Abstract
Given the increase in population and energy demand worldwide, alternative methods have been adopted for the production of hydrogen as a clean energy source. This energy offers an alternative energy source due to its high energy content, and without emissions to the environment. In this bibliometric analysis of energy production using electrolysis and taking into account the different forms of energy production. In this analysis, it was possible to evaluate the research trends based on the literature in the Scopus database during the years 2011–2021. The results showed a growing interest in hydrogen production from electrolysis and other mechanisms, with China being the country with the highest number of publications and the United States TOP in citations. The trend shows that during the first four years of this study (2011–2014), the average number of publications was 74 articles per year, from 2015 to 2021 where the growth is an average of 209 articles, the journal that published the most on this topic is Applied Energy, followed by Energy, contributing with almost 33% in the research area. Lastly, the keyword analysis identified six important research points for future discussions, which we have termed clusters. The study concludes that new perspectives on clean hydrogen energy generation, environmental impacts, and social acceptance could contribute to the positive evolution of the hydrogen energy industry.
1. Introduction
The growth of the world’s population and energy demand has had a considerable impact [1,2] emissions. Therefore, a continuous energy supply is sought to help mitigate the negative impacts caused by the burning of fossil fuels, such as greenhouse gas emissions. Currently, one of the opportunities to maintain this supply is through different forms, among them, renewable sources, such as solar, wind, and hydrogen, are especially promising [3]. In the same vein, countries are moving towards the energy transition from conventional sources to renewable energies, considered a viable option for the management of environmental problems. For this reason, technologies such as wind turbines, solar farms, and recently, the production of hydrogen on different bases, etc., have been developed. These are capable of producing clean energy; however, it has been verified that they are dependent on intermittent resources Chi-Wei Su [2].
On the other hand, in the hydrogen sector, it has been studied by different authors such as Seddiq sebbahi [4] statists that electrolysis is a method that was developed for the production of hydrogen, consists of using electric current to break down water molecules (H2O), in hydrogen gases (H2) and oxygen (O), on a large scale can be produced by renewable energy. Electrolysis is useful in the energy field for electricity production, transportation, heating, and chemicals. In addition, the hydrogen resulting from water electrolysis is considered pure. Giovanni Nicoletti [5] studied hydrogen as a possible replacement for fossil fuels, the research makes a comparison between hydrogen (H2) and fossil fuels. Minli Yu cites in her article [6] that there are several processes to obtain Hydrogen (H2), they are classified into two, one is produced by thermal and chemical technology, which is known as blue Hydrogen, and the green one is by renewable energies for water electrolysis. Wei Deng conducts research [7] on the analysis of the factors that can affect the production of Hydrogen and the theoretical verification of this analysis is carried out on the power of renewable energy technologies available for the production of Hydrogen, to integrate this new form of energy into the system without affecting it. It is concluded that the production is proportional to the increase of the renewable energy connection and also to the DC capacitance of the system, demonstrating that no variation affects it negatively. D. Wei [8] The comparison of which technology produces the most hydrogen under conditions established in different scenarios based on the information gathered was carried out using it was subsequently concluded that solar panels have much more potential than wind turbines for hydrogen production.
Boyang Ma [9] in his article mentions that H2 is considered energy, and likewise, Meiling Yue [10] says that hydrogen has become a resource for a sustainable energy transition worldwide. Therefore, some trends of systems that work with H2 are exposed and the role it has in an energy system is analyzed, it is used in order to obtain electricity, and it can be produced for immediate use and stored for the future. Generally speaking, H2 is produced by electrolysis of water or any raw material containing it in its composition and is stored in a fuel cell as a future reserve source. On the other hand, Thomas Bacquart [11] mentions that in the industrial sector such as the transport market, hydrogen as a transport fuel can be produced from renewable energy sources and can be implemented in electric vehicles with fuel cells, which has a positive impact on the environment as it can reduce emissions of polluting gases. According to Iain Staffell [12], vehicles in Europe use PEM fuel cells, as they offer high efficiency and contribute to the reduction of CO2 impact and decarbonization. In the same way, according to Sofia G. Simoes [12], the aim is to decarbonize Europe and the whole world with the production of H2 and thus reduce emissions and mitigate the growth of the greenhouse effect, for this study carried out the study of the potential of water sources in Portugal, taking into account for the analysis of ocean water, wastewater, rainwater, surface, public network, etc. Sofia G. Simoes carried out the study in two places in Portugal, one near the coast and the other in a rural area, a characterization of the potential for the production of green H2 between ocean water, wastewater, rainwater, surface water, public network, rural areas vs. coastal areas, and it was obtained that public network water proved to be the most suitable for electrolysis in both places.
The production of hydrogen at the industrial level since its high chemical potential was first discovered, as quoted by Manna [13] who cites that much of the hydrogen produced and consumed by industries comes from fossil fuels and to a lesser extent from renewable hydrogen. On the other hand, Wenguo Liu mentions that [14] in the steel industries of China, in its steel production process, the conversion of a blast furnace is given with an energy structure called coal, and this causes these companies to generate globally contribute with 5% of emissions and nationally 15%. Additionally, for this reason, most of the global industries that generate these emissions are committed to mitigating and reducing Co2 emissions, which is why in the above-mentioned articles [13,14], the challenge is to identify the energy potential required to use renewable hydrogen to replace fossil hydrogen and thus mitigate pollutant gas emissions, ensuring sustainable development and environmental sustainability. Avishek Paul y Mark D.Symes in [15], that the uncoupled electrolysis of water consists of separating the reactions of H2 and oxygen so that the splitting of both does not occur at the same time and in the same cell, this method of uncoupling is usually much faster than the original method, which is the coupled one; however, after its proposal, other improved mechanisms were developed, the article presents a summary of the most recent uncoupled electrolysis methods due to the fact that the model first proposed presents intermittency, and through a search between the years 2018–2020 it was found that the updated uncoupled electrolysis methods are soluble and solution-dispersed decoupling agents, and solid-state decoupling agents; these are of great potential where high gas purity is desired and where gas mixing is of particular concern. The TaoLiua [16] article also compares this type of electrolysis with traditional electrolysis and presents its progress over seventy years. This has proven to be a promising technology that dominates the field of research at present, and each of the techniques of pulsed electrolysis has been presented in a summarized manner, and it was shown that pulsing the potential is more efficient than maintaining a constant potential for the electrolysis of water. On the other hand, Rafael d’Amore-Domenech carried out [17] a multi-criteria analysis in his article, comparing the different electrolysis technologies to determine which one has the best response in the application of seawater for the production of green hydrogen. Taking into account the different study criteria, it was found that the best option for seawater electrolysis technology is the proton exchange membrane. Nowadays we find studies that make valuable contributions to our research such as Yang Sui talks about hydrogen energy from mining wastewater. His research talks about how well wastewater could be used to produce anaerobic hydrogen. Using coupling of sewage sludge suspensions and mining waste, it was measured by electrodialysis methods at 50 and 100 mA [18]. In the research, Tom Terlouw discusses large-scale hydrogen production through the electrolysis of water and different renewable sources and shows grid-connected, hybrid, and stand-alone hydrogen production configurations [19]; proposed an efficient hydrogen production method using low-power electrolysis in order to innovate the field to a theoretical basis for the selection of hydrogen production parameters using pulsed current electrolysis [20]. Sofia G. Simoes’ research on the availability of water and water use solutions for electrolysis mentions that Europe is involved in a growth strategy that will transform the union into a modern, resource-efficient, and competitive economy, aiming at CO2 neutrality by 2050 with the implementation of water electrolysis for the production of green hydrogen [21]. This bibliometric analysis approaches hydrogen production by electrolysis from a scientometric point of view, comparing it with other current energy generation routes.
Carrying out a study or analysis of bibliometrics is of great contribution to the research community because through bibliometrics, a valuable amount of information can be collected regarding a topic; in the case of this research, it is the “hydrogen production” that can be captured, and researchers can come to know trends of the topic over time and, in the same way, provide readers with its evolution and quality of information, through the frequency with which keywords are used, number of citations, and number of times an author and co-authorship are cited over time [21,22]. This research is carried out because of the current need for the use of this clean energy as a source of electricity generation, and also serves as an instrument for the academic and industrial sector to carry out a global analysis of the research on this subject, which is a current topic for generating companies and researchers in the area. This article allows the study of the recent advances in hydrogen prospecting through the use of statistical and data mining tools. This research can help industry and academia to understand the current dynamics of hydrogen as a promising source of clean energy.
2. Methods
Bibliometric analysis is a quantitative method to analyze data from different areas of study, evaluating aspects such as publications and citations; this type of study began in the 1950s [23]. This research is based on the concept of scientific mapping based on the quantitative approach and bibliometric methods to analyze the structure and development of scientific fields and disciplines [24] and consists of analyzing scientific databases using methods that match the information in scientific databases [25]. This analysis makes it possible to generate network associations to structure the information in scientific maps under the parameters of graph theory to visualize their conceptual subdomains (general areas or particular topics), their thematic evolution, trends, and research agendas. A bibliometric analysis focused on the study of articles, citations, publications, and most relevant authors on the topic of hydrogen production by means of water electrolysis was adopted. The Scopus database was used and keywords such as “hydrogen production”, “hydrogen”, and “renewable energies” were introduced. Thus, we used an exhaustive search in order to seek further theoretical argumentation and to obtain a clearer evaluation in our data extraction. After the extraction of data in Bibtex format which will allow the reading of the data in the software. The progress and contributions made on the topic of hydrogen production by means of electrolysis will be evaluated all using analysis of the research trends and the area of hydrogen production by means of electrolysis and the future growth of the research field (Figure 1).
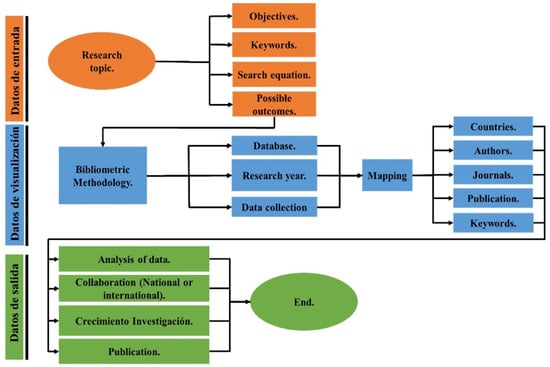
Figure 1.
Bibliometric methodology.
This article is developed in three sections: Section 1 and Section 2 data visualization, in this section the bibliometric mapping of the evaluated aspects is performed, e.g., countries with the highest contribution, publications, authors, among others. Finally, in Section 3 the output data analysis is performed, projecting research trends and conclusion, as described in Figure 1.
Some of the most commonly used programs to perform this type of computer study are VOSviewer (Visualization Of Similarities viewer), Bibexcel, HistCite, R, and Python which have been developed for this purpose and are generally used by researchers as shown in the following Figure 2. These special features usually result in a graphical representation of extensive, complex data and accurate analysis of the information. For this study, these tools were used as follows.
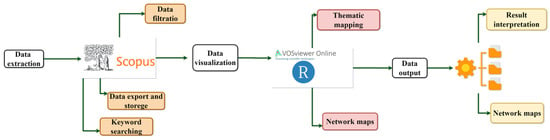
Figure 2.
Analysis tools.
2.1. Data Analysis
The study conducted the extraction of scientific data using the Scopus database on 12 August 2022. This database is chosen as it is a widely accepted tool for data mining and scientific search after the Web of Science. The study aims to map the trends in the area of hydrogen energy generation from electrolysis along with its research achievements. Literature was analyzed between the years 2011–2022 using the advanced search options “title, abstract, and keywords” to evaluate the advances and contributions made in the topic as shown in Figure 3 The data was filtered from the discipline of engineering and power generation.
Figure 3.
Time-lapse items.
2.2. Content Analysis
For data extraction and analysis, the R bibliometric package and Microsoft Excel 365 are used, providing the analysis of document types, collaborations, number of publications, keywords, and countries that make a significant contribution to the research topic. In theory, data extraction by R study is based on a method that has contributed to the development of the scientific community, it is practical, and used to analyze published texts and possible current trends regarding the research topic. Refining data to extract valuable information, and identify patterns and trends verbatim [26,27].
2.3. Network Analysis
VOSviewer software is used in the research as a mechanism for the investigation of bibliographic information. Although it is free software, it is a practical tool for background representation, it is a robust software, which allows data tabulation and manipulation of these, and the representation of these depends on what the researcher wants to analyze [21]. In addition, compared to other programs, this one allows the analysis by data groups; however, it presents similarities in terms of showing parameters such as collaboration with authors, keywords, co-occurrences, journals or country links in the form of networks, density, and heat maps [28]. VOSviewer has a color code that allows the researcher to see the similarities of the chosen research topic between countries. The clustering allows performing a mapping in which research trends towards a topic grouping similarity based on keyword and citation occurrence can be appreciated. For clustering, the software is based on the principle of a multidimensional scaling approach that performs the evaluations by calculating similarity indices to build clustering strength. The mapping is generated by thinking about the articles accordingly. This is due to useful features, as well as the promising interface systems, VOSview is set up to evaluate correlation and relationships [29].
3. Bibliometric Results
3.1. General Publication Trends
The annual number of publications from 2011 to 2022 is presented. The analysis shows that during the first four years of this study (2011–2014) the average number of publications related to the topic of energy generation by means of electrolysis was 74 articles per year, tending to increase. The trend cussing from 2015 to 2021 where the growth is an average of 209 published articles (blue line) as shown in the following Figure 4, in the case of the year 2022 at the end of the research, 235 are shown as of August 12 (date of data extraction). The analysis leads to consider that possibly due to tax incentives and/or financial support generated by different corporate, industrial, and governmental entities, more researchers support this area by publishing their findings. Another reason could be the growing demand for alternative forms of energy that are ecological, environmentally friendly, and sustainable. Thus, both publications and citations among authors increase annually with an average of 133 citations per year in relation to the topic of study.
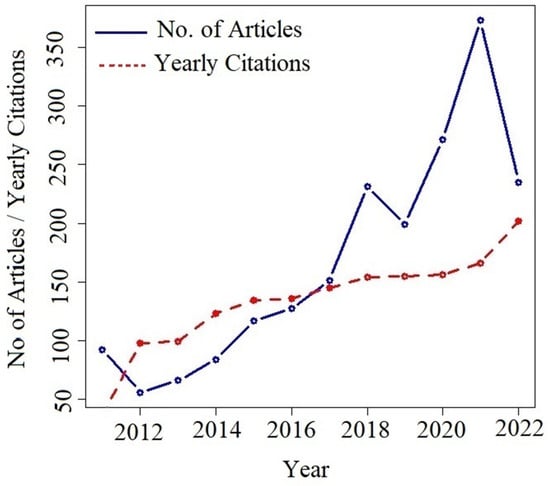
Figure 4.
Trend analysis of about publications.
There was also a remarkable worldwide collaboration between international authors as shown in Figure 5, the number of international collaborations and patents has increased significantly. Although a limited number of articles have been published under the title of hydrogen generation from electrolysis conversion of food waste into hydrogen energy, several investigations have been carried out on the use of hydrogen as a fuel, with emphasis on production technologies, as compared to conventional fossil fuels.
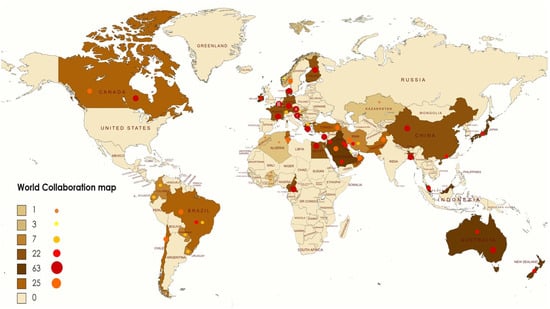
Figure 5.
World map of collaboration.
3.2. Performance of Countries/Territories and Institutions
For the creation of the network mapping of the countries and their publications, the citation analysis that can be seen on Figure 6a,b VOSviewer software was used for the construction and visualization of the data, such as the analysis of co-occurrence of keywords, number of citations and countries with more citations. To implement the analysis, the circles on the maps that describe the countries with the most publications are called “nodes”, which are represented on a bibliometric map and the presence of the characteristic being worked on.

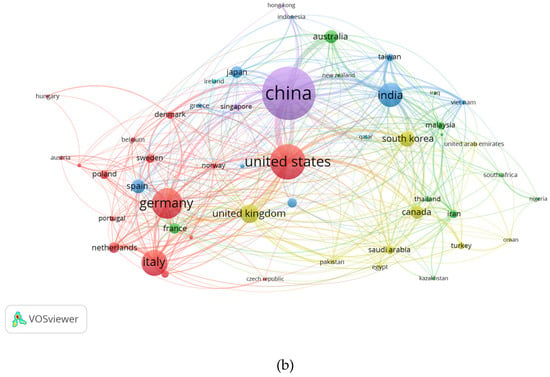
Figure 6.
(a,b) Performance of countries.
The larger the node is, the higher the number of citations in that country correlates positively with the number of citations in that country, and when the node is in the center, this means that the subject is closely related to the other nodes and research articles published in that country (47 ART).
In turn, the total strength of the link marks the impact of research between countries worldwide. However, for our study the production of hydrogen by electrolysis of water. The cluster data showed that the maximum number of research articles from Chinese authors (380), followed by the United States (234), United Kingdom (111), India (147) and Italy (161). In this case, China has the highest index of SCP: single-country publications; MCP: publications with collaborations. as the country with the most activity in this field, followed by Italy and India.
3.3. Performance of Journals
A total of 224 journals, 11 books, and 191 conferences have been published linked to the topic of hydrogen production by electrolysis in the last few years. Table 1 exhibits the five trending journals on hydrogen production by electrolysis, CiteScore, SNP, and journal ranking of origin (JSR) are metrics that help to quantify the citation impact and development of the journals. A total (228 papers) has been published in Applied Energy followed by Energy (217 papers), ACS Energy Letters (22 papers), and Energy and Environmental Science (17 papers), which are considered famous among the list of high-impact factor journals, Table 1 is the publication of articles on the production of hydrogen from electrolysis.

Table 1.
Journal published.
The most cited articles are described below in Table 2, which will allow for some frame of reference in relation to the topic of hydrogen generation from the electrolysis:

Table 2.
Top 5 most cited articles.
3.4. Institutions
Germany and China surpass the other countries in terms of the number of articles published related to hydrogen production by electrolysis (Table 3). On the other hand, the United States, whose publications are more cited (9279) in relation to the other countries, within this order, the possible reason for the number of publications and citations is due to the fact that the countries are looking for new forms of clean energy generation-polluting and thus reducing dependence on fossil fuels. Achieved through sustainable energy sources, with zero emissions and continuously replenished resources, hydrogen can be a sustainable energy carrier. Thanks to its high conversion efficiency of energy produced from water with zero emissions, hydrogen can be a sustainable energy carrier.

Table 3.
Institutions with the highest citation rate.
3.5. Keywords for Analysis
For the co-occurrence of keywords, the following analysis of the words and their classification was performed Table 4 shows a Sankey diagram Figure 7 consisting of three parameters and their relationships between authors, keywords, and journals (Figure 7). Where n is the number of elements in that variable or data set. The links between the components are connected, with gray links indicating the intensity of the network. As for the width of the flow, these indicate the importance of hydrogen production by electrolysis “hydrogen”, “hydrogen production”, and “electrolysis” have been the most used words by the authors in various journals in Table 4 The analysis shows the number of occurrences 291 (30% of articles) used “hydrogen” as a keyword, followed by “renewable energy” 199 (21% of articles), “hydrogen production” 150 (15% of articles) and “electrolysis” 64 (7% of articles).

Table 4.
Keyword analysis.
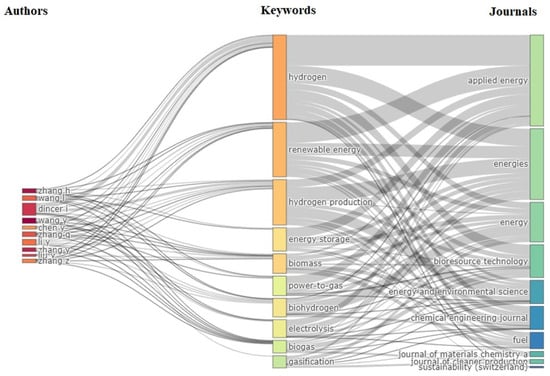
Figure 7.
Tree fields plot illustrating the relationship between authors, keywords, and journals.
Also, in this analysis, the most relevant research topics to be addressed in the near future have been demonstrated. As a result, a minimum of five words per document were, taking the areas as a categorical limit, implementing the visualization tool VOSviewer, professional software for visualization designed by Nees y Waltman [30]. This represents the number of similar areas and their network links under which they are working, in the Figure 7 the list of authors who have published at least five articles is displayed.
3.6. Author Analysis
The map shows those researchers who have contributed with at least five articles to the Figure 8 illustrates a list of authors who have written or published articles related to the topic of hydrogen production by water electrolysis.
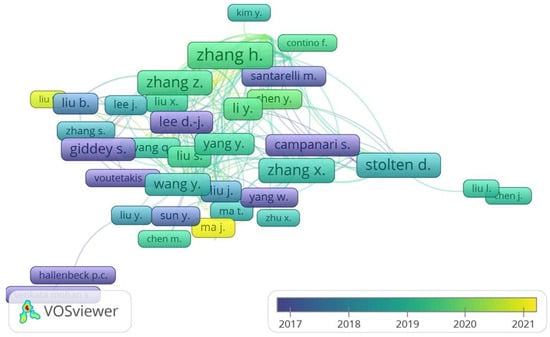
Figure 8.
Overlay visualization for the list of authors who have published a minimum of five articles.
On this map, the colors indicate the impact factors of the researchers; the impact of the author is indicated by the intensity of the color. The visualization by citation analysis with a unit of authors with more contribution is of China with a greater strength being thus the researchers who occupy the first positions in terms of articles and citations followed by the other countries.
4. Research Hotspots for Future Discussion
Figure 9 illustrates the cluster of keywords with their critical areas. A total of six clusters were inferred from the thematic research. In this case, we have the first cluster which has (39 items) which is the red color zone and is hydrogen production, the next cluster has (35 items) and is renewable energy followed by hydrogen storage technology with (31 items) and the zone is blue, biohydrogen would be the yellow color zone which has a total of (29items) followed by energy efficiency and electrolysis zones which (17 items) and (11 items).
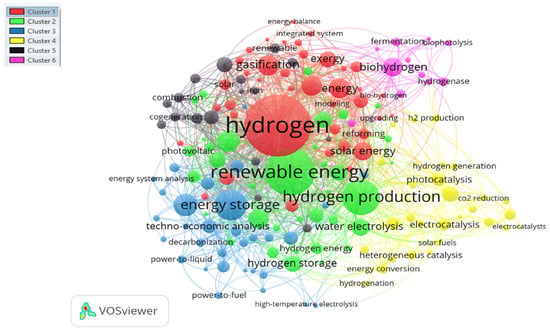
Figure 9.
Keywords and keyword clusters with their research hotspots requiring further emphasis.
4.1. Cluster I: Hydrogen Production
Según AG.Olavi [31] mentions in his publication that in the face of the environmental problems caused by traditional energy sources such as those that work thanks to the burning of fossil fuels, hydrogen (H2) is an energy source that can contribute to mitigate the carbonization stain caused by the production of energy through fossil fuels, hydrogen (H2) shows a better performance if its production is obtained through renewable energies and can be obtained in any material in which it is present in its chemical composition (Figure 10). J.O.Abe [32] says that compared to other raw materials for energy generation, hydrogen (H2) offers a better energy potential, in addition [32,33,34,35,36,37,38,39,40] cites that in the face of the energy crisis due to the increase in population and the negative environmental impact, the scientific community and engineers have been committed to finding another mechanism or way to generate energy that is sustainable and complies with environmental standards and regulations, which represents a challenge for engineering and science.
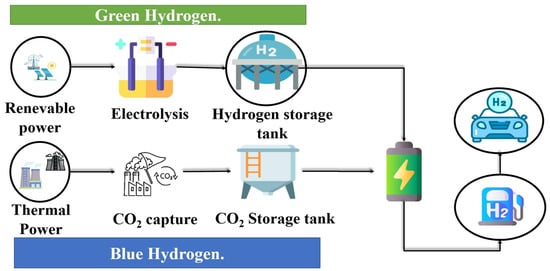
Figure 10.
Abundant renewable source of energy.
Gonzales mentioned in [41] Table 5 that producing hydrogen is economical only by adding costs such as storage and transportation the price of this is affected, also its large-scale production is limited because initially it is assumed that its demand will not be much, i.e., it will not be very centralized, but it will be more decentralized to meet the need for distributed generation for self-consumption, then in Table 5 a summary of each of the technologies for hydrogen production is shown, as well as the level of efficiency of each one, availability and level of CO2 emissions [25].

Table 5.
Efficiency and availability of generation mechanisms for hydrogen production [42].
Table 5 shows the technologies that liberate more CO2 emissions in the production of hydrogen, which is why we want to avoid those based on fossil fuels. These technologies have a high efficiency compared to some mechanisms of hydrogen production that are based on renewable energy, but despite this, hydrogen is still a green resource that provides, compared to the rest of the mechanisms, energy mostly free of emissions. In addition, it is evident that these technologies are not fully available so it is confirmed that so far, they are only used for self-consumption momentarily, due to the low demand for hydrogen [41]. Table 6 below shows some advantages and disadvantages of green and blue hydrogen according to other research.

Table 6.
Advantages and disadvantages of green and blue hydrogen.
4.2. Cluster II: Renewable Energy
The World Energy Outlook 2020 vision 2050 for achieving net zero emissions calls for sustainable development based on clean energy and provides guidance on the measures to be implemented in the next generations to achieve net zero emissions [44]. The power sector is expected to play a vital role in reducing emissions, but a low-carbon fuel such as hydrogen is also essential to achieve zero emissions through the introduction of clean energy technologies [45]. Hydrogen is not only the most abundant gas but also serves as an environmentally friendly fuel since energy production from hydrogen generates only heat and water, which reduces greenhouse gas emissions [46]. Figure 11 of hydrogen production [47].
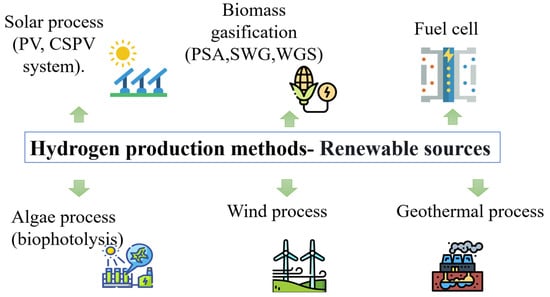
Figure 11.
Hydrogen Production through Renewable Energies.
Renewable energies contribute a small fraction of hydrogen production, which is why recent research has focused on developing environmentally friendly and pollution-free hydrogen from these sources (Table 7).

Table 7.
Reviews the benefits, drawbacks, and challenges associated with hydrogen production processes from renewable sources.
4.3. Cluster III: Hydrogen Storage Technologies
H2 hydrogen has different ways of generating it from various sources, H2 as a new form of clean energy, requires methods for its storage and subsequent use. It has been verified that H2 is a fuel with a higher gravimetric density, because it has one of the lowest densities, due to this different storage strategies are applied to overcome the low density of H2, either in its gaseous, liquid, or solid-state [63].
4.3.1. Liquefied Hydrogen Storage
The cryogenic or liquefied hydrogen (LH2), which has a higher density, indicates that the volumetric energy density increases greatly, and the density of liquid hydrogen reaches around 71 g/L a −253 °C [64]. Its critical temperature is around −240 °C, which is why, in order to work with it, it is necessary to cool it below its critical temperature. In order to make liquefaction, for its storage in liquid form (Figure 12) this technology is also very developed and also very to the hydrogen compression [65].
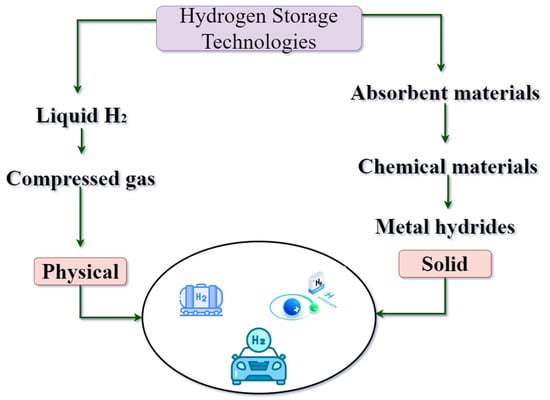
Figure 12.
Hydrogen storage technologies.
4.3.2. Metal Hydrides
It is clear that hydrogen storage systems have serious characteristics such as safety, efficiency, economy, lightness, and compactness, based on this metal hydride is a chemical reaction, but acts as a physical storage method Table 8. [66]. Metal hydride is formed when a hydrogen molecule dissociates into atomic hydrogen at the surface and then diffuses into the bulk and is chemically absorbed into the metal or alloy structure; this occurs through a direct reaction of the hydrogen with the metal electrochemical dissociation of the water molecule [67].

Table 8.
Describes the main methods of hydrogen storage systems and their advantages and disadvantages of the technologies.
4.4. Cluster IV: Electrolysis
Growing global energy demand (increasing 1.3% per year through 2040), largely due to technological advances, population, and economic growth, has necessitated reliance on fossil fuels, a major source of greenhouse gas emissions, which are projected to dominate the energy sector until at least 2050 [71].
Today, about 95% of the hydrogen generated is based on fossil fuels, mainly natural gas [72]. An environmentally friendly way of producing hydrogen is the electrolysis of water with the help of renewable electrical energy. Electricity is necessary for the endothermic water-splitting reaction.
On the one hand, a detailed understanding of the electrode mechanisms is required to achieve high-purity hydrogen with minimal energy input [73]. On the other hand, economical and reliable fuel cells and electrolyzes are needed for large-scale production, as shown in the following figure. Table 9 shows some processes that enable the electrolysis process.

Table 9.
Description of the electrolysis processes.
4.5. Cluster V: Energy Efficiency
Some authors such as Qiang Cui quote in [79] that energy efficiency is defined as a mechanism for verifying how efficiently energy has been used [80,81]. Others such as Xing Zhou [82] cite that hydrogen has been considered as an alternative fossil energy that plays a crucial role in the process of decarbonization and reduction of CO2 emissions and this has been recognized worldwide [83,84,85].
N Burton [86] cites in his research that hydrogen is an excellent energy vector that can become a great competitor to other existing energy vectors, however, there are aspects to improve in order to bring out its maximum energy yields, such as the feasibility and efficiency of production processes, electrolyzers, photocathode photovoltaic panels, the former must improve their coupling and therefore their efficiency as they are low [87,88]. Below, in Table 10 are some studies performed in other research consulted with their advantages and disadvantages.

Table 10.
Energy efficiency—electrolysis.
Then, in Figure 13, the cluster, energy savings, emissions, and consumption reduction, as well as improvements in the hydrogen production process are illustratively represented.
Figure 13.
Efficiency energy.
4.6. Cluster VI: Biohydrogen
Biohydrogen is defined as all types of feedstock or sources such as fossil fuels, biomass, and wastewater. From renewable resources, food waste, and other variants, it has become increasingly established due to its efficiency compared to thermochemical technologies [94,95].
Dark fermentation is a process that involves the conversion of food waste or organic compounds into hydrogen, during the process of dark fermentation glucose is broken down anaerobically [96,97]. In this sense, it is understood that enzymatic hydrolysis is generated by a group of enzymes called hydrolases, which favor high molecular weight compounds to degenerate in order to produce hydrogen and volatile fatty acids as main products [98]. The difference between the two processes is that the latter occurs to light.
4.6.1. Alkaline Electrolysis
Alkaline electrolysis of water stands out among other forms of hydrogen production as one of the most commercially viable. This system consists of electrodes immersed in an aqueous alkaline solution of potassium hydroxide (KoH) or potassium hydroxide (NaOH) base, with a concentration range of 25–30%. At the cathode, water is fractionated to form H2 and hydroxide anions which are passed through the diaphragm and recombined at the anode to form O2. The following equations show the reactions [99,100]:
4.6.2. Proton Exchange Membrane Electrolysis
The electrolyzes (PEM) is a polymeric membrane that allows the exchange of protons () hence its name. These have established themselves as a good industrially viable technology. They operate at low cell voltages and higher current densities as well as at high temperatures and pressures is 80–90% [101].
4.6.3. Solid Oxide Electrolysis (SOE)
This, unlike those already mentioned, is known as a High-Temperature Electrolyzer due to its particular process (Figure 14), which is made from water vapor at high temperatures. This offers higher efficiency compared to those already mentioned and also uses waste heat instead of part of the electricity needed. The technology is not yet viable for the market due to its durability due to the severe conditions and the short lifetime of the materials. The reactions that occur at the cathode and anode can be seen in the following equations [102]
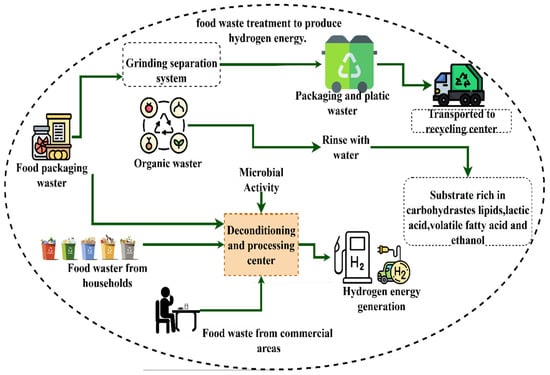
Figure 14.
Overview of the most common biohydrogen production technology, main advantages, and problems of the different technologies.
5. Conclusions
The bibliometric analysis is an effective and efficient route or tool to know the qualitative and qualitative advances of a particular topic, thus helping the academic, research, and industrial sectors to know the trend in technologies, tools, researchers, countries, and others working on a topic.
To meet the research objectives, the WoS database was used to study 2002 articles related to hydrogen energy production with electrolysis, which lays the foundation for the comprehensive study. The paper first provides descriptive statistics on terminologies of high frequency or use by researchers or authors, as well as evidence of the geographical distribution of the literature on the topic of study, the network of co-occurrence of authors, as well as popular and influential journals. The evolution path of hydrogen energy knowledge from electrolysis is analyzed using timeline mapping and the strong citation burst of the database through RStudio.
- Among the findings found, it was possible to identify that A total of 224 journals, 11 books, and 191 conferences have been published linked to the topic of hydrogen production by electrolysis in the last few years and Applied Energy, and Applied Energy is the top in publishing this kind of topic.
- The annual number of publications from 2011 to 2022 is presented. The analysis shows that during the first four years of this study (2011–2014) the average number of publications related to the topic of energy generation by means of electrolysis was 74 articles per year, tending to increase.
- China surpasses other countries in terms of the number of articles published related to hydrogen products using electrolysis. On the other hand, the United States’ publications are more cited (9279).
- The analysis of the clusters made it possible to show the trends of keywords in the research.
Through the synthesis analysis of the bibliometric results, findings such as stakeholder participation network, theories related to the topic, and processes of technology and biotechnology generation are revealed. Secondly, a time frame of publication years is constructed to evaluate the behavior of publications. Third, the evolutionary path analysis reveals how research related to hydrogen energy production has evolved over time, which is confirmed by the results of the cluster analysis, and identifies certain main keywords that help this type of analysis. Fourth, it summarizes research characteristics of the topic of study and possible research opportunities, which include (1) coordinated stakeholder involvement, (2) joint development of environmental, social, and economic performance, as well as (3) cross-cultural cooperation in clean energy production between regions. Finally, a global analysis is elaborated by studying possible research opportunities and research gaps for those in need.
In addition, the complementary application of bibliometric tools provides references for a more comprehensive analysis. This strategy enhances the existing methodology by combining the advantages of several tools to extract more valuable information from the massive literature and provide implications for future research.
However, given the rapidly updating literature in the field of hydrogen energy production, it is important to update the literature sources and deepen the literature contents in the future. Therefore, the integration of other suitable bibliometric tools (e.g., VOSviewer and Gephi) may be a way out for a comprehensive review based on another database.
Author Contributions
Conceptualization, Y.C.E. and G.C.C.; methodology, Y.C.E.; software, L.C.; validation, D.C., L.C. and I.P.; formal analysis, A.A.-M.; research, L.C.; resources, A.A.-M.; data preservation, D.C.; original draft-writing, L.C.; drafting-revising and editing, Y.C.E.; visualization, G.C.C.; supervision, A.A.-M.; project administration, Y.C.E.; obtaining funding, A.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
Research Group en Deterioro de Materiales, Transición Energética y Ciencia de datos DANT3, Facultad de Ingeniería, Arquitectura y Urbanismo, Universidad Señor de Sipán, Chiclayo 14820, Perú.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Temiz, M.; Dincer, I. Concentrated solar driven thermochemical hydrogen production plant with thermal energy storage and geothermal systems. Energy 2020, 219, 119554. [Google Scholar] [CrossRef]
- Su, C.-W.; Khan, K.; Umar, M.; Chang, T. Renewable energy in prism of technological innovation and economic uncertainty. Renew. Energy 2022, 189, 467–478. [Google Scholar] [CrossRef]
- Sajjad, U.; Abbas, N.; Hamid, K.; Abbas, S.; Hussain, I.; Ammar, S.M.; Sultan, M.; Ali, H.M.; Hussain, M.; Rehman, T.U.; et al. A review of recent advances in indirect evaporative cooling technology. Int. Commun. Heat Mass Transf. 2021, 122, 105140. [Google Scholar] [CrossRef]
- Sebbahi, S.; Nabil, N.; Alaoui-Belghiti, A.; Laasri, S.; Rachidi, S.; Hajjaji, A. Assessment of the three most developed water electrolysis technologies: Alkaline Water Electrolysis, Proton Exchange Membrane and Solid-Oxide Electrolysis. Mater. Today Proc. 2022, 66, 140–145. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Yu, M.; Wang, K.; Vredenburg, H. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrog. Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Deng, W.; Pei, W.; Yi, Y.; Zhuang, Y.; Kong, L. Study on enhancing hydrogen production potential from renewable energy in multi-terminal DC system. Energy Rep. 2021, 7, 395–404. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, L.; Alotaibi, A.A.; Fang, J.; Alshahri, A.H.; Almitani, K.H. Transient simulation and comparative assessment of a hydrogen production and storage system with solar and wind energy using TRNSYS. Int. J. Hydrog. Energy 2022, 47, 26646–26653. [Google Scholar] [CrossRef]
- Ma, B.; Liu, S.; Pei, F.; Su, Z.; Yu, J.; Hao, C.; Li, Q.; Jiang, L.; Zhang, J.; Gan, Z. Development of Hydrogen Energy Storage Industry and Research Progress of Hydrogen Production Technology. In Proceedings of the 2021 IEEE 4th International Electrical and Energy Conference, CIEEC 2021, Wuhan, China, 28–30 May 2021. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Bacquart, T.; Arrhenius, K.; Persijn, S.; Rojo, A.; Auprêtre, F.; Gozlan, B.; Moore, N.; Morris, A.; Fischer, A.; Murugan, A.; et al. Hydrogen fuel quality from two main production processes: Steam methane reforming and proton exchange membrane water electrolysis. J. Power Sources 2019, 444, 227170. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; di Berardino, S.; Amorim, F.; Gírio, F.; Rangel, C.; de Leão, T.P. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Manna, J.; Jha, P.; Sarkhel, R.; Banerjee, C.; Tripathi, A.; Nouni, M. Opportunities for green hydrogen production in petroleum refining and ammonia synthesis industries in India. Int. J. Hydrog. Energy 2021, 46, 38212–38231. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrog. Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Paul, A.; Symes, M.D. Decoupled electrolysis for water splitting. Curr. Opin. Green Sustain. Chem. 2021, 29, 100453. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Yang, X.; Gong, M. A review of pulse electrolysis for efficient energy conversion and chemical production. J. Energy Chem. 2020, 59, 69–82. [Google Scholar] [CrossRef]
- D’Amore-Domenech, R.; Santiago, Ó.; Leo, T.J. Multicriteria analysis of seawater electrolysis technologies for green hydrogen production at sea. Renew. Sustain. Energy Rev. 2020, 133, 110166. [Google Scholar] [CrossRef]
- Sui, Y.; Al-Huqail, A.A.; Suhatril, M.; Abed, A.M.; Zhao, Y.; Assilzadeh, H.; Khadimallah, M.A.; Ali, H.E. Hydrogen energy of mining waste waters: Extraction and analysis of solving issues. Fuel 2023, 331, 125685. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-scale hydrogen production via water electrolysis—A techno-economic and environmental assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Zheng, X.; Liu, C.; Chen, Q. Hydrogen Production by Water Electrolysis with Low Power and High Efficiency Based on Pre-Magnetic Polarization. Energies 2022, 15, 1878. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.; Nabgan, W.; Hamid, M.; Bahari, M.; Ikram, M. Bibliometric studies and impediments to valorization of dry reforming of methane for hydrogen production. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Raman, R.; Nair, V.K.; Prakash, V.; Patwardhan, A.; Nedungadi, P. Green-hydrogen research: What have we achieved, and where are we going? Bibliometrics analysis. Energy Rep. 2022, 8, 9242–9260. [Google Scholar] [CrossRef]
- Bakır, M.; Özdemir, E.; Akan, Ş.; Atalık, Ö. A bibliometric analysis of airport service quality. J. Air Transp. Manag. 2022, 104, 102273. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Agbodjan, Y.S.; Wang, J.; Cui, Y.; Liu, Z.; Luo, Z. Bibliometric analysis of zero energy building research, challenges and solutions. Sol. Energy 2022, 244, 414–433. [Google Scholar] [CrossRef]
- AChoudhary, A.; Oluikpe, P.; Harding, J.; Carrillo, P. The needs and benefits of Text Mining applications on Post-Project Reviews. Comput. Ind. 2009, 60, 728–740. [Google Scholar] [CrossRef]
- Ghazinoory, S.; Ameri, F.; Farnoodi, S. An application of the text mining approach to select technology centers of excellence. Technol. Forecast. Soc. Change 2013, 80, 918–931. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Xiao, L. Progress in the production of hydrogen energy from food waste: A bibliometric analysis. Int. J. Hydrog. Energy 2021, 47, 26326–26354. [Google Scholar] [CrossRef]
- Au-Yong-Oliveira, M.; Pesqueira, A.; Sousa, M.J.; Mas, F.D.; Soliman, M. The Potential of Big Data Research in HealthCare for Medical Doctors’ Learning. J. Med. Syst. 2021, 45, 13. [Google Scholar] [CrossRef]
- Olabi, A.; Bahri, A.S.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrog. Energy 2020, 46, 23498–23528. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Chamoun, R.; Demirci, U.B.; Miele, P. Cyclic Dehydrogenation-(Re)Hydrogenation with Hydrogen-Storage Materials: An Overview. Energy Technol. 2015, 3, 100–117. [Google Scholar] [CrossRef]
- Veziroglu, T.N. Conversion to Hydrogen Economy. Energy Procedia 2012, 29, 654–656. [Google Scholar] [CrossRef]
- Sahaym, U.; Norton, M.G. Advances in the application of nanotechnology in enabling a ‘hydrogen economy’. J. Mater. Sci. 2008, 43, 5395–5429. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Jia, Z.-C.; Yuan, Z.-M.; Yang, T.; Qi, Y.; Zhao, D.-L. Development and Application of Hydrogen Storage. J. Iron Steel Res. Int. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Veziroǧlu, T.N.; Şahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Rand, D.A.J. A journey on the electrochemical road to sustainability. J. Solid State Electrochem. 2011, 15, 1579–1622. [Google Scholar] [CrossRef]
- Sahlberg, M. Light-Metal Hydrides for Hydrogen Storage. PhD Thesis, Uppsala Universitet, Uppsala, Sweden, 2009. [Google Scholar]
- Marbán, G.; Valdes-Solis, T. Towards the hydrogen economy? Int. J. Hydrog. Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Antonio González García-Conde. Producción, Almacenamiento y Distribución de Hidrógeno. España. Available online: http://www2.udg.edu/Portals/88/proc_industrials/5%20-%20Otros%20Combustibles-Hidrogeno.pdf (accessed on 25 October 2022).
- Mazzeo, D.; Herdem, M.S.; Matera, N.; Wen, J.Z. Green hydrogen production: Analysis for different single or combined large-scale photovoltaic and wind renewable systems. Renew. Energy 2022, 200, 360–378. [Google Scholar] [CrossRef]
- Cloete, S.; del Pozo, C.A.; Álvaro, Á.J. System-friendly process design: Optimizing blue hydrogen production for future energy systems. Energy 2022, 259, 124954. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). International Energy Outlook 2016. Volume 0484. 2016. Available online: www.eia.gov (accessed on 26 June 2022).
- Brockway, P.E.; Owen, A.; Brand-Correa, L.I.; Hardt, L. Estimation of global final-stage energy-return-on-investment for fossil fuels with comparison to renewable energy sources. Nat. Energy 2019, 4, 612–621. [Google Scholar] [CrossRef]
- Ruocco, C.; Palma, V.; Ricca, A. Kinetics of Oxidative Steam Reforming of Ethanol Over Bimetallic Catalysts Supported on CeO2–SiO2: A Comparative Study. Top. Catal. 2019, 62, 467–478. [Google Scholar] [CrossRef]
- Palhares, D.D.D.F.; Vieira, L.; Damasceno, J.J.R. Hydrogen production by a low-cost electrolyzer developed through the combination of alkaline water electrolysis and solar energy use. Int. J. Hydrog. Energy 2018, 43, 4265–4275. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Salhi, B.; Wudil, Y.S.; Hossain, M.K.; Al-Ahmed, A.; Al-Sulaiman, F.A. Review of recent developments and persistent challenges in stability of perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 90, 210–222. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Chen, X.; Lin, G.; Chen, J. Configuration design and performance optimum analysis of a solar-driven high temperature steam electrolysis system for hydrogen production. Int. J. Hydrog. Energy 2013, 38, 4298–4307. [Google Scholar] [CrossRef]
- Sivabalan, K.; Hassan, S.; Ya, H.; Pasupuleti, J. A review on the characteristic of biomass and classification of bioenergy through direct combustion and gasification as an alternative power supply. J. Phys. Conf. Ser. 2021, 1831, 012033. [Google Scholar] [CrossRef]
- Kim, J.; Jun, A.; Gwon, O.; Yoo, S.; Liu, M.; Shin, J.; Lim, T.-H.; Kim, G. Hybrid-solid oxide electrolysis cell: A new strategy for efficient hydrogen production. Nano Energy 2017, 44, 121–126. [Google Scholar] [CrossRef]
- Saleem, A.H.F.; Harris, J.; Shang, K. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification—A critical review. Chem. Eng. J. 2020, 382, 122761. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Xu, P.; Liu, B.; Shuai, Y.; Li, B. Hydrogen production through biomass gasification in supercritical water: A review from exergy aspect. Int. J. Hydrog. Energy 2019, 44, 15727–15736. [Google Scholar] [CrossRef]
- Almutairi, K.; Dehshiri, S.S.H.; Mostafaeipour, A.; Jahangiri, M.; Techato, K. Technical, economic, carbon footprint assessment, and prioritizing stations for hydrogen production using wind energy: A case study. Energy Strategy Rev. 2021, 36, 100684. [Google Scholar] [CrossRef]
- Li, Z.; Guo, P.; Han, R.; Sun, H. Current status and development trend of wind power generation-based hydrogen production technology. Energy Explor. Exploit. 2018, 37, 5–25. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Murphy, J.D. Inhibition of thermochemical treatment on biological hydrogen and methane co-production from algae-derived glucose/glycine. Energy Convers. Manag. 2018, 158, 201–209. [Google Scholar] [CrossRef]
- Show, K.-Y.; Yan, Y.; Ling, M.; Ye, G.; Li, T.; Lee, D.-J. Hydrogen production from algal biomass—Advances, challenges and prospects. Bioresour. Technol. 2018, 257, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.J.; Junior, C.B.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Escamilla, A.; Sánchez, D.; García-Rodríguez, L. Assessment of power-to-power renewable energy storage based on the smart integration of hydrogen and micro gas turbine technologies. Int. J. Hydrog. Energy 2022, 47, 17505–17525. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Prabhukhot Prachi, R.; Wagh Mahesh, M.; Gangal Aneesh, C. A Review on Solid State Hydrogen Storage Material. Adv. Energy Power 2016, 4, 11–22. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Dong, X.; Yang, J.; Guo, H.; Gong, M. Thermodynamic analysis of low-temperature and high-pressure (cryo-compressed) hydrogen storage processes cooled by mixed-refrigerants. Int. J. Hydrog. Energy 2022, 47, 28932–28944. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, N.; Hillmansen, S.; Roberts, C.; Yan, Y. Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review. Energies 2022, 15, 6467. [Google Scholar] [CrossRef]
- Sapre, S.; Vyas, M.; Pareek, K. Impact of refueling parameters on storage density of compressed hydrogen storage Tank. Int. J. Hydrog. Energy 2020, 46, 16685–16692. [Google Scholar] [CrossRef]
- Jain, I.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrog. Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Yang, X.; Bulushev, D.A.; Yang, J.; Zhang, Q. New Liquid Chemical Hydrogen Storage Technology. Energies 2022, 15, 6360. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E.; Atyabi, S. Thermal and electrochemical analysis of different flow field patterns in a PEM electrolyzer. Electrochim. Acta 2018, 267, 234–245. [Google Scholar] [CrossRef]
- Ngoh, S.K.; Njomo, D. An overview of hydrogen gas production from solar energy. Renew. Sustain. Energy Rev. 2012, 16, 6782–6792. [Google Scholar] [CrossRef]
- Jørgensen, C.; Ropenus, S. Production price of hydrogen from grid connected electrolysis in a power market with high wind penetration. Int. J. Hydrog. Energy 2008, 33, 5335–5344. [Google Scholar] [CrossRef]
- Yang, Y.; De La Torre, B.; Stewart, K.; Lair, L.; Phan, N.L.; Das, R.; Gonzalez, D.; Lo, R.C. The scheduling of alkaline water electrolysis for hydrogen production using hybrid energy sources. Energy Convers. Manag. 2022, 257, 115408. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Speckmann, F.-W.; Bintz, S.; Birke, K.P. Influence of rectifiers on the energy demand and gas quality of alkaline electrolysis systems in dynamic operation. Appl. Energy 2019, 250, 855–863. [Google Scholar] [CrossRef]
- Nie, J.; Chen, Y. Numerical modeling of three-dimensional two-phase gas–liquid flow in the flow field plate of a PEM electrolysis cell. Int. J. Hydrog. Energy 2010, 35, 3183–3197. [Google Scholar] [CrossRef]
- Lee, H.-S.; Xin, W.; Katakojwala, R.; Mohan, S.V.; Tabish, N.M. Microbial electrolysis cells for the production of biohydrogen in dark fermentation—A review. Bioresour. Technol. 2022, 363, 127934. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Kuang, H.-B.; Wu, C.-Y.; Li, Y. The changing trend and influencing factors of energy efficiency: The case of nine countries. Energy 2014, 64, 1026–1034. [Google Scholar] [CrossRef]
- Clinch, J.P.; Healy, J.D.; King, C. Modelling Improvements in Domestic Energy Efficiency. 2001. Available online: www.elsevier.com/locate/envsoft (accessed on 16 July 2022).
- Blomberg, J.; Henriksson, E.; Lundmark, R. Energy efficiency and policy in Swedish pulp and paper mills: A data envelopment analysis approach. Energy Policy 2012, 42, 569–579. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, H.; Ma, Z.; Li, N.; Li, G.; Zhang, T.; Lu, P.; Gong, X. Biochar sacrificial anode assisted water electrolysis for hydrogen production. Int. J. Hydrog. Energy 2022, 47, 36482–36492. [Google Scholar] [CrossRef]
- Anniwaer, A.; Chaihad, N.; Zahra, A.C.A.; Yu, T.; Kasai, Y.; Kongparakul, S.; Samart, C.; Abudula, A.; Guan, G. Steam co-gasification of Japanese cedarwood and its commercial biochar for hydrogen-rich gas production. Int. J. Hydrog. Energy 2021, 46, 34587–34598. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Marzbali, M.H.; Chiang, K.; Surapaneni, A.; Shah, K. Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int. J. Hydrog. Energy 2020, 45, 29978–29992. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X.; Li, J.; Zhao, J.; Li, C.; Du, M.; Yu, Z.; Fang, Y. Pressurized catalytic calcium looping hydrogen generation from coal with in-situ CO2 capture. Energy Convers. Manag. 2019, 198, 111899. [Google Scholar] [CrossRef]
- Burton, N.; Padilla, R.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2020, 135, 110255. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Shanmugam, S. Recent advances in methods and technologies for enhancing bubble detachment during electrochemical water splitting. Renew. Sustain. Energy Rev. 2019, 114, 109300. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.S. Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- HLiu, H.-B.; Xu, H.; Pan, L.-M.; Zhong, D.-H.; Liu, Y. Porous electrode improving energy efficiency under electrode-normal magnetic field in water electrolysis. Int. J. Hydrog. Energy 2019, 44, 22780–22786. [Google Scholar] [CrossRef]
- Li, K.; Gao, Y.; Zhang, S.; Liu, G. Study on the energy efficiency of bioethanol-based liquid hydrogen production process. Energy 2021, 238, 122032. [Google Scholar] [CrossRef]
- Cho, K.; Hoffmann, M.R. Molecular hydrogen production from wastewater electrolysis cell with multi-junction BiOx/TiO2 anode and stainless steel cathode: Current and energy efficiency. Appl. Catal. B Environ. 2017, 202, 671–682. [Google Scholar] [CrossRef]
- Salemme, L.; Simeone, M.; Chirone, R.; Salatino, P. Analysis of the energy efficiency of solar aided biomass gasification for pure hydrogen production. Int. J. Hydrog. Energy 2014, 39, 14622–14632. [Google Scholar] [CrossRef]
- Lei, J.; Chen, X.; Liu, X.; Feng, W.; Zhang, J.; Li, H.; Zhang, Y. Under-brine superaerophobic perfluorinated ion exchange membrane with re-entrant superficial microstructures for high energy efficiency of NaCl aqueous solution electrolysis. J. Membr. Sci. 2020, 619, 118801. [Google Scholar] [CrossRef]
- Yun, Y.-M.; Lee, M.-K.; Im, S.-W.; Marone, A.; Trably, E.; Shin, S.-R.; Kim, M.-G.; Cho, S.-K.; Kim, D.-H. Biohydrogen production from food waste: Current status, limitations, and future perspectives. Bioresour. Technol. 2018, 248, 79–87. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Sillero, L.; Sganzerla, W.G.; Forster-Carneiro, T.; Solera, R.; Perez, M. A bibliometric analysis of the hydrogen production from dark fermentation. Int. J. Hydrog. Energy 2022, 47, 27397–27420. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by Enterobacter aerogenes 2822. Int. J. Hydrog. Energy 2020, 46, 1777–1800. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Khairilbinibrahim, M. Biohydrogen generation from jackfruit peel using anaerobic contact filter. Int. J. Hydrog. Energy 2006, 31, 569–579. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sanchez-Pena, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Badwal, S.P.; Giddey, S.; Munnings, C. Hydrogen production via solid electrolytic routes. WIREs Energy Environ. 2012, 2, 473–487. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).