Viable Recycling of Polystyrene via Hydrothermal Liquefaction and Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Liquefaction (HTL)
2.3. Pyrolysis
2.4. Product Characterization
3. Results and Discussion
3.1. PS HTL
3.1.1. Degradation of PS during the HTL
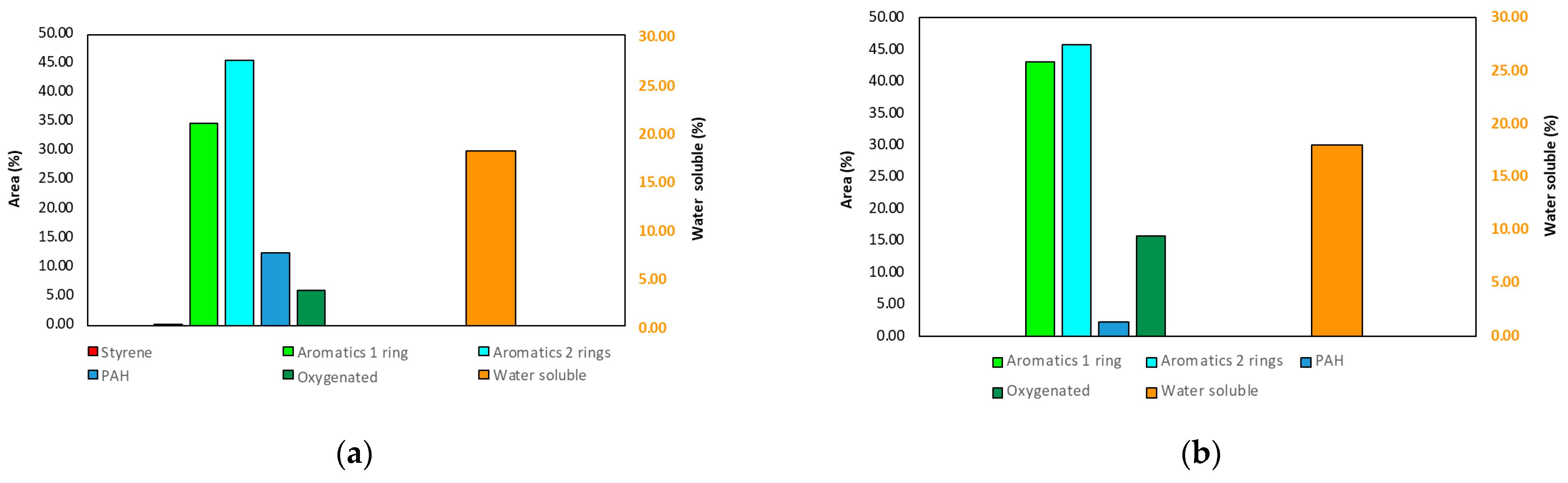
3.1.2. Characterization of the Liquid Products of PS HTL
3.1.3. Characterization of the Solid Product of PS HTL
3.2. PS Pyrolysis
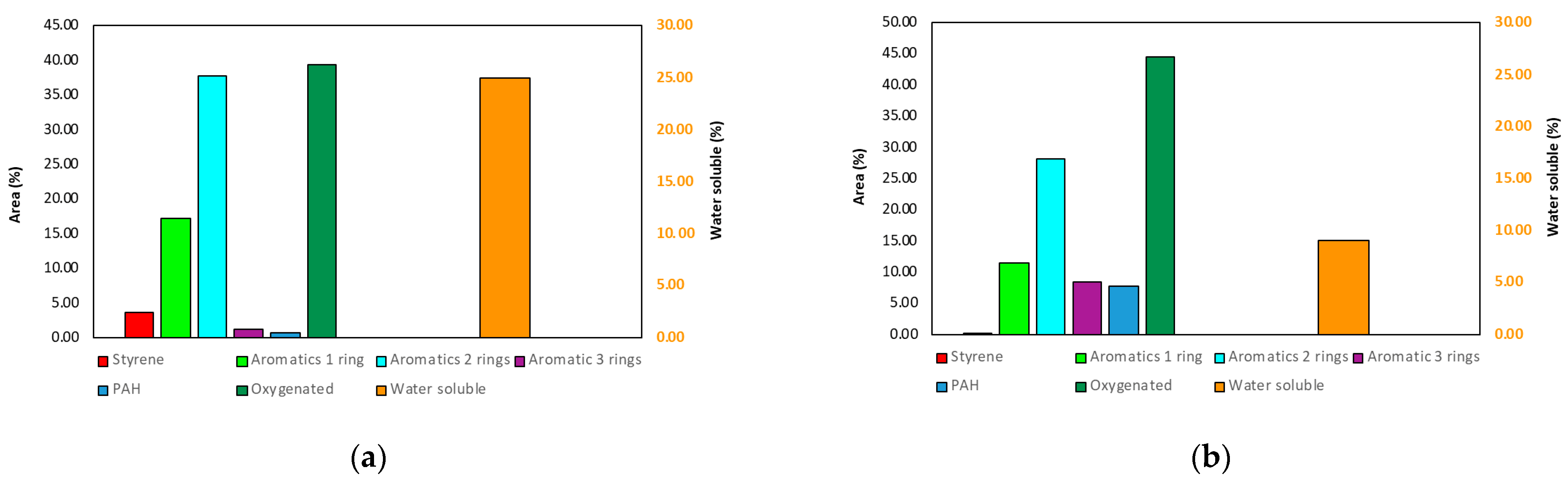
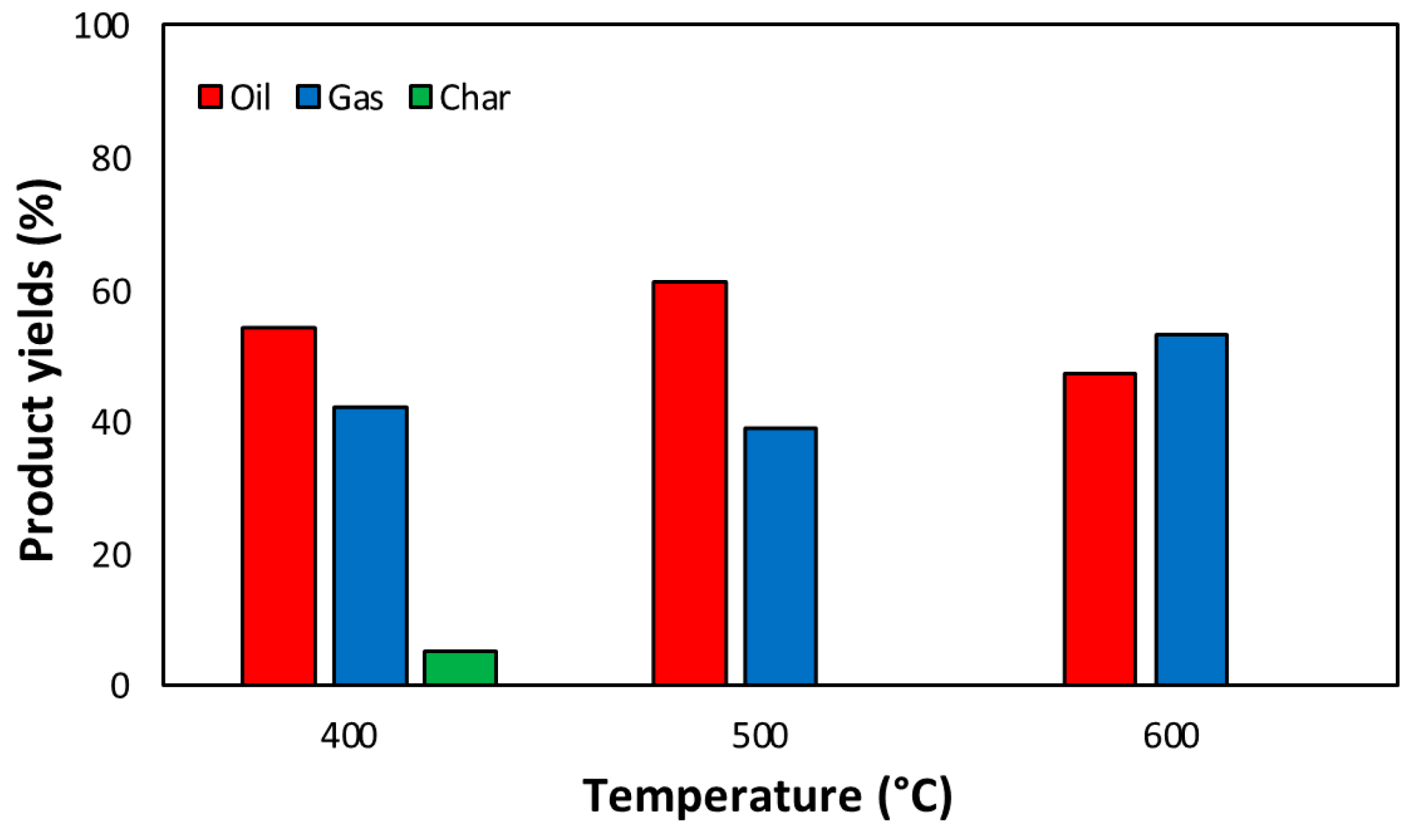
3.2.1. Product Distribution of PS Pyrolysis
3.2.2. Chemical Composition of PS Pyrolysis Oil
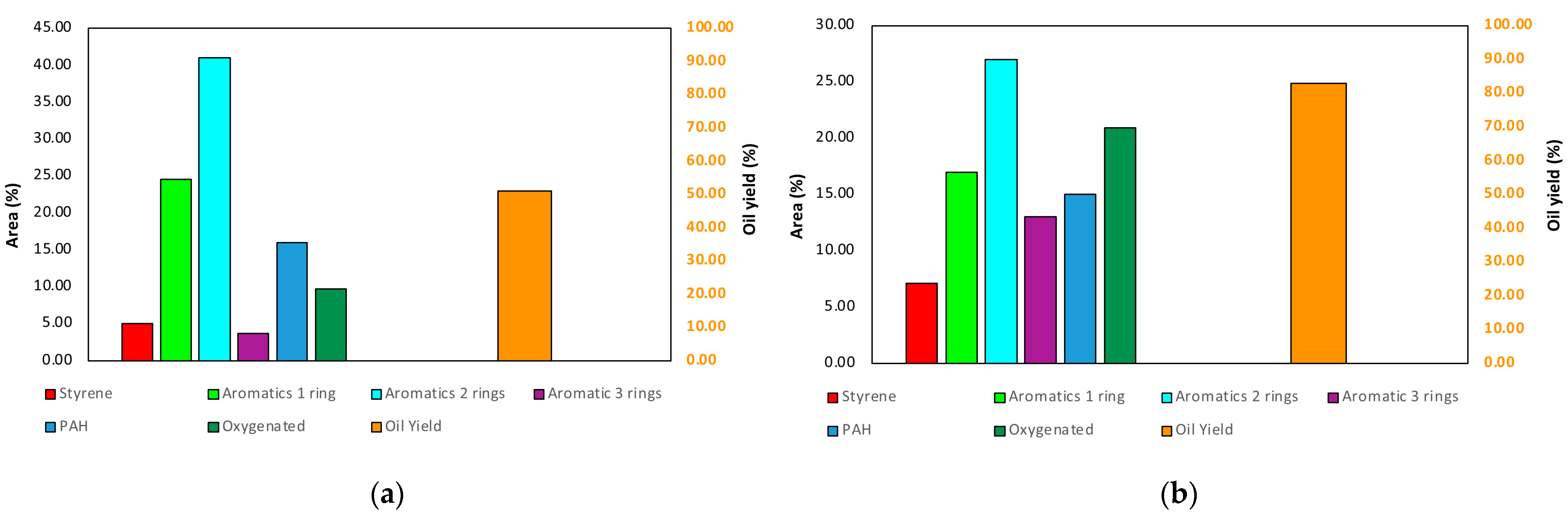
3.3. Pyrolysis vs. HTL: Summary
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jahirul, M.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Hasan, M.M.; Hazrat, M.A. Transport fuel from waste plastics pyrolysis—A review on technologies, challenges and opportunities. Energy Convers. Manag. 2022, 258, 115451. [Google Scholar] [CrossRef]
- Queiroz, A.; Pedroso, G.B.; Kuriyama, S.N.; Fidalgo-Neto, A.A. Subcritical and supercritical water for chemical recycling of plastic waste. Curr. Opin. Green Sustain. Chem. 2020, 25, 100364. [Google Scholar] [CrossRef]
- Phanisankar, B.S.S.; Vasudeva Rao, N.; Manikanta, J.E. Conversion of waste plastic to fuel products. Mater Today Proc. 2020, 33, 5190–5195. [Google Scholar] [CrossRef]
- Okoro, O.V.; Faloye, F.D. Comparative Assessment of Thermo-Syngas Fermentative and Liquefaction Technologies as Waste Plastics Repurposing Strategies. AgriEngineering 2020, 2, 378–392. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Zhang, C.; Xie, Y.; Wang, Y.; Wang, J.; Zhang, R. Aromatic hydrocarbons production and synergistic effect of plastics and biomass via one-pot catalytic co-hydropyrolysis on HZSM-5. J. Anal. Appl. Pyrolysis 2020, 147, 104800. [Google Scholar] [CrossRef]
- Seshasayee, M.S.; Savage, P.E. Oil from plastic via hydrothermal liquefaction: Production and characterization. Appl. Energy 2020, 278, 115673. [Google Scholar] [CrossRef]
- Baeyens, J.; Brems, A.; Dewil, R. Recovery and recycling of post-consumer waste materials. Part 2. Target wastes (glass beverage bottles, plastics, scrap metal and steel cans, end-of-life tyres, batteries and household hazardous waste). Int. J. Sustain. Eng. 2010, 3, 232–245. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Laredo, G.C.; Reza, J.; Meneses Ruiz, E. Hydrothermal liquefaction processes for plastics recycling: A review. Clean. Chem. Eng. 2023, 5, 100094. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, G.; Shah, A.; Iqbal, M.; Khan, R.A.; Sirajuddin; Anwar, F.; Ullah, R.; Akhter, M.S. Fuel production from waste polystyrene via pyrolysis: Kinetics and products distribution. Waste Manag. 2019, 88, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Tai, L.; Musivand, S.; de Caprariis, B.; Damizia, M.; Hamidi, R.; Ma, W.; De Filippis, P. Co-treatment of plastics with subcritical water for valuable chemical and clean solid fuel production. J. Clean. Prod. 2022, 337, 130529. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Jin, L.; Ni, P.; Liu, G.; He, H.; Zhang, J.; Dong, J.; Ruan, R. Catalytic hydrothermal depolymerization of nylon 6. J. Mater. Cycles Waste Manag. 2010, 12, 321–325. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Faravelli, T.; Pinciroli, M.; Pisano, F.; Bozzano, G.; Dente, M.; Ranzi, E. Thermal degradation of polystyrene. J. Anal. Appl. Pyrolysis 2001, 60, 103–121. [Google Scholar] [CrossRef]
- Park, K.B.; Jeong, Y.S.; Guzelciftci, B.; Kim, J.S. Two-stage pyrolysis of polystyrene: Pyrolysis oil as a source of fuels or benzene, toluene, ethylbenzene, and xylenes. Appl. Energy 2020, 259, 114240. [Google Scholar] [CrossRef]
- Ge, S.; Shi, Y.; Xia, C.; Huang, Z.; Manzo, M.; Cai, L.; Ma, H.; Zhang, S.; Jiang, J.; Sonne, C.; et al. Progress in pyrolysis conversion of waste into value-added liquid pyro-oil, with focus on heating source and machine learning analysis. Energy Convers. Manag. 2021, 245, 114638. [Google Scholar] [CrossRef]
- Rahman, W.U.; Patel, M.; Kurian, V.; Kumar, A. A comparative techno-economic assessment of fast pyrolysis, hydrothermal liquefaction, and intermediate pyrolysis of municipal solid waste for liquid transportation fuels production. Energy Convers. Manag. 2022, 267, 115877. [Google Scholar] [CrossRef]
- Larrain, M.; Van Passel, S.; Thomassen, G.; Kresovic, U.; Alderweireldt, N.; Moerman, E.; Billen, P. Economic performance of pyrolysis of mixed plastic waste: Open-loop versus closed-loop recycling. J. Clean. Prod. 2020, 270, 122442. [Google Scholar] [CrossRef]
- Haig, S.; Morrish, L.; Morton, R.; Onwuamaegbu, U.; Speller, P.; Wilkinson, S. Plastic to Oil IFM002 Final Report 2. Available online: www.zerowastescotland.org.uk (accessed on 27 January 2023).
- Savoldelli, J.; Tomback, D.; Savoldelli, H. Breaking down polystyrene through the application of a two-step thermal degradation and bacterial method to produce usable byproducts. Waste Manag. 2017, 60, 123–126. [Google Scholar] [CrossRef]
- dos Passos, J.S.; Glasius, M.; Biller, P. Screening of common synthetic polymers for depolymerization by subcritical hydrothermal liquefaction. Process. Saf. Environ. Prot. 2020, 139, 371–379. [Google Scholar] [CrossRef]
- Shin, H.Y.; Bae, S.Y. Thermal decomposition of polystyrene in supercritical methanol. J. Appl. Polym. Sci. 2008, 108, 3467–3472. [Google Scholar] [CrossRef]
- Karaduman, A. Pyrolysis of Polystyrene Plastic Wastes with Some Organic Compounds for Enhancing Styrene Yield. Energy Sources 2010, 24, 667–674. [Google Scholar] [CrossRef]
- Maryudi Salamah, S.; Aktawan, A. Product distribution of pyrolysis of polystyrene foam waste using catalyst of natural zeolite and nickel/silica. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012012. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; El-Fatah Abomohra, A.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manag. 2019, 89, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, H.; Hentschel, J.; Hornung, A.; Hornung, U. Environmental engineering: Stepwise pyrolysis of plastic waste. Chem. Eng. Sci. 1999, 54, 3043–3051. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Feitosa, L.F.; Berhault, G.; Laurenti, D.; Teixeira Da Silva, V. Effect of the Nature of the Carbon Support on the Guaiacol Hydrodeoxygenation Performance of Nickel Phosphide: Comparison between Carbon Nanotubes and a Mesoporous Carbon Support. Ind. Eng. Chem. Res. 2019, 58, 16164–16181. [Google Scholar] [CrossRef]
- Park, Y.; Hool, J.N.; Curtis, C.W.; Roberts, C.B. Depolymerization of styrene-butadiene copolymer in near-critical and supercritical water. Ind Eng Chem Res. 2001, 40, 756–767. [Google Scholar] [CrossRef]
- Kwak, H.; Shin, H.Y.; Bae, S.Y.; Kumazawa, H. Characteristics and kinetics of degradation of polystyrene in supercritical water. J. Appl. Polym. Sci. 2006, 101, 695–700. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, N.; Maafa, I.M.; Ahmed, U.; Akhter, P.; Shehzad, N.; Amjad, U.-E.; Hussain, M. Thermal conversion of polystyrene plastic waste to liquid fuel via ethanolysis. Fuel 2020, 279, 118498. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, Y.; Zhan, L.; Xie, B.; Gao, B.; Wang, J. Hydrothermal Treatment of E-Waste Plastics for Tertiary Recycling: Product Slate and Decomposition Mechanisms. ACS Sustain. Chem. Eng. 2019, 7, 1464–1473. [Google Scholar] [CrossRef]
- Huang, K.; Tang, L.H.; Zhu ZBin Zhang, C.F. Reaction mechanism of styrene monomer recovery from waste polystyrene by supercritical solvents. Polym. Degrad. Stab. 2005, 89, 312–316. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Degradation of brominated flame-retarded plastics (Br-ABS and Br-HIPS) in supercritical water. J. Supercrit. Fluids 2009, 49, 356–368. [Google Scholar] [CrossRef]
- Khaobang, C.; Areeprasert, C. Investigation on thermal decomposition and kinetics study of recovered oil from electronic waste by thermogravimetric analysis. In Energy Procedia; Elsevier: Amsterdam, The Netherlands, 2017; Volume 138, pp. 506–511. [Google Scholar] [CrossRef]
- Yao, Q.; Wilkie, C.A. Thermal degradation of blends of polystyrene and poly(sodium 4-styrenesulfonate) and the copolymer, poly(styrene-co-sodium 4-styrenesulfonate). Polym. Degrad. Stab. 1999, 66, 379–384. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Novianti, A.; Hakim, I.I.; Putra, N.; Koestoer, R.A. Influence of temperature on conversion of plastics waste (polystyrene) to liquid oil using pyrolysis process. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012033. [Google Scholar] [CrossRef]
- Levine, S.E.; Broadbelt, L.J. Reaction pathways to dimer in polystyrene pyrolysis: A mechanistic modeling study. Polym. Degrad. Stab. 2008, 93, 941–951. [Google Scholar] [CrossRef]

| Oil (%) | Water Organic (%) | Solid (%) | Gas (%) | |
|---|---|---|---|---|
| 360 °C-4 h | 83 | 9 | 3 | 5 |
| 360 °C-3 h | 51 | 25 | 20 | 4 |
| 330 °C-4 h | 38 | 14.2 | 43.8 | 4 |
| 330 °C-3 h | 34 | 14.2 | 48.8 | 3 |
| Main results of published works | ||||
| 350 °C-30 min [6] | 86 | 2 | 2 | n.r. |
| 350 °C-1 h (1) [36] | 89 | n.r | 10 | 1 |
| 350 °C-1 h [36] | 74.2 | n.r | n.r | n.r |
| 350 °C-1 h (2) [35] | 84.7 | n.r | 3.8 | 11.5 |
| 350 °C-30 (3) min [37] | 83 | n.r | 13 | 4 |
| C (wt%) | H (wt%) | O (wt%) | HHV (MJ/kg) | ER (%) | |
|---|---|---|---|---|---|
| Oil 360 °C-3 h | 89.3 | 8.4 | 2.3 | 42.0 | 51 |
| Oil 360 °C-4 h | 89.8 | 7.5 | 2.7 | 40.81 | 95 |
| Td (°C) | T50% (°C) | Tmax (°C) | rmax (%/°C) | |
|---|---|---|---|---|
| PS | 380.9 | 415.5 | 417.4 | −2.62 |
| HTL_330 °C-3 h | 278.8 | 411.6 | 415.9 | −2.52 |
| HTL_360 °C-3 h | 209.2 | 399.5 | 418.7 | −1.44 |
| Compounds | Vol (%) |
|---|---|
| H2 | 13.5 |
| CH4 | 10.2 |
| C2H6 | 3.5 |
| C2H4 | 36.9 |
| C3H6 | 19.8 |
| Others (MW > C3H6) | 16.1 |
| Temperature (°C) | 400 | 500 | 600 |
|---|---|---|---|
| Benzene (%) | 0.19 | 0.23 | 0.28 |
| Toluene (%) | 0.20 | 0.20 | 0.14 |
| Styrene (%) | 36.65 | 40.16 | 34.6 |
| Alpha-methyl styrene (%) | 0.13 | 0.19 | 0.33 |
| 1,2-diphenylethan (%) | 0.64 | 0.59 | 0.47 |
| 1,3-diphenylpropane (%) | 0.19 | 0.22 | 0.41 |
| Dimer (%) | 11.90 | 12.05 | 8.18 |
| 2,4-diphenyl-1pentene (%) | 0.15 | 0.20 | 0.33 |
| Trimer (%) | 5.19 | 5.31 | 3.4 |
| Degradation (%) | Oil Yield (%) | Styrene Yield (%) | Oil HHV (MJ/kg) | |
|---|---|---|---|---|
| HTL-360 °C-4 h | 97 | 83 | 4 | 40.81 |
| Py-500 °C-30 min | 100 | 55 | 20 | 42.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musivand, S.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; de Caprariis, B. Viable Recycling of Polystyrene via Hydrothermal Liquefaction and Pyrolysis. Energies 2023, 16, 4917. https://doi.org/10.3390/en16134917
Musivand S, Bracciale MP, Damizia M, De Filippis P, de Caprariis B. Viable Recycling of Polystyrene via Hydrothermal Liquefaction and Pyrolysis. Energies. 2023; 16(13):4917. https://doi.org/10.3390/en16134917
Chicago/Turabian StyleMusivand, Sogand, Maria Paola Bracciale, Martina Damizia, Paolo De Filippis, and Benedetta de Caprariis. 2023. "Viable Recycling of Polystyrene via Hydrothermal Liquefaction and Pyrolysis" Energies 16, no. 13: 4917. https://doi.org/10.3390/en16134917
APA StyleMusivand, S., Bracciale, M. P., Damizia, M., De Filippis, P., & de Caprariis, B. (2023). Viable Recycling of Polystyrene via Hydrothermal Liquefaction and Pyrolysis. Energies, 16(13), 4917. https://doi.org/10.3390/en16134917







