Efficiency Enhancement of the Single Line Multi-Stage Gasification of Hungarian Low-Rank Coal: Effects of Gasification Temperature and Steam/Carbon (S/C) Ratio
Abstract
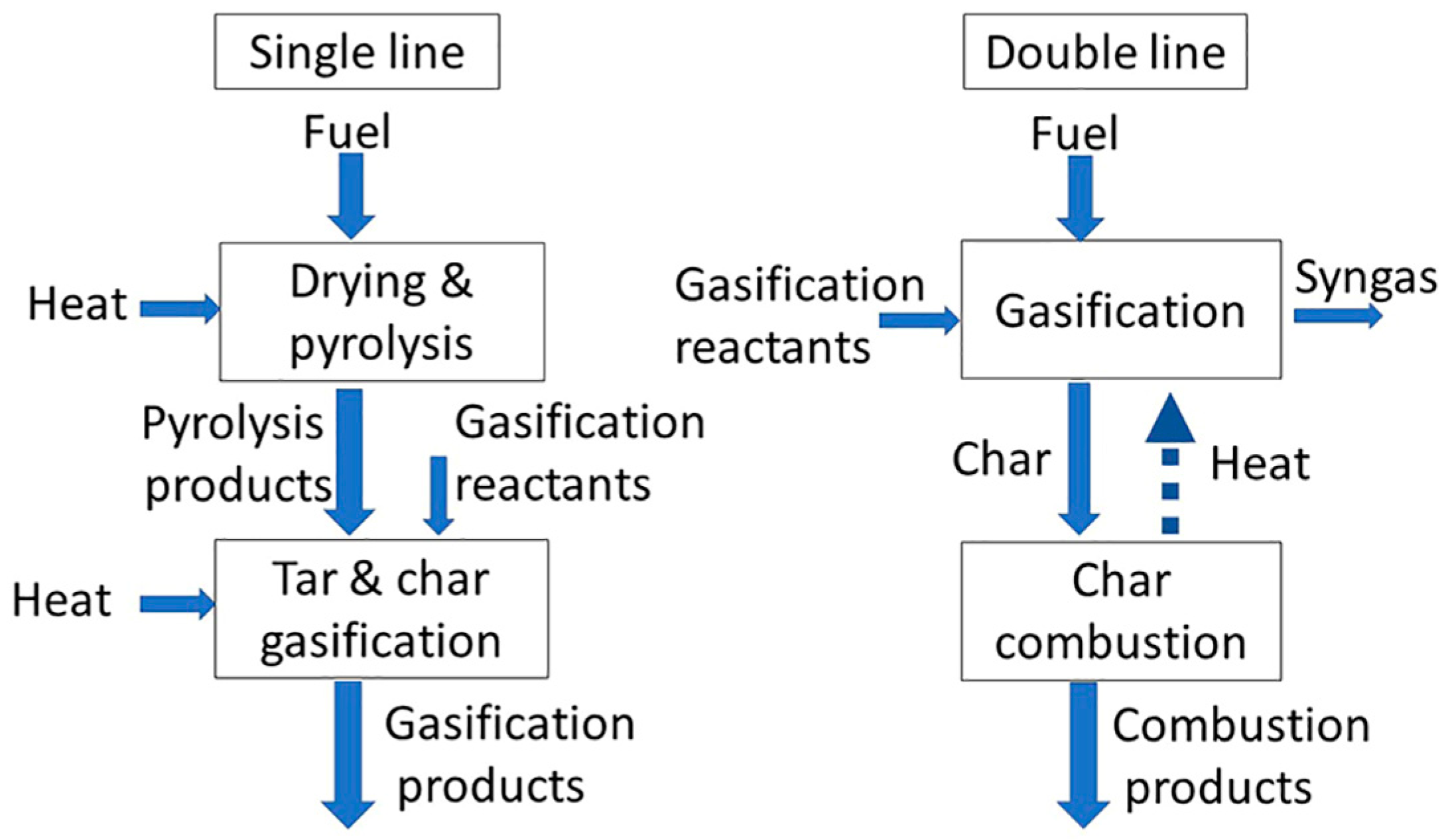
1. Introduction
2. Materials and Methods
2.1. Material
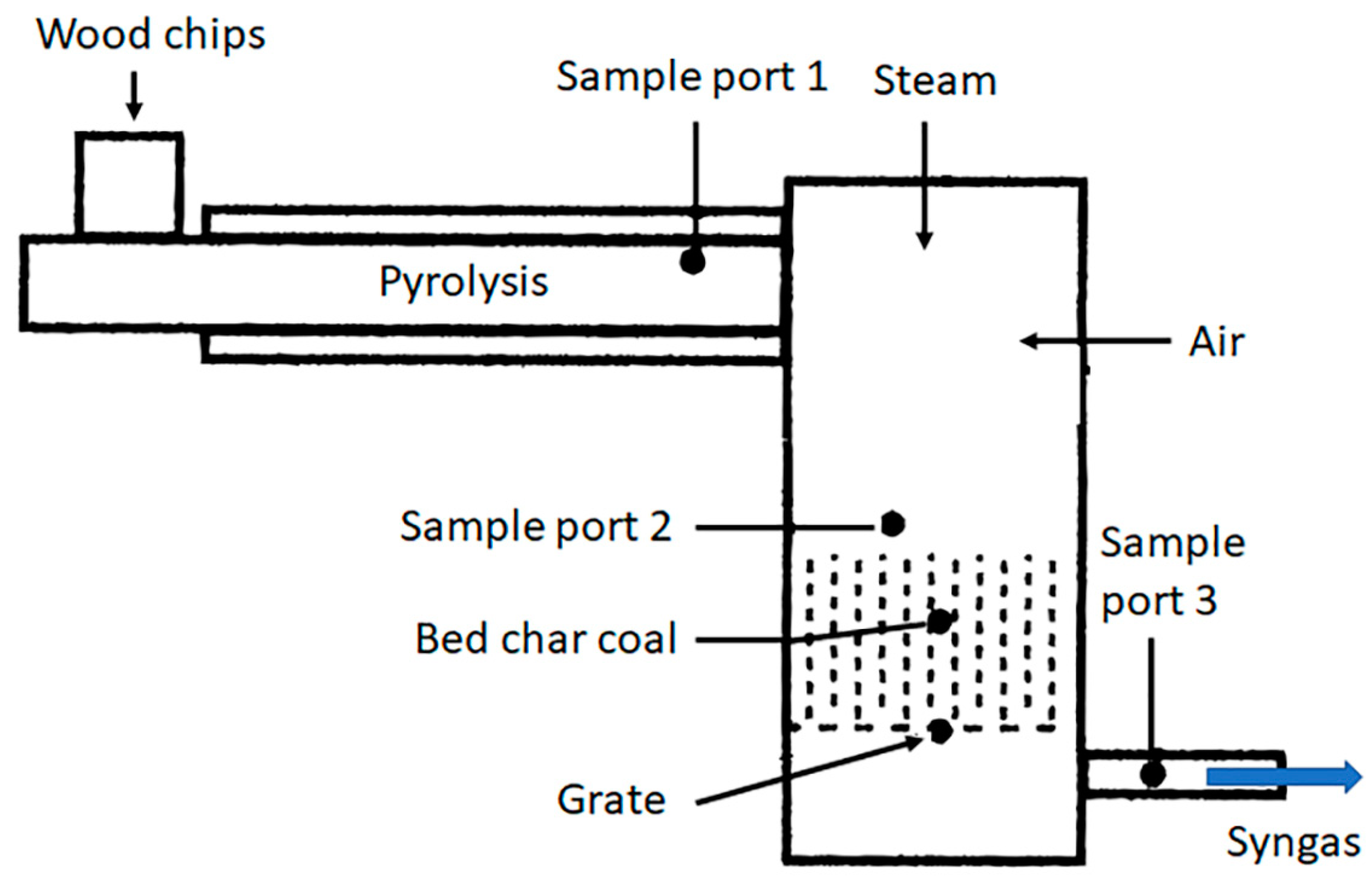
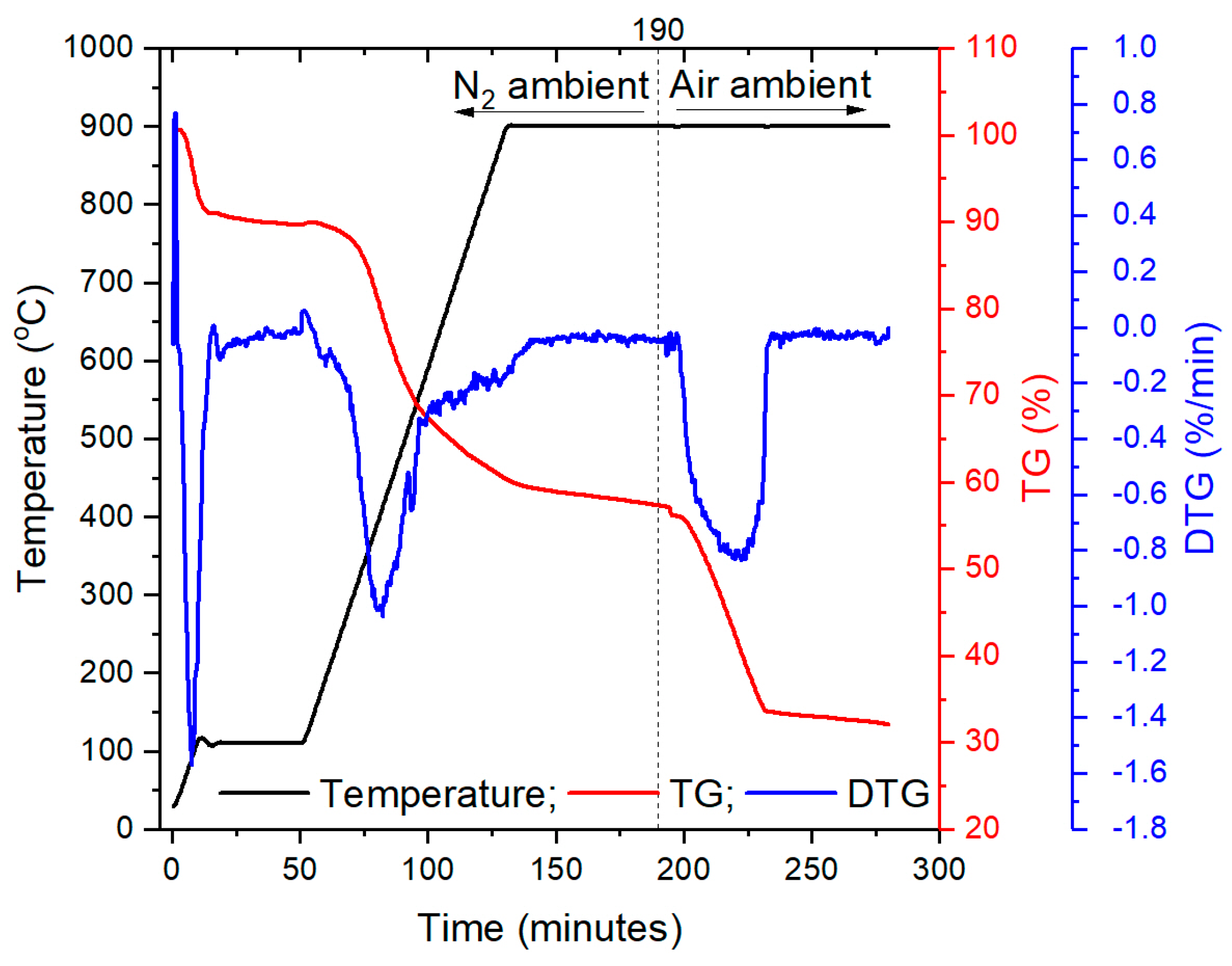
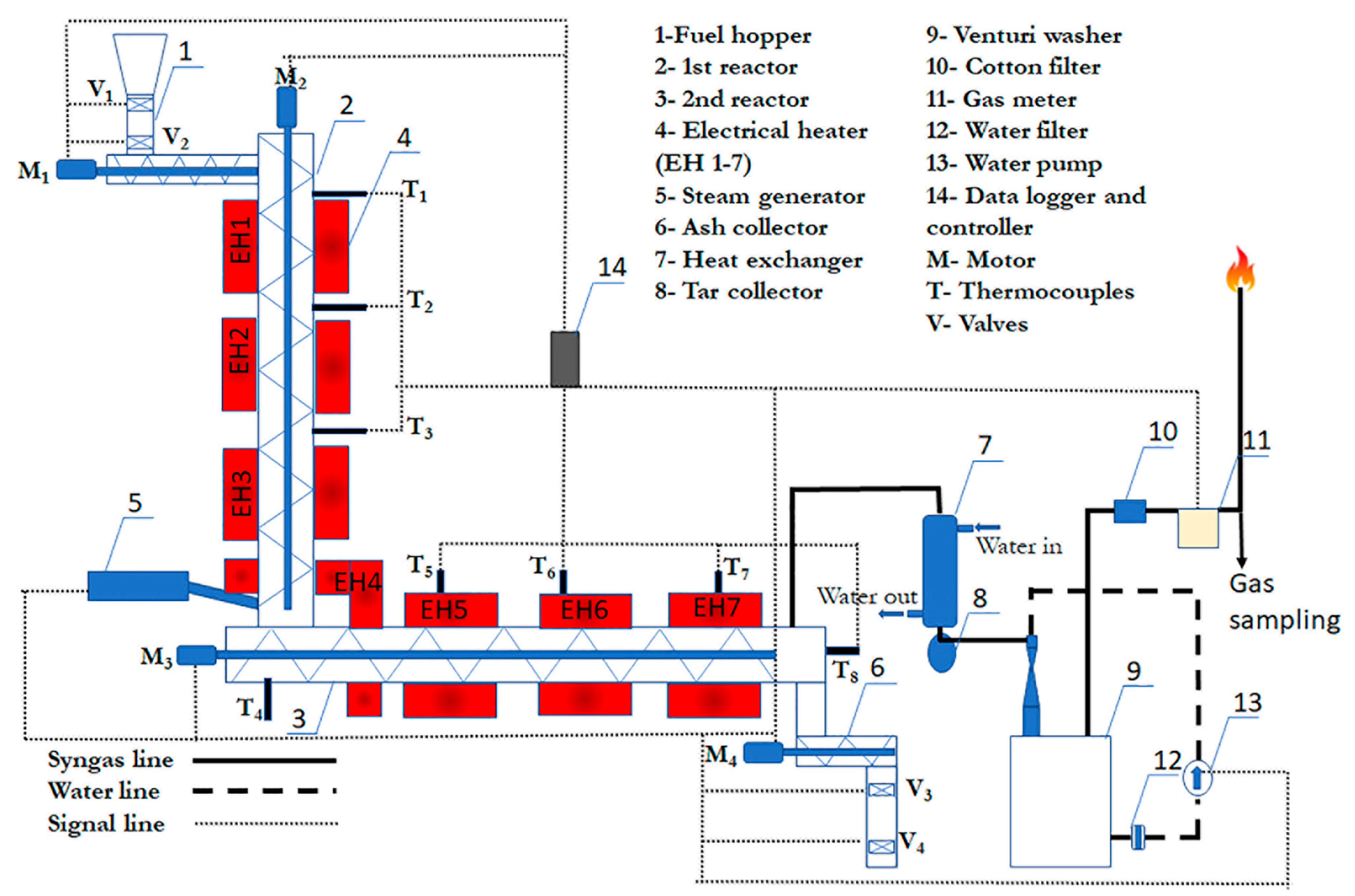
2.2. Experimental Apparatus
2.3. Methods of Data Processing
3. Results and Discussions
3.1. Effects of Gasification Temperature and Steam Flow Rate on Gasification Products
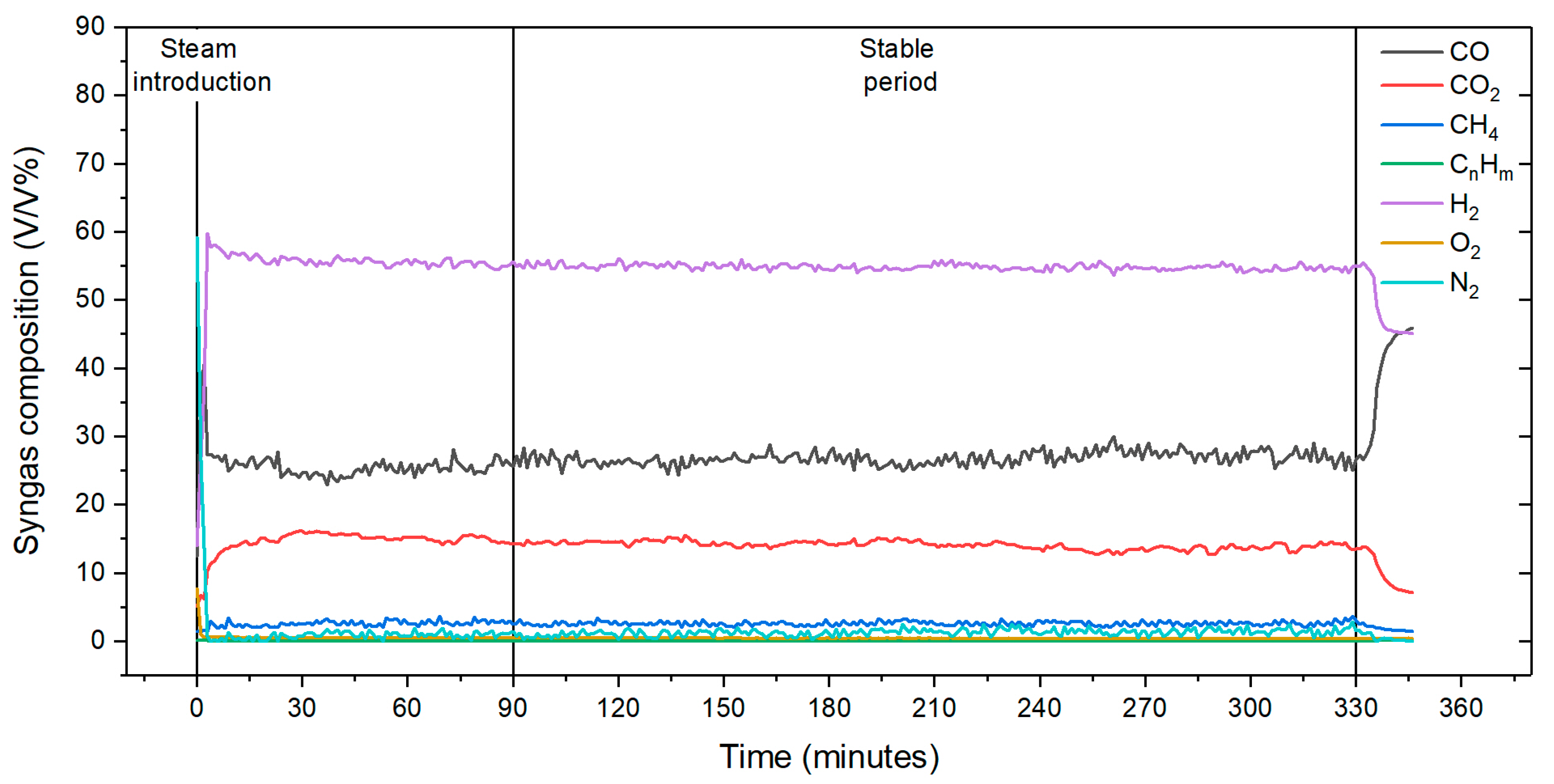
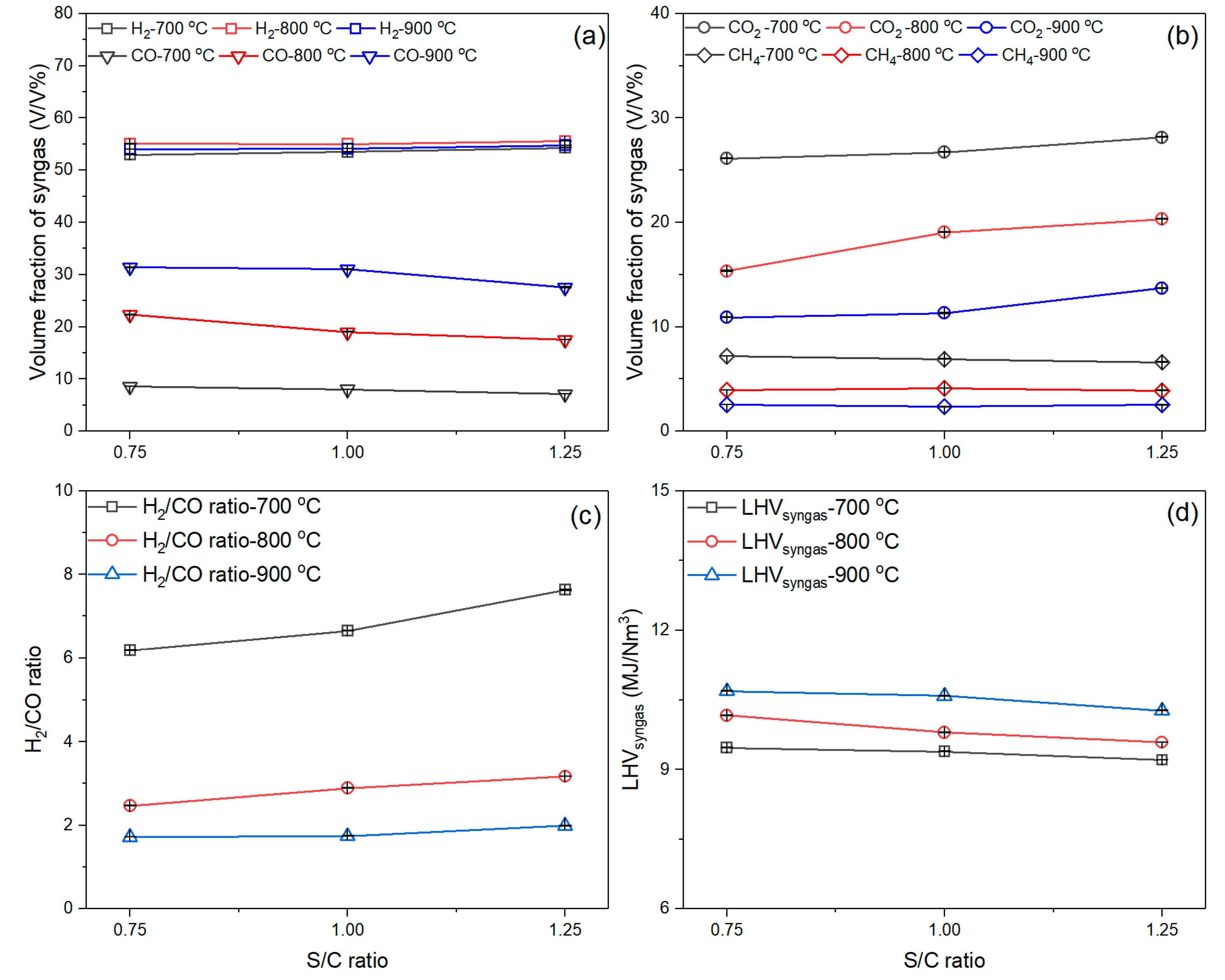
3.2. Effects of Gasification Temperature and Steam Flow Rate on Syngas Composition
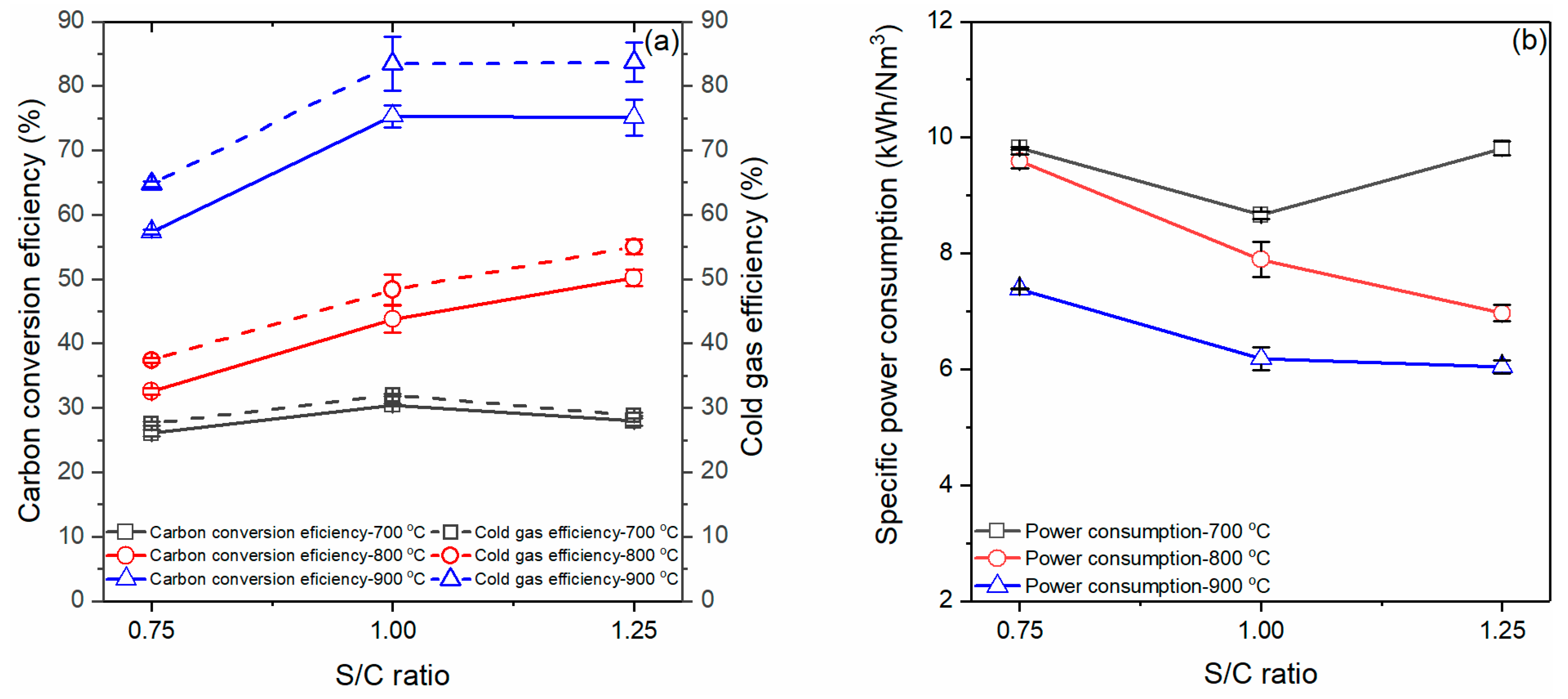
3.3. Effects of Gasification Temperature and Steam Flow Rate on Gasification Efficiency
4. Conclusions
- The higher gasification temperature significantly improved the gasification efficiency, as a decrease in char yield and an increase in dry syngas yield. The positive effects of the increasing S/C ratio on char gasification were limited, especially at 700 and 900 °C. The excess steam lowered the dry gas yield in the case of 700 °C of the gasification temperature. The highest dry syngas yield was 1.14 Nm3/kgcoal at 900 °C and the S/C ratio of 1.25.
- The increase in the gasification temperature had a significant effect on the syngas composition. Meanwhile, the syngas concentration varied slightly when the S/C ratio increased from 0.75 to 1.25. From a chemical utilization point of view, the gasification temperature at 900 °C and the S/C ratio of 1.25 resulted in the most promising H2/CO ratio of 1.99. From an energetic point of view, the highest LHVsyngas was observed at 900 °C and the S/C ratio of 0.75, about 10.69 MJ/Nm3.
- The carbon conversion also reached the highest values at 900 °C and the S/C ratio of 1.00, around 75.32%. The highest value of cold gas efficiency was 83.69% at 900 °C and the S/C ratio of 1.25.
- The experimental results showed that the multi-stage gasification process is a viable solution to improve not only the use value of Hungarian brown coal but also the carbon emission reduction by producing a synthesis gas that is readily usable for methanol synthesis.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Gasification Temperature | |||||||
|---|---|---|---|---|---|---|---|
| 700 °C | 800 °C | 900 °C | |||||
| S/C ratio | Char (g/h) | Liquid (g/h) | Char (g/h) | Liquid (g/h) | Char (g/h) | Liquid (g/h) | |
| 0.75 | 1 | 625.70 | 329.50 | 547.30 | 141.10 | 470.00 | 90.60 |
| 2 | 629.80 | 326.30 | 621.05 | 151.50 | 502.90 | 83.40 | |
| 3 | 612.70 | 327.90 | 630.60 | 132.30 | 491.90 | 81.30 | |
| Mean value | 622.73 | 327.90 | 599.65 | 141.90 | 488.27 | 85.10 | |
| 1.00 | 1 | 568.00 | 410.80 | 568.90 | 260.40 | 417.00 | 169.10 |
| 2 | 588.80 | 409.60 | 584.50 | 273.60 | 401.60 | 185.60 | |
| 3 | 585.80 | 417.10 | 550.50 | 273.50 | 402.70 | 170.60 | |
| Mean value | 580.87 | 412.50 | 567.97 | 269.17 | 407.10 | 175.10 | |
| 1.25 | 1 | 593.60 | 431.30 | 499.70 | 359.90 | 409.00 | 277.40 |
| 2 | 596.30 | 435.00 | 484.00 | 338.00 | 363.60 | 263.50 | |
| 3 | 613.40 | 431.40 | 490.00 | 310.70 | 346.50 | 266.90 | |
| Mean value | 601.10 | 432.57 | 491.23 | 336.20 | 373.03 | 269.27 | |
| Gasification Temperature | S/C Ratio | Syngas Composition (V/V%) | LHVsyngas | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO | CO2 | CH4 | CnHm | H2 | O2 | N2 | MJ/Nm3 | |||
| 700 | 0.75 | Mean | 8.56 | 26.12 | 7.18 | 0.13 | 52.96 | 0.57 | 4.48 | 9.47 |
| Max | 9.47 | 27.53 | 9.04 | 0.23 | 55.49 | 0.66 | 5.91 | 9.99 | ||
| Min | 7.70 | 24.38 | 5.68 | 0.05 | 50.32 | 0.50 | 3.26 | 9.00 | ||
| SD | 0.30 | 0.59 | 0.61 | 0.03 | 0.90 | 0.03 | 0.68 | 0.17 | ||
| 1.00 | Mean | 8.04 | 26.72 | 6.92 | 0.15 | 53.48 | 0.61 | 4.08 | 9.39 | |
| Max | 8.81 | 27.91 | 8.87 | 0.25 | 55.54 | 0.84 | 5.92 | 9.92 | ||
| Min | 7.15 | 25.87 | 5.53 | 0.08 | 51.21 | 0.53 | 3.02 | 8.99 | ||
| SD | 0.27 | 0.37 | 0.55 | 0.03 | 0.85 | 0.05 | 0.43 | 0.15 | ||
| 1.25 | Mean | 7.12 | 28.15 | 6.61 | 0.11 | 54.29 | 0.63 | 3.08 | 9.21 | |
| Max | 7.68 | 30.39 | 7.82 | 0.19 | 56.68 | 0.76 | 4.56 | 9.59 | ||
| Min | 6.19 | 26.48 | 5.19 | 0.02 | 51.95 | 0.55 | 1.44 | 8.70 | ||
| SD | 0.28 | 0.72 | 0.52 | 0.03 | 0.76 | 0.04 | 0.55 | 0.15 | ||
| CO | CO2 | CH4 | CnHm | H2 | O2 | N2 | ||||
| 800 | 0.75 | Mean | 22.32 | 15.34 | 3.94 | 0.01 | 55.04 | 0.52 | 2.79 | 10.17 |
| Max | 25.51 | 18.78 | 5.52 | 0.06 | 57.53 | 0.60 | 4.35 | 10.62 | ||
| Min | 18.18 | 11.75 | 2.93 | 0.00 | 52.18 | 0.47 | 1.30 | 9.71 | ||
| SD | 1.56 | 1.37 | 0.44 | 0.01 | 0.77 | 0.03 | 0.52 | 0.17 | ||
| 1.00 | Mean | 19.01 | 19.04 | 4.11 | 0.01 | 55.00 | 0.53 | 2.30 | 9.80 | |
| Max | 21.26 | 21.77 | 5.26 | 0.07 | 56.65 | 0.65 | 2.96 | 10.15 | ||
| Min | 15.88 | 17.46 | 3.26 | 0.00 | 53.33 | 0.46 | 1.38 | 9.47 | ||
| SD | 0.98 | 0.77 | 0.37 | 0.01 | 0.47 | 0.05 | 0.30 | 0.11 | ||
| 1.25 | Mean | 17.52 | 20.31 | 3.88 | 0.00 | 55.57 | 0.64 | 2.06 | 9.59 | |
| Max | 19.23 | 22.37 | 5.04 | 0.06 | 57.05 | 0.78 | 4.73 | 9.87 | ||
| Min | 15.47 | 18.91 | 3.04 | 0.00 | 50.08 | 0.58 | 1.10 | 9.10 | ||
| SD | 0.74 | 0.60 | 0.37 | 0.01 | 0.60 | 0.04 | 0.42 | 0.10 | ||
| CO | CO2 | CH4 | CnHm | H2 | O2 | N2 | ||||
| 900 | 0.75 | Mean | 31.38 | 10.90 | 2.54 | 0.00 | 54.03 | 0.52 | 0.59 | 10.69 |
| Max | 35.19 | 14.00 | 3.56 | 0.15 | 56.00 | 0.66 | 2.51 | 10.93 | ||
| Min | 23.39 | 8.99 | 1.82 | 0.00 | 52.27 | 0.43 | 0.00 | 10.32 | ||
| SD | 1.70 | 0.96 | 0.31 | 0.02 | 0.66 | 0.06 | 0.51 | 0.11 | ||
| 1.00 | Mean | 30.99 | 11.30 | 2.35 | 0.01 | 54.10 | 0.43 | 0.79 | 10.59 | |
| Max | 36.55 | 13.54 | 3.21 | 0.16 | 56.17 | 0.59 | 2.75 | 10.98 | ||
| Min | 26.01 | 8.95 | 1.65 | 0.00 | 51.01 | 0.36 | 0.00 | 10.20 | ||
| SD | 1.92 | 0.99 | 0.31 | 0.04 | 0.75 | 0.03 | 0.64 | 0.14 | ||
| 1.25 | Mean | 27.55 | 13.70 | 2.52 | 0.00 | 54.76 | 0.40 | 1.06 | 10.28 | |
| Max | 30.79 | 15.47 | 3.74 | 0.13 | 56.15 | 0.47 | 2.85 | 10.51 | ||
| Min | 24.34 | 12.29 | 1.86 | 0.00 | 53.57 | 0.35 | 0.00 | 9.96 | ||
| SD | 1.23 | 0.61 | 0.32 | 0.01 | 0.46 | 0.03 | 0.58 | 0.10 | ||
References
- Song, Y.; Wang, N. Exploring Temporal and Spatial Evolution of Global Coal Supply-Demand and Flow Structure. Energy 2019, 168, 1073–1080. [Google Scholar] [CrossRef]
- Zang, G.; Zhang, J.; Ratner, A.; Shi, Y. Techno-Economic Analysis of a Cooling, Heating, and Electricity Trigeneration System Based on Downdraft Fixed Bed Wood and Tire Gasification: Case Study of a Campus Office Building. Sustain. Energy Technol. Assess. 2023, 55, 102939. [Google Scholar] [CrossRef]
- Xie, S.; Qin, P.; Zhang, M.; Xu, J.; Ouyang, T. A High-Efficiency and Eco-Friendly Design for Coal-Fired Power Plants: Combined Waste Heat Recovery and Electron Beam Irradiation. Energy 2022, 258, 124884. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Wu, J.; Ma, C. Exergy and Exergoeconomic Analysis of a Combined Cooling, Heating, and Power System Based on Solar Thermal Biomass Gasification. Energies 2019, 12, 2418. [Google Scholar] [CrossRef]
- Prestipino, M.; Piccolo, A.; Polito, M.F.; Galvagno, A. Combined Bio-Hydrogen, Heat, and Power Production Based on Residual Biomass Gasification: Energy, Exergy, and Renewability Assessment of an Alternative Process Configuration. Energies 2022, 15, 5524. [Google Scholar] [CrossRef]
- Li, C.Y.; Deethayat, T.; Wu, J.Y.; Kiatsiriroat, T.; Wang, R.Z. Simulation and Evaluation of a Biomass Gasification-Based Combined Cooling, Heating, and Power System Integrated with an Organic Rankine Cycle. Energy 2018, 158, 238–255. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, J.Y.; Shen, Y.; Kan, X.; Dai, Y.J.; Wang, C.-H. Evaluation of a Combined Cooling, Heating, and Power System Based on Biomass Gasification in Different Climate Zones in the U.S. Energy 2018, 144, 326–340. [Google Scholar] [CrossRef]
- Jie, P.; Yan, F.; Wen, Z.; Li, J. Evaluation of the Biomass Gasification-Based Combined Cooling, Heating and Power System Using the Maximum Generalized Entropy Principle. Energy Convers. Manag. 2019, 192, 150–160. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Gasification Technology; Wiley: Hoboken, NJ, USA, 2020; ISBN 9781118773536. [Google Scholar]
- Lamprecht, D.; Nel, R.; Leckel, D. Production of On-Specification Fuels in Coal-to-Liquid (CTL) Fischer−Tropsch Plants Based on Fixed-Bed Dry Bottom Coal Gasification. Energy Fuels 2010, 24, 1479–1486. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, S.; Yu, P.; Yang, Q.; Zhang, D. Thermodynamic and Techno-Economic Analysis of Coal to Ethylene Glycol Process (CtEG) with Different Coal Gasifiers. Energy Convers. Manag. 2019, 191, 80–92. [Google Scholar] [CrossRef]
- Frilund, C.; Kurkela, E.; Hiltunen, I. Development of a Simplified Gas Ultracleaning Process: Experiments in Biomass Residue-Based Fixed-Bed Gasification Syngas. Biomass Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Kurkela, E.; Kurkela, M.; Hiltunen, I. Pilot-Scale Development of Pressurized Fixed-Bed Gasification for Synthesis Gas Production from Biomass Residues. Biomass Convers. Biorefinery 2021, 1–22. [Google Scholar] [CrossRef]
- Bany Ata, A.; Seufert, P.M.; Heinze, C.; Alobaid, F.; Epple, B. Optimization of Integrated Gasification Combined-Cycle Power Plant for Polygeneration of Power and Chemicals. Energies 2021, 14, 7285. [Google Scholar] [CrossRef]
- Alves, O.; Garcia, B.; Rijo, B.; Lourinho, G.; Nobre, C. Market Opportunities in Portugal for the Water-and-Waste Sector Using Sludge Gasification. Energies 2022, 15, 6600. [Google Scholar] [CrossRef]
- Wang, J.-J.; Yang, K.; Xu, Z.-L.; Fu, C.; Li, L.; Zhou, Z.-K. Combined Methodology of Optimization and Life Cycle Inventory for a Biomass Gasification Based BCHP System. Biomass Bioenergy 2014, 67, 32–45. [Google Scholar] [CrossRef]
- Hamel, S.; Hasselbach, H.; Weil, S.; Krumm, W. Autothermal Two-Stage Gasification of Low-Density Waste-Derived Fuels. Energy 2007, 32, 95–107. [Google Scholar] [CrossRef]
- Bui, T.; Loof, R.; Bhattacharya, S.C. Multi-Stage Reactor for Thermal Gasification of Wood. Energy 1994, 19, 397–404. [Google Scholar] [CrossRef]
- Galindo, A.L.; Lora, E.S.; Andrade, R.V.; Giraldo, S.Y.; Jaén, R.L.; Cobas, V.M. Biomass Gasification in a Downdraft Gasifier with a Two-Stage Air Supply: Effect of Operating Conditions on Gas Quality. Biomass Bioenergy 2014, 61, 236–244. [Google Scholar] [CrossRef]
- Saleh, A.R.; Sudarmanta, B.; Fansuri, H.; Muraza, O. Syngas Production from Municipal Solid Waste with a Reduced Tar Yield by Three-Stages of Air Inlet to a Downdraft Gasifier. Fuel 2020, 263, 116509. [Google Scholar] [CrossRef]
- Brandt, P.; Larson, E.; Henriksen, U. High Tar Reduction in a Two Stage Gasifier. Energy Fuels 2000, 14, 816–819. [Google Scholar] [CrossRef]
- Ahrenfeldt, J.; Henriksen, U.; Jensen, T.K.; Gøbel, B.; Wiese, L.; Kather, A.; Egsgaard, H. Validation of a Continuous Combined Heat and Power (CHP) Operation of a Two-Stage Biomass Gasifier. Energy Fuels 2006, 20, 2672–2680. [Google Scholar] [CrossRef]
- Gadsbøll, R.Ø.; Sárossy, Z.; Jørgensen, L.; Ahrenfeldt, J.; Henriksen, U.B. Oxygen-Blown Operation of the TwoStage Viking Gasifier. Energy 2018, 158, 495–503. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Qin, J.; Wu, J.; Li, J.; Zi, Z.; Liu, G.; Wu, J.; Sun, L. Gasification of Biomass with Oxygen-Enriched Air in a Pilot Scale Two-Stage Gasifier. Fuel 2015, 150, 386–393. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, Y.; Yi, C. Syngas Production by Catalytic Steam Gasification of Municipal Solid Waste in Fixed-Bed Reactor. Energy 2012, 44, 391–395. [Google Scholar] [CrossRef]
- Soni, C.G.; Wang, Z.; Dalai, A.K.; Pugsley, T.; Fonstad, T. Hydrogen Production via Gasification of Meat and Bone Meal in Two-Stage Fixed Bed Reactor System. Fuel 2009, 88, 920–925. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Cheng, Z.; Yan, B.; Dan, Z.; Ma, W. Air Gasification of Biogas-Derived Digestate in a Downdraft Fixed Bed Gasifier. Waste Manag. 2017, 69, 162–169. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, S.; Song, Y.; Wang, C.; Ouyang, S. Gasification of Low-Rank Coal for Hydrogen-Rich Gas Production in a Dual Loop Gasification System. Fuel Process. Technol. 2018, 171, 110–116. [Google Scholar] [CrossRef]
- Yuan, X.; Namkung, H.; Kang, T.J.; Kim, H.-T. K2CO3-Catalyzed Steam Gasification of Indonesian Low-Rank Coal for H2-Rich Gas Production in a Fixed Bed Reactor. Energy Technol. 2015, 3, 527–534. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Cao, L.; Ma, L.; You, S.; Li, W. Co-Gasification of Digestate and Lignite in a Downdraft Fixed Bed Gasifier: Effect of Temperature. Energy Convers. Manag. 2020, 213, 112798. [Google Scholar] [CrossRef]
- Fan, S.; Yuan, X.; Zhao, L.; Xu, L.H.; Kang, T.J.; Kim, H.T. Experimental and Kinetic Study of Catalytic Steam Gasification of Low Rank Coal with an Environmentally Friendly, Inexpensive Composite K2CO3-Eggshell Derived CaO Catalyst. Fuel 2016, 165, 397–404. [Google Scholar] [CrossRef]
- Chen, Z.; Dun, Q.; Shi, Y.; Lai, D.; Zhou, Y.; Gao, S.; Xu, G. High Quality Syngas Production from Catalytic Coal Gasification Using Disposable Ca(OH)2 Catalyst. Chem. Eng. J. 2017, 316, 842–849. [Google Scholar] [CrossRef]
- Li, K.; Zhang, R.; Bi, J. Experimental Study on Syngas Production by Co-Gasification of Coal and Biomass in a Fluidized Bed. Int. J. Hydrogen Energy 2010, 35, 2722–2726. [Google Scholar] [CrossRef]
- Lin, C.-L.; Weng, W.-C. Effects of Different Operating Parameters on the Syngas Composition in a Two-Stage Gasification Process. Renew. Energy 2017, 109, 135–143. [Google Scholar] [CrossRef]
- Mondal, K.; Piotrowski, K.; Dasgupta, D.; Hippo, E.; Wiltowski, T. Hydrogen from Coal in a Single Step. Ind. Eng. Chem. Res. 2005, 44, 5508–5517. [Google Scholar] [CrossRef]




| Ultimate Analysis | Proximate Analysis | ||||
|---|---|---|---|---|---|
| N | 0.72 | wt% | Moisture | 10.37 | wt% |
| C | 35.60 | wt% | Volatile | 32.33 | wt% |
| H | 3.39 | wt% | Fixed carbon | 25.22 | wt% |
| S | 3.07 | wt% | Ash | 32.08 | wt% |
| O a | 25.14 | wt% | Higher heating value | 15.03 | MJ/kg |
| Mass Flow Rate of Coal | Temperature at 2nd Stage | Steam/C Ratio |
|---|---|---|
| g/h | °C | mol/mol |
| 1084 | 700 | 0.75 |
| 1.00 | ||
| 1.25 | ||
| 1084 | 800 | 0.75 |
| 1.00 | ||
| 1.25 | ||
| 1084 | 900 | 0.75 |
| 1.00 | ||
| 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, T.D.; Koós, T.; Sebe, E.; Siménfalvi, Z.; Kállay, A.A. Efficiency Enhancement of the Single Line Multi-Stage Gasification of Hungarian Low-Rank Coal: Effects of Gasification Temperature and Steam/Carbon (S/C) Ratio. Energies 2023, 16, 4427. https://doi.org/10.3390/en16114427
Mai TD, Koós T, Sebe E, Siménfalvi Z, Kállay AA. Efficiency Enhancement of the Single Line Multi-Stage Gasification of Hungarian Low-Rank Coal: Effects of Gasification Temperature and Steam/Carbon (S/C) Ratio. Energies. 2023; 16(11):4427. https://doi.org/10.3390/en16114427
Chicago/Turabian StyleMai, Thuan Duc, Tamás Koós, Emese Sebe, Zoltán Siménfalvi, and András Arnold Kállay. 2023. "Efficiency Enhancement of the Single Line Multi-Stage Gasification of Hungarian Low-Rank Coal: Effects of Gasification Temperature and Steam/Carbon (S/C) Ratio" Energies 16, no. 11: 4427. https://doi.org/10.3390/en16114427
APA StyleMai, T. D., Koós, T., Sebe, E., Siménfalvi, Z., & Kállay, A. A. (2023). Efficiency Enhancement of the Single Line Multi-Stage Gasification of Hungarian Low-Rank Coal: Effects of Gasification Temperature and Steam/Carbon (S/C) Ratio. Energies, 16(11), 4427. https://doi.org/10.3390/en16114427






