Abstract
Calcium borohydride (Ca(BH4)2) is a complex hydride that has been less investigated compared to its lighter counterpart, magnesium borohydride. While offering slightly lower hydrogen storage capacity (11.5 wt% theoretical maximum, 9.6 wt% under actual dehydrogenation conditions), there are many improvement avenues for maximizing the reversible hydrogen storage that have been explored recently, from DFT calculations and polymorph investigations to reactive hydride composites (RHCs) and catalytic and nanosizing effects. The stability of Ca(BH4)2, the possibility of regeneration from spent products, and the relatively mild dehydrogenation conditions make calcium borohydride an attractive compound for hydrogen storage purposes. The ionic conductivity enhancements brought about by the rich speciation of borohydride anions can extend the use of Ca(BH4)2 to battery applications, considering the abundance of Ca relative to alkali metal borohydrides typically used for this purpose. The current work aims to review the synthetic strategies, structural considerations of various polymorphs and adducts, and hydrogen storage capacity of composites based on calcium borohydrides and related complex hydrides (mixed anions, mixed cations, additives, catalysts, etc.). Additional applications related to batteries, organic and organometallic chemistry, and catalysis have been briefly described.
1. Introduction
The road to sustainable energy has an ultimate endpoint: the hydrogen economy. Hydrogen remains the only energy source producing a high amount of clean energy with no carbon-containing by-products [1,2,3,4,5,6,7,8,9,10,11,12]. However, vehicular and stationary applications relying on hydrogen technology still face major drawbacks and important technological challenges, along with safety risks associated with H2 storage tanks where pressure spikes to 700 bar H2 [1].
In this context, solid-state hydrogen storage represents a safer alternative to classical high pressure H2 tanks, although the strict requirements of the US DOE have yet to be met [2]. The current target for 2025 is a material capable of storing 5.5 wt% hydrogen (1.8 kWh/kg, 1.3 kWh/L, with an estimated cost of $9/kWh). Perhaps the most important aspect of solid-state hydrogen storage is the material’s capability to reversibly store H2 without continual loss of this storage capacity. To this end, most experimental tanks have been fueled by various intermetallics (AB2-type or AB5-type, such as LaNi5H6 with 1.38 wt%), which offer a storage capacity not exceeding, at best, 2 wt% hydrogen. With a storage capacity of up to 20 wt% (Be(BH4)2) and high gravimetric densities (110–150 g H2/L), complex hydrides are the most promising solid-state hydrogen storage materials, and the amount of research in this area has been staggering. Still, the kinetic and thermodynamic factors of absorption/desorption processes hinder their wider use, while the potentially toxic boron-containing gases (diborane or higher homologues) are of concern for the environment.
Light metal borohydrides M(BH4)n (M = Li, Na, Mg, Ca) and alanates have received the most attention not only for their increased theoretical hydrogen storage capacity, but also due to promising results pointing to the possibility of reversibility during successive a/d cycles. With a high storage capacity of about 10 wt% (actually attainable under experimental conditions) and a relatively low dehydrogenation temperature, Ca(BH4)2 holds promise for a viable and reversible hydrogen storage material under suitable conditions. While dehydrogenation of Ca(BH4)2 has been reported in the range 350–500 °C, this is still lower than other light metal borohydrides. Driven by the high hydrogen storage capacity of calcium borohydride needed for energy storage applications, particularly in the field of hydrogen storage and fuel cells, researchers have investigated various methods to enhance the hydrogen release kinetics and lower the dehydrogenation temperature. Ca(BH4)2 has the potential to offer high energy density, enabling the development of compact and efficient energy storage systems. Calcium borohydride can serve as both a source of energy and a hydrogen reservoir within energy storage devices, such as lithium-ion batteries and sodium-ion batteries.
However, despite its potential, there are several challenges that need to be addressed before the wide acceptance of Ca(BH4)2 as an energy storage material. The kinetics of hydrogen release from calcium borohydride are relatively slow, limiting its practical application. Enhancing hydrogen release kinetics is a key research focus. The dehydrogenation reaction mechanisms of calcium borohydride are not yet fully understood, and further investigation is required to gain detailed insights into the underlying processes. Another obstacle comes from an economic point of view. The cost of Ca(BH4)2 is high; therefore, the development of cost-effective and scalable synthesis routes for calcium borohydride is another area that requires more research results.
The current review aims to describe the most significant aspects concerning Ca(BH4)2, from the synthesis routes, crystal structure and polymorphs, structural variability and modifications (DFT computations, nanoconfinement, destabilization, RHCs, solvates/adducts), characterization methods, hydrogen storage properties, and further applications in battery technology, organic synthesis, and organometallic chemistry. Optimizing the overall performance, stability, and efficiency of energy storage devices utilizing calcium borohydride is a critical research challenge.
2. Synthesis Routes
There are various methods reported for the synthesis of borohydrides [5,13] and, in particular, of Ca(BH4)2. For instance, Barkhordarian et al. reported the hydrogenation of the RHC: CaH2 + MgB2 at 350 °C and 140 bar H2 to produce Ca(BH4)2 + MgH2 (Equation (1)) [13].
However, the layered structure of B in MgB2 was essential for Equation (1) to occur, as no reactivity was observed when substituting MgB2 for B. The reasoning seemed to lie in the structural features of MgB2, where layers of B are located between layers of Mg, a structure bearing similarities to graphite. The authors also reported enhanced kinetics of de-/rehydrogenation when using Ti(OiPr)4 as an additive [13].
Dodecahydroborates have been obtained by desolvation at ~120 °C of the MB12H12 solvates, or in their pure form, by the following Equation (2) [14], The mixture of M(BH4)2 and B10H14 is typically milled in a planetary ball mill (10 steel balls, 7 mm diameter, 0.1 MPa Ar, 5 h milling), followed by sintering in stainless steel crucibles at 380 °C for 2 h in the case of CaB12H12 [14].
Nanostructuring has long been utilized to achieve significantly better yields for reactions where classical synthetic routes have failed [6], while stabilization of reactive species has often been attained via complex formation, i.e., through ligand coordination, as is the case for ammine solvates of M(BH4)2 (M = Ca, Sr) [15]. Jepsen et al. reported the synthesis of four such solvates in the case of calcium-based borohydrides: Ca(NH3)n(BH4)2 (n = 1, 2, 4, and 6) (Equation (3)) [15]. The pure Ca(BH4)2 absorbs gaseous ammonia, forming the unstable hexa-ammine calcium borohydride Ca(NH3)6(BH4)2, which undergoes a rapid decomposition equilibrium to form the tetra-ammine calcium borohydride Ca(NH3)4(BH4)2.
Karabulut et al. synthesized Ca(BH4)2 in a solid-state reaction starting from anhydrous, synthetic colemanite, a calcium borate mineral of formula Ca2B6O11.5H2O [16]. By milling Ca2B6O11 and CaH2 in a 1:12 molar ratio in a spex-mill, calcium borohydride was obtained with a sole by-product in the form of CaO (Equation (4)). Using colemanite as the raw material has a clear economic advantage, as the cost of 1 g Ca(BH4)2 would be ~$4, compared to the commercial version’s (~$200).
It is also interesting to note that the hydrogen content of Ca(BH4)2 could effectively double when subjected to hydrolysis, as Ca(BO2)2 is the main product along with H2 (Equation (5)).
Given the reactivity of metal borohydrides towards water, it follows that the formation of a bimetallic borohydride in the form of LiCa3(BH4)(BO3)2, as isolated and characterized by Lee et al., is not that surprising after all [17]. The mixed borohydride was isolated from hydrogenation studies carried out on RHC based on 0.75 LiBH4–0.25 Ca(BH4)2 embedded in a mesoporous carbon matrix (Vp = 1.15 cm3/g), and most likely originates from oxygen-containing impurities (oxygen or water) [17]. The formation of LiCa3(BH4)(BO3)2 was tracked by XRD and was found to proceed through the intermediacy of mixed anion calcium borohydride, Ca3(BH4)3(BO3) (Equation (6)).
The general route to prepare Ca(BH4)2 starts from CaH2 or alkoxide Ca(OR)2 and B2H6, or more conveniently by wet chemistry in a metathesis reaction, where CaCl2 and NaBH4 are ball milled in THF (tetrahydrofuran), producing the commercially available THF adduct of calcium borohydride, namely, Ca(BH4)2.2THF [18,19] (Equation (7)).
Other adducts of Ca(BH4)2 are also reported; for instance, desolvation of Ca(BH4)2.2THF at 80 °C led to the formation of Ca(BH4)2·THF, as reported by Richter et al. [20].
Møller et al. reported the synthesis of M(BH4)n from the systems AlB2–MHx (M = Li, Na, Ca, but not Mg), where AlB2 served as a boron source, and H2 was introduced at a pressure in the range of 100–600 bar [21] (Equation (8)).
The inclusion of Al in the case of Ca(BH4)2 can produce 6.3 wt% with a reaction enthalpy of 64.9 kJ/mol H2 and an entropy of 156.5 J/k mol H2, requiring a temperature of 140 °C at an equilibrium H2 pressure of 1 bar (Equation (9)). This alone is a marked improvement compared to neat Ca(BH4)2 (87.2 kJ/mol H2, 158.3 J/K mol H2, 280 °C) [21].
Nakagawa et al. used ball milling of CaH2–CaB6 mixtures to obtain Ca(BH4)2 near room temperature, noting the important role of crystalline CaB6 in order to achieve reasonably high rates for hydrogenation [22].
Several experimental aspects have to be considered when synthesizing Ca(BH4)2, along with strong safety protocols. For instance, the influence of chlorides was illustrated in the mixture CaCl2:LiBH4, when the reactant ratio needs to be carefully optimized, otherwise solid solutions Li(BH4)1-xClx/Ca(BH4)2-yCly (milling performed under an Ar atmosphere) are obtained, and CaHCl forms instead of Ca(BH4)2 [23]. Ca(BH4)2 was also produced by milling CaB6 with CaH2 in a 1:2 molar ratio under 700 bar H2 at 400–440 °C, as evidenced by Ronnebro and Majzoub [11].
Ca(BH4)2 can serve as as borohydride source for other metathesis reactions, for instance with M2(SO4)x metal sulfates, with the driving force for obtaining M(BH4)x being the formation of the precipitate of CaSO4 [18]. Other authors have reported a mixed adduct Ca(BH4)2·Al(BH4)3 resulted by mixing the unsolvated starting borohydrides, decomposing into starting borohydrides upon heating, and having a postulated structure [Ca(BH4)]+[Al(BH4)4]− supported by IR spectrum analysis [24].
3. Structural Variability, Polymorphs, Crystal Structure, DFT Predictions, and Phase Transitions
The structural stability aspects of many representative simple and complex hydrides (alanates, borohydrides) have been reviewed in the recent past [25], along with Ca-based RHCs ([Ca(BH4)2 + MgH2 and Ca(BH4)2 + MgH2 + 0.1NbF5]) [26]. Karimi et al. have highlighted the catalytic role of NbF5 on the hydrogen sorption kinetics of Ca(BH4)2 + MgH2 (10.5 wt% H2), when ~10 nm diameter NbB2 was evidenced to form and to remain stable during a/d cycles, while also being responsible for producing ~50% finer Ca-RHC + 0.1NbF5 nanocomposites and preventing agglomeration of calcium-RHCs [26].
The structural features of Ca(BH4)2 have been described by Llamas-Jansa et al., who reported the different behavior between α, β, and γ polymorphs of Ca(BH4)2 during hydrogenation studies [27]. Vibrational spectroscopy data was employed to correlate the increase in wavenumber to the increased decomposition temperature (15 °C between the α and γ polymorphs). The most effective H2 release was observed when ramping at 10 °C/min a sample of pure α-Ca(BH4)2 [27]. Moreover, Sharma et al. shed light on the B-H bond breaking/formation, using isotope exchange reactions with a D2 pressure of 20 bar, concluding an activation energy Ea = 82.1 ± 2.7 kJ mol−1 for the transformation of Ca(BH4)2 into Ca(BD4)2, and proposing a mechanistic scheme involving the formation of an activated complex: [28]. Considering all the literature data, it seems that the dehydrogenation behavior of Ca(BH4)2 depends greatly on the polymorph composition and their respective ratio in the starting sample material, especially considering the typical co-existence of α and β phases during hydrogenation experiments [29]. Soloninin et al. studied the reorientation motion of BH4 tetrahedra in M(BH4)2 (M = Mg, Ca) by proton and 11B spin-lattice relaxation rates and found that at low temperatures, the reorientation in the β phase is faster (Ea = 100–116(5) meV for β-, Ea = 286(7) meV for α-Ca(BH4)2), and depends on the changes in the local environment of BH4 groups [29].
3.1. Crystal Structure, Polymorphs, and Phase Transitions
The two most common phases of calcium borohydride are α-Ca(BH4)2 (lattice parameters: a = 8.7782(2) Å, b = 13.129(1) Å, c = 7.4887(9) Å; 300 K) and β-Ca(BH4)2 (a = 6.9509(5) Å, c = 4.3688(3) Å, 433 K). The alpha phase is stable from 0 K to 440 K, while from 440 K, the high temperature phase, β-Ca(BH4)2 becomes dominant. The metastable γ-Ca(BD4)2 crystal structure was reported by combined X-ray and neutron measurements, and was refined in the orthorhombic space group Pbca (a = 7.525(1) Å, b = 13.109(2) Å, c = 8.403(1) Å) [30,31]. The γ phase is less stable than the α phase, and its stability decreases with temperature; the expected phase transitions are expected to follow the trend α-Ca(BH4)2 → γ-Ca(BH4)2 → β-Ca(BH4)2 [30]. There have been some debates regarding the space group of both α and β phases; for instance, DFT computations conducted by Le et al. [32] over the hydrostatic pressure range 0–40 GPa showed that the F2dd space group seems to be the preferred structure of α-Ca(BH4)2 at low temperatures [32]. Crystal structures and total energy have been calculated based on the general gradient approximation (GGA) method for density functional theory (DFT), implemented by the ABINIT simulation package, while Hartwigsen–Goedecker–Hutter pseudopotentials have been used for calculations, and the exchange–correlation interactions were described by the Perdew–Burke–Ernzerhof (PBE) GGA functional [32]. The transition to the beta phase at higher temperatures was supported by calculations performed by Lee et al. [33], who pointed out a vibrational entropy excess of β-Ca(BH4)2 over α-Ca(BH4)2 of 16 J/(mol K). A total of nine possible crystal structures have been proposed in the literature for calcium borohydride: three α phases, one α’ phase, four β phases, and one γ phase [34]. The most agreed-upon structures are, however, the α and β phases [35] (Table 1).

Table 1.
Crystal structure of calcium borohydride with corresponding space groups, decomposition temperatures (and rehydrogenation temperatures, where available) (°C), and crystal systems.
Other reports have dealt with phase variation occurring via desolvation from the adduct Ca(BH4)2.2THF when a mixture of α-Ca(BH4)2 and γ-Ca(BH4)2 polymorphs was observed experimentally [35]. Filinchuk et al. have also reported a second-order transition, α-Ca(BH4)2 (F2dd) → α’-Ca(BH4)2 (I-42d), occurring at ~495 K [35]. The various polymorphs of α-Ca(BH4)2 (prepared by desolvation of commercially available Ca(BH4)2.2THF at 433 K for 1 h) have also been investigated under high pressure (10.4 GPa, room temperature) by Liu et al. [38] by combined Raman and IR spectroscopies, and it was found that the transformations are reversible throughout the pressure region investigated [38]. The authors also pointed out the need to start the compression from phase-pure Ca(BH4)2, otherwise H2 release and interaction in mixtures α-Ca(BH4)2–β-Ca(BH4)2 (which are the typical starting point) would further complicate the analysis of lattice, bending, and stretching modes [38]. In order to avoid the α-Ca(BH4)2 → β-Ca(BH4)2 transition, a more conservative approach was proposed by Paolone et al., who heated Ca(BH4)2∙2THF at 125 °C for 20 h (incomplete conversion of THF adduct to α-Ca(BH4)2), followed by the α → α’ structural phase transition at 180–220 °C, and finally the α’→β transformation at 320–330 °C [39]. Monitoring the vibration frequency of Ca(BH4)2 via anelastic spectroscopy measurements, the authors also noted that upon further cooling the sample from 320 °C to room temperature, the borohydride did not undergo any phase transitions, consistent with irreversible transformations occurring during heating (α → α’ → β). Although not detected by DSC, evaluation of the relative variation/decrease of Young’s modulus upon heating can indirectly point out these transitions [39]. Some debate still exists in the literature, as some authors claim another phase in the C2/c space group for Ca(BH4)2 near ambient conditions [40].
Stoichiometric Equations (1)–(7) can describe several possible decomposition pathways for Ca(BH4)2 (Figure 1).
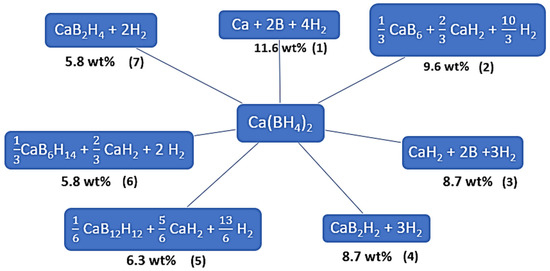
Figure 1.
Decomposition pathways possible for calcium borohydride (Equations (1)–(7)).
Judging by how widespread dodecahydro-closo-dodecaborates (B12H122−) are during the dehydrogenation of metal borohydrides M(BH4)n, it seemed reasonable to further study the implications of these intermediate species on hydrogen storage properties as well as on the unexpected increase in ionic conductivity of related alkali and alkali-metal compounds [41]. Some representative examples of polymorphs of Ca(BH4)2, mixed anion borohydrides, and dodecaborate anion are depicted in Figure 2.
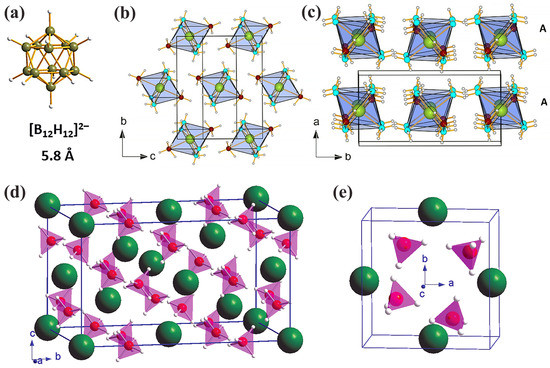
Figure 2.
(a) Representation of the dodecahedral [B12H12]2− ion of the icosahedral frame. Boron atoms (green), hydrogen atoms (white). (b) Crystal structure of Ca(NH3)4(BH4)2. Ca, B, N, and H are represented by yellow, red, blue, and gray spheres, respectively; the molecular [Ca(NH3)4(BH4)2] octahedra form hexagonal patterns in the bc plane. (c) The layers are stacked in the order AAA along the a direction (reproduced with permission from Ref. [15]). (d) Crystal structures of α-Ca(BH4)2 in space group Fddd and (e) β-Ca(BH4)2 in space group P. The color codes for atoms are green (calcium), pink (boron), and white (hydrogen). Each BH4− unit is shown by a tetrahedron. Reprinted with permission from Ref. [38].
The thermal stability of MB12H12 is rather high, but a tendency towards amorphization (perhaps of the tentative formula B12H12–x2−) has been recorded upon heating to 400–450 °C and further, when anion polymerization may occur (CaByHz)n, but elemental B was not observed even at 750 °C [41].
Jepsen et al. have recently described the DFT-optimized and experimental crystal structures (synchrotron radiation powder XRD data) of Ca(NH3)n(BH4)2 (n = 1, 2, 4, and 6) (Figure 3) [15]. DFT calculations were performed to optimize the structures from the SR-PXD data by using the Vienna Ab-initio Simulation Package (VASP) in the Perdew–Burke–Ernzerhof generalized-gradient approximation [15]. Ca(NH3)4(BH4)2 (P21/c) has a molecular structure connected by dihydrogen bonds, and all ammine complexes release ammonia upon gentle heating (at a low partial pressure of NH3), or H2-NH3 (high partial pressure of NH3). These findings suggest metal ammine borohydrides can be used to store ammonia, or to release H2 by catalytic ammonia splitting [15].
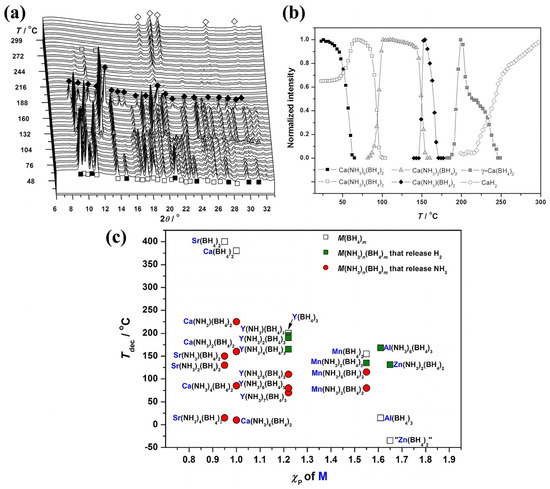
Figure 3.
(a) In situ SR-PXD pattern of Ca(NH3)6(BH4)2 heated from RT to 350 °C (5 °C min−1, p(Ar) = 1 bar, λ = 0.9941 Å). (b) Normalized integrated diffraction intensities plotted as a function of temperature. Reprinted with permission from Ref. [15]. (c) Decomposition temperatures for selected ammine metal borohydrides and the respective metal borohydrides under an Ar atmosphere, as a function of the metal M electronegativity. Zn(BH4)2 is not observed experimentally and is considered unstable. Reprinted with permission from Ref. [15].
Ca2+ is octahedrally coordinated to six equivalent [BH4] tetrahedra, featuring Ca-B bond distances of 2.82–2.97 Å. The divalent cations in α-Ca(BH4)2 form a close-packed, diamond-type structure where the tetrahydroborate groups exhibit T-shape coordination.
Another interesting intermediate that can be observed during thermal decomposition of Ca(11BD4)2; after initially being described as belonging to “δ-Ca(BH4)2”, it has been properly assigned to Ca3(11BD4)3(11BO3) and its structure was deduced by Riktor et al. based on synchrotron radiation powder XRD and supported by IR measurements [42].
With a decomposition enthalpy in the range 40.6–87 kJ/(mol H2) and an onset decomposition temperature of 278 °C under 1 bar equilibrium pressure, bulk Ca(BH4)2 requires considerable modifications in order to conform to DOE’s restrictions. Overcoming sluggish kinetics, problematic mass transfer, and high reaction enthalpies remain key steps needing further improvement.
3.2. Cation Substitution
Cation substitution has been pursued in dual-cation borohydrides in order to decrease the energy barriers in hydrogenation studies of calcium borohydride [43,44,45,46,47]. For instance, Fang et al. studied the dual-cation (Li, Ca) borohydride LiCa(BH4)3 [43]. The mixed borohydride was synthesized by ball milling a 1:1 mixture of LiBH4:Ca(BH4)2, when an intermediate phase Li0.9Ca(BH4)2.9 was observed to transform into the final stoichiometric mixed-cation borohydride LiCa(BH4)3 upon heating. The dual cation Li0.9Ca(BH4)2.9 was produced most likely by incorporation of dissolved LiBH4 into β-Ca(BH4)2 (Equation (10)).
Contrary to the desorption of individual borohydrides, the mixed-phase LiCa(BH4)3 started to desorb H2 at 200 °C, considerably lower than the onset of LiBH4 (400 °C) or Ca(BH4)2 (300 °C), and lost ~9.6 wt% after heating to 500 °C [43]. The Li-Ca-B-H system also bypassed the formation of the [B12H12]2− anion, following a two-step reaction that yielded 1.2 wt% and 8.1 wt% H2, respectively (Equation (11)). The reversibility of the second dehydrogenation step was, however, only moderate, affording 5.3 wt% H2 recharging [43].
Jiang et al. observed a new mixed-borohydride adduct when mixing 3 LiBH4:1 CaCl2 in THF, namely, LiBH4·Ca(BH4)2·2THF, which released a mixture of H2-B2H6 upon heating in a complex, 4-step process involving the detection of yet-unknown Li-Ca-B-H phases [44]. Although typically mixing metal halides such as MCln with LiBH4 can yield mixed alkali metal-transition metal borohydrides (Equation (12)), a different reaction pathway was revealed when tuning the CaCl2:LiBH4 molar ration, while also using THF as reaction media.
Additionally, LiBH4·Ca(BH4)2·2THF was shown to store reversibly up to 5.63 wt.% H2 in the second a/d cycle [44]. Switching LiBH4 with NaBH4, Mattox et al. have reported the influence of the borohydride starting material on the additive used (40% CaCl2), concluding that NaBH4 might interpenetrate the CaCl2 lattice, expanding it [46]. The hypothesized product was marked as CaNa(BH4)1-xClx, akin to the expected product based on Equation (12).
Lang et al. reported the decomposition of another substituted borohydride, the perovskite-type NH4Ca(BH4)3 (15.7 wt.% hydrogen capacity, Equation (13)) [45]. The origin of the low desorption temperature (65 °C) was found to reside in the destabilization of H+ in NH4+ and H− in BH4− [45].
The low onset correlates well with the reaction between NH4+ and BH4−, which takes place at 85 °C according to Equation (14).
Upon heating, NH4Ca(BH4)3 releases H2 with the formation of Ca(BH4)2NH3BH3, an adduct that further desorbs ammonia borane AB: NH3BH3 (which undergoes a complex decomposition process also releasing H2), and Ca(BH4)2 that can further react with LiCl to form anion-substituted borohydrides (Equation (15)) [45].
It appears that ~3.8 wt% H2 can be released upon heating to 140 °C for 25 min, with very little other impurity gases such as NH3 or B2H6 (Figure 4).
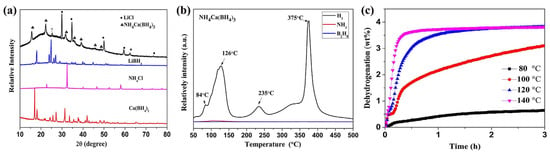
Figure 4.
(a) XRD patterns of starting materials and as-milled samples; (b) MS profiles of NH4Ca(BH4)3; (c) isothermal hydrogen desorption curves of NH4Ca(BH4)3 at varied temperatures. Reprinted with permission from Ref. [45].
The previous study on NH4Ca(BH4)3 in relation to the proposed destabilization borohydride-ammonium system, was reported by Schouwink et al. [47]. In addition to Equation (13), Schouwink used a novel approach: soft milling Ca(BH4)2 (30 min, 500 rpm) with temperature-stabilized NH4BH4 (cooling at 243 K) (Equation (16)).
The crystal structure of NH4Ca(BH4)3 was also reported with a structural model in space group P-43m [47] (Figure 5).
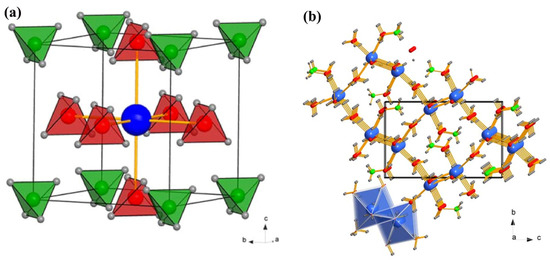
Figure 5.
Structural models for (a) NH4Ca(BH4)3 in space group P-43m; NH4+ (green), BH4− (red), Ca (blue0; and (b) Ca(BH4)2·AB; N (green), B (red), H (grey), Ca (blue). Reprinted with permission from reference [47].
3.3. Anion Substitution
Grove et al. have studied halide substitution in Ca(BH4)2 by ball milling the mixtures Ca(BH4)2:x CaX2 (X = F, Cl, Br) with various molar ratios (x = 0.5, 1, 2) [48]. The compositions from x = 0–0.6 yield solid solutions of composition β-Ca((BH4)1-xClx)2 as revealed by Rietveld analysis of diffraction data, but there was no substitution of X− for BH4− occurring for X = F or Br, possibly due to the positive enthalpy of mixing (X = F) or the lack of orthorhombic-to-tetragonal phase transition (X = Br) (Equation (17)) [48].
Even though DFT calculations predicted Br− substitution to be possible, this possibility was experimentally discarded. Additionally, DSC of β-Ca((BH4)0.5Cl0.5)2 showed a higher decomposition temperature compared to pure Ca(BH4)2. Interestingly, no substitution was observed at temperatures below 250 °C or in the case of α-Ca(BH4)2, the only polymorph undergoing this substitution being β-Ca(BH4)2 (Figure 6) [48]. The calculations were performed applying the periodic quantum-mechanical software CRYSTAL09 within the density functional theory, PBE functional [48].
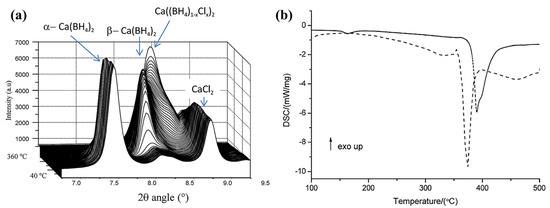
Figure 6.
(a) In situ SR-PXD measured for Ca(BH4)2+CaCl2 in molar ratio 1:1, heating rate 3 K min−1. The temperature increases from 40 to 360 °C. (a) 3D plot of the selected 2θ area. Λ = 0.703511 Å; (b) DSC data for Ca(BH4)2 (dashed) and Ca(BH4)2–CaCl2 (1:1) (solid) with a heating rate of 10 K min−1. Reproduced with permission from Ref. [48].
Extending the range of calcium halides used for anion substitution in calcium borohydride, Rude et al. have employed CaI2 in the system Ca(BH4)2–CaI2 and found three new components formed by halide substitution: a solid solution Ca((BH4)1-xIx)2 with x~0.3, the trigonal tri-Ca((BH4)0.70I0.30)2, and the orthorhombic ort-Ca((BH4)0.64I0.36)2 [49]. The hydrogen release occurs from tetragonal Ca((BH4)1–xIx)2 via CaHI; however, the anion substitution strategy affords borohydrides with a decomposition temperature similar to that of Ca(BH4)2 [49].
3.4. DFT Computation and Predictions
Several DFT computation studies have surfaced related to calcium borohydride, from crystal structure under high pressure [50], predictive evaluation of mixed-anion Ca(BH4)(NH2) [51], simulation of the nanosizing effect of Ca(BH4)2 [52], to the inclusion of metal borohydrides in a combined multidisciplinary approach aimed to further adjust hydride materials for vehicular applications [1]. Aidhy et al. have used a combination of density functional theory (DFT) calculations and a Monte Carlo (MC)-based crystal structure prediction tool, the Prototype Electrostatic Ground State (PEGS) method [51]. Albanese et al. have utilized for the theoretical investigation of Ca (BH4)2 the periodic density functional theory (DFT) calculations employing the PBE functionals as implemented in the CRYSTAL program, with the following all-electron basis set used for all the atoms, namely, 865-11G (2d) for Ca, 6-21G (d) for B, and 31G (p) for H [52]. Other studies have investigated the metal electronegativity role in the M(BH4)x–LiNH2 system as a means to enhance dehydrogenation thermodynamics and kinetics in the RHC [53]. Blanchard et al. have combined quasielastic neutron scattering (QENS) and DFT calculations to elucidate the role of hydrogen rotational and translational diffusion in calcium borohydride as an important factor of hydrogen dynamics in crystalline Ca(BH4)2 [54]. The calculations were performed by using the atomic simulation environment (ASE) package, and the DACAPO plane-wave basis-set implementation was used to solve the electronic structure problem within the DFT formalism; the ion cores were described by ultrasoft pseudopotentials, and the exchange and correlation effects were described by the PW91 functional [54].
Alkali-earth metal borohydride stability M(BH4)2 (M=Be, Mg, Ca) has been studied since the 1990s by Bonaccorsi et al. by systematic nonempirical calculations performed on M(BH4)2, HMBH4, and MBH4+, resulting in optimized geometries [3]. Interestingly, the authors employed the effect of electron correlation in the study of the multi-step decomposition leading to borane elimination, an experimental detail observed in the case of many TM-based borohydrides (Equation (18)).
However, a critical point regarding DFT computations is that the agreement with experimental data must be reasonably good; with this goal in mind, Franco et al. used Quantum ESPRESSO to perform DFT computations on seven simple and complex hydrides (including Ca(BH4)2), and found that coupling the GIPAW (gauge-including projected augmented-wave) ab initio method with solid-state NMR experiments led to a good agreement among all investigated samples [55]. For example, 11B MAS (Hahn echo) spectra of Ca(BH4)2 as a mixture of α and β phases showed corresponding signals at −32.5 pp (β phase) and −29.9 ppm (α phase), and 1H SSNMR data also showed good correlation with prediction shifts (PAW: 0.48 ppm error, QE: 0.35 ppm error) (Figure 7) [55].
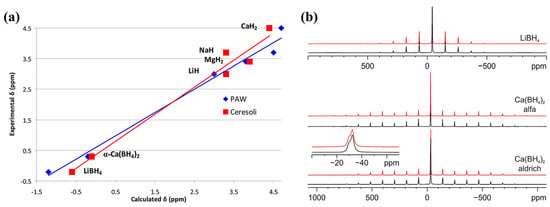
Figure 7.
(a) Experimental vs. calculated 1H chemical shifts (TMS as reference). (b) Experimental (black) and simulated (red) 11B MAS (Hahn echo) spectra of α-Ca(BH4)2, and Ca(BH4)2 Aldrich (α + β form) recorded with a spinning speed of 14 kHz. Reproduced with permission from Ref. [55].
There is, however, an important need to develop new systems for energy storage, and Wolverton et al. employed an atomic scale computational approach using Perdew–Wang GGA (generalized gradient approximation) in this regard, with three-fold advances being recorded: the prediction of hydriding enthalpies and free energies, the prediction of favorable decomposition pathways, and the prediction of low-energy crystal structures of complex hydrides [12]. The results suggest that the DFT approach is useful in evaluating potential decomposition pathways, as was exemplified for Li4BN3H10, a metastable phase with three potential decomposition pathways for T < 300 K and thermodynamically possible with ΔG < 0 [12].
4. Characterization and Stability
As previously mentioned, there are several early reports related to the structure and stability of calcium borohydrides [3,35,56]. For instance, Filinchuk et al. [35] and Bosenberg et al. [57] reported the characterization of metal hydride using in situ synchrotron radiation powder X-ray diffraction (SR-PXD). SR-PXD is a powerful tool to track solid-gas reactions, and hydrogenation studies made use of a cell that allows control over wide pressure and temperature ranges (up to 200 bar, 550 °C) [57,58,59]. The authors reported that β-Ca(BH4)2 and MgH2 were observed when a mixture of CaH2-MgB2 was heated, as well as the β → α phase transition of calcium borohydride upon cooling [57]. Dematteis et al. deduced the heat capacities and thermodynamic properties of complex hydrides, which will further ease the thermodynamic data computations (ΔS, ΔH and ΔG) [60].
Other physical characterization methods include inelastic neutron scattering [61], high-resolution laser excitation spectroscopy (albeit for hypothetical, gas phase CaBH4 molecule) [62], vibrational spectra (IR and Raman, for α-, β-, and mixed (β,γ)-Ca(BH4)2) [38,63,64], solid-state NMR (1H, 11B MAS for α-, β-Ca(BH4)2 [29,55,65], confirmation of [B12H12]2− species during borohydride dehydrogenation [66], 11B spin-lattice relaxation [67], analysis of RHCs based on calcium borohydride like LiBH4–Ca(BH4)2 [68]), and various DFT computations (excluding CaB2H2 as dehydrogenation intermediate, while favoring CaB12H12 as an intermediate, as argued by Frankcombe, Equation (19) [69], or evaluating [Ca(BH4)2]n=1–4 clusters for hydrogen storage by Han et al. [70]).
Hattrick-Simpers et al. described the use of high-throughput backscattering Raman spectroscopic measurements for combinatorial in situ Raman spectroscopy of Ca(BH4)2 and other RHCs (up to 10 Mpa and 823 K) [71].
The stability of Ca(BH4)2 has been investigated in many reports, including Miwa et al. [72], Nakamori et al. [73], Riktor et al. [58], and others. Since the decomposition of complex hydrides is related to their hydrogen storage capacity, these results will be discussed in Section 5.
4.1. Neat vs. Nanoconfined
Several aspects of nanosizing are regarded as beneficial for enhancing de-/rehydrogenation of complex hydrides and related RHCs. For instance, when impregnating LiBH4 into ferrite-catalyzed-graphene, a synergistic effect was observed and an important reduction in activation energy was recorded [74].
4.2. DFT Simulation of the Nanosizing Effect
Albanese et al. have studied the nanostructuring of β-Ca(BH4)2 by atomistic thin film models to mimic nanosizing effects (DFT, using CRYSTAL software) in order to evaluate the reduction of dehydrogenation enthalpy of calcium borohydride, showing that for thickness in the range 5–20 Å, the reduction on enthalpy reaches 30–35 kJ/mol H2, making calcium borohydride an appealing candidate for hydrogen storage under nanosizing conditions [52]. The decomposition pathways for Ca(BH4)2 have been investigated by Zhang et al. by means of DFT calculations of free energy, predicting a new CaB2H6 compound as a decomposition intermediate [75]. Other groups have evaluated the effectiveness of DFT computations in establishing metastable reaction pathways [76].
Dual-cation borohydrides of form M1M2(BH4)3(NH3)2–6 have been investigated by Emdadi et al., finding several potentially promising new solvates and stable alloys, including M2=Ca [77]. Nanosizing was invoked in the case of LiBH4–Ca(BH4)2 RHC when catalyzed by LaMg3, with an improvement registered in hydrogenation behavior due to nanoparticulate alloy addition affording a dehydrogenation onset at ~200 °C, 100° lower than pristine LiBH4–Ca(BH4)2 and rehydrogenation proceeding at 150 °C [78].
Guo et al. performed first-principles calculations on the system KBH4/Ca(BH4)2 and proposed three new reactions in the mentioned RHC (20), (21) and (22), two of them involving the formation of the [B12H12]2− anion [79,80].
Moreover, Kulkarni et al. described by first principles the dodecaborane, amorphous phase CaB12H12 as a result of an overlap of structurally distinct crystallites [81].
Huang et al. performed first-principles calculations involving Cr-doping of α-Ca(BH4)2 and found half-metallic behavior (0.98 eV for Ca doped sites, 0.63 eV for B) useful for further use in spintronic devices [82]. Computational thermodynamics coupled with CALPHAD (CALculation of PHAse Diagram) modeling revealed the formation of Ca(BH4)2 as a result of Ca addition on LiBH4, along with CaB6, which significantly reduces the H2 releasing temperature of the system [83].
The utilization of Ca(BH4)2 in electrolyte solution for Ca-ion batteries has been shown feasible by a computational approach called AIMD (ab initio molecular dynamics) performed on ether- and ester-based electrolytes on a calcium anode, revealing an aspect that was confirmed experimentally, namely, that the best combination for an electrolyte solution is that of Ca(BH4)2 and the polar, aprotic THF solvent [84]. Calcium borohydride (Ca(BH4)2) has shown potential for use in both lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs) due to its high hydrogen storage capacity and favorable electrochemical properties. Regarding LIBs, it must be noted that Ca(BH4)2 can release H2 through a reversible electrochemical process, enabling its use as an energy and H2 reservoir within the battery. The high capacity of Ca(BH4)2 for Li storage (by theoretical calculations, one mole of Ca(BH4)2 can potentially store four moles of lithium, leading to a high energy density) makes it an attractive solution for LIB applications. However, compared to Mg(BH4)2, which afforded 540 mAh/g (first discharge)/250 mAh/g (recharge), the reported values for Ca(BH4)2 were lower. With good chemical stability, calcium borohydride showed good stability during lithium insertion/extraction, therefore showing good potential for long term cycling stability. Several downsides should be addressed, such as developing the proper electrolyte system that would afford better overall battery performance and stability. As with LIBs, SIBs show similar advantages and downsides for Ca(BH4)2 use in battery applications.
Mikhailin et al. employed potential energy surfaces along minimal energy pathways to study the decomposition of M(BH4)2(NH3)1–2 and the [M(BH4)2(NH3)2]2 dimers (M=Be, Mg, Ca, and Zn) using the B3LYP method (DFT). The dimeric structures have almost no effect on the energy barriers, while coordination of the ammonia molecule to the metal center reduced these barriers considerably, affording dehydrogenation at temperatures much lower than those of their corresponding pristine counterparts [85]. The potential decomposition pathways of calcium ammine borohydride complexes would lead to Ca(BH2NH)(BHN), Ca(BH4)(BHN) or Ca2(BH4)2(NH3)2(BH3NH2)2 in the case of the dimer [85]. The case of decomposition of Ca(BH4)2·NH3 was further investigated by Zhang et al. by means of DFT and showed a reasonable agreement with decomposition in the range 190–250 °C, releasing 11.3 wt% hydrogen [86]. Notably, the interaction N-H…H-B was found to play an essential role in favoring H2 release, recognizing the unique role of dihydrogen bonding in H2 release from solid compounds such as complex hydrides and their adducts [86]. Various complementary predictions have been made on the basis of thermodynamic computations by Siegel et al. [87], and Vajeeston et al. [88,89].
4.3. Destabilization Strategies
Destabilization of boron-based materials can be achieved by using additives (various compounds based on Mg, B, Al, C, S, Ti, intermetallics, etc.), chemical modifications, and nanosizing effects [90,91,92,93,94,95,96,97,98]. Various RHCs have been employed, and most showed improved desorption temperatures compared to pristine components; for instance, Mg(BH4)2/Ca(BH4)2, Mg(BH4)2/CaH2, or Mg(BH4)2/CaH2/3NaH (Equations (23)–(25)) (Figure 8) [92,94].
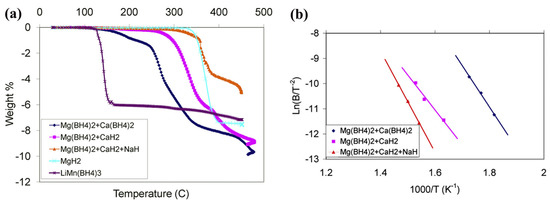
Figure 8.
(a) TPD profiles comparing the desorption temperatures of several solid-state hydrogen storage materials. (b) Activation energy plots using the Kissinger equation for several mixtures. Reprinted with permission from Ref. [92].
Ibikunle et al. also studied the RHC Mg(BH4)2/Ca(BH4)2 and found the H2 release to be diffusion- and phase boundary-controlled (Figure 9) [94,95].
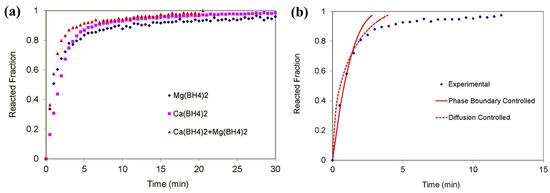
Figure 9.
(a) Plots of reacted fraction versus time for Mg(BH4)2, Ca(BH4)2, and the Mg(BH4)2/Ca(BH4)2 mixture at 450 °C. (b) Modeling curves for Mg(BH4)2/Ca(BH4)2. Reprinted with permission from reference [94].
An extension of the destabilization strategy proposed by Durojaiye in 2010 [92] was employed by Huang et al. in 2016, who used Mg(AlH4)2 in order to destabilize Ca(BH4)2 instead of magnesium borohydride [93]. The destabilization effect of magnesium alanate Mg(AlH4)2 in the 1 Mg(AlH4)2:1 Ca(BH4)2 RHC was quantified and shown to exhibit a 10-fold faster desorption at 300 °C (0.337 wt.% H2/min) compared to pristine Ca(BH4)2 (2.6 wt% H2 at the same temperature), releasing 8.4 wt% H2 at 330 °C in a three-step reaction (with release peaks at 130, 280, and 310 °C) [93]. A key step in the enhancement observed was attributed to the formation of the Al (Mg) solid solution, effectively destabilizing Ca(BH4)2 [93].
Destabilization in the system NaBH4/Ca(BH4)2 has also been reported (Equation (26)), and the TPD release curves were altered by a decomposition product of Ca(BH4)2; the system behaved reversibly under ~5.5 Mpa H2 at 400 °C when recharged for 10 h [97].
An important aspect of the reversibility reaction of many metal borohydrides is the regeneration of B–H bonds present in the [BH4]− anion [90]. This issue was tackled by Cai et al., who proposed a general route potentially applicable to M(BH4)2 as well (M=Mg, Ca), based on the reaction of SiB4 with MHn=1–2 to form M(BH4)n=1–2 under mild conditions in the case of LiBH4 (10 Mpa, 250 °C) (Equation (27)). The chemical bonding of boron in the reagent (SiB4) was found essential for the best hydrogenation performance; for comparison, the synthesis of LiBH4 from the elements requires 700 °C, while the mildest conditions are required by the solid-gas reaction between LiH and B2H6, yielding LiBH4 at 120 °C.
5. Hydrogen Storage Properties
Various aspects related to hydrogen desorption behavior have been investigated in recent years, from the detailed decomposition of pristine calcium borohydride by Kim et al. [99,100], to the kinetic and thermodynamic investigation of Ca(BH4)2 decomposition [101], to the further decomposition behavior of the CaB12H12 intermediate [66] or the debated formation of the CaB2Hx intermediate reported by Riktor et al. [102]. Other reports shed light on the H2 backpressure effect [100] or the dehydrogenation temperature used (350 °C vs. 400 °C), which can allow different pathways for the decomposition reaction (via CaB2H6 at 350 °C, or via B and CaH2, leading to CaB6 when heated at 400 °C) [103,104]. Further experimental details related to the H2 desorption potential of Ca(BH4)2 will be discussed in the following sections (Section 5.1,Section 5.2,Section 5.3,Section 5.4,Section 5.5 and Section 5.6), as well as the applicability of calcium borohydride to ion conductivity studies and applications (Section 5.7).
5.1. Bulk Ca(BH4)2
As early as 2008, Aoki et al. investigated the hydrogen release properties of Ca(BH4)2 and reported the phase transition (LT, low temperature, to HT, high temperature), while also identifying an unassigned intermediate compound and CaH2 as the final phase identified by XRD data analysis [105]. Decomposition of pristine calcium borohydride has been thought and further demonstrated to proceed through the formation of CaB12H12 [14,66] or another intermediate type of the general formula CaB2Hx (DFT computations) [34]. A pressure investigation performed on a mixture α- and β-Ca(BH4)2 led George et al. to identify a novel phase in which the β– phase transforms when reaching 10.2 Gpa, but no α-to-β transition was observed around 5.3 Gpa, as theoretically predicted [106]. Other DFT computations were run on [Ca(BH4)2]n=1–4 clusters and found the smallest dissociation energy to belong to the tetramer [Ca(BH4)2]4, indicative of it being the better hydrogen storage candidate among the investigated clusters [70].
The dehydrogenation of Ca(BH4)2 and that of the destabilized system Ca(BH4)2–MgH2 were investigated by Kim et al., highlighting the essential role of the CaB6 product in achieving system reversibility [107], as corroborated by the findings of Sahle et al. a few years later [108]. It was also suggested that CaB6 forms as the final product of the reaction from intermediate phases likely containing B and CaH2 [108].
As for the decomposition of CaB12H12, the decomposition pathway differs from that of Ca(BH4)2, producing no CaB6, and can be described by Equation (28) [14]:
Moreover, although there are conflicting reports regarding the role of CaB12H12 in the dehydrogenation of Ca(BH4)2, it seems unlikely that the dodecaborate is a stable dehydrogenation intermediate [14,37].
5.2. Nanoconfined
Nanoconfinement remains a viable route for tuning the thermodynamic parameters of hydrogen storage materials and of Ca(BH4)2 in particular, with several reports describing marked improvements over the pristine compounds’ thermal behavior [68,74,96,109,110,111,112,113,114,115,116,117,118]. Some of the most important characteristics of these systems are summarized in Table 2. A porous borohydride, CaB2H7, was obtained by heating Ca(BH4)2 with Ti(Oet)4 at 160 °C for 1 h and showed improved release and uptake of hydrogen (Equation (29)) [112].

Table 2.
Hydrogen storage characteristics of nanocomfined Ca(BH4)2 and its RHCs.
The desorption of the porous borohydride occurred in the 300–420 °C interval, following a two-step dehydrogenation pathway (Equation (30)) [112].
When LiBH4 is used in the eutectic mixture 0.7 Li(BH4)2-0.3 Ca(BH4)2 displaying eutectic melting, the reversible coupled RHC system obeys the reversible pathway described by Equation (31).
Other studies have focused on the infiltration and interaction between the eutectic LiBH4–Ca(BH4)2 and mesoporous scaffolds (CMK–3, MCM–41) by means of NMR investigation, including the lack of surface modification of the inert carbon scaffold CMK-3, as revealed by 13C MAS and 1H-13C CPMAS (cross-polarization MAS) spectra [68]. Other studies have used mesoporous silica as the host (MCM–41 and SBA–15) for LiBH4–Ca(BH4)2 infiltration and found that the mesoporous structure of the scaffold was altered even with low-energy ball milling [115] or reported on the infiltration of the same RHC into mesoporous carbon [116]. One should also note the affinity of the borohydride for the SiO2 and surface silanol groups, which may lead to another side reaction [115].
5.3. Additives Used to Improve H2 Storage Features of Calcium Borohydride
While still holding the highest theoretical hydrogen storage capacity of all known materials, complex hydrides still face many shortcomings related to sluggish kinetic and/or thermodynamic barriers that are hard to bypass. To tackle this issue, several additives have been used, with important improvements recorded: MgB2 yielded Ca(BH4)2 + MgB2 composite with 8.3 wt% gravimetric hydrogen capacity [119]; Co ion-loaded poly(acrylamide-co-acrylic acid) (p(Aam-co-Aac)-Co) hydrogel when a decrease in conversion rate from 98.5 to 83.5% was reported over ten a/d cycles [120,121]; urea yielded a new complex hydride Ca(BH4)2·4CO(NH2)2, releasing 5.2 wt% hydrogen below 250 °C [122]; 5 wt% CoCl2 doping in Ca(BH4)2-4LiNH2 afforded catalyzed RHC (Co NPs formed during ball milling as active catalytic species), releasing 7 wt% hydrogen at 178 °C [123]; CoCl2-doped Ca(BH4)2·2NH3 desorbed at 200 °C, releasing 7.6 wt% H2 with high purity [124]; Mg(BH4)2 yielded corresponding RHCs Mg(BH4)2/Ca(BH4)2 [92], MgF2 in Ca(BH4)2-MgF2 reversible system [125]; in situ generated TiO2 to afford novel CaB2H7/0.1TiO2 catalyzed hydride system [112]; particulate LaMg3 additive in the RHC Ca(BH4)2 + LiBH4 [78]; DFT investigation on Cr-doped α-Ca(BH4)2 [82]; TiF3 introduced via ball milling in Ca(BH4)2 [126]; NbF5 doping in Ca(BH4)2 + MgH2 [127]; Nb and Ti doping on Ca(BH4)2 investigated by DFT [128]; Co-Ni-Fe-P alloy as hydrolysis catalyst [129]; TiCl3 doping of Ca(BH4)2 yielded 2 wt% H2 reversible at 300 °C and 9 Mpa, while also suppressing borohydride melting [103]; 10 mol% addition of CaX2 (X=F, Cl) produced Ca(BH4)2–CaX2 mixtures, where CaX2 changed the dehydrogenation pathway [130].
Other reports deal with the additive role of calcium borohydride in the Mg(NH2)2–2LiH–0.1Ca(BH4)2 system, yielding CaH2 and LiBH4 during ball milling to account for 4.5 wt% storage capacity [131]; TiF4 and NbF5 doped in Ca(BH4)2-MgH2 RHC, where a slight improvement was seen in the case of NbF5 doping, and there was evidence of CaB12H12 formation along with the formation of active metal boride nanoparticulate species TiB2 and NbB2 (XANES data) with partial reversibility reported [132,133,134]; Ti(OiPr)4 and CaF2 [134]; TiF3 additive in the 2NaAlH4 + Ca(BH4)2 system generating Al3Ti and CaF2 with a synergistic catalytic role and affording improved a/d kinetics [135]; investigation of the boron effect in the Ca(BH4)2 + B system, showing only minor differences in the maximum rate of H2 evolution [136]; CaF2 replacement of CaH2 in CaH2 + MgB2 yielding the nonstoichiometric CaF2–xHx solid solution with a direct influence over a/d behavior of the system [137]; TiX3 (X=F, Cl) showing a superior effect of TiF3 on reversibility (57% yield) but not of TiCl3, despite their electronic similarities [138].
The catalytic role of additives in the synthesis of calcium borohydride from caB6 and CaH2 was confirmed by Ronnenbro et al. utilizing RuCl3 or, even better, mixtures of TiCl3 + Pd [11]. Another synthesis route of calcium borohydride starts from the CaH2 + MgB2 system, where the beneficial role of the boride additive AlB2 was reported by Schiavo et al. [139], or the role of fluoride-based additives in the same CaH2 + MgB2 system, as shown by Suarez-Alcantara et al., who investigated the systems 9CaH2 + CaF2 + 10MgB2, 10Ca(BH4)2 + 9MgH2 + MgF2, and 9Ca(BH4)2 + Ca(BF4)2 + 10MgH2 [59].
5.4. RHCs—Reactive Hydride Composites Containing Ca(BH4)2 or Its Precursors
There have been many attempts to catalyze Ca(BH4)2 and its RHCs; however, not all of them showed palpable results. For instance, while MgH2 was considerably improved using Ni (5 wt%) and Ni5Zr2 (5 wt%) catalysts, these catalysts showed very little influence over Ca(BH4)2 [140]. On the other hand, the system Ca(BH4)2–MgH2 was shown to produce the species Ca[B12H12] [141]. The same Ca(BH4)2–MgH2 RHC (10.5 wt% H2 theoretical, 6.4 wt% H2 desorbed within 3 h) has been characterized when catalyzed by 0.05 mol TiF4 and NbF5, yet the catalyst employed did not suppress dodecaborane anion formation, and only some minor improvements were recorded in the case of the NbF5 catalyst’s usage of TM fluorides, as shown by XANES data to form TM boride nanoparticles acting as active catalysts [132]. Minella et al. have also extended the study of Ca(BH4)2–MgH2 RHC by using Mg, which behaves as an adjuvant for heterogeneous nucleation of CaB6 formed during desorption [142]. CaB6 formation was also reported in the RHC 6LiBH4 + CaH2 (11.7 wt% theoretical), which afforded 9.1 wt% reversible storage capacity when catalyzed by 0.25 mol TiCl3 [143,144]. Other authors have investigated the role of the boron source (SiB4, FeB, and TiB2) on the production of Ca(BH4)2 from the hydrogenation of SiB4 and CaH2 [90].
The hydridic–protic interaction has been exploited in a series of RHCs containing borohydride–amide mixtures [145,146]. When the 1:4 molar ratio mixture Ca(BH4)2-4LiNH2 was ball milled, Equation (32) occurred, leading to the formation of Ca(NH2)2, LiBH4, and Li3(NH2)2BH4, affording a 26.2% reduction in EA compared to LiBH4-2LiNH2 chosen as reference [53].
Other reports describe a slightly different reaction pathway for the system Ca(BH4)2-4LiNH2, corresponding to Equation (33) [147]. The formation of mixed anion Ca(BH4)(NH2)2-x was also found by Morelle et al. in the RHC system Ca(BH4)2–xNaNH2 (x = 1, 2, and 3), showing release of NH3 (300 °C) and H2 (above 350 °C), and a decreasing decomposition temperature as the amide fraction of the composite increased, which advocates for the destabilization of starting Ca(BH4)2 by the amide compound (NaNH2) [148].
Other molar ratios of Ca(BH4)2-xLiNH2 have also been investigated, for instance, for x = 3 [149]. The intramolecular destabilization in the systems Ca(BH4)2-MNH2 (M=Li, Na) has also been evidenced by Poonyayant et al., when a metathetical reaction led to the formation of cation-mixed, anion-mixed complex hydrides of type mCa(BH4)2(NH2) with a release profile starting at ca. 150 °C through hydridic-protic interactions in a two-step release accounting for 9.3 wt% [98]. The Mn(BH4)2–CaH2 mixture system (1:1 molar, manual grinding) led to the more stable Ca(BH4)2 through a double exchange reaction, which effectively suppressed the formation of diborane in the utilized system [150].
Investigation of the Ca(BH4)2-2.5 Mg2NiH4 RHC was shown to proceed through three different pathways, depending on the hydrogen backpressure, which again highlights the aspects of chemical equilibrium (Equations (34) - 2.3 wt%, (35) - 4.2 wt%, (36) - 4.6 wt%) [151]. At 1 bar, the reaction proceeds via Equation (36), whereas at 20 or 50 bar, all three reactions occur simultaneously.
The influence of alkali metal amides on the desorption properties of calcium borohydride was studies by Chu et al. [152], concluding that the driving force of reactions (37) and (38) must lie in the interaction B–H and N–H present in the milled RHC M(NH2)2–Ca(BH4)2 (M=Mg, Ca).
Binary, ternary and quaternary mixtures in the LiBH4-NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2 system have been investigated by Dematteis et al. showing that new phases occurring might originate from the interaction Mg(BH4)2-Ca(BH4)2 [153,154,155]. This interaction in the Mg(BH4)2-Ca(BH4)2 was studied in the 5:1 molar ratio showing a destabilization effect of Ca(BH4)2 over Mg(BH4)2 [94,95].
Another improvement strategy regarding H2 storage performance is utilization of eutectic mixture, and 0.68 LiBH4-0.32 Ca(BH4)2 is a known and tested model system [156,157]. Interestingly, the eutectic mixture of borohydrides can release H2 below each individual components, and even a release onset below the melting of pristine complex hydride [157]. When used as an additive, Ca(BH4)2 can produce very notable improvements; Mg(NH2)2–2LiH–0.1Ca(BH4)2 for instance can release 4.5 wt% H2 with onset at 90 °C, and a rehydrogenation temperature of 60 °C [131]. The synergistic effect of the formed CaH2 and LiBH4 probably played a role in the improvement recorded [131]. Li et al. have studied the system 2LiNH2–MgH2–xCa(BH4)2 with a very low desorption onset of 80 °C and a 8.2 wt% hydrogen storage content (x = 0.3); the system 2LiNH2–MgH2–0.1Ca(BH4)2 was also studied. [158]. It’s worth noting that a tentative metathesis reaction of NaBH4 and CaCl3 did not yield the expected Ca(BH4)2 product, and il led to CaNa(BH4)1-xClx instead, which forms as a result of a diffusional process of one reagent into the other [46].
Other additives potentially leading to calcium borohydride have also been employed: 2LiBH4–CaH5 (featuring a lower 1.1 wt% storage capacity at a lower temperature of 270 °C) [159]. A combination of different complex hydrides (alanate–borohydride) was proposed by Moller et al., namely, NaAlH4 + Ca(BH4)2 [160]. This approach led to NaBH4 and Ca(AlH4)2 through a metathesis reaction, releasing ca. 6 wt% H2 below 400 °C [160]. The system NaAlH4 + Ca(BH4)2 has been investigated mechanistically by Mustafa et al., who identified various decomposition intermediates in the multi-step desorption of the RHC, like CaAlH5, Al, CaH2, Al4Ca, and Al2Ca (Figure 10) [135,161].
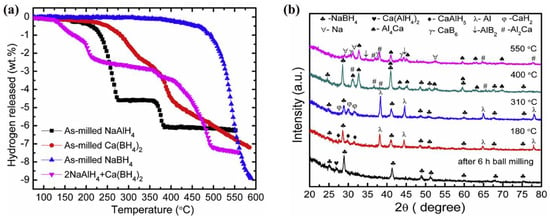
Figure 10.
(a) TPD curves of the as-milled NaAlH4, as-milled Ca(BH4)2, as-milled NaBH4, and 2NaAlH4 + Ca(BH4)2; (b) XRD patterns of the 2NaAlH4 + Ca (BH4)2 composite after 6 h of ball milling and after dehydrogenation at 180 °C, 310 °C, 400 °C, and 550 °C. Reprinted with permission from Ref. [161].
The ternary system Ca(BH4)2–LiBH4–MgH2 was reported to exhibit long-term cycling stability [162], the precursors CaH2–MgB2 thin films allowed identification of individual steps leading to the formation of Ca(BH4)2 [163], the effect of MgF2 additive was studied in the system Ca(BH4)2-MgF2 with up to 5.8 wt% H2 uptake at 330 °C after 2.5 h during the first three a/d cycles [125], the formation of β–Ca(BH4)2 in the γ-Mg(BH4)2-CaH2 RHC system [164], LaMg3-catalyzed Ca(BH4)2–LiBH4 with H2 release onset at 150 °C and good capacity retention of 70% after five a/d cycles [78], or DFT investigations in the KBH4–Ca(BH4)2 system [79].
When MgH2 is used, destabilization occurs, and a system with a total H2 capacity of 9.1 wt% is generated (Equation (39)) [165]. This type of reactivity was also proven by Kim et al., who demonstrated the reversibility of the Ca(BH4)2+MgH2 RHC, where the formation of CaB2 boride seems to be essential while the formation of a-B is a major downside [107].
Other RHCs like LiBH4-Ca(BH4)2 were investigated over a wide compositional domain by Lee et al. in xLiBH4+(1-x)Ca(BH4)2 (0 < x < 1) [166], by Yan et al. in the eutectic composite LiBH4+Ca(BH4)2 [167], or when nanoconfined in mesoporous scaffolds of type CMK-3 [68,116,118] or SBA-15 as support, with enhanced interface interaction [115]. In fact, combining nanocatalysts (NbF5) and nanoconfinement (CMK-3 ordered mesoporous carbon) afforded a total capacity of 13.3 wt% for the system LiBH4–Ca(BH4)2 in the resulting nanocomposite 0.68LiBH4–0.32Ca(BH4)2–0.05NbF5@CMK-3 [118].
xNaBH4-(1-x)Ca(BH4)2 composite, however, unlike the Mg(BH4)2 counterpart, yielded no eutectic melting, hence there was no improvement over H2 release temperature [168]. Employing organic borohydrides, such as guanidinium borohydride (GBH), led to a marked improvement in the Ca(BH4)2/C(NH2)3]+[BH4]− coupled system, which suppressed ammonia release and afforded ca. 10 wt% H2 evolution between 60 and 300 °C [169].
There have been attempts to increase the stability of Al(BH4)3 (volatile), and compounding it with Ca(BH4)2 by Titov et al. was one of them, leading to the formation of the complex Ca(BH4)2·Al(BH4)3 (slow reaction: 3–4 days) with a postulated structure [Ca(BH4)]+[Al(BH4)4−], in line with experimental IR data [24]. Some representative RHCs and their hydrogen storage performance are summarized in Table 3.

Table 3.
Hydrogen storage characteristics of RHCs based on Ca(BH4)2 and its precursors.
5.5. Adduct Formation and Dihydrogen Bonding in Solvates
From molecular insights into the dihydrogen bonding in borohydride ammoniates [174] to DFT modeling of chemical bonding [175,176] or the dehydrogenation mechanism in Ca(BH4)2·2NH3 [177,178], as well as experimental confirmation of the theoretical aspects [179], various adducts of Ca(BH4)2 have been described and investigated [39,179,180], including calcium tetradecahydroundecaborate Ca(B11H14)2·4Dg (Dg = diglyme) [181]. The first ammoniate of calcium borohydride was synthesized and characterized by Chu et al., who isolated it by direct synthesis from components Ca(BH4)2·2NH3 (space group Pbcn) featuring longer B–H and N–H bond lengths due to dihydrogen bonding, which facilitates the release of 11.3 wt% H2 at 250 °C [182]. DFT studies performed on Ca(BH4)2·2NH3 reveal that the Ea of 1.41 eV for thermal decomposition can be tuned by using metal dopants (Fe, Co, and Ni) that can modify the Fermi level [183]. While the adduct Ca(BH4)2·NH3 typically releases NH3 upon heating at 162–250 °C, when a multi-cation strategy was employed by Tang et al., the RHC Ca(BH4)2·NH3/LiBH4 released 12 wt% H2 of high purity (>99%) at 250 °C [184]. The reaction leading to such a behavior was a two-step process described by Equation (40) [184].
Furthermore, the addition of Mg(BH4)2 over Ca(BH4)2.nNH3 (n = 1, 2, 4) revealed an increase in H2 purity and storage capacity in the system Ca(BH4)2.4NH3–Mg(BH4)2, a synergy that inhibited ammonia release to yield >99% pure H2 upon thermal treatment at 500 °C [179].
Extending the scope of borohydride adducts of calcium borohydride, Li et al. studied the hydrogen release properties of calcium borohydride hydrazinates Ca(BH4)2.nN2H4 (n = 1, 4/3, 2, 3). Among these compounds, the monohydrazinate Ca(BH4)2.4/3 N2H4 showed 10.8 wt% H2 release with a dehydrogenation temperature of 140 °C when utilizing an Fe-based catalyst (2 wt% FeCl3) [185].
Other complexes have also been reported, such as the AB (ammonia borane) adducts of complex hydrides, mainly Ca(BH4)2(NH3BH3)2 and its lithium counterpart [186]. The AB adduct of calcium borohydride showed partially reversible behavior (2.4 wt%) under moderate conditions (82 bar H2, 400 °C), with a release of 11 wt% H2. Guadinate adducts of metal borohydrides have been reported by Wu et al. in the case of Li, Mg, and Ca complex hydrides MBH4·nCN3H5 [187]. The low contamination of gaseous decomposition products with ammonia and diborane was based on the C–N bonds of guanidine and H− anion of borohydrides. Ca(BH4)2·2CN3H5 released about 10 wt% H2 under modest conditions [187].
5.6. Reversibility Assessment
As a key element in advancing hydrogen storage materials, reversibility remains one of the toughest problems in solid-state energy storage [11,104,188,189,190]. The influence that metal fluorides exert on Ca(BH4)2 dehydrogenation/rehydrogenation was studied in the case of VF4, TiF4, and NbF5 [133]. Several systems were investigated and showed various degrees of reversibility, such as pristine [191] and TiF4- and NbF5–catalyzed Ca(BH4)2–MgH2 [132], Ca(BH4)2–Mg2NiH4 [151,192], LiBH4–Ca(BH4)2 [166], ternary RHCs with higher reversibility Ca(BH4)2–LiBH4–MgH2 [162], Ca(BH4)2–MgF2 [125], the implications of CaB12H12 on cycling capacity of calcium-based RHCs [41,193,194,195], eutectic mixture LiBH4–Ca(BH4)2 [156], the use of catalyst (TiCl3 [196]; TiF3, NbF5, NbCl5 [197]) on the reversibility of Ca(BH4)2 (rehydrogenation under 90 bar H2, 623 K, 3.8 wt% H2) [196].
The catalytic effect of Ca(BH4)2 was explored in a RHC Mg(NH2)2–2LiH–0.1Ca(BH4)2 composite (4.5 wt% H2 reversible, onset at 90 °C and release at 140 °C, and rehydrogenation with onset at 60 °C) [131]. Other RHCs serve as precursors for calcium borohydride, and these systems already showed promising results regarding cycling behavior, like LiBH4–Ca(AlH4)2 (4.5 wt% reversible, 450 °C) [198]. TiX3 (X=F, Cl) has been successfully used to lower the thermodynamic barriers of Ca(BH4)2 [138] or in RHCs 6LiBH4–CaH2 systems [143]. A critical step for the reversibility of Ca(BH4)2 is the formation of CaB6, presumably from a CaB2H6 precursor that can only be produced between 320 and 350 °C; otherwise, amorphous B would result, which is a clear bottleneck in the cycling pathway of calcium borohydride [104,199].
5.7. Ionic Conductivity
Ionic conductivity [173,200,201,202,203,204,205,206,207] and superconductivity [208,209] have also been investigated recently, with some surprising results and direct implications in the applicability of Ca(BH4)2 in electrolytes for batteries or even as high-temperature superconductors when properly doped, as DFT computations have revealed.
It has been hypothesized that Ca2+ might be too big for migration in Ca-based electrolytes, preferring an octahedral coordination by binding six ligands, but recent reports imply that using weaker coordinating ligands, such as B12H122− might increase Ca2+ mobility.
6. Ca(BH4)2 in Organic Synthesis and Organometallic Chemistry
6.1. Catalyst/Initiator
Calcium borohydride extends its utility in organic synthesis as well. For instance, the THF adduct [Ca(BH4)2(THF)2] was prepared from Ca(oMe)2 and BH3.THF reacting in THF solvent and used for the polymerization ε-caprolactone and L-lactide in a combined DFT and experimental investigation [210]. The active catalysts employed were produced by the treatment of [Ca(BH4)2(THF)2] with KCp* (Cp* = (η5-C5Me5)) and K{(Me3SiNPPh2)2CH}, namely producing dimeric heteroleptic mono-borohydride derivatives [Cp*Ca(BH4)(THF)n]2 and [{(Me3SiNPPh2)2CH}Ca(BH4)(THF)2] (Figure 11). These heteroleptic borohydrides were used as initiators for the ring-opening polymerization (ROP) of ε-caprolactone and L-lactide in good yields [210].
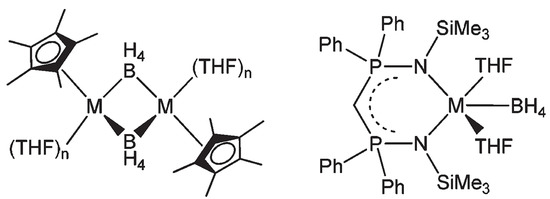
Figure 11.
Ca-based (M=Ca) borohydride complexes used for ROP reactions [210].
6.2. Reducing Agent
Borohydride compounds can typically be used for reduction reactions, given their [BH4]− anion-reducing characteristics. For example, Ca(BH4)2 afforded the reduction of the corresponding organic precursor to β-vanillyl-γ-butyrolactone [211] (Figure 12).
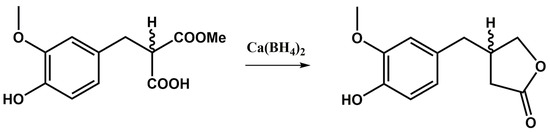
Figure 12.
Chemical reduction of organic esters and acids performed by calcium borohydride [211].
The reduction of β–keto esters was also shown to be effective when using Ca(BH4)2, yielding corresponding hydroxy-acids and, further on, water elimination to yield α, β—unsaturated acids [212]. Aliphatic and aromatic esters R(Ar)COOEt were fully reduced to alcohols E(Ar)CH2OH by Ca(BH4)2 in the presence of alkene catalysts such as 1-decene, cyclohexene [213,214], or cyclooctadiene (COD) [215].
A sulfurated modified calcium borohydride complex, Ca(BH2S3)2, was synthesized and used for the reduction of aryl azides and aryl nitro derivatives to their corresponding Ar-NH2 amino compounds when refluxed in THF, resulting in high yields [216]. The synthesis of Ca(BH2S3)2 proceeds via a metathetical reaction of NaBH2S3 and CaCl2 in THF, the driving force being the higher stability of the calcium-sulfurated borohydride compared to the sodium counterpart, which undergoes decomposition when exposed to air and moisture (Equation (41)) [216]. Additionally, Ca(BH2S3)2 can easily reduce carbonyl compounds (aldehydes, ketones, acyloins, α-diketones, α, β—unsaturated carbonyl compounds, azides, and carboxylic acid chlorides) to their corresponding alcohols in high yields [217].
With a lower reactivity towards esters compared to lithium borohydride, Ca(BH4)2 was used together with Grignard reagents in a molar ratio of 0.25 Ca(BH4)2:4 EtMgBr to produce the reduction of methyl esters RCOOMe in THF at room temperature to RCH2OH alcohol (6%), RCH(OH)Et (83%), and RC(OH)Et2 (11%) [218]. Both Ca(BH4)2 and Zn(BH4)2 were investigated in this reduction and showed similar results.
Another modified borohydride complex, calcium amidoborane Ca(NH2BH3)2 was used successfully to reduce α, β–unsaturated aldehydes and ketones (carbonyl compounds) to allylic alcohols [219]. Its synthesis involves the reaction of CaH2 and AB (ammonia borane) in THF (Equation (42)) [219].
6.3. Promoter of Cyclization in Various Reactions
Calcium borohydride was also involved as an active catalyst in σ-bond metathesis, a fundamental reaction in organic chemistry [220]. Notably, Bellham et al. reacted amine-borane t-BuNH2·BH3 with a β-diketiminate-supported silylamido calcium complex with elimination of HN(SiMe3)2 while also isolating the active catalyst, characterized by XRD and shown to belong to a polymeric infinite chain of calcium borohydride, in soluble in organic media, namely, [Ca(BH4)2·THF]∞ [221]. Access to polylactide macrocycles by cyclo-polymerization of L-lactide was reported to proceed in good yield (up to 77%, 20 min) when catalyzed by Ca(BH4)2 by intramolecular transesterification occurring during ROP [222,223,224].
6.4. Reaction Inhibitor and Miscellaneous Reactivity
There are reports of other derivatives of calcium borohydride, like the cyclopentadienyl complex Cp2Ca(THF)2 (obtained by mixing Ca(BH4)2 and CpNa in THF) and the methyl-substituted analog, (MeCp)2Ca(THF)2 (MeCp = ηS-CH3C5H4) [225]. Similarly, [BmMeBenz]2Ca(THF)2 was also obtained and characterized by the reaction of Ca(BH4)2·2THF with 1-methyl-1,3-dihydro-2H-benzimidazole-2-thione, where Ca is eight-coordinate and features two Ca…H-B interactions [226].
Ca(BH4)2 has served as the starting point for the synthesis of thorium and uranium metallocene borohydride complexes, allowing the isolation and structure determination of (C5Me5)2Th(η3-H3BH)2 [227].
Alternatively, calcium borohydride found its use as an inhibitor for the synthesis of fluorine-modified polysilazanes, leading to a solid, soluble fluorinated polysilazane suitable for metal substrates coating [228], or as a catalyst for regioselective hydroboration of terminal alkenes [214].
7. Remaining Challenges and Future Prospects
While bearing some similarities to its lighter counterpart Mg(BH4)2, calcium borohydride features some unique features like the relative ease of desolvation from synthesis adducts, different de-/rehydrogenation enthalpies, a lower and thus more feasible activation energy Ea, and different decomposition pathways. This decomposition could be further tuned, allowing the generation of high-purity hydrogen with small amounts of boranes.
The use of calcium borohydride as an energy storage material has the potential to impact the environment in several ways. It is essential to consider both the potential benefits and the hazards associated with the production, handling, and disposal of this complex hydride and its related composites. Regarding the production route, hazardous chemicals are involved in the synthesis of calcium borohydride as well as energy-intensive processes such as ball milling. These processes may involve the use of solvents, reagents, and catalysts that could have adverse effects on human health and the environment if not properly handled and managed. The production of calcium borohydride requires significant energy inputs, which, depending on the energy source, could contribute to greenhouse gas emissions. Handling complex hydrides involves some strict safety precautions needed to prevent accidental exposure and ensure safety. It is important to follow established safety protocols to minimize risks during transportation, storage, and usage of Ca(BH4)2. Being reactive towards moisture with the subsequent release of hydrogen gas, calcium borohydride can be flammable and potentially lead to fire or explosion hazards; therefore, adequate measures must be taken to prevent accidental ignition and control the release of hydrogen gas.
An important aspect linked to the circular economy and a sustainable hydrogen economy is the disposal of end-of-life hydrogen storage materials based on Ca(BH4)2. Since a reaction with water would lead to the release of hydrogen, proper disposal methods should be followed to ensure the safe handling of any H2 generated during disposal processes. The impact associated with the disposal of calcium borohydride waste, if not properly managed, could be linked to adverse environmental effects. It is important to adhere to local regulations and guidelines for the disposal of hazardous waste to prevent contamination of soil, water, or air. Furthermore, the overall environmental impact of calcium borohydride as an energy storage material can be evaluated by life cycle assessment studies that consider the potential environmental impact throughout the entire life cycle of the compound, including production, usage, and disposal, and could guide the development of more sustainable processes.
Reversibility perhaps remains the highest barrier to adopting Ca(BH4)2 as a mainstream hydrogen storage fuel, along with the currently rather cumbersome synthesis procedure. The formation of various rock-stable boron by-products (CaB6, for instance) or other very stable compounds still represents important research avenues to explore. The multistep decomposition can be altered by using catalysts/additives or by conducting the reaction in a suitable solvent capable of decreasing reaction enthalpies by forming borohydride adducts. Among TM-based catalysts, Nb and Ti showed the best results, lowering rehydrogenation conditions (350 °C, 24 h, 90 bar H2). These results add to other reports where nanoconfined Ca(BH4)2 in various nanosized supports, such as carbonaceous hosts [229], achieved a reliable H2 storage capacity, although much lower than the theoretical one.
Additionally, research efforts should focus on developing environmentally friendly synthesis routes, optimizing energy consumption during production, and exploring recycling methods for calcium borohydride waste. Adherence to regulations, responsible waste management, and continuous improvement in production and disposal practices are essential to minimizing the environmental footprint of calcium borohydride and ensuring its safe and sustainable use.
8. Conclusions
With a high production cost, calcium borohydride sits in an awkward place among solid-state hydrogen storage materials; it offers nearly 10 wt% H2 storage but with restricted reversibility and sluggish kinetics, which require the use of catalysts and potentially novel additives. Nanostructuring may be another avenue for researchers to follow in order to achieve improved behavior of Ca(BH4)2 during hydrogenation studies. Calcium borohydride Ca(BH4)2 remains one of the most promising tetrahydridoborates due to its high hydrogen storage capacity, the relative abundance of starting raw materials, and its pivotal role in catalysis for many decades.
The current study has highlighted the synthesis routes for Ca(BH4)2, the identified polymorphs, and various adducts that could potentially yield high-capacity hydrogen storage systems while also significantly reducing production costs. Catalyst/additive compounding is another route to improving a/d kinetics, together with nanosizing and/or nanoconfinement. The wide range of applications of Ca(BH4)2, from energy storage systems to batteries, organic synthesis, organometallic chemistry, and catalysis, have been reviewed.
Understanding the key steps and intermediates of the decomposition pathways of Ca(BH4)2 under different experimental conditions could allow further advances by tuning the thermodynamics and kinetics of hydrogen release/uptake. Other strategies focused on using RHCs containing Ca(BH4)2, while species containing B12H122− anion might be suitable in ion conductivity studies. Addressing the challenges related to synthesis methods, reaction mechanisms, hydrogen release kinetics, and overall device performance is essential for realizing its full potential. Further research is needed to bridge the existing knowledge gaps and unlock the practical applications of calcium borohydride in energy storage systems. Additionally, several reports of using Ca(BH4)2 in catalysis and organic synthesis as a reductant reaffirm the advantages of the plurivalent calcium borohydride.
Funding
This work was supported by the Romanian Ministry of Research and Innovation through Project No. PN-III-P1-1.1-TE-2021-1657 (TE 84/2022) and by the Core Program of the National Institute of Materials Physics, granted by the Romanian Ministry of Research, Innovation and Digitization through the Project PC3-PN23080303.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Romanian Ministry of Research and Innovation through Project No. PN-III-P1-1.1-TE-2021-1657 (TE 84/2022) and PN19-03 (contract no. PN21N/2019).
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Amieiro-Fonseca, A.; Ellis, S.R.; Nuttall, C.J.; Hayden, B.E.; Guerin, S.; Purdy, G.; Soulié, J.-P.; Callear, S.K.; Culligan, S.D.; David, W.I.F.; et al. A multidisciplinary combinatorial approach for tuning promising hydrogen storage materials towards automotive applications. Faraday Discuss. 2011, 151, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Comanescu, C. Recent Development in Nanoconfined Hydrides for Energy Storage. Int. J. Mol. Sci. 2022, 23, 7111. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, R.; Charkin, O.P.; Tomasi, J. Nonempirical study of the structure and stability of beryllium, magnesium, and calcium borohydrides. Inorg. Chem. 1991, 30, 2964–2969. [Google Scholar] [CrossRef]
- Comanescu, C. Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials 2022, 15, 2286. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Amdisen, M.B.; Skov, L.N.; Moller, K.T.; Kristensen, L.G.; Polanski, M.; Heere, M.; Jensen, T.R. New perspectives of functional metal borohydrides. J. Alloys Compd. 2022, 896, 163014. [Google Scholar] [CrossRef]
- Comanescu, C. Paving the Way to the Fuel of the Future—Nanostructured Complex Hydrides. Int. J. Mol. Sci. 2023, 24, 143. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Nakamori, Y.; Li, H.W.; Matsuo, M.; Miwa, K.; Towata, S.; Orimo, S. Development of metal borohydrides for hydrogen storage. J. Phys. Chem. Solids 2008, 69, 2292–2296. [Google Scholar] [CrossRef]
- Ronnebro, E. High-pressure techniques for discovering and re-hydrogenation of metal hydride materials. J. Phys. Chem. Solids 2010, 71, 1154–1158. [Google Scholar] [CrossRef]
- Ronnebro, E. Development of group II borohydrides as hydrogen storage materials. Curr. Opin. Solid State Mater. Sci. 2011, 15, 44–51. [Google Scholar] [CrossRef]
- Ronnebro, E.; Majzoub, E.H. Calcium borohydride for hydrogen storage: Catalysis and reversibility. J. Phys. Chem. B 2007, 111, 12045–12047. [Google Scholar] [CrossRef] [PubMed]
- Wolverton, C.; Siegel, D.J.; Akbarzadeh, A.R.; Ozolins, V. Discovery of novel hydrogen storage materials: An atomic scale computational approach. J. Phys.-Condens. Matter 2008, 20, 064228. [Google Scholar] [CrossRef] [PubMed]
- Barkhordarian, G.; Jensen, T.R.; Doppiu, S.; Bosenberg, U.; Borgschulte, A.; Gremaud, R.; Cerenius, Y.; Dornheim, M.; Klassen, T.; Bormann, R. Formation of Ca(BH4)2 from hydrogenation of CaH2 + MgB2 composite. J. Phys. Chem. C 2008, 112, 2743–2749. [Google Scholar] [CrossRef]
- He, L.Q.; Li, H.W.; Tumanov, N.; Filinchuk, Y.; Akiba, E. Facile synthesis of anhydrous alkaline earth metal dodecaborates MB12H12 (M = Mg, Ca) from M(BH4)2. Dalton Trans. 2015, 44, 15882–15887. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Lee, Y.S.; Cerny, R.; Sarusie, R.S.; Cho, Y.W.; Besenbacher, F.; Jensen, T.R. Ammine Calcium and Strontium Borohydrides: Syntheses, Structures, and Properties. Chemsuschem 2015, 8, 3472–3482. [Google Scholar] [CrossRef]
- Karabulut, A.F.; Guru, M.; Boynuegri, T.A.; Aydin, M.Y. Synthesis of Ca(BH4)2 from Synthetic Colemanite Used in Hydrogen Storage by Mechanochemical Reaction. J. Electron. Mater. 2016, 45, 3957–3963. [Google Scholar] [CrossRef]
- Lee, Y.S.; Filinchuk, Y.; Lee, H.S.; Suh, J.Y.; Kim, J.W.; Yu, J.S.; Cho, Y.W. On the Formation and the Structure of the First Bimetallic Borohydride Borate, LiCa3(BH4)(BO3)2. J. Phys. Chem. C 2011, 115, 10298–10304. [Google Scholar] [CrossRef]
- Titov, L.V. The synthesis of calcium borohydride. Dokl. Akad. Nauk 1964, 154, 654–656. [Google Scholar]
- Titov, L.V. Synthesis and chemical transformations of ionic octahydrotriborates: Cleavage of the B3H8-anion. Russ. J. Inorg. Chem. 2003, 48, 1471–1479. [Google Scholar]
- Richter, B.; Grinderslev, J.B.; Moller, K.T.; Paskevicius, M.; Jensen, T.R. From Metal Hydrides to Metal Borohydrides. Inorg. Chem. 2018, 57, 10768–10780. [Google Scholar] [CrossRef]
- Moller, K.T.; Fogh, A.S.; Paskevicius, M.; Skibsted, J.; Jensen, T.R. Metal borohydride formation from aluminium boride and metal hydrides. Phys. Chem. Chem. Phys. 2016, 18, 27545–27553. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ichikawa, T.; Miyaoka, H.; Tsubota, M.; Kojima, Y. Synthesis of Calcium Borohydride by Milling Hydrogenation of Hydride and Boride. J. Jpn. Inst. Met. Mater. 2013, 77, 609–614. [Google Scholar] [CrossRef]
- Rongeat, C.; Lindemann, I.; Borgschulte, A.; Schultz, L.; Gutfleisch, O. Effect of the presence of chlorides on the synthesis and decomposition of Ca(BH4)2. Int. J. Hydrogen Energy 2011, 36, 247–253. [Google Scholar] [CrossRef]
- Titov, L.V.; Eremin, E.R. Complex of aluminum borohydride with calcium borohydride. Bull. Acad. Sci. Ussr Div. Chem. Sci. 1975, 24, 1095–1096. [Google Scholar] [CrossRef]
- George, L.; Saxena, S.K. Structural stability of metal hydrides, alanates and borohydrides of alkali and alkali-earth elements: A review. Int. J. Hydrogen Energy 2010, 35, 5454–5470. [Google Scholar] [CrossRef]
- Karimi, F.; Pranzas, P.K.; Hoell, A.; Vainio, U.; Welter, E.; Raghuwanshi, V.S.; Pistidda, C.; Dornheim, M.; Klassen, T.; Schreyer, A. Structural analysis of calcium reactive hydride composite for solid state hydrogen storage. J. Appl. Crystallogr. 2014, 47, 67–75. [Google Scholar] [CrossRef]
- Llamas-Jansa, I.; Friedrichs, O.; Fichtner, M.; Bardaji, E.G.; Zuttel, A.; Hauback, B.C. The Role of Ca(BH4)2 Polymorphs. J. Phys. Chem. C 2012, 116, 13472–13479. [Google Scholar] [CrossRef]
- Sharma, M.; Sethio, D.; D’Anna, V.; Fallas, J.C.; Schouwink, P.; Cerny, R.; Hagemann, H. Isotope Exchange Reactions in Ca(BH4)2. J. Phys. Chem. C 2015, 119, 29–32. [Google Scholar] [CrossRef]
- Soloninin, A.V.; Babanova, O.A.; Skripov, A.V.; Hagemann, H.; Richter, B.; Jensen, T.R.; Filinchuk, Y. NMR Study of Reorientational Motion in Alkaline-Earth Borohydrides: Beta and gamma Phases of Mg(BH4)2 and alpha and beta Phases of Ca(BH4)2. J. Phys. Chem. C 2012, 116, 4913–4920. [Google Scholar] [CrossRef]
- Buchter, F.; Lodziana, Z.; Remhof, A.; Friedrichs, O.; Borgschulte, A.; Mauron, P.; Zuttel, A.; Sheptyakov, D.; Palatinus, L.; Chlopek, K.; et al. Structure of the Orthorhombic gamma-Phase and Phase Transitions of Ca(BD4)2. J. Phys. Chem. C 2009, 113, 17223–17230. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Matsumoto, M.; Miwa, K.; Towata, S.; Li, H.W.; Orimo, S. Crystal structure and charge density analysis of Ca(BH4)2. J. Alloys Compd. 2010, 491, 57–62. [Google Scholar] [CrossRef]
- Le, T.; Do, P.M. The Preferable F2dd Phase for Ca(BH4)2 Crystal Under Hydrostatic Pressures. J. Electron. Mater. 2017, 46, 3479–3483. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, Y.; Cho, Y.W.; Shapiro, D.; Wolverton, C.; Ozolins, V. Crystal structure and phonon instability of high-temperature β-Ca(BH4)2. Phys. Rev. B 2009, 79, 9. [Google Scholar] [CrossRef]
- Frankcombe, T.J. Calcium Borohydride for Hydrogen Storage: A Computational Study of Ca(BH4)2 Crystal Structures and the CaB2Hx Intermediate. J. Phys. Chem. C 2010, 114, 9503–9509. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Ronnebro, E.; Chandra, D. Crystal structures and phase transformations in Ca(BH4)2. Acta Mater. 2009, 57, 732–738. [Google Scholar] [CrossRef]
- Majzoub, E.H.; Ronnebro, E. Crystal Structures of Calcium Borohydride: Theory and Experiment. J. Phys. Chem. C 2009, 113, 3352–3358. [Google Scholar] [CrossRef]
- Stavila, V.; Her, J.H.; Zhou, W.; Hwang, S.J.; Kim, C.; Ottley, L.A.M.; Udovic, T.J. Probing the structure, stability and hydrogen storage properties of calcium dodecahydro-closo-dodecaborate. J. Solid State Chem. 2010, 183, 1133–1140. [Google Scholar] [CrossRef]
- Liu, A.; Xie, S.T.; Dabiran-Zohoory, S.; Song, Y. High-Pressure Structures and Transformations of Calcium Borohydride Probed by Combined Raman and Infrared Spectroscopies. J. Phys. Chem. C 2010, 114, 11635–11642. [Google Scholar] [CrossRef]
- Paolone, A.; Palumbo, O.; Rispoli, P.; Miriametro, A.; Cantelli, R.; Luedtke, A.; Ronnebro, E.; Chandra, D. Structural phase transitions and adduct release in calcium borohydride. J. Alloys Compd. 2011, 509, S691–S693. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.P.; Wei, S.L.; Zhou, D.; Wang, Y.C.; Wang, X.; Li, F.F.; Zhou, Q.; Liu, B.B.; Huang, X.L.; et al. New Phase of Ca(BH4)2 at Near Ambient Conditions. J. Phys. Chem. C 2018, 122, 14272–14276. [Google Scholar] [CrossRef]
- Grahame, A.; Aguey-Zinsou, K.-F. Properties and Applications of Metal (M) dodecahydro-closo-dodecaborates (Mn=1,2B12H12) and Their Implications for Reversible Hydrogen Storage in the Borohydrides. Inorganics 2018, 6, 106. [Google Scholar] [CrossRef]
- Riktor, M.D.; Filinchuk, Y.; Vajeeston, P.; Bardaji, E.G.; Fichtner, M.; Fjellvag, H.; Sorby, M.H.; Hauback, B.C. The crystal structure of the first borohydride borate, Ca3(BD4)3(BO3). J. Mater. Chem. 2011, 21, 7188–7193. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Kang, X.D.; Luo, J.H.; Wang, P.; Li, H.W.; Orimo, S. Formation and Hydrogen Storage Properties of Dual-Cation (Li, Ca) Borohydride. J. Phys. Chem. C 2010, 114, 22736–22741. [Google Scholar] [CrossRef]
- Jiang, K.; Xiao, X.Z.; Deng, S.S.; Zhang, M.; Li, S.Q.; Ge, H.W.; Chen, L.X. A Novel Li-Ca-B-H Complex Borohydride: Its Synthesis and Hydrogen Storage Properties. J. Phys. Chem. C 2011, 115, 19986–19993. [Google Scholar] [CrossRef]
- Lang, C.G.; Jia, Y.; Liu, J.W.; Wang, H.; Ouyang, L.Z.; Zhu, M.; Yao, X.D. Dehydrogenation and reaction pathway of Perovskite-Type NH4Ca(BH4)3. Prog. Nat. Sci. 2018, 28, 194–199. [Google Scholar] [CrossRef]
- Mattox, T.M.; Bolek, G.; Pham, A.L.; Kunz, M.; Liu, Y.S.; Fakra, S.C.; Gordon, M.P.; Doran, A.; Guo, J.H.; Urban, J.J. Calcium chloride substitution in sodium borohydride. J. Solid State Chem. 2020, 290, 121499. [Google Scholar] [CrossRef]
- Schouwink, P.; Morelle, F.; Sadikin, Y.; Filinchuk, Y.; Cerny, R. Increasing Hydrogen Density with the Cation-Anion Pair BH4−-NH4+ in Perovskite-Type NH4Ca(BH4)3. Energies 2015, 8, 8286–8299. [Google Scholar] [CrossRef]
- Grove, H.; Rude, L.H.; Jensen, T.R.; Corno, M.; Ugliengo, P.; Baricco, M.; Sorby, M.H.; Hauback, B.C. Halide substitution in Ca(BH4)2. Rsc Adv. 2014, 4, 4736–4742. [Google Scholar] [CrossRef]
- Rude, L.H.; Filinchuk, Y.; Sorby, M.H.; Hauback, B.C.; Besenbacher, F.; Jensen, T.R. Anion Substitution in Ca(BH4)2-CaI2: Synthesis, Structure and Stability of Three New Compounds. J. Phys. Chem. C 2011, 115, 7768–7777. [Google Scholar] [CrossRef]
- Aeberhard, P.C.; Refson, K.; Edwards, P.P.; David, W.I.F. High-pressure crystal structure prediction of calcium borohydride using density functional theory. Phys. Rev. B 2011, 83, 174102. [Google Scholar] [CrossRef]
- Aidhy, D.S.; Zhang, Y.S.; Wolverton, C. Prediction of a Ca(BH4)(NH2) quaternary hydrogen storage compound from first-principles calculations. Phys. Rev. B 2011, 84, 134103. [Google Scholar] [CrossRef]
- Albanese, E.; Corno, M.; Baricco, M.; Civalleri, B. Simulation of nanosizing effects in the decomposition of Ca(BH4)2 through atomistic thin film models. Res. Chem. Intermed. 2021, 47, 345–356. [Google Scholar] [CrossRef]
- Bai, Y.; Pei, Z.W.; Wu, F.; Wu, C. Role of Metal Electronegativity in the Dehydrogenation Thermodynamics and Kinetics of Composite Metal Borohydride-LiNH2 Hydrogen Storage Materials. ACS Appl. Mater. Interfaces 2018, 10, 9514–9521. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.; Riktor, M.D.; Maronsson, J.B.; Jacobsen, H.S.; Kehres, J.; Sveinbjornsson, D.; Bardaji, E.G.; Leon, A.; Juranyi, F.; Wuttke, J.; et al. Hydrogen Rotational and Translational Diffusion in Calcium Borohydride from Quasielastic Neutron Scattering and DFT Calculations. J. Phys. Chem. C 2010, 114, 20249–20257. [Google Scholar] [CrossRef]
- Franco, F.; Baricco, M.; Chierotti, M.R.; Gobetto, R.; Nervi, C. Coupling Solid-State NMR with GIPAW ab Initio Calculations in Metal Hydrides and Borohydrides. J. Phys. Chem. C 2013, 117, 9991–9998. [Google Scholar] [CrossRef]
- Borgschulte, A.; Gremaud, R.; Zuttel, A.; Martelli, P.; Remhof, A.; Ramirez-Cuesta, A.J.; Refson, K.; Bardaji, E.G.; Lohstroh, W.; Fichtner, M.; et al. Experimental evidence of librational vibrations determining the stability of calcium borohydride. Phys. Rev. B 2011, 83, 024102. [Google Scholar] [CrossRef]
- Bosenberg, U.; Pistidda, C.; Tolkiehn, M.; Busch, N.; Saldan, I.; Suarez-Alcantara, K.; Arendarska, A.; Klassen, T.; Dornheim, M. Characterization of metal hydrides by in-situ XRD. Int. J. Hydrogen Energy 2014, 39, 9899–9903. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sorby, M.H.; Chlopek, K.; Fichtner, M.; Buchter, F.; Zuettel, A.; Hauback, B.C. In situ synchrotron diffraction studies of phase transitions and thermal decomposition of Mg(BH4)2 and Ca(BH4)2. J. Mater. Chem. 2007, 17, 4939–4942. [Google Scholar] [CrossRef]
- Suarez-Alcantara, K.; Sorby, M.H.; Pistidda, C.; Karimi, F.; Saldan, I.; Hauback, B.C.; Klassen, T.; Dornheim, M. Synchrotron Diffraction Studies of Hydrogen Absorption/Desorption on CaH2 + MgB2 Reactive Hydride Composite Mixed With Fluorinated Compounds. J. Phys. Chem. C 2015, 119, 11430–11437. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Jensen, S.R.; Jensen, T.R.; Baricco, M. Heat capacity and thermodynamic properties of alkali and alkali-earth borohydrides. J. Chem. Thermodyn. 2020, 143, 106055. [Google Scholar] [CrossRef]
- Tomkinson, J.; Waddington, T.C. Inelastic Neutron Scattering from the Alkali Metal Borohydrides and Calcium Borohydride. J. Chem. Soc. Faraday Trans. 2 1976, 72, 528–538. [Google Scholar] [CrossRef]
- Dick, M.J.; Sheridan, P.M.; Wang, J.G.; Bernath, P.F. High resolution laser excitation spectroscopy of the B2E-X2A1 transitions of calcium and strontium monoborohydride. J. Chem. Phys. 2007, 126, 164311. [Google Scholar] [CrossRef]
- Fichtner, M.; Chlopek, K.; Longhini, M.; Hagemann, H. Vibrational spectra of Ca(BH4)2. J. Phys. Chem. C 2008, 112, 11575–11579. [Google Scholar] [CrossRef]
- Reed, D.; Book, D. Recent applications of Raman spectroscopy to the study of complex hydrides for hydrogen storage. Curr. Opin. Solid State Mater. Sci. 2011, 15, 62–72. [Google Scholar] [CrossRef]
- Kumar, S.; Nakajima, K.; Singh, A.; Kojima, Y.; Dey, G.K. Assessment of hydrogen storage property of Ca-Mg-B-H system using NMR and thermal analysis techniques. Int. J. Hydrogen Energy 2017, 42, 26007–26012. [Google Scholar] [CrossRef]
- Hwang, S.J.; Bowman, R.C.; Reiter, J.W.; Rijssenbeek, J.; Soloveichik, G.L.; Zhao, J.C.; Kabbour, H.; Ahn, C.C. NMR confirmation for formation of [B12H12]2− complexes during hydrogen desorption from metal borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Kim, C. Symmetrized Normal Mode Analysis of the Spin-Lattice Relaxation in Solid Calcium Borohydride. Bull. Korean Chem. Soc. 2021, 42, 792–797. [Google Scholar] [CrossRef]
- Lee, H.S.; Hwang, S.J.; Kim, H.K.; Lee, Y.S.; Park, J.; Yu, J.S.; Cho, Y.W. In Situ NMR Study on the Interaction between LiBH4-Ca(BH4)2 and Mesoporous Scaffolds. J. Phys. Chem. Lett. 2012, 3, 2922–2927. [Google Scholar] [CrossRef]
- Frankcombe, T.J. A comment on “Prediction of crystal structure, lattice dynamical, and mechanical properties of CaB2H2” by Vajeeston et al., Int. J. Hydrogen Energy 36 (2011) 10149–10158. Int. J. Hydrogen Energy 2012, 37, 2709–2710. [Google Scholar] [CrossRef]
- Han, C.L.; Dong, Y.Y.; Wang, B.Q.; Zhang, C.Y. [Ca(BH4)2]n Clusters as Hydrogen Storage Material: A DFT Study. Russ. J. Phys. Chem. A 2016, 90, 1997–2005. [Google Scholar] [CrossRef]
- Hattrick-Simpers, J.R.; Hurst, W.S.; Srinivasan, S.S.; Maslar, J.E. Optical cell for combinatorial in situ Raman spectroscopic measurements of hydrogen storage materials at high pressures and temperatures. Rev. Sci. Instrum. 2011, 82, 033103. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Aoki, M.; Noritake, T.; Ohba, N.; Nakamori, Y.; Towata, S.; Zuttel, A.; Orimo, S. Thermodynamical stability of calcium borohydride Ca(BH4)2. Phys. Rev. B 2006, 74, 155122. [Google Scholar] [CrossRef]
- Nakamori, Y.; Li, H.W.; Kikuchi, K.; Aoki, M.; Miwa, K.; Towata, S.; Orimo, S. Thermodynamical stabilities of metal-borohydrides. J. Alloys Compd. 2007, 446, 296–300. [Google Scholar] [CrossRef]
- Palade, P.; Comanescu, C.; Radu, C. Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride. Materials 2023, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Majzoub, E.; Ozolins, V.; Wolverton, C. Theoretical prediction of different decomposition paths for Ca(BH4)2 and Mg(BH4)2. Phys. Rev. B 2010, 82, 174107. [Google Scholar] [CrossRef]
- Kim, K.C.; Kulkarni, A.D.; Johnson, J.K.; Sholl, D.S. Examining the robustness of first-principles calculations for metal hydride reaction thermodynamics by detection of metastable reaction pathways. Phys. Chem. Chem. Phys. 2011, 13, 21520–21529. [Google Scholar] [CrossRef]
- Emdadi, A.; Demir, S.; Kislak, Y.; Tekin, A. Computational Screening of Dual-Cation Metal Ammine Borohydrides by Density Functional Theory. J. Phys. Chem. C 2016, 120, 13340–13350. [Google Scholar] [CrossRef]
- Gu, J.; Gao, M.X.; Wen, L.J.; Huang, J.J.; Liu, Y.F.; Pan, H.G. Improved hydrogen storage properties of combined Ca(BH4)2 and LiBH4 system motivated by addition of LaMg3 assisted with ball milling in H2. Int. J. Hydrogen Energy 2015, 40, 12325–12335. [Google Scholar] [CrossRef]
- Guo, Y.J.; Jia, J.F.; Wang, X.H.; Ren, Y.; Wu, H.S. Prediction of thermodynamically reversible hydrogen storage reactions in the KBH4/M(M = Li, Na, Ca)(BH4)n(n=1,2) system from first-principles calculation. Chem. Phys. 2013, 418, 22–27. [Google Scholar] [CrossRef]
- Guo, Y.J.; Ren, Y.; Wu, H.S.; Jia, J.F. Prediction of thermodynamically reversible hydrogen storage reactions utilizing Ca-M(M = Li, Na, K)-B-H systems: A first-principles study. J. Mol. Model. 2013, 19, 5135–5142. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Wang, L.L.; Johnson, D.D.; Sholl, D.S.; Johnson, J.K. First-Principles Characterization of Amorphous Phases of MB12H12, M = Mg, Ca. J. Phys. Chem. C 2010, 114, 14601–14605. [Google Scholar] [CrossRef]
- Huang, H.M.; Luo, S.J.; Xiong, Y.C. First principles investigations on the electronic properties of Cr doped α-Ca(BH4)2. Chin. J. Phys. 2017, 55, 870–875. [Google Scholar] [CrossRef]
- Lee, S.H.; Manga, V.R.; Liu, Z.K. Effect of Mg, Ca, and Zn on stability of LiBH4 through computational thermodynamics. Int. J. Hydrogen Energy 2010, 35, 6812–6821. [Google Scholar] [CrossRef]
- Liepinya, D.; Smeu, M. A Computational Comparison of Ether and Ester Electrolyte Stability on a Ca Metal Anode. Energy Mater. Adv. 2021, 2021, 9769347. [Google Scholar] [CrossRef]
- Mikhailin, A.A.; Charkin, D.O.; Klimenko, N.M.; Charkin, O.P. Theoretical study of elementary reactions of dehydrogenation of magnesium, calcium, zinc, and beryllium amminoborate and amminoalanate complexes. Russ. J. Inorg. Chem. 2013, 58, 416–426. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Yang, J.Z.; Fu, H.; Zheng, J.; Li, Y.; Li, X.G. Structural and electronic properties of the hydrogen storage compound Ca(BH4)2 2NH3 from first-principles. Comput. Mater. Sci. 2012, 54, 345–349. [Google Scholar] [CrossRef]
- Siegel, D.J.; Wolverton, C.; Ozolins, V. Thermodynamic guidelines for the prediction of hydrogen storage reactions and their application to destabilized hydride mixtures. Phys. Rev. B 2007, 76, 134102. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Fjellvåg, H. A new series of high hydrogen content hydrogen-storage materials—A theoretical prediction. J. Alloys Compd. 2007, 446, 44–47. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Fjellvag, H. Reply to “A comment on ‘Prediction of crystal structure, lattice dynamical, and mechanical properties of CaB2H2’ by Vajeeston et al., Int J Hydrogen Energy 36 (2011) 10149–10158” Discussion. Int. J. Hydrogen Energy 2012, 37, 2711–2712. [Google Scholar] [CrossRef]
- Cai, W.T.; Hou, J.M.; Huang, S.Y.; Chen, J.N.; Yang, Y.Z.; Tao, P.J.; Ouyang, L.Z.; Wang, H.; Yang, X.S. Altering the chemical state of boron towards the facile synthesis of LiBH4 via hydrogenating lithium compound-metal boride mixture. Renew. Energy 2019, 134, 235–240. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Moury, R.; Ould-Amara, S.; Demirci, U.B. Destabilization of Boron-Based Compounds for Hydrogen Storage in the Solid-State: Recent Advances. Energies 2021, 14, 7003. [Google Scholar] [CrossRef]
- Durojaiye, T.; Ibikunle, A.; Goudy, A.J. Hydrogen storage in destabilized borohydride materials. Int. J. Hydrogen Energy 2010, 35, 10329–10333. [Google Scholar] [CrossRef]
- Huang, J.J.; Gao, M.X.; Li, Z.L.; Cheng, X.B.; Gu, J.; Liu, Y.F.; Pan, H.G. Destabilization of combined Ca(BH4)2 and Mg(AlH4)2 for improved hydrogen storage properties. J. Alloys Compd. 2016, 670, 135–143. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Goudy, A.J. Kinetics and modeling study of a Mg(BH4)2/Ca(BH4)2 destabilized system. Int. J. Hydrogen Energy 2012, 37, 12420–12424. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Sabitu, S.T.; Goudy, A.J. Kinetics and modeling studies of the CaH2/LiBH4, MgH2/LiBH4, Ca(BH4)2 and Mg(BH4)2 systems. J. Alloys Compd. 2013, 556, 45–50. [Google Scholar] [CrossRef]
- Lai, Q.W.; Aguey-Zinsou, K.F. Destabilisation of Ca(BH4)2 and Mg(BH4)2 via confinement in nanoporous Cu2S hollow spheres. Sustain. Energy Fuels 2017, 1, 1308–1319. [Google Scholar] [CrossRef]
- Mao, J.F.; Guo, Z.P.; Yu, X.B.; Liu, H.K. Improved Hydrogen Storage Properties of NaBH4 Destabilized by CaH2 and Ca(BH4)2. J. Phys. Chem. C 2011, 115, 9283–9290. [Google Scholar] [CrossRef]
- Poonyayant, N.; Stavila, V.; Majzoub, E.H.; Klebanoff, L.E.; Behrens, R.; Angboonpong, N.; Ulutagay-Kartin, M.; Pakawatpanurut, P.; Hecht, E.S.; Breit, J.S. An Investigation into the Hydrogen Storage Characteristics of Ca(BH4)2/LiNH2 and Ca(BH4)2/NaNH2: Evidence of Intramolecular Destabilization. J. Phys. Chem. C 2014, 118, 14759–14769. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, S.A.; Shim, J.H.; Cho, Y.W. Thermal decomposition behavior of calcium borohydride Ca(BH4)2. J. Alloys Compd. 2008, 461, L20–L22. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.J.; Lee, Y.S.; Suh, J.Y.; Han, H.N.; Cho, Y.W. Hydrogen Back-Pressure Effects on the Dehydrogenation Reactions of Ca(BH4)2. J. Phys. Chem. C 2012, 116, 25715–25720. [Google Scholar] [CrossRef]
- Mao, J.F.; Guo, Z.P.; Poh, C.K.; Ranjbar, A.; Guo, Y.H.; Yu, X.B.; Liu, H.K. Study on the dehydrogenation kinetics and thermodynamics of Ca(BH4)2. J. Alloys Compd. 2010, 500, 200–205. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sorby, M.H.; Chlopek, K.; Fichtner, M.; Hauback, B.C. The identification of a hitherto unknown intermediate phase CaB2Hx from decomposition of Ca(BH4)2. J. Mater. Chem. 2009, 19, 2754–2759. [Google Scholar] [CrossRef]
- Lai, Q.W.; Milanese, C.; Aguey-Zinsou, K.F. Stabilization of Nanosized Borohydrides for Hydrogen Storage: Suppressing the Melting with TiCl3 Doping. ACS Appl. Energy Mater. 2018, 1, 421–430. [Google Scholar] [CrossRef]
- Yan, Y.; Rentsch, D.; Remhof, A. Controllable decomposition of Ca(BH4)2 for reversible hydrogen storage. Phys. Chem. Chem. Phys. 2017, 19, 7788–7792. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Miwa, K.; Noritake, T.; Ohba, N.; Matsumoto, M.; Li, H.W.; Nakamori, Y.; Towata, S.; Orimo, S. Structural and dehydriding properties of Ca(BH4)2. Appl. Phys. A 2008, 92, 601–605. [Google Scholar] [CrossRef]
- George, L.; Drozd, V.; Saxena, S.K.; Bardaji, E.G.; Fichtner, M. High-Pressure Investigation on Calcium Borohydride. J. Phys. Chem. C 2009, 113, 15087–15090. [Google Scholar] [CrossRef]
- Kim, Y.; Reed, D.; Lee, Y.S.; Lee, J.Y.; Shim, J.H.; Book, D.; Cho, Y.W. Identification of the Dehydrogenated Product of Ca(BH4)2. J. Phys. Chem. C 2009, 113, 5865–5871. [Google Scholar] [CrossRef]
- Sahle, C.J.; Sternemann, C.; Giacobbe, C.; Yan, Y.G.; Weis, C.; Harder, M.; Forov, Y.; Spiekermann, G.; Tolan, M.; Krisch, M.; et al. Formation of CaB6 in the thermal decomposition of the hydrogen storage material Ca(BH4)2. Phys. Chem. Chem. Phys. 2016, 18, 19866–19872. [Google Scholar] [CrossRef]
- Ampoumogli, A.; Charalambopoulou, G.; Javadian, P.; Richter, B.; Jensen, T.R.; Steriotis, T. Hydrogen desorption and cycling properties of composites based on mesoporous carbons and a LiBH4-Ca(BH4)2 eutectic mixture. J. Alloys Compd. 2015, 645, S480–S484. [Google Scholar] [CrossRef]
- Ampoumogli, A.; Steriotis, T.; Trikalitis, P.; Bardaji, E.G.; Fichtner, M.; Stubos, A.; Charalambopoulou, G. Synthesis and characterisation of a mesoporous carbon/calcium borohydride nanocomposite for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 16631–16635. [Google Scholar] [CrossRef]
- Comanescu, C.; Capurso, G.; Maddalena, A. Nanoconfinement in activated mesoporous carbon of calcium borohydride for improved reversible hydrogen storage. Nanotechnology 2012, 23, 385401. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gao, M.X.; Pan, H.G.; Liu, Y.F.; Li, B.; Yang, Y.J.; Liang, C.; Fu, H.L.; Guo, Z.X. Improved hydrogen storage performance of Ca(BH4)2: A synergetic effect of porous morphology and in situ formed TiO2. Eneryg Environ. Sci. 2013, 6, 847–858. [Google Scholar] [CrossRef]
- Hwang, S.J.; Lee, H.S.; To, M.; Lee, Y.S.; Cho, Y.W.; Choi, H.; Kim, C. Probing molecular dynamics of metal borohydrides on the surface of mesoporous scaffolds by multinuclear high resolution solid state NMR. J. Alloys Compd. 2015, 645, S316–S319. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4-Ca(BH4)2. Nano Energy 2015, 11, 96–103. [Google Scholar] [CrossRef]
- Lee, H.S.; Hwang, S.J.; To, M.; Lee, Y.S.; Cho, Y.W. Discovery of Fluidic LiBH4 on Scaffold Surfaces and Its Application for Fast Co-confinement of LiBH4-Ca(BH4)2 into Mesopores. J. Phys. Chem. C 2015, 119, 9025–9035. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, Y.S.; Suh, J.Y.; Kim, M.; Yu, J.S.; Cho, Y.W. Enhanced Desorption and Absorption Properties of Eutectic LiBH4-Ca(BH4)2 Infiltrated into Mesoporous Carbon. J. Phys. Chem. C 2011, 115, 20027–20035. [Google Scholar] [CrossRef]
- Nale, A.; Pendolino, F.; Maddalena, A.; Colombo, P. Enhanced hydrogen release of metal borohydrides M(BH4)n (M = Li, Na, Mg, Ca) mixed with reduced graphene oxide. Int. J. Hydrogen Energy 2016, 41, 11225–11231. [Google Scholar] [CrossRef]
- Zhai, B.; Xiao, X.; Lin, W.; Huang, X.; Fan, X.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Enhanced hydrogen desorption properties of LiBH4-Ca(BH4)2 by a synergetic effect of nanoconfinement and catalysis. Int. J. Hydrogen Energy 2016, 41, 17462–17470. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Boynuegri, T.A.; Guru, M. Catalytic dehydrogenation of calcium borohydride by using hydrogel catalyst. Int. J. Hydrogen Energy 2017, 42, 17869–17873. [Google Scholar] [CrossRef]
- Boynuegri, T.A.; Karabulut, A.F.; Guru, M. Synthesis of Borohydride and Catalytic Dehydrogenation by Hydrogel Based Catalyst. J. Electron. Mater. 2016, 45, 3949–3956. [Google Scholar] [CrossRef]
- Chu, H.L.; Qiu, S.J.; Liu, L.; Zou, Y.J.; Xiang, C.L.; Zhang, H.Z.; Xu, F.; Sun, L.X.; Zhou, H.Y.; Wu, G.T. Significantly enhanced dehydrogenation properties of calcium borohydride combined with urea. Dalton Trans. 2014, 43, 15291–15294. [Google Scholar] [CrossRef]
- Chu, H.L.; Qiu, S.; Sun, L.; Wu, G. Improved hydrogen desorption properties of Li-Ca-B-N-H system catalyzed by cobalt containing species. J. Renew. Sustain. Energy 2014, 6, 013105. [Google Scholar] [CrossRef]
- Chu, H.L.; Qiu, S.J.; Zou, Y.J.; Xiang, C.L.; Zhang, H.Z.; Xu, F.; Sun, L.X.; Zhou, H.Y. Improvement on Hydrogen Desorption Performance of Calcium Borohydride Diammoniate Doped with Transition Metal Chlorides. J. Phys. Chem. C 2015, 119, 913–918. [Google Scholar] [CrossRef]
- Gosalawit-Utke, R.; Suarez, K.; Von Colbe, J.M.B.; Bösenberg, U.; Jensen, T.R.; Cerenius, Y.; Minella, C.B.; Pistidda, C.; Barkhordarian, G.; Schulze, M.; et al. Ca(BH4)2-MgF2 reversible hydrogen storage: Reaction mechanisms and kinetic properties. J. Phys. Chem. C 2011, 115, 3762–3768. [Google Scholar] [CrossRef]
- Jansa, I.L.; Friedrichs, O.; Fichtner, M.; Bardají, E.G.; Züttel, A.; Hauback, B.C. Effects of Ball Milling and TiF3 Addition on the Dehydrogenation Temperature of Ca(BH4)2 Polymorphs. Energies 2020, 13, 4828. [Google Scholar] [CrossRef]
- Karimi, F.; Pranzas, P.K.; Pistidda, C.; Puszkiel, J.A.; Milanese, C.; Vainio, U.; Paskevicius, M.; Emmler, T.; Santoru, A.; Utke, R.; et al. Structural and kinetic investigation of the hydride composite Ca(BH4)2 + MgH2 system doped with NbF5 for solid-state hydrogen storage. Phys. Chem. Chem. Phys. 2015, 17, 27328–27342. [Google Scholar] [CrossRef]
- Wang, Z.G.; Hui, Q.; Wang, C.L.; Cheng, N.P. First-principle study of the effects of Nb and Ti doping on the dehydrogenation properties of Ca(BH4)2. Comput. Mater. Sci. 2011, 50, 3114–3118. [Google Scholar] [CrossRef]
- Kim, D.H.; Jo, S.; Kwon, J.; Lee, S.; Eom, K. Effect of iron content on the hydrogen production kinetics of electroless-deposited Co-Ni-Fe-P alloy catalysts from the hydrolysis of sodium borohydride, and a study of its feasibility in a new hydrolysis using magnesium and calcium borohydrides. Int. J. Hydrogen Energy 2019, 44, 15228–15238. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.S.; Suh, J.Y.; Shim, J.H.; Cho, Y.W. Metal halide doped metal borohydrides for hydrogen storage: The case of Ca(BH4)2-CaX2 (X = F, Cl) mixture. J. Alloys Compd. 2010, 506, 721–727. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.F.; Gu, J.; Gao, M.X.; Pan, H.G. Synergetic Effects of In Situ Formed CaH2 and LiBH4 on Hydrogen Storage Properties of the Li-Mg-N-H System. Chem.-Asian J. 2013, 8, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Minella, C.B.; Garroni, S.; Pistidda, C.; Baro, M.D.; Gutfleisch, O.; Klassen, T.; Dornheim, M. Sorption properties and reversibility of Ti(IV) and Nb(V)-fluoride doped—Ca(BH4)2-MgH2 system. J. Alloys Compd. 2015, 622, 989–994. [Google Scholar] [CrossRef]
- Minella, C.B.; Garroni, S.; Pistidda, C.; Gosalawit-Utke, R.; Barkhordarian, G.; Rongeat, C.; Lindemann, I.; Gutfleisch, O.; Jensen, T.R.; Cerenius, Y.; et al. Effect of Transition Metal Fluorides on the Sorption Properties and Reversible Formation of Ca(BH4)2. J. Phys. Chem. C 2011, 115, 2497–2504. [Google Scholar] [CrossRef]
- Minella, C.B.; Pellicer, E.; Rossinyol, E.; Karimi, F.; Pistidda, C.; Garroni, S.; Milanese, C.; Nolis, P.; Baro, M.D.; Gutfleisch, O.; et al. Chemical State, Distribution, and Role of Ti- and Nb-Based Additives on the Ca(BH4)2 System. J. Phys. Chem. C 2013, 117, 4394–4403. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Significant effect of TiF3 on the performance of 2NaAlH4+Ca(BH4)2 hydrogen storage properties. Int. J. Hydrogen Energy 2019, 44, 21979–21987. [Google Scholar] [CrossRef]
- Pendolino, F. “Boron Effect” on the Thermal Decomposition of Light Metal Borohydrides MBH4 (M = Li, Na, Ca). J. Phys. Chem. C 2012, 116, 1390–1394. [Google Scholar] [CrossRef]
- Pistidda, C.; Karimi, F.; Garroni, S.; Rzeszutek, A.; Minella, C.B.; Milanese, C.; Le, T.T.; Rude, L.H.; Skibsted, J.; Jensen, T.R.; et al. Effect of the Partial Replacement of CaH2 with CaF2 in the Mixed System CaH2 + MgB2. J. Phys. Chem. C 2014, 118, 28409–28417. [Google Scholar] [CrossRef]
- Rongeat, C.; D’Anna, V.; Hagemann, H.; Borgschulte, A.; Zuttel, A.; Schultz, L.; Gutfleisch, O. Effect of additives on the synthesis and reversibility of Ca(BH4)2. J. Alloys Compd. 2010, 493, 281–287. [Google Scholar] [CrossRef]
- Schiavo, B.; Girella, A.; Agresti, F.; Capurso, G.; Milanese, C. Ball-milling and AlB2 addition effects on the hydrogen sorption properties of the CaH2 + MgB2 system. J. Alloys Compd. 2011, 509, S714–S718. [Google Scholar] [CrossRef]
- Amama, P.B.; Spowart, J.E.; Voevodin, A.A.; Fisher, T.S. Modified Magnesium Hydride and Calcium Borohydride for High-Capacity Thermal Energy Storage. In Proceedings of the 8th ASME/JSME Thermal Engineering Joint Conference, Honolulu, HI, USA, 13–17 March 2011; American Society of Mechanical Engineers: Honolulu, HI, USA, 2011; pp. 1617–1623. [Google Scholar] [CrossRef]
- Minella, C.B.; Garroni, S.; Olid, D.; Teixidor, F.; Pistidda, C.; Lindemann, I.; Gutfleisch, O.; Baro, M.D.; Bormann, R.; Klassen, T.; et al. Experimental Evidence of Ca[B12H12] Formation During Decomposition of a Ca(BH4)2 + MgH2 Based Reactive Hydride Composite. J. Phys. Chem. C 2011, 115, 18010–18014. [Google Scholar] [CrossRef]
- Minella, C.B.; Pistidda, C.; Garroni, S.; Nolis, P.; Baro, M.D.; Gutfleisch, O.; Klassen, T.; Bormann, R.; Dornheim, M. Ca(BH4)2 + MgH2: Desorption Reaction and Role of Mg on Its Reversibility. J. Phys. Chem. C 2013, 117, 3846–3852. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S. Reversible hydrogen storage in the lithium borohydride—Calcium hydride coupled system. J. Alloys Compd. 2008, 464, L1–L4. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Qu, X.H. Investigation on LiBH4-CaH2 composite and its potential for thermal energy storage. Sci. Rep. 2017, 7, srep41754. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.J.; Chu, H.L.; Zou, Y.J.; Xiang, C.L.; Xu, F.; Sun, L.X. Light metal borohydrides/amides combined hydrogen storage systems: Composition, structure and properties. J. Mater. Chem. A 2017, 5, 25112–25130. [Google Scholar] [CrossRef]
- Yu, X.B.; Yang, Z.X.; Guo, Y.H.; Li, S.G. Thermal decomposition performance of Ca(BH4)2/LiNH2 mixtures. J. Alloys Compd. 2011, 509, S724–S727. [Google Scholar] [CrossRef]
- Chu, H.; Xiong, Z.; Wu, G.; Guo, J.; Zheng, X.; He, T.; Wu, C.; Chen, P. Hydrogen storage properties of Ca(BH4)2-LiNH2 system. Chem. Asian J. 2010, 5, 1594–1599. [Google Scholar] [CrossRef]
- Morelle, F.; Jepsen, L.H.; Jensen, T.R.; Sharma, M.; Hagemann, H.; Filinchuk, Y. Reaction Pathways in Ca(BH4)2-NaNH2 and Mg(BH4)2-NaNH2 Hydrogen-Rich Systems. J. Phys. Chem. C 2016, 120, 8428–8435. [Google Scholar] [CrossRef]
- Chu, H.L.; Xiong, Z.; Wu, G.; Guo, J.; He, T.; Chen, P. Improved dehydrogenation properties of Ca(BH4)2-LiNH2 combined system. Dalton Trans. 2010, 39, 10585–10587. [Google Scholar] [CrossRef]
- Roedern, E.; Jensen, T.R. Thermal Decomposition of Mn(BH4)2-M(BH4)x and Mn(BH4)2-MHx Composites with M = Li, Na, Mg, and Ca. J. Phys. Chem. C 2014, 118, 23567–23574. [Google Scholar] [CrossRef]
- Bergemann, N.; Pistidda, C.; Milanese, C.; Aramini, M.; Huotari, S.; Nolis, P.; Santoru, A.; Chierotti, M.R.; Chaudhary, A.L.; Baro, M.D.; et al. A hydride composite featuring mutual destabilisation and reversible boron exchange: Ca(BH4)2-Mg2NiH4. J. Mater. Chem. A 2018, 6, 17929–17946. [Google Scholar] [CrossRef]
- Chu, H.L.; Wu, G.T.; Zhang, Y.; Xiong, Z.T.; Guo, J.P.; He, T.; Chen, P. Improved Dehydrogenation Properties of Calcium Borohydride Combined with Alkaline-Earth Metal Amides. J. Phys. Chem. C 2011, 115, 18035–18041. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Baricco, M. Hydrogen Desorption in Mg(BH4)2-Ca(BH4)2 System. Energies 2019, 12, 3230. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Pistidda, C.; Dornheim, M.; Baricco, M. Exploring Ternary and Quaternary Mixtures in the LiBH4-NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2 System. Chemphyschem 2019, 20, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Dematteis, E.M.; Santoru, A.; Poletti, M.G.; Pistidda, C.; Klassen, T.; Dornheim, M.; Baricco, M. Phase stability and hydrogen desorption in a quinary equimolar mixture of light-metals borohydrides. Int. J. Hydrogen Energy 2018, 43, 16793–16803. [Google Scholar] [CrossRef]
- Javadian, P.; GharibDoust, S.P.; Li, H.W.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Reversibility of LiBH4 Facilitated by the LiBH4-Ca(BH4)2 Eutectic. J. Phys. Chem. C 2017, 121, 18439–18449. [Google Scholar] [CrossRef]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774–19789. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.F.; Gu, J.; Gu, Y.J.; Gao, M.X.; Pan, H.G. Mechanistic investigations on significantly improved hydrogen storage performance of the Ca(BH4)2-added 2LiNH2/MgH2 system. Int. J. Hydrogen Energy 2013, 38, 5030–5038. [Google Scholar] [CrossRef]
- Meggouh, M.; Grant, D.M.; Deavin, O.; Brunelli, M.; Hansen, T.C.; Walker, G.S. Investigation of the dehydrogenation behavior of the 2LiBH4:CaNi5 multicomponent hydride system. Int. J. Hydrogen Energy 2015, 40, 2989–2996. [Google Scholar] [CrossRef]
- Moller, K.T.; Grinderslev, J.B.; Jensen, T.R. A NaAlH4-Ca(BH4)2 composite system for hydrogen storage. J. Alloys Compd. 2017, 720, 497–501. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Yap, F.A.H.; Yahya, M.S.; Ismail, M. The hydrogen storage properties and reaction mechanism of the NaAlH4 + Ca(BH4)2 composite system. Int. J. Hydrogen Energy 2018, 43, 11132–11140. [Google Scholar] [CrossRef]
- Gao, M.; Gu, J.; Pan, H.; Wang, Y.; Liu, Y.; Liang, C.; Guo, Z. Ca(BH4)2-LiBH4-MgH2: A novel ternary hydrogen storage system with superior long-term cycling performance. J. Mater. Chem. A 2013, 1, 12285–12292. [Google Scholar] [CrossRef]
- Gonzalez-Silveira, M.; Gremaud, R.; Schreuders, H.; van Setten, M.J.; Batyrev, E.; Rougier, A.; Dupont, L.; Bardaji, E.G.; Lohstroh, W.; Dam, B. In-Situ Deposition of Alkali and Alkaline Earth Hydride Thin Films To Investigate the Formation of Reactive Hydride Composites. J. Phys. Chem. C 2010, 114, 13895–13901. [Google Scholar] [CrossRef]
- Grube, E.; Jensen, S.R.H.; Nielsen, U.G.; Jensen, T.R. Reactivity of magnesium borohydride—Metal hydride composites, gamma-Mg(BH4)2-MHx, M = Li, Na, Mg, Ca. J. Alloys Compd. 2019, 770, 1155–1163. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, Y.; Shim, J.H.; Lee, Y.S.; Suh, J.Y.; Ahn, J.P.; Kim, G.H.; Cho, Y.W. Microstructural Analysis of Dehydrogenation Products of the Ca(BH4)2-MgH2 Composite. Microsc. Microanal. 2013, 19, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Ravnsbaek, D.; Lee, Y.S.; Kim, Y.; Cerenius, Y.; Shim, J.H.; Jensen, T.R.; Hur, N.H.; Cho, Y.W. Decomposition Reactions and Reversibility of the LiBH4-Ca(BH4)2 Composite. J. Phys. Chem. C 2009, 113, 15080–15086. [Google Scholar] [CrossRef]
- Yan, Y.G.; Remhof, A.; Mauron, P.; Rentsch, D.; Lodziana, Z.; Lee, Y.S.; Lee, H.S.; Cho, Y.W.; Zuttel, A. Controlling the Dehydrogenation Reaction toward Reversibility of the LiBH4-Ca(BH4)2 Eutectic System. J. Phys. Chem. C 2013, 117, 8878–8886. [Google Scholar] [CrossRef]
- Ley, M.B.; Roedern, E.; Thygesen, P.M.M.; Jensen, T.R. Melting Behavior and Thermolysis of NaBH4-Mg(BH4)2 and NaBH4-Ca(BH4)2 Composites. Energies 2015, 8, 2701–2713. [Google Scholar] [CrossRef]
- Tang, Z.W.; Guo, Y.H.; Li, S.F.; Yu, X.B. Low-Temperature Hydrogen Generation and Ammonia Suppression from Calcium Borohydride Combined with Guanidinium Borohydride. J. Phys. Chem. C 2011, 115, 3188–3193. [Google Scholar] [CrossRef]
- Alcantara, K.S.; Boesenberg, U.; Zavorotynska, O.; von Colbe, J.B.; Taube, K.; Baricco, M.; Klassen, T.; Dornheim, M. Sorption and desorption properties of a CaH2/MgB2/CaF2 reactive hydride composite as potential hydrogen storage material. J. Solid State Chem. 2011, 184, 3104–3109. [Google Scholar] [CrossRef]
- Alcantara, K.S.; Ramallo-Lopez, J.M.; Boesenberg, U.; Saldan, I.; Pistidda, C.; Requejo, F.G.; Jensen, T.; Cerenius, Y.; Sorby, M.; Avila, J.; et al. 3CaH2 + 4MgB2 + CaF2 Reactive Hydride Composite as a Potential Hydrogen Storage Material: Hydrogenation and Dehydrogenation Pathway. J. Phys. Chem. C 2012, 116, 7207–7212. [Google Scholar] [CrossRef]
- Chaudhary, A.L.; Li, G.Q.; Matsuo, M.; Orimo, S.; Deledda, S.; Sorby, M.H.; Hauback, B.C.; Pistidda, C.; Klassen, T.; Dornheim, M. Simultaneous desorption behavior of M borohydrides and Mg2FeH6 reactive hydride composites (M = Mg, then Li, Na, K, Ca). Appl. Phys. Lett. 2015, 107, 073905. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Vaunois, S.; Pistidda, C.; Dornheim, M.; Baricco, M. Reactive Hydride Composite of Mg2NiH4 with Borohydrides Eutectic Mixtures. Crystals 2018, 8, 90. [Google Scholar] [CrossRef]
- Arumugam, S.; Angamuthu, A.; Gopalan, P. Molecular Insights on the Dihydrogen Bond Properties of Metal Borohydride Complexes upon Ammoniation. ECS J. Solid State Sci. Technol. 2021, 10, 091006. [Google Scholar] [CrossRef]
- Kiruthika, S.; Ravindran, P. Chemical Bonding Analysis on Amphoteric Hydrogen-Alkaline Earth Ammine Borohydrides. In Proceedings of the 62nd DAE Solid State Physics Symposium, Mumbai, India, 26–30 December 2017; American Institute of Physics: Mumbai, India, 2017. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.G.; Jiao, J.S.; Zhang, T.L.; Zhou, Z.N. A First-Principles Study: Structure and Decomposition of Mono-/Bimetallic Ammine Borohydrides. J. Phys. Chem. C 2014, 118, 8271–8279. [Google Scholar] [CrossRef]
- Chen, X.W.; Yu, X.B. Electronic Structure and Initial Dehydrogenation Mechanism of M(BH4)2∙2NH3 (M = Mg, Ca, and Zn): A First-Principles Investigation. J. Phys. Chem. C 2012, 116, 11900–11906. [Google Scholar] [CrossRef]
- Yuan, P.-F.; Wang, F.; Sun, Q.; Jia, Y.; Guo, Z.-X. Structural, energetic and thermodynamic analyses of Ca(BH4)2∙2NH3 from first principles calculations. J. Solid State Chem. 2012, 185, 206–212. [Google Scholar] [CrossRef]
- Chen, X.W.; Yuan, F.; Tan, Y.B.; Tang, Z.W.; Yu, X.B. Improved Dehydrogenation Properties of Ca(BH4)2·nNH3 (n = 1, 2, and 4) Combined with Mg(BH4)2. J. Phys. Chem. C 2012, 116, 21162–21168. [Google Scholar] [CrossRef]
- Lobkovskii, E.B.; Chekhlov, A.N.; Levicheva, M.D.; Titov, L.V. Crystalline and molecular-structure of calcium borohydride complex with dimethyl ether of diethyleneglycole-Ca(BH4)2 2DG. Koord. Khimiya 1988, 14, 543–550. [Google Scholar]
- Titov, L.V.; Petrovskii, P.V. Synthesis and Some Properties of Calcium Tetradecahydroundecaborate Ca(B11H14)2·4Dg (Dg = Diglyme). Russ. J. Inorg. Chem. 2011, 56, 1032–1033. [Google Scholar] [CrossRef]
- Chu, H.L.; Wu, G.T.; Xiong, Z.T.; Guo, J.P.; He, T.; Chen, P. Structure and Hydrogen Storage Properties of Calcium Borohydride Diammoniate. Chem. Mater. 2010, 22, 6021–6028. [Google Scholar] [CrossRef]
- Huang, Z.N.; Wang, Y.Q.; Zheng, L.; Wang, D.; Yang, F.S.; Wu, Z.; Wu, L.; Zhang, Z.X. Decomposition mechanisms and the role of transition metal impurities in the kinetics of Ca(BH4)2∙2NH3. Int. J. Hydrogen Energy 2020, 45, 2999–3007. [Google Scholar] [CrossRef]
- Tang, Z.W.; Tan, Y.B.; Gu, Q.F.; Yu, X.B. A novel aided-cation strategy to advance the dehydrogenation of calcium borohydride monoammoniate. J. Mater. Chem. 2012, 22, 5312–5318. [Google Scholar] [CrossRef]
- Li, Z.; He, T.; Wu, G.T.; Ju, X.H.; Chen, P. The synthesis, structure and dehydrogenation of calcium borohydride hydrazinates. Int. J. Hydrogen Energy 2015, 40, 5333–5339. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Pinkerton, F.E.; Meyer, M.S.; Srinivas, G.; Yildirim, T.; Udovic, T.J.; Rush, J.J. A new family of metal borohydride ammonia borane complexes: Synthesis, structures, and hydrogen storage properties. J. Mater. Chem. 2010, 20, 6550–6556. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.Q.; Rodriguez, E.E.; Zhou, W.; Udovic, T.J.; Yildirim, T.; Rush, J.J. A new family of metal borohydride guanidinate complexes: Synthesis, structures and hydrogen-storage properties. J. Solid State Chem. 2016, 242, 186–192. [Google Scholar] [CrossRef]
- Kim, Y.; Reed, D.; Lee, Y.S.; Shim, J.H.; Han, H.N.; Book, D.; Cho, Y.W. Hydrogenation reaction of CaH2-CaB6-Mg mixture. J. Alloys Compd. 2010, 492, 597–600. [Google Scholar] [CrossRef]
- Li, H.W.; Akiba, E.; Orimo, S. Comparative study on the reversibility of pure metal borohydrides. J. Alloys Compd. 2013, 580, S292–S295. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sorby, M.H.; Muller, J.; Bardaji, E.G.; Fichtner, M.; Hauback, B.C. On the rehydrogenation of decomposed Ca(BH4)2. J. Alloys Compd. 2015, 632, 800–804. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Tan, Q.W.; Zhang, Z.L.; Wan, Q.; Liu, Z.W.; Subramanian, A.; Qu, X.H. Thermal properties and cycling performance of Ca(BH4)2/MgH2 composite for energy storage. Chem. Phys. Lett. 2018, 700, 44–49. [Google Scholar] [CrossRef]
- Bergemann, N.; Pistidda, C.; Milanese, C.; Emmler, T.; Karimi, F.; Chaudhary, A.L.; Chierotti, M.R.; Klassen, T.; Dornheim, M. Ca(BH4)2-Mg2NiH4: On the pathway to a Ca(BH4)2 system with a reversible hydrogen cycle. Chem. Commun. 2016, 52, 4836–4839. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.J.; Shim, J.H.; Lee, Y.S.; Han, H.N.; Cho, Y.W. Investigation of the Dehydrogenation Reaction Pathway of Ca(BH4)2 and Reversibility of Intermediate Phases. J. Phys. Chem. C 2012, 116, 4330–4334. [Google Scholar] [CrossRef]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-Principles Prediction of Thermodynamically Reversible Hydrogen Storage Reactions in the Li-Mg-Ca-B-H System. J. Am. Chem. Soc. 2009, 131, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Graham, D.D.; Robertson, I.M.; Johnson, D.D. On the Reversibility of Hydrogen-Storage Reactions in Ca(BH4)2: Characterization via Experiment and Theory. J. Phys. Chem. C 2009, 113, 20088–20096. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, S.A.; Shim, J.H.; Cho, Y.W. Reversible hydrogen storage in calcium borohydride Ca(BH4)2. Scr. Mater. 2008, 58, 481–483. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, J.H.; Cho, Y.W. On the reversibility of hydrogen storage in Ti- and Nb-catalyzed Ca(BH4)2. J. Power Sources 2008, 181, 140–143. [Google Scholar] [CrossRef]
- Liu, D.M.; Gao, C.; Qian, Z.X.; Si, T.Z.; Zhang, Q.A. Reversible hydrogen storage in LiBH4/Ca(AlH4)2 systems. Int. J. Hydrogen Energy 2013, 38, 3291–3296. [Google Scholar] [CrossRef]
- Yan, Y.; Remhof, A.; Rentsch, D.; Züttel, A.; Giri, S.; Jena, P. A novel strategy for reversible hydrogen storage in Ca(BH4)2. Chem. Commun. 2015, 51, 11008–11011. [Google Scholar] [CrossRef]
- Didelot, E.; Cerny, R. Ionic conduction in bimetallic borohydride borate, LiCa3(BH4)(BO3)2. Solid State Ion. 2017, 305, 16–22. [Google Scholar] [CrossRef]
- Hahn, N.T.; Self, J.; Han, K.S.; Murugesan, V.; Mueller, K.T.; Persson, K.A.; Zavadil, K.R. Quantifying Species Populations in Multivalent Borohydride Electrolytes. J. Phys. Chem. B 2021, 125, 3644–3652. [Google Scholar] [CrossRef]
- Hahn, N.T.; Self, J.; Seguin, T.J.; Driscoll, D.M.; Rodriguez, M.A.; Balasubramanian, M.; Persson, K.A.; Zavadil, K.R. The critical role of configurational flexibility in facilitating reversible reactive metal deposition from borohydride solutions. J. Mater. Chem. A 2020, 8, 7235–7244. [Google Scholar] [CrossRef]
- Melemed, A.M.; Skiba, D.A.; Gallant, B.M. Toggling Calcium Plating Activity and Reversibility through Modulation of Ca2+ Speciation in Borohydride-Based Electrolytes. J. Phys. Chem. C 2022, 126, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Mezaki, T.; Kuronuma, Y.; Oikawa, I.; Kamegawa, A.; Takamura, H. Li-Ion Conductivity and Phase Stability of Ca-Doped LiBH4 under High Pressure. Inorg Chem 2016, 55, 10484–10489. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsson, D.; Blanchard, D.; Myrdal, J.S.G.; Younesi, R.; Viskinde, R.; Riktor, M.D.; Norby, P.; Vegge, T. Ionic conductivity and the formation of cubic CaH2 in the LiBH4-Ca(BH4)2 composite. J. Solid State Chem. 2014, 211, 81–89. [Google Scholar] [CrossRef]
- Yang, F.; Feng, X.; Zhuo, Z.; Vallez, L.; Liu, Y.-S.; McClary, S.A.; Hahn, N.T.; Glans, P.-A.; Zavadil, K.R.; Guo, J. Ca2+ Solvation and Electrochemical Solid/Electrolyte Interphase Formation toward the Multivalent-Ion Batteries. Arab. J. Sci. Eng. 2023, 48, 7243–7262. [Google Scholar] [CrossRef]
- Yang, J.; Jiao, Y.; Han, Y.-H.; Li, J. Pressure-Induced Metallization and Electrical Phase Diagram for Polycrystalline CaB6 under High Pressure and Low Temperature. Chin. Phys. Lett. 2016, 33, 086201. [Google Scholar] [CrossRef]
- Di Cataldo, S.; Boeri, L. Metal borohydrides as ambient-pressure high-Tc superconductors. Phys. Rev. B 2023, 107, L060501. [Google Scholar] [CrossRef]
- Yang, W.-H.; Lu, W.-C.; Qin, W.; Sun, H.-J.; Xue, X.-Y.; Ho, K.M.; Wang, C.Z. Pressure-induced superconductivity in the hydrogen-rich pseudobinary CaB-Hn compounds. Phys. Rev. B 2021, 104, 174106. [Google Scholar] [CrossRef]
- Kuzdrowska, M.; Annunziata, L.; Marks, S.; Schmid, M.; Jaffredo, C.G.; Roesky, P.W.; Guillaume, S.M.; Maron, L. Organometallic calcium and strontium borohydrides as initiators for the polymerization of epsilon-caprolactone and L-lactide: Combined experimental and computational investigations. Dalton Trans. 2013, 42, 9352–9360. [Google Scholar] [CrossRef]
- Daugan, A.; Brown, E. Lignanes, 11. Preparation of (R)-(+)-Beta-Vanyllyl-Gamma-Butyrolactone and Its Use in Total Synthesis of Natural Lignanes. J. Nat. Prod. 1991, 54, 110–118. [Google Scholar] [CrossRef]
- Matsui, M.; Miyano, M.; Tomita, K. Calcium Borohydride Reduction of Beta-Ketoesters. Bull. Agric. Chem. Soc. Jpn. 1956, 20, 139–140. [Google Scholar] [CrossRef]
- Narasimhan, S.; Prasad, K.G.; Madhavan, S. Calcium Borohydride—A Reagent for Facile Conversion of Carboxylic Esters to Alcohols and Aldehydes. Synth. Commun. 1995, 25, 1689–1697. [Google Scholar] [CrossRef]
- Narasimhan, S.; Prasad, K.G.; Madhavan, S. Remarkable Regioselective Hydroboration of Terminal Alkenes by Calcium Borohydride. Tetrahedron Lett. 1995, 36, 1141–1144. [Google Scholar] [CrossRef]
- Narasimhan, S.; Prasad, K.G.; Prasanna, R. Alkene-catalyzed reductions of esters by calcium borohydride. ChemInform 1993, 24, 102. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Tamami, B.; Kiasat, A.R. Efficient reduction of aryl azides and aryl nitro compounds to their corresponding amines with sulfurated calcium borohydride Ca(BH2S3)2. A new modified borohydride agent. Synth. Commun. 2000, 30, 587–596. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Tamami, B.; Kiasat, A.R. Efficient reduction of organic compounds with sulfurated calcium borohydride Ca(S3BH2)2, a new and stable modified borohydride reagent. Phosphorus Sulfur Silicon Relat. Elem. 2000, 159, 99–108. [Google Scholar] [CrossRef]
- Hallouis, S.; Saluzzo, C.; Amouroux, R. Grignard reagent/borohydride combinations alkylation/reduction of esters. Synth. Commun. 2000, 30, 313–324. [Google Scholar] [CrossRef]
- Xu, W.L.; Wang, R.M.; Wu, G.T.; Chen, P. Calcium amidoborane, a new reagent for chemoselective reduction of alpha, beta-unsaturated aldehydes and ketones to allylic alcohols. Rsc Adv. 2012, 2, 6005–6010. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Crimmin, M.R.; Hill, M.S.; Hitchcock, P.B.; Procopiou, P.A. Reactions of beta-diketiminate-stabilized calcium amides with 9-borabicyclo [3.3.1] nonane (9-BBN). Organometallics 2007, 26, 4076–4079. [Google Scholar] [CrossRef]
- Bellham, P.; Hill, M.S.; Kociok-Kohn, G. Stoichiometric and Catalytic Reactivity of tert-Butylamine Borane with Calcium Silylamides. Organometallics 2014, 33, 5716–5721. [Google Scholar] [CrossRef]
- Bonnet, F.; Stoffelbach, F.; Fontaine, G.; Bourbigot, S. Continuous cyclo-polymerisation of L-lactide by reactive extrusion using atoxic metal-based catalysts: Easy access to well-defined polylactide macrocycles. RSC Adv. 2015, 5, 31303–31310. [Google Scholar] [CrossRef]
- Collins, R.A.; Unruangsri, J.; Mountford, P. Synthesis and rac-lactide ring-opening polymerisation studies of new alkaline earth tetrahydroborate complexes. Dalton Trans. 2013, 42, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Cushion, M.G.; Mountford, P. Cationic and charge-neutral calcium tetrahydroborate complexes and their use in the controlled ring-opening polymerisation of rac-lactide. Chem. Commun. 2011, 47, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.P.; Makhaev, V.D. Preparation of calcium cyclopentadienyl complexes using calcium borohydride. Russ. Chem. Bull. 1993, 42, 339–340. [Google Scholar] [CrossRef]
- Chakrabarti, N.; Quinlivan, P.J.; Parkin, G. Synthesis and Structural Characterization of [BmMeBenz)2Ca(THF)2: Ca⋯H-B interactions in a Sulfur-Rich Coordination Environment. J. Mex. Chem. Soc. 2017, 61, 90–96. [Google Scholar] [CrossRef]
- Erickson, K.A.; Scott, B.L.; Kiplinger, J.L. Ca(BH4)2 as a simple tool for the preparation of thorium and uranium metallocene borohydride complexes: First synthesis and crystal structure of (C5Me5)2Th(η3-H3BH)2. Inorg. Chem. Commun. 2017, 77, 44–46. [Google Scholar] [CrossRef]
- Furtat, P.; Lenz-Leite, M.; Ionescu, E.; Machado, R.A.F.; Motz, G. Synthesis of fluorine-modified polysilazanes via Si-H bond activation and their application as protective hydrophobic coatings. J. Mater. Chem. A 2017, 5, 25509–25521. [Google Scholar] [CrossRef]
- Comanescu, C. Graphene Supports for Metal Hydride and Energy Storage Applications. Crystals 2023, 13, 878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).