The Modeling of Fuel Auto-Ignition Delay and Its Verification Using Diesel Engines Fueled with Oils with Standard or Increased Cetane Numbers
Abstract
1. Introduction
2. Modeling of Auto-Ignition Delay
2.1. Modeling of Single Droplet Auto-Ignition Delay
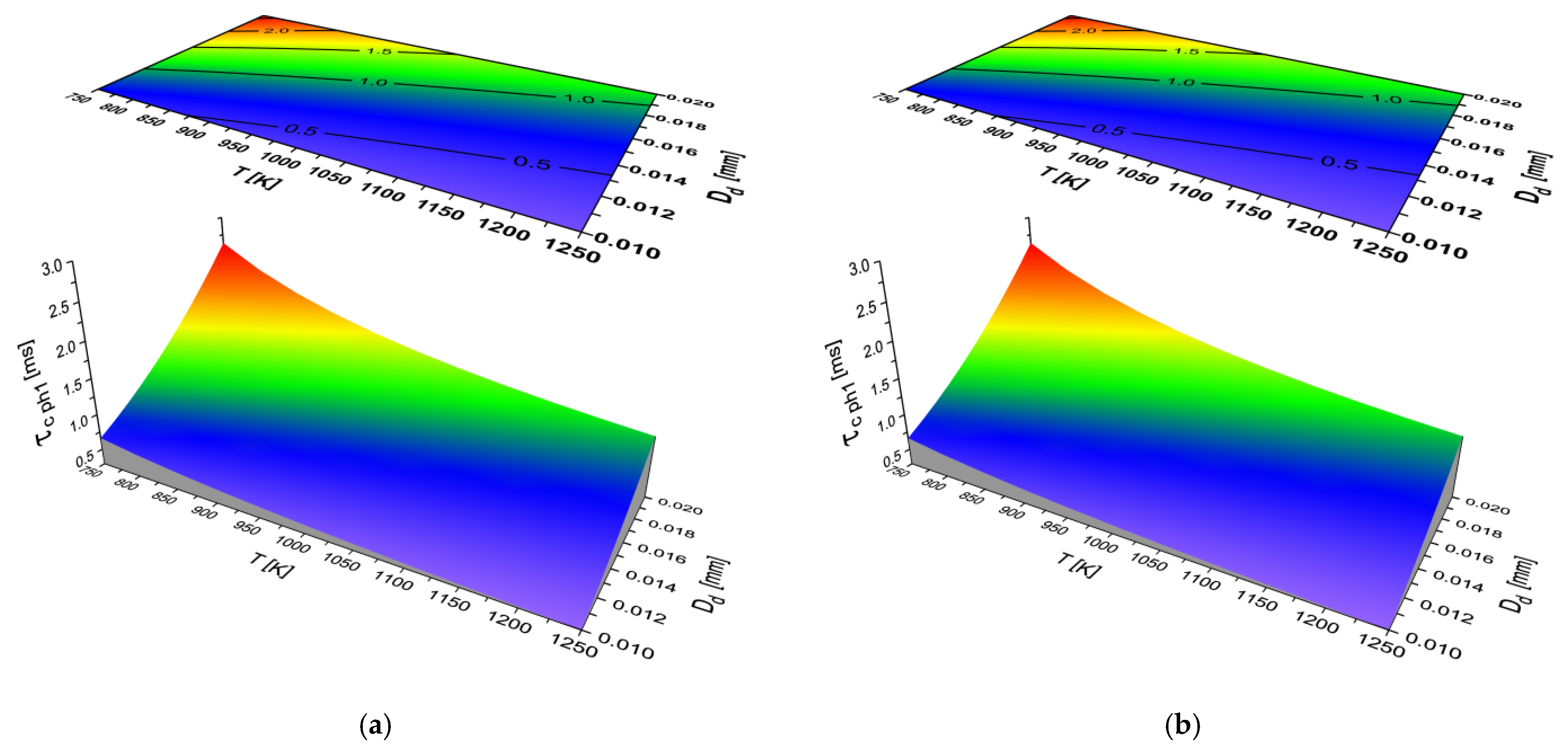
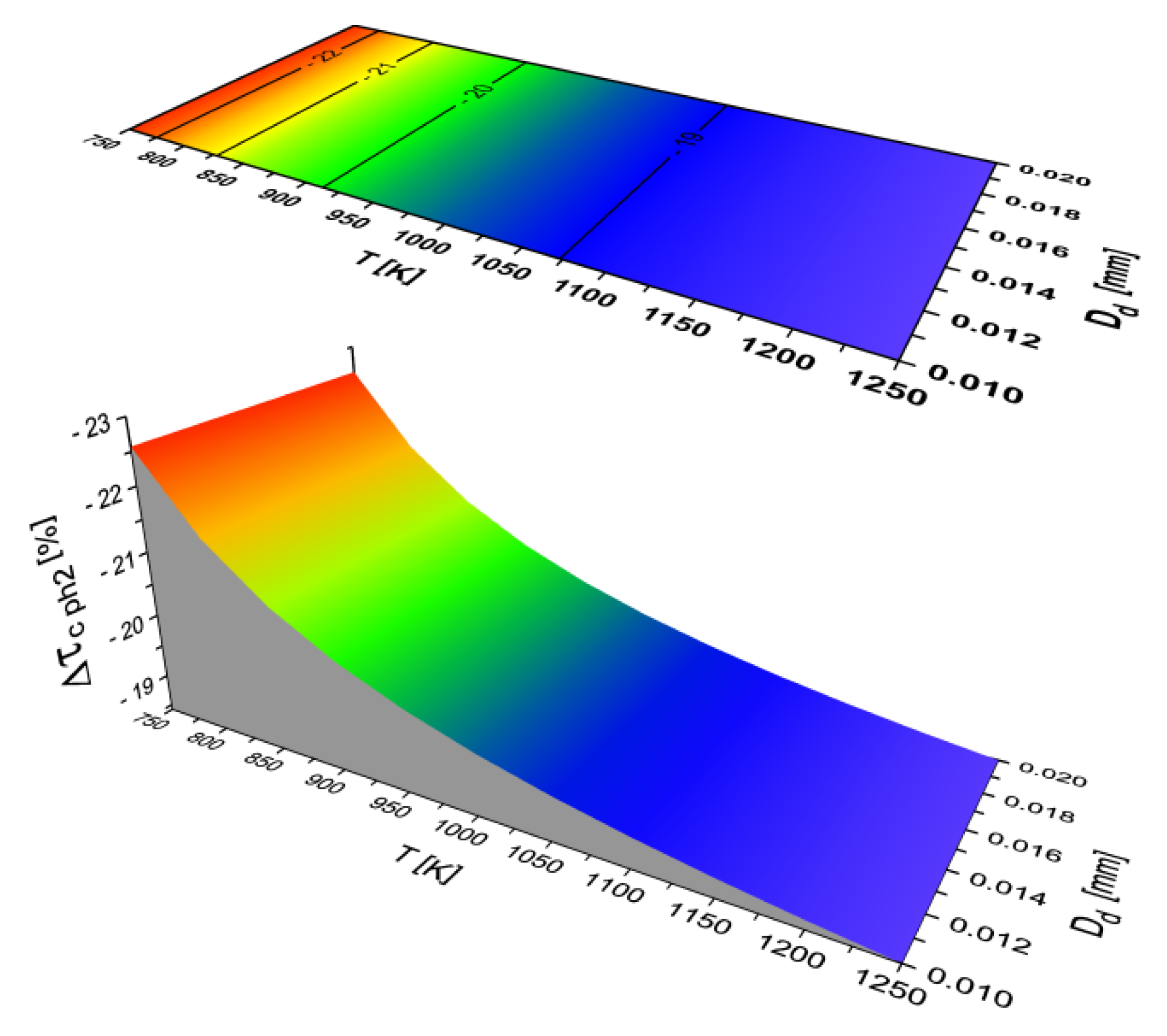
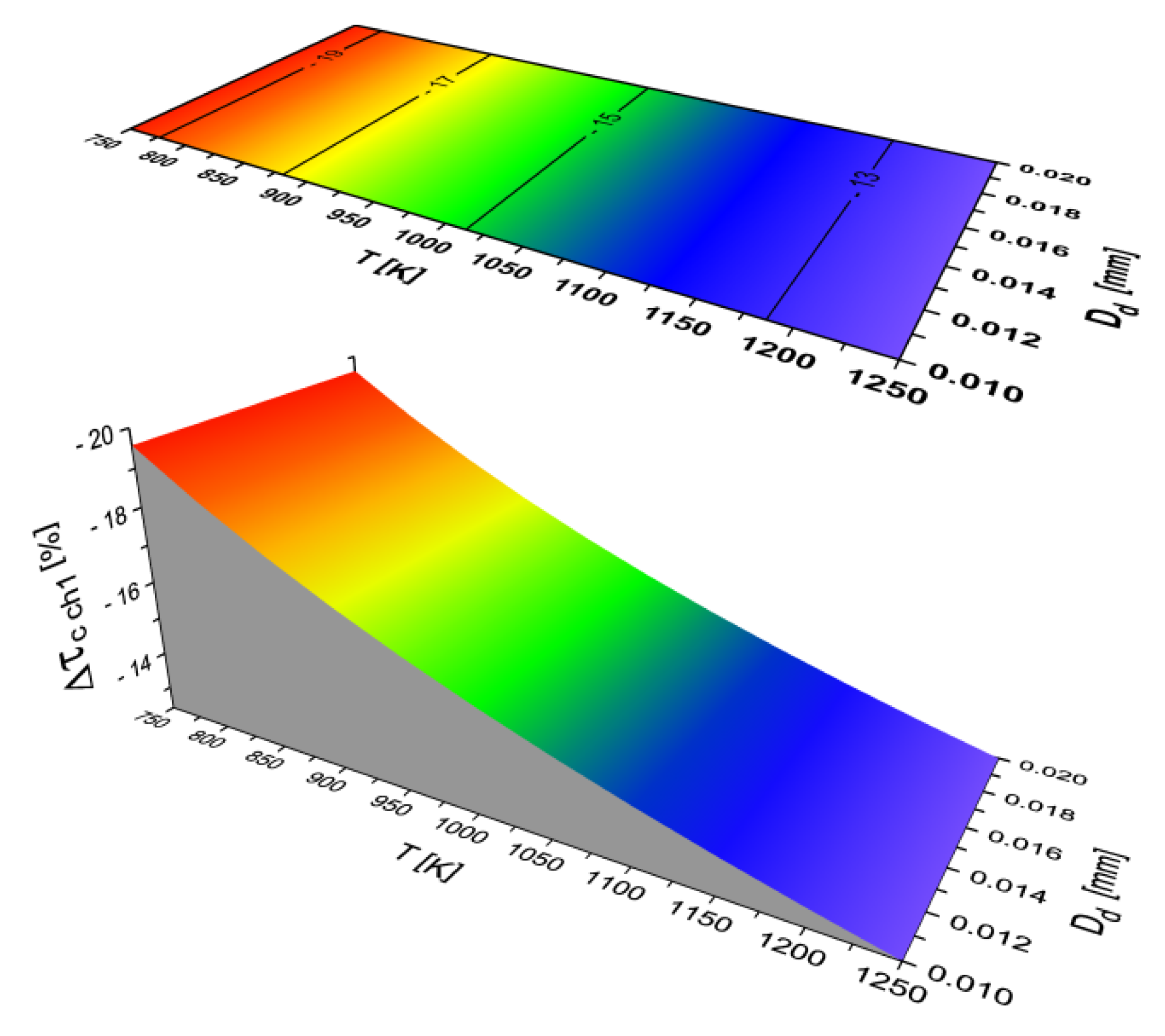
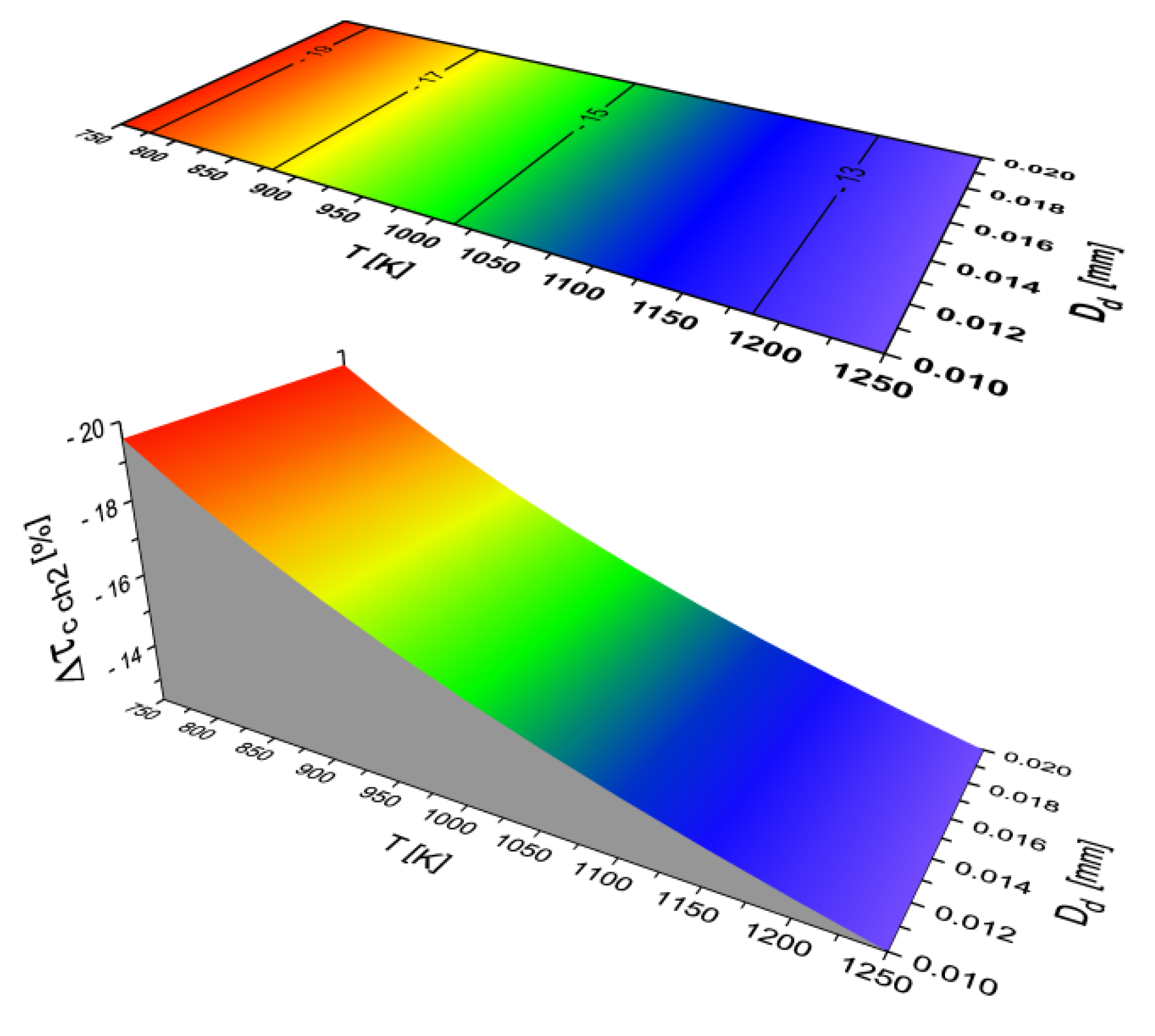
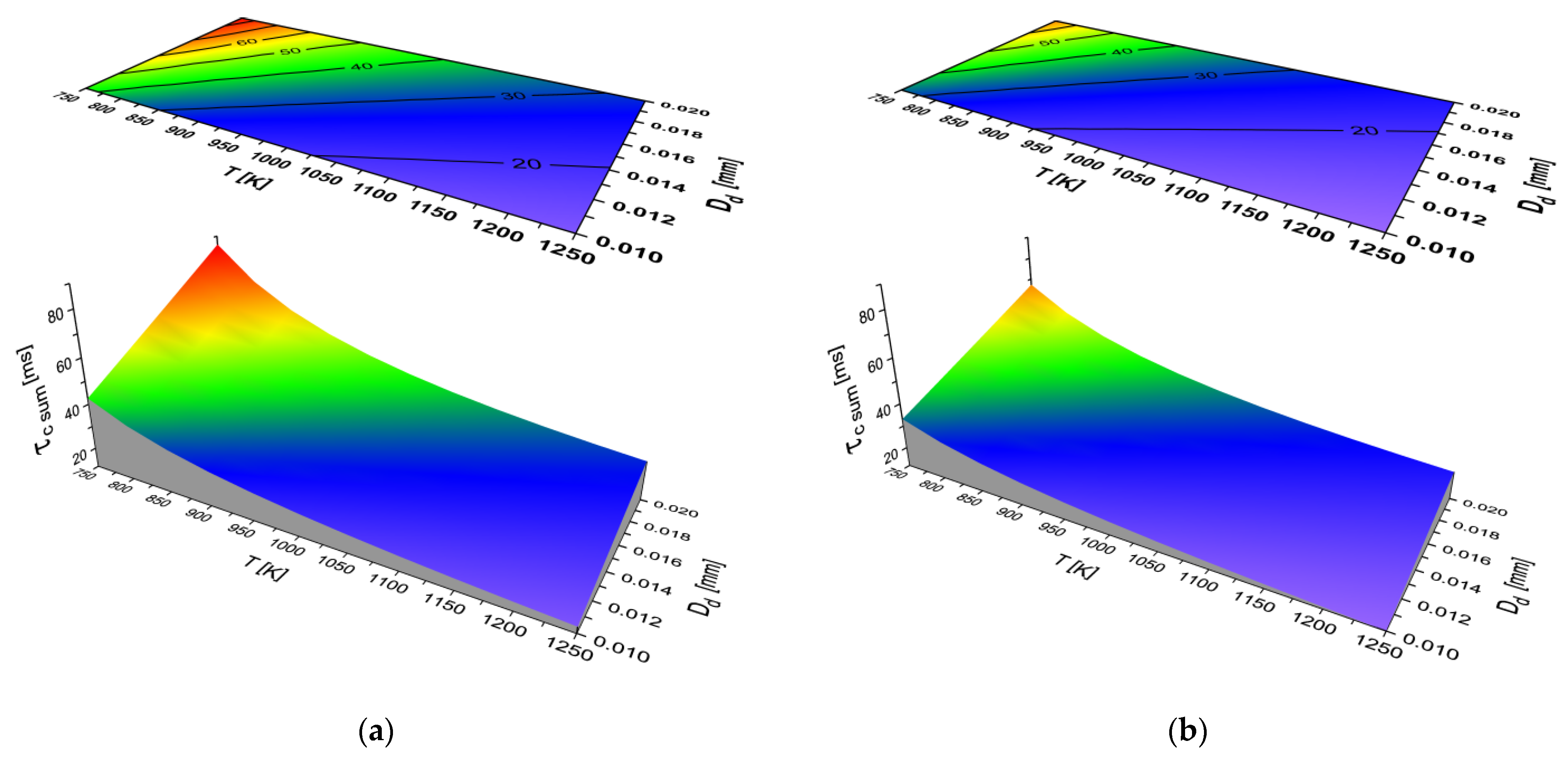
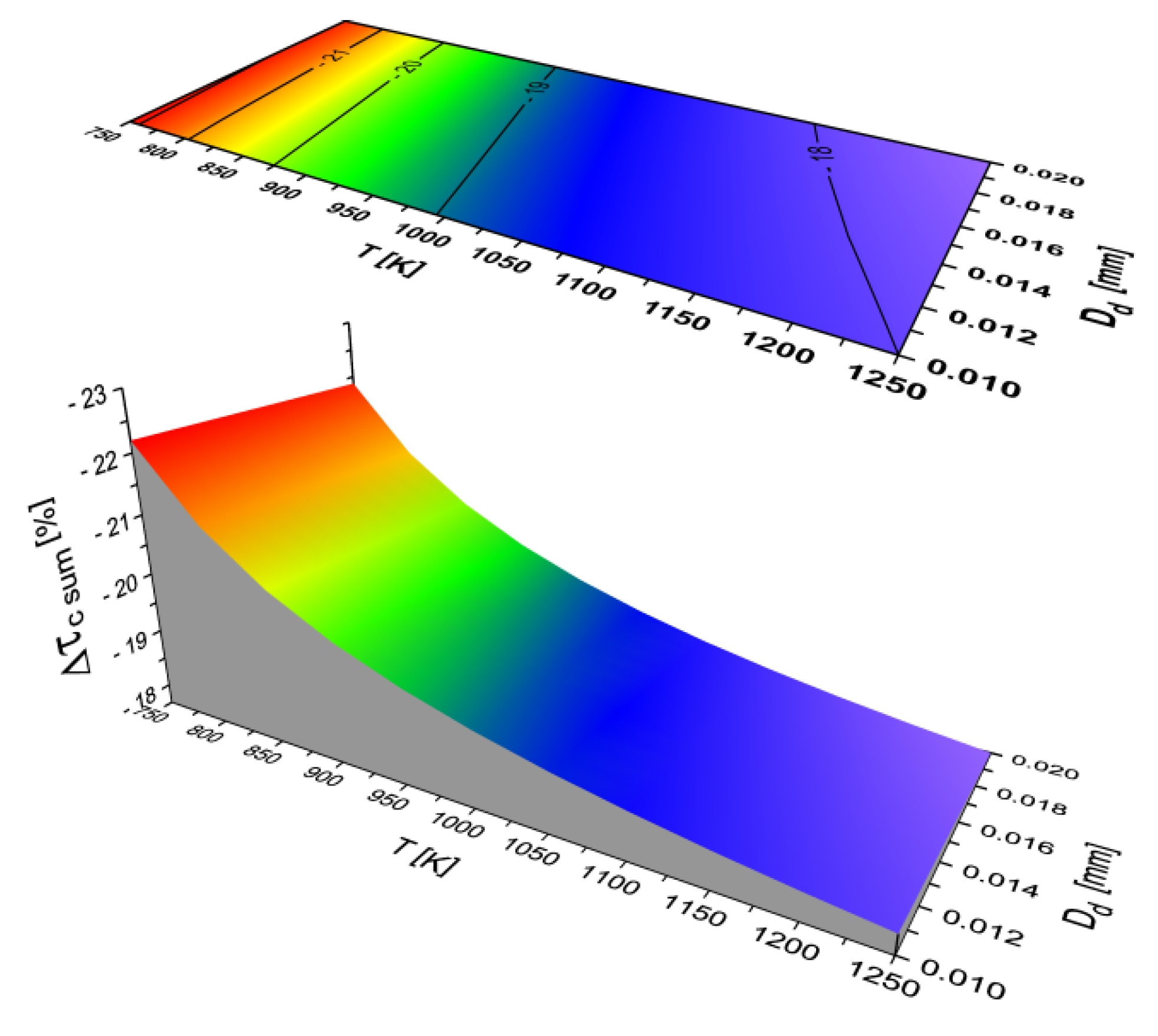
2.2. Results of Auto-Ignition Delay Modeling for Fuels with Different Cetane Number
| Fuel | DFB | DFKA | Rapeseed Oil | Ethanol | |
|---|---|---|---|---|---|
| Parameter | |||||
| Diesel fuels | Alternative fuels | ||||
| cetane numer CN [–] | 52 | 58 | 50 | 25 | |
| fuel boiling point [°C] | 150 | 150 | 200 | 78 | |
| temperature of auto-ignition [°C] | 330 | 300 | 357 | 425 | |
| original droplet temperature [°C] | 20 | 20 | 20 | 20 | |
| fuel density [kg/m3] | 833 | 833 | 930 | 798 | |
| mixture density [kg/m3] | 0.462 | 0.462 | 0.462 | 0.462 | |
| kinematic viscosity (20 °C) [mm2/s] | 4 | 4 | 70 | 1.5 | |
| surface tension (20 °C) [N/m] | 2.4 × 10−2 | 2.4 × 10−2 | 3.5 × 10−2 | 2.3 × 10−2 | |
| specific heat of the fuel [J/kg·°C] | 1930 | 1930 | 2390 | 1125 | |
| specific heat of the mixture [J/kg·°C] | 1030 | 1030 | 1030 | 1030 | |
| thermal conductivity of the fuel [W/m·°C] | 0.116 | 0.116 | 0.110 | 0.167 | |
| thermal conductivity in the mixture [W/m·°C] | 0.0416 | 0.0416 | 0.400 | 0.0416 | |
| latent heat of vaporization [J/kg] | 2.51 × 105 | 2.51 × 105 | 8.37 × 105 | 8.54 × 105 | |
| diffusion coefficient [m2/s] | 2.14 × 105 | 2.14 × 105 | 2.14 × 105 | 2.14 × 105 | |
| coefficient of volumetric expansion of the mixture (air, 250 °C) [1/°C] | 3.66 × 10−3 | 3.66 × 10−3 | 3.66 × 10−3 | 3.66 × 10−3 | |
| coefficient of kinematic expansion of the mixture (air, 250 °C) [m2/s] | 4.26 × 10−5 | 4.26 × 10−5 | 4.26 × 10−5 | 4.26 × 10−5 | |
| the Prandtl number of the mixture [–] | 0.688 | 0.688 | 0.688 | 0.688 | |
| activation energy [J/kmol] | 2.09 × 104 | 1.95 × 104 | 2.26 × 104 | 5.76 × 104 | |
3. Experimental Studies Verifying the Results of the Fuel Auto-Ignition Delay Modelling
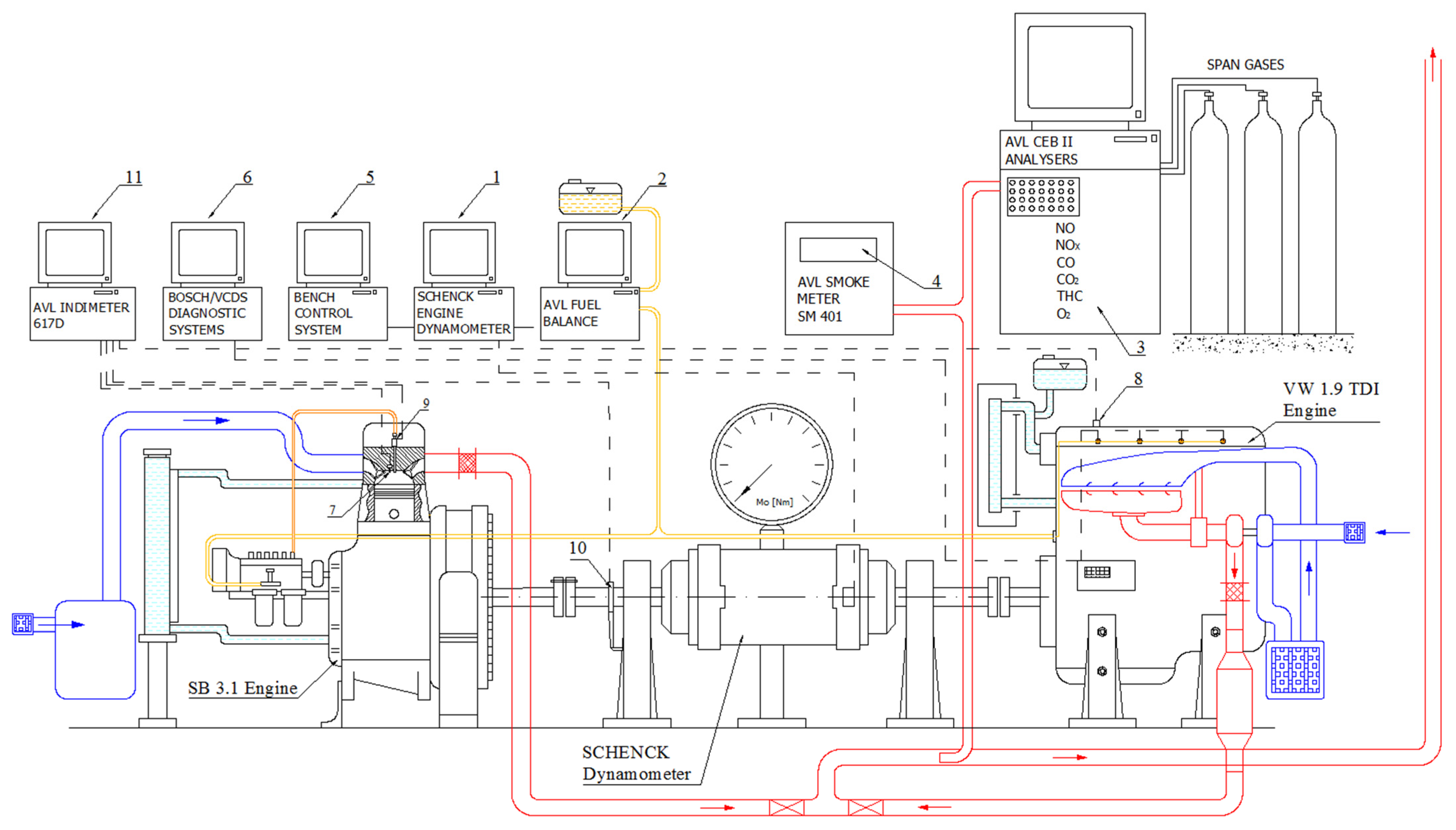
3.1. Methodology and Measuring Stand
3.2. Results of the Engine Tests of Auto-Ignition Delay for the Used Fuels
4. Summary and Conclusions
- The modeling of the difference in self-ignition delay (Δτc sum) of single drops of two hydrocarbon fuels (with different cetane numbers) is consistent with actual engine measurements, in a way sufficient for practical analysis of the τc of different fuels.
- DFKA fuel with an increased cetane number shortens the second physical and the first and second chemical stages relative to DFB fuel, leading to a significant, beneficial shortening of the total delay of self-ignition.
- The character of the function τc (T, Dd) is the same for all the analyzed fuels.
- The total self-ignition delay (τc sum) decreases for all the analyzed liquid fuels with an increase in the ambient temperature of the droplet and a decrease in the equivalent diameter of the fuel droplet.
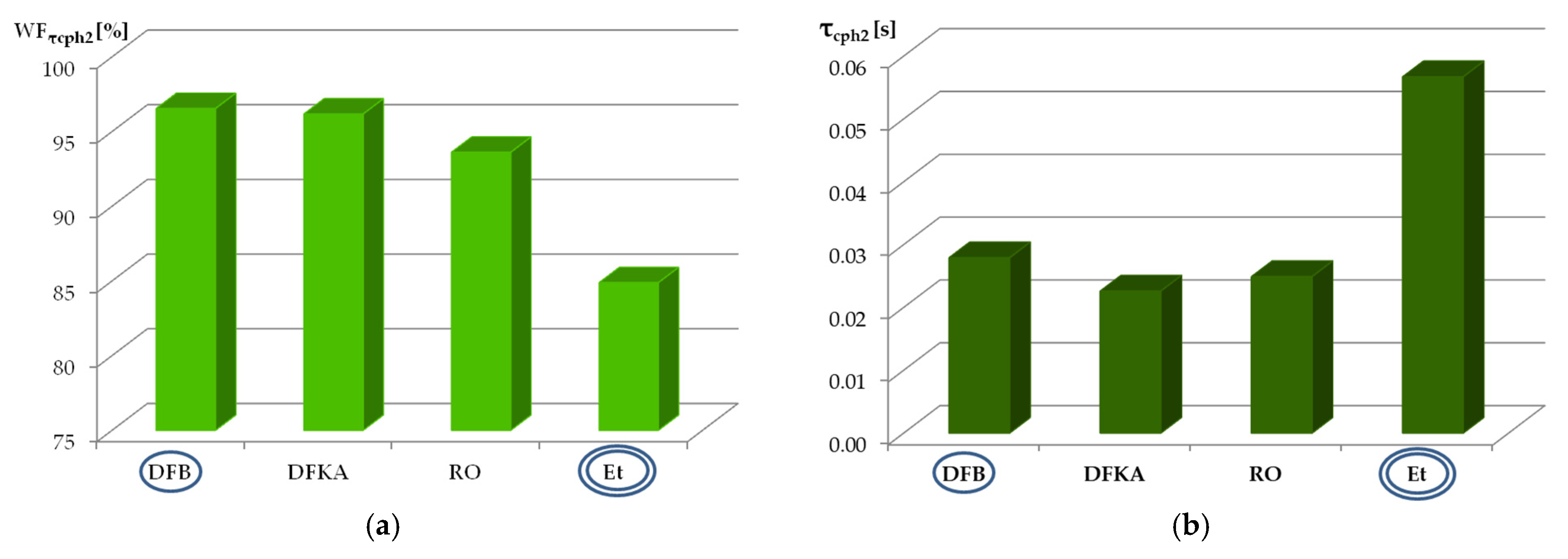
- The largest share in the total delay of self-ignition (τc) for all the modeled liquid fuels is taken by the second physical phase (τcph2)—the process of fuel evaporation.
- Both the physical parts of self-ignition delay (τcphi) depend on both the diameter and the ambient temperature of the fuel droplet. As the temperature increases and the diameter of the fuel droplet decreases, the time (τcphi) decreases.
- Both the chemical parts of self-ignition delay (τcchi) depend only on the ambient temperature of the fuel droplet (they do not depend on the diameter of the droplet). As the temperature increases, the time (τcchi) also decreases,
- In the case of using two different fuels, a decrease in the percentage share of any phase of the self-ignition delay (WFτi) does not necessarily mean that the time (τci) of this phase is shortened. An example is the WFτcph2 and τcph2 values for diesel fuel and ethanol.
- The self-ignition delay for a mixture of two fuels does not have to be the arithmetic mean of the delay of each of these fuels (an example is a mixture of diesel oil and ethanol).
- The difference in self-ignition delay (τc sum) for two different fuels need not be a constant value. Under some conditions of temperature and fuel droplet diameter, τc sum may be longer for the base fuel, whereas under other conditions τc sum will be shorter for this fuel compared with the second analyzed fuel. A typical example of this is the comparison of the total self-ignition delay for diesel oil and crude vegetable oil: with low ambient temperatures and large droplets, crude rapeseed oil shows a greater self-ignition delay than diesel oil, but at higher ambient temperatures (occurring at medium and high engine speeds and loads), rapeseed oil drops have a lower self-ignition delay compared with diesel oil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| τc | self-ignition delay (engine test), [s] |
| αsi | start of fuel injection, [deg a. TDC] |
| αsc | start of fuel combustion, [deg a. TDC] |
| τcph | the physical part of the self-ignition delay (modeling), [s] |
| τcch | the chemical part of the self-ignition delay (modeling), [s] |
| τc sum | self-ignition delay (modeling), [s] |
| τcph1 | fuel droplet heating time, [s] |
| τcph2 | evaporation time, [s] |
| τcch1 | time to the appearance of a low-temperature flame, [s] |
| τcch2 | time to the formation of an explosive flame, [s] |
| tf | original temperature of droplet, [°C] |
| tv | boiling point, [°C] |
| Q1 | amount of heat delivered to the surface of the fuel droplet, [J] |
| Qtk | heat used to raise the temperature of the droplet, [J] |
| Qp | heat needed to vaporize the fuel, [J] |
| τ | time, [s] |
| t | drop temperature, [°C] |
| t∞ | ambient temperature of the drop, [°C] |
| α | thermal conductivity of the droplet, [W/m2.°C] |
| L | latent heat of vaporization, [J/kg] |
| evaporation rate, [kg/s] | |
| ρf | fuel density, [kg/m3] |
| cf | specific heat of the fuel, [J/kg · °C] |
| d | droplet diameter, [m] |
| λm | thermal conductivity in the mixture, [W/m · °C] |
| Gr | Grashof number, [-] |
| g | gravitational acceleration, [m/s2] |
| βm | coefficient of volumetric expansion of the mixture, [1/°C] |
| νm | coefficient of kinematic expansion of the mixture, [m2/s] |
| Pr | Prandtl number, [-] |
| μm | viscosity coefficient of the mixture, [Pa · s] |
| ρm | mixture density, [kg/m3] |
| D | diffusion coefficient, [m2/s] |
| ωw | vapor concentration on the surface of the drop, [kg/kg] |
| ω∞ | vapor concentration around the droplet, [kg/kg] |
| Sc | Schmidt number = νm/D, [-] |
| Q2 | heat absorbed by the air mixture, [J] |
| Qtm | heat needed to raise the temperature of the mixture, [J] |
| Qd | heat received from the mixture due to mass transfer (diffusion), [J] |
| dm | diameter of the mixture area, [m] |
| αm | heat conductivity coefficient of the mixture, [W/m2.°C] |
| cm | specific heat of the mixture at constant pressure, [J/kg · °C] |
| τcph | physical delay of auto-ignition, [s] |
| p | pressure, [Pa] |
| E | activation energy (minimum energy required for a chemical reaction), [J/kmol] |
| R | gas constant = 6.28, [J/kmol] |
| T | ambient temperature of the drop, [°C, K] |
| DFB | diesel fuel (base) |
| DFKA | diesel fuel with kinetic additive |
| RO | rapeseed oil |
| Et | ethyl alcohol (ethanol) |
| CN | cetane number, [-] |
| WFτ | weighting factor for auto-ignition delay, [%] |
| TDC | top dead center of a piston |
| LDV | light duty vehicles |
| HDV | heavy duty vehicles |
References
- Hill, L. Global trends in emissions legislation. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Bielaczyc, P. Global trends in vehicular emissions regulations, current “hot topics” and their impact on powertrain technology—Introduction to the subject. In Proceedings of the VIII International PTNSS Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Joshi, A. Overview of technologies for near-zero tailpipe emissions from light- and heavy-duty vehicles. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Merkisz, J. Development trends in road transport powertrains. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Engeljehringer, K. LD vehicle emission challenge from legislation to testbed. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Bielaczyc, P.; Woodburn, J. Global trends in vehicular emissions legislation with a special focus on further stages (EU 7/VII and China 7/VII)—Associated development of ICE/hybrid/EV powertrain technologies and testing methodologies for LD, HD and NRMM sectors. In Proceedings of the IX International PTNSS Congress on Combustion Engines, Lublin, Poland, 27–28 June 2021. [Google Scholar]
- Neugebauer, S. Technological scenarios for the decarbonization of road transport. In Proceedings of the 30th International AVL Conference “Engine & Environment”, Graz, Austria, 7–8 June 2018. [Google Scholar]
- Winkler, S.L.; Anderson, J.E.; Garza, L. Vehicle criteria pollutant (PM, NOx, CO, HCs) emissions: How low should we go? npj Clim. Atmos Sci. 2018, 1, 26. [Google Scholar] [CrossRef]
- Bielaczyc, P. Global development of emissions reduction strategies from light duty vehicles. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 12139. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engines Fundamentals; McCraw-Hill Book Co.: New York, NY, USA, 1988. [Google Scholar]
- Kolakoti, A.; Rao, B. A Comprehensive Review of Biodiesel Application in IDI Engines with Property Improving Additives. i-manager’s J. Mech. Eng. 2015, 5, 35–45. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B. A comprehensive review of biodiesel as an alternative fuel for compression ignition engine. Renew. Sustain. Energy Rev. 2016, 57, 799–821. [Google Scholar] [CrossRef]
- Damanik, N.; Ong, H.C.; Tong, C.W.; Mahlia, T.M.I.; Silitonga, A.S. A review on the engine performance and exhaust emission characteristics of diesel engines fueled with biodiesel blends. Environ. Sci. Pollut. Res. 2018, 25, 15307–15325. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.; Nanthagopal, K.; Jeevanantham, A.; Bhowmick, P.; Malhotra, D.; Agarwal, P. An assessment of calophyllum inophyllum biodiesel fuelled diesel engine characteristics using novel antioxidant additives. Energy Convers. Manag. 2017, 148, 935–943. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Subbarao, R.; Johny, A.; Mohan, A.; Tamilarasu, A. Experimental studies on the effect of metal oxide and antioxidant additives with Calophyllum Inophyllum Methyl ester in compression ignition engine. J. Clean. Prod. 2017, 166, 474–484. [Google Scholar] [CrossRef]
- Pisac, C. An Experimental Study of Combustion Characteristics of Fatty Acid Methyl Ester Biodiesel. Ph.D. Dissertation, University of Hertfordshire, Hertfordshire, UK, 2014. [Google Scholar]
- Kale, P.; Ragit, S. An Overview of Ignition Delay and Combustion of Biodiesel Fuelled in CI Engine. In Proceedings of the International Conference On Emanations in Modern Technology and Engineering (ICEMTE-2017), Mumbai, India, 4–5 March 2017. [Google Scholar]
- Subramaniam, D.; Murugesan, A.; Avinash, A.; Kumaravel, A. Bio-diesel production and its engine characteristics—An expatiate view. Renew. Sustain. Energy Rev. 2013, 22, 361–370. [Google Scholar] [CrossRef]
- Yasin, M.M.; Yusaf, T.; Mamat, R.; Yusop, A.F. Characterization of a diesel engine operating with a small proportion of methanol as a fuel additive in biodiesel blend. Appl. Energy 2014, 114, 865–873. [Google Scholar] [CrossRef]
- Ong, H.; Mahlia, T.; Masjuki, H.; Norhasyima, R. Comparison of palm oil, Jatropha curcas and Calophyllum inophyllum for biodiesel: A review. Renew. Sustain. Energy Rev. 2011, 15, 3501–3515. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.; Mahlia, T.; Silitonga, A.; Chong, W.; Leong, K. Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers. Manag. 2014, 81, 30–40. [Google Scholar] [CrossRef]
- Pirouzfar, V.; Fayyazbakhsh, A. Diesel Fuel Additives; ECOpoint Inc.: St. Rifle, CO, USA, 2016. [Google Scholar]
- Li, H.; Lea-Langton, A.; Biller, P.; Andrews, G.E.; Hadavi, S.; Charlton, A.; Richards, P. Effect of Multifunctional Fuel Additive Package on Fuel Injector Deposit, Combustion and Emissions using Pure Rape Seed Oil for a DI Diesel. SAE Int. J. Fuels Lubr. 2009, 2, 54–65. [Google Scholar] [CrossRef]
- Imtenan, S.; Varman, M.; Masjuki, H.; Kalam, M.; Sajjad, H.; Arbab, M.; Fattah, I.R. Impact of low temperature combustion attaining strategies on diesel engine emissions for diesel and biodiesels: A review. Energy Convers. Manag. 2014, 80, 329–356. [Google Scholar] [CrossRef]
- Imtenan, S.; Masjuki, H.; Varman, M.; Fattah, I.R.; Sajjad, H.; Arbab, M. Effect of n-butanol and diethyl ether as oxygenated additives on combustion–emission-performance characteristics of a multiple cylinder diesel engine fuelled with diesel–jatropha biodiesel blend. Energy Convers. Manag. 2015, 94, 84–94. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Ong, H.; Mahlia, T.; Masjuki, H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Kawre, P.; Chaudhary, R.; Badholiya, S. Optimization of pme biodiesel and ethanol blended fuel to reduce co emission in diesel engine. Int. J. Eng. Sci. Res. Technol. 2018, 7, 155–173. [Google Scholar]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Sidik, N.A.C.; Azmi, W.H. The effect of combustion management on diesel engine emissions fueled with biodiesel-diesel blends. Renew. Sustain. Energy Rev. 2017, 73, 307–331. [Google Scholar] [CrossRef]
- Kumar, M.V.; Babu, A.V.; Kumar, P.R. The impacts on combustion, performance and emissions of biodiesel by using additives in direct injection diesel engine. Alex. Eng. J. 2017, 57, 509–516. [Google Scholar] [CrossRef]
- Raman, R.; Kumar, N. The utilization of n-butanol/diesel blends in Acetylene Dual Fuel Engine. Energy Rep. 2019, 5, 1030–1040. [Google Scholar] [CrossRef]
- Araya, K.; Yoshida, T. Analysis of ignitability under Low Temperature of Diesel Engines Using Sunflower Oil as a Renewable Fuel (Part 2). Ignition Lag of Injected Fuel Particles. J. Jpn. Soc. Agric. Mach. 1985, 47, 429–434. [Google Scholar]
- Tanazawa, S. Diesel Engines I; Sankaido Co.: Tokyo, Japan, 1956. [Google Scholar]
- Sitokei, G. Kraft Stoffaufbereitung und Verbrennung bei Dieselmotoren; Springer: Berlin, German, 1964. [Google Scholar]
- Obata, T. Fuels for Internal Combustion Engines; Yokendo Co.: Tokyo, Japan, 1966. [Google Scholar]
- Araya, K.; Tsunematsu, S. Single Droplet Combustion of Sunflower Oil; SAE-Papar 870590; SAE International: Warrendale, PA, USA, 1987. [Google Scholar]
- Koto, Y. Theory of Heat Transfer; Yokendo Co.: Tokyo, Japan, 1964. [Google Scholar]
- Kowalewicz, A. Podstawy Procesów Spalania; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 2000. [Google Scholar]
- Cisek, J. Wpływ Oleju Rzepakowego i Jego Mieszanin z Olejem Napędowym na Własności Silnika Wysokoprężnego. Ph.D. Thesis, Politechnika Krakowska, Kraków Polska, 1998. [Google Scholar]
- Lu, G.-B.; Yang, T.; Chen, L.-P.; Zhou, Y.-S.; Chen, W.-H. Thermal decomposition kinetics of 2-ethylhexyl nitrate under non-isothermal and isothermal conditions. J. Therm. Anal. Calorim. 2016, 124, 471–478. [Google Scholar] [CrossRef]
- Bornemann, H.; Scheidt, F.; Sander, W. Thermal decomposition of 2-ethylhexyl nitrate (2-EHN). Int. J. Chem. Kinet. 2002, 34, 34–38. [Google Scholar] [CrossRef]
- Almodovar, C.A.; Goldsmith, C.F. Laser schlieren study of the thermal decomposition of 2-ethylhexyl-nitrate. Proc. Combust. Inst. 2021, 38, 997–1005. [Google Scholar] [CrossRef]
- Kusy, P. Transestryfication of Vegetable Oils for Fuels. In Proceedings of the ASAE International Conference, Fargo, North Dakota, 2–4 August 1982. [Google Scholar]
- Tahir, A.R. Sunflower Oil as a Fuel for Compression Ignition Engines. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 1982. [Google Scholar]
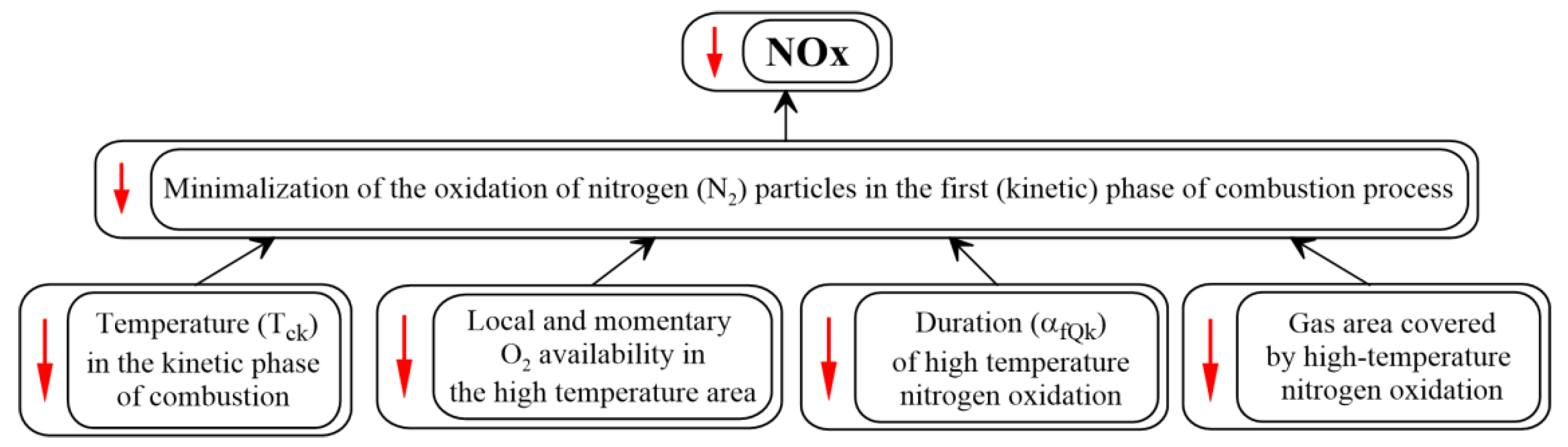
- Cisek, J.; Borowski, A. Sposób Zmniejszania Stężenia I Emisji Tlenków Azotu w Spalinach Silnika o Zapłonie Samoczynnym; Zgłoszenie nr. P.443866; Urząd Patentowy Rzeczpospolitej Polskiej: Warszawa, Polska, 2023. [Google Scholar]
- Cisek, J.; Lesniak, S.; Stanik, W.; Przybylski, W. The Synergy of Two Biofuel Additives on Combustion Process to Simultaneously Reduce NOx and PM Emissions. Energies 2021, 14, 2784. [Google Scholar] [CrossRef]
- Cisek, J.; Leśniak, S.; Borowski, A.; Przybylski, W.; Mokretskyy, V. Visualisation and Thermovision of Fuel Combustion Affecting Heat Release to Reduce NOx and PM Diesel Engine Emissions. Energies 2022, 15, 4882. [Google Scholar] [CrossRef]








| Engine Type | SB 3.1 (Research Engine) | VW 1.9 TDI (Serial Engine) | |
|---|---|---|---|
| Parameter | |||
| combustion system | direct fuel injection to open combustion chamber in piston | direct fuel injection to open combustion chamber in piston | |
| fuel supply system | piston injection pump | unit injectors | |
| injector | hydraulic | electromagnetic | |
| sprayer | 4-hole, φ = 0.35 mm | 5-hole, φ = 0.20 mm | |
| max. fuel injection pressure | 95 MPa | 200 Mpa | |
| air supply system | undercharged | turbocharged with intercooler | |
| displacement | 1850 cm3 | 1896 cm3 | |
| number of cylinders | 1 | 4 | |
| piston diameter | 127.0 mm | 79.5 mm | |
| piston stroke | 146.0 mm | 95.5 mm | |
| compression ratio | 15.75 | 18.00 | |
| rated power | 23 kW | 85 kW | |
| engine speed at max. power | 2200 rpm | 4000 rpm | |
| maximum engine torque | 110 Nm | 285 Nm | |
| engine speed at max. torque | 1600 rpm | 1900 rpm | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisek, J.; Leśniak, S. The Modeling of Fuel Auto-Ignition Delay and Its Verification Using Diesel Engines Fueled with Oils with Standard or Increased Cetane Numbers. Energies 2023, 16, 5273. https://doi.org/10.3390/en16145273
Cisek J, Leśniak S. The Modeling of Fuel Auto-Ignition Delay and Its Verification Using Diesel Engines Fueled with Oils with Standard or Increased Cetane Numbers. Energies. 2023; 16(14):5273. https://doi.org/10.3390/en16145273
Chicago/Turabian StyleCisek, Jerzy, and Szymon Leśniak. 2023. "The Modeling of Fuel Auto-Ignition Delay and Its Verification Using Diesel Engines Fueled with Oils with Standard or Increased Cetane Numbers" Energies 16, no. 14: 5273. https://doi.org/10.3390/en16145273
APA StyleCisek, J., & Leśniak, S. (2023). The Modeling of Fuel Auto-Ignition Delay and Its Verification Using Diesel Engines Fueled with Oils with Standard or Increased Cetane Numbers. Energies, 16(14), 5273. https://doi.org/10.3390/en16145273




