An Overview of Developments In Silica Gel Matrix Composite Sorbents for Adsorption Chillers with Desalination Function
Abstract
1. Introduction
2. Adsorption Chillers—An Overview
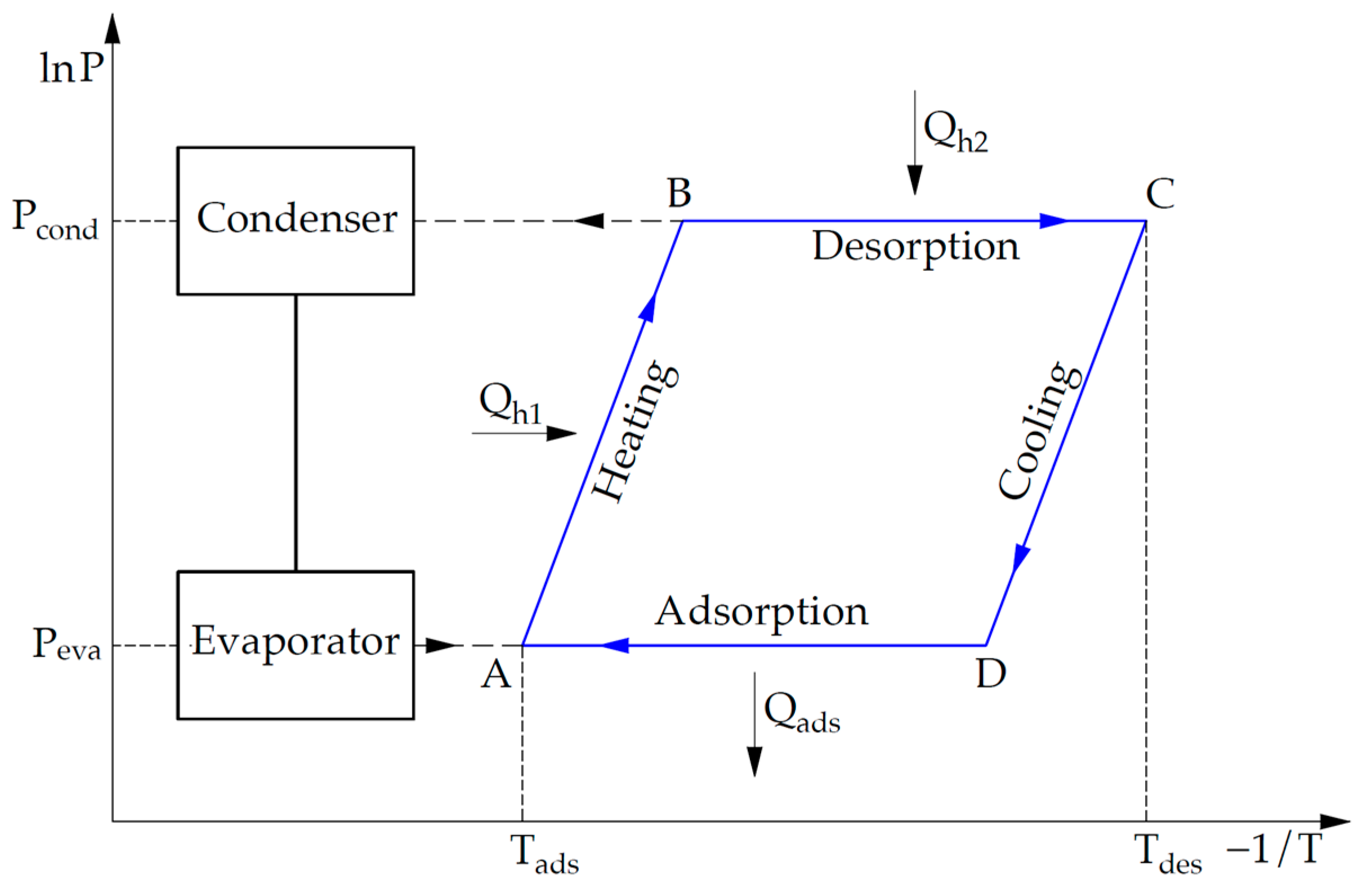
2.1. Theoretical Background
2.2. Principle of Operation of an Adsorption Cooling System
2.3. Improvement of Performance Parameters of Adsorption Chillers
- Improvement of adsorbent properties that are beneficial for their use in adsorption chillers;
- Modification in the design of the heat exchanger in the adsorbent bed;
- Optimisation of the operating parameters of the adsorption unit;
- Modification in the cooling system, e.g., by using two or more adsorbent beds, using multistage systems, using either one or more refrigerant evaporation stages and using a redesigned cooling cycle.
2.3.1. Optimisation of Operating Parameters of an Adsorption Chiller
- A lower condenser cooling water temperature with constant heating and chilled-water temperatures results in higher SCP and COP values [45].
- A higher chilled-water temperature at the evaporator inlet has a positive effect on the cooling capacity value and COP of the adsorption chiller under constant heating and chilled-water temperatures [46].
- A higher chilled-water flow rate results in an increase in COP and cooling capacity [47].
- A higher heating-water flow rate results in an increase in cooling capacity and a decrease in COP [47].
2.3.2. Modification in the Design of Heat Exchanger in the Bed
2.3.3. Multibed Adsorption Chillers
2.3.4. Multistage Chillers
2.3.5. Process Modifications to Adsorption Chiller Operation
2.3.6. Selection of the Working Pair
2.3.7. Improving the Properties of Adsorbent Materials
3. Adsorbents, Adsorbates and Working Pairs—An Overview
3.1. Adsorbents
- Good affinity of the adsorbent to the adsorbate;
- Low regeneration temperature (evaporation of the adsorbate from the surface of the adsorbent);
- Adsorption capability of the adsorbate at low temperatures and low relative pressures;
- Large specific surface area;
- High porosity;
- High thermal conductivity coefficient;
- Ability to maintain stability of properties over time;
- Ability to adsorb a maximum amount of adsorbate per unit mass of sorbent;
- Nontoxicity;
- Nonflammability.
3.1.1. Silica Gel
3.1.2. Zeolites
3.1.3. Activated Carbon
3.1.4. Metal–Organic Frameworks
3.2. Adsorbent–Adsorbate Working Pairs
- High latent heat per unit volume;
- Low evaporation temperature;
- Small particle size;
- Low viscosity;
- Low specific heat;
- High thermal conductivity;
- Small volume in liquid state;
- Chemical and thermal stability;
- No negative impact on the environment;
- Nontoxicity and nonflammability.
4. Sorption Composite Materials with Silica Gel
4.1. Methods of Synthesis
4.2. Mechanism of Adsorption
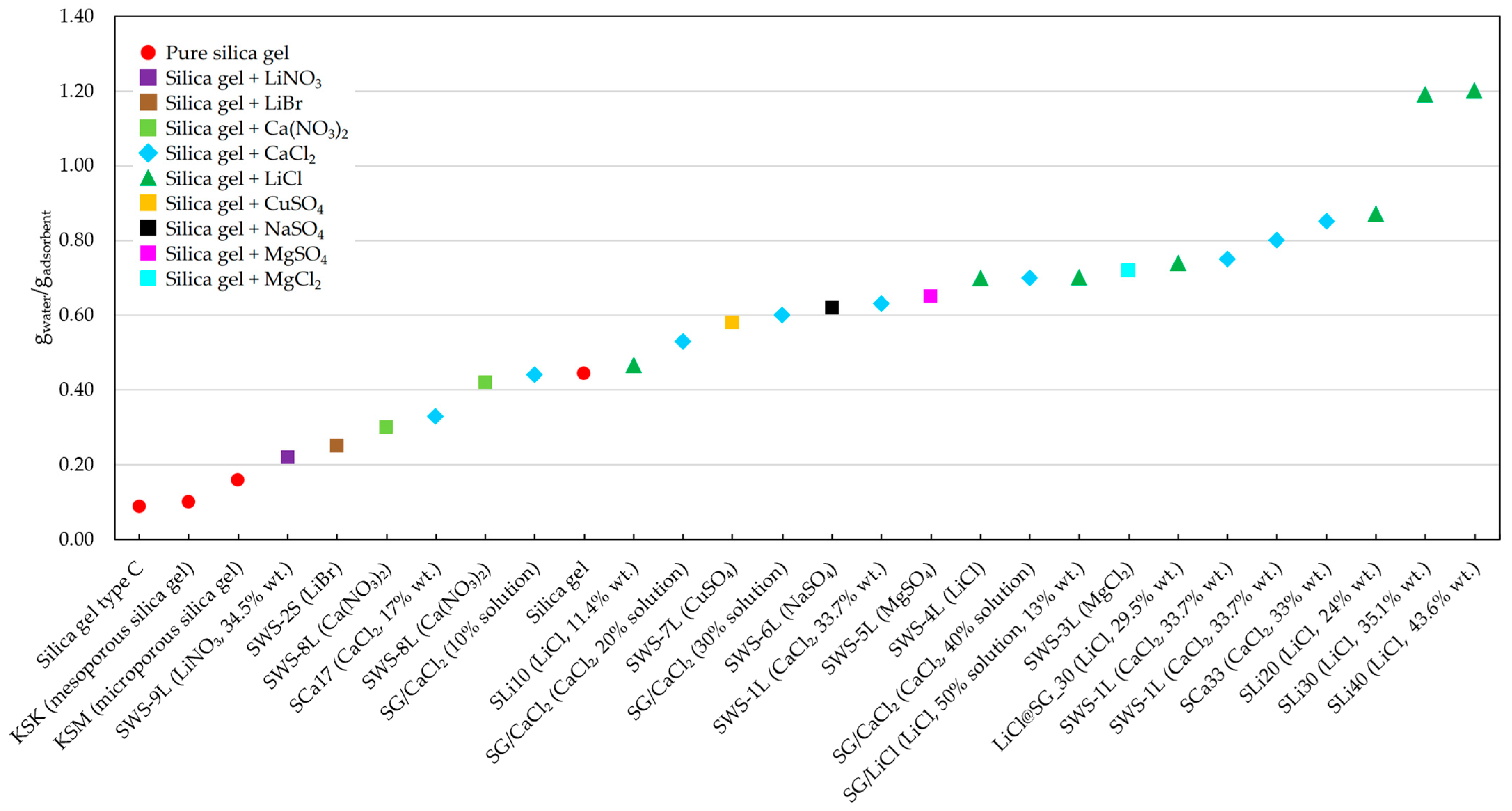
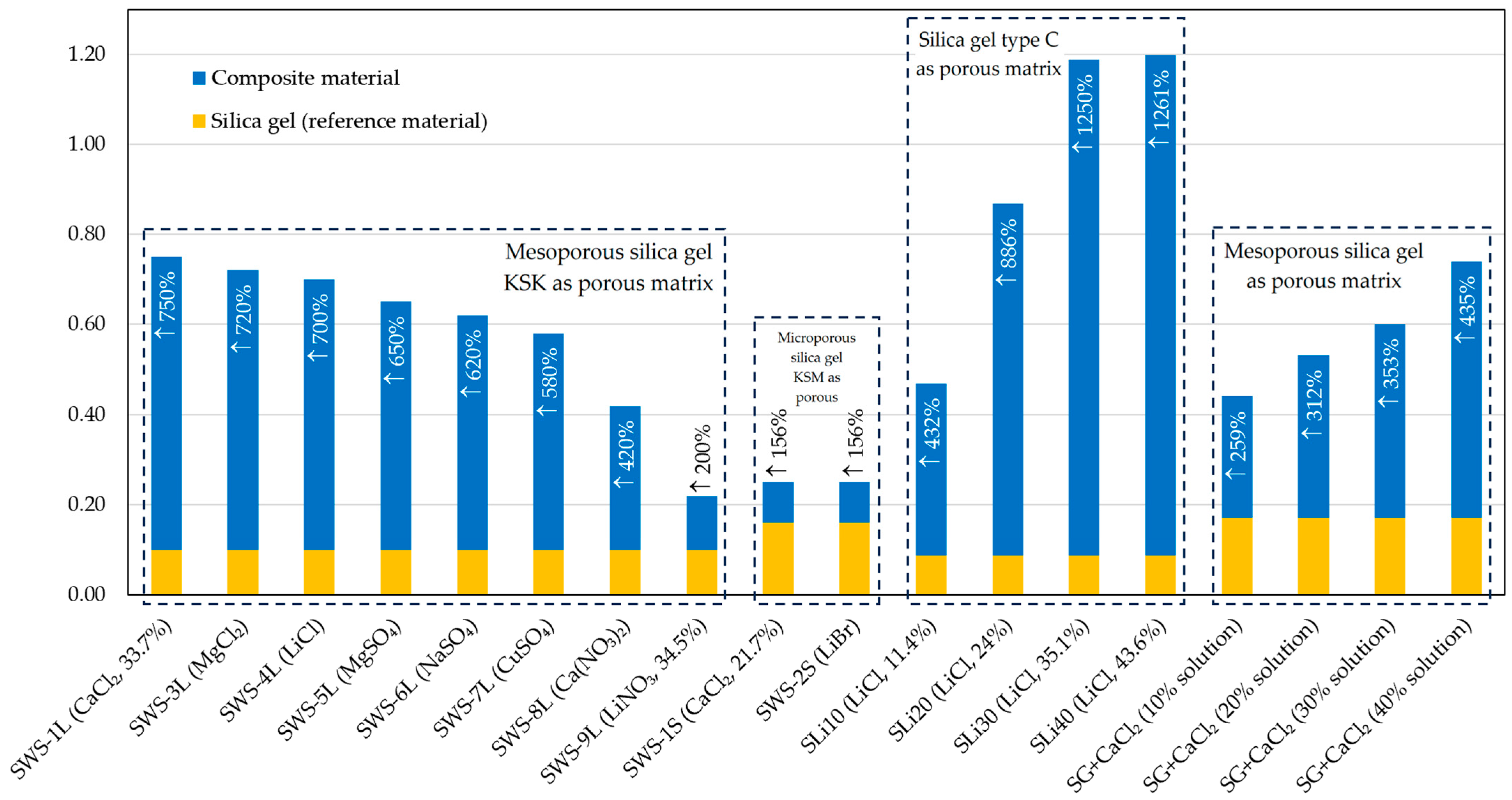
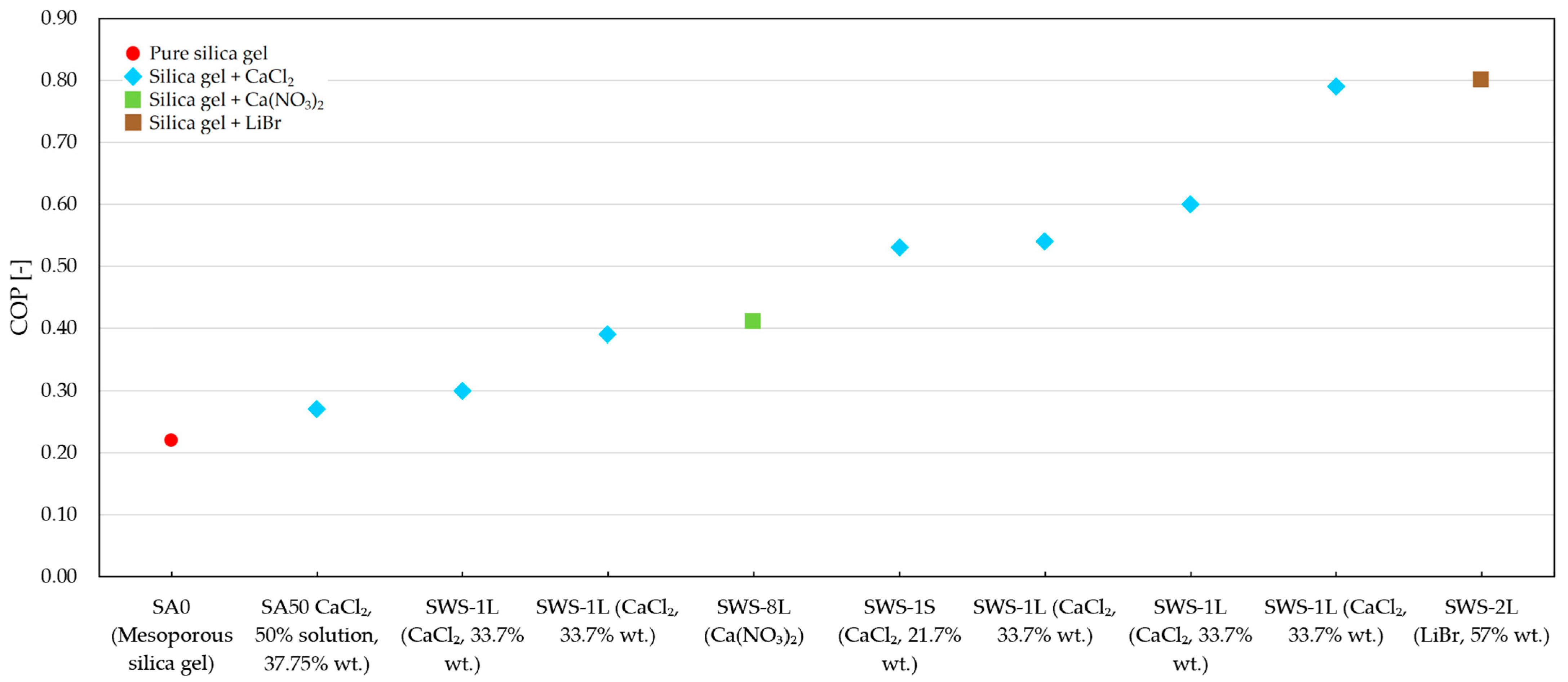
4.3. Overview of Tests and Materials Tested
4.4. The Authors’ Own Research
| Material | Porous Matrix | Addition | Percentage of Salt (%) | BET Specific Surface Area (m2/g) | BJH Pore Volume (cm3/g) | Water Uptake (g/g) | Synthesis Procedure | Desorption Temperature (°C) | COP in Adsorption Chiller | SCP in Adsorption Chiller (W/kg) | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWS-1L | Silica gel KSK | Inorganic salt CaCl2 | 33.7 | - | - | 0.8 | Dry impregnation by saturation of mesoporous silica gel KSK with CaCl2 solution and drying at 150 °C. | 80–100 | max. 0.54 | - | [130] |
| KSK * | Mesoporous silica gel | - | - | - | 1 | 0.1 | - | - | approx. 0.5 (model, for a desorption temp. of 95 °C) | - | [131,133] |
| KSM * | Microporous silica gel | - | - | - | 0.35 | approx. 0.16 | - | - | - | - | |
| SWS-1L | Silica gel KSK | Inorganic salt CaCl2 | 33.7 | - | - | 0.75 | Silica gel granules were filled with a solution of appropriate inorganic salts and dried to constant weight at 200 °C. | - | 0.79 (model, for a desorption temp. of 95 °C) | - | |
| SWS-1S | Silica gel KSM | Inorganic salt CaCl2 | 21.7 | - | - | approx. 0.25 | Temperatures of 70–130 °C were tested | 0.53 (model, for a desorption temp. of 90–100 °C) | - | ||
| SWS-2L | Silica gel KSK | Inorganic salt LiBr | 32 | - | - | - | - | - | |||
| SWS-2L | Silica gel KSK | Inorganic salt LiBr | 57 | - | - | - | 0.8 (at a desorption temp. of 95 °C) | - | |||
| SWS-1L | Silica gel KSK | Inorganic salt CaCl2 | 33 | - | - | - | Saturation of the pores of silica gel KSK with 40% CaCl2 solution and then drying to constant weight at 200 °C. | 80–100 | 0.6 (at a heat-source temperature of 90–95 °C) | 20 | [129,133] |
| SWS-1L | Silica gel KSK | Inorganic salt CaCl2 with added binder (25 wt%) | 33.7 | - | - | - | - | 90–100 | 0.15–0.30 | 150–200 | [141] |
| SWS-1L | Silica gel KSK | Inorganic salt CaCl2 | 33.7 | - | - | 0.63 | - | 80 | 0.39 | 510 | [160] |
| SWS-2S | Silica gel KSM | Inorganic salt LiBr | - | - | - | 0.25 | - | - | - | - | [140,161] |
| SWS-3L | Silica gel KSK | Inorganic salt MgCl2 | - | - | - | 0.72 | - | - | - | - | [140,161] |
| SWS-4L | Silica gel KSK | Inorganic salt LiCl | - | - | - | 0.7 | - | - | - | - | [140,161] |
| SWS-5L | Silica gel KSK | Inorganic salt MgSO4 | - | - | - | 0.65 | - | - | - | - | [140,162] |
| SWS-6L | Silica gel KSK | Inorganic salt NaSO4 | - | - | - | 0.62 | - | - | - | - | [140,162] |
| SWS-7L | Silica gel KSK | Inorganic salt CuSO4 | - | - | - | 0.58 | - | - | - | - | [140,162] |
| SWS-8L | Silica gel KSK | Inorganic salt Ca(NO3)2 | - | - | - | 0.42 | - | - | - | - | [140,163] |
| SGA * | Microporous silica gel | - | - | 706 | 0.4 | - | - | - | - | - | [144] |
| SGB * | Silica gel type B | - | - | 487 | 0.82 | - | - | - | - | - | |
| SGC * | Mesoporous silica gel | - | - | 395 | 0.93 | - | - | - | - | - | |
| SGA/LiCl | Microporous silica gel | Inorganic salt LiCl | 20 | 296 | 0.18 | - | Saturation of silica gel with an aqueous salt solution for 24 h, then a vacuum filter was used and the samples were dried for at least 4 h (to constant weight) at 120 °C. | - | - | - | |
| SGB/LiCl | Silica gel type B | 36 | 205 | 0.46 | - | - | - | - | |||
| SGC/LiCl | Mesoporous silica gel | 39 | 219 | 0.53 | - | - | - | - | |||
| SGA/LiBr | Microporous silica gel | Inorganic salt LiBr | 27 | 148 | 0.08 | - | - | - | - | ||
| SGB/LiBr | Silica gel type B | 42 | 127 | 0.26 | - | - | - | - | |||
| SGC/LiBr | Mesoporous silica gel | 48 | 143 | 0.36 | - | - | - | - | |||
| SGA/CaCl2 | Microporous silica gel | Inorganic salt CaCl2 | 19 | 327 | 0.20 | - | - | - | - | ||
| SGB/CaCl2 | Silica gel type B | 37 | 180 | 0.44 | - | - | - | - | |||
| SGC/CaCl2 | Mesoporous silica gel | 41 | 165 | 0.42 | - | - | - | - | |||
| Silica KSK * | Mesoporous silica gel KSK | - | - | 260 | 1 | - | - | - | - | - | [150,164] |
| SWS-8L | Mesoporous silica gel KSK | Ca(NO3)2 | - | 60 | 0.24 | 0.2–0.3 | Dry impregnation by saturation of mesoporous silica gel with an aqueous solution of calcium nitrate (45 wt% of salt). The material was dried at 200 °C. | 90–95 °C | 0.18–0.41 (depending on cycle time) | 190–389 (depending on cycle time) | |
| SG * | Silica gel type C (pore size 2–3 nm) | - | - | 348 | 0.99 | 0.088 | - | - | - | - | [138] |
| - | Silica gel type B (pore size 2–3 nm) | LiCl (10% solution) | 6.5 | - | - | - | First, the silica gel was dried at 120 °C. The silica gel was then immersed in aqueous LiCl solutions of 10–40% at 25 °C for 12 h. The sample was then dried to constant weight at 120 °C. | - | - | - | |
| - | LiCl (20% solution) | 19.8 | - | - | - | - | - | - | |||
| - | LiCl (30% solution) | 24.3 | - | - | - | - | - | - | |||
| - | LiCl (40% solution) | 25,6 | - | - | - | - | - | - | |||
| SLi10 | Silica gel type C (pore size 2–3 nm) | LiCl (10% solution) | 11.4 | 293 | 0.91 | 0.467 | - | - | - | ||
| SLi20 | LiCl (20% solution) | 24.0 | 242 | 0.75 | 0.87 | - | - | - | |||
| SLi30 | LiCl (30% solution) | 35.1 | 204 | 0.65 | 1.19 | - | - | - | |||
| SLi40 | LiCl (40% solution) | 43.6 | 179 | 0.55 | 1.2 | - | - | - | |||
| - | Mesoporous silica gel KSK | - | - | 350 | 1 | - | - | - | - | - | [132] |
| SWS-9L | Mesoporous silica gel KSK | LiNO3 | 34.5 | - | - | 0.22 | Drying of silica gel at 200 °C for 2 h. Then saturation of the silica gel with LiNO3 solution at 25 °C and drying to constant sorbent weight at 200 °C. | - | - | - | |
| SCa17 | Mesoporous silica gel | Inorganic salt CaCl2 | 17 | - | - | 0.33 | Impregnation with an aqueous salt solution and drying at 120 °C. | - | - | - | [145] |
| SCa33 | Mesoporous silica gel | Inorganic salt CaCl2 | 33 | - | - | 0.85 | - | - | - | ||
| SG * | Silica gel | - | - | 481 | 0.844 | 0.444 | - | - | - | - | [137] |
| SG + LiCl | Silica gel | Inorganic salt LiCl (50% solution) | 13 | 350 | 0.613 | 0.702 | Silica gel was dried in a vacuum dryer at 85 °C, and then a salt solution was added in the chamber and heated. The whole was then kept at room temp. for 24 h to saturate the matrix. The composite was dried at 120 °C to dry mass. This was followed by a procedure of removing excess salt (at 30 °C, RH 90%). | - | - | - | |
| - | Mesoporous silica gel * | - | - | 529 | 0.806 | approx. 0.17 | - | - | - | - | [165] |
| - | Mesoporous silica gel | Inorganic salt CaCl2 (10% solution) | - | - | 0.698 | approx. 0.44 | First, the silica gel was dried at 120 °C in an oven and then cooled in a vacuum dryer. Then the silica gel was immersed in a salt solution. During impregnation, the temperature was 25–80 °C and implementation lasted 1–8 h. | - | - | - | |
| - | Mesoporous silica gel | Inorganic salt CaCl2 (20% solution) | - | - | 0.567 | 0.53 | - | - | - | ||
| - | Mesoporous silica gel | Inorganic salt CaCl2 (30% solution) | - | - | 0.529 | approx. 0.6 | - | - | - | ||
| - | Mesoporous silica gel | Inorganic salt CaCl2 (40% solution) | - | - | 0.395 | 0.74 | - | - | - | ||
| SA0 * | Mesoporous silica gel | - | - | 300–400 | 0.75–1.0 | 0.02 (RH 20%) 0.059 (RH 50%) | - | 90 | 0.22 | 41.5 | [152] |
| SB0 * | Silica gel type B | - | - | 450–600 | 0.5–0.8 | 0.019 (RH 20%) 0.072 (RH 50%) | - | - | - | - | |
| SC0 * | Microporous silica gel | - | - | ≥600 | 0.35–0.45 | 0.047 (RH 20%) 0.228 (RH 50%) | - | - | - | - | |
| SA50 | Mesoporous silica gel | Inorganic salt CaCl2 (50% solution) | 37.75 | - | - | 0.157 (RH 20%) 0.376 (RH 50%) | Silica gel was dried to constant weight at 120 °C. It was then cooled in a vacuum chamber to ambient temperature. The silica gel was impregnated with a 50% CalCl2 salt solution with deionised water for 48 h at 25 °C. The sample was washed with deionised water to remove salt from the surface and dried to constant weight at in a vacuum dryer at 120 °C. | 90 | 0.27 | 128.3 | |
| SB50 | Silica gel type B | 41.3 | - | - | 0.156 (RH 20%) 0.376 (RH 50%) | - | - | - | |||
| SC50 | Microporous silica gel | 23.08 | - | - | 0.07 (RH 20%) 0.214 (RH 50%) | - | - | - | |||
| SA20 | Mesoporous silica gel | Inorganic salt CaCl2 (50% solution) | 15.38 | - | - | 0.084 (RH 20%) 0.234 (RH 50%) | - | - | - | ||
| SB20 | Silica gel type B | 14.09 | - | - | 0.07 (RH 20%) 0.232 (RH 50%) | - | - | - | |||
| SC20 | Microporous silica gel | 5.18 | - | - | 0.047 (RH 20%) 0.172 (RH 50%) | - | - | - | |||
| SG | Mesoporous silica gel | - | - | 417.18 | 0.884 | - | - | - | - | - | [157] |
| LiCl@SG_30 | Mesoporous silica gel | Inorganic salt LiCl | 29.5% | - | - | up to 0.74 | First, the porous matrix was dried at 150 °C. The matrix was then mixed with a LiCl solution (dry impregnation). After mixing, the material was left at room temperature for 24 h and then dried in an oven at 150 °C for 12 h. | - | - | - |
5. Discussion
- The operating parameters of the adsorption chiller (primarily the cycle time, the hot, cooling and chilled-water temperatures and the water flow rates in the hot, cooling and chilled-water circuits);
- The methods of synthesis and the materials/substances used in the synthesis that directly affect the properties of the composite sorbent.
- Chemical nature of the impregnating substance;
- Concentration of salt in the impregnating aqueous solution;
- Chemical nature of the porous matrix;
- Porosity of the matrix;
- Process conditions for the synthesis of the composite material.
- Preparation of a salt solution with appropriate mass concentration;
- Drying of the porous matrix to remove water (Aristov [140] suggests 120–180 °C);
- Wet or dry impregnation of porous matrix with salt;
- Drying of the composite material to remove water (Aristov [140] suggests 120–150 °C);
- Possible treatment of the material after synthesis.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Population Prospects 2022. Available online: https://population.un.org/wpp/ (accessed on 5 May 2023).
- International Energy Outlook 2021. Available online: https://www.eia.gov/outlooks/ieo/ (accessed on 5 May 2023).
- Gonzalez-Torres, M.; Perez-Lombard, L.; Coronel, J.F.; Maestre, I.R.; Yan, D. A review on buildings energy information: Trends, end-uses, fuels and drivers. Energy Rep. 2022, 8, 626–637. [Google Scholar] [CrossRef]
- Dupont, J.L.; Domanski, P.; Lebrun, P.; Ziegler, F. The Role of Refrigeration in the Global Economy (2019), 38th Note on Refrigeration Technologies; Informatory note of the International Institute of Refrigeration; The International Institute of Refrigeration: Paris, France, June 2019. [Google Scholar] [CrossRef]
- Elsaid, K.; Sayed, E.T.; Yousef, B.A.A.; Rabaia, M.K.H.; Abdelkareem, M.A.; Olabi, A.G. Recent progress on the utilization of waste heat for desalination: A review. Energy Convers. Manag. 2020, 221, 113105. [Google Scholar] [CrossRef]
- Askalany, A.A.; Salem, M.; Ismael, I.M.; Ali, A.H.H.; Morsy, M.G.; Saha, B.B. An overview on adsorption pairs for cooling. Renew. Sustain. Energy Rev. 2013, 19, 565–572. [Google Scholar] [CrossRef]
- Zajaczkowski, B. Optimizing performance of a three-bed adsorption chiller using new cycle time allocation and mass recovery. Appl. Therm. Eng. 2016, 100, 744–752. [Google Scholar] [CrossRef]
- Ng, K.C.; Thu, K.; Kim, Y.; Chakraborty, A.; Amy, G. Adsorption desalination: An emerging low-cost thermal desalination method. Desalination 2013, 308, 161–179. [Google Scholar] [CrossRef]
- Olkis, C.; Brandani, S.; Santori, G. Cycle and performance analysis of a small-scale adsorption heat transformer for desalination and cooling applications. Chem. Eng. J. 2019, 378, 122104. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Brites, G.J.V.N.; Costa, J.J.; Gaspar, A.R.; Costa, V.A.F. Review and future trends of solar adsorption refrigeration systems. Renew. Sustain. Energy Rev. 2014, 39, 102–123. [Google Scholar] [CrossRef]
- Rouf, R.A.; Jahan, N.; Alam, K.C.A.; Sultan, A.A.; Saha, B.B.; Saha, S.C. Improved cooling capacity of a solar heat driven adsorption chiller. Case Stud. Therm. Eng. 2020, 17, 100568. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Wang, R. Application analysis of adsorption refrigeration system for solar and data center waste heat utilization. Energy Convers. Manag. 2021, 228, 113564. [Google Scholar] [CrossRef]
- Patel, J.K.; Mehta, N.; Dabhi, J. Adsorption Refrigeration System: Design and Analysis. Mater. Today Proc. 2017, 4, 10278–10282. [Google Scholar] [CrossRef]
- Hassan, A.A.; Elwardany, A.E.; Ookawara, S.; Ahmed, M.; El-Sharkawy, I.I. Integrated adsorption-based multigeneration systems: A critical review and future trends. Int. J. Refrig. 2020, 116, 129–145. [Google Scholar] [CrossRef]
- Rogala, Z. Adsorption chiller using flat-tube adsorbers—Performance assessment and optimization. Appl. Therm. Eng. 2017, 121, 431–442. [Google Scholar] [CrossRef]
- Babamiri, A.; Gharib, M.; Ebrahimi, M. Multi-criteria evaluation of a novel micro-trigeneration cycle based on α-type Stirling engine, organic Rankine cycle, and adsorption chiller. Energy Convers. Manag. 2022, 253, 115162. [Google Scholar] [CrossRef]
- Naseem, M.; Park, S.; Lee, S. Experimental and theoretical analysis of a trigeneration system consisting of adsorption chiller and high temperature PEMFC. Energy Convers. Manag. 2022, 251, 114977. [Google Scholar] [CrossRef]
- Grisel, R.J.H.; Smeding, S.F.; de Boer, R. Waste heat driven silica gel/water adsorption cooling in trigeneration. Appl. Therm. Eng. 2010, 30, 1039–1046. [Google Scholar] [CrossRef]
- Al-Mousawi, F.; Al-Dadah, R.; Mahmoud, S. Low grade heat driven adsorption system for cooling and power generation using advanced adsorbent materials. Energy Convers. Manag. 2016, 126, 373–384. [Google Scholar] [CrossRef]
- Pan, Q.W.; Wang, R.Z. Study on operation strategy of a silica gel-water adsorption chiller in solar cooling application. Solar Energy 2018, 172, 24–31. [Google Scholar] [CrossRef]
- Baiju, V.; Asif Sha, A.; Shajahan, C.A.; Chindhu, V.G. Energy and exergy based assessment of a two bed solar adsorption cooling system. Int. J. Refrig. 2022, 141, 90–101. [Google Scholar] [CrossRef]
- Hassan, A.A.; Elwardany, A.E.; Ookawara, S.; Elwardany, A.E.; El-Sharkawy, I.I. Performance investigation of a solar-powered adsorption-based trigeneration system for cooling, electricity, and domestic hot water production. Appl. Therm. Eng. 2021, 199, 117553. [Google Scholar] [CrossRef]
- Robbins, T.; Garimella, S. An autonomous solar driven adsorption cooling system. Sol. Energy 2020, 211, 1318–1324. [Google Scholar] [CrossRef]
- Gupta, R.; Puri, I.K. Waste heat recovery in a data center with an adsorption chiller: Technical and economic analysis. Energy Convers. Manag. 2021, 245, 114576. [Google Scholar] [CrossRef]
- Verde, M.; Harby, K.; de Boer, R.; Corberán, J.M. Performance evaluation of a waste-heat driven adsorption system for automotive air-conditioning: Part I—Modeling and experimental validation. Energy 2016, 116, 526–538. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Wang, R.Z.; Zhang, M.; Jiangzhou, S. Adsorption cold storage system with zeolite–water working pair used for locomotive air conditioning. Energy Convers. Manag. 2003, 44, 1733–1743. [Google Scholar] [CrossRef]
- Zhu, R.; Han, B.; Lin, M.; Yu, Y. Experimental investigation on an adsorption system for producing chilled water. Int. J. Refrig. 1992, 15, 31–34. [Google Scholar] [CrossRef]
- Alsaman, A.S.; Askalany, A.A.; Harby, K.; Ahmed, M.S. Performance evaluation of a solar-driven adsorption desalination-cooling system. Energy 2017, 128, 196–207. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, J.; Liu, C.; Lin, X.; Sun, Y. Experimental investigation on an adsorption desalination system with heat and mass recovery between adsorber and desorber beds. Desalination 2018, 446, 42–50. [Google Scholar] [CrossRef]
- Thu, K.; Yanagi, H.; Saha, B.B.; Ng, K.C. Performance analysis of a low-temperature waste heat-driven adsorption desalination prototype. Int. J. Heat Mass Transf. 2013, 65, 662–669. [Google Scholar] [CrossRef]
- Thu, K.; Saha, B.B.; Chua, K.J.; Ng, K.C. Performance investigation of a waste heat-driven 3-bed 2-evaporator adsorption cycle for cooling and desalination. Int. J. Heat Mass Transf. 2016, 101, 1111–1122. [Google Scholar] [CrossRef]
- Alsaman, A.S.; Askalany, A.A.; Harby, K.; Ahmed, M.S. A state of the art of hybrid adsorption desalination–cooling systems. Renew. Sustain. Energy Rev. 2016, 58, 692–703. [Google Scholar] [CrossRef]
- Wang, X.; Ng, K.C. Experimental investigation of an adsorption desalination plant using low-temperature waste heat. Appl. Therm. Eng. 2005, 25, 2780–2789. [Google Scholar] [CrossRef]
- Ościk, J. Adsorpcja, Wydawnictwo Naukowe; PWN: Warszawa, Poland, 1979. [Google Scholar]
- Stefański, S.; Mika, Ł.; Sztekler, K.; Kalawa, W.; Lis, Ł.; Nowak, W. Adsorption bed configurations for adsorption cooling application. E3S Web Conf. 2019, 108, 01010. [Google Scholar] [CrossRef]
- Goyal, P.; Baredar, P.; Mittal, A.; Siddiqui, A.R. Adsorption refrigeration technology—An overview of theory and its solar energy applications. Renew. Sustain. Energy Rev. 2016, 53, 1389–1410. [Google Scholar] [CrossRef]
- Alahmer, A.; Ajib, S.; Wang, X. Comprehensive strategies for performance improvement of adsorption air conditioning systems: A review Renew. Sustain. Energy Rev. 2019, 99, 138–158. [Google Scholar] [CrossRef]
- Iguchi, K.; Nakayama, M.; Akisawa, A. Method for estimating optimum cycle time based on adsorption chiller parameters. Int. J. Refrig. 2019, 105, 66–71. [Google Scholar] [CrossRef]
- Youssef, P.G.; Mahmoud, S.M.; Al-Dadah, R. Performance analysis of four bed adsorption water desalination/refrigeration system, comparison of AQSOA-Z02 to silica-gel. Desalination 2015, 375, 100–107. [Google Scholar] [CrossRef]
- Sarbak, Z. Adsorpcja i Adsorbenty; Wydawnictwo Naukowe UAM: Poznań, Poland, 2000. [Google Scholar]
- Feng, C.; Jiaquang, E.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X.; Han, D. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954. [Google Scholar] [CrossRef]
- Chorowski, M.; Pyrka, P. Modelling and experimental investigation of an adsorption chiller using low-temperature heat from cogeneration. Energy 2015, 92, 221–229. [Google Scholar] [CrossRef]
- Wang, R.Z.; Oliveira, R.G. Adsorption refrigeration—An efficient way to make good use of waste heat and solar energy. Prog. Energy Combust. Sci. 2006, 32, 424–458. [Google Scholar] [CrossRef]
- Gado, M.; Elgendy, E.; Elsayed, K.; Fatouh, M. Performance enhancement of an adsorption chiller by optimum cycle time allocation at different operating conditions. Adv. Mech. Eng. 2019, 11. [Google Scholar] [CrossRef]
- Saha, B.B.; Koyama, S.; Lee, J.B.; Kuwahara, K.; Alam, K.C.A.; Hamamoto, Y.; Akisawa, A.; Kashiwagi, T. Performance evaluation of a low-temperature waste heat driven multi-bed adsorption chiller. Int. J. Multiph. Flow 2003, 29, 1249–1263. [Google Scholar] [CrossRef]
- Luo, H.; Wang, R.; Dai, Y. The effects of operation parameter on the performance of a solar-powered adsorption chiller. Appl. Energy 2010, 87, 3018–3022. [Google Scholar] [CrossRef]
- Chang, W.-S.; Wang, C.-C.; Shieh, C.-C. Experimental study of a solid adsorption cooling system using flat-tube heat exchangers as adsorption bed. Appl. Therm. Eng. 2007, 27, 2195–2199. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Herman, F.; Pyrka, P.; Sosnowski, M.; Prauzner, T.; Nowak, W. Optimization of a three-bed adsorption chiller by genetic algorithms and neural networks. Energy Convers. Manag. 2017, 153, 313–322. [Google Scholar] [CrossRef]
- Chen, W.D.; Hasanien, H.M.; Chua, K.J. Towards a digital twin approach—Experimental analysis and energy optimization of a multi-bed adsorption system. Energy Convers. Manag. 2022, 271, 116346. [Google Scholar] [CrossRef]
- Elsheniti, M.B.; Hassab, M.A.; Attia, A.-E. Examination of effects of operating and geometric parameters on the performance of a two-bed adsorption chiller. Appl. Therm. Eng. 2019, 146, 674–687. [Google Scholar] [CrossRef]
- Niazmand, H.; Talebian, H.; Mahdavikhah, M. Bed geometrical specifications effects on the performance of silica/water adsorption chillers. Int. J. Refrig. 2012, 35, 2261–2274. [Google Scholar] [CrossRef]
- Verde, M.; Harby, K.; Corberán, J.M. Optimization of thermal design and geometrical parameters of a flat tube-fin adsorbent bed for automobile air-conditioning. Appl. Therm. Eng. 2017, 111, 489–502. [Google Scholar] [CrossRef]
- Rezk, A.R.M.; Al-Dadah, R.K. Physical and operating conditions effects on silica gel/water adsorption chiller performance. Appl. Energy 2012, 89, 142–149. [Google Scholar] [CrossRef]
- Li, X.H.; Hou, X.H.; Zhang, X.; Yuan, Z.X. A review on development of adsorption cooling–Novel beds and advanced cycles. Energy Convers. Manag. 2015, 94, 221–232. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.K.; Mahmoud, S.; Elsayed, A. Effects of contact resistance and metal additives in finned-tube adsorbent beds on the performance of silica gel/water adsorption chiller. Appl. Therm. Eng. 2013, 53, 278–284. [Google Scholar] [CrossRef]
- Grabowska, K.; Krzywanski, J.; Nowak, W.; Wesolowska, M. Construction of an innovative adsorbent bed configuration in the adsorption chiller—Selection criteria for effective sorbent-glue pair. Energy 2018, 151, 317–323. [Google Scholar] [CrossRef]
- Tatlier, M. Performances of MOF vs. zeolite coatings in adsorption cooling applications. Appl. Therm. Eng. 2017, 113, 290–297. [Google Scholar] [CrossRef]
- Boruta, P.; Bujok, T.; Mika, Ł.; Sztekler, K. Adsorbents, Working Pairs and Coated Beds for Natural Refrigerants in Adsorption Chillers—State of the Art. Energies 2021, 14, 4707. [Google Scholar] [CrossRef]
- Duong, X.Q.; Cao, N.V.; Lee, W.S.; Park, M.Y.; Chung, J.D.; Bae, K.J.; Kwon, O.K. Effect of coating thickness, binder and cycle time in adsorption cooling applications. Appl. Therm. Eng. 2021, 184, 116265. [Google Scholar] [CrossRef]
- Girnik, I.S.; Grekova, A.D.; Gordeeva, L.G.; Aristov, Y.I. Dynamic optimization of adsorptive chillers: Compact layer vs. bed of loose grains. Appl. Therm. Eng. 2017, 125, 823–829. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Caprì, A.; Frazzica, A.; Sapienza, A. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Chua, H.T.; Ng, K.C.; Malek, A.; Kashiwagi, T.; Akisawa, A.; Saha, B.B. Multi-bed regenerative adsorption chiller—Improving the utilization of waste heat and reducing the chilled water outlet temperature fluctuation. Int. J. Refrig. 2001, 24, 124–136. [Google Scholar] [CrossRef]
- Sztekler, K.; Kalawa, W.; Nowak, W.; Mika, L.; Gradziel, S.; Krzywanski, J.; Radomska, E. Experimental Study of Three-Bed Adsorption Chiller with Desalination Function. Energies 2020, 13, 5827. [Google Scholar] [CrossRef]
- Mitra, S.; Kumar, P.; Srinivasan, K.; Dutta, P. Simulation study of a two-stage adsorber system. Appl. Therm. Eng. 2014, 72, 283–288. [Google Scholar] [CrossRef]
- Mitra, S.; Thu, K.; Saha, B.B.; Dutta, P. Performance evaluation and determination of minimum desorption temperature of a two-stage air cooled silica gel/water adsorption system. Appl. Therm. Eng. 2017, 206, 507–518. [Google Scholar] [CrossRef]
- Wang, D.C.; Li, Y.H.; Li, D.; Xia, Y.Z.; Zhang, J.P. A review on adsorption refrigeration technology and adsorption deterioration in physical adsorption systems. Renew. Sustain. Energy Rev. 2010, 14, 344–353. [Google Scholar] [CrossRef]
- Wang, X.; Ng, K.C.; Chakarborty, A.; Saha, B.B. How Heat and Mass Recovery Strategies Impact the Performance of Adsorption Desalination Plant: Theory and Experiments. Heat. Transf. Eng. 2007, 28, 147–153. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Tian, X.; Liu, D.; Sumathy, K. Progress in silica gel–water adsorption refrigeration technology. Renew. Sustain. Energy Rev. 2014, 30, 85–104. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Wang, R. Experimental study of an adsorption chiller for extra low temperature waste heat utilization. Appl. Therm. Eng. 2019, 163, 114341. [Google Scholar] [CrossRef]
- Ng, K.C.; Wang, X.; Lim, Y.S.; Saha, B.B.; Chakarborty, A.; Koyama, S.; Akisawa, A.; Kashiwagi, T. Experimental study on performance improvement of a four-bed adsorption chiller by using heat and mass recovery. Int. J. Heat Mass Transf. 2006, 49, 3343–3348. [Google Scholar] [CrossRef]
- Wang, R.Z. Performance improvement of adsorption cooling by heat and mass recovery operation. Int. J. Refrig. 2001, 24, 602–611. [Google Scholar] [CrossRef]
- Choudhury, B.; Saha, B.B.; Chatterjee, P.K.; Sarkar, J.P. An overview of developments in adsorption refrigeration systems towards a sustainable way of cooling. Appl. Energy 2013, 104, 554–567. [Google Scholar] [CrossRef]
- Liu, Y.; Leong, K.C. Numerical study of a novel cascading adsorption cycle. Int. J. Refrig. 2006, 29, 250–259. [Google Scholar] [CrossRef]
- Basdanis, T.; Tsimpoukis, A.; Valougeorgis, D. Performance optimization of a solar adsorption chiller by dynamically adjusting the half-cycle time. Renew. Energy 2021, 164, 362–374. [Google Scholar] [CrossRef]
- Sztekler, K.; Kalawa, W.; Mika, Ł.; Sowa, M. Effect of Metal Additives in the Bed on the Performance Parameters of an Adsorption Chiller with Desalination Function. Energies 2021, 14, 7226. [Google Scholar] [CrossRef]
- Glaznev, I.S.; Aristov, Y.I. The effect of cycle boundary conditions and adsorbent grain size on the water sorption dynamics in adsorption chillers. Int. J. Heat Mass Transf. 2010, 53, 1893–1898. [Google Scholar] [CrossRef]
- Mohammed, R.H.; Askalany, A.A. Productivity Improvements of Adsorption Desalination Systems. In Solar Desalination Technology. Green Energy and Technology; Kumar, A., Prakash, O., Eds.; Springer: Singapore, 2019; pp. 325–357. [Google Scholar] [CrossRef]
- Ilis, G.G.; Demir, H.; Saha, B.B. Innovative approach in adsorption chiller: Combination of condenser-adsorber for improving performance. Appl. Therm. Eng. 2021, 192, 116958. [Google Scholar] [CrossRef]
- Demir, H.; Mobedi, M.; Ülkü, S. Effects of porosity on heat and mass transfer in a granular adsorbent bed. Int. Commun. Heat Mass Transf. 2009, 36, 372–377. [Google Scholar] [CrossRef]
- Tso, C.Y.; Chao, C.Y.H. Activated carbon, silica-gel and calcium chloride composite adsorbents for energy efficient solar adsorption cooling and dehumidification systems. Int. J. Refrig. 2012, 35, 1626–1638. [Google Scholar] [CrossRef]
- Demir, H.; Mobedi, M.; Ülkü, S. The use of metal piece additives to enhance heat transfer rate through an unconsolidated adsorbent bed. Int. J. Refrig. 2010, 33, 714–720. [Google Scholar] [CrossRef]
- Askalany, A.A.; Henninger, S.K.; Ghazy, M.; Saha, B.B. Effect of improving thermal conductivity of the adsorbent on performance of adsorption cooling system. Appl. Therm. Eng. 2017, 110, 695–702. [Google Scholar] [CrossRef]
- Sztekler, K.; Kalawa, W.; Mika, Ł.; Mlonka-Medrala, A.; Sowa, M.; Nowak, W. Effect of Additives on the Sorption Kinetics of a Silica Gel Bed in Adsorption Chiller. Energies 2021, 14, 1083. [Google Scholar] [CrossRef]
- Eun, T.-H.; Song, H.-K.; Hun Han, J.; Lee, K.-H.; Kim, J.-N. Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps: Part I. Characterization of the composite blocks. Int. J. Refrig. 2000, 23, 164–173. [Google Scholar] [CrossRef]
- Eun, T.-H.; Song, H.-K.; Hun Han, J.; Lee, K.-H.; Kim, J.-N. Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps. Part II. Cooling system using the composite blocks. Int. J. Refrig. 2000, 23, 74–81. [Google Scholar] [CrossRef]
- Hua, W.; Xie, W.; Zhang, X.; Gao, L.; Que, L.; Ding, X. Synthesis and characterization of silica gel composite with thermal conductivity enhancers and polymer binders for adsorption desalination and cooling system. Int. J. Refrig. 2022, 139, 93–103. [Google Scholar] [CrossRef]
- Younes, M.M.; El-Sharkawy, I.A.; Kabeel, A.E.; Uddin, K.; Pal, A.; Mitra, S.; Thu, K.; Saha, B.B. Synthesis and characterization of silica gel composite with polymer binders for adsorption cooling applications. Int. J. Refrig. 2019, 98, 161–170. [Google Scholar] [CrossRef]
- Sumathy, K.; Yeung, K.H.; Yong, L. Technology development in the solar adsorption refrigeration systems. Prog. Energy Combust. Sci 2003, 29, 301–327. [Google Scholar] [CrossRef]
- Alghoul, M.A.; Sulaiman, M.Y.; Azmi, B.Z.; Wahab, M.A. Advances on multi-purpose solar adsorption systems for domestic refrigeration and water heating. Appl. Therm. Eng. 2007, 27, 813–822. [Google Scholar] [CrossRef]
- Thu, K.; Chakraborty, A.; Saha, B.B.; Ng, K.C. Thermo-physical properties of silica gel for adsorption desalination cycle. Appl. Therm. Eng. 2013, 50, 1596–1602. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, R.Z.; Oliveira, R.G. A review on adsorption working pairs for refrigeration. Renew. Sustain. Energy Rev. 2009, 3, 518–534. [Google Scholar] [CrossRef]
- Ng, K.C.; Chua, H.T.; Chung, C.Y.; Loke, C.H.; Kashiwagi, T.; Akisawa, A.; Saha, B.B. Experimental investigation of the silica gel–water adsorption isotherm characteristics. Appl. Therm. Eng. 2001, 21, 1631–1642. [Google Scholar] [CrossRef]
- Sharma, C.S.; Harriott, P.; Hughes, R. Thermal conductivity of catalyst pellets and other porous particles: Part II: Experimental measurements. Chem. Eng. J. 1975, 10, 73–80. [Google Scholar] [CrossRef]
- Wang, R.Z.; Ge, T.S.; Chen, C.J.; Ma, Q.; Xiong, Z.Q. Solar sorption cooling systems for residential applications: Options and guidelines. Int. J. Refrig. 2009, 32, 638–660. [Google Scholar] [CrossRef]
- Shabir, F.; Sultan, M.; Miyazaki, T.; Saha, B.B.; Askalany, A.; Ali, I.; Zhou, Y.; Ahmad, R.; Shamshiri, R.R. Recent updates on the adsorption capacities of adsorbent-adsorbate pairs for heat transformation applications. Renew. Sustain. Energy Rev. 2020, 119, 109630. [Google Scholar] [CrossRef]
- Product Information: Silica Gel. Available online: https://www.sigmaaldrich.com/product/sial/236845 (accessed on 2 June 2023).
- Gordeeva, L.; Aristov, Y. Novel sorbents of ethanol “salt confined to porous matrix” for adsorptive cooling. Energy 2010, 35, 2703–2708. [Google Scholar] [CrossRef]
- AL-Hasni, S.; Santori, G. The cost of manufacturing adsorption chillers. Therm. Sci. Eng. Prog. 2023, 39, 101685. [Google Scholar] [CrossRef]
- Saha, B.B.; Akisawa, A.; Kashiwagi, T. Solar/waste heat driven two-stage adsorption chiller: The prototype. Renew. Energ. 2001, 23, 93–101. [Google Scholar] [CrossRef]
- Zhai, X.Q.; Wang, R.Z.; Dai, Y.J.; Wu, J.Y.; Xu, Y.X.; Ma, Q. Solar integrated energy system for a green building. Energy Build. 2007, 39, 985–993. [Google Scholar] [CrossRef]
- Palomba, V.; Ferraro, M.; Frazzica, A.; Vasta, S.; Sergi, F.; Antonucci, V. Experimental and numerical analysis of a SOFC-CHP system with adsorption and hybrid chillers for telecommunication applications. Appl. Energy 2018, 216, 620–633. [Google Scholar] [CrossRef]
- Freni, A.; Maggio, G.; Sapienza, A.; Frazzica, A.; Restuccia, G.; Vasta, S. Comparative analysis of promising adsorbent/adsorbate pairs for adsorptive heat pumping, air conditioning and refrigeration. Appl. Therm. Eng. 2016, 104, 85–95. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Cacciola, G.; Restuccia, G.; Giordano, N. Fast simple and accurate measurement of zeolite thermal conductivity. Zeolites 1990, 10, 565–570. [Google Scholar] [CrossRef]
- Hu, P.; Yao, J.-J.; Chen, Z.-S. Analysis for composite zeolite/foam aluminum–water mass recovery adsorption refrigeration system driven by engine exhaust heat. Energy Convers. Manag. 2009, 50, 255–261. [Google Scholar] [CrossRef]
- Sayilgan, Ş.Ç.; Mobedi, M.; Ülkü, S. Effect of regeneration temperature on adsorption equilibria and mass diffusivity of zeolite 13x-water pair. Microporous Mesoporous Mater. 2016, 224, 9–16. [Google Scholar] [CrossRef]
- Chan, K.C.; Tso, C.Y.; Wu, C.; Chao, C.Y.H. Enhancing the performance of a zeolite 13X/CaCl2–water adsorption cooling system by improving adsorber design and operation sequence. Energy Build. 2018, 158, 1368–1378. [Google Scholar] [CrossRef]
- Chan, K.C.; Chao, C.Y.H.; Sze-To, G.N.; Hui, K.N. Performance predictions for a new zeolite 13X/CaCl2 composite adsorbent for adsorption cooling systems. Int. J. Heat Mass Transf. 2012, 55, 3214–3224. [Google Scholar] [CrossRef]
- Kossmann, J.; Rothe, R.; Heil, T.; Antonietti, M.; López-Salas, N. Ultrahigh water sorption on highly nitrogen doped carbonaceous materials derived from uric acid. J. Colloid Interface Sci. 2021, 602, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Myat, A.; Choon, N.K.; Thu, K.; Kim, Y.-D. Experimental investigation on the optimal performance of Zeolite–water adsorption chiller. Appl. Energy 2013, 102, 582–590. [Google Scholar] [CrossRef]
- Younes, M.M.; El-Sharkawy, I.I.; Kabeel, A.E.; Saha, B.B. A review on adsorbent-adsorbate pairs for cooling applications. Appl. Therm. Eng. 2017, 114, 394–414. [Google Scholar] [CrossRef]
- Wang, L.W.; Tamainot-Telto, Z.; Thorpe, R.; Critoph, R.E.; Metcalf, S.J.; Wang, R.Z. Study of thermal conductivity, permeability, and adsorption performance of consolidated composite activated carbon adsorbent for refrigeration. Renew. Energy 2011, 36, 2062–2066. [Google Scholar] [CrossRef]
- Hua, W.S.; Xu, H.J.; Xie, W.H. Review on adsorption materials and system configurations of the adsorption desalination applications. Appl. Energy 2022, 204, 117958. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, S.; Zhao, B.; Qi, X.; Sun, H. Performance prediction of adsorbent in an adsorption refrigeration system based on variable temperature and constant pressure experiment and dynamic model. Int. J. Refrig. 2021, 131, 909–919. [Google Scholar] [CrossRef]
- Henninger, S.K.; Ernst, S.-J.; Gordeeva, L.; Bendix, P.; Fröhlich, D.; Grekova, A.D.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New materials for adsorption heat transformation and storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Elsayed, E.; AL-Dadah, R.; Mahmoud, S.; Anderson, P.; Elsayed, A. Experimental testing of aluminium fumarate MOF for adsorption desalination. Desalination 2020, 475, 114170. [Google Scholar] [CrossRef]
- Tannert, N.; Jansen, C.; Nießing, S.; Janiak, C. Robust Synthesis Routes and Porosity of the Al-Based Metal–Organic Frameworks Al-Fumarate, CAU-10-H and MIL-160. Dalton Trans. 2019, 48, 2967–2976. [Google Scholar] [CrossRef]
- Ma, L.; Rui, Z.; Wu, Q.; Yang, H.; Yin, Y.; Liu, Z.; Cui, Q.; Wang, H. Performance evaluation of shaped MIL-101–ethanol working pair for adsorption refrigeration. Appl. Therm. Eng. 2016, 95, 223–228. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Miyazaki, T.; Koyama, S.; Henninger, S.K.; Herbst, A.; Janiak, C. Ethanol adsorption onto metal organic framework: Theory and experiments. Energy 2015, 79, 363–370. [Google Scholar] [CrossRef]
- Janiak, C.; Henninger, S.K. Porous Coordination Polymers as Novel Sorption Materials for Heat Transformation Processes. Chimia 2013, 67, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Dakkama, H.J.; Youssef, P.G.; Al-Dadah, R.K.; Mahmoud, S. Adsorption ice making and water desalination system using metal organic frameworks/water pair. Energy Convers. Manag. 2017, 142, 53–61. [Google Scholar] [CrossRef]
- Gwadera, M. Adsorpcja wody na silikażelu w adsorpcyjnych urządzeniach chłodniczych. Inż. Ap. Chem. 2013, 52, 317–318. [Google Scholar]
- Srivastava, N.C.; Eames, I.W. A review of adsorbents and adsorbates in solid–vapour adsorption heat pump systems. Appl. Therm. Eng. 1998, 18, 707–714. [Google Scholar] [CrossRef]
- Hu, E.J. A study of thermal decomposition of methanol in solar powered adsorption refrigeration systems. Sol. Energy 1998, 62, 325–329. [Google Scholar] [CrossRef]
- El-Sharkawy, I.A.; Hassan, M.; Saha, B.B.; Koyama, S.; Nasr, M.M. Study on adsorption of methanol onto carbon based adsorbents. Int. J. Refrig. 2009, 32, 1579–1586. [Google Scholar] [CrossRef]
- El-Sharkawy, I.A.; Saha, B.B.; Koyama, S.; He, J.; Ng, K.C.; Yap, C. Experimental investigation on activated carbon–ethanol pair for solar powered adsorption cooling applications. Int. J. Refrig. 2008, 31, 1407–1413. [Google Scholar] [CrossRef]
- Shmroukh, A.N.; Ali, A.H.H.; Ookawara, S. Adsorption working pairs for adsorption cooling chillers: A review based on adsorption capacity and environmental impact. Renew. Sustain. Energy Rev. 2015, 50, 445–456. [Google Scholar] [CrossRef]
- Scopus Database. Available online: https://www.scopus.com/search/form.uri?display=basic#basic (accessed on 22 May 2023).
- Restuccia, G.; Freni, A.; Aristov, Y. Selective water sorbent for solid sorption chiller: Experimental results and modelling. Int. J. Refrig. 2004, 27, 284–293. [Google Scholar] [CrossRef]
- Saha, B.B.; Chakraborty, A.; Koyama, S.; Aristov, Y.I. A new generation cooling device employing CaCl2-in-silica gel–water system. Int. J. Heat Mass Transf. 2009, 52, 516–524. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Restuccia, G.; Cacciola, G.; Parmon, V.N. A family of new working materials for solid sorption air conditioning systems. Appl. Therm. Eng. 2002, 22, 191–204. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Sapienza, A.; Ovoshchnikov, D.S.; Freni, A.; Restuccia, G. Reallocation of adsorption and desorption times for optimisation of cooling cycles. Int. J. Refrig. 2012, 35, 525–531. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Cacciola, G.; Restuccia, G. Selective water sorbents for multiple applications, 1. CaCl2 confined in mesopores of silica gel: Sorption properties. React. Kinet. Catal. Lett. 1996, 59, 325–333. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites ‘salt inside porous matrix’ for adsorption heat transformation: A current state-of-the-art and new trends. Int. J. Low Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. The Role of the Liquid-Solid Interface in the Preparation of Supported Catalysts. Catal. Rev. 2006, 48, 363–444. [Google Scholar] [CrossRef]
- Khattak, A.K.; Afzal, M.; Saleem, M.; Yasmeen, G.; Ahmad, R. Surface modification of alumina by metal doping. Colloids Surf. A Physicochem. Eng. Asp. 2000, 162, 99–106. [Google Scholar] [CrossRef]
- Gong, L.X.; Wang, R.Z.; Xia, Z.Z.; Chen, C.J. Adsorption Equilibrium of Water on a Composite Adsorbent Employing Lithium Chloride in Silica Gel. J. Chem. Eng. Data 2010, 55, 2920–2923. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W. Development and characterization of silica gel–LiCl composite sorbents for thermal energy storage. Chem. Eng. Sci. 2014, 111, 73–84. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Glaznev, I.S.; Savchenko, E.V.; Malakhov, V.V.; Aristov, Y.I. Impact of phase composition on water adsorption on inorganic hybrids “salt/silica”. J. Colloid Interface Sci. 2006, 301, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Aristov, Y.I. New family of solid sorbents for adsorptive cooling: Material scientist approach. J. Eng. Thermophys. 2007, 16, 63–72. [Google Scholar] [CrossRef]
- Freni, A.; Russo, F.; Vasta, S.; Tokarev, M.; Aristov, Y.I.; Restuccia, G. An advanced solid sorption chiller using SWS-1L. Appl. Therm. Eng. 2007, 27, 2200–2204. [Google Scholar] [CrossRef]
- Aristov, Y.I. Optimal adsorbent for adsorptive heat transformers: Dynamic considerations. Int. J. Refrig. 2009, 32, 675–686. [Google Scholar] [CrossRef]
- Gong, L.; Wang, R.; Xia, Z.Z.; Chen, C.J. Design and performance prediction of a new generation adsorption chiller using composite adsorbent. Energy Convers. Manag. 2011, 52, 2345–2350. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, T.; Wang, R.; Hu, L.M. Performance study of composite silica gels with different pore sizes and different impregnating hygroscopic salts. Chem. Eng. Sci. 2014, 120, 1–9. [Google Scholar] [CrossRef]
- Cortés, F.B.; Chejne, F.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Moreno-Castilla, C. Water sorption on silica- and zeolite-supported hygroscopic salts for cooling system applications. Energy Convers. Manag. 2012, 53, 219–223. [Google Scholar] [CrossRef]
- Gordeeva, L.; Aristov, Y.I. Composite sorbent of methanol “LiCl in mesoporous silica gel” for adsorption cooling: Dynamic optimization. Energy 2011, 36, 1273–1279. [Google Scholar] [CrossRef]
- Maggio, G.; Gordeeva, L.G.; Freni, A.; Aristov, Y.I.; Santori, G.; Polonara, F.; Restuccia, G. Simulation of a solid sorption ice-maker based on the novel composite sorbent “lithium chloride in silica gel pores”. Appl. Therm. Eng. 2009, 29, 1714–1720. [Google Scholar] [CrossRef]
- Lu, Z.S.; Wang, R.Z. Study of the new composite adsorbent of salt LiCl/silica gel–methanol used in an innovative adsorption cooling machine driven by low temperature heat source. Renew. Energy 2014, 63, 445–451. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Freni, A.; Aristov, Y.I.; Restuccia, G. Composite Sorbent of Methanol “Lithium Chloride in Mesoporous Silica Gel” for Adsorption Cooling Machines: Performance and Stability Evaluation. Ind. Eng. Chem. Res. 2009, 48, 13, 6197–6202. [Google Scholar] [CrossRef]
- Freni, A.; Sapienza, A.; Glaznev, I.S.; Aristov, Y.I. Experimental testing of a lab-scale adsorption chiller using a novel selective water sorbent “silica modified by calcium nitrate”. Int. J. Refrig. 2012, 35, 518–524. [Google Scholar] [CrossRef]
- Daou, K.; Wang, R.Z.; Xia, Z.Z. Development of a new synthesized adsorbent for refrigeration and air conditioning applications. Appl. Therm. Eng. 2006, 26, 56–65. [Google Scholar] [CrossRef]
- Bu, X.; Wang, L.; Huang, Y. Effect of pore size on the performance of composite adsorbent. Adsorption 2013, 19, 929–935. [Google Scholar] [CrossRef]
- Daou, K.; Wang, R.Z.; Xia, Z.Z.; Yang, G.Z. Experimental comparison of the sorption and refrigerating performances of a CaCl2 impregnated composite adsorbent and those of the host silica gel. Int. J. Refrig. 2007, 30, 68–75. [Google Scholar] [CrossRef]
- Tanashev, Y.Y.; Krainov, A.V.; Aristov, Y.I. Thermal conductivity of composite sorbents “salt in porous matrix” for heat storage and transformation. Appl. Therm. Eng. 2013, 61, 401–407. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Huo, S.; Li, Z. Dynamics and isotherms of water vapor sorption on mesoporous silica gels modified by different salts. Kinet. Catal. 2010, 51, 754–761. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Sun, C.; Wu, Y. Fluidisable mesoporous silica composites for thermochemical energy storage. Energy 2023, 275, 127255. [Google Scholar] [CrossRef]
- Zhang, Y.; Palomba, V.; Frazzica, A. Development and characterization of LiCl supported composite sorbents for adsorption desalination. Appl. Therm. Eng. 2022, 203, 117953. [Google Scholar] [CrossRef]
- Zhang, Y.; Palamara, D.; Palomba, V.; Calabrese, L.; Frazzica, A. Performance analysis of a lab-scale adsorption desalination system using silica gel/LiCl composite. Desalination 2023, 548, 116278. [Google Scholar] [CrossRef]
- Xie, W.; Hua, W.; Zhang, X.; Xu, H.; Gao, L.; Zhang, L. Preparation and characteristics analysis of the new-type silica gel/CaCl2 adsorbents with nanoparticles for adsorption desalination and cooling system. J. Sol. Gel Sci. Technol. 2023, 105, 525–536. [Google Scholar] [CrossRef]
- Ali, S.M.; Chakraborty, A. Thermodynamic modelling and performance study of an engine waste heat driven adsorption cooling for automotive air-conditioning. Appl. Therm. Eng. 2015, 90, 54–63. [Google Scholar] [CrossRef]
- Gordeeva, L.G. New Materials for Thermochemical Energy Storage. Ph.D. Thesis, Boreskov Institute of Catalysis, Novosibirsk, Russia, 2000. [Google Scholar]
- Gordeeva, L.G.; Glaznev, I.S.; Aristov, Y.I. Sorption of water by sodium, copper, and magnesium sulfates dispersed into mesopores of silica gel and alumina. Russ. J. Phys. Chem. A 2003, 77, 1715–1720. [Google Scholar]
- Simonova, I.A.; Aristov, Y.I. Sorption properties of calcium nitrate dispersed in silica gel: The effect of pore size. Russ. J. Phys. Chem. A 2005, 79, 1307–1311. [Google Scholar]
- Simonova, I.A.; Freni, A.; Restuccia, G.; Aristov, Y.I. Water sorption on composite “silica modified by calcium nitrate”. Microporous. Mesoporous Mater. 2009, 122, 223–228. [Google Scholar] [CrossRef]
- Wu, H.; Wang, S.; Zhu, D. Effects of impregnating variables on dynamic sorption characteristics and storage properties of composite sorbent for solar heat storage. Sol. Energy 2007, 81, 864–871. [Google Scholar] [CrossRef]
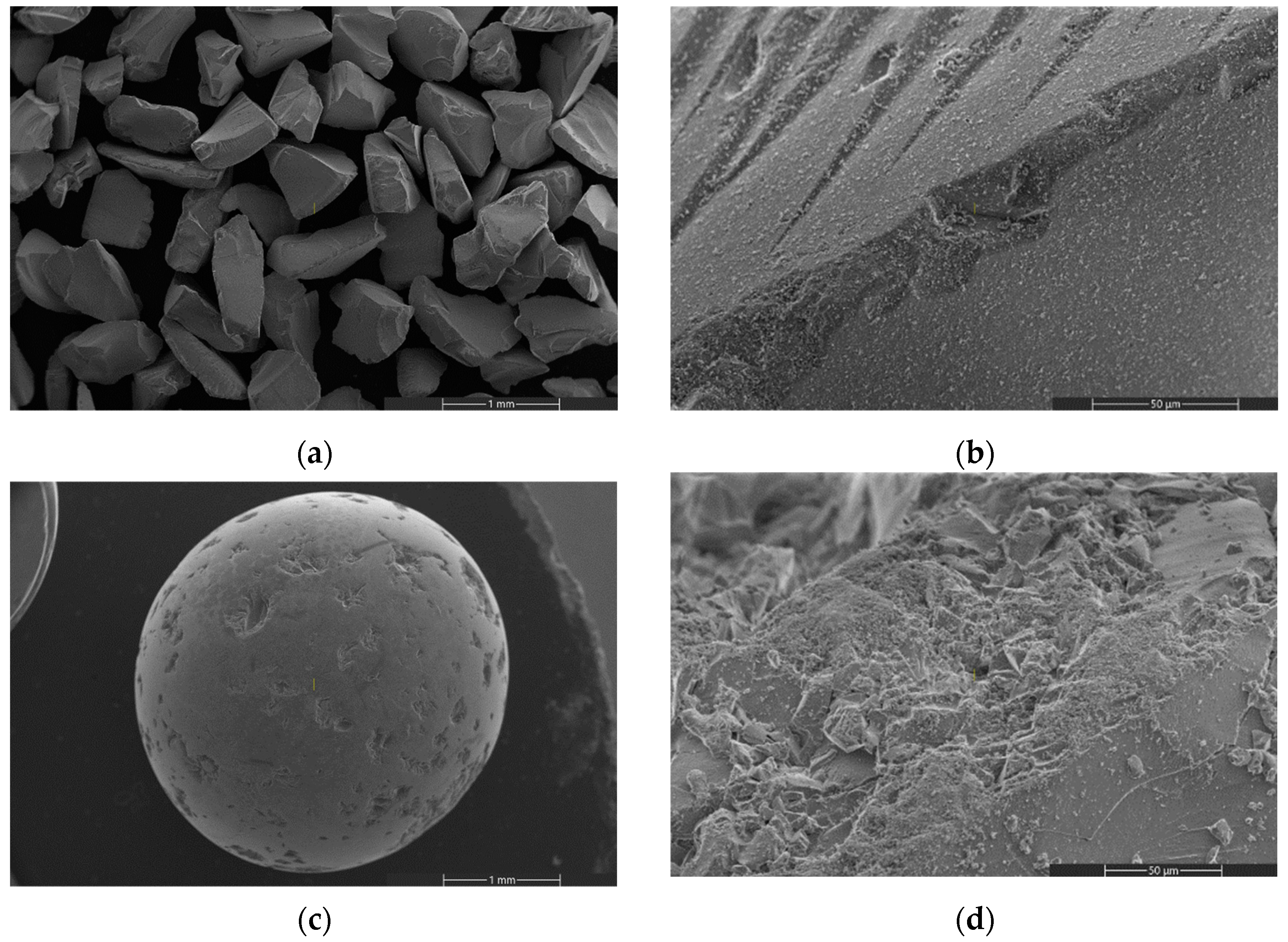
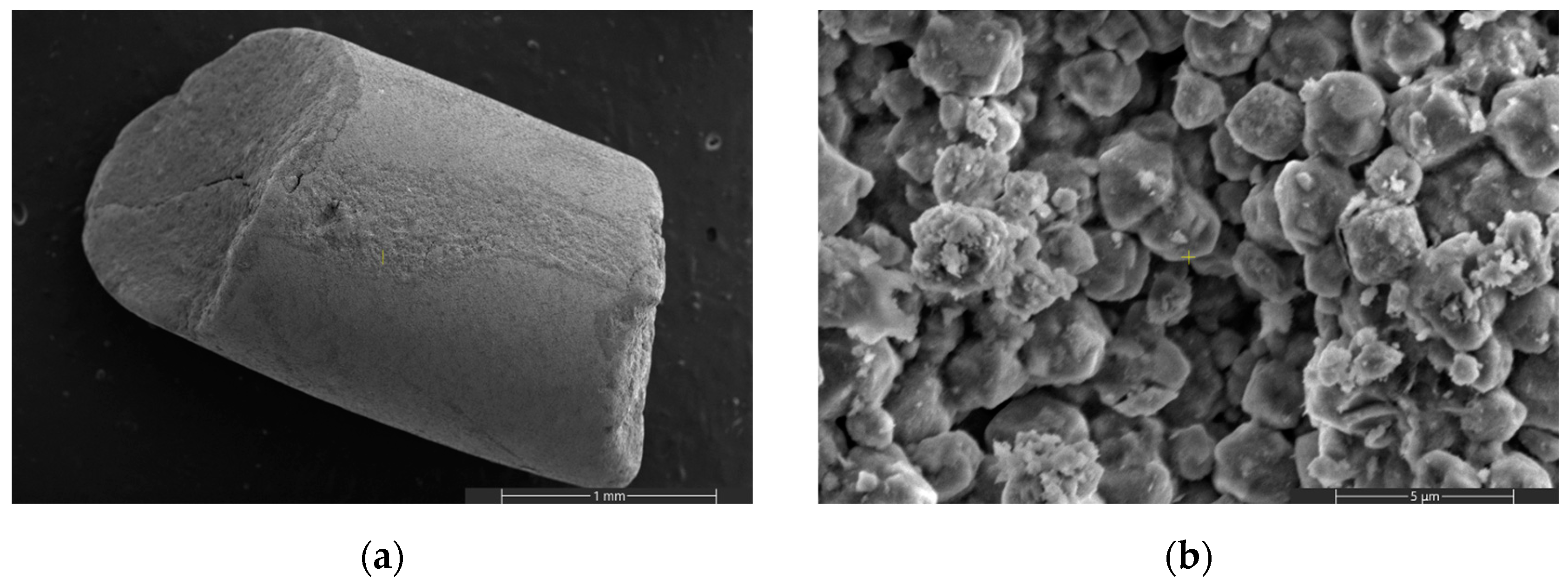
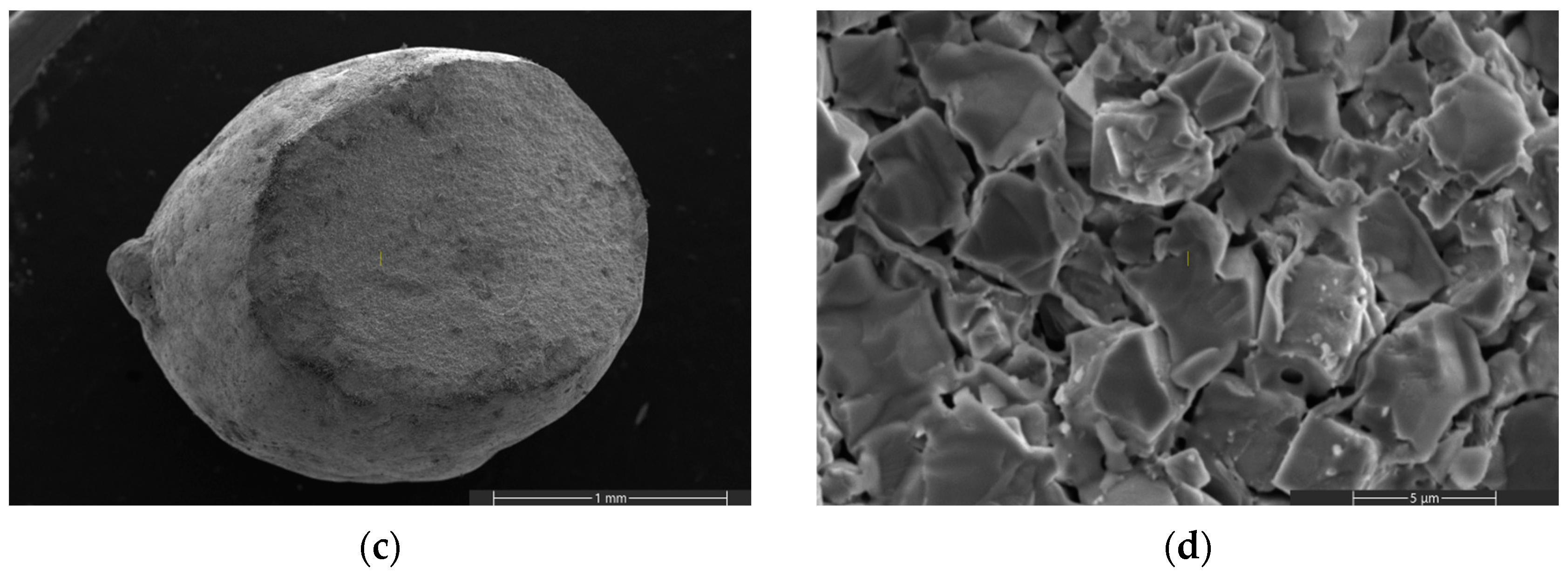
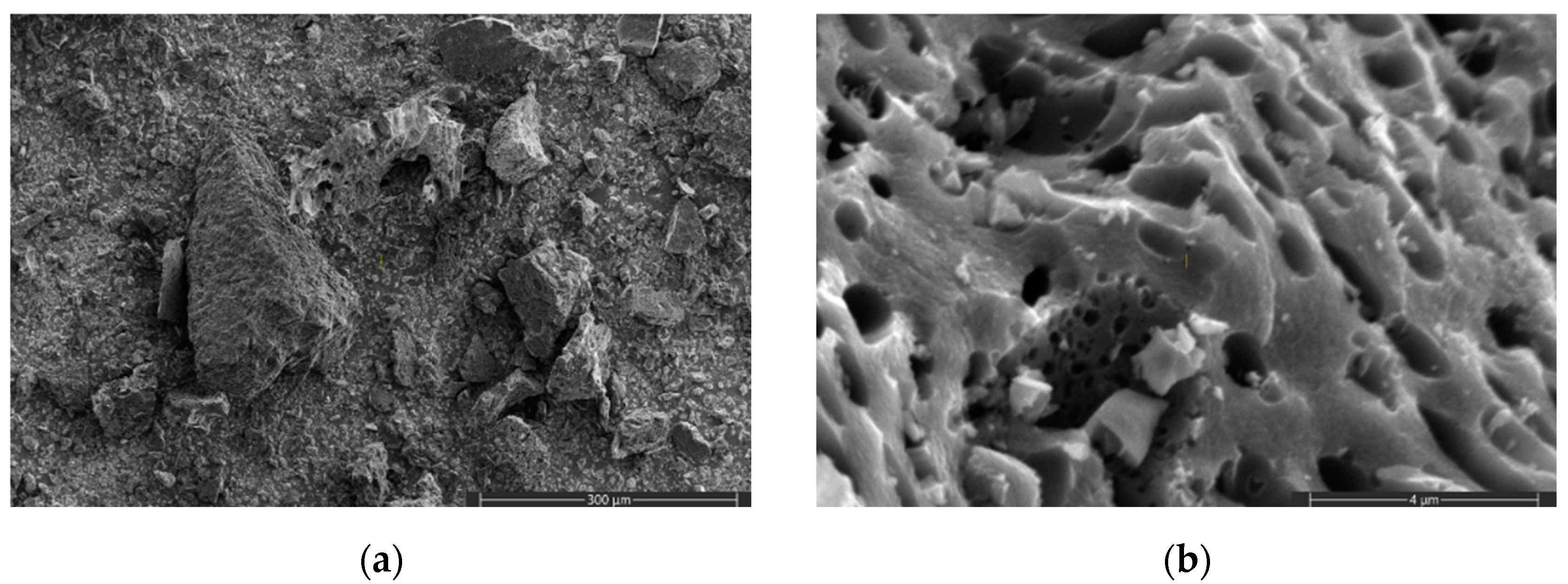
| Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Source | |
|---|---|---|---|
| Silica gel Fuji Davison Type A | 650 | 0.36 | [92] |
| Silica gel Fuji Davison Type 3A | 606 | 0.45 | [92] |
| Silica gel Fuji Davison Type RD | 650 | 0.35 | [92] |
| Silica gel Type-RD 2560, Fuji Silysia | 636.4 | 0.314 | [90] |
| Silica gel Type-A5BW, KD Corporation | 769.1 | 0.446 | [90] |
| Silica gel Type-A++, Mayekawa | 863.6 | 0.476 | [90] |
| Silica gel Davisil Grade 646 | 300 | 1.15 | [96] |
| Silica gel Grace SP2-8506 | 340 | 0.9 | [97] |
| Location | Working Pair | Cooling Capacity (kW) | COP (-) | Heat-Source Temperature (°C) | Source |
|---|---|---|---|---|---|
| Cracow, Poland | Silica gel– water | 1.1 | 0.6 | 75–85 | [75] |
| Tokyo, Japan | Silica gel– water | 3.54 | 0.34 | 55 | [99] |
| Shanghai, China | Silica gel– water | 8 | 0.4 | 85 | [100] |
| Messina, Italy | Silica gel– water | 10 | up to 0.6 | 65–85 | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowa, M.; Sztekler, K.; Mlonka-Mędrala, A.; Mika, Ł. An Overview of Developments In Silica Gel Matrix Composite Sorbents for Adsorption Chillers with Desalination Function. Energies 2023, 16, 5808. https://doi.org/10.3390/en16155808
Sowa M, Sztekler K, Mlonka-Mędrala A, Mika Ł. An Overview of Developments In Silica Gel Matrix Composite Sorbents for Adsorption Chillers with Desalination Function. Energies. 2023; 16(15):5808. https://doi.org/10.3390/en16155808
Chicago/Turabian StyleSowa, Marcin, Karol Sztekler, Agata Mlonka-Mędrala, and Łukasz Mika. 2023. "An Overview of Developments In Silica Gel Matrix Composite Sorbents for Adsorption Chillers with Desalination Function" Energies 16, no. 15: 5808. https://doi.org/10.3390/en16155808
APA StyleSowa, M., Sztekler, K., Mlonka-Mędrala, A., & Mika, Ł. (2023). An Overview of Developments In Silica Gel Matrix Composite Sorbents for Adsorption Chillers with Desalination Function. Energies, 16(15), 5808. https://doi.org/10.3390/en16155808







