Bibliometric Analysis of Global Trends around Hydrogen Production Based on the Scopus Database in the Period 2011–2021
Abstract
1. Introduction
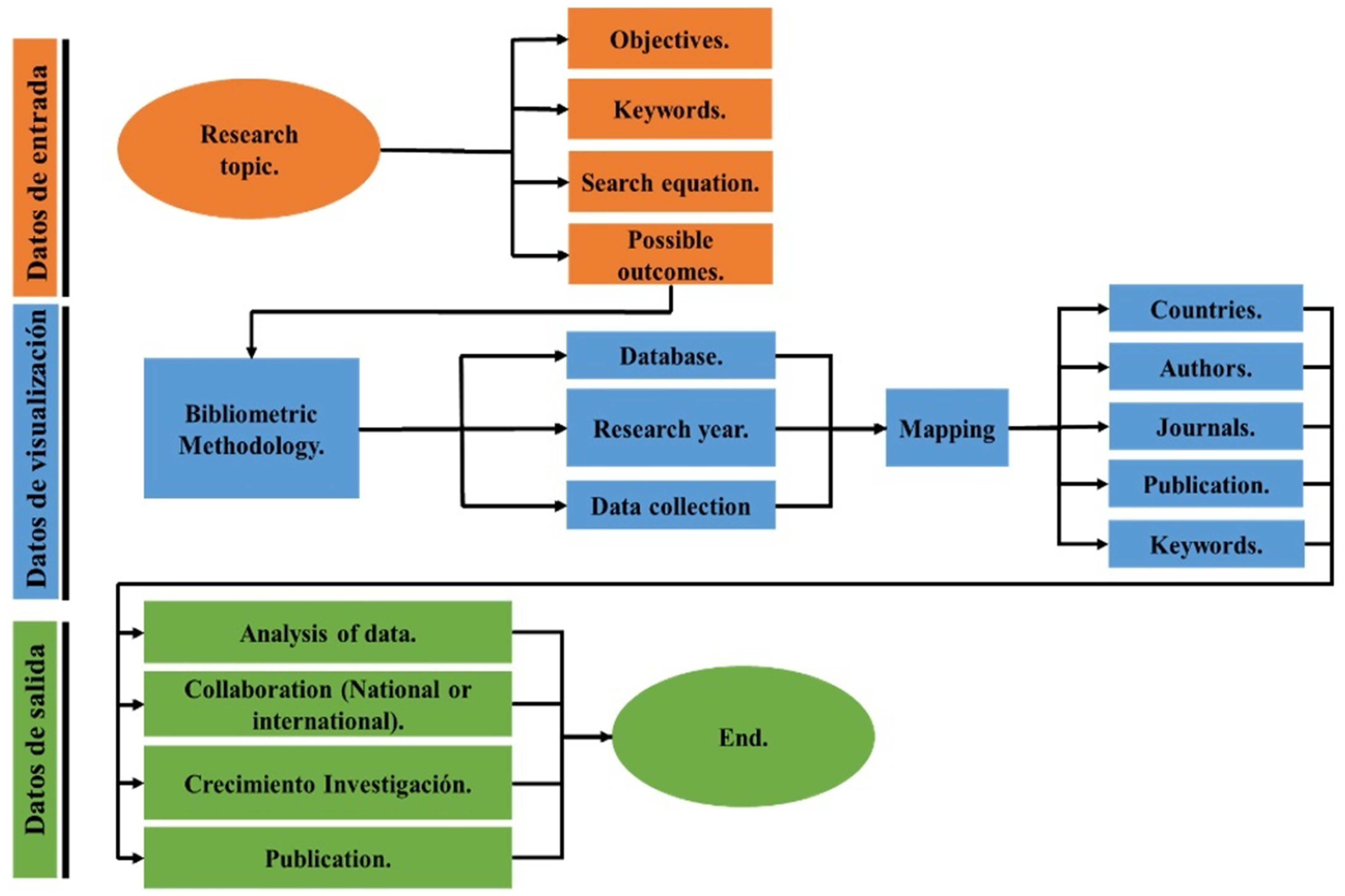
2. Methods
2.1. Data Analysis
2.2. Content Analysis
2.3. Network Analysis
3. Bibliometric Results
3.1. General Publication Trends
3.2. Performance of Countries/Territories and Institutions
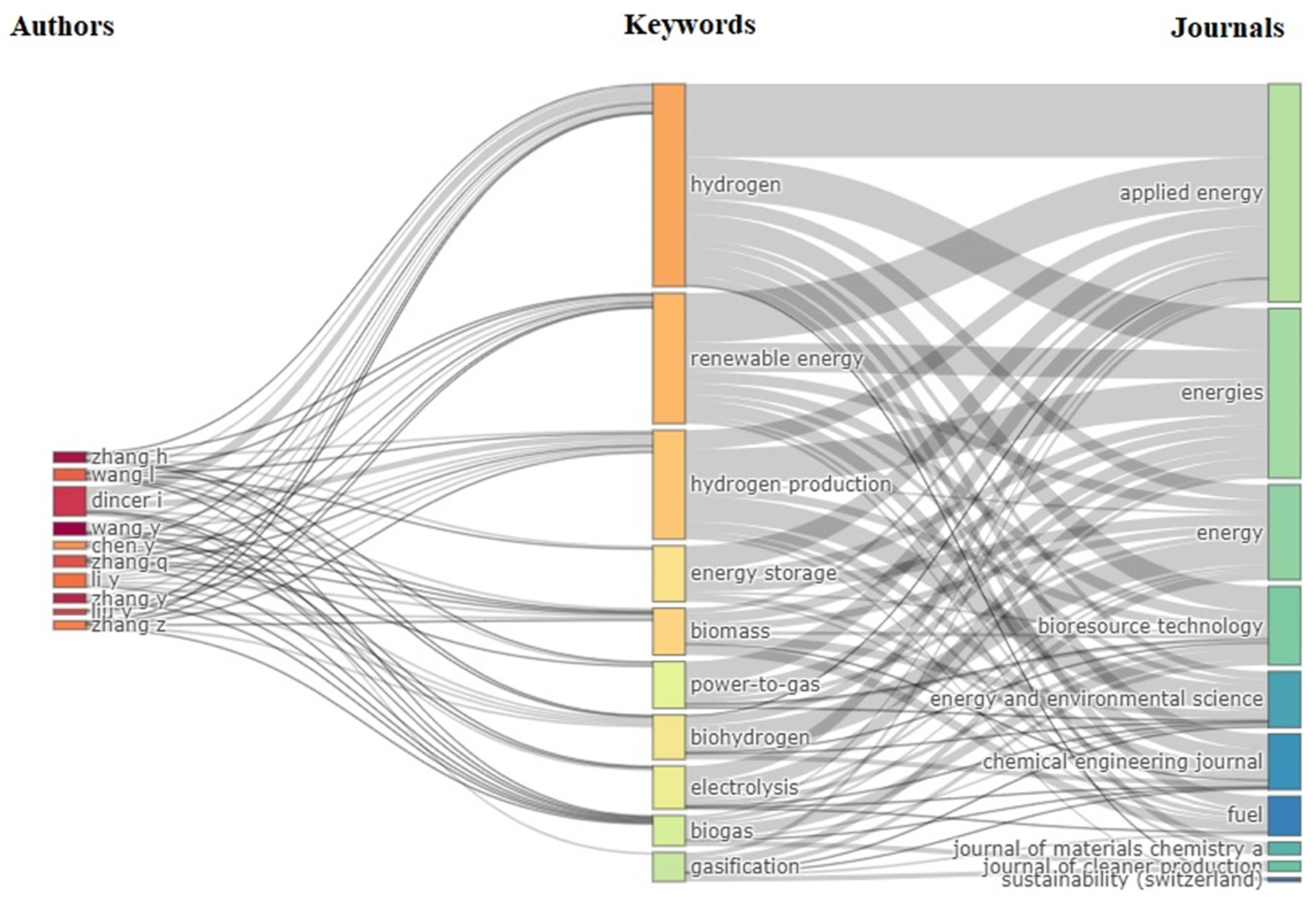
3.3. Performance of Journals
3.4. Institutions
3.5. Keywords for Analysis
3.6. Author Analysis
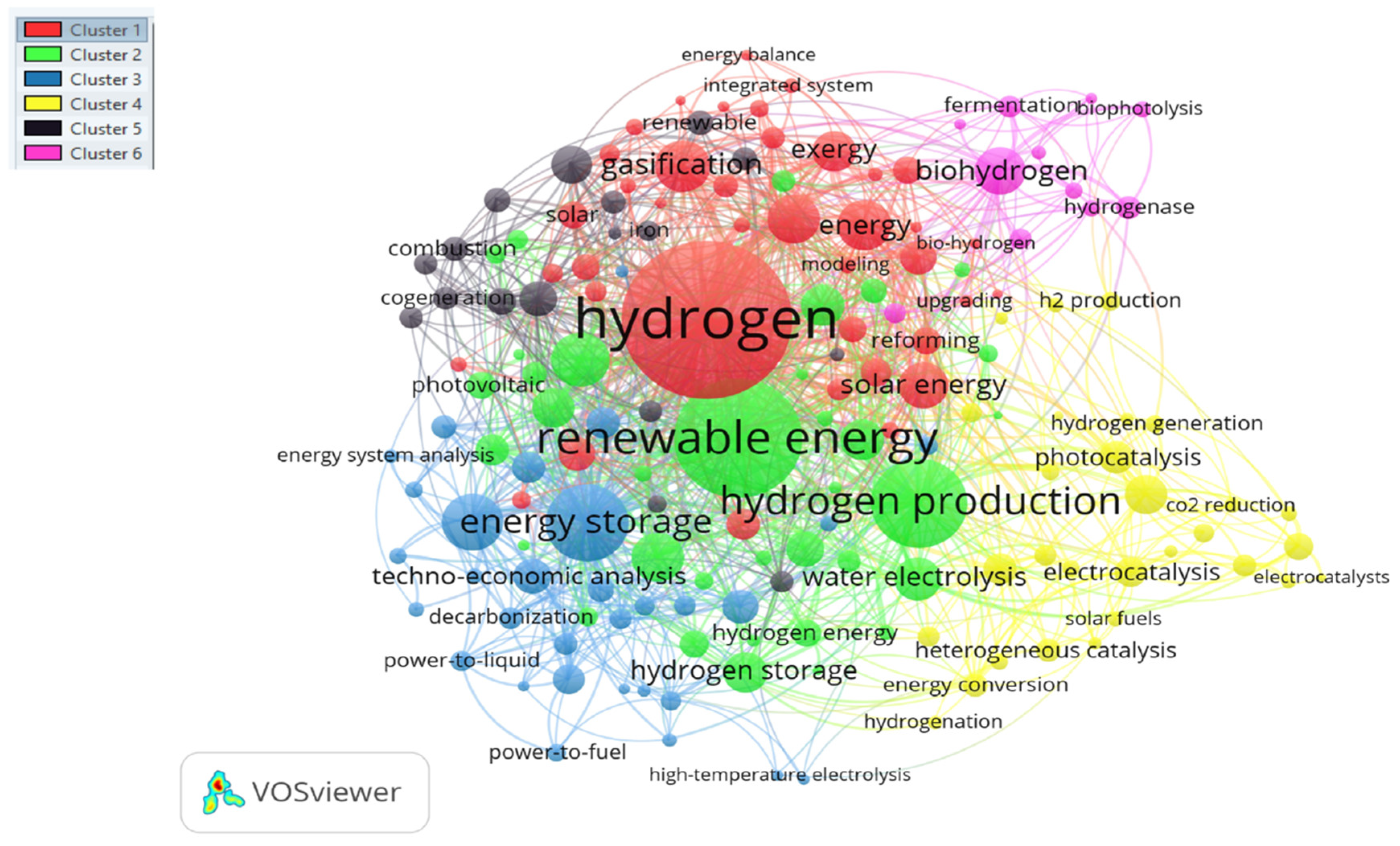
4. Research Hotspots for Future Discussion
4.1. Cluster I: Hydrogen Production
4.2. Cluster II: Renewable Energy
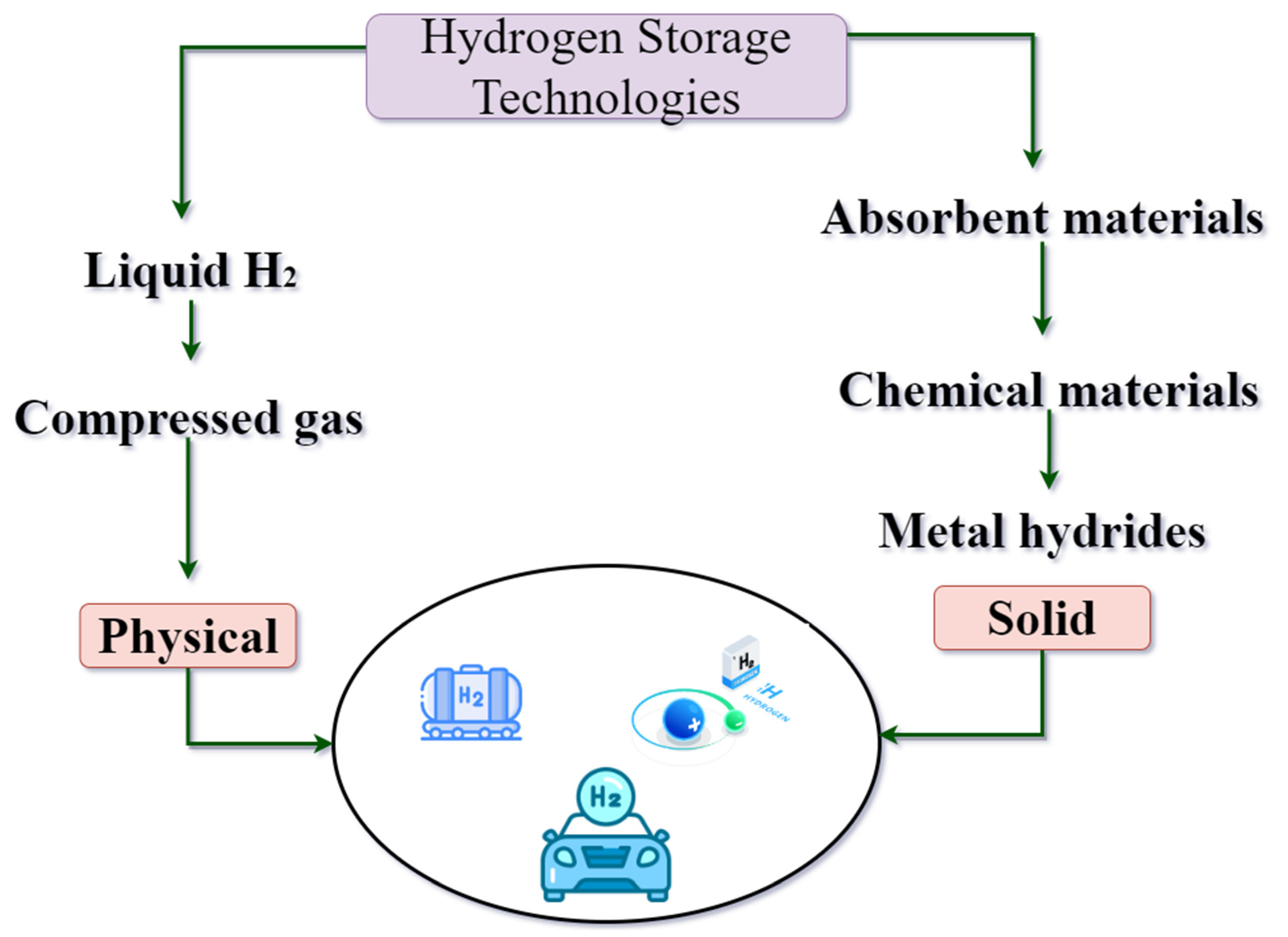
4.3. Cluster III: Hydrogen Storage Technologies
4.3.1. Liquefied Hydrogen Storage
4.3.2. Metal Hydrides
4.4. Cluster IV: Electrolysis
4.5. Cluster V: Energy Efficiency
4.6. Cluster VI: Biohydrogen
4.6.1. Alkaline Electrolysis
4.6.2. Proton Exchange Membrane Electrolysis
4.6.3. Solid Oxide Electrolysis (SOE)
5. Conclusions
- Among the findings found, it was possible to identify that A total of 224 journals, 11 books, and 191 conferences have been published linked to the topic of hydrogen production by electrolysis in the last few years and Applied Energy, and Applied Energy is the top in publishing this kind of topic.
- The annual number of publications from 2011 to 2022 is presented. The analysis shows that during the first four years of this study (2011–2014) the average number of publications related to the topic of energy generation by means of electrolysis was 74 articles per year, tending to increase.
- China surpasses other countries in terms of the number of articles published related to hydrogen products using electrolysis. On the other hand, the United States’ publications are more cited (9279).
- The analysis of the clusters made it possible to show the trends of keywords in the research.
Author Contributions
Funding
Conflicts of Interest
References
- Temiz, M.; Dincer, I. Concentrated solar driven thermochemical hydrogen production plant with thermal energy storage and geothermal systems. Energy 2020, 219, 119554. [Google Scholar] [CrossRef]
- Su, C.-W.; Khan, K.; Umar, M.; Chang, T. Renewable energy in prism of technological innovation and economic uncertainty. Renew. Energy 2022, 189, 467–478. [Google Scholar] [CrossRef]
- Sajjad, U.; Abbas, N.; Hamid, K.; Abbas, S.; Hussain, I.; Ammar, S.M.; Sultan, M.; Ali, H.M.; Hussain, M.; Rehman, T.U.; et al. A review of recent advances in indirect evaporative cooling technology. Int. Commun. Heat Mass Transf. 2021, 122, 105140. [Google Scholar] [CrossRef]
- Sebbahi, S.; Nabil, N.; Alaoui-Belghiti, A.; Laasri, S.; Rachidi, S.; Hajjaji, A. Assessment of the three most developed water electrolysis technologies: Alkaline Water Electrolysis, Proton Exchange Membrane and Solid-Oxide Electrolysis. Mater. Today Proc. 2022, 66, 140–145. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Yu, M.; Wang, K.; Vredenburg, H. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrog. Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Deng, W.; Pei, W.; Yi, Y.; Zhuang, Y.; Kong, L. Study on enhancing hydrogen production potential from renewable energy in multi-terminal DC system. Energy Rep. 2021, 7, 395–404. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, L.; Alotaibi, A.A.; Fang, J.; Alshahri, A.H.; Almitani, K.H. Transient simulation and comparative assessment of a hydrogen production and storage system with solar and wind energy using TRNSYS. Int. J. Hydrog. Energy 2022, 47, 26646–26653. [Google Scholar] [CrossRef]
- Ma, B.; Liu, S.; Pei, F.; Su, Z.; Yu, J.; Hao, C.; Li, Q.; Jiang, L.; Zhang, J.; Gan, Z. Development of Hydrogen Energy Storage Industry and Research Progress of Hydrogen Production Technology. In Proceedings of the 2021 IEEE 4th International Electrical and Energy Conference, CIEEC 2021, Wuhan, China, 28–30 May 2021. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Bacquart, T.; Arrhenius, K.; Persijn, S.; Rojo, A.; Auprêtre, F.; Gozlan, B.; Moore, N.; Morris, A.; Fischer, A.; Murugan, A.; et al. Hydrogen fuel quality from two main production processes: Steam methane reforming and proton exchange membrane water electrolysis. J. Power Sources 2019, 444, 227170. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; di Berardino, S.; Amorim, F.; Gírio, F.; Rangel, C.; de Leão, T.P. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Manna, J.; Jha, P.; Sarkhel, R.; Banerjee, C.; Tripathi, A.; Nouni, M. Opportunities for green hydrogen production in petroleum refining and ammonia synthesis industries in India. Int. J. Hydrog. Energy 2021, 46, 38212–38231. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrog. Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Paul, A.; Symes, M.D. Decoupled electrolysis for water splitting. Curr. Opin. Green Sustain. Chem. 2021, 29, 100453. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Yang, X.; Gong, M. A review of pulse electrolysis for efficient energy conversion and chemical production. J. Energy Chem. 2020, 59, 69–82. [Google Scholar] [CrossRef]
- D’Amore-Domenech, R.; Santiago, Ó.; Leo, T.J. Multicriteria analysis of seawater electrolysis technologies for green hydrogen production at sea. Renew. Sustain. Energy Rev. 2020, 133, 110166. [Google Scholar] [CrossRef]
- Sui, Y.; Al-Huqail, A.A.; Suhatril, M.; Abed, A.M.; Zhao, Y.; Assilzadeh, H.; Khadimallah, M.A.; Ali, H.E. Hydrogen energy of mining waste waters: Extraction and analysis of solving issues. Fuel 2023, 331, 125685. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-scale hydrogen production via water electrolysis—A techno-economic and environmental assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Zheng, X.; Liu, C.; Chen, Q. Hydrogen Production by Water Electrolysis with Low Power and High Efficiency Based on Pre-Magnetic Polarization. Energies 2022, 15, 1878. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.; Nabgan, W.; Hamid, M.; Bahari, M.; Ikram, M. Bibliometric studies and impediments to valorization of dry reforming of methane for hydrogen production. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Raman, R.; Nair, V.K.; Prakash, V.; Patwardhan, A.; Nedungadi, P. Green-hydrogen research: What have we achieved, and where are we going? Bibliometrics analysis. Energy Rep. 2022, 8, 9242–9260. [Google Scholar] [CrossRef]
- Bakır, M.; Özdemir, E.; Akan, Ş.; Atalık, Ö. A bibliometric analysis of airport service quality. J. Air Transp. Manag. 2022, 104, 102273. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Agbodjan, Y.S.; Wang, J.; Cui, Y.; Liu, Z.; Luo, Z. Bibliometric analysis of zero energy building research, challenges and solutions. Sol. Energy 2022, 244, 414–433. [Google Scholar] [CrossRef]
- AChoudhary, A.; Oluikpe, P.; Harding, J.; Carrillo, P. The needs and benefits of Text Mining applications on Post-Project Reviews. Comput. Ind. 2009, 60, 728–740. [Google Scholar] [CrossRef]
- Ghazinoory, S.; Ameri, F.; Farnoodi, S. An application of the text mining approach to select technology centers of excellence. Technol. Forecast. Soc. Change 2013, 80, 918–931. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Xiao, L. Progress in the production of hydrogen energy from food waste: A bibliometric analysis. Int. J. Hydrog. Energy 2021, 47, 26326–26354. [Google Scholar] [CrossRef]
- Au-Yong-Oliveira, M.; Pesqueira, A.; Sousa, M.J.; Mas, F.D.; Soliman, M. The Potential of Big Data Research in HealthCare for Medical Doctors’ Learning. J. Med. Syst. 2021, 45, 13. [Google Scholar] [CrossRef]
- Olabi, A.; Bahri, A.S.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrog. Energy 2020, 46, 23498–23528. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Chamoun, R.; Demirci, U.B.; Miele, P. Cyclic Dehydrogenation-(Re)Hydrogenation with Hydrogen-Storage Materials: An Overview. Energy Technol. 2015, 3, 100–117. [Google Scholar] [CrossRef]
- Veziroglu, T.N. Conversion to Hydrogen Economy. Energy Procedia 2012, 29, 654–656. [Google Scholar] [CrossRef]
- Sahaym, U.; Norton, M.G. Advances in the application of nanotechnology in enabling a ‘hydrogen economy’. J. Mater. Sci. 2008, 43, 5395–5429. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Jia, Z.-C.; Yuan, Z.-M.; Yang, T.; Qi, Y.; Zhao, D.-L. Development and Application of Hydrogen Storage. J. Iron Steel Res. Int. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Veziroǧlu, T.N.; Şahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Rand, D.A.J. A journey on the electrochemical road to sustainability. J. Solid State Electrochem. 2011, 15, 1579–1622. [Google Scholar] [CrossRef]
- Sahlberg, M. Light-Metal Hydrides for Hydrogen Storage. PhD Thesis, Uppsala Universitet, Uppsala, Sweden, 2009. [Google Scholar]
- Marbán, G.; Valdes-Solis, T. Towards the hydrogen economy? Int. J. Hydrog. Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Antonio González García-Conde. Producción, Almacenamiento y Distribución de Hidrógeno. España. Available online: http://www2.udg.edu/Portals/88/proc_industrials/5%20-%20Otros%20Combustibles-Hidrogeno.pdf (accessed on 25 October 2022).
- Mazzeo, D.; Herdem, M.S.; Matera, N.; Wen, J.Z. Green hydrogen production: Analysis for different single or combined large-scale photovoltaic and wind renewable systems. Renew. Energy 2022, 200, 360–378. [Google Scholar] [CrossRef]
- Cloete, S.; del Pozo, C.A.; Álvaro, Á.J. System-friendly process design: Optimizing blue hydrogen production for future energy systems. Energy 2022, 259, 124954. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). International Energy Outlook 2016. Volume 0484. 2016. Available online: www.eia.gov (accessed on 26 June 2022).
- Brockway, P.E.; Owen, A.; Brand-Correa, L.I.; Hardt, L. Estimation of global final-stage energy-return-on-investment for fossil fuels with comparison to renewable energy sources. Nat. Energy 2019, 4, 612–621. [Google Scholar] [CrossRef]
- Ruocco, C.; Palma, V.; Ricca, A. Kinetics of Oxidative Steam Reforming of Ethanol Over Bimetallic Catalysts Supported on CeO2–SiO2: A Comparative Study. Top. Catal. 2019, 62, 467–478. [Google Scholar] [CrossRef]
- Palhares, D.D.D.F.; Vieira, L.; Damasceno, J.J.R. Hydrogen production by a low-cost electrolyzer developed through the combination of alkaline water electrolysis and solar energy use. Int. J. Hydrog. Energy 2018, 43, 4265–4275. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Salhi, B.; Wudil, Y.S.; Hossain, M.K.; Al-Ahmed, A.; Al-Sulaiman, F.A. Review of recent developments and persistent challenges in stability of perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 90, 210–222. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Chen, X.; Lin, G.; Chen, J. Configuration design and performance optimum analysis of a solar-driven high temperature steam electrolysis system for hydrogen production. Int. J. Hydrog. Energy 2013, 38, 4298–4307. [Google Scholar] [CrossRef]
- Sivabalan, K.; Hassan, S.; Ya, H.; Pasupuleti, J. A review on the characteristic of biomass and classification of bioenergy through direct combustion and gasification as an alternative power supply. J. Phys. Conf. Ser. 2021, 1831, 012033. [Google Scholar] [CrossRef]
- Kim, J.; Jun, A.; Gwon, O.; Yoo, S.; Liu, M.; Shin, J.; Lim, T.-H.; Kim, G. Hybrid-solid oxide electrolysis cell: A new strategy for efficient hydrogen production. Nano Energy 2017, 44, 121–126. [Google Scholar] [CrossRef]
- Saleem, A.H.F.; Harris, J.; Shang, K. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification—A critical review. Chem. Eng. J. 2020, 382, 122761. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Xu, P.; Liu, B.; Shuai, Y.; Li, B. Hydrogen production through biomass gasification in supercritical water: A review from exergy aspect. Int. J. Hydrog. Energy 2019, 44, 15727–15736. [Google Scholar] [CrossRef]
- Almutairi, K.; Dehshiri, S.S.H.; Mostafaeipour, A.; Jahangiri, M.; Techato, K. Technical, economic, carbon footprint assessment, and prioritizing stations for hydrogen production using wind energy: A case study. Energy Strategy Rev. 2021, 36, 100684. [Google Scholar] [CrossRef]
- Li, Z.; Guo, P.; Han, R.; Sun, H. Current status and development trend of wind power generation-based hydrogen production technology. Energy Explor. Exploit. 2018, 37, 5–25. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Murphy, J.D. Inhibition of thermochemical treatment on biological hydrogen and methane co-production from algae-derived glucose/glycine. Energy Convers. Manag. 2018, 158, 201–209. [Google Scholar] [CrossRef]
- Show, K.-Y.; Yan, Y.; Ling, M.; Ye, G.; Li, T.; Lee, D.-J. Hydrogen production from algal biomass—Advances, challenges and prospects. Bioresour. Technol. 2018, 257, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.J.; Junior, C.B.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Escamilla, A.; Sánchez, D.; García-Rodríguez, L. Assessment of power-to-power renewable energy storage based on the smart integration of hydrogen and micro gas turbine technologies. Int. J. Hydrog. Energy 2022, 47, 17505–17525. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Prabhukhot Prachi, R.; Wagh Mahesh, M.; Gangal Aneesh, C. A Review on Solid State Hydrogen Storage Material. Adv. Energy Power 2016, 4, 11–22. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Dong, X.; Yang, J.; Guo, H.; Gong, M. Thermodynamic analysis of low-temperature and high-pressure (cryo-compressed) hydrogen storage processes cooled by mixed-refrigerants. Int. J. Hydrog. Energy 2022, 47, 28932–28944. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, N.; Hillmansen, S.; Roberts, C.; Yan, Y. Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review. Energies 2022, 15, 6467. [Google Scholar] [CrossRef]
- Sapre, S.; Vyas, M.; Pareek, K. Impact of refueling parameters on storage density of compressed hydrogen storage Tank. Int. J. Hydrog. Energy 2020, 46, 16685–16692. [Google Scholar] [CrossRef]
- Jain, I.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrog. Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Yang, X.; Bulushev, D.A.; Yang, J.; Zhang, Q. New Liquid Chemical Hydrogen Storage Technology. Energies 2022, 15, 6360. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E.; Atyabi, S. Thermal and electrochemical analysis of different flow field patterns in a PEM electrolyzer. Electrochim. Acta 2018, 267, 234–245. [Google Scholar] [CrossRef]
- Ngoh, S.K.; Njomo, D. An overview of hydrogen gas production from solar energy. Renew. Sustain. Energy Rev. 2012, 16, 6782–6792. [Google Scholar] [CrossRef]
- Jørgensen, C.; Ropenus, S. Production price of hydrogen from grid connected electrolysis in a power market with high wind penetration. Int. J. Hydrog. Energy 2008, 33, 5335–5344. [Google Scholar] [CrossRef]
- Yang, Y.; De La Torre, B.; Stewart, K.; Lair, L.; Phan, N.L.; Das, R.; Gonzalez, D.; Lo, R.C. The scheduling of alkaline water electrolysis for hydrogen production using hybrid energy sources. Energy Convers. Manag. 2022, 257, 115408. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Speckmann, F.-W.; Bintz, S.; Birke, K.P. Influence of rectifiers on the energy demand and gas quality of alkaline electrolysis systems in dynamic operation. Appl. Energy 2019, 250, 855–863. [Google Scholar] [CrossRef]
- Nie, J.; Chen, Y. Numerical modeling of three-dimensional two-phase gas–liquid flow in the flow field plate of a PEM electrolysis cell. Int. J. Hydrog. Energy 2010, 35, 3183–3197. [Google Scholar] [CrossRef]
- Lee, H.-S.; Xin, W.; Katakojwala, R.; Mohan, S.V.; Tabish, N.M. Microbial electrolysis cells for the production of biohydrogen in dark fermentation—A review. Bioresour. Technol. 2022, 363, 127934. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Kuang, H.-B.; Wu, C.-Y.; Li, Y. The changing trend and influencing factors of energy efficiency: The case of nine countries. Energy 2014, 64, 1026–1034. [Google Scholar] [CrossRef]
- Clinch, J.P.; Healy, J.D.; King, C. Modelling Improvements in Domestic Energy Efficiency. 2001. Available online: www.elsevier.com/locate/envsoft (accessed on 16 July 2022).
- Blomberg, J.; Henriksson, E.; Lundmark, R. Energy efficiency and policy in Swedish pulp and paper mills: A data envelopment analysis approach. Energy Policy 2012, 42, 569–579. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, H.; Ma, Z.; Li, N.; Li, G.; Zhang, T.; Lu, P.; Gong, X. Biochar sacrificial anode assisted water electrolysis for hydrogen production. Int. J. Hydrog. Energy 2022, 47, 36482–36492. [Google Scholar] [CrossRef]
- Anniwaer, A.; Chaihad, N.; Zahra, A.C.A.; Yu, T.; Kasai, Y.; Kongparakul, S.; Samart, C.; Abudula, A.; Guan, G. Steam co-gasification of Japanese cedarwood and its commercial biochar for hydrogen-rich gas production. Int. J. Hydrog. Energy 2021, 46, 34587–34598. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Marzbali, M.H.; Chiang, K.; Surapaneni, A.; Shah, K. Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int. J. Hydrog. Energy 2020, 45, 29978–29992. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X.; Li, J.; Zhao, J.; Li, C.; Du, M.; Yu, Z.; Fang, Y. Pressurized catalytic calcium looping hydrogen generation from coal with in-situ CO2 capture. Energy Convers. Manag. 2019, 198, 111899. [Google Scholar] [CrossRef]
- Burton, N.; Padilla, R.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2020, 135, 110255. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Shanmugam, S. Recent advances in methods and technologies for enhancing bubble detachment during electrochemical water splitting. Renew. Sustain. Energy Rev. 2019, 114, 109300. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.S. Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- HLiu, H.-B.; Xu, H.; Pan, L.-M.; Zhong, D.-H.; Liu, Y. Porous electrode improving energy efficiency under electrode-normal magnetic field in water electrolysis. Int. J. Hydrog. Energy 2019, 44, 22780–22786. [Google Scholar] [CrossRef]
- Li, K.; Gao, Y.; Zhang, S.; Liu, G. Study on the energy efficiency of bioethanol-based liquid hydrogen production process. Energy 2021, 238, 122032. [Google Scholar] [CrossRef]
- Cho, K.; Hoffmann, M.R. Molecular hydrogen production from wastewater electrolysis cell with multi-junction BiOx/TiO2 anode and stainless steel cathode: Current and energy efficiency. Appl. Catal. B Environ. 2017, 202, 671–682. [Google Scholar] [CrossRef]
- Salemme, L.; Simeone, M.; Chirone, R.; Salatino, P. Analysis of the energy efficiency of solar aided biomass gasification for pure hydrogen production. Int. J. Hydrog. Energy 2014, 39, 14622–14632. [Google Scholar] [CrossRef]
- Lei, J.; Chen, X.; Liu, X.; Feng, W.; Zhang, J.; Li, H.; Zhang, Y. Under-brine superaerophobic perfluorinated ion exchange membrane with re-entrant superficial microstructures for high energy efficiency of NaCl aqueous solution electrolysis. J. Membr. Sci. 2020, 619, 118801. [Google Scholar] [CrossRef]
- Yun, Y.-M.; Lee, M.-K.; Im, S.-W.; Marone, A.; Trably, E.; Shin, S.-R.; Kim, M.-G.; Cho, S.-K.; Kim, D.-H. Biohydrogen production from food waste: Current status, limitations, and future perspectives. Bioresour. Technol. 2018, 248, 79–87. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Sillero, L.; Sganzerla, W.G.; Forster-Carneiro, T.; Solera, R.; Perez, M. A bibliometric analysis of the hydrogen production from dark fermentation. Int. J. Hydrog. Energy 2022, 47, 27397–27420. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by Enterobacter aerogenes 2822. Int. J. Hydrog. Energy 2020, 46, 1777–1800. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Khairilbinibrahim, M. Biohydrogen generation from jackfruit peel using anaerobic contact filter. Int. J. Hydrog. Energy 2006, 31, 569–579. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sanchez-Pena, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Badwal, S.P.; Giddey, S.; Munnings, C. Hydrogen production via solid electrolytic routes. WIREs Energy Environ. 2012, 2, 473–487. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]











| Journal Published | TP (R %) | h-Index | CiteScore 2021 | SNIP | SJR | Publisher |
|---|---|---|---|---|---|---|
| Applied Energy | 1 (211) | 235 | 20.4 | 2652 | 3.062 | Elsevier |
| Energy | 5 (303) | 212 | 13.4 | 2038 | 2.041 | Elsevier |
| ACS Energy Letters | 1 (50) | 134 | 33.2 | 2599 | 7.362 | American Chemical Society |
| Energy and Environmental Science | 1 (144) | 376 | 54.0 | 4761 | 11.558 | Royal Society of Chemistry |
| Journal of Materials Chemistry A | 13 (455) | 240 | 21.0 | 1619 | 3099 | Royal Society of Chemistry |
| Article Title | Author Name | Journal Published | Citations | Publication Year |
|---|---|---|---|---|
| (as of 2022) | ||||
| Hydrogen production by PEM water electrolysis—A review | Shiva Kumar S.; Himabindu V. | Materials Science for Energy Technologies | 145 | 2019 |
| Life cycle assessment of hydrogen production via electrolysis—a review | Ramchandra Bhandari, Clemens A. Trudewind, Petra Zapp | Journal of Cleaner Production | 26 | 2014 |
| Development of a multigenerational energy system for clean hydrogen generation | Aras Karapekmez; Ibrahim Dincer | Journal of Cleaner Production | 10 | 2021 |
| Hydrogen production via solid electrolytic routes | Sukhvinder P.S. Badwal; Sarbjit Giddey, Christopher Munnings | Wiley Interdisciplinary Reviews: Energy and Environment | 52 | 2013 |
| Renewable hydrogen production by dark-fermentation: Current status, challenges, and perspectives | Shikha Dahiyab, Sulogna Chatterjee, b, Omprakash Sarkar a S. Venkata Mohan a | Bioresource Technology | 45 | 2021 |
| Affiliations | Articles | Rank (Weightage %) | Country or Region Located |
|---|---|---|---|
| RWTH Aachen University | 33 | 30 | Germany |
| Tsinghua University | 20 | 20 | China |
| Delft University of Technology | 17 | 15 | Netherlands |
| Imperial College London | 16 | 10 | England |
| Indian Institute of Technology | 16 | 10 | Indian |
| Words | Occurrences | Keyword Usage by Authors (%) | Rank |
|---|---|---|---|
| Hydrogen | 291 | 30 | 1 |
| Renewable energy | 199 | 21 | 2 |
| Hydrogen production | 150 | 15 | 3 |
| Energy storage | 75 | 8 | 4 |
| Electrolysis | 64 | 7 | 5 |
| Fuel cell | 43 | 4 | 6 |
| Water splitting | 41 | 4 | 7 |
| Hydrogen storage | 38 | 4 | 8 |
| Green hydrogen | 37 | 4 | 9 |
| Water electrolysis | 33 | 3 | 10 |
| Technology | Efficiency | CO2 Emissions | Availability |
|---|---|---|---|
| Natural gas (SMR with CCS) | 60 | 42.7 | Medium Term |
| Natural gas (SMR without CCS) | 70–75 | 288–292 | Available |
| Coal (without CCS) | 60–50 | 659 | Available |
| Coal (with CCS) | 50–40 | 20.3 | Medium Term |
| Gasification Biomass | 56 | 0 | Medium Term |
| Electrolysis (Wind) | 65–70 | 0 | Short Term |
| Electrolysis (Grid) | 30 | 440 | Available |
| Thermochemical Cycle (Solar Energy) | 30 | 0 | Long Term |
| Thermochemical cycle (Nuclear energy) | 30 | 0 | Long Term |
| Type of Hydrogen | System of Study | Objective | Advantage | Disadvantage |
|---|---|---|---|---|
| Green [42] | Wind and photovoltaic systems [42] | Propose a methodology for the energetic comparison of wind and solar systems for hydrogen production [42] | The procedure is like a simulation for the annual energy analysis [42] | Due to limited wind and solar resources, it is not very profitable to install hybrid systems in some areas [42] |
| It is applicable to any wind and solar system, under any conditions [26] | ||||
| Reports the annual and monthly performance of the system [42] | ||||
| Allows you to select an ideal place to install a system WT y ST [42] | ||||
| Blue [43] | Hydrogen production optimization [43] | Development of an optimization methodology for the design of a flexible plant within the design and modeling of energy and hydrogen systems [43] | Cost reduction with the use of natural gas reforming and water changeover to membrane-assisted gas fueled by coal and biomass [27] | Operates on fossil fuels [27] |
| It allows the use of resources in a more efficient way and within sustainable ranges [43] | High cost [43] |
| Process | Method | Advantage | Disadvantage | Challenges |
|---|---|---|---|---|
| Solar | Solar energy to hydrogen via electrolysis of various types | Several processes are available for the conversion of solar energy into hydrogen fuel. | Low efficiency of solar cells. | Thermal dissociation of water through a solar cell requires a high temperature, which makes it difficult to implement the process [48] |
| Concentrating Solar Photovoltaic Systems (CSPV) using parabolic troughs and dishes has a high efficiency among all solar-to-hydrogen processes. Hydrogen production can be maximized using reflective mirrors and concentrated solar technologies [49] | Daylight intermittency and radiation intensity at all locations [50,51] | Higher temperature causing decrease in solar panel efficiency [47]. | ||
| Biomass gasification | Supercritical Water Gasification (SWG) | Sources are abundant, such as wood, agricultural wastes, plants, animal wastes, etc., etc. [52] | Process associated with a large amount of GHG emissions [53] | Due to the variation in feedstock and biomass composition, the calorific value of the resulting gas is always affected. |
| New plasma-assisted biomass gasification technology available for use [54] | During the process, tar formation can lead to clogging and breakage of the equipment [55] | |||
| Wind | Hydrogen wind power (use of AC/DC converter, power controllers, and alkaline electrolyzers) | Hydrogen storage and transportation in remote locations. | ||
| One of the cleanest processes with no harmful emissions [56] | Requirements for other components, such as AC/DC converter, power controller, etc. [57] | |||
| Geothermal | Geothermal to Hydrogen | Independent of environmental conditions. In the geothermal power plant (GPP) it is possible to produce additional electricity for water splitting through electrolyzers [58] | Purities such as H2S and other toxic gases emitted by the geothermal plant [59] | Higher geothermal fluid temperature is needed for better efficiency and cost-effective hydrogen production. |
| Algae | Dark fermentation | Abundant renewable source of energy [60] | Photoautotrophic organisms need so-lar light, nutrients, and organic sources to grow and produce bio-hydrogen. Presence of oxygen leading to low hydrogen production [60] | The high sugar content of the liquid fuel requires further processing of the gas [61] |
| Photo-fermentation | Algae-derived products, such as glucose and glycine, are also capable of thermochemical hydrogen production. | |||
| Fuel cell | Combined heat and power process (CHP) | Higher electrical conversion efficiency up to 95%. | CO gas formation and loss of catalyst activity during the internal reforming process [62] | As long as there is a need for hydrocarbon fuel to produce hydrogen internally in the fuel cell, it is not a fully decarbonized technology. |
| Electrolysis process | Direct use of biogas in high-temperature fuel cells, such as SOFCs and MOFCs, is possible. |
| Method | Advantages | Disadvantages | Storage Material | H-átoms per cm3 (×10 22) | % by Weight of Hydrogen |
|---|---|---|---|---|---|
| Compressed hydrogen | Ease of transport [68] | Containers are heavy [69] | |||
| Fuel cell vehicles [70] | Fairly slow development [69] | H2 Gas (200 bar) | 0.99 | 100 [70] | |
| It is the most developed technology | The amount of hydrogen is small at low pressures [69] | H2 liquid (20 k) | 4.2 | 100 [70] | |
| H2 solid (4.2) | 5.3 | 100 [70] | |||
| Liquid hydrogen | It is the most economical method [71] | Flash evaporation | MgH2_ | 6.3 | 7.6 [70] |
| Avoiding damage when H2 suddenly leaks through an opening [71] | Energy used for liquefaction is high | Mg 2 NiH 4 | 5.9 | 3.6 [70] | |
| It offers high hydrogen release rates [71] | The energy stored per unit volume is lower compared to fossil fuels. | FeTiH 1.95 | 6.0 | 1.89 [70] |
| Electrolysis Process | Advantage | Disadvantage |
|---|---|---|
| Alkaline Electrolysis | Marketed 70–80% energy efficiency Low-cost technology Electrocatalysts based on non-noble metals Highly developed technology [74,75] | Low dynamic operation low operating pressure (3–30 bar) Low gas purity Formation of carbonates on the electrode reduces electrolyze performance [76] |
| Highly dynamic operation 80–90% energy efficiency Highest H2 production rate with high-purity gases (99.99%) Compact system design Fast response and high current densities [72] | Low durability Acidic environment High cost of components Partially and newly established Commercialization is imminent [75,77] | |
| Solid Oxide Electrolysis | The operating pressure is high Electrocatalysts based on non-noble metals Efficiency is high (90–100%) high efficiency (90–100%) | Low durability The design of the system is on a large laboratory scale |
| Microbial Electrolysis | He used various organic water wastes to produce hydrogen hydrogen under the influence of a low external voltage [78] | Complicated design high internal resistance high operating and manufacturing costs under development [75] |
| Study System | Target | Advantage | Disadvantage |
|---|---|---|---|
| Porous electrode for water electrolysis [89]. | Improved efficiency in the magnetic field of water electrolysis [89]. | Reduction of consumption is proportional to the improvement of energy efficiency [89]. | There is not much hydrogen production with this system [89]. |
| Production of liquid hydrogen with bioethanol [90]. | The hydrogen liquefaction process with nitrogen precooling and cryogenic helium cycles, and the liquefied cryogenic hydrogen is recycled as a cold source to achieve 100% liquefaction of the process [90]. | The inferred relationships between the hydrogen liquefaction ratio and energy efficiency can be applied to analyze the nitrogen precooling cycle [90]. | It does not take into account possible variations in hydrogen temperature and pressure parameters [90]. |
| Molecular hydrogen production from wastewater cells [91]. | Method using a multiple junction semiconductor anode coupled with a stainless-steel cathode [91]. | Method used to optimize energy efficiency during electrochemical wastewater treatment | Impacts are only seen on decentralized production since the demand for hydrogen by electrolysis is not very high [92]. |
| Analysis of the impact of the integration of low- temperature solar heat in power generation processes based on the production of pure hydrogen through biomass gasification [92]. | For the integration of solar heat, membrane reactors are used to improve the efficiency of the gasification process [92]. | ||
| The reactors are common gasification, super critical, and water gas reactor, followed by a water gas displacement reactor with an integrated membrane. | |||
| Energy efficiency for electrolysis of NaCl aqueous solution [93] | To construct a superoleophobicity per fluorinated ion exchange membrane (SPIEM) to improve the efficiency of aqueous electrolysis of chlor-alkali (NaCL) process by ZrO2 nanoparticle sputtering process [93] | This strategy is used in order to improve the efficiency of the aqueous solution electrolysis process by reducing consumption and is intended for electrolysis, too. | The aqueous electrolysis process is an energy-intensive process [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, L.; Comas, D.; Escorcia, Y.C.; Alviz-Meza, A.; Carrillo Caballero, G.; Portnoy, I. Bibliometric Analysis of Global Trends around Hydrogen Production Based on the Scopus Database in the Period 2011–2021. Energies 2023, 16, 87. https://doi.org/10.3390/en16010087
Camargo L, Comas D, Escorcia YC, Alviz-Meza A, Carrillo Caballero G, Portnoy I. Bibliometric Analysis of Global Trends around Hydrogen Production Based on the Scopus Database in the Period 2011–2021. Energies. 2023; 16(1):87. https://doi.org/10.3390/en16010087
Chicago/Turabian StyleCamargo, Luis, Daniel Comas, Yulineth Cardenas Escorcia, Anibal Alviz-Meza, Gaylord Carrillo Caballero, and Ivan Portnoy. 2023. "Bibliometric Analysis of Global Trends around Hydrogen Production Based on the Scopus Database in the Period 2011–2021" Energies 16, no. 1: 87. https://doi.org/10.3390/en16010087
APA StyleCamargo, L., Comas, D., Escorcia, Y. C., Alviz-Meza, A., Carrillo Caballero, G., & Portnoy, I. (2023). Bibliometric Analysis of Global Trends around Hydrogen Production Based on the Scopus Database in the Period 2011–2021. Energies, 16(1), 87. https://doi.org/10.3390/en16010087






