Abstract
The persistent fight against global environmental threats and energy catastrophe is currently a major concern for the economic development of bioenergy across the entire country. Hence, traditional fuel-based reserves are overburdened to cope with the rapid energy crisis, necessitating an urgent need for an innovative carbon-neutral green-energy resource. In order to address these critical bottlenecks, microalgae with incredible metabolic versatility have paved the way for a pivotal attention towards sustainable biofuel production. However, due to high operational costs and low lipid productivity, the microalgae-based biofuel resource is still in its infancy. As a result, this problem can be overcome by incorporating engineered microalgal strains which can pave the way for significant lipid augmentation for biofuel production. Thus, our current review depicts an in-depth understanding of a multi-omics approach to microalgae, the broad scope of self-sustaining microalgae cultivation, lipid-extraction strategies, and conversion processes to improve economic commercialization in the bioenergy framework. The present review also provides a detailed analysis of the international and national status of bioenergy development by several federal agencies.
1. Introduction
Global climate catastrophe, energy conflicts, and greenhouse gas emissions caused by anthropogenic activities in the biogeochemical cycle are addressed as alarming vulnerabilities in the recent epoch [1,2,3]. The burning of petroleum-derived fuel (PDF) reserves and the release of noxious air pollutants in large quantities have a negative impact on humans and our environmental community [4,5,6]. Burning of petroleum-derived fuel has contributed 35,300 million tonnes of CO2 to the atmosphere as of 2018 although the anticipated daily atmospheric CO2 output is 29,000 megatons of CO2 [3,7,8]. This negative scenario is expected to accelerate to around 9 billion by 2050 [9,10]. Hence, in order to combat all these loopholes, most developing countries are putting forth their best efforts to transition fossil-fuel reserves to sustainable and renewable energy production. As a result of the emphasis on current and future green energy needs, biomass-based feedstocks have received significant consideration. As a result, a variety of biomass resources, including edible and inedible crops, wood residues, plants, agricultural husks, and microalgae, have been used to advance the development of eco-friendly bioenergy.
1.1. Microalgae vs. Plants
Photosynthetic microalgae, which have high metabolic activity, are widely used to remove contaminants from waste streams and remarkably assimilate lipid conglomerates, which are promisingly applied for bioenergy, primarily biofuel generation [4,11]. Microalgae-derived biofuel production has been revealed to be of utmost importance due to its small environmental impact, high biomass yield, non-arable land requirement, simplicity of cultivation conditions, and enhanced lipid productivity. In contrast to the surrounding environment, microalgae are capable of capturing inorganic carbon in the cytoplasm, which is crucial for the mechanism of carbon concentration [12]. Earlier literature depicted that the accumulation of green microalgae biomass involves approximately 1.83 kg of CO2 per kg [3,13]. Although their photosynthetic abilities are comparable to those of land plants, microalgae significantly increase the capability of converting solar energy into biomass when compared to plants [14]. In this quest, previous literature revealed that the utmost performance of biomass production from solar energy was recorded as 4.6% for C3 plants and 6.0% for C4 plants at a temperature of 30 °C, which decreased considerably to 2.9% and 4.2%, when evaluated in the field [14]. Hence, instead of plant crops, it has been emphasised that the remarkable record production of microalgae cultivation at temperate latitudes is denoted as >5 times. Thus, it is believed that microalgae have a high capacity to transform atmospheric CO2 into stored carbon and oxygen, thereby acting as a carbon sink by utilising solar energy. On the other hand, in comparison to plant-based lipids such as soybean (0.4-ton ha−1 y−1), rapeseed (0.7-ton ha−1 y−1), and jatropha (4.1-ton ha−1 y−1), microalgal biomass has a higher lipid yield (4.5–7.5-ton ha−1), boosting economic biofuel assimilation [15].
There are several contributions of microalgae in determining their applications:
- i.
- Under photoautotrophic conditions, microalgae maintain a simple unicellular structural configuration that allows the entire biomass to be photosynthetically reactive without the use of heterotrophic organelles. They are naturally occurring and exist across a variety of habitats from fresh water to marine without regard for a seasonal life cycle.
- ii.
- The efficient metabolic activity of microalgal cells is influenced by the availability of several trophic modes, namely photoautotrophic, mixotrophic, and heterotrophic, which are dependent on physicochemical balance and environmental adaptation. Over intrinsic photoautotrophy, the organic carbon and light-interceded mixotrophic cultivation conditions provide a supplementary benefit to the entire microalgal structure for increasing biomass and accumulation of essential by-products. As a result, microalgae have a quicker doubling time and a flexible metabolism.
- iii.
- Microalgae are not involved in the food vs. fuel debate because they can grow in both non-fertile land and wastewater. Accordingly, the use of microalgae would evolve as an environmentally sustainable option to plant-based crops.
- iv.
- Different microalgal strains can be designated for specific growth conditions, which are suitable and easily adaptable to terrestrial climatic behaviour, which is portrayed as more difficult with conventional crops.
1.2. Microalgae Biorefinery
The combination of numerous unit operations in the ever-expanding field of microalgae biorefinery accelerates resource recovery, process performance, and sustainability, with the goal of producing numerous types of bioenergy and other valuable products (Figure 1). Therefore, it is a convincing fact that microalgae-derived biomass can be accredited as a useful feedstock when combined with other bioprocessing parameters to generate secondary products. As a result, several biorefinery schemes have been implemented in order to focus on target products [15,16].
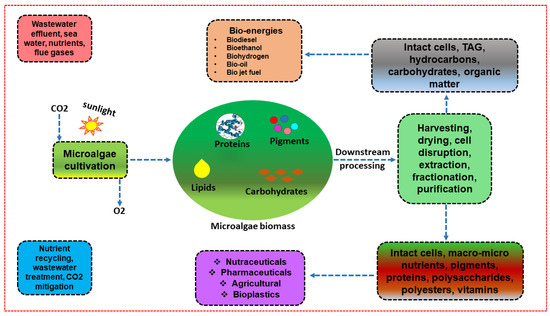
Figure 1.
Conceptualization of microalgae biorefinery.
Individual product valorisation from microalgal biomass, on the other hand, has made the entire bioprocessing process more time-consuming, expensive, and less profitable [17]. In order to maximise the economic advantage and minimise waste release, the focus has recently switched to cascading extraction of various products from the same microalgal biomass. Even if the idea of cascading extraction for the production of several products has been widely used, it is still in its infancy and needs a lot of research to be realised [18]. A critical technical bottleneck is associated with large-scale microalgae culture, which occurs frequently in modular operations, resulting in cost reduction [19]. Aside from that, to assess the feasibility of microalgae-derived biomass, energy and cost-inducive cell harvesting have emerged as additional challenges, increasing processing costs by at least 20–30% [20]. To improve biomass recovery, a number of cell-harvesting methods have been used [2]. Among them, a few microalgal strains are harvested using autoflocculation, which is considered to be the most important and economical method [21]. However, the self-flocculation technique is entirely dependent on species specificity and does not always meet economic robustness. Microalgal responses to inorganic flocculants, on the other hand, are most likely strain-dependent [22]. Furthermore, the addition of such synthetic inorganic flocculants results in an additional operating cost. As a result, the use of biopolymers and nanomaterials derived from microalgal strains may increase cell-harvesting efficiency for bioenergy production. Henceforth, by analysing all the aforementioned bottlenecks and their potential solutions, we herein provide the conceptual phenomenon of “use and throw—liner” towards a “use, treat, and reuse-circular” bioeconomical approach, with the goal of closing the energy–environmental nexus gap. The novel perspective of sequential extraction technology from microalgae for multiproduct synthesis addresses the entire waste valorisation prospect and supports the zero-waste discharge principle. As a result, we believe that this novel concept of recycled technology contributes to a low carbon footprint and can reduce reliance on fossil-fuel-based resources in order to live a sustainable life. As a result, the entire phenomenon is considered to be a possible way of creating an innovative boulevard within the context of a circular bioeconomy and possible zero-waste technology development.
2. Overview of Research Status of Bioenergy Generation
According to global estimates, bioenergy currently has a high potential for increasing to approximately 10% of the world’s energy supply [23]. Forecasts show that the rate of production of renewable energy sources will increase to 145 EJ by the year 2060, aiming for advanced bioenergy to play a significant role than conventional sources. A critical discussion has been elaborated about the current status (international and national) of microalgae-derived bioenergy by taking into account all of these factors.
2.1. International Status
China has experienced incredible population growth over the last four decades, necessitating an urgent focus on renewable energy to address the environmental crisis. According to the National Bureau of Statistics of China (NBSC), total energy utilisation increased to 4300 metric tonnes in 2016, while imported energy increased to 774 metric tonnes [24]. Furthermore, according to IEA (International Energy Agency) estimates, China was one of the top CO2 emitting countries in 2010 [25]. In light of this, it has been determined that the rate of CO2 emissions increased by up to 4.2%. As a result, there is a strong need for renewable energy production for economic stability, both from an ecological standpoint and to decrease the cost of coal-based oil manufacturing. As a result, the Chinese government implemented various environmental policies to promote the sustainability of bioenergy and reduce carbon emissions. According to the current state of China’s biofuel scenario, bioethanol plays an important role, and a few regions, such as Henan, Anhui, Shandong, and Heilongjiang, have extensive infrastructure for bioethanol transportation [23,26]. Aside from bioethanol, China has focused on biogas production by installing biogas digestors in various locations. Though the rate of biodiesel generation in China is currently low, it is expected to rise to more than 54% in the coming years. Contrarily, Russia is well-known for having the world’s largest biomass reserves. When the availability of large biomass production is considered, bioenergy demonstrates significant potential in the Russian economic sector as well as the international energy market [27]. However, in the current scenario, Russia’s bioenergy sector is losing ground in terms of commercialization. As a result, the concern of technocrats is required for further development in the bioenergy sector. Despite the accelerated growth of the wind and solar energy sectors in European countries in recent years, the bioenergy sector has emerged as the pivotal and primary source of renewable and biodegradable energy. According to the analysis, European nations increased approximately 18% in total gross energy consumption, among others. Few countries, such as Germany, Italy, and the United Kingdom, have achieved the highest levels of bioenergy production, with 328,840, 78,355, and 94,303 TJ, respectively. Taking all factors into account, it has been determined that the European Union is one of the world’s major biodiesel producers, accounting for 31% of global biodiesel generation [28]. A report from the US Energy Administration claims that the use of domestic energy sources would increase by 32% between 2006 and 2012 [29]. In 2012, renewable energy accounted for approximately 9% of total energy consumption. The Energy Act (1992) was primarily recognised in the United States, focusing on the generation of alternative energy resources. In recent years, the demand for biodiesel in the United States has increased significantly. According to the EIA 2021 report, in 2020 due to rising demand, diesel imports grew by nearly 12% in United States. Imports of biodiesel into the United States were predicted to rise by 43% and 49% in 2021 and 2022, respectively. Recently, Asian countries have shown promising utilization of bioenergy sources such as bagasse and husk. Biofuel production in ASEAN countries has changed dramatically in recent years. The Malaysia government implemented a biofuel policy in 2006 with the goal of reducing the consumption of fossil fuels. The government organisation intended to increase the blending ratio from 7% to 10% by 2008. (B7:B10). Malaysia produced 1250 million litres of biofuel with a 16% reduction in 2020. In mid-2021, the government planned to increase B20 blending by 20% [30].
2.2. National Status
India is regarded as the world’s most energy-intensive country. One of the primary resources for bioenergy development has been biomass. According to bioenergy-generation analysis, Punjab produces the most bioenergy (16,860 MJ), followed by Haryana (11,559 MJ), Gujarat (6660 MJ), and Uttar Pradesh (3716 MJ), among other states. Furthermore, India’s northeast region has enormous potential in the bioenergy sector [31]. According to India’s bioenergy survey, New National Biogas and Organic Manure Programme, Thermal Energy Application, and Biogas Power Plant are a few of the recent initiatives that the Indian government has introduced to increase the production of bioenergy. The Indian government has also contributed to a few joint ventures and investments that have benefited the biofuels sector. The National Policy for Bioenergy Development has permitted a maximum of 100% direct investment from foreign countries for the automatic upgrade of biofuel generation, with the condition that all generated biofuels be used for inland applications. A few state governments have also enthusiastically participated in order to explore further implementation with other states and countries. However, the question of infrastructure and land requirements for developing biofuel industries remains unclear. Thus, the government is concerned about meeting the need for bioenergy development.
3. Photosynthetic Carbon Sequestration Efficiency in Microalgae
3.1. Microalgae Based Composition
Basic microalgae are a combination of sugars, lipids, and protein, which can all be converted into a variety of products. Microalgae are chemically composed of primary metabolites that can be transformed into high-value products [32]. The chemical composition of microalgae, on the other hand, varies depending on species and strain. Certain microalgae strains produce only lipids or carbohydrates. For example, oleaginous microalgae from the genus Nanochloropsis and Trachydiscus are incapable of producing carbohydrates [33]. To maximise production for the conversion process, it is critical to select a strain with a high lipid and carbohydrate level. Lipid composition is a significant consideration in the production of microalgae-based biofuel [34]. Saturated and unsaturated fatty acids with carbon-chain lengths ranging from 12 to 24 make up microalgae lipids. Microalgae also contain significant amounts of proteins and carbohydrates. Starch is a form of storage for carbohydrates in the plastids, and it also serves as the main building block of cell walls (cellulose, pectin, and sulphated polysaccharides) [35]. Although microalgal carbohydrate composition varies by species, microalgae usually have certain polysaccharides like pectin, agar, and alginate in their outer cell wall. In contrast, cellulose, hemicellulose, and glycoprotein make up the majority of the inner cell wall [36].
3.2. Factors Influencing Microalgae Growth
Certain factors, such as nutritional content, light, mixing, and temperature, have a significant impact on microalgae biomass by directly affecting the photosystem.
3.2.1. Nutrients
Nitrogen is a crucial nutrient for microalgae development as well as a key component of basic metabolism [35]. For the synthesis of biomolecules such as proteins and DNA, nitrogen is necessary [37]. The ability of microalgae to synthesis proteins, lipids, and carbohydrates will be impacted in low nitrogen environments [38]. Chlorella minutissima was cultivated in BMM medium with a 50% drop in nitrogen, and a light intensity of 33.75 mol m −2s −1, yielding a carbohydrate content of 60.3% [39]. Stress conditions associated with nitrogen scarcity are expected to have an impact on biomass and carbohydrate production [40]. Chlorella produced 3430 mg/L of biomass with an optimal nitrogen concentration of 0.05%. Phosphorus has an impact on biomass production, specifically the amount of lipids and carbohydrates [41,42]. For heterotrophic cultivation, the carbon sources are fructose, glucose, and glycerol. Mixotrophic culture employs a combination of inorganic and organic carbon sources [41]. Because of OH formation during the photosynthesis process, the pH of the medium frequently rises to 11, affecting the growth of microalgae, so CO2 must be continuously supplied into the system [43].
3.2.2. Culture Conditions
- (a)
- Light and Temperature
It has been discovered that the amount of light has a substantial influence on the generation of biomass from microalgae. The chemical composition and growth of cells are directly influenced by their photosynthetic system [44]. In the case of the microalgae Scenedesmus abundans, higher light intensities appear to increase photosystem efficiency [45]. Furthermore, several findings suggested that dark and light phases can be changed for photosynthetic efficiency [46]. Variations in biomass output and growth rate have been empirically demonstrated by studying the cell development of similar species under different light and time conditions [47]. According to the literature, the intensity of light varies from species to species, but in general, the ideal conditions for microalgae development are 16:8 (light and dark). Temperature control is critical in biological and microalgae growth [48,49]. Different microalgae species have different optimal growth temperatures. Any temperature difference, whether higher or lower, can restrict or even stop microalgal activity and growth, lowering biomass output [50]. pH and CO2, both of which are influenced by temperature, are two important factors associated with biomass production. The ideal atmospheric condition has been determined to be between 20 and30 °C [51], but carbohydrate and lipid levels have been found to rise at 25 °C in Chlorella [52].
- (b)
- Magnetic fields
Magnetic fields (MF) have long been studied in microalgal cultures. The intensity and duration of MF vary depending on the microalga species [53,54]. MF application is a toxin-free, cost-effective alternative for increasing biomass, depending on the timing and technique of treatment. In one study, high carbohydrate production was observed for Chlorella minutissima when it was cultivated for 12 days. To increase carbohydrate yield, three factors were used, which were limiting nitrogen content, adding pentose, and MF studies [55]. Generally speaking, the aforementioned combination of several factors leads to synthesis of biomolecules by microalgae. Thus, to improve carbohydrate yield, the strain and ideal cell-culture growing conditions should be prioritised, followed by technical measures such as nutrient starvation, irradiation, and MF treatments.
- (c)
- pH and salinity
Variations in the pH of the medium affect photosynthesis and the production of microalgal biomass. Microalgae prefer optimal pH and salinity [56]. Using atmospheric CO2 to grow microalgae in alkaline conditions promotes biomass development [57]. It has been discovered that the optimal pH range for microalgal growth is pH 6–9 [41]. However, when the pH was raised to 9.5, the amount of chlorophyll in microalgae was found to decrease [58]. The microalga Chlorella can adapt to a wide pH range of 4–10, and biomass was obtained at pH 9–10 [59]. In addition, fungal contamination can be avoided by cultivating in alkaline conditions [60].
3.2.3. Operational Mode
Biomass production is significantly impacted by the culturing medium’s nutrient load and operating mode. Batch, semicontinuous, continuous, and two-stage operations are the most typical modes of operation [61]. These processes have the potential to influence and promote cell development [62]. Rosa, et al. [63] and Qu, et al. [64] elevated carbohydrate production using a semi-continuous system for Spirulina and Chlamydomonas sp. Scenedesmus obliquus was cultivated in batch, semi continuous, followed by a continuous system, in the presence of atmospheric CO2 and nitrogen -limiting conditions. The semi-continuous mode increased the CO2 fixation rate and carbohydrate productivity by 1989 mg/l/d and 468 mg/l/d, respectively. Several cultivation methods can be used, followed by various culture processes that are classified as autotrophic, heterotrophic, or mixotrophic [65]. The modes are used to increase biomass concentration in order to increase yield of biofuel-relevant components, primarily microalgal sugars. Autotrophic cultivation is based on sunlight and inorganic carbon, which stimulates the photosynthetic system and thus increases biomass. Because organic carbon is limited in the autotrophic medium, microorganism contamination is reduced. In the absence of sunlight, the heterotrophic culture relies solely on organic substances for carbon supply. In general, heterotrophic cultivation is less expensive and easier to scale-up. Mixotrophic culture is a promising method for increasing biomass yield and metabolites [66,67,68]. The most promising method for boosting biomass production among the other cultivation methods mentioned is mixotrophic cultivation. Recently, attention has switched away from two-stage operations or bi-phasic microalgal cultivation towards a multi-phasic mode that triggers microalgal lipid productivity without reducing microalgal biomass [1,11]. During the multi-stage operation, the ratio of nutrients along with light radiation were implemented as the major significant considerations for increasing biomass, lipids, and other valuable metabolites. As a result, we anticipate that the multi-phasic culture condition of microalgae by incorporating different cultivation methods such as fed-batch or semi-continuous would benefit multi-stage operations towards the massive production of biomass and lipids.
3.2.4. Reactor Types
Microalgae can be grown in a variety of reactors to increase productivity and thus macromolecule concentration, with the goal of increasing production scale. Cultivation systems are classified into two types: open (raceway ponds) and closed (tubular photobioreactors). Open culture systems are designed to provide continuous mixing via paddlewheels, preventing cell settling. When compared to photobioreactors, they perform well in natural settings, are widely used for mass production, and are less expensive to manufacture and maintain. These cultivation systems are commonly used for the production of liquid fuels such as biohydrogen and bioethanol, but they are not suitable for pharmaceutic applications due to the increased risk of contaminants and water loss due to evaporation. Despite this, raceways are frequently used for large-scale microalgae growth due to their low cost. The two most popular kinds of photobioreactors are stirred tanks and vertical or horizontal tubular reactors. They work with both natural and artificial lighting. Vertical and horizontal tubular photobioreactors are more frequently studied for microalgal biomass because of their large surface areas. Horizontal photobioreactors are widely used for mass procedures due to their variety of layouts and combinations [65]. However, their scalability, pH, temperature control, and mixing can increase operational costs. Gonçalves, et al. [69] used a photobioreactor to grow the microalgae Pseudoneochloris marina for carbohydrate production and investigation for biofuel synthesis in their study [69]. Furthermore, there are several cultivation stages, such as single-stage and two-stage strategies, which increase biomass and lipid production. Due to the high stress levels associated with this cultivation system, a comparison of the characteristics of other single-stage techniques reveals that the high lipid output can be easily achieved using a semi-continuous strategy [70]. To produce the most biomass, the first step of the two-step cultivation system employs a nutrient-rich growth medium. Once an adequate concentration of algal biomass has been created, the medium condition transforms into a stress-induction condition in the second stage.
4. Microalgae Lipids—Production and Relevance
Microalgae is a potential source of feedstock for the creation of carbon-neutral biofuels [70]. Over the years, green microalgae-derived biofuels have received considerable attention as an alternative green energy source to traditional fuel reserves around the world [71]. Among these, lipid is a well-studied form of primary metabolite in microalgae, nourishing a specific membrane characterization and cell signalling pathway [72]. Technocrats have used a variety of approaches to augment the necessary lipid biosynthesis in order to accelerate the accumulation of microalgae-associated biofuels.
4.1. Technologies in Lipid Extraction
In view of this, the technologies used in the lipid-extraction strategy are regarded as a critical step that must be optimised in order to recover all of the neutral lipids for biofuel generation [70,73]. A comprehensive examination of various lipid-extraction technologies is presented in Table 1. Aside from a variety of lipid-extraction techniques, organic solvents-mediated extraction is considered as one of the most convenient methods when applied to the microalgal cell system [74,75,76]. As a result, it is known that lipid biomolecules are transported from wet or dried cell mass in the presence of organic solvents [70]. Traditional lipid-extraction technology generally emphasises the integration of organic solvents and neutral lipids present within each microalgae cell [77,78]. The lipid bodies are desorbed from the cellular matrix and further dissolved in the applied solvent when such an association becomes a dominant component. Thus, we believe that the various types of organic solvents are highly influential due to their potential specificity for neutral lipid release [70]. Furthermore, the use of highly volatile organic solvents facilitates product formation with less energy distillation followed by extraction technique [79,80]. To investigate the lipid-extraction efficiency from a microalgae cell, a wide range of polar (methanol, ethanol, acetone, etc.) and non-polar organic solvents (chloroform, hexane, toluene, diethyl ether, etc.) have been studied [81]. However, the hydrogen and electrostatic interactions of neutral lipid bodies with other biomolecules found in the cell membrane, such as proteins and polar lipids, would not be sufficiently disrupted by the non-polar class of organic solvents. In the future, less polar organic solvents will be mixed with non-polar solvents to form a strong association with membrane-based neutral lipids, facilitating lipid removal efficacy. In most cases, the incorporation of polar organic solvents stimulates the co-extraction of undesirable polar lipids.

Table 1.
Comparison of solvent-mediated lipid-extraction technologies, costs, and energy efficacy.
4.1.1. Traditional Methods: Folch and Bligh-Dyer
- (a)
- Folch Method
Several organic solvents or combinations of them have typically been preferred to specifically extract total lipids by disrupting the microalgal cell membrane [2,11,70]. The Folch method extracts lipids from prospective endogenous cells using a mixture of chloroform and methanol in a 2:1 ratio. One of the major advantages is the ease of processing and rapid extraction of many samples. Various organic solvent-based lipid-extraction technologies are depicted in Table 1.
- (b)
- Bligh and Dyer
Bligh and Dyer is the most effectively used protocol for microalgal lipid-extraction technology [93], where protein molecules settle at the interfacial state of bi-phasic liquid–liquid separation. This method is fairly comparable to the Folch method. The main distinctions are the solvent–solvent and solvent–tissue ratios. The methanol-to-chloroform ratio for this method is 2:1. However, due to the toxic nature of conventional solvents (methanol and chloroform), other organic solvent combinations such as hexane–ether and hexane–isopropanol, have been proposed [94]. According to Lee, Ong, Gan, Chen, and Mahlia [32], the combination of hexane and isopropanol has been proven to be efficient in removing neutral lipids from Chlorella sp. However, the presence of glycolipids reduces the algal cell’s ability to recover lipids.
4.1.2. Accelerated Solvent Extraction Procedure (ASE)
By utilising organic solvents, lipid extraction’s effectiveness can be increased. Richter, et al. [95] developed a novel technique known as ASE, in which the use of organic solvents was performed at elevated temperatures (50–200 °C) and pressure (500–3000 psi). The primary advantages of this extraction procedure are as follows:
- Organic solvents become much less viscous at high temperatures, increasing solvent diffusion [96];
- As the temperature rises from 20 to 150 °C, the diffusion rate increases [95];
- The high pressure of the entire ASE-related lipid-extraction system emphasises the organic solvents’ deep penetration into the cell membrane, causing cell disruption;
- A smaller volume of organic solvent and a shorter extraction time are both highly advantageous for the ASE technique.
By analysing all of the aforementioned benefits of ASE over the traditional one, Mulbry, et al. [97] reported that, in comparison to the Folch method, the ASE method was able to yield 85–95% of lipids from green algae Rhizoclonium sp.
4.1.3. Supercritical Fluids
Toxic, volatile, and exhaustible organic solvents are undesirable for large-scale lipid extraction for environmental and security reasons. As a result, instead of toxin-mediated organic based solvents, supercritical fluids are used as one of the most efficient green solvents. CO2 is the most commonly used green solvent in this quest, with a modest critical pressure of 72 bar and a lower critical temperature of 320 °C [85]. SC-CO2 has contributed a number of advantages for suitable lipid extraction, such as:
- Easy and fast penetration into solid matrices to release the lipid biomolecules;
- Extracted or released lipids will be recovered rapidly by the evaporation of SC-CO2 at the gaseous stage via depressurization, yielding a solvent-free lipid.
However, the non-polar properties of SC-CO2 show a lower dissolution capacity. To produce polar and membrane-bound neutral lipids such as phospholipids and glycolipids, all polar organic solvents are added to the mixture. In view of this, McKennedy, Önenç, Pala, and Maguire [83] showed that when methanol was used as the solvent for the SC-CO2-based extraction, there was a greater increase in long chain fatty acids than when hexane was used as a co-solvent [83]. Moreover, on the other hand, Choi, et al. [98] reported that the SC-CO2-extraction process improved the neutral lipids as well as a very small fraction of glycolipids from Scenedesmus obliquus, but not phospholipids [98]. However, when compared to Bligh and Dyer, lipid productivity was relatively low.
4.1.4. Soxhlet Extraction of Lipids
Soxhlet-based lipid extraction yields more lipids; however, a few researchers have demonstrated conflicting reports [99]. According to the literature, the Soxhlet extraction technique is typically ineffective for biomass with a high moisture content [100]. During this process, lipid diffusion takes place through the microalgal cell wall [101,102]. Proper identification and selection of the organic solvent is thought to play a significant role in carrying out this process. Hence, several polar-based organic solvents such as ethanol, chloroform, hexane, and others produced the greatest amount of lipid biomolecules, whereas acetone produced the least amount. Among all binary solvents, the combinations of chloroform–hexane, ethanol–hexane, and chloroform–ethanol have been demonstrated as 1:1, 1:2, 1:3, and 3:1 ratios, with the chloroform–ethanol (1:1, v/v) yielding the highest amount of lipid. Soxhlet extraction yields the highest lipid yield; however, continuous heating generated during the boiling process could potentially result in lipid oxidation and degradation of heat-accountable amalgams [103,104].
4.2. Lipid Processing and Conversion to Fuel
There are several lipid-processing and conversion technologies available for fuel conversion. Figure 2 illustrates the various methods of producing lipids from microalgae using various conversion processes to bioenergy.
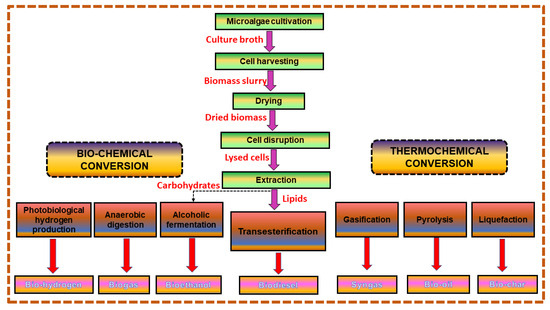
Figure 2.
Generation of biofuels from microalgae by employing several conversion techniques.
4.2.1. Thermochemical Conversion
Heat and chemicals are typically used in the thermochemical conversion process to generate energy. Microalgae biomass has been transferred into biochar as well as bio-oil with other accessories based on the chemical constituents and moisture content present in the microalgal cells [5]. An overview of this process and the operational conditions of all the thermochemical conversion are illustrated in Table 2.

Table 2.
Operational modes of different thermochemical conversion techniques [15].
- (a)
- Combustion process
The combustion process is regarded as one of the most important lipid-conversion technologies, in which microalgal biomass is ignited at elevated temperatures ranging from 800 to 10,000 °C in the presence of atmospheric O2. Using this combustion process, steam generated from dried cell mass with lower moisture content can generate bioelectricity, allowing steam turbines to run [105]. Combustion plant locations range from domestic to industrial (100–3000 MW). However, bioproducts, which emit NOX, CO2, CO, ash, dust, and other pollutants, are the major bottleneck of the entire combustion process. As a result of its maximum energy conversion efficiency, the co-combustion process is a highly influential and striking option, particularly in coal-based power plants [5]. Several European countries, including Spain, the Netherlands, and Germany, favour combustion as the sole and fastest method of biomass conversion [106]. When extremely packed and dense pellets are used as fuels, the main benefits are increased electric efficiency, lower speculation costs, and direct emission avoidance. Aside from that, combustion can help reduce CO2 emissions.
- (b)
- Pyrolysis
One of the most widely used thermochemical conversion techniques is pyrolysis. During this process, biomass is converted into solid (charcoal), liquid (bio-oil), and gas (fuel) phases using a vacuum and a 5000 °C heat treatment. The hydrocarbon chain lengths found in microalgal biomass are broken down into smaller ones using a variety of strategies. Hemicellulose molecules, which have oxygen side chains, allow for a slight breakdown of long hydrocarbon chains in order to assimilate acetic acids and other organic acids, which is considered the initial and first step. The second step deals with cellulose, which requires a little more heat to degrade into levoglucosan and other accessories. Lignin molecules, on the other hand, are split to form monomers and oligomers of polyphenols. By maintaining the temperature, rate of heating, and other parameters, all by-products are transported between the solid and liquid phases. In India, pyrolysis is recognised as a simple application for bioelectricity generation.
- (c)
- Gasification
In an endothermic process called gasification, biomass is transformed into highly exhaustible gases at temperatures ranging from 800 to 10,000 °C [5]. During this process, the biomass reacts with gaseous phases, for example steam, oxygen-rich atmospheric air, or a combination of the two. Typically, the behaviour and quantity of the gaseous phase instigate the qualitative phenomenon and energy features of gas; thus, such generated gas is referred to as syngas to generate methanol. As a result, we believe that the biomass-amalgamated gasification process should be considered as a potential application for converting the gaseous phase into electricity via gas turbines.
4.2.2. Biochemical Conversion
Biochemical conversion is the most well-established approach for transforming biomass into oil by utilising various microalgal strains and catalysts. The major conversion routes during this process are depicted here.
- (a)
- Transesterification
Transesterification is a biochemical process that converts TAG derived from microalgae to FAME, which is required for biodiesel production. In this process, various types of homogeneous, heterogeneous, and enzymatic catalysts are used. FAME has long been thought of as a primary ingredient in the production of biodiesel. A few European nations use biodiesel as a transportation fuel. Aside from that, numerous private and public sector organisations in India are actively engaged in biodiesel production. FAME can also be utilised as an alternative green fuel when mixed with diesel.
- (b)
- Anaerobic digestion (AD)
During the AD process, organic constituents are converted into biogas, which produces CH4, CO2, and a small quantity of H2S. This is a slightly complicated procedure with a total transformation efficiency of 21%. Following the release of CO2, the biogas is efficiently converted into high-impact natural gas and methanol. To improve the waste heat generated by turbines, a combination of heat and power machineries has been implemented. The sludge residue is used in organic farming, making AD a potentially useful and promising technique with numerous applications. The main aspects for improving AD effectiveness are the types of biomass, operational conditions, and reactor design.
5. Biochemical Conversion for Bioenergy Production
5.1. Biodiesel
One of the most important biofuels produced is biodiesel, which has a high demand due to its biodegradable and non-toxic properties, allowing it to be used in regular transportation without modification. It can be utilised without modification in both diesel and gasoline engines [107]. Using a catalyst, the chemical process of transesterification causes an interaction between oil and alcohol to create biodiesel [108]. Prior to transesterification, the usual process involves isolating and purifying the microalgal biomass. Cell harvesting and lipid extraction are examples of refining phases [109]. Because oil extraction and cellular disruption techniques for some microalgae require higher energy output, these refinement stages severely limit the commercialization of biodiesel. Second, wet biomass affects oil extraction due to the available water, necessitating time-consuming drying. The catalyst type used in the traditional transesterification process has an impact on the various transesterification pathways [110]. Several studies have been conducted to determine the optimal operational conditions for biodiesel production using microalgae via an enzymatic and homogeneous alkaline catalyst or a heterogeneous catalyst [111,112].
5.2. Bioethanol
The biomass from microalgae can also be utilized to synthesis bioethanol via fermentation [113]. The cellulose/starch-rich algal residues left over after extraction of lipid can be used to make bioethanol. A crucial element in the generation of bioethanol is the amount of carbohydrates in the biomass [114,115]. The microalgae cultivation parameters can influence bioethanol production. In fact, changing the culture conditions allows for the determination of the biochemical composition. The primary environmental variables that influence biochemical composition are illumination, pH, and temperature. In general, efforts to limit nutrition sources can be used to develop microalgae in order to increase carbohydrate concentration [116]. When nitrogen and phosphorus are limited during microalgae cultivation, the biomass contains more carbohydrates. Before extracting carbohydrates from microalgae, a pre-treatment step is needed. According to Velazquez-Lucio, et al. [117], the starch or cellulose extracted from cells can be fermented to produce bioethanol using water or organic solvents. Because of their ability to convert carbohydrates into alcohol, yeasts are the most used microorganisms for fermentation. The advantages of using microalgae biomass for bioethanol synthesis are that algae biomass does not contain components such as hemicellulose and lignin, making extraction easier. The main barriers to industrial application are a lack of understanding about genetically altered cyanobacteria and research into fermentation technologies [118].
5.3. Biohydrogen
Microalgae and cyanobacteria have the necessary characteristics for producing bioH2 gas. Given that the only by-product of hydrogen combustion is water, this energy source is both renewable and clean. With a converting power that is 2.75 times that of combustible hydrocarbons, H= is regarded as a future fuel [119,120]. Although the earth does not naturally have access to this resource, it has recently been used to generate power via internal combustion engines or fuel cells. Compared to conventional thermochemical and electrochemical processes like gasification and water electrolysis, biological hydrogen production (bioH2) is more energy-efficient and less damaging to the environment [121]. Traditional techniques require high temperatures and are energy-intensive (970–1100 K). Furthermore, because they produce significant CO2 emissions, they are not recognised as environmentally friendly processes on a commercial level. The first four types of procedures for producing bioH2 from microalgae are direct bio-photolysis, indirect bio-photolysis, photo-fermentation, and dark fermentation. Protons (H+) and oxygen (O2) from water are separated by microalgae in the presence of light in direct bio-photolysis. H+ is converted into H2 by hydrogenase, an enzyme that generates H2 [122,123]. The production of H2 is low in this process due to the simultaneous production of H2 and O2 and the subsequent mixing of the two gases, which produces water as a by-product. Furthermore, the sensitivity of hydrogenase to oxygen slows H2 generation rates [124]. This inhibiting effect can be overcome by using indirect bio-photolysis. Indirect bio-photolysis has two stages. Cells produce oxygen and engage in photosynthesis in phase 1 to accumulate organic molecules, primarily glucose. This stage is also known as the aerobic phase. In phase 2, cells break down organic molecules that have been stored anaerobically. In addition, nutritional stress is applied to the microalgae to increase sugar synthesis. Later, they are used as a substrate for dark fermentation by an Enterobacter aerogenes strain. Following nutritional stress, the sugar contents of the three microalgae are 42.6, 21.9, and 28.6%, respectively, while producing 57, 41, and 47 mL H2/g of H2. According to the literature, the amount of sugar in microalgae has a direct impact on H2 production. However, the commercialization of large-scale H2 production has only been considered in a few studies. The energy sector discourages commercial production of H2 gas because it is expensive in comparison to other fuels. Metabolic engineering is being applied to conduct techno-economic analyses and optimise processes to attract investments and make it more affordable.
5.4. Bio Oil
Crude bio-oil, a dark, viscous, and potent fuel, is one popular use for algae energy. Bio-oil from microalgae can be produced using pyrolysis and hydrothermal liquefaction processes. According to reports, many microalgae produced bio-oil yields that were 5 to 25% higher than their lipid content [125]. Because it requires less energy than drying, digesting microalgal biomass through aquaculture is appealing. The three primary subcategories of pyrolysis are slow, intermediate, and fast pyrolysis. Chemically, bio-oils are made up of acids, cresols, and aldehydes. Bio-oils produced by pyrolyzing lignocellulose biomass are complex and viscous, but high in oxygen [126]. Hydrogenation and cracking of bio-oils are thus required to improve them. The liquefaction process is sped up using catalysts. The primary end products are bio-oils; however, during the conversion process, gaseous, aqueous, and solid bi-products are also created [110]. Before analysis, a liquefaction procedure in an autoclave is completed and cooled at room temperature.
5.5. Bio Jet Fuel
Researchers are attempting to turn biodiesel into bio-jet fuel in order to raise the quality of the by-product [127]. Many studies on the production of biodiesel from microalgae have been published [68,128]. A catalyst (meso-Y) proved effective in converting heavy microalgae biodiesel to bio-jet fuel with aromatic hydrocarbon selectivity (4.47%) in a study. Numerous studies have reported on the conversion of microalgal biomass to biofuel, but studies on bio-jet fuels remain unexplored. The properties of bio-jet fuel must meet American standards. Table 3 illustrates various types of biomass used for bio-jet fuel, including soybean. Advanced and additive-based processes will be required to create a bio-jet fuel that meets ASTM criteria. Table 4 depicted various microalgae capable of producing various types of biofuel and eventually replacing fossil fuels in the future.

Table 3.
Properties of microalgal bio-jet fuel compared to conventional bio-jet fuel.

Table 4.
Microalgae-based bioenergy production.
6. Energy Kinetics—TGA
Thermogravimetric analysis (TGA) is frequently used to observe the thermodynamic behaviour of a substance in a controlled environment [148]. TGA has been used extensively to demonstrate the essential features of microalgae-derived bioenergy production. The rate of chemical reaction is known to be influenced by reactor configuration and thermochemical operation [149]. Chemical kinetics are critical in the production of microalgae bioenergy. Determination of the thermal characteristics of microalgae is critical for recognising thermal degradation or stability and the mass’s efficacy in product formation. The Arrhenius law has been used to define energy kinetics or thermochemical properties:
Here, is determined as conversion degree, t expresses the time of conversion, A is denoted as the pre-exponential factor, describes as the activation energy, R demonstrates the universal gas constant, and T determines the absolute temperature of the entire reaction. The degree of conversion is usually indicated as the mass fractionation of degraded solid residues and expressed as the following equation:
Here, m0 and mf are described as the initial and final masses of solid substrates and m is expressed as the mass of the solid at a given time; is described as the entire mass of all free volatiles and v determines the mass of free volatile substrates.
If we convert Equation (1) in non-isothermal form, then the reaction describes the rate of conversion as a function of temperature by maintaining the heating constant value β. In view of that, we are developing the chemical equation:
By doing the substitution of Equation (1) into Equation (3), the non-isothermal rate has been determined as
Different types of kinetic modelling have been successfully applied to microalgal feedstocks by observing all of the above-mentioned chemical equations. The study of TGA analysis is presented in Table 5.

Table 5.
TGA-analysis condition for energy kinetics study of different microalgal strains.
7. Algomics Approach for Microalgae-Based Bioenergy Synthesis
Significant biotechnological interventions can now improve green microalgae-allied biomass and lipid assimilation. These changes in microalgae are influenced by advanced technologies based on nuclear, chloroplast, and mitochondrial genomes. In this review, we discuss multi-omics technologies for estimating microalgal lipid accumulation toward bioenergy yield (Figure 3).
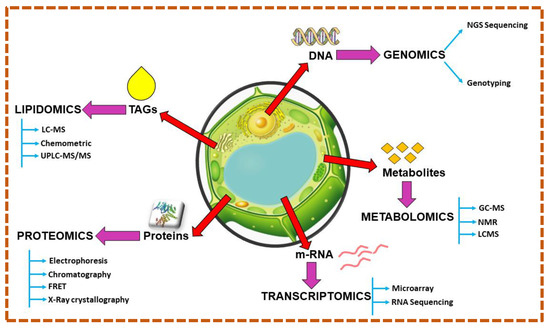
Figure 3.
Overview of multi-omics approach in microalgae.
7.1. Genomics
Genomics is concerned with the evolutionary development of various microalgae strains and their ability to adapt to environmental stress conditions [157]. It is well known that increasing lipid productivity in microalgae is entirely dependent on the expression and silencing of lipid-producing genes or genomes. Previous research demonstrated that the Chlorophyceae microalgae Chlamydomonas reinhardtii and green microalgae Nannocholoropsis sp. were modified or altered for maximum lipid yield by examining genome editing tools such as CRISPR/Cas 9 [158,159,160]. In order to investigate the lipid biosynthetic metabolism toward bioenergy production, the Kennedy pathway is recognised as one of the primary routes for TAG accumulation within the microalgal cell, which employs the sequential transmission of acyl groups from the acyl-CoA with the assistance of acyltransferases [161]. Furthermore, DGAT (diacylglycerol acyl transferase) overexpression influences the enhancement of neutral lipid globules. This has been confirmed in the microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum, which showed an increase of at least 20–44% neutral lipids [162,163]. As a result, we anticipate that the enhanced oil bodies may trigger the neutral lipid content due to DGAT 2 overexpression, which could be used directly for biofuel estimation. Furthermore, a study of its FAME components revealed a significant increase in PUFA, primarily the EPA (76.2%). The covalent relationship of DAG in the presence of DGAT enzyme is the most important step in TAG assimilation. In light of this, DGAT 1 and DGAT 2 have been isolated; however, the gene sequences do not share a similarity index. The genes expressed in the DGAT 1 family have a homology to ACAT and the genes in the DGAT2 family have a similar sequence to monoester synthase [163,164]. Henceforth, it is convincible that the overexpression of the aforementioned genes can play a significant role in the accumulation of TAG in green microalgae.
7.2. Proteomics
Proteomics is a computational biology approach that focuses on the large-scale characterization of the entire protein complex in a tissue or organism. Essentially, the concept phenomenon of proteomics demonstrates protein expression by using appropriate conditions at a specific time period [165]. In view of this, the selection of a target protein molecule may be able to aid in understanding the characteristics of an organism in order to improve it. As a result, we believe that proteomics study using model microalgae strains, for instance Synechocystis sp. PCC 6803 and Chlamydomons reinhardtii, is particularly useful for evaluating the protein profile under regular or environmentally stressed conditions [166,167]. Previously, the Chlorophyceae unicellular green microalgae Chlamydomons reinhardtii was linked to the presence of closely aggregated protein molecules, which could aid in the synthesis of oil bodies [168]. It is known to all that the specific enzyme, diacylglyceryl-N, N, N-trimethylhomoserine synthase, is already present in many microalgal strains and is thus responsible for the accumulation of DGTS (diacylglyceryltrimethylhomo-Ser, extraplastidial membrane lipid). The enzymes that assimilate DGTS are usually associated with the formation of membranous lipid bodies. Aside from that, scientists and researchers have discovered the existence of additional lipid-associated proteins such as lipid-trafficking proteins, acyltransferases, lipases, and other protein molecules involved in sterol-based metabolic activity. Cao, et al. [169] reported that the use and expression of a specific microalgae-based protein Chlre4|400527 (GPAT9) ortho-log, associating with glycerol-3-phosphate acyltransferase (MmGPAT3) enzyme, stimulated lipid production, thereby initiating the oil-synthesis metabolic route [169]. On the other hand, Trentacoste, et al. [170] demonstrated the selection, description, and knock-down of specific lipase-based enzymatic reactivity in Thalassiosira pseudonana genome version 3 (protein ID 264297, Thaps3 264297). They addressed a hypothesis to target the knock-down of lipase-catalysing enzymes, which could potentially increase the generation of free fatty acids (FFA) from lipid bodies, in the direction of the lipid catabolism approach [170]. Moreover, they emphasised that involving the knock-down responses of lipid catabolism will not impede microalgal biomass production. Furthermore, carbohydrate synthesis would be unaffected, and thus the functionality of microalgal growth, which is linked to a metabolic pathway, would not be affected. Apart from that, the research team of Trentacoste, Shrestha, Smith, Glé, Hartmann, Hildebrand and Gerwick [170] discovered that mutated microalgal strains were able to improve lipid assimilation under nutrient replete and deplete conditions, in contrast with wild-type strains. It is a well-known fact that nitrogen-enriched or limited conditions stimulate lipid accumulation in microalgae cells, and this entire phenomenon has been extensively demonstrated for the change of microalgae proteome.
7.3. Transcriptomics
Transcriptomics, a high throughput technology, reveals structural and functional characterization of an entire transcript under specific conditions. Transcriptomics is concerned with the appearance and abundance of RNA transcripts [171]. The transcriptomic approach is used in microalgae to identify or explain the mechanistic behaviour of specific metabolic pathways in microalgae cells. According to Peng, Wu and Tu [153], transcriptome estimation of green microalgae Coccomyxa subellipsoidea C-169 had demonstrated the ability to generate carbon flux by maintaining the C–N ratio at an elevated CO2 level for growth and lipid accumulation. [153].
7.4. Metabolomics
Furthermore, the study of metabolomics refers to low molecular biomolecules produced during cellular metabolic developments, where the responses of such biological processes have been altered for both genetic and environmental reasons. Microalgae-associated metabolomics studies are primarily concerned with the accumulation of qualitative and quantitative determinations of economically derived secondary metabolites such as fatty acids, pigments, polysaccharides, and so on, which can be used in nutraceutical and pharmaceutical frameworks [157]. In microalgal metabolism, a variety of strategies such as flux-balance estimation, photosynthesis-efficiency modification, lipid biosynthesis and statistical optimization have been used [172]. In view of that, to focus on microbial metabolism, NMR-assisted evaluation is playing an important role, as proved by Abreu, et al. [173], where they demonstrated enhanced secondary metabolite production in the microalgae Amphidinium carterae by modulating the culture medium as well as light irradiation [173]. Recently, Zhang, et al. [174] investigated the integration of transcriptomics and metabolomics phenomena in metabolic regulation of Chlamydomonas reinhardtii at elevated CO2 levels. Table 6 depicts a number of microalgal strains and the omics technology used to indicate microalgal metabolism and associated lipid assimilation on behalf of the multi-omics approach.

Table 6.
Microalgal strains used for the study of algomics approach towards their improvement in lipid biosynthesis.
7.5. In Silico Metabolic Approach
The study of secondary metabolites in microalgae includes fatty acids, carotenoids, and polysaccharides. Various bioinformatics tools have been developed to help researchers better understand gene-regulation networks and metabolic activity in different microalgae species. Metabolic flux analysis (MFA) is one of the approaches that combines stoichiometric constraints and a radio-labelled tracer to provide information on the flux distribution among microorganisms [182]. NMR-based metabolic study has also revealed that changing of the parameters (nutrient and light) results in increased fatty acid and carotenoid production. Genome-scale modelling has recently been explored in several areas such as medicine and the environment for studying metabolic pathways. Metabolic model construction frequently necessitates knowledge of biochemical reactions (S matrix), species-specific data from genomic annotations, and high-throughput experimental data. Using annotated sequences, a new genome-scale model has been developed for evaluating environmental disturbance and genetic manipulations among different species.
8. Economic Aspect of Microalgae-Derived Biofuels
8.1. Life Cycle Assessment (LCA)
LCA is regarded as a statistical technique for analysing the economic viability of microalgae-based biofuels as well as their environmental implications throughout the bioprocessing process [183]. A variety of microalgae-cultivation strategies are used to assess the feasibility of supporting the biorefinery approach for commercialization. To advance the ecological sustainability of the entire microalgae system, it is critical to optimise metabolic conditions, extract different bioproducts using low-cost technologies, and reutilize the media and co-products [184]. As a result, we believe that LCA can assist in identifying hotspot conditions in a structure and drive to demonstrate a few technological modernizations affording reduced energy as well as environmental impact [185]. Numerous LCA-analysis studies, including energy, water use, and environmental prospects, are currently being conducted that emphasise the production of microalgae biomass, biofuels, and other valuable metabolites. [185,186]. As we all know, the current cost of microalgae-derived biofuels is significantly higher than that of traditional fossil-based fuel resources. However, forecasts show that technological advancements, conversion techniques, and large-scale microalgae production will significantly reduce production costs in the near future. Despite the promising potential of microalgae as a feedstock for renewable energy production, a thorough technology assessment is required to make the entire system more cost-effective and sustainable long-term.
8.2. Economics of Microalgae Based Biofuels
Technocrats must involve modern technologies that support economic considerations in order to focus on effective development of the microalgae biorefinery model. We know that microalgae have enormous potential to produce maximum amounts of lipids and an inept ability to grow even on non-arable land [184]. This simple distinguishing feature has engrossed many efforts to improve the entire bioprocessing of microalgae, which could be used to harvest bioenergy in a costly manner. As a result, the race of nanobiotechnology has now given peak momentum for microalgal growth and cost-effective harvesting [187]. In today’s scenario, there is a strong emphasis on the advancement and large-scale development of microalgae-derived feedstock for bioenergy production. The conformation of microalgae-based biomass and its metamorphosis into various fuel sources is regarded as an ideal and critical feature for effective biorefineries. Thus, there is an urgent need to maintain technoeconomic analysis (TEA) in order to focus on the development of the entire biofuel generation from a laboratory scale, up to pilot, and to an industrial level. According to a survey of annual operational costs to produce biofuels from microalgae, raw feedstock is critical, and biomass degradation to yield fermentable sugars determines the total cost. According to the TEA, microalgae feedstock contributes approximately 32% of the total processing cost of several biofuels.
9. Research Needs and Future Implications
The versatility of photosynthetic green microalgae for bioenergy production has been cultivated as a potential and promising alternative to traditional fossil fuels to meet the world’s ever-increasing energy demand. We are confident that multi-omics approaches will be extremely useful in predicting metabolic pathways for detecting lipid-based genome sequencing, which may exhibit the conceptual phenomenon for lipid enhancement towards significant biofuel generation.
Despite research advances in microalgae-based biofuel systems, improvements are still needed to establish an economically viable biofuel-production system. Several bioprocessing strategies are used in the biochemical conversion of microalgal biomass and derived lipids, including acid hydrolysis, microalgae fermentation, enzyme saccharification, distillation, purification, and other pre-treatment steps. Future research should concentrate on the low-cost pre-treatment methods and efficient fermentation to avoid sugar loss during subsequent biofuel production from microalgae. Furthermore, one of the principal difficulties for commercialization of the microalgae-based biofuel system has emerged as lipid enhancement without compromising the lethal effects of microalgal growth. Thus, research is needed to advance the study of lipid assimilation, metabolism, and genome sequencing at the molecular and genetic levels. As a result, next-generation computational biology-based sequencing is required to determine the genetic hotspots associated with lipid accumulation in microalgae. Furthermore, researchers should demonstrate the lipid-processing mechanism for future economically viable bioenergy production.
10. Conclusions
The achievement of carbon-neutral biofuel production from microalgae demonstrates promising potential for environmentally friendly energy evolution on an industrial scale. Microalgae’s expanded opportunities as a sustainable and renewable feedstock in the bioenergy sector to support the circular bioeconomy are quite promising. Lipid-extraction strategies and multi-omics-based approaches can increase biofuel yield in microalgae, resulting in a cost-effective system with a high eco-sustainability index.
Author Contributions
Conceptualization, writing—original draft preparation: S.V., A.K., M.P.R. and S.A.K.; writing—review and editing: D.Y.Y.T., H.S.H.M. and Z.M.; supervision, funding acquisition: P.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Grant Scheme, Malaysia [FRGS/1/2019/STG05/UNIM/02/2], National Natural Science Foundation (No. 41876124) and Zhejiang Provincial Natural Science Foundation of China (No. LZ21C030001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Khanra, A.; Vasistha, S.; Rai, M.P.; Cheah, W.Y.; Khoo, K.S.; Chew, K.W.; Chuah, L.F.; Show, P.L. Green bioprocessing and applications of microalgae-derived biopolymers as a renewable feedstock: Circular bioeconomy approach. Environ. Technol. Innov. 2022, 28, 102872. [Google Scholar] [CrossRef]
- Vasistha, S.; Khanra, A.; Clifford, M.; Rai, M.P. Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Renew. Sust. Energ. Rev. 2021, 137, 110498. [Google Scholar] [CrossRef]
- Sharma, G.K.; Khan, S.A.; Shrivastava, M.; Gupta, N.; Kumar, S.; Malav, L.C.; Nogiya, M.; Dubey, S.K. Bioremediation of sewage wastewater through microalgae (Chlorella minutissima). Indian J. Agric. Sci. 2020, 90, 2024–2028. [Google Scholar] [CrossRef]
- Rai, M.P.; Gupta, S. Effect of media composition and light supply on biomass, lipid content and FAME profile for quality biofuel production from Scenedesmus abundans. Energy Convers. Manag. 2017, 141, 85–92. [Google Scholar] [CrossRef]
- Yadav, K.K.; Krishnan, S.; Gupta, N.; Prasad, S.; Amin, M.A.; Cabral-Pinto, M.M.S.; Sharma, G.K.; Marzouki, R.; Jeon, B.-H.; Kumar, S.; et al. Review on Evaluation of Renewable Bioenergy Potential for Sustainable Development: Bright Future in Energy Practice in India. ACS Sustain. Chem. Eng. 2021, 9, 16007–16030. [Google Scholar] [CrossRef]
- Khan, M.; Salman, M.; Bashir, U.; Malik, M.S.; Ikram, A. Joint external evaluation of IHR core capacities of the Islamic Republic of Pakistan, 2016. Int. J. Infect. Dis. 2018, 73, 36–37. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Parthiba Karthikeyan, O.; Rene, E.R.; Kumar, G.; Rajesh Banu, J. A review on anaerobic digestion of energy and cost effective microalgae pretreatment for biogas production. Bioresour. Technol. 2021, 332, 125055. [Google Scholar] [CrossRef]
- Khan, S.A.; Sharma, G.K.; Malla, F.A.; Kumar, A.; Gupta, N. Microalgae based biofertilizers: A biorefinery approach to phycoremediate wastewater and harvest biodiesel and manure. J. Clean. Prod. 2019, 211, 1412–1419. [Google Scholar] [CrossRef]
- Baldos, U.L.C.; Fuglie, K.O.; Hertel, T.W. The research cost of adapting agriculture to climate change: A global analysis to 2050. Agric. Econ. 2020, 51, 207–220. [Google Scholar] [CrossRef]
- Malla, F.A.; Khan, S.A.; Rashmi; Sharma, G.K.; Gupta, N.; Abraham, G. Phycoremediation potential of Chlorella minutissima on primary and tertiary treated wastewater for nutrient removal and biodiesel production. Ecol. Eng. 2015, 75, 343–349. [Google Scholar] [CrossRef]
- Khanra, A.; Vasistha, S.; Kumar, S.; Rai, M.P. Cultivation of microalgae on unhydrolysed waste molasses syrup using mass cultivation strategy for improved biodiesel. 3 Biotech 2021, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.-P.; Probert, I.; Michaud, P. What Is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Venkata Mohan, S. Microalgae-biorefinery with cascading resource recovery design associated to dairy wastewater treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Erbland, P.; Caron, S.; Peterson, M.; Alyokhin, A. Design and performance of a low-cost, automated, large-scale photobioreactor for microalgae production. Aquac. Eng. 2020, 90, 102103. [Google Scholar] [CrossRef]
- ElFar, O.A.; Chang, C.-K.; Leong, H.Y.; Peter, A.P.; Chew, K.W.; Show, P.L. Prospects of Industry 5.0 in algae: Customization of production and new advance technology for clean bioenergy generation. Energy Convers. Manag. X 2021, 10, 100048. [Google Scholar] [CrossRef]
- Young, P.; Phasey, J.; Wallis, I.; Vandamme, D.; Fallowfield, H. Autoflocculation of microalgae, via magnesium hydroxide precipitation, in a high rate algal pond treating municipal wastewater in the South Australian Riverland. Algal Res. 2021, 59, 102418. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Shobana, S.; Bakonyi, P.; Nemestóthy, N.; Xia, A.; Banu, J.R.; Kumar, G. A review on chemical mechanism of microalgae flocculation via polymers. Biotechnol. Rep. 2019, 21, e00302. [Google Scholar] [CrossRef] [PubMed]
- Duarah, P.; Haldar, D.; Patel, A.K.; Dong, C.-D.; Singhania, R.R.; Purkait, M.K. A review on global perspectives of sustainable development in bioenergy generation. Bioresour. Technol. 2022, 348, 126791. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Ke, J.H.; Zhou, S.; Xie, G.H. Estimation of the quantity and availability of forestry residue for bioenergy production in China. Resour. Conserv. Recycl. 2020, 162, 104993. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, P. Focus on bioenergy industry development and energy security in China. Renew. Sust. Energ. Rev. 2014, 32, 302–312. [Google Scholar] [CrossRef]
- Peidong, Z.; Yanli, Y.; Yongsheng, T.; Xutong, Y.; Yongkai, Z.; Yonghong, Z.; Lisheng, W. Bioenergy industries development in China: Dilemma and solution. Renew. Sust. Energ. Rev. 2009, 13, 2571–2579. [Google Scholar] [CrossRef]
- Namsaraev, Z.B.; Gotovtsev, P.M.; Komova, A.V.; Vasilov, R.G. Current status and potential of bioenergy in the Russian Federation. Renew. Sust. Energ. Rev. 2018, 81, 625–634. [Google Scholar] [CrossRef]
- Oecd FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD: Paris, France, 2022. [Google Scholar]
- Energy, E.T. US Energy Information Administration (EIA) Monthly Energy Review; U.S. Department of Energy: Washington, DC, USA, 2021. [Google Scholar]
- Wahab, A.G. Biofuels Annual; MY2020-0013; Office of Agricultural Affairs: Wilayah Persekutuan Kuala Lumpur, Malaysia, 2020. [Google Scholar]
- Hiloidhari, M.; Das, D.; Baruah, D.C. Bioenergy potential from crop residue biomass in India. Renew. Sust. Energ. Rev. 2014, 32, 504–512. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Hildebrand, M.; Abbriano, R.M.; Polle, J.E.W.; Traller, J.C.; Trentacoste, E.M.; Smith, S.R.; Davis, A.K. Metabolic and cellular organization in evolutionarily diverse microalgae as related to biofuels production. Curr. Opin. Chem. Biol. 2013, 17, 506–514. [Google Scholar] [CrossRef]
- Lim, J.H.K.; Gan, Y.Y.; Ong, H.C.; Lau, B.F.; Chen, W.-H.; Chong, C.T.; Ling, T.C.; Klemeš, J.J. Utilization of microalgae for bio-jet fuel production in the aviation sector: Challenges and perspective. Renew. Sust. Energ. Rev. 2021, 149, 111396. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Yamada, T.; Sakaguchi, K. Comparative studies onChlorella cell walls: Induction of protoplast formation. Arch. Microbiol. 1982, 132, 10–13. [Google Scholar] [CrossRef]
- Muñoz, R.; Gonzalez-Fernandez, C. Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- de Carvalho Silvello, M.A.; Severo Gonçalves, I.; Patrícia Held Azambuja, S.; Silva Costa, S.; Garcia Pereira Silva, P.; Oliveira Santos, L.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef] [PubMed]
- Freitas, B.C.B.; Cassuriaga, A.P.A.; Morais, M.G.; Costa, J.A.V. Pentoses and light intensity increase the growth and carbohydrate production and alter the protein profile of Chlorella minutissima. Bioresour. Technol. 2017, 238, 248–253. [Google Scholar] [CrossRef]
- Solís-Salinas, C.E.; Patlán-Juárez, G.; Okoye, P.U.; Guillén-Garcés, A.; Sebastian, P.J.; Arias, D.M. Long-term semi-continuous production of carbohydrate-enriched microalgae biomass cultivated in low-loaded domestic wastewater. Sci. Total Environ. 2021, 798, 149227. [Google Scholar] [CrossRef]
- Khan, S.A.; Malla, F.A.; Malav, L.C.; Gupta, N.; Kumar, A. Potential of wastewater treating Chlorella minutissima for methane enrichment and CO2 sequestration of biogas and producing lipids. Energy 2018, 150, 153–163. [Google Scholar] [CrossRef]
- Sonkar, S.; Mallick, N. An alternative strategy for enhancing lipid accumulation in chlorophycean microalgae for biodiesel production. J. Appl. Phycol. 2018, 30, 2179–2192. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.-H. Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sust. Energ. Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Mandotra, S.K.; Kumar, P.; Suseela, M.R.; Nayaka, S.; Ramteke, P.W. Evaluation of fatty acid profile and biodiesel properties of microalga Scenedesmus abundans under the influence of phosphorus, pH and light intensities. Bioresour. Technol. 2016, 201, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Dunker, S.; Wilhelm, C. Cell Wall Structure of Coccoid Green Algae as an Important Trade-Off Between Biotic Interference Mechanisms and Multidimensional Cell Growth. Front. Microbiol. 2018, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. The effects of light and temperature on microalgal growth and nutrient removal: An experimental and mathematical approach. RSC Adv. 2016, 6, 22896–22907. [Google Scholar] [CrossRef]
- Wang, L.; Addy, M.; Lu, Q.; Cobb, K.; Chen, P.; Chen, X.; Liu, Y.; Wang, H.; Ruan, R. Cultivation of Chlorella vulgaris in sludge extracts: Nutrient removal and algal utilization. Bioresour. Technol. 2019, 280, 505–510. [Google Scholar] [CrossRef]
- Huang, J.; Hankamer, B.; Yarnold, J. Design scenarios of outdoor arrayed cylindrical photobioreactors for microalgae cultivation considering solar radiation and temperature. Algal Res. 2019, 41, 101515. [Google Scholar] [CrossRef]
- Suthar, S.; Verma, R. Production of Chlorella vulgaris under varying nutrient and abiotic conditions: A potential microalga for bioenergy feedstock. Process Saf. Environ. Prot. 2018, 113, 141–148. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Santos, L.O.; Deamici, K.M.; Menestrino, B.C.; Garda-Buffon, J.; Costa, J.A.V. Magnetic treatment of microalgae for enhanced product formation. World J. Microbiol. Biotechnol. 2017, 33, 169. [Google Scholar] [CrossRef]
- Huo, S.; Chen, X.; Zhu, F.; Zhang, W.; Chen, D.; Jin, N.; Cobb, K.; Cheng, Y.; Wang, L.; Ruan, R. Magnetic field intervention on growth of the filamentous microalgae Tribonema sp. in starch wastewater for algal biomass production and nutrients removal: Influence of ambient temperature and operational strategy. Bioresour. Technol. 2020, 303, 122884. [Google Scholar] [CrossRef]
- Menestrino, B.d.C.; Pintos, T.H.C.; Sala, L.; Costa, J.A.V.; Santos, L.O. Application of Static Magnetic Fields on the Mixotrophic Culture of Chlorella minutissima for Carbohydrate Production. Appl. Biochem. Biotechnol. 2020, 192, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Ren, T. Primary Factors Affecting Growth of Microalgae Optimal Light Exposure Duration and Frequency. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Maizatul, A.Y.; Radin Mohamed, R.M.S.; Al-Gheethi, A.A.; Hashim, M.K.A. An overview of the utilisation of microalgae biomass derived from nutrient recycling of wet market wastewater and slaughterhouse wastewater. Int. Aquat. Res. 2017, 9, 177–193. [Google Scholar] [CrossRef]
- Daliry, S.; Hallajisani, A.; Mohammadi Roshandeh, J.; Nouri, H.; Golzary, A. Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob. J. Environ. Sci. 2017, 3, 217–230. [Google Scholar]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Ho, S.-H.; Kondo, A.; Hasunuma, T.; Chang, J.-S. Engineering strategies for improving the CO2 fixation and carbohydrate productivity of Scenedesmus obliquus CNW-N used for bioethanol fermentation. Bioresour. Technol. 2013, 143, 163–171. [Google Scholar] [CrossRef]
- Rosa, G.M.d.; Moraes, L.; Cardias, B.B.; Souza, M.d.R.A.Z.d.; Costa, J.A.V. Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour. Technol. 2015, 192, 321–327. [Google Scholar] [CrossRef]
- Qu, W.; Loke Show, P.; Hasunuma, T.; Ho, S.-H. Optimizing real swine wastewater treatment efficiency and carbohydrate productivity of newly microalga Chlamydomonas sp. QWY37 used for cell-displayed bioethanol production. Bioresour. Technol. 2020, 305, 123072. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, Q.; Han, P.; Li, J. Chapter 3—Microalgae Cultivation and Photobioreactor Design. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 31–50. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Zhai, J.; Wei, H. Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresour. Technol. 2018, 263, 555–561. [Google Scholar] [CrossRef]
- Verma, R.; Kumari, K.V.L.K.; Srivastava, A.; Kumar, A. Photoautotrophic, mixotrophic, and heterotrophic culture media optimization for enhanced microalgae production. J. Environ. Chem. Eng. 2020, 8, 104149. [Google Scholar] [CrossRef]
- Li, T.; Cheng, J.; Zhang, X.; Liu, J.; Huang, R.; Zhou, J. Jet range hydrocarbons converted from microalgal biodiesel over mesoporous zeolite-based catalysts. Int. J. Hydrog. Energy 2018, 43, 9988–9993. [Google Scholar] [CrossRef]
- Gonçalves, C.F.; Menegol, T.; Rech, R. Biochemical composition of green microalgae Pseudoneochloris marina grown under different temperature and light conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101032. [Google Scholar] [CrossRef]
- Lee, S.Y.; Khoiroh, I.; Vo, D.-V.N.; Senthil Kumar, P.; Show, P.L. Techniques of lipid extraction from microalgae for biofuel production: A review. Environ. Chem. Lett. 2021, 19, 231–251. [Google Scholar] [CrossRef]
- Yap, J.K.; Sankaran, R.; Chew, K.W.; Halimatul Munawaroh, H.S.; Ho, S.-H.; Rajesh Banu, J.; Show, P.L. Advancement of green technologies: A comprehensive review on the potential application of microalgae biomass. Chemosphere 2021, 281, 130886. [Google Scholar] [CrossRef]
- Mishra, S.; Maiti, A. The efficiency of Eichhornia crassipes in the removal of organic and inorganic pollutants from wastewater: A review. Environ. Sci. Pollut. Res. 2017, 24, 7921–7937. [Google Scholar] [CrossRef]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2, 61. [Google Scholar] [CrossRef]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Schaub, T.; Holguin, F.O.; Cooke, P.; Lammers, P.; Nirmalakhandan, N.; Li, Y.; Lu, X.; et al. In situ ethyl ester production from wet algal biomass under microwave-mediated supercritical ethanol conditions. Bioresour. Technol. 2013, 139, 308–315. [Google Scholar] [CrossRef]
- Amaro, H.M.; Fernandes, F.; Valentão, P.; Andrade, P.B.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Effect of Solvent System on Extractability of Lipidic Components of Scenedesmus obliquus (M2-1) and Gloeothece sp. on Antioxidant Scavenging Capacity Thereof. Marine Drugs 2015, 13, 6453–6471. [Google Scholar] [CrossRef]
- Qiu, C.; He, Y.; Huang, Z.; Li, S.; Huang, J.; Wang, M.; Chen, B. Lipid extraction from wet Nannochloropsis biomass via enzyme-assisted three phase partitioning. Bioresour. Technol. 2019, 284, 381–390. [Google Scholar] [CrossRef]
- Karemore, A.; Sen, R. Downstream processing of microalgal feedstock for lipid and carbohydrate in a biorefinery concept: A holistic approach for biofuel applications. RSC Adv. 2016, 6, 29486–29496. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Du, T.-B.; Pi, H.-C.; Jang, S.-M.; Lin, Y.-H.; Lee, H.-T. Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2. Bioresour. Technol. 2011, 102, 10151–10153. [Google Scholar] [CrossRef] [PubMed]
- Sati, H.; Mitra, M.; Mishra, S.; Baredar, P. Microalgal lipid extraction strategies for biodiesel production: A review. Algal Res. 2019, 38, 101413. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Lorenzen, J.; Igl, N.; Tippelt, M.; Stege, A.; Qoura, F.; Sohling, U.; Brück, T. Extraction of microalgae derived lipids with supercritical carbon dioxide in an industrial relevant pilot plant. Bioprocess Biosyst Eng. 2017, 40, 911–918. [Google Scholar] [CrossRef]
- McKennedy, J.; Önenç, S.; Pala, M.; Maguire, J. Supercritical carbon dioxide treatment of the microalgae Nannochloropsis oculata for the production of fatty acid methyl esters. J. Supercrit. Fluids 2016, 116, 264–270. [Google Scholar] [CrossRef]
- Obeid, S.; Beaufils, N.; Camy, S.; Takache, H.; Ismail, A.; Pontalier, P.-Y. Supercritical carbon dioxide extraction and fractionation of lipids from freeze-dried microalgae Nannochloropsis oculata and Chlorella vulgaris. Algal Res. 2018, 34, 49–56. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Sartaj, K.; Chandra, R. Chapter 2—Extraction of lipids from algae using supercritical carbon dioxide. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin Asiri, A.M., Isloor, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–39. [Google Scholar] [CrossRef]
- Patil, P.D.; Dandamudi, K.P.R.; Wang, J.; Deng, Q.; Deng, S. Extraction of bio-oils from algae with supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2018, 135, 60–68. [Google Scholar] [CrossRef]
- Cicci, A.; Sed, G.; Bravi, M. Potential of choline chloride–based natural deep eutectic solvents (NaDES) in the extraction of microalgal metabolites. Chem. Eng. Trans. 2017, 57, 61–66. [Google Scholar]
- Lu, W.; Alam, M.A.; Pan, Y.; Wu, J.; Wang, Z.; Yuan, Z. A new approach of microalgal biomass pretreatment using deep eutectic solvents for enhanced lipid recovery for biodiesel production. Bioresour. Technol. 2016, 218, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Alam, M.A.; Wang, Z.; Huang, D.; Hu, K.; Chen, H.; Yuan, Z. One-step production of biodiesel from wet and unbroken microalgae biomass using deep eutectic solvent. Bioresour. Technol. 2017, 238, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Sed, G.; Cicci, A.; Jessop, P.G.; Bravi, M. A novel switchable-hydrophilicity, natural deep eutectic solvent (NaDES)-based system for bio-safe biorefinery. RSC Adv. 2018, 8, 37092–37097. [Google Scholar] [CrossRef]
- Tommasi, E.; Cravotto, G.; Galletti, P.; Grillo, G.; Mazzotti, M.; Sacchetti, G.; Samorì, C.; Tabasso, S.; Tacchini, M.; Tagliavini, E. Enhanced and Selective Lipid Extraction from the Microalga P. tricornutum by Dimethyl Carbonate and Supercritical CO2 Using Deep Eutectic Solvents and Microwaves as Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 8316–8322. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś, P.; Gębicki, J. Theoretical and Economic Evaluation of Low-Cost Deep Eutectic Solvents for Effective Biogas Upgrading to Bio-Methane. Energies 2020, 13, 3379. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Nagle, N.; Lemke, P. Production of methyl ester fuel from microalgae. Appl. Biochem. Biotechnol. 1990, 24, 355–361. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Bu, X.; Pang, M.; Wang, B.; Zhang, Y.; Xie, K.; Zhao, X.; Wang, Y.; Guo, Y.; Liu, C.; Wang, R.; et al. Determination of Piperazine in Eggs Using Accelerated Solvent Extraction (ASE) and Solid Phase Extraction (SPE) with High-Performance Liquid Chromatography—Fluorescence Detection (HPLC-FLD) and Pre-Column Derivatization with Dansyl Chloride. Anal. Lett. 2020, 53, 53–71. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Buyer, J.; Luthria, D.L. Optimization of an Oil Extraction Process for Algae from the Treatment of Manure Effluent. J. Am. Oil Chem. Soc. 2009, 86, 909–915. [Google Scholar] [CrossRef]
- Choi, K.J.; Nakhost, Z.; Krukonis, V.J.; Karel, M. Supercritical fluid extraction and characterization of lipids from algae scenedesmus obliquus. Food Biotechnol. 1987, 1, 263–281. [Google Scholar] [CrossRef]
- Li, Y.; Ghasemi Naghdi, F.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A comparative study: The impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Factories 2014, 13, 14. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Quinn, M.; Van Wychen, S.; Templeton, D.W.; Wolfrum, E.J. Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification. Anal. Bioanal. Chem. 2012, 403, 167–178. [Google Scholar] [CrossRef]
- Ríos, S.D.; Castañeda, J.; Torras, C.; Farriol, X.; Salvadó, J. Lipid extraction methods from microalgal biomass harvested by two different paths: Screening studies toward biodiesel production. Bioresour. Technol. 2013, 133, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Patnaik, R.; Singh, A.K.; Mallick, N. Comparative assessment of various lipid extraction protocols and optimization of transesterification process for microalgal biodiesel production. Environ. Technol. 2013, 34, 2009–2018. [Google Scholar] [CrossRef]
- Hajinajaf, N.; Rabbani, Y.; Mehrabadi, A.; Tavakoli, O. Experimental and modeling assessment of large-scale cultivation of microalgae Nannochloropsis sp. PTCC 6016 to reach high efficiency lipid extraction. Int. J. Environ. Sci. Technol. 2022, 19, 5511–5528. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; John Wiley & Sons: New York, NY, USA, 2019. [Google Scholar]
- Boumanchar, I.; Chhiti, Y.; M’Hamdi Alaoui, F.E.; Elkhouakhi, M.; Sahibed-dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Investigation of (co)-combustion kinetics of biomass, coal and municipal solid wastes. Waste Manag. 2019, 97, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bhuiya, M.M.K.; Rasul, M.; Khan, M.; Ashwath, N.; Mofijur, M. Comparison of oil extraction between screw press and solvent (n-hexane) extraction technique from beauty leaf (Calophyllum inophyllum L.) feedstock. Ind. Crops Prod. 2020, 144, 112024. [Google Scholar] [CrossRef]
- Sajid, Z.; Khan, F.; Zhang, Y. Process simulation and life cycle analysis of biodiesel production. Renew. Energy 2016, 85, 945–952. [Google Scholar] [CrossRef]
- Hidalgo, P.; Toro, C.; Ciudad, G.; Navia, R. Advances in direct transesterification of microalgal biomass for biodiesel production. Rev. Environ. Sci. Biotechnol. 2013, 12, 179–199. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Teo, S.H.; Islam, A.; Ng, F.L.; Taufiq-Yap, Y.H. Biodiesel synthesis from photoautotrophic cultivated oleoginous microalgae using a sand dollar catalyst. RSC Adv. 2015, 5, 47140–47152. [Google Scholar] [CrossRef]
- Umdu, E.S.; Tuncer, M.; Seker, E. Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al
2O
3 supported CaO and MgO catalysts. Bioresour. Technol. 2009, 100, 2828–2831. [Google Scholar] [CrossRef] [PubMed] - Catone, C.M.; Ripa, M.; Geremia, E.; Ulgiati, S. Bio-products from algae-based biorefinery on wastewater: A review. J. Environ. Manag. 2021, 293, 112792. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vladut, V.; Biris, S.-S. Capitalization of wastewater-grown algae in bioethanol production. In Proceedings of the 19th International Scientific Conference, Jelgava, Latvia, 20–22 May 2020; Engineering for Rural Development, Latvia University of Life Sciences and Technologies: Jelgava, Latvia, 2020; pp. 1859–1864. [Google Scholar]
- Kim, S.W.; Koo, B.S.; Lee, D.H. A comparative study of bio-oils from pyrolysis of microalgae and oil seed waste in a fluidized bed. Bioresour. Technol. 2014, 162, 96–102. [Google Scholar] [CrossRef]
- Velazquez-Lucio, J.; Rodríguez-Jasso, R.M.; Colla, L.M.; Sáenz-Galindo, A.; Cervantes-Cisneros, D.E.; Aguilar, C.N.; Fernandes, B.D.; Ruiz, H.A. Microalgal biomass pretreatment for bioethanol production: A review. Biofuel Res. J. 2018, 5, 780–791. [Google Scholar] [CrossRef]
- Ramos Tercero, E.A.; Domenicali, G.; Bertucco, A. Autotrophic production of biodiesel from microalgae: An updated process and economic analysis. Energy 2014, 76, 807–815. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kumar, P.; Mehariya, S.; Purohit, H.J.; Lee, J.-K.; Kalia, V.C. Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int. J. Hydrog. Energy 2014, 39, 14663–14668. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Memon, S.; Ur Rahman, Z.; Lee, K.; Han, J.-I. Current status, barriers and developments in biohydrogen production by microalgae. Renew. Sust. Energ. Rev. 2013, 22, 571–579. [Google Scholar] [CrossRef]
- Batista, A.P.; Ambrosano, L.; Graça, S.; Sousa, C.; Marques, P.A.S.S.; Ribeiro, B.; Botrel, E.P.; Castro Neto, P.; Gouveia, L. Combining urban wastewater treatment with biohydrogen production—An integrated microalgae-based approach. Bioresour. Technol. 2015, 184, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Tavakoli, O. Improving hydrogen production using co-cultivation of bacteria with Chlamydomonas reinhardtii microalga. Mater. Sci. Energy Technol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Vargas, S.R.; Santos, P.V.d.; Zaiat, M.; Calijuri, M.d.C. Optimization of biomass and hydrogen production by Anabaena sp. (UTEX 1448) in nitrogen-deprived cultures. Biomass Bioenergy 2018, 111, 70–76. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J.; Chang, J.-S. Bioreactor and process design for biohydrogen production. Bioresour. Technol. 2011, 102, 8524–8533. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Harman-Ware, A.E.; Morgan, T.; Wilson, M.; Crocker, M.; Zhang, J.; Liu, K.; Stork, J.; Debolt, S. Microalgae as a renewable fuel source: Fast pyrolysis of Scenedesmus sp. Renew. Energy 2013, 60, 625–632. [Google Scholar] [CrossRef]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Z.; Zhang, X.; Liu, J.; Zhou, J.; Cen, K. Sulfonated mesoporous Y zeolite with nickel to catalyze hydrocracking of microalgae biodiesel into jet fuel range hydrocarbons. Int. J. Hydrog. Energy 2019, 44, 1650–1658. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Microalgae processing for jet fuel production. Biofuels Bioprod. Biorefin. 2018, 12, 522–535. [Google Scholar] [CrossRef]
- Li, L.; Coppola, E.; Rine, J.; Miller, J.L.; Walker, D. Catalytic Hydrothermal Conversion of Triglycerides to Non-ester Biofuels. Energy Fuels 2010, 24, 1305–1315. [Google Scholar] [CrossRef]
- McAfee, E.A. The Aemetis Biorefinery: 100% Replacement, Renewable Jet and Diesel Fuels by Conversion of Existing Biofuels Refinery Facilities. In Proceedings of the Advanced Biofuels Marketing Conference, Amsterdam, The Netherlands, 28–29 June 2012. [Google Scholar]
- Corporan, E.; Edwards, T.; Shafer, L.; DeWitt, M.J.; Klingshirn, C.; Zabarnick, S.; West, Z.; Striebich, R.; Graham, J.; Klein, J. Chemical, Thermal Stability, Seal Swell, and Emissions Studies of Alternative Jet Fuels. Energy Fuels 2011, 25, 955–966. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Q.; Guan, Q.; He, L.; Li, W. Bio-aviation fuel production from hydroprocessing castor oil promoted by the nickel-based bifunctional catalysts. Bioresour. Technol. 2015, 183, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, S.S.; Forster, S.; Eibl, S. In-Depth Interpretation of Mid-Infrared Spectra of Various Synthetic Fuels for the Chemometric Prediction of Aviation Fuel Blend Properties. Energy Fuels 2017, 31, 2934–2943. [Google Scholar] [CrossRef]
- Buffi, M.; Valera-Medina, A.; Marsh, R.; Pugh, D.; Giles, A.; Runyon, J.; Chiaramonti, D. Emissions characterization tests for hydrotreated renewable jet fuel from used cooking oil and its blends. Appl. Energy 2017, 201, 84–93. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; de Lira-Flores, J.A.; Hernández, S. A review on the production processes of renewable jet fuel. Renew. Sust. Energ. Rev. 2017, 79, 709–729. [Google Scholar] [CrossRef]
- Gómez-De la Cruz, A.; Romero-Izquierdo, A.G.; Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; Hernández, S. Modelling of the hydrotreating process to produce renewable aviation fuel from micro-algae oil. In Computer Aided Chemical Engineering; Espuña, A., Graells, M., Puigjaner, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 40, pp. 655–660. [Google Scholar]
- Garcia Alba, L.; Torri, C.; Samorì, C.; van der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal Treatment (HTT) of Microalgae: Evaluation of the Process As Conversion Method in an Algae Biorefinery Concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
- Samiee-Zafarghandi, R.; Karimi-Sabet, J.; Abdoli, M.A.; Karbassi, A. Supercritical water gasification of microalga Chlorella PTCC 6010 for hydrogen production: Box-Behnken optimization and evaluating catalytic effect of MnO2/SiO2 and NiO/SiO2. Renew. Energy 2018, 126, 189–201. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, K.; Oh, B.-R.; Lee, M.-E.; Seo, M.; Li, S.; Kim, J.-K.; Choi, M.; Chang, Y.K. A novel process for the coproduction of biojet fuel and high-value polyunsaturated fatty acid esters from heterotrophic microalgae Schizochytrium sp. ABC101. Renew. Energy 2021, 165, 481–490. [Google Scholar] [CrossRef]
- Ismail, M.M.; Ismail, G.A.; El-Sheekh, M.M. Potential assessment of some micro- and macroalgal species for bioethanol and biodiesel production. Energy Sources Part A: Recovery Util. Environ. Eff. 2020, 1–17. [Google Scholar] [CrossRef]
- Reyimu, Z.; Özçimen, D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Seo, J.-W.; Oh, B.-R.; Lee, E.Y. Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, Y.; Viamajala, S.; Varanasi, S. In situ and Ex situ Catalytic Pyrolysis of Microalgae and Integration With Pyrolytic Fractionation. Front. Chem. 2020, 8, 786. [Google Scholar] [CrossRef]
- Hidalgo, P.; Ciudad, G.; Schober, S.; Mittelbach, M.; Navia, R. Biodiesel synthesis by direct transesterification of microalga Botryococcus braunii with continuous methanol reflux. Bioresour. Technol. 2015, 181, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Kim, B.; Lee, J.W. Concurrent production of biodiesel and chemicals through wet in situ transesterification of microalgae. Bioresour. Technol. 2015, 193, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.B.; Pinnarat, T.; Savage, P.E. Biodiesel Production from Wet Algal Biomass through in Situ Lipid Hydrolysis and Supercritical Transesterification. Energy Fuels 2010, 24, 5235–5243. [Google Scholar] [CrossRef]
- Jadhao, P.R.; Vuppaladadiyam, A.K.; Prakash, A.; Pant, K.K. Co-pyrolysis characteristics and kinetics of electronic waste and macroalgae: A synergy study based on thermogravimetric analysis. Algal Res. 2022, 61, 102601. [Google Scholar] [CrossRef]
- Rasam, S.; Azizi, K.; Moraveji, M.K.; Akbari, A.; Soria-Verdugo, A. Insights into the co–pyrolysis of olive stone, waste polyvinyl chloride and Spirulina microalgae blends through thermogravimetric analysis. Algal Res. 2022, 62, 102635. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Prussi, M.; Bettucci, L.; Libelli, I.M.; Chiaramonti, D. Characterization of microalga Chlorella as a fuel and its thermogravimetric behavior. Appl. Energy 2013, 102, 24–31. [Google Scholar] [CrossRef]
- Agrawal, A.; Chakraborty, S. A kinetic study of pyrolysis and combustion of microalgae Chlorella vulgaris using thermo-gravimetric analysis. Bioresour. Technol. 2013, 128, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wu, Q.; Tu, P. Pyrolytic characteristics of heterotrophic Chlorella protothecoides for renewable bio-fuel production. J. Appl. Phycol. 2001, 13, 5–12. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, I.S.; Kim, H.M.; Wi, S.G.; Bae, H.-J. Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour. Technol. 2014, 153, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Silva, L.; López-González, D.; Garcia-Minguillan, A.M.; Valverde, J.L. Pyrolysis, combustion and gasification characteristics of Nannochloropsis gaditana microalgae. Bioresour. Technol. 2013, 130, 321–331. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.; Yang, X. Co-pyrolysis characteristics of microalgae Isochrysis and Chlorella: Kinetics, biocrude yield and interaction. Bioresour. Technol. 2015, 198, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.S.; Chang, J.-S. Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef]
- Jiang, W.; Brueggeman Andrew, J.; Horken Kempton, M.; Plucinak Thomas, M.; Weeks Donald, P. Successful Transient Expression of Cas9 and Single Guide RNA Genes in Chlamydomonas reinhardtii. Eukaryot. Cell 2014, 13, 1465–1469. [Google Scholar] [CrossRef]
- Baek, K.; Kim, D.H.; Jeong, J.; Sim, S.J.; Melis, A.; Kim, J.-S.; Jin, E.; Bae, S. DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Sci. Rep. 2016, 6, 30620. [Google Scholar] [CrossRef]
- Ajjawi, I.; Verruto, J.; Aqui, M.; Soriaga, L.B.; Coppersmith, J.; Kwok, K.; Peach, L.; Orchard, E.; Kalb, R.; Xu, W.; et al. Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P. Acyl-lipid metabolism. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0161. [Google Scholar] [CrossRef]
- Deng, X.-D.; Gu, B.; Li, Y.-J.; Hu, X.-W.; Guo, J.-C.; Fei, X.-W. The roles of acyl-CoA: Diacylglycerol acyltransferase 2 genes in the biosynthesis of triacylglycerols by the green algae Chlamydomonas reinhardtii. Mol. Plant 2012, 5, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-F.; Zhang, M.-H.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Bai, W.-B.; Li, H.-Y. Improvement of Neutral Lipid and Polyunsaturated Fatty Acid Biosynthesis by Overexpressing a Type 2 Diacylglycerol Acyltransferase in Marine Diatom Phaeodactylum tricornutum. Marine Drugs 2013, 11, 4558–4569. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Smith, S.J.; Zheng, Y.-W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [PubMed]
- Ndimba, B.K.; Ndimba, R.J.; Johnson, T.S.; Waditee-Sirisattha, R.; Baba, M.; Sirisattha, S.; Shiraiwa, Y.; Agrawal, G.K.; Rakwal, R. Biofuels as a sustainable energy source: An update of the applications of proteomics in bioenergy crops and algae. J. Proteom. 2013, 93, 234–244. [Google Scholar] [CrossRef]
- Stauber, E.J.; Hippler, M. Chlamydomonas reinhardtii proteomics. Plant Physiol. Biochem. 2004, 42, 989–1001. [Google Scholar] [CrossRef]
- Pandhal, J.; Wright, P.C.; Biggs, C.A. Proteomics with a pinch of salt: A cyanobacterial perspective. Saline Syst. 2008, 4, 1. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Baudet, M.; Cuiné, S.; Adriano, J.-M.; Barthe, D.; Billon, E.; Bruley, C.; Beisson, F.; Peltier, G.; Ferro, M.; et al. Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: With focus on proteins involved in lipid metabolism. Proteomics 2011, 11, 4266–4273. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.-L.; Li, D.; Tobin, J.F.; Gimeno, R.E. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19695–19700. [Google Scholar] [CrossRef]
- Trentacoste, E.M.; Shrestha, R.P.; Smith, S.R.; Glé, C.; Hartmann, A.C.; Hildebrand, M.; Gerwick, W.H. Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19748–19753. [Google Scholar] [CrossRef]
- Jamers, A.; Blust, R.; De Coen, W. Omics in algae: Paving the way for a systems biological understanding of algal stress phenomena? Aquat. Toxicol. 2009, 92, 114–121. [Google Scholar] [CrossRef]
- Banerjee, C.; Dubey, K.K.; Shukla, P. Metabolic Engineering of Microalgal Based Biofuel Production: Prospects and Challenges. Front. Microbiol. 2016, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Molina-Miras, A.; Aguilera-Sáez, L.M.; López-Rosales, L.; Cerón-García, M.d.C.; Sánchez-Mirón, A.; Olmo-García, L.; Carrasco-Pancorbo, A.; García-Camacho, F.; Molina-Grima, E.; et al. Production of Amphidinols and Other Bioproducts of Interest by the Marine Microalga Amphidinium carterae Unraveled by Nuclear Magnetic Resonance Metabolomics Approach Coupled to Multivariate Data Analysis. J. Agric. Food Chem. 2019, 67, 9667–9682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Z.; Ren, Y.; Wang, L.; Zhang, J.; Liang, C.; Tong, S.; Wang, Y.; Xu, D.; Zhang, X.; et al. Integrating Transcriptomics and Metabolomics to Characterize Metabolic Regulation to Elevated CO2 in Chlamydomonas Reinhardtii. Mar. Biotechnol. 2021, 23, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, L.; Huang, R.; Wang, Q. Improved biohydrogen production with an expression of codon-optimized hemH and lba genes in the chloroplast of Chlamydomonas reinhardtii. Bioresour. Technol. 2011, 102, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Liu, W.-J.; Hu, D.-X.; Wang, X.; Balamurugan, S.; Alimujiang, A.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Identification of a malonyl CoA-acyl carrier protein transacylase and its regulatory role in fatty acid biosynthesis in oleaginous microalga Nannochloropsis oceanica. Biotechnol. Appl. Biochem. 2017, 64, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yuan, C.; Jin, Y.; Hu, G.-R.; Li, F.-L. Characterization of 3-ketoacyl-coA synthase in a nervonic acid producing oleaginous microalgae Mychonastes afer. Algal Res. 2018, 31, 225–231. [Google Scholar] [CrossRef]
- Deng, X.; Cai, J.; Fei, X. Effect of the expression and knockdown of citrate synthase gene on carbon flux during triacylglycerol biosynthesis by green algae Chlamydomonas reinhardtii. BMC Biochem. 2013, 14, 38. [Google Scholar] [CrossRef]
- Shang, C.; Zhu, S.; Wang, Z.; Qin, L.; Alam, M.A.; Xie, J.; Yuan, Z. Proteome response of Dunaliella parva induced by nitrogen limitation. Algal Res. 2017, 23, 196–202. [Google Scholar] [CrossRef]
- Arora, N.; Kumari, P.; Kumar, A.; Gangwar, R.; Gulati, K.; Pruthi, P.A.; Prasad, R.; Kumar, D.; Pruthi, V.; Poluri, K.M. Delineating the molecular responses of a halotolerant microalga using integrated omics approach to identify genetic engineering targets for enhanced TAG production. Biotechnol. Biofuels 2019, 12, 2. [Google Scholar] [CrossRef]
- Balamurugan, S.; Wang, X.; Wang, H.-L.; An, C.-J.; Li, H.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Occurrence of plastidial triacylglycerol synthesis and the potential regulatory role of AGPAT in the model diatom Phaeodactylum tricornutum. Biotechnol. Biofuels 2017, 10, 97. [Google Scholar] [CrossRef]
- Babu, S.S.; Gondi, R.; Vincent, G.S.; JohnSamuel, G.C.; Jeyakumar, R.B. Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies. Sustainability 2022, 14, 15070. [Google Scholar] [CrossRef]
- Ubando, A.T.; Anderson, S.; Ng, E.; Chen, W.-H.; Culaba, A.B.; Kwon, E.E. Life cycle assessment of microalgal biorefinery: A state-of-the-art review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Huang, Z.; Liu, T.; Li, H. Photothermal technique-enabled ambient production of microalgae biodiesel: Mechanism and life cycle assessment. Bioresour. Technol. 2023, 369, 128390. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Priya, A.K.; Dutta, K.; Rajendran, S.; Vasseghian, Y. Engineering strategies and opportunities of next generation biofuel from microalgae: A perspective review on the potential bioenergy feedstock. Fuel 2022, 312, 122827. [Google Scholar] [CrossRef]
- Huang, R.; Li, J.; Tang, Y.; Song, W.; Yu, Y.; Yang, W.; Cheng, J. Comparative life-cycle assessment of microalgal biodiesel production via various emerging wet scenarios: Energy conversion characteristics and environmental impacts. Energy Convers. Manag. 2022, 257, 115427. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Upadhyaya, H.M.; Gupta, V.K.; Verma, P. Microalgae conversion to alternative energy, operating environment and economic footprint: An influential approach towards energy conversion, and management. Energy Convers. Manag. 2022, 269, 116118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).