Methane Dry Reforming Catalysts Based on Pr-Doped Ceria–Zirconia Synthesized in Supercritical Propanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis Methods
2.2. Characterizations
2.3. Catalytic Tests
3. Results
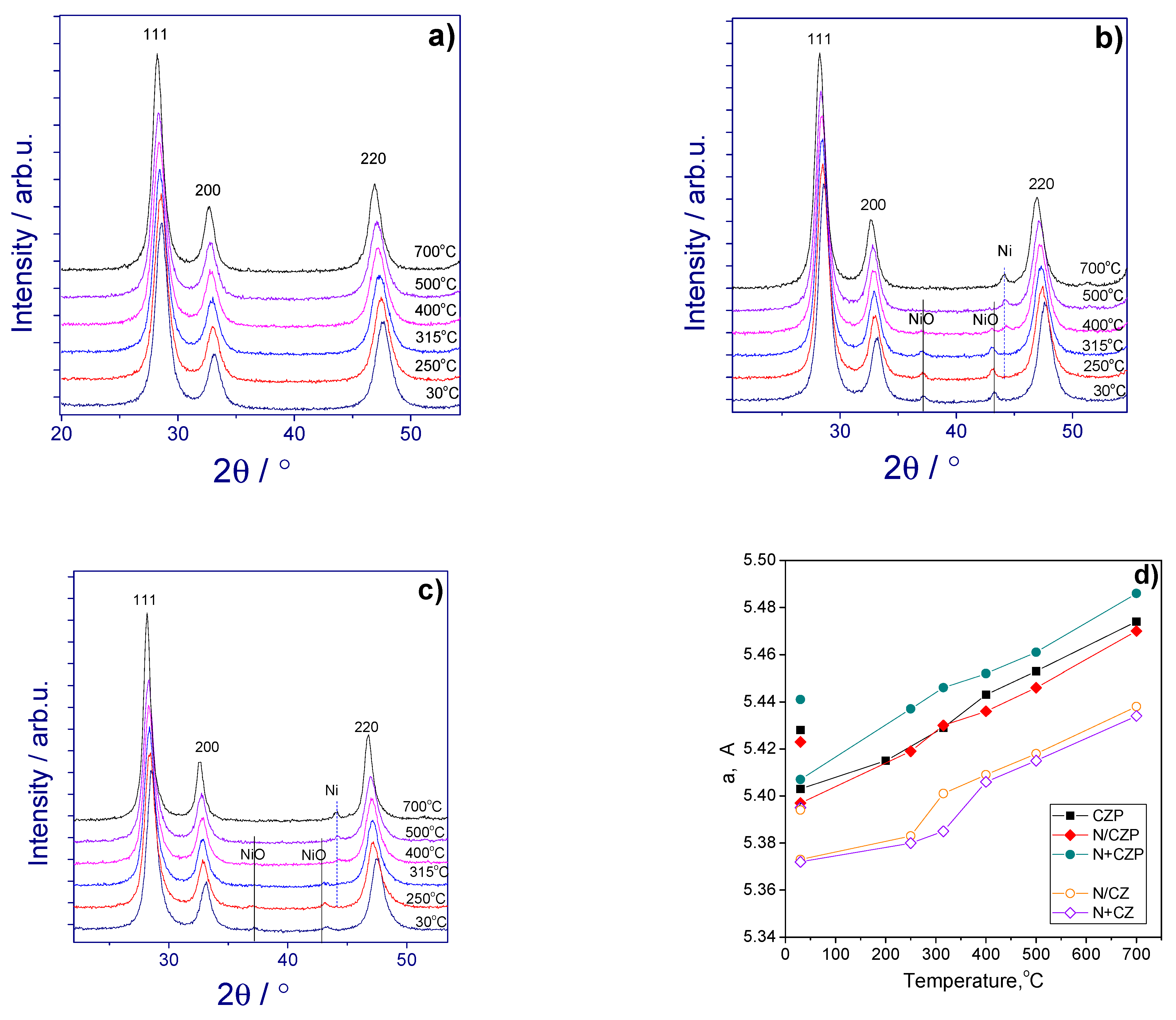
3.1. Textural and Structural Features
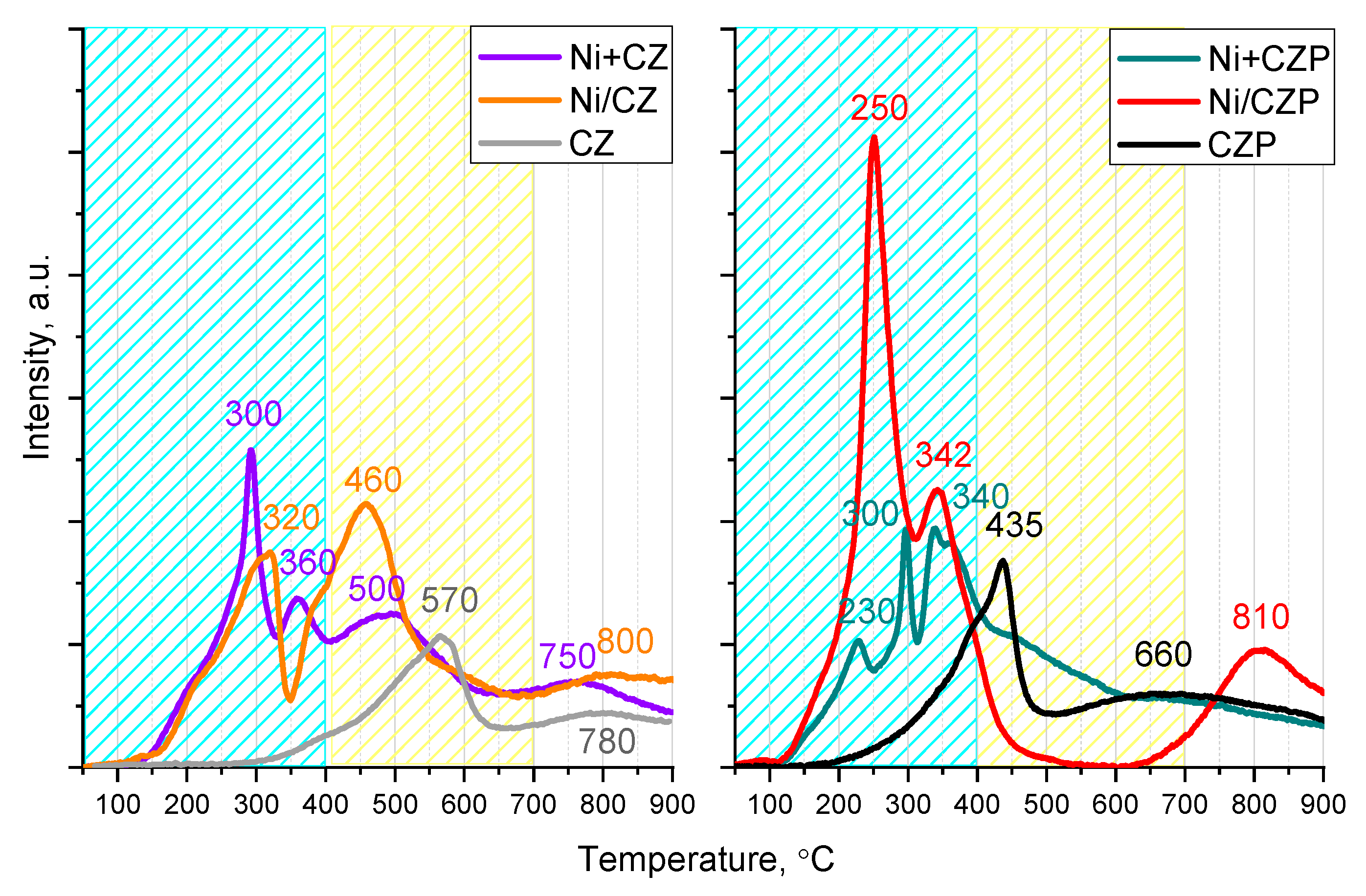
3.2. Reducibility and Oxygen Reactivity
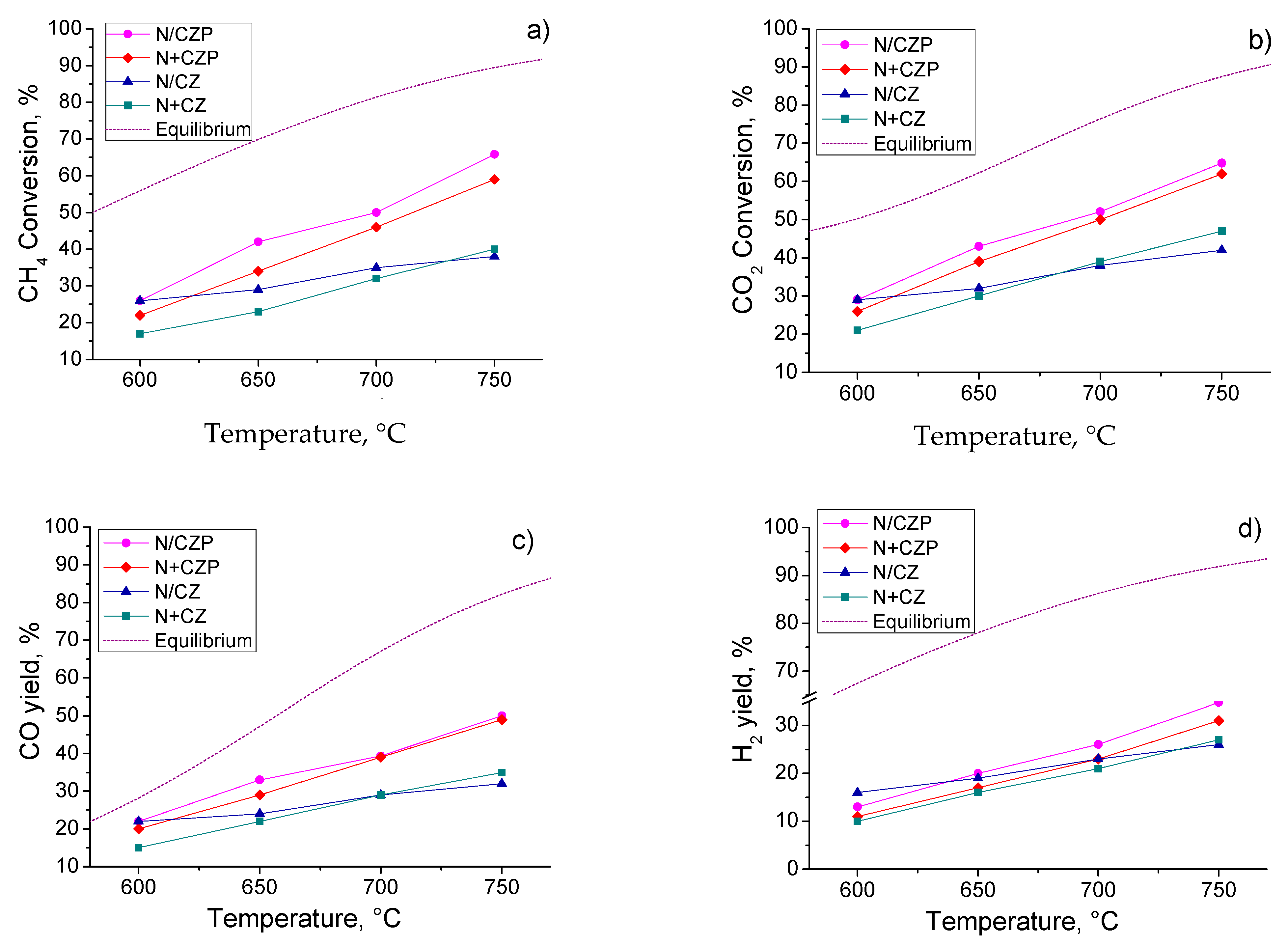
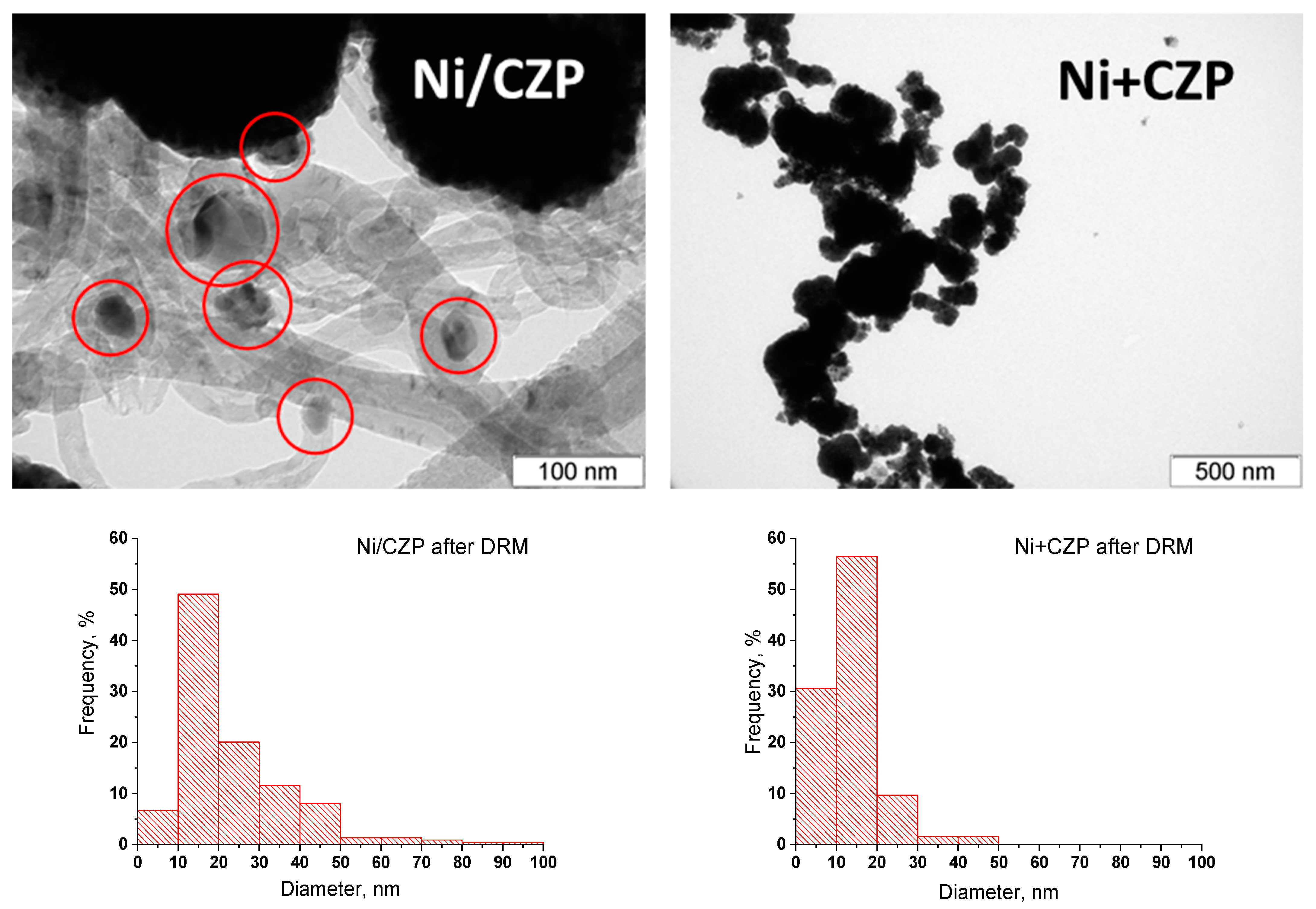
3.3. Catalytic Activity in MDR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Verykios, X.E. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Int. J. Hydrog Energy 2003, 28, 1045–1063. [Google Scholar] [CrossRef]
- Su, B.; Wang, Y.; Xu, Z.; Han, W.; Jin, H.; Wang, H. Novel ways for hydrogen production based on methane steam and dry reforming integrated with carbon capture. Energy Convers. Manag. 2022, 270, 116199. [Google Scholar] [CrossRef]
- Alipour, Z.; Borugadda, V.B.; Wang, H.; Dalai, A.K. Syngas production through dry reforming: A review on catalysts and their materials, preparation methods and reactor type. Chem. Eng. J. 2023, 452 Pt 3, 139416. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahima, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulation. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Lan, T.; Shen, T.; Zhong, Q.; Ren, W.; Zhang, D. Promoting Methane Dry Reforming over Ni Catalysts via Modulating Surface Electronic Structures of BN Supports by Doping Carbon. ACS Catal. 2022, 12, 14152–14161. [Google Scholar] [CrossRef]
- Rosli, S.N.A.; Abidin, S.Z.; Osazuwa, O.U.; Fan, X.; Jiao, Y. The effect of oxygen mobility/vacancy on carbon gasification in nano catalytic dry reforming of methane: A review. J. CO2 Util. 2022, 63, 102109. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Al-Fatesh, A.S.; Kumar, R.; Lanre, M.S.; Frusteri, F.; AlReshaidan, S.B.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. Promotional effect of addition of ceria over yttria-zirconia supported Ni based catalyst system for hydrogen production through dry reforming of methane. Int. J. Hydrog Energy 2022, 47, 20838–20850. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, R.; Gao, Y.; Lin, Y.; Liu, A.; Wang, X.; Zheng, A.; Huang, Z.; Zhao, Z. High syngas selectivity and near pure hydrogen production in perovskite oxygen carriers for chemical looping steam methane reforming. Fuel Process. Technol. 2022, 236, 107398. [Google Scholar] [CrossRef]
- Li, R.-J.; Zhang, J.-P.; Shi, J.; Li, K.-Z.; Liu, H.-L.; Zhu, X. Regulation of metal-support interface of Ni/CeO2 catalyst and the performance of low temperature chemical looping dry reforming of methane. J. Fuel Chem. Technol. 2022, 50, 1458–1470. [Google Scholar] [CrossRef]
- Vijay, S.; Ju, W.; Brückner, S.; Tsang, S.C.; Strasser, P.; Chan, K. Unified mechanistic understanding of CO2 reduction to CO on transition metal and single atom catalysts. Nat. Catal. 2021, 4, 1024–1031. [Google Scholar] [CrossRef]
- Yu, K.; Lou, L.; Liu, S.; Zhou, W. Asymmetric Oxygen Vacancies: The Intrinsic Redox Active Sites in Metal Oxide Catalyst. Adv. Sci. 2020, 7, 1901970. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Fang, Y.; Hoang, S.; Li, L.; Yang, W.; Liang, Z.; Wu, J.; Hu, J.; Xiao, W.; et al. Oxygen Vacancy Promoted O2 Activation over Perovskite Oxide for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Ren, H.; Gao, X. A short review on nickel-based catalysts in dry reforming of methane: Influences of oxygen defects on anti-coking property. Mater. Today Proc. 2021, 42, 153–160. [Google Scholar] [CrossRef]
- Salcedo, A.; Lustemberg, P.G.; Rui, N.; Palomino, R.M.; Liu, Z.; Nemsak, S.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V.; Irigoyen, B. Reaction Pathway for Coke-Free Methane Steam Reforming on a Ni/CeO2 Catalyst: Active Sites and the Role of Metal−Support Interactions. ACS Catal. 2021, 11, 8327–8337. [Google Scholar] [CrossRef]
- McFarland, E.W.; Metiu, H. Catalysis by Doped Oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef]
- Shi, J.; Li, H.; Genest, A.; Zhao, W.; Qi, P.; Wang, T.; Rupprechter, G. High-performance water gas shift induced by asymmetric oxygen vacancies: Gold clusters supported by ceria-praseodymia mixed oxides. Appl. Catal. B Environ. 2022, 301, 120789. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Djinović, P.; Davlyatova, L.F.; Pintar, A.; Efstathiou, A.M. Origin and reactivity of active and inactive carbon formed during DRM over Ni/Ce0.38Zr0.62O2-δ studied by transient isotopic techniques. Catal. Today 2018, 299, 201–211. [Google Scholar] [CrossRef]
- Puigdollers, A.R.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent progress in ceria-based catalysts for the dry reforming of methane: A review. Chem. Eng. Sci. 2021, 239, 116606. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. Int. J. Hydrog. Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt%Ni/Ce1−xMxO2 (M = Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Lanre, M.S.; Abasaeed, A.E.; Fakeeha, A.H.; Ibrahim, A.A.; Al-Awadi, A.S.; bin Jumah, A.; Al-Mubaddel, F.S.; Al-Fatesh, A.S. Lanthanum–Cerium-Modified Nickel Catalysts for Dry Reforming of Methane. Catalysts 2022, 12, 715. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Zhang, S.; Akter, N.; Palomino, R.M.; Vovchok, D.; Orozco, I.; Salazar, D.; Rodriguez, J.A.; Llorca, J.; et al. In situ elucidation of the active state of Co–CeOx catalysts in the dry reforming of methane: The important role of the reducible oxide support and interactions with cobalt. ACS Catal. 2018, 8, 3550–3560. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Deng, J.; Kuboon, S.; Faungnawakij, K.; Xiao, S.; Zhang, D. Coking resistant dry reforming of methane over BN–nanoceria interface-confined Ni catalysts. Catal. Sci. Technol. 2020, 10, 4237–4244. [Google Scholar] [CrossRef]
- Boaro, M.; Colussi, S.; Trovarelli, A. Ceria-Based Materials in Hydrogenation and Reforming Reactions for CO2 Valorization. Front. Chem. 2019, 7, 28. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Song, W.; Liu, J. Carbonate-mediated Mars–van Krevelen mechanism for CO oxidation on cobalt-doped ceria catalysts: Facet-dependence and coordination-dependence. Phys. Chem. Chem. Phys. 2018, 20, 16045–16059. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Simonov, M.N.; Mezentseva, N.V.; Pavlova, S.N.; Fedorova, Y.E.; Bobin, A.S.; Bespalko, Y.N.; Ishchenko, A.V.; Krieger, T.A.; Glazneva, T.S.; et al. Ni-loaded nanocrystalline ceria-zirconia solid solutions prepared via modified Pechini route as stable to coking catalysts of CH4 dry reforming. Open Chem. 2016, 14, 363–376. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Pavlova, S.N.; Krieger, T.A.; Bespalko, Y.N.; Anikeev, V.I.; Chesalov, Y.A.; Kaichev, V.V.; Mezentseva, N.V.; Sadykov, V.A. The synthesis of Ce1–xZrxO2 oxides in supercritical alcohols and catalysts for carbon dioxide reforming of methane on their basis. Russ. J. Phys. Chem. B 2017, 12, 15–28. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Makri, M.M.; Djinović, P.; Erjavec, B.; Pintar, A.; Efstathiou, A.M. Dry reforming of methane over 5 wt% Ni/Ce1-xPrxO2-catalysts: Performance and characterisation of active and inactive carbon by transient isotopic techniques. Appl. Catal. B Environ. 2016, 197, 168–183. [Google Scholar] [CrossRef]
- Sinev, M.Y.; Graham, G.W.; Haack, L.P.; Shelef, M. Kinetic and structural studies of oxygen availability of the mixed oxides Pr1-xMxOy (M = Ce, Zr). J. Mater. Res. 1996, 11, 1960–1971. [Google Scholar] [CrossRef]
- Niu, G.; Zoellner, M.H.; Schroeder, T.; Schaefer, A.; Jhang, J.H.; Zielasek, V.; Bäumer, M.; Wilkens, H.; Wollschlager, J.; Olbrich, R.; et al. Controlling the physics and chemistry of binary and ternary praseodymium and cerium oxide systems. Phys. Chem. Chem. Phys. 2015, 17, 24513–24540. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A. Papadopoulou, Ch. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Hirano, M.; Hirai, K. Effect of hydrolysis conditions on the direct formation of nanoparticles of ceria–zirconia solid solutions from acidic aqueous solutions. J. Nanopart. Res. 2003, 5, 147–156. [Google Scholar] [CrossRef]
- Pradeep, E.; Habu, T.; Tooriyama, H.; Ohtani, M.; Kobiro, K. Ultra-simple synthetic approach to the fabrication of CeO2–ZrO2 mixed nanoparticles into homogeneous, domain, and core–shell structures in mesoporous spherical morphologies using supercritical alcohols. J. Supercrit. Fluids 2015, 97, 217–223. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzón, A. Methane reforming with CO2 over Ni/ZrO2-CeO2 catalysts prepared by sol-gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.S.; Selvakumar, K. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Kuznetsova, T.G.; Sadykov, V.A.; Moroz, E.M.; Trukhan, S.N.; Paukshtis, E.A.; Kolomiichuk, V.N.; Burgina, E.B.; Zaikovskii, V.I.; Fedotov, M.A.; Lunin, V.V.; et al. Preparation of Ce-Zr-O composites by a polymerized complex method. Stud. Surf. Sci. Catal. 2002, 143, 659–667. [Google Scholar] [CrossRef]
- Basile, F.; Mafessanti, R.; Fasolini, A.; Fornasari, G.; Lombardi, E.; Vaccari, A. Effect of synthetic method on CeZr support and catalytic activity of related Rh catalyst in the oxidative reforming reaction. J. Eur. Ceram. 2019, 39, 41–52. [Google Scholar] [CrossRef]
- Cai, W.; Dong, J.; Chen, Q.; Xu, T.; Zhai, S.; Liu, X.; Cui, L.; Zhang, S. One-pot microwave-assisted synthesis of Cu-Ce0.8Zr0.2O2 with flower-like morphology: Enhanced stability for ethanol dry reforming. Adv. Powder Technol. 2020, 31, 3874–3881. [Google Scholar] [CrossRef]
- Manjunatha, S.; Dharmaprakash, M.S. Thermal stability, optical and Photoluminescence properties of spherical CexZr1−xO2 (x = 0.05) crystalline blue-emitting nanophosphors synthesized by microwave method. Mater. Res. Express 2018, 5, 035043. [Google Scholar] [CrossRef]
- Guo, J.; Xin, X.; Zhang, X.; Zhang, S. Ultrasonic-induced synthesis of high surface area colloids CeO2–ZrO2. J. Nanopart. Res. 2009, 11, 737–741. [Google Scholar] [CrossRef]
- Khani, Y.; Bahadoran, F.; Shariatinia, Z.; Varmazyari, M.; Safari, N. Synthesis of highly efficient and stable Ni/CexZr1-xGdxO4 and Ni/X-Al2O3 (X = Ce, Zr, Gd, Ce-Zr-Gd) nanocatalysts applied in methane reforming reactions. Ceram. Int. 2020, 46, 25122–25135. [Google Scholar] [CrossRef]
- Guo, T.; Du, J.; Wu, J.; Li, J. Enhanced properties of solid solution (CeZr)O2 modified with metal oxides for catalytic oxidation of low-concentration methane. Chin. J. Chem. Eng. 2017, 25, 187–192. [Google Scholar] [CrossRef]
- Lovell, E.; Horlyck, J.; Scott, J.; Amal, R. Flame spray pyrolysis-designed silica/ceria-zirconia supports for the carbon dioxide reforming of methane. Appl. Catal. 2017, 546, 47–57. [Google Scholar] [CrossRef]
- Veriansyah, B.; Park, H.; Kim, J.D.; Min, B.K.; Shin, Y.H.; Lee, Y.W.; Kim, J. Characterization of surface-modified ceria oxide nanoparticles synthesized continuously in supercritical methanol. J. Supercrit. Fluids 2009, 50, 283–291. [Google Scholar] [CrossRef]
- Hakuta, Y.; Hayashi, H.; Arai, K. Fine particle formation using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 341–351. [Google Scholar] [CrossRef]
- Cabanas, A.; Darr, J.A.; Lester, E.; Poliakoff, M. A continuous and clean one-step synthesis of nano-particulate Ce12xZrxO2 solid solutions in near-critical water. Chem. Commun. 2000, 11, 901–902. [Google Scholar] [CrossRef]
- Darr, J.A.; Poliakoff, M. New Directions in Inorganic and Metal-Organic Coordination Chemistry in Supercritical Fluids. Chem. Rev. 1999, 99, 495–541. [Google Scholar] [CrossRef]
- Simonov, M.; Bespalko, Y.; Smal, E.; Valeev, K.; Fedorova, V.; Krieger, T.; Sadykov, V. Nickel-containing ceria-zirconia doped with Ti and Nb. Effect of support composition and preparation method on catalytic activity in methane dry reforming. Nanomaterials 2020, 10, 1281. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Mao, Z.; Salcedo, A.; Irigoyen, B.; Ganduglia-Pirovano, M.V.; Campbell, C.T. Nature of the active sites on Ni/CeO2 catalysts for methane conversions. ACS Catal. 2021, 11, 10604–10613. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Veen, A.C. Tuning the catalytic performance of Ni-catalysed dry reforming of methane and carbon deposition via Ni-CeO2-x interaction. Appl. Catal. B Environ. 2018, 237, 641–648. [Google Scholar] [CrossRef]
- Anchieta, C.G.; Assaf, E.M.; Assaf, J.M. Syngas production by methane tri-reforming: Effect of Ni/CeO2 synthesis method on oxygen vacancies and coke formation. J. CO2 Util. 2022, 56, 101853. [Google Scholar] [CrossRef]
- Lyu, Y.; Jocz, J.N.; Xu, R.; Stavitski, E.; Sievers, C. Nickel speciation and methane dry reforming performance of Ni/CexZr1−xO2 prepared by different synthesis methods. ACS Catal. 2020, 10, 11235–11252. [Google Scholar] [CrossRef]
- Bespalko, Y.; Smal, E.; Simonov, M.; Valeev, K.; Fedorova, V.; Krieger, T.; Cherepanova, S.; Ishchenko, A.; Rogov, V.; Sadykov, V. Novel Ni/Ce(Ti)ZrO2 catalysts for methane dry reforming prepared in supercritical alcohol media. Energies 2020, 13, 3365. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Ballauri, S.; Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Fino, D.; Russo, N.; Bensaid, S. Wide range temperature stability of palladium on ceria-praseodymia catalysts for complete methane oxidation. Catal. Today 2022, 390–391, 185–197. [Google Scholar] [CrossRef]
- Rajendran, M.; Mallick, K.K.; Bhattacharya, A.K. Combustion synthesis, powder characteristics and crystal structure of phases in Ce–Pr–O system. J. Mater. Sci. 1998, 33, 5001–5006. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Yuan, Q.; Feng, W.; Xu, J.; Sun, L.; Song, W.; Yan, C. Sustainable and Facile Route to Nearly Monodisperse Spherical Aggregates of CeO2 Nanocrystals with Ionic Liquids and Their Catalytic Activities for CO Oxidation. J. Phys. Chem. C 2008, 112, 18405–18411. [Google Scholar] [CrossRef]
- Takeguchi, T.; Furukawa, S.N.; Inoue, M. Hydrogen spillover from NiO to the large surface area CeO2-ZrO2 solid solutions and activity of the NiO/CeO2-ZrO2 catalysts for partial oxidation of methane. J. Catal. 2001, 202, 14–24. [Google Scholar] [CrossRef]
- Iglesias, I.; Baronetti, G.; Marino, F. Ni/Ce0.95M0.05O2-d (M = Zr, Pr, La) for methane steam reforming at mild conditions. Int. J. Hydrog. Energy 2017, 42, 29735–29744. [Google Scholar] [CrossRef]
- Jalowiecki-Duhamel, L.; Zarrou, H.; D’Huysser, A. Hydrogen production at low temperature from methane on cerium and nickel based mixed oxides. Int. J. Hydrog. Energy 2008, 33, 5527–5534. [Google Scholar] [CrossRef]
- Smal, E.; Bespalko, Y.; Arapova, M.; Fedorova, V.; Valeev, K.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Glazneva, T.; Sadykov, V.; et al. Carbon formation during methane dry reforming over Ni-containing ceria-zirconia catalysts. Nanomaterials 2022, 12, 3676. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Kurdi, A.; Al-Baqmaa, Y.A.; Ibrahim, A.A.; Abasaeed, A.E.; Al-Fatesh, A.S. Performance Study of Methane Dry Reforming on Ni/ZrO2 Catalyst. Energies 2022, 15, 3841. [Google Scholar] [CrossRef]
- Shine, S.; Su, N.Y.; Hoon, H.G.; In, P.J.; Tae, S.H.; Kwan-Young, L.; Ju, M.D. Dry reforming of methane over Ni/ZrO2-Al2O3 catalysts: Effect of preparation methods. J. Taiwan Inst. Chem. Eng. 2018, 90, 25–32. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Lanre, M.S.; Al-Awadi, A.S.; Alreshaidan, S.B.; Albaqmaa, Y.A.; Adil, S.F.; Al-Zahrani, A.A.; Abasaeed, A.E.; Al-Fatesh, A.S. The Effect of Calcination Temperature on Various Sources of ZrO2 Supported Ni Catalyst for Dry Reforming of Methane. Catalysts 2022, 12, 361. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Kasim, S.O.; Alharthi, A.; Fakeeha, A.H.; Abasaeed, A.E.; Bonura, G.; Frusteri, F. Catalytic Behaviour of Ce-Doped Ni Systems Supported on Stabilized Zirconia under Dry Reforming Conditions. Catalysts 2019, 9, 473. [Google Scholar] [CrossRef]
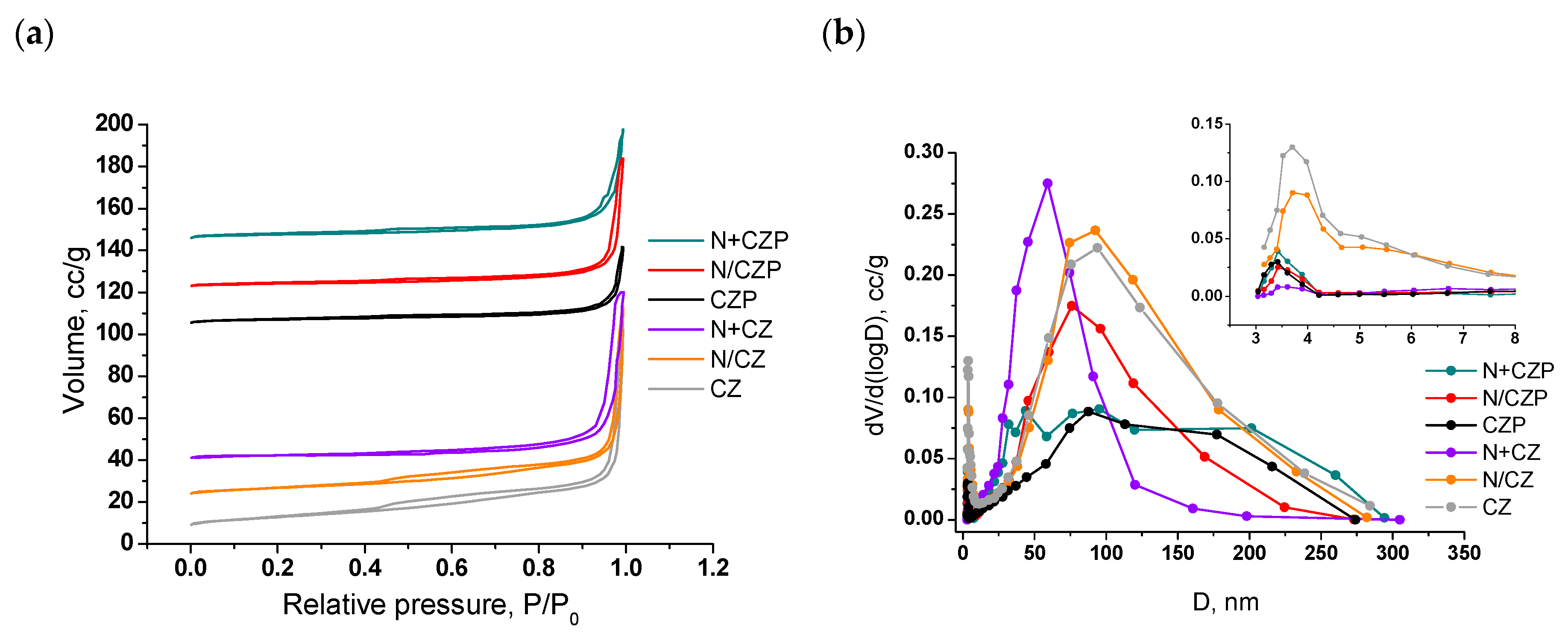
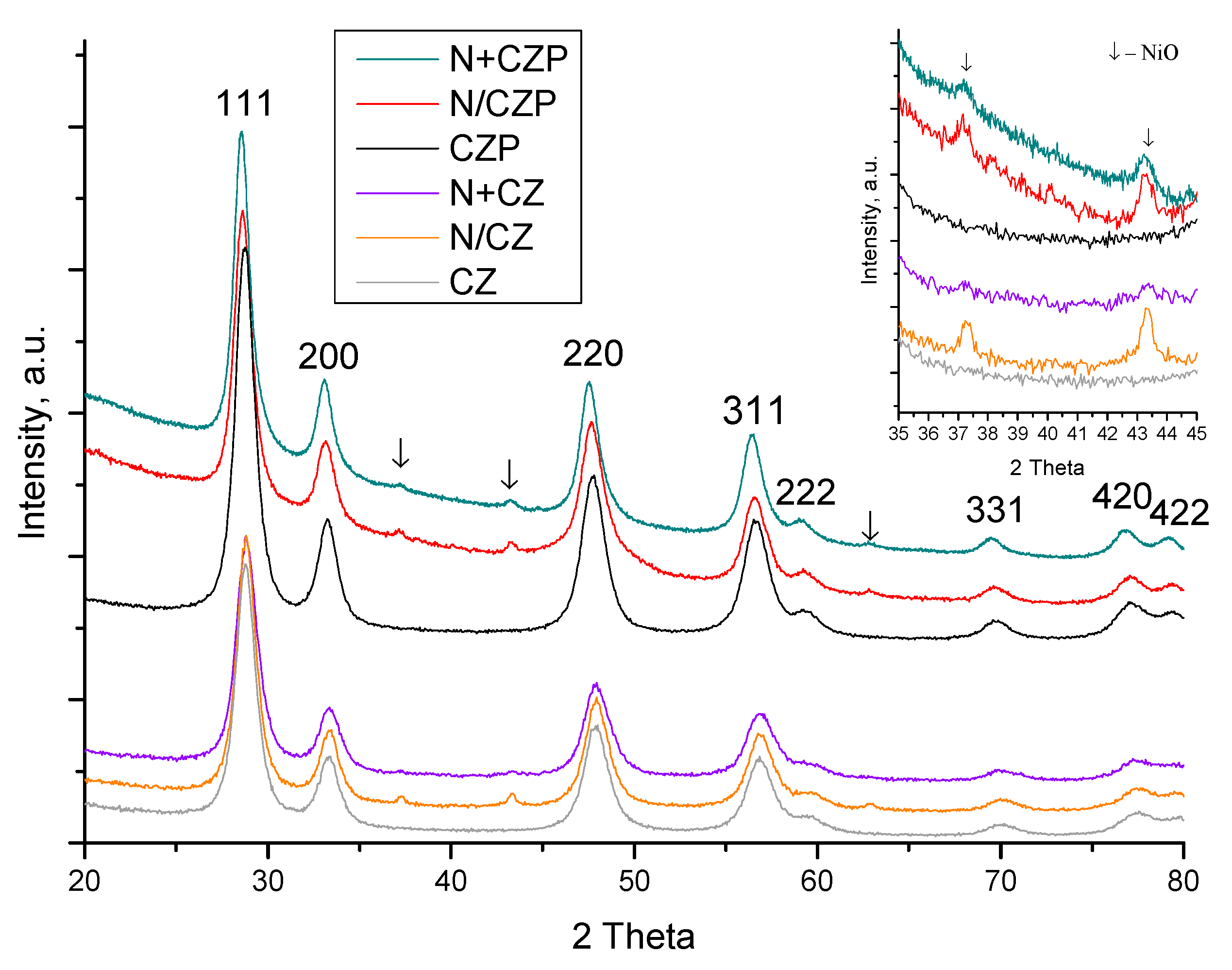
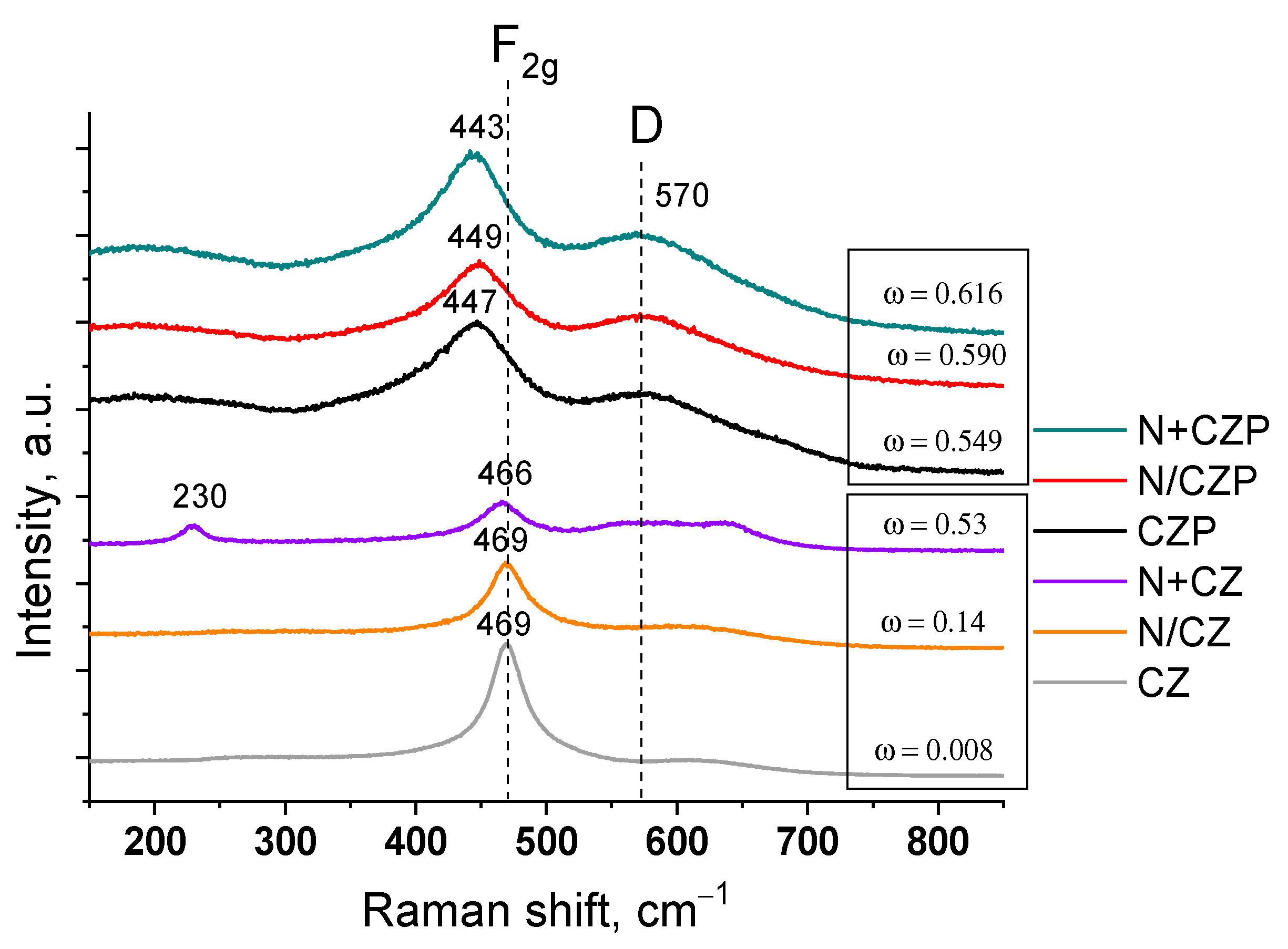

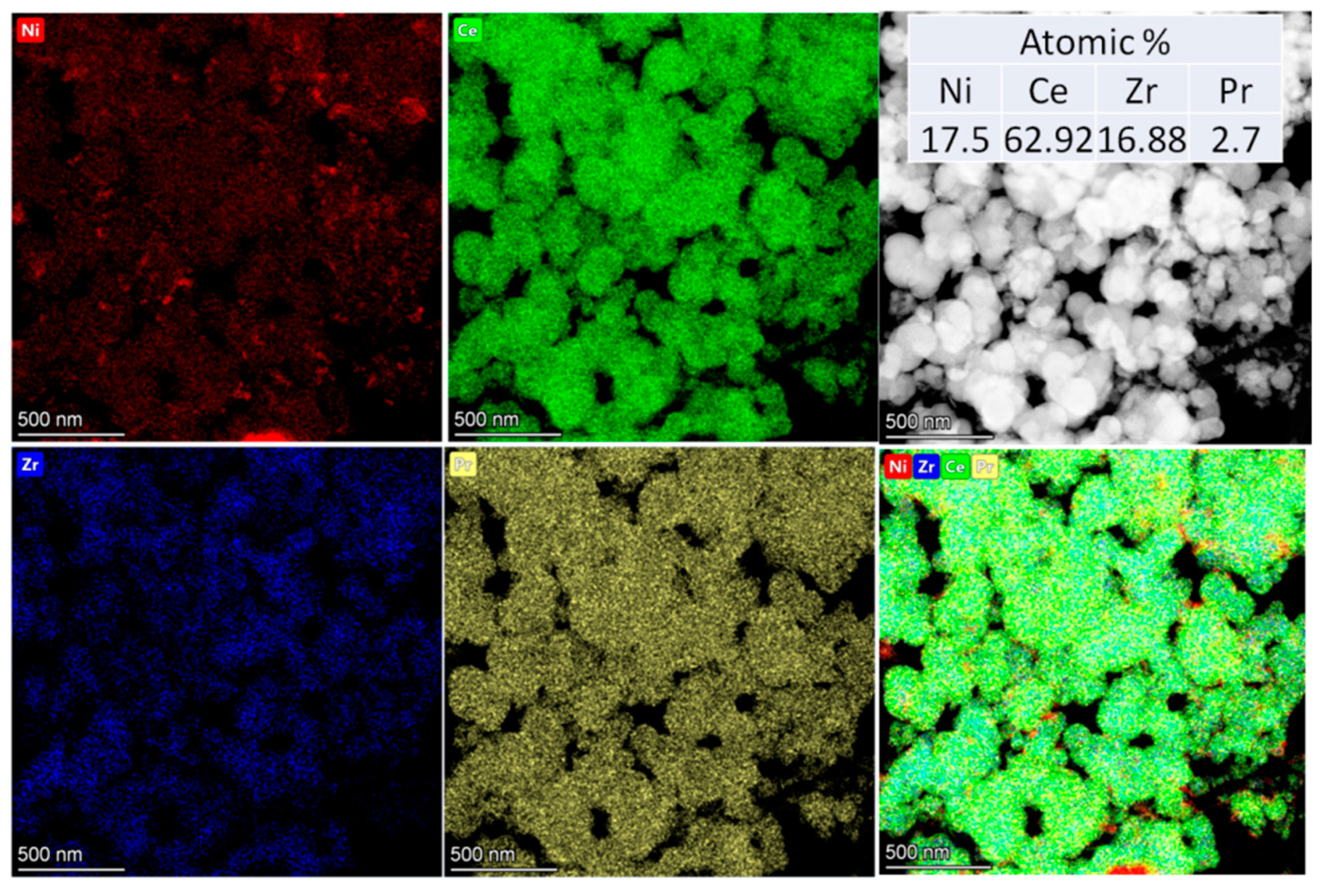

| Designation | Ni Addition Method | Composition | SSA, m2/g | Vpore, cm3/g | a *, Å |
|---|---|---|---|---|---|
| CZP | - | Ce0.75Zr0.15Pr0.1O2 | 14 | 0.055 | 5.403(1) |
| N/CZP | Impregnation | 5%Ni/Ce0.75Zr0.15Pr0.1O2 | 9 | 0.095 | 5.397(1) |
| N+CZP | One-pot | 5%Ni+Ce0.75Zr0.15Pr0.1O2 | 14 | 0.081 | 5.407(1) |
| CZ | - | Ce0.75Zr0.25O2 | 29 | 0.150 | 5.369(1) |
| N/CZ | Impregnation | 5%Ni/Ce0.75Zr0.25O2 | 21 | 0.144 | 5.373(1) |
| N+CZ | One-pot | 5%Ni+Ce0.75Zr0.25O2 | 13 | 0.126 | 5.372(1) |
| Sample | H2 Consumption, mmol H2/gcat | Ni Reducibility, % |
|---|---|---|
| CZP | 1.21 | - |
| N/CZP | 2.14 | 117 |
| N+CZP | 1.84 | 81 |
| CZ | 1.36 | - |
| N/CZ | 2.21 | 108 |
| N+CZ | 2.03 | 87 |
| Catalyst | Reaction Conditions | τ, ms | X(CH4) at 700 °C, % | Ref |
|---|---|---|---|---|
| Ni/Ce0.5Zr0.5O2 | CH4 = 20% CO2 = 20% N2 = 60% | 120 | 15 | [25] |
| Ni/Ce0.75Ti0.05Nb0.05Zr0.15O2 | CH4 = 15% CO2 = 15% N2 = 70% | 10 | 30 | [53] |
| Ni/ZrO2 | CH4:CO2:N2 = 3:3:1 | ~90 | 56 | [69] |
| Ni/ZrO2-Al2O3 | CH4:CO2 = 1 | ~150 | 38 | [70] |
| 5%Ni/Y+Zr | CH4:CO2:N2 = 3:3:1 | ~90 | 67 | [71] |
| 5%Ni/La+Zr | CH4:CO2:N2 = 6:6:1 | ~90 | 66 | [72] |
| Ni/Ce0.75Pr0.1Zr0.15O2 | CH4 = 15% CO2 = 15% N2 = 70% | 10 | 39 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arapova, M.; Smal, E.; Bespalko, Y.; Valeev, K.; Fedorova, V.; Hassan, A.; Bulavchenko, O.; Sadykov, V.; Simonov, M. Methane Dry Reforming Catalysts Based on Pr-Doped Ceria–Zirconia Synthesized in Supercritical Propanol. Energies 2023, 16, 4729. https://doi.org/10.3390/en16124729
Arapova M, Smal E, Bespalko Y, Valeev K, Fedorova V, Hassan A, Bulavchenko O, Sadykov V, Simonov M. Methane Dry Reforming Catalysts Based on Pr-Doped Ceria–Zirconia Synthesized in Supercritical Propanol. Energies. 2023; 16(12):4729. https://doi.org/10.3390/en16124729
Chicago/Turabian StyleArapova, Marina, Ekaterina Smal, Yuliya Bespalko, Konstantin Valeev, Valeria Fedorova, Amir Hassan, Olga Bulavchenko, Vladislav Sadykov, and Mikhail Simonov. 2023. "Methane Dry Reforming Catalysts Based on Pr-Doped Ceria–Zirconia Synthesized in Supercritical Propanol" Energies 16, no. 12: 4729. https://doi.org/10.3390/en16124729
APA StyleArapova, M., Smal, E., Bespalko, Y., Valeev, K., Fedorova, V., Hassan, A., Bulavchenko, O., Sadykov, V., & Simonov, M. (2023). Methane Dry Reforming Catalysts Based on Pr-Doped Ceria–Zirconia Synthesized in Supercritical Propanol. Energies, 16(12), 4729. https://doi.org/10.3390/en16124729








