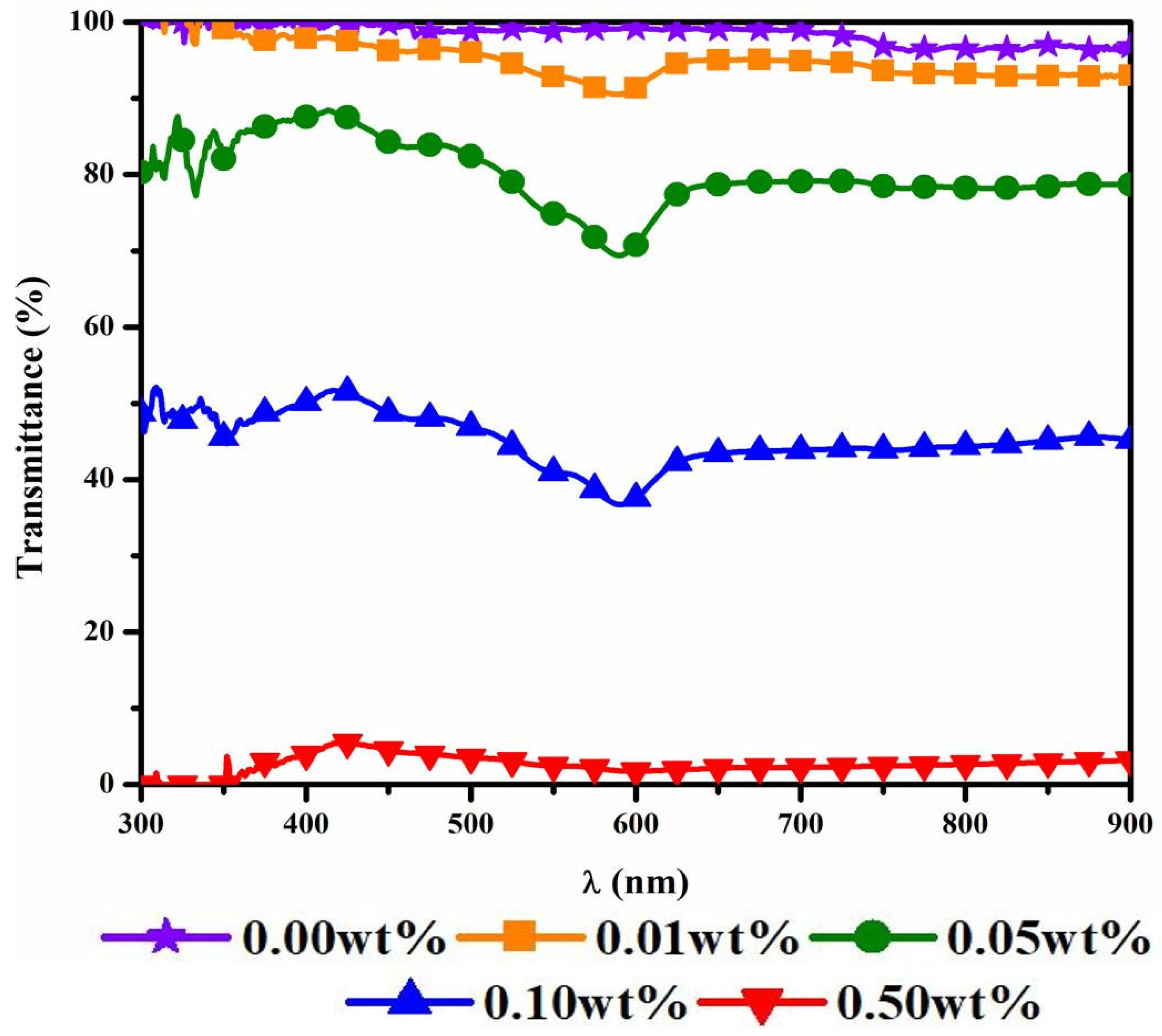
3.1. Optical Transmittance of the Working Fluids
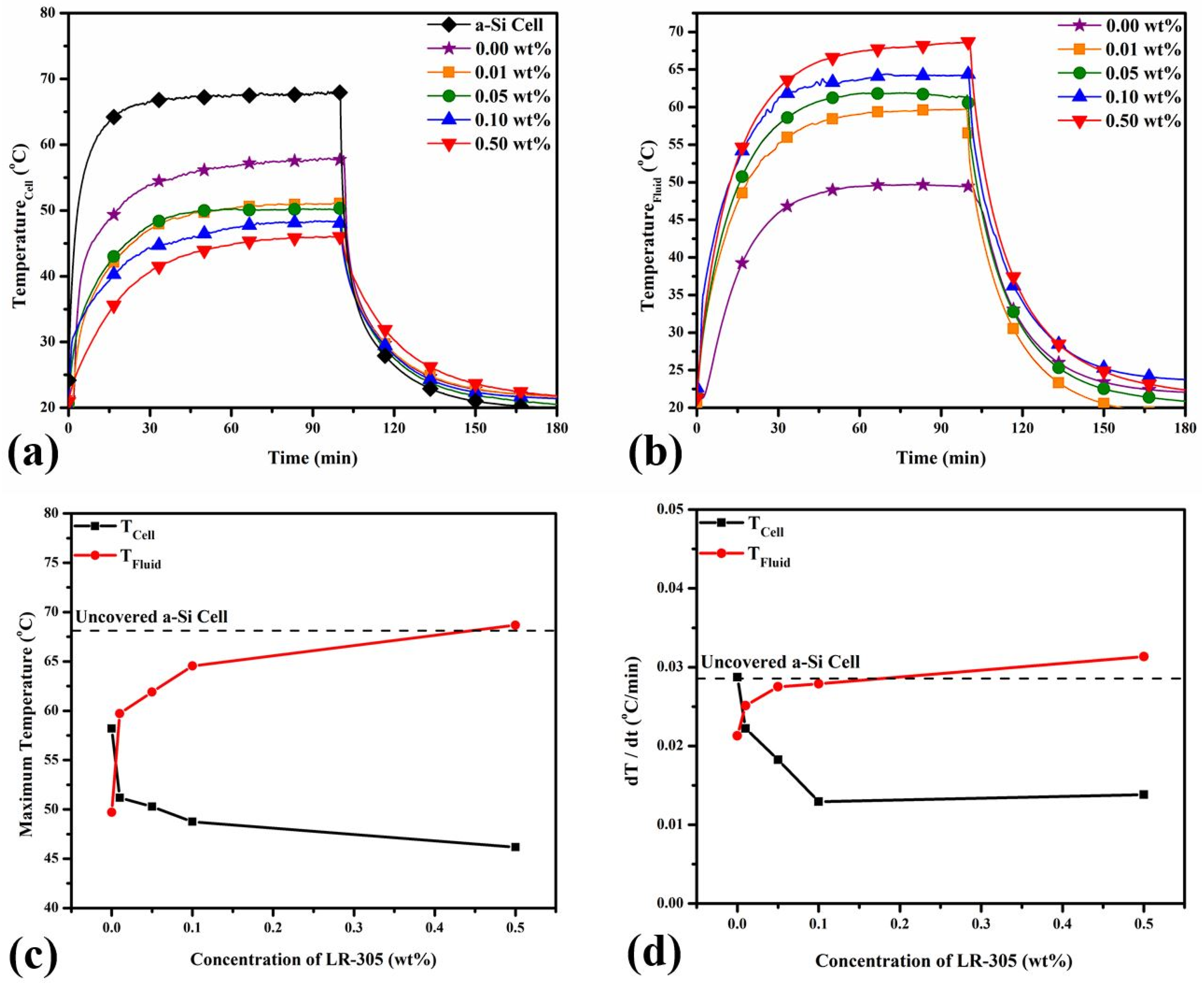
3.2. Thermal Response of the PV Cell & the Working Fluid
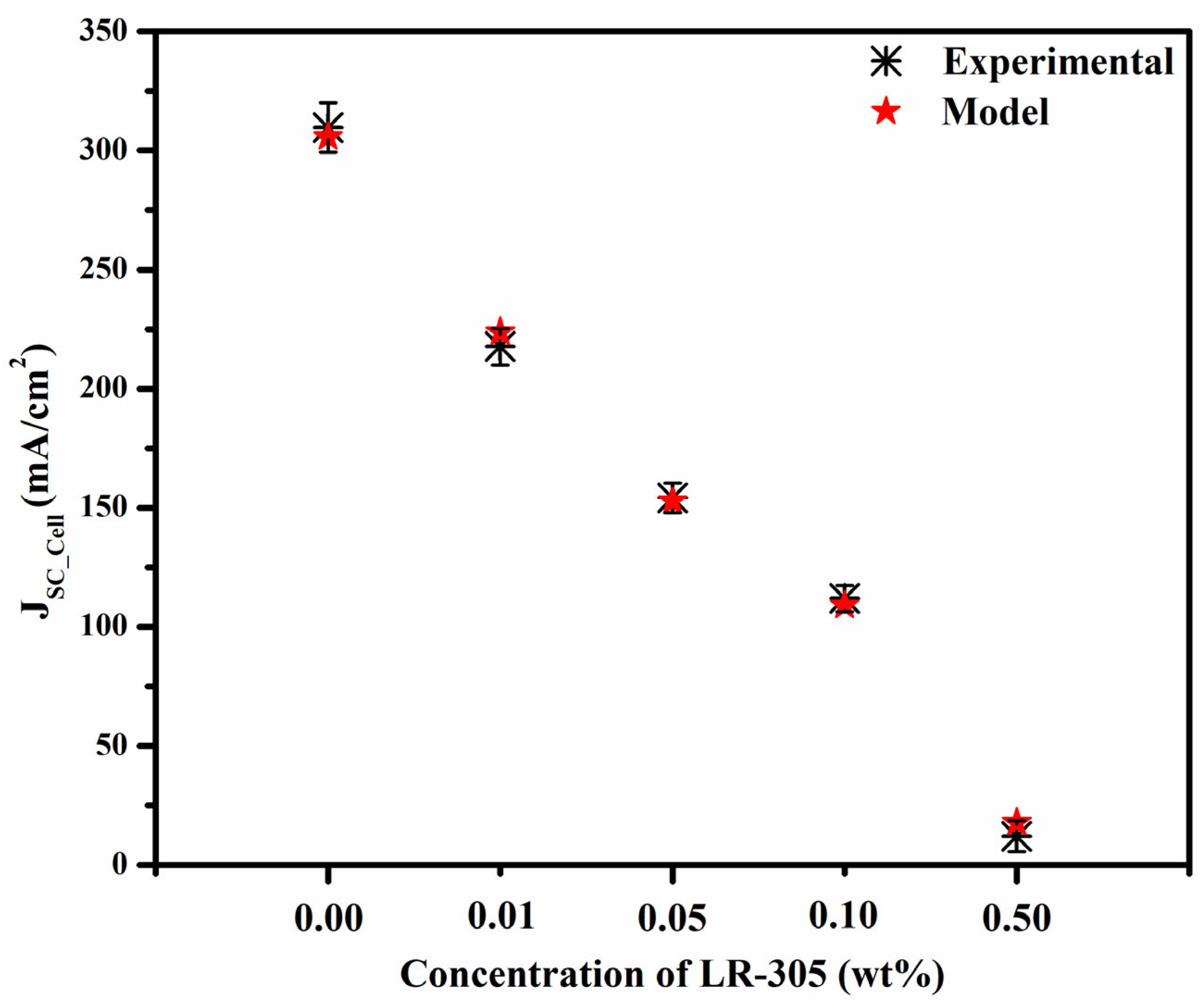
3.3. Electrical Characteristics of the PV Cell
3.4. Monte Carlo Raytracing Model
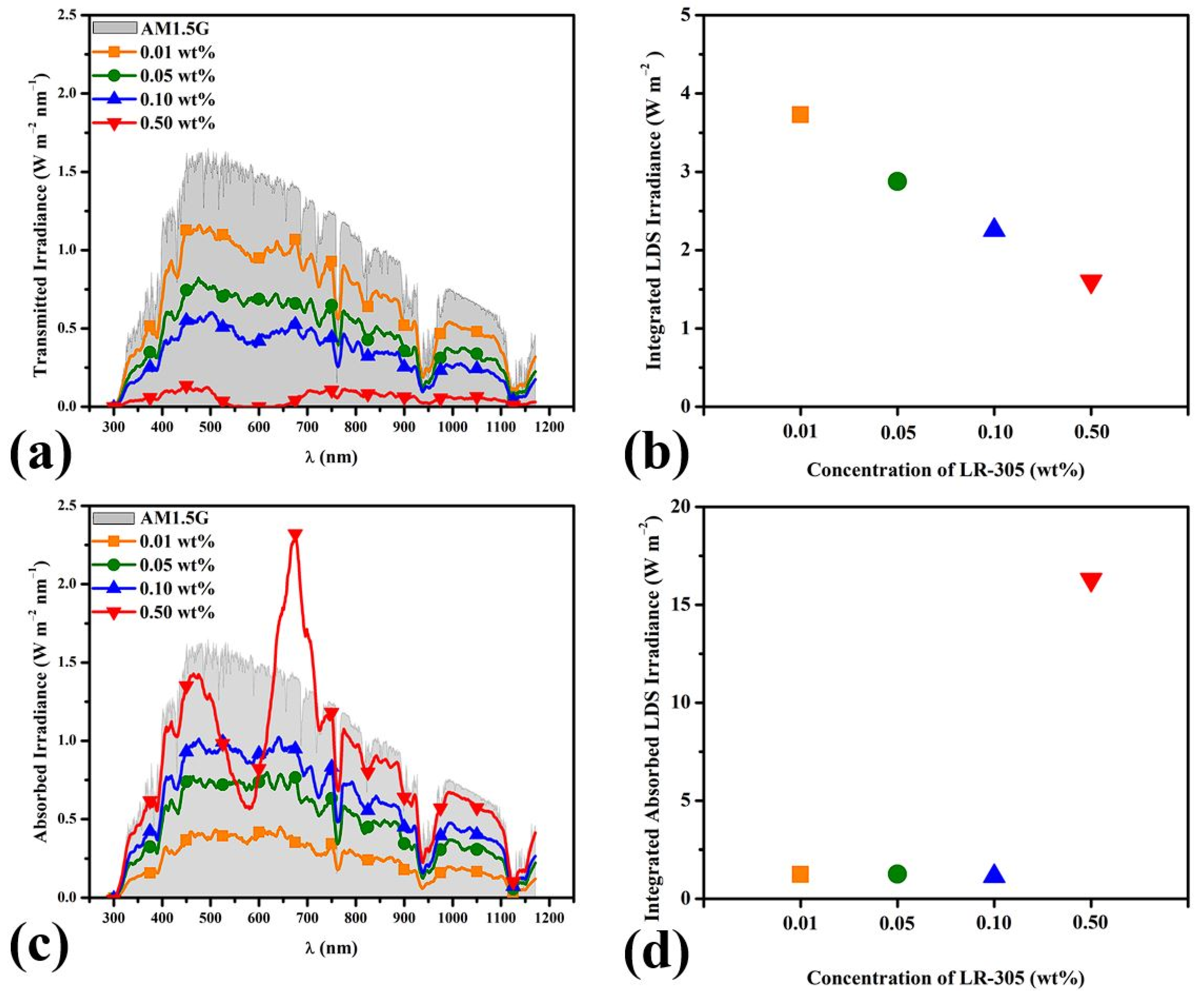
3.4.1. Photons Incident on Solar Cell
3.4.2. Absorbed Photons
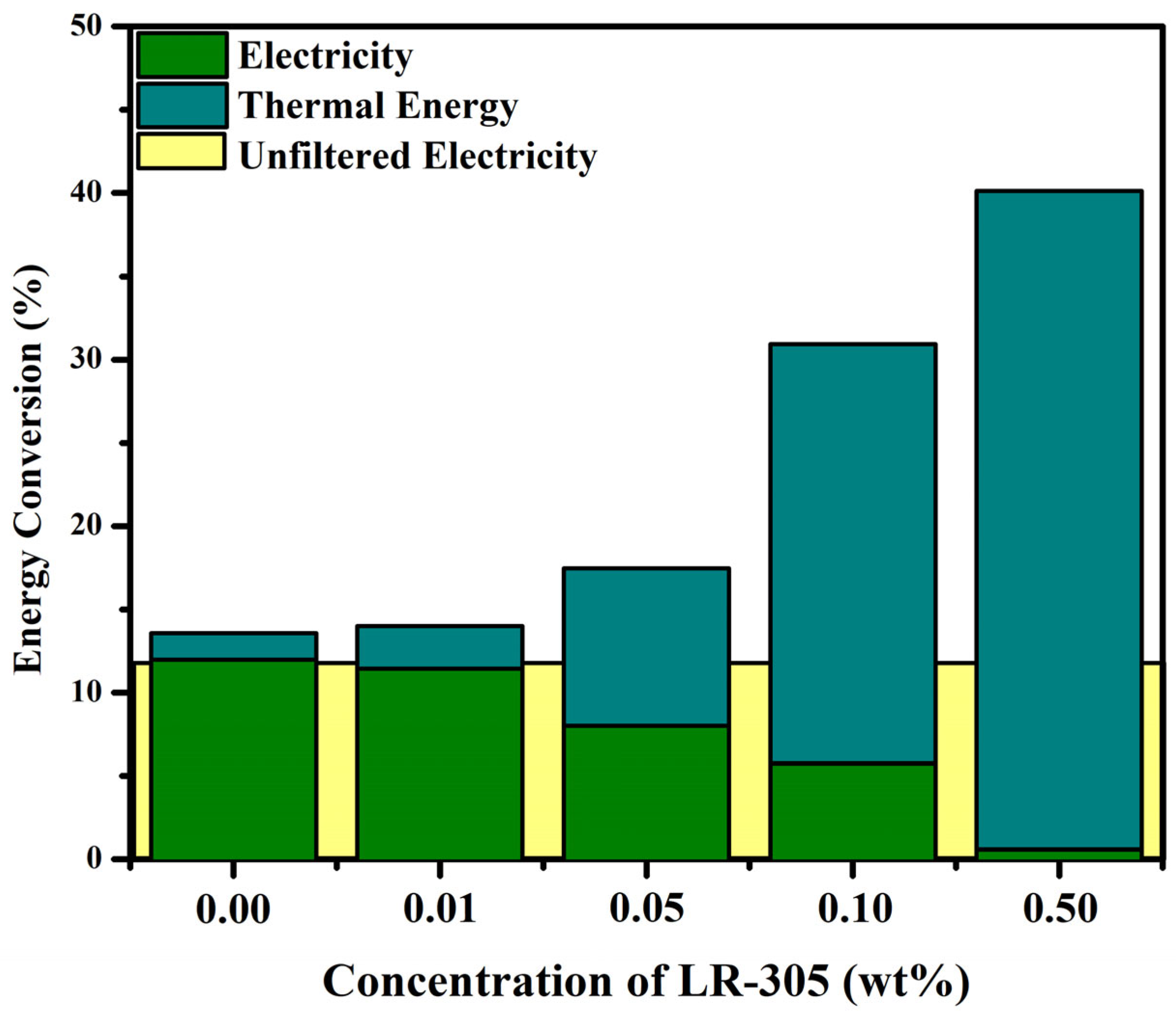
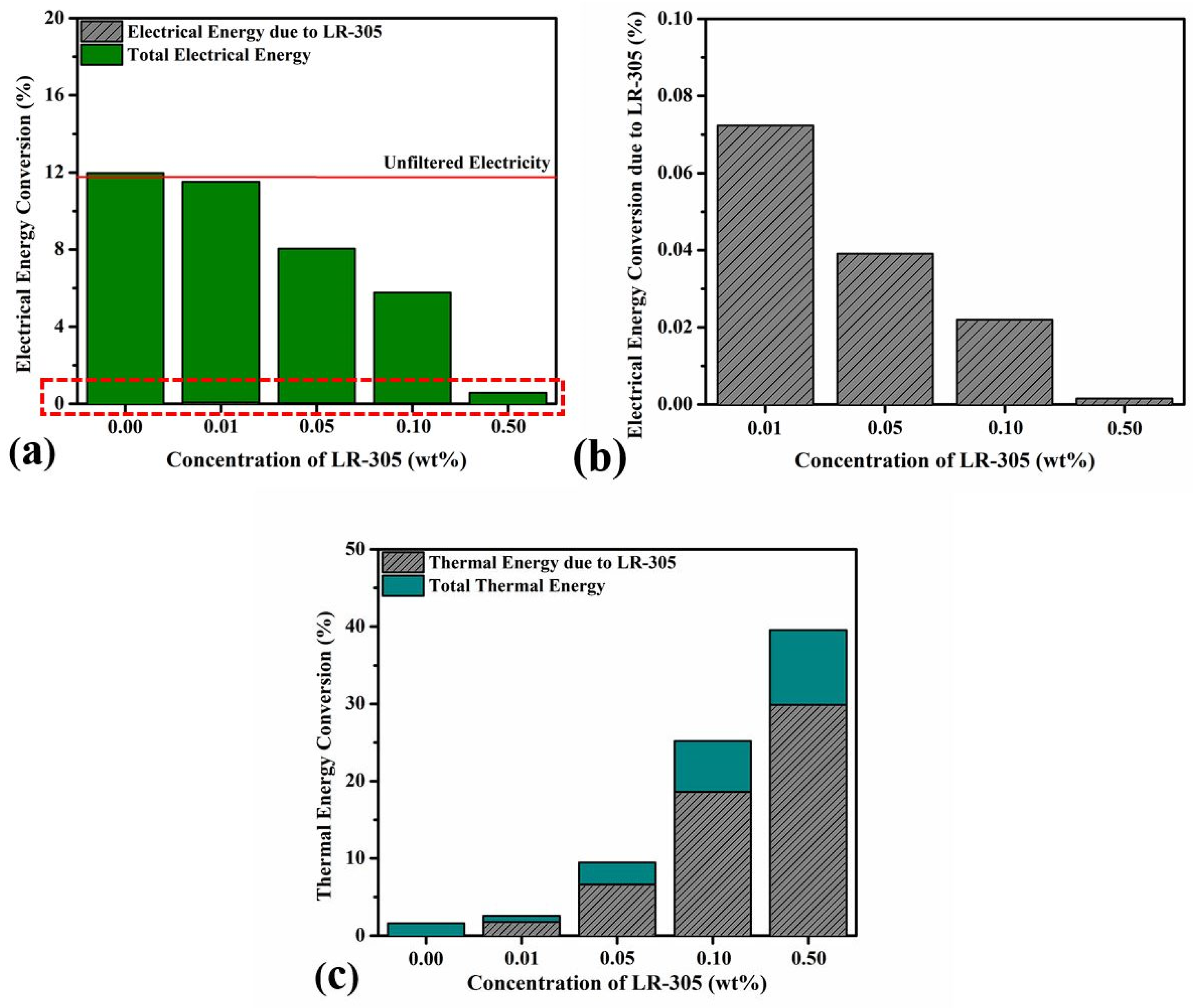
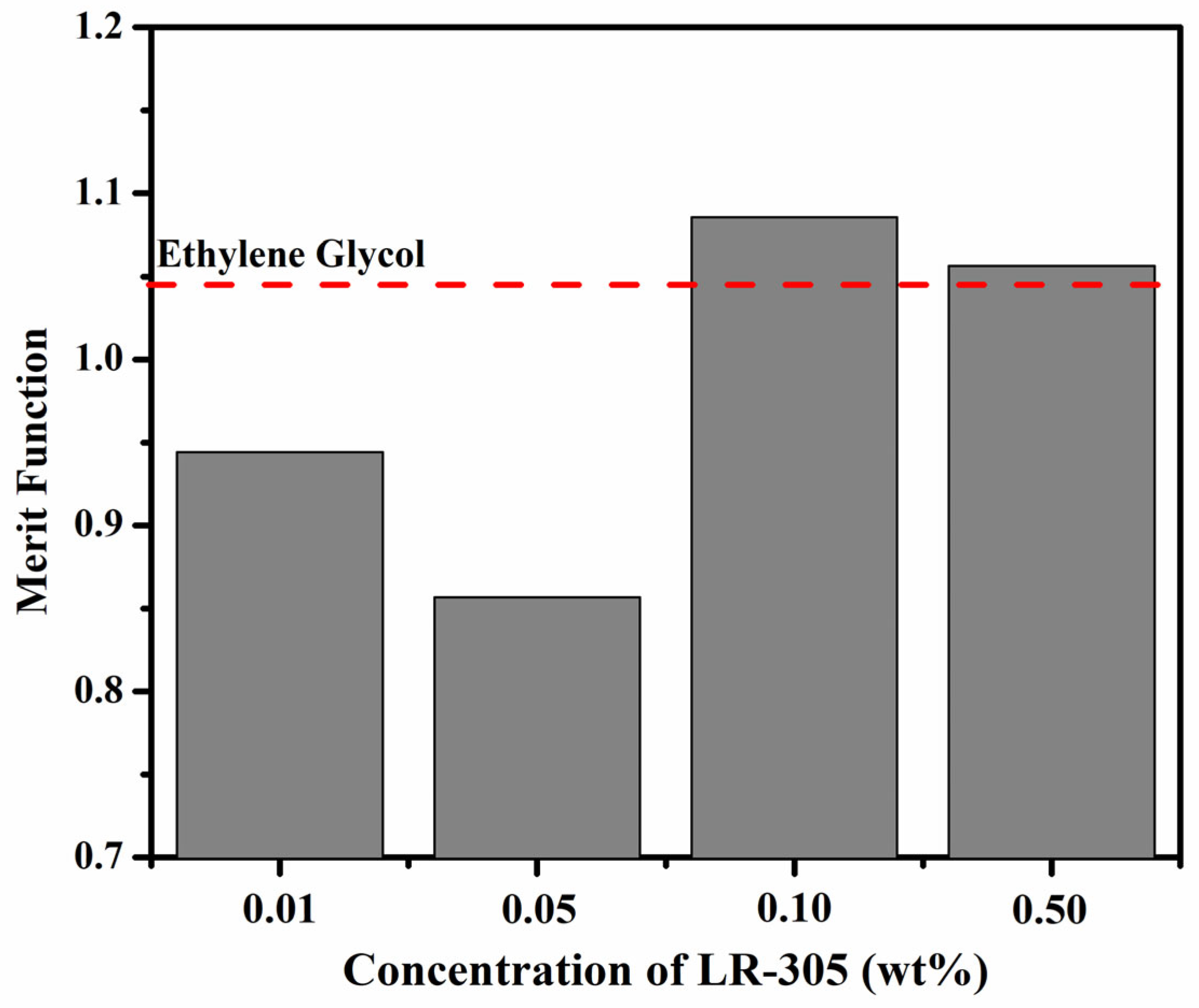
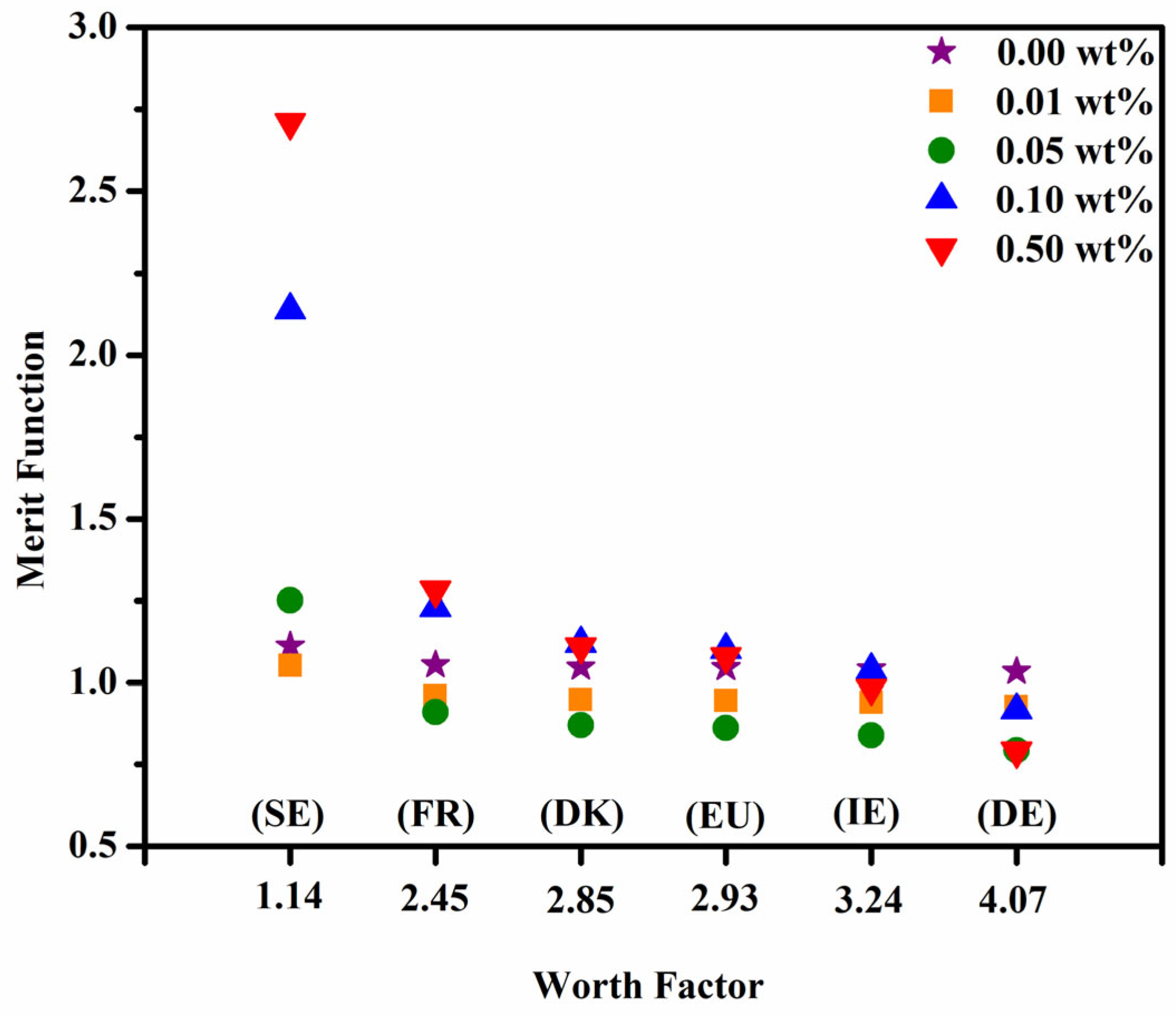
3.5. PVT Energy Conversion Efficiency and Merit Function Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapilan, N.; Nithin, K.C.; Chiranth, K.N. Challenges and Opportunities in Solar Photovoltaic System. Mater. Today Proc. 2022, 62, 3538–3543. [Google Scholar] [CrossRef]
- Shamim, M.M.H.; Silmee, S.M.; Sikder, M.M.; Shamim, M.M.H.; Silmee, S.M.; Sikder, M.M. Optimization and Cost-Benefit Analysis of a Grid-Connected Solar Photovoltaic System. AIMS Energy 2022, 10, 434–457. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Al-Othman, A.; Kafiah, F.; Abdelsalam, E.; Almomani, F.; Alkasrawi, M. Environmental Impacts of Solar Photovoltaic Systems: A Critical Review of Recent Progress and Future Outlook. Sci. Total Environ. 2021, 759, 143528. [Google Scholar] [CrossRef]
- IEA. Solar. Available online: https://www.iea.org/energy-system/renewables/solar-pv (accessed on 16 July 2023).
- Wydra, K.; Vollmer, V.; Busch, C.; Prichta, S.; Wydra, K.; Vollmer, V.; Busch, C.; Prichta, S. Agrivoltaic: Solar Radiation for Clean Energy and Sustainable Agriculture with Positive Impact on Nature. In Solar Radiation—Enabling Technologies, Recent Innovations, and Advancements for Energy Transition; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Radziemska, E. The Effect of Temperature on the Power Drop in Crystalline Silicon Solar Cells. Renew. Energy 2003, 28, 1–12. [Google Scholar] [CrossRef]
- Divitini, G.; Cacovich, S.; Matteocci, F.; Cinà, L.; Di Carlo, A.; Ducati, C. In Situ Observation of Heat-Induced Degradation of Perovskite Solar Cells. Nat. Energy 2016, 1, 15012. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, S.D.; Jain, L.; Al Khaimah, R. Standalone Photovoltaic (PV) Module Outdoor Testing Facility for UAE Climate; CSEM-UAE Innovation Centre LLC: Dubai, United Arab Emirates, 2007. [Google Scholar]
- Haidar, Z.A.; Orfi, J.; Kaneesamkandi, Z. Experimental Investigation of Evaporative Cooling for Enhancing Photovoltaic Panels Efficiency. Results Phys. 2018, 11, 690–697. [Google Scholar] [CrossRef]
- Kherici, Z.; Kahoul, N.; Cheghib, H.; Younes, M.; Chekal Affari, B. Main Degradation Mechanisms of Silicon Solar Cells in Algerian Desert Climates. Sol. Energy 2021, 224, 279–284. [Google Scholar] [CrossRef]
- Sato, D.; Yamada, N. Review of Photovoltaic Module Cooling Methods and Performance Evaluation of the Radiative Cooling Method. Renew. Sustain. Energy Rev. 2019, 104, 151–166. [Google Scholar] [CrossRef]
- Park, N.C.; Oh, W.W.; Kim, D.H. Effect of Temperature and Humidity on the Degradation Rate of Multicrystalline Silicon Photovoltaic Module. Int. J. Photoenergy 2013, 2013, 925280. [Google Scholar] [CrossRef]
- Kaplani, E. Detection of Degradation Effects in Field-Aged c-Si Solar Cells through IR Thermography and Digital Image Processing. Int. J. Photoenergy 2012, 2012, 396792. [Google Scholar] [CrossRef]
- Kokul, S.R.; Bhowmik, S. Recycling of Crystalline Silicon Photovoltaic Solar Panel Waste to Modified Composite Products. Prog. Rubber Plast. Recycl. Technol. 2021, 37, 327–339. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Chen, X.; Ren, L.; Geng, C. A Review of End-of-Life Crystalline Silicon Solar Photovoltaic Panel Recycling Technology. Sol. Energy Mater. Sol. Cells 2022, 248, 111976. [Google Scholar] [CrossRef]
- Tao, M.; Fthenakis, V.; Ebin, B.; Steenari, B.M.; Butler, E.; Sinha, P.; Corkish, R.; Wambach, K.; Simon, E.S. Major Challenges and Opportunities in Silicon Solar Module Recycling. Prog. Photovolt. Res. Appl. 2020, 28, 1077–1088. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Yu, J.; Xu, C.; Wu, Y.; Pan, D.; Senthil, R.A. An Overview of the Comprehensive Utilization of Silicon-Based Solid Waste Related to PV Industry. Resour. Conserv. Recycl. 2021, 169, 105450. [Google Scholar] [CrossRef]
- Kwak, J.-I.; Nam, S.H.; Kim, L.; An, Y.J. Potential Environmental Risk of Solar Cells: Current Knowledge and Future Challenges. J. Hazard. Mater. 2020, 392, 122297. [Google Scholar] [CrossRef]
- Venkatachary, S.K.; Samikannu, R.; Murugesan, S.; Dasari, N.R.; Subramaniyam, R.U. Economics and Impact of Recycling Solar Waste Materials on the Environment and Health Care. Environ. Technol. Innov. 2020, 20, 10113. [Google Scholar] [CrossRef]
- Lunardi, M.M.; Alvarez-Gaitan, J.P.; Bilbao, J.I.; Corkish, R. Comparative Life Cycle Assessment of End-of-Life Silicon Solar Photovoltaic Modules. Appl. Sci. 2018, 8, 1396. [Google Scholar] [CrossRef]
- Cuce, E.; Bali, T.; Sekucoglu, S.A. Effects of Passive Cooling on Performance of Silicon Photovoltaic Cells. Int. J. Low-Carbon Technol. 2011, 6, 299–308. [Google Scholar] [CrossRef]
- Perrakis, G.; Tasolamprou, A.C.; Kenanakis, G.; Economou, E.N.; Tzortzakis, S.; Kafesaki, M. Submicron Organic-Inorganic Hybrid Radiative Cooling Coatings for Stable, Ultrathin, and Lightweight Solar Cells. ACS Photonics 2022, 9, 1327–1337. [Google Scholar] [CrossRef]
- Yang, X.; Yan, K.; Kang, Z.; Tan, X.; Qi, G.; Tu, Y. Antireflection and Radiative Cooling Difunctional Coating Design for Silicon Solar Cells. Opt. Express 2023, 31, 22296–22307. [Google Scholar] [CrossRef]
- Hirose, F.; Kubota, S.; Ahmmad, B. Optical Design of Multilayer Antireflection Coatings for Indoor Solar Cell Applications. Appl. Opt. 2023, 62, 2117–2123. [Google Scholar] [CrossRef]
- Walshe, J.; Girtan, M.; McCormack, S.; Doran, J.; Amarandei, G. Combined Experimental and Modeling Analysis for the Development of Optical Materials Suitable to Enhance the Implementation of Plasmonic-Enhanced Luminescent Down-Shifting Solutions on Existing Silicon-Based Photovoltaic Devices. ACS Appl. Electron. Mater. 2021, 3, 2512–2525. [Google Scholar] [CrossRef]
- Walshe, J.; Girtan, M.; McCormack, S.; Doran, J.; Amarandei, G. Exploring the Development of Nanocomposite Encapsulation Solutions for Enhancing the Efficiency of PV Systems Using Optical Modelling. Opt. Mater. 2021, 111, 110654. [Google Scholar] [CrossRef]
- Cazzaniga, R.; Rosa-Clot, M.; Rosa-Clot, P.; Tina, G.M. Floating Tracking Cooling Concentrating (FTCC) Systems. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 514–519. [Google Scholar] [CrossRef]
- Calise, F.; Dentice d’Accadia, M.; Palombo, A.; Vanoli, L. Dynamic Simulation of a Novel High-Temperature Solar Trigeneration System Based on Concentrating Photovoltaic/Thermal Collectors. Energy 2013, 61, 72–86. [Google Scholar] [CrossRef]
- Fudholi, A.; Sopian, K.; Yazdi, M.H.; Ruslan, M.H.; Ibrahim, A.; Kazem, H.A. Performance Analysis of Photovoltaic Thermal (PVT) Water Collectors. Energy Convers. Manag. 2014, 78, 641–651. [Google Scholar] [CrossRef]
- Joshi, S.S.; Dhoble, A.S. Photovoltaic -Thermal Systems (PVT): Technology Review and Future Trends. Renew. Sustain. Energy Rev. 2018, 92, 848–882. [Google Scholar] [CrossRef]
- Zondag, H.A.; de Vries, D.W.; van Helden, W.G.J.; van Zolingen, R.J.C.; van Steenhoven, A.A. The Yield of Different Combined PV-Thermal Collector Designs. Sol. Energy 2003, 74, 253–269. [Google Scholar] [CrossRef]
- Walshe, J.; Carron, P.M.; McCormack, S.; Doran, J.; Amarandei, G. Organic Luminescent Down-Shifting Liquid Beam Splitters for Hybrid Photovoltaic-Thermal (PVT) Applications. Sol. Energy Mater. Sol. Cells 2021, 219, 110818. [Google Scholar] [CrossRef]
- Sharma, M.K.; Bhattacharya, J. Dependence of Spectral Factor on Angle of Incidence for Monocrystalline Silicon Based Photovoltaic Solar Panel. Renew. Energy 2022, 184, 820–829. [Google Scholar] [CrossRef]
- Fernández-Solas, Á.; Micheli, L.; Almonacid, F.; Fernández, E.F. Optical Degradation Impact on the Spectral Performance of Photovoltaic Technology. Renew. Sustain. Energy Rev. 2021, 141, 110782. [Google Scholar] [CrossRef]
- Singh, A.; Umakanth, V.; Tyagi, N.; Baghel, A.K.; Kumar, S. Comparative Study of Commercial Crystalline Solar Cells. Results Opt. 2023, 11, 100379. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Zeng, J.; Chen, J.; Chen, H. Enhance Short-Wavelength Response of CIGS Solar Cell by CdSe Quantum Disks as Luminescent down-Shifting Material. Sol. Energy 2019, 193, 303–308. [Google Scholar] [CrossRef]
- Klampaftis, E.; Ross, D.; McIntosh, K.R.; Richards, B.S. Enhancing the Performance of Solar Cells via Luminescent Down-Shifting of the Incident Spectrum: A Review. Sol. Energy Mater. Sol. Cells 2009, 93, 1182–1194. [Google Scholar] [CrossRef]
- Sanguineti, A.; Sassi, M.; Turrisi, R.; Ruffo, R.; Vaccaro, G.; Meinardi, F.; Beverina, L. High Stokes Shift Perylene Dyes for Luminescent Solar Concentrators. Chem. Commun. 2013, 49, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Debije, M.G.; Verbunt, P.P.C.; Nadkarni, P.J.; Velate, S.; Bhaumik, K.; Nedumbamana, S.; Rowan, B.C.; Richards, B.S.; Hoeks, T.L. Promising Fluorescent Dye for Solar Energy Conversion Based on a Perylene Perinone. Appl. Opt. 2011, 50, 163–169. [Google Scholar] [CrossRef]
- Li, Y.; Olsen, J.; Nunez-Ortega, K.; Dong, W.J. A Structurally Modified Perylene Dye for Efficient Luminescent Solar Concentrators. Sol. Energy 2016, 136, 668–674. [Google Scholar] [CrossRef]
- Jo, K.; Hong, S.; Kim, H.J. Optical Properties of EVA Films Including V570 for Transparent Luminescent Solar Concentrator. Appl. Sci. Converg. Technol. 2020, 29, 14–18. [Google Scholar] [CrossRef]
- Ahmed, H.; Doran, J.; McCormack, S.J. External Quantum Efficiency Measurements and Outdoor Characterisation for PV Luminescent Downshifting Devices. In Renewable Energy and Sustainable Buildings. Innovative Renewable Energy; Springer International Publishing: Cham, Switzerland, 2020; pp. 727–733. [Google Scholar] [CrossRef]
- Mojiri, A.; Taylor, R.; Thomsen, E.; Rosengarten, G. Spectral Beam Splitting for Efficient Conversion of Solar Energy—A Review. Renew. Sustain. Energy Rev. 2013, 28, 654–663. [Google Scholar] [CrossRef]
- Ni, J.; Li, J.; An, W.; Zhu, T. Performance Analysis of Nanofluid-Based Spectral Splitting PV/T System in Combined Heating and Power Application. Appl. Therm. Eng. 2018, 129, 1160–1170. [Google Scholar] [CrossRef]
- Vivar, M.; Everett, V. A Review of Optical and Thermal Transfer Fluids Used for Optical Adaptation or Beam-Splitting in Concentrating Solar Systems. Prog. Photovolt. Res. Appl. 2014, 22, 612–633. [Google Scholar] [CrossRef]
- Tiwari, G.N.; Gaur, A. Photovoltaic Thermal (PVT) Systems and Its Applications. In Proceedings of the 2014 2nd International Conference on Green Energy and Technology (ICGET 2014), Dhaka, Bangladesh, 5–6 September 2014; pp. 132–138. [Google Scholar] [CrossRef]
- Proell, M.; Karrer, H.; Brabec, C.J.; Hauer, A. The Influence of CPC Reflectors on the Electrical Incidence Angle Modifier of C-Si Cells in a PVT Hybrid Collector. Sol. Energy 2016, 126, 220–230. [Google Scholar] [CrossRef]
- Joshi, S.S.; Dhoble, A.S.; Jiwanapurkar, P.R. Investigations of Different Liquid Based Spectrum Beam Splitters for Combined Solar Photovoltaic Thermal Systems. J. Sol. Energy Eng. 2016, 138, 021003. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Yuan, X.; Lin, J.; Chen, Z. Novel Design and Analysis of a Solar PVT System Using LFR Concentrator and Nano-Fluids Optical Filter. Case Stud. Therm. Eng. 2021, 27, 101328. [Google Scholar] [CrossRef]
- Chemisana, D.; Fernandez, E.F.; Riverola, A.; Moreno, A. Fluid-Based Spectrally Selective Filters for Direct Immersed PVT Solar Systems in Building Applications. Renew. Energy 2018, 123, 263–272. [Google Scholar] [CrossRef]
- Coldrick, K.; Walshe, J.; Doran, J.; Amarandei, G. Evaluation of the Photon Contributions to the Solar Energy Conversion for Organic Luminescent Down-Shifting Liquid Beam Splitters in Hybrid Photovoltaic-Thermal (PVT) Applications Using Raytracing Monte Carlo Simulations. Sol. Energy Mater. Sol. Cells 2023, 254, 112201. [Google Scholar] [CrossRef]
- Kang, H. Crystalline Silicon vs. Amorphous Silicon: The Significance of Structural Differences in Photovoltaic Applications. IOP Conf. Ser. Earth Environ. Sci. 2021, 726, 012001. [Google Scholar] [CrossRef]
- GitHub. Danieljfarrell/Pvtrace: Optical Ray Tracing for Luminescent Materials and Spectral Converter Photovoltaic Devices. Available online: https://github.com/danieljfarrell/pvtrace (accessed on 16 July 2023).
- Slooff, L.H.; Bakker, N.J.; Sommeling, P.M.; Büchtemann, A.; Wedel, A.; Van Sark, W.G.J.H.M. Long-Term Optical Stability of Fluorescent Solar Concentrator Plates. Phys. Status Solidi A 2014, 211, 1150–1154. [Google Scholar] [CrossRef]
- Grandes, J.; Illarramendi, M.A.; Arrospide, E.; Bikandi, I.; Aramburu, I.; Guarrotxena, N.; García, O.; Zubia, J. Temperature Effects on the Emission of Polymer Optical Fibers Doped with Lumogen Dyes. Opt. Fiber Technol. 2022, 72, 102980. [Google Scholar] [CrossRef]
- De La Gree, G.D.; Papadopoulos, A.; Debije, M.; Cox, M.; Krumer, Z.; Reinders, A.; Rosemann, A. A New Design for Luminescent Solar Concentrating PV Roof Tiles. In Proceedings of the 2015 IEEE 42nd Photovoltaic Specialist Conference (PVSC 2015), New Orleans, LA, USA, 14–19 June 2015. [Google Scholar] [CrossRef]
- Hashemian, M.; Jafarmadar, S.; Khalilarya, S.; Faraji, M. Energy Harvesting Feasibility from Photovoltaic/Thermal (PV/T) Hybrid System with Ag/Cr2O3-Glycerol Nanofluid Optical Filter. Renew. Energy 2022, 198, 426–439. [Google Scholar] [CrossRef]
- Han, X.; Sun, Y.; Huang, J.; Zheng, J. Design and Analysis of a CPV/T Solar Receiver with Volumetric Absorption Combined Spectral Splitter. Int. J. Energy Res. 2020, 44, 4837–4850. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Wang, Q.; Alelyani, S.M.; Qu, J. Investigation of CoSO4-Based Ag Nanofluids as Spectral Beam Splitters for Hybrid PV/T Applications. Sol. Energy 2019, 177, 387–394. [Google Scholar] [CrossRef]
- Buffa, M.; Carturan, S.; Debije, M.G.; Quaranta, A.; Maggioni, G. Dye-Doped Polysiloxane Rubbers for Luminescent Solar Concentrator Systems. Sol. Energy Mater. Sol. Cells 2012, 103, 114–118. [Google Scholar] [CrossRef]
- Carbone, I.A.; Frawley, K.R.; McCann, M.K. Flexible, Front-Facing Luminescent Solar Concentrators Fabricated from Lumogen f Red 305 and Polydimethylsiloxane. Int. J. Photoenergy 2019, 2019, 8680931. [Google Scholar] [CrossRef]
- Krumer, Z.; van Sark, W.G.J.H.M.; Schropp, R.E.I.; de Mello Donegá, C. Compensation of Self-Absorption Losses in Luminescent Solar Concentrators by Increasing Luminophore Concentration. Sol. Energy Mater. Sol. Cells 2017, 167, 133–139. [Google Scholar] [CrossRef]
- Xiao, Y.; Bao, Y.; Yu, L.; Zheng, X.; Qin, G.; Chen, M.; He, M. Ultra-Stable Carbon Quantum Dot Nanofluids as Excellent Spectral Beam Splitters in PV/T Applications. Energy 2023, 273, 127159. [Google Scholar] [CrossRef]
- Natural Gas Price Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Natural_gas_price_statistics (accessed on 16 July 2023).
- Electricity Price Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Electricity_price_statistics (accessed on 16 July 2023).
- Walshe, J.; Doran, J.; Amarandei, G. Evaluation of the Potential of Nanofluids Containing Different Ag Nanoparticle Size Distributions for Enhanced Solar Energy Conversion in Hybrid Photovoltaic-Thermal (PVT) Applications. Nano Express 2022, 3, 015001. [Google Scholar] [CrossRef]
- Han, X.; Zhao, X.; Huang, J.; Qu, J. Optical Properties Optimization of Plasmonic Nanofluid to Enhance the Performance of Spectral Splitting Photovoltaic/Thermal Systems. Renew. Energy 2022, 188, 573–587. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coldrick, K.; Walshe, J.; McCormack, S.J.; Doran, J.; Amarandei, G. Experimental and Theoretical Evaluation of a Commercial Luminescent Dye for PVT Systems. Energies 2023, 16, 6294. https://doi.org/10.3390/en16176294
Coldrick K, Walshe J, McCormack SJ, Doran J, Amarandei G. Experimental and Theoretical Evaluation of a Commercial Luminescent Dye for PVT Systems. Energies. 2023; 16(17):6294. https://doi.org/10.3390/en16176294
Chicago/Turabian StyleColdrick, Kenneth, James Walshe, Sarah J. McCormack, John Doran, and George Amarandei. 2023. "Experimental and Theoretical Evaluation of a Commercial Luminescent Dye for PVT Systems" Energies 16, no. 17: 6294. https://doi.org/10.3390/en16176294
APA StyleColdrick, K., Walshe, J., McCormack, S. J., Doran, J., & Amarandei, G. (2023). Experimental and Theoretical Evaluation of a Commercial Luminescent Dye for PVT Systems. Energies, 16(17), 6294. https://doi.org/10.3390/en16176294






