Abstract
Palm oil is, from an economic, environmental, and social point of view, a vegetable oil with great potential and the state of Pará-Brazil is Brazil’s great producer. In addition, soap phase residue or palm oil neutralization sludge (PONS), a byproduct of the neutralization step of the chemical refinement of palm oil, is produced, posing a huge problem for waste disposal and management in the production process of refined palm oil (RPO). In this context, this work aims to systematically investigate the economic analysis of the thermal–catalytic process of crude palm oil (CPO) and palm oil neutralization sludge (PONS). The thermocatalytic processes of CPO and PONS carried out at pilot scale and their economic feasibility were analyzed. The yields of biofuels produced by fractional distillation were also presented. The physicochemical properties of CPO and PONS, as well as those of organic liquid products obtained by the thermal–catalytic process of CPO and PONS were taken into account in the economic analysis. In addition, the chemical composition organic liquid products obtained by thermal–catalytic process of CPO and PONS, as well as its distillation fractions (green gasoline, green kerosene, green light diesel and heavy diesel), used as key factors/indicators on the economic analysis. The analysis of the key factors/indicators from the thermocatalytic processes of CPO and PONS showed economic viability for both crude palm oil (Elaeis guineensis, Jacq) and palm oil neutralization sludge. The minimum fuel selling price (MFSP) obtained in this work for the biofuels was 1.59 USD/L using crude palm oil (CPO) and 1.34 USD/L using palm oil neutralization sludge (PONS). The best breakeven point obtained was of 1.24 USD/L considering the PONS. The sensibility analysis demonstrated that the pyrolysis and distillation yields are the most important variables that affect the minimum fuel-selling price (MFSP) in both economic analyses.
1. Introduction
More than 8.1 million people worldwide are now employed by the renewable energy industry—a 5% increase since last year—according to a report released by the International Renewable Energy Agency (IRENA) during its 11th council meeting. The countries with the highest number of renewable energy jobs in 2015 are China, Brazil, the United States, India, Japan and Germany. Within the renewable energy sector, the photovoltaic (PV) solar energy segment remains the largest employer in the world, with 2.8 million jobs (up from 2.5 million at last count) with manufacturing, installation and operations and maintenance jobs. This increase is being driven by the fall in renewable energy technology costs and more favorable public policies. Liquid biofuels account for the world’s second-largest employer with 1.7 million jobs worldwide [1].
According to a report published recently by the International Renewable Energy Agency (IRENA), nearly half of all jobs in the world generated by the biofuels industry are in Brazil. The Brazilian ethanol and biodiesel industries employed a total of 845.000 workers last year [2].
The necessities for energy of the modern consuming society have grown tremendously in the last decades. Energy is used to run industrial processes, for domestic consumption in homes, for transportation and for various other purposes. This energy, coming from different sources, forms a system referred to as the energy matrix. The energy matrix represents all the sources available in a country, state or in the world to supply the need (demand) for energy [3].
The world’s energy matrix is mainly composed of nonrenewable sources, such as coal, oil and natural gas. Figure 1A shows the world energy matrix in 2020 [3]. Renewable sources such as solar, wind and geothermal energy, account for only 2.5% of the world energy matrix (marked as “others” in the pie chart of Figure 1A). Combined with hydraulic energy and biomass, only about 15.0% of the global energy sources are renewable.
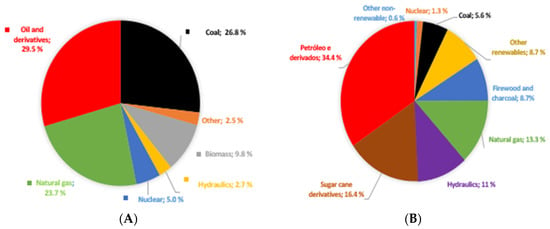
Figure 1.
(A)—world energy matrix 2020 and (B)—Brazilian energy matrix 2021 [3].
Brazil’s energy matrix is very different from that of the rest of the world. In Brazil, although energy consumption from nonrenewable sources exceeds that from renewable sources, it uses more renewable energy than the rest of the world.
Brazil’s consumption of renewable energy is higher than the rest of the world’s renewable energy consumption, with Brazil’s present energy matrix being 48.4% of renewable energy (51.6% of nonrenewable) and the rest of the world’s being 15% of renewable energy (85% non-renewable) [3].
This characteristic of Brazil’s energy matrix is important. Nonrenewable energy sources are mainly responsible for the emission of greenhouse gas emissions (GHGs). Given that Brazil consumes more energy from renewable sources than other countries, it emits less GHG per inhabitant than most other countries [3].
The oil crisis that has taken place in recent decades, coupled with the increased demand for fuels and growing concern for the environment, advocates the search for alternative sources in the production of energy [4]. Alternative sources of renewable energy, such as biomass, are favored over the use of petroleum products because they reduce the emission of gases that cause the greenhouse gas emissions effect.
The main advantages of the renewable energy use (such as palm oil) to produce biofuels are that the plants absorb carbon dioxide in this cycle of production, are biodegradable and present low content of sulfur (S) in the product. The available territorial and climatic conditions in Brazil are favorable to the planting of raw materials necessary for the production of biofuels, which has encouraged investments in public policies in the social sphere to harness regional potential, income generation and employment [5].
One example of a governmental incentive to produce biofuel (such as biodiesel) in Brazil it is that diesel of fossil origin must have the mandatory addition of 13% of biodiesel, in replacement of the 12% applied since March of 2020. This action was planned in Resolution 16 of 2018 of the national energy policy council (CNPE), that has authorized the ANP to increase this percentage to 15%, which should occur by 2023 [6].
The are other technologies applied to producing biofuels using palm oil and palm oil neutralization sludge. In the first case, it can be used the transesterification or enzymatic transesterification. In the second case (palm oil neutralization sludge as a raw material), it also can be applied the enzymatic transesterification or microbiological degradation to produce biogas (this may be accomplished due to the characteristics of soap and consequently high pH).
In recent years, several works have investigated the economic feasibility analysis of biofuel production using biomass as raw material (such as palm oil) and residues (such as palm oil neutralization sludge) through pyrolysis followed by the distillation process. A summary of the most recent studies reported in the literature on techno-economic evaluation is presented synthetically as follows.
Abnisa et al. [7] presented a study on bio-oil production by pyrolysis using palm shell as feedstock in a fluidized-bed reactor at 400, 500, 600 and 800 °C and with N2 carrier gas at 1, 2, 3, 4 and 5 L/min. In order to optimize palm shell production, the effects of temperature, flow rate of N2, particle size and reaction time were measured. The maximum yield of bio-oil obtained was 47.3 wt.% (500 °C, 2 L/min of N2 flow, 60 min reaction time). The most important parameter was the temperature over the bio-oil yield.
Abnisa et al. [8] investigated the production of bio-oil and biochar using three palm oil residues by pyrolysis in a fixed-bed reactor at 500 °C, N2 flow rate of 2 L/min and reaction time of 60 min. The yields obtained from bio-oil production were 16.58–43.50 wt.% and of biochar 28.63–36.75 wt.%. These results are affected by the characteristics of the samples, such as ash, fixed carbon, volatiles, hemicellulose and cellulose. The energy density of the biochar was found to be higher than that of the bio-oil. The highest energy density of the biochar was obtained from a palm leaf sample (23.32 MJ/kg), while that of the bio-oil was obtained from a frond sample (15.41 MJ/kg).
Do et al. [9] presented a study of bio-oil production using empty fruit bunches (EFB) as feedstock, a main residue of the palm oil industry, via fast pyrolysis in a fluidized-bed reactor. It was used a model for commercial process simulations. The total capital investment (TCI) was estimated for five different plant sizes. The EFB bio-oil plant was analyzed in terms of the payback period (PBP), return on investment (ROI), specific capital cost (SCC) and the product value (PV). It was found that 20 kton-dry EFB/yr is the minimum profitable plant size, resulting in a PV of 0.47 USD/kg of bio-oil including 39% water. Sensitivity analysis demonstrated that the plant size and the bio-oil yield are a major influence on the PV. In the most optimistic scenario analyzed, bio-oil can be produced at a PV of 0.27 USD/kg.
Moncada et al. [10] presented a study on biodiesel, ethanol and poly-3-hydroxybutyrate production using a bio-refinery based palm oil, evaluating the techno-economic and environmental aspects. The results demonstrated an economic margin of 64.5% (1.33-fold higher than standalone ethanol production); the potential environmental impact (PEI) was 156.42 PEI/t products and 0.51 t CO2 eq/m3 of biodiesel. In order to measure the footprint indicator and economic impact, feedstock transportation was also included in the analysis. Considering the distance of 300 km, the carbon footprint increased up to 0.59 t CO2 eq/m3 of biodiesel and the economic margin was reduced by 1.31%.
Peryoga et al. [11] investigated bio-oil production using empty fruit bunches (EFBs) as feedstock by pyrolysis. The cost evaluation considered a palm oil mill of 30 metric tons FFB/h capacity. The steps considered in this economic evaluation were chopping, drying, grinding, pyrolysis, solid removal, bio-oil recovery and storage. Sensitivity analysis demonstrated that the raw material price is the most important parameter that affects production cost. The results indicate a promising viability to implement this process in Indonesia. The optimum alternative is to have a bio-oil plant integrated with a palm oil mill.
Mabrouki et al. [12] investigated the biofuel production using three palm oil residues, namely empty fruit bunch (EFB), palm shell (PS) and mesocarp fiber (MF), using a process simulator called SuperPro Designer (SPD). The simulation includes pretreatment, fast pyrolysis, product collection and upgrading sections. The results were validated with data from the literature of wood oak in a fluidized-bed reactor. Maximum bio-oil production was obtained at 550 °C. The results demonstrated that the PS produced a higher yield of char, while a higher yield of liquid fraction was obtained from the EFB and MF.
Thangalazhy-Gopakumar et al. [13] studied biochar and bio-oil production using, as feedstock, palm oil sludge by pyrolysis. The bio-oil presented a heating value of 22.2 ± 3.7 MJ/kg, a yield of 27.4 ± 1.7 wt.% and negligible ash content of 0.23 ± 0.01 wt.%. The biochar was investigated for sorption efficiency by adsorbing (Cd2+ ions) from water and presented a yield of 49.9 ± 0.3 wt.% in the pyrolysis. The removal efficiency of Cd2+ was similar to a commercially activated carbon (89.4 ± 2%). The adsorption isotherm was well-described by the Langmuir model.
Lee et al. [14] investigated biochar production using palm oil sludge (POS), palm kernel shell (PKS) and empty fruit bunch (EFB), residues from the palm oil industry, as feedstock by slow pyrolysis (50 mL min−1 of N2 at 500 °C). It was identified that higher lignin, carbon, volatiles and HHV were present in PKS and EFB when compared with POS (which also presented lower ash). The thermogravimetric was used to analyze the kinetics of pyrolysis at different heating rates (10–40 °C). The results demonstrated that PKS and EFB are promising sources of biochars.
Li et al. [15] presented a study of biofuel and biochar production using different biomasses by fast pyrolysis. The raw materials were grouped into five types: husk/shell/pit, organic residue/product, grass/plant, woody, stalk/cob/ear. The results demonstrated that the biochar yield increases from 0.13 to 0.16 kg/kg with ash content (0.3–7.7 wt.%) in the biomass and decreases biofuel yields from 87.3 to 40.7 gallons per ton. The MFSP increases with higher ash content in biomass.
Shemfe et al. [16] investigated bio-oil production via biomass fast pyrolysis and subsequent bio-oil upgrading via zeolite cracking. The techno-economic assessment was accomplished with two conceptual catalysts’ regeneration for the zeolite cracking process. In the simulations, using the software Aspen Plus, the production of 72 t/day of pine wood was considered. The sensitivity analysis indicates that the operating cost, income tax and fuel yield affect the MFSP the most.
Lam et al. [17] presented a study of biochar production using banana and orange peels as feedstocks by pyrolysis at 400–500 °C. The pyrolysis generated 30.7–47.7 wt.% yield of a dark biochar, which contained no sulfur, low volatile content (34 wt.%) and high amounts of fixed carbon (72 wt.%). The use of the biochar as adsorbent to treat the palm oil mill effluent (POME) demonstrated a reduction of chemical oxygen demand (COD), concentration of biochemical oxygen demand (BOD) reduction of 57%, oil and grease (O&G) and total suspended solid (TSS) of POME to acceptable standards. The superficial area obtained from the biochar was of 105 m²/g (mesopores), indicating applicability to be used as an adsorbent.
Giwa et al. [18] presented a study of biochar production using date palm waste and simulations made with SuperPro Designer v8.5. A comparison between production with the conventional process (electric heating-based pyrolysis) and concentrated solar energy was accomplished by analyzing economic and environmental sustainability aspects. Economic analysis demonstrated the most viability from the use of solar energy, with an internal return (IRR) of 14.8% and payback time (PBT) of 4 years and 132 days, and gross margin of 35.5% and return on investment (ROI) of 22.9%. The CO2 emissions are minor in solar use (38%) when compared with conventional pyrolysis. Sensitivity analysis demonstrated that the cost of date palm waste presents a lower impact in the PBT than changes in biochar sale price.
Lama et al. [19] investigated fuel production by microwave vacuum pyrolysis compared with conventional pyrolysis, using waste plastic and used cooking oil simultaneously. The results demonstrated short processing time (20 min) and low electrical energy consumption (0.38 kWh). The bio-oil yield obtained was 84 wt., containing higher heating value (49 MJ/kg) than diesel and gasoline, as well as high content of light hydrocarbons. The economic analysis showed a production cost of USD 0.25/L (where in Malaysia the diesel price is USD 0.523/L).
Batlle et al. [20] studied the utilization of a bio refinery starting from a palm oil mill. Three scenarios were applied: the first (I), the base case, was a traditional palm oil mill; the second (II) was bio-oil and biochar production by fast pyrolysis; and the third (III) considers a bio refinery for biodiesel and glycerin production in a palm oil mill by an extraction/transesterification stage, as well as incorporating the pyrolysis process. The environmental evaluation applied to the scenario (III) demonstrated environmental impacts of 32.5% (climate change category) lower than those when producing electricity and 14.2% lower than environmental impacts from producing biodiesel (resources category), demonstrating that bio-oil production by fast pyrolysis results in lower environmental impacts compared with the other products obtained in a bio refinery. For the three scenarios, the surplus electricity index was calculated, where scenario (III) was the most favorable, achieving 110.23 kW per ton of fresh fruit bunch with an overall efficiency of 82.69%. Finally, the net energy ratio (for the best scenario regarding the thermodynamic performance—scenario (III): 21.17) was calculated and compared with the literature data, resulting in a gain of total energy flow of up to 17.77.
Vasu et al. [21] presented a study that produced bio-oil with improved pH using blends of palm kernel shell (PKS) and palm oil sludge (POS). The pyrolysis temperature applied was 507 ± 13 °C in a fixed-bed reactor. A reduction in bio-oil yield with the increase of POS in the blend from 0 to 100 wt.% was observed. At a PKS-to-POS mass ratio of 50:50, the pH value of the bio-oil produced was 4.6 ± 0.1. The total acid number (TAN) of bio-oils decreased with increasing POS ratio in the blends.
Yeo et al. [22] presented a study to develop a bio refinery using as biomass from palm oil industry as feedstock. Techno-economic evaluation of the sustainable circular economy was applied. Three resources were analyzed using Process Graph (P-graph): steam, fertilizer and electricity for regeneration and recycling of biomass. The circular economy model demonstrated potential in reducing 13.469% of the imported electricity and 39.292% of the imported steam, while also presenting 0.642% lower in terms of gross profit.
Yahya et al. [23] investigated bio-oil, char and burnable gas production using date palm biomass waste of by fast pyrolysis at 525 °C, with results of bio-oil, bio char and gases of 38.8, 37.2 and 24 wt.%, respectively. An economic analysis presented the results of 2.57 years of payback time, with a net saving of USD 556.8 per ton of the date palm waste processed in the pyrolysis unit. It was further estimated that Saudi Arabia could earn USD 44.77 million per annum, approximately, if 50% of the total date palm waste was processed through fast pyrolysis. In addition to that, it would incur a reduction of 2029 tons of greenhouse gas emissions annually.
Kaniapan et al. [24] presented a review regarding transportation fuel use and energy production of biomass from the palm oil industry (and its residues). A feasibility analysis of palm oil and its residues was made using current valorization methods such as biochemical and thermochemical techniques.
Terry et al. [25] presented a review of bio-oil production using oil palm biomass (OPB) as lignocellulosic feedstock, as well as presenting a discussion of the chemical compositions of different OPBs and their effects on the bio-oil yield and quality obtained from the pyrolysis process, followed by a discussion on the addition of catalysts and plastics into the pyrolysis process for bio-oil upgrading, and lastly presenting environmental studies and techno-economic analyses regarding the potential use of the process’ integration. Low-density polyethylene (LDPE), high-density polyethylene (HDPE) and polypropene (PP) have commonly been used in the co-pyrolysis of OPB, which can increase the heating value of bio-oil up to 80.0% compared to that of diesel.
Pires et al. [26] investigated fuel and chemical production by pyrolysis using biomass as feedstock and the process graph (P-graph) methodology for synthesis in pyrolysis oil refineries. The study demonstrates the profitability of various bio refinery designs. It studies the addition of new unit operations such as a centrifuge for water extraction and a wet oxidation system for acetic acid production, which have profitability ranging from USD 1.650/h to USD 23.66/h with acetic acid and levoglucosan as the main products, respectively.
Detchusananard et al. [27] presented a study of techno-economic assessment on multibiofuel production using empty fruit brunch (EFB) biomass residual from the palm oil milling process with a pyrolysis–gasification-integrated process. The modeling was accomplished using the Aspen Energy Analyzer and economic performance indicators such as internal rate of return (IRR), payback period (PB) and net present value (NPV). The economic analyses indicate that the proposed process is economically feasible and attractive with 22.0% on IRR, 5.98 years of PB and a USD 249,951.964 NPV.
Attasophonwattana et al. [28] presented a study of hydrochar’s production using empty fruit bunch (EFB) as feedstock by pilot-scale hydrothermal carbonization (HTC), gasification and washing processes, as well as anaerobic digestion of the HTC’s liquid product. The products regarding gasification were of char (22.7–33.8%) and tar (17.3–28.8%); CO2/O2 gasification resulted in 31.3–36.6% for char and 8.5–30.8% for tar. The lower heating value of syngas was in the range of 4.7–6.6 MJ/Nm³ and cold gas efficiency was approximately 39.1–55.1%. Syngas products from air gasification of hydro chars were 39.9–56.5%, 11.4–21.4% and 9.0–14.4% for CO, H2 and CH4, respectively, while CO2/O2 gasification products yielded 45.1–56.6%, 11.6–24.3% and 9.4–14.0% for CO, H2 and CH4, respectively.
Parthasarathy et al. [29] presented a study of bio-oil production using oil palm wastes (OPW) such as empty fruit bunches (EFB), palm kernel shell (PKS), oil palm frond (OPF) and their blends by pyrolysis. According to the economic analysis, EFB-based pyrolysis has the lowest CAPEX of all tested feedstocks and PKS-based pyrolysis has the highest capital expenses (CAPEX). Furthermore, PKS has the highest operating expenses (OPEX) due to its higher market price as well as higher moisture content. Among the feedstocks, OPF delivers the highest profit at USD 17 M/year, with a 22.0% return on investment (ROI). Regarding the payback period, all OPW feedstocks demonstrated a period of 4–6 years.
In this work, the economic feasibility of the thermal–catalytic process of crude palm oil (CPO) and palm oil neutralization sludge (PONE) followed by the distillation of organic liquid products to produce biofuels was studied not only to evaluate the best investment alternative, but also to evaluate the viability of the project according to the criteria of project evaluation [30]. The economic feasibility analysis is based on the following economic methods: simple payback criterion, discounted payback, net present value (NPV), internal rate of return (IRR) and index of profitability (IP), as described in detail by Rocha et al. [30]. The breakeven-point, the minimum sale price (MFSP) and an analysis of sensibility for each process [30] was also calculated.
2. Materials and Methods
2.1. Materials
2.1.1. Palm Oil (Elaeis guineensis, Jacq)
Palm and palm kernel oils have low concentrations of polyunsaturated carboxylic acids. Palm oil contains saturated (44% palmitic acid, 5% stearic acid) and unsaturated fatty acids (39% oleic acid). In comparison, palm kernel oil is 54–70% unsaturated, and includes a large proportion of lauric acid content [31,32]. Table 1 shows the chemical composition, in terms of fatty acids, of palm oil and palm kernel oil [32].

Table 1.
Chemical composition of common fatty acids of the palm oil and of the palm kernel [32].
Palm oil can be broken down into two fractions after refining, namely olein (60%) and stearin (40%). Olein is a liquid oil intended for cooking and stearin can be used as a fat in the cake and biscuit industry, also serving as a raw material for the manufacture of margarine, mayonnaise and ice cream. In addition, it can replace tallow in the production of soaps [32]. Table 2 shows the physicochemical properties of the crude palm oil [33,34,35,36,37,38].

Table 2.
Physicochemical characterization of crude palm oil (CPO) used as raw renewable material by thermal–catalytic processes at pilot scale [31,33,34,35,36,37,38].
The physicochemical properties of the crude palm oil (CPO) are described in Table 3 according to those reported in the literature [31,33,34,35,36,37,38].

Table 3.
Physicochemical properties range of crude palm oil (CPO) [31,33,34,35].
2.1.2. Palm Oil Neutralization Sludge
Palm oil neutralization sludge, i.e., soap phase residue from the neutralization process of palm oil, is an aqueous alkaline lipid emulsion that contains approximately 50% (wt.) water, with free fatty acids, phosphatides, triglycerides, pigments and other nonpolar compounds [39,40,41,42]. Neutralization sludge is generated at a rate of approximately 6% (vol.) of the refined volume of the crude palm oil [40,41,42]. The low added cost of the neutralization sludge, and the environmental characteristics of its waste make it a technically alternative viable for the production of biofuels. The cracking process allows the sludge to transform into hydrocarbon mixtures [40,41]. Table 4 presents the results of physicochemical analysis of palm oil neutralization sludge after the dehydration process was accomplished in an agitated tank reactor, as well as the water percentage obtained in the pyrolysis process up to 100 °C.

Table 4.
Physicochemical characterization of palm oil neutralization sludge (soap phase residue from the neutralization process of palm oil) used as raw material in the pyrolysis pilot plant [40,41].
2.2. Thermal Cracking, Thermal–Catalytic Cracking and Distillation Process
The thermal–catalytic cracking and distillation processes of crude palm oil (Elaeis guineensis, Jacq) and palm oil neutralization sludge are described in detail elsewhere [31,33,34,35,36,37,38,39,40,41].
2.3. Project Evaluation Criteria
The indicators of project evaluation were described in detail by Amaral et al. [30]. The indicators used were the discounted payback, net present value simple payback (NPV), index of profitability (IP) and internal rate return (IRR). For each indicator, the criteria for project evaluation were also presented. The breakeven-point, the minimum fuel sale price (MFSP) and the analysis of sensibility were also calculated. Figure 2A presents the scheme used in the process to convert the palm oil into bio-oil, coke and methane gas by pyrolysis process using 10% (wt.%) sodium carbonate as a catalyst at 450 °C and 1 atm, followed by distillation to obtain biofuels (biogasoline, biokerosene, green diesel, heavy diesel). Figure 2B illustrates the scheme used to convert palm oil neutralization sludge into bio-oil, coke and methane gas though pyrolysis process at 450 °C and 1 atm followed by distillation to obtain biofuels (biogasoline, biokerosene, green diesel, heavy diesel).
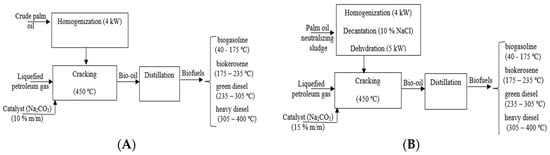
Figure 2.
(A)—process scheme of palm oil (Elaeis guineensis, Jacq) conversion into in biofuels and (B)—process scheme for conversion of palm oil neutralization sludge in biofuel.
2.4. Calculation Methodology
The calculation methodology, described in detail by Amaral et al. [30], was applied to compute revenues and expenses using crude palm oil (Elaeis guineensis, Jacq) and using palm oil neutralization sludge as a raw material. The additional process steps for including the cost of desiccant and dehydration are provided in Section 2.4.1 and Section 2.4.2, respectively. Table 5 illustrates the process parameters used to compute revenues and expenses.

Table 5.
Process parameters used in the equations.
2.4.1. Cost of Desiccant (NaCl_10%)
CNaCl = cost of sodium chloride (USD/L); %NaCl = percentage of sodium chloride (10) in m/m (%); PNaCl = price of sodium chloride (0.005) (USD/L).
2.4.2. Dehydration
= cost of dehydration (USD/L); = factor of conversion from calories (cal) to kWh in (kWh/cal); = price of kWh in USD/kWh; = power of the boiler in (cal); = time of dehydration per batch (h); = number of dehydration batches per day (-).
3. Results
3.1. Palm Oil (Elaeis guineensis, Jacq)
The results presented is this work were those described in the literature for thermal–catalytic cracking of crude palm oil with 10% of catalyst (Na2CO3) in the pilot unit [27,29,30,31,32,33,34]. The results for the yields of the thermal–catalytic cracking of crude palm oil and distillation of organic liquid products [27,29], [31,33] are shown in Supplementary Table S1 An important point to note is that only an estimated value of the distillation yield is shared due to problems encountered during the pilot unit distillation experiments (the result of yield distillation used was around 60% to the stand experiments according [30,31,32,33,34,35]). Supplementary Table S2 presents the characterization of crude palm oil used as a raw material by thermal–catalytic cracking with 10% (wt.) of catalyst (Na2CO3) at pilot scale [31,33]. Supplementary Table S3 presents the chemical composition of green gasoline in terms of hydrocarbons and oxygenates. Among the identified and quantified hydrocarbons are normal paraffins, branched paraffins, naphthenics, aromatics and olefins, the main components present in distillation fractions of fossil fuels (petroleum), as reported elsewhere [43,44].
Supplementary Table S4 presents the chemical compositional of the distillation fraction at 175–235 °C (bio kerosene), in terms of oxygenates and hydrocarbons. The biokerosene is composed of hydrocarbons including aromatics, normal paraffins, naphthenics and mainly olefins; in addition, there are oxygenated compounds. The percentage of hydrocarbons and of oxygenated compounds correspond to 86.37% (area) and 13.63% (area), respectively.
Supplementary Table S5 presents the results of the distillation fraction at 235–305 °C, corresponding to green diesel. It has been identified a mixture rich in hydrocarbons, composed mainly of normal paraffins and olefins. The total percentage of hydrocarbons corresponds to 91.38% (area) and that of oxygenated compounds to 8.62% (area).
Supplementary Table S6 presents the results of the distillation fraction at 305–400 °C, corresponding to heavy diesel. It has been identified a mixture rich in hydrocarbons composed mainly of normal paraffins and olefins. The total percentage of hydrocarbons correspond to 70.78% (area) and to 29.22% (area) for that of oxygenated compounds. The results are in agreement with those reported in the literature [43,44].
Table 6 demonstrated the economic parameters for discounted cash flow analysis. The total project investment is USD 195,760.67 (one hundred and ninety-five thousand seven hundred and sixty and sixty-seven cents) and corresponds to the initial investment of the project.

Table 6.
Economic parameters for discounted cash flow analysis.
Table 7 demonstrated the total revenue, total expense and the annual profit of USD 52,500.2 (fifty-two thousand five hundred and two cents) per year. The MFSP obtained in this work for the biofuels was 1.59 USD/L. The literature presented in Amaral et al. [30] shows values of 0.68 up to 1.34 USD/L.

Table 7.
Revenues and expenses from using crude palm oil (Elaeis guineensis, Jacq) as raw material.
Table 8 presents an investment analysis of the cash flow using the simple payback criterion. It can be confirmed that in the fourth year, the total investment is recovered, totaling USD 14,240.31 (fourteen thousand two hundred and forty and thirty-one cents). This way, taking into account the analysis horizon of 5 years, the project is considered economically feasible.

Table 8.
Annual cash flow for the crude palm oil (Elaeis guineensis, Jacq) and simple payback analysis.
Table 9 shows the cash flow considering the discounted payback criterion. It can be observed that in the fifth year, the total investment is recovered. This way, taking into accounting the analysis horizon of 5 years, the project is considerable feasible. The discounted cash flow rate was considered at 10% per year.

Table 9.
Annual cash flow for crude palm oil (Elaeis guineensis, Jacq) and discounted payback analysis, profitability index, internal rate of return (IRR) and net present value (NPV) analyses.
Table 9 shows the cash flow considering the net present value (NPV) criterion. It can be confirmed that in the fifth year, there is a capital increase of USD 3256.57 (three thousand two hundred fifty-six and fifty-seven cents). This way, the result of NPV is positive, indicating the feasibility of the project. The discounted cash flow rate was considered at 10% per year.
Table 9 demonstrates the cash flow for the investment analysis considering the internal rate of return (IRR) criterion. It can be observed that in the fourth to fifth year inversion of the signal occurs, which represents the IRR of the project as 10.0% per year. This way, the IRR is equal to the minimum attractiveness of the project (10% per year), which indicates that the project is economically feasible. Thilakaratne et al. [46] obtained an MFSP of USD 3.69 per gal (0.98 USD/L) and Amaral et al. [30] of 1.34 USD, assuming 10% IRR.
Table 9 demonstrates the cash flow considering the profitability index. Based on the results of profitability index, equal to 1.02, for each dollar invested in the project a return of 1.02 dollars it will occur within the analysis horizon of 5 years. This way, the criteria indicate the feasibility of the project.
Figure 3 is the sensitivity analysis for 900 L/day to reach the MFSP of 1.59 USD/L and 10% of IRR. The results demonstrated that the distillation yield and bio-oil yield are the most significative parameters that affect the MFSP. These results are in agreement with Amaral et al. [30] and Brown et al. [47].
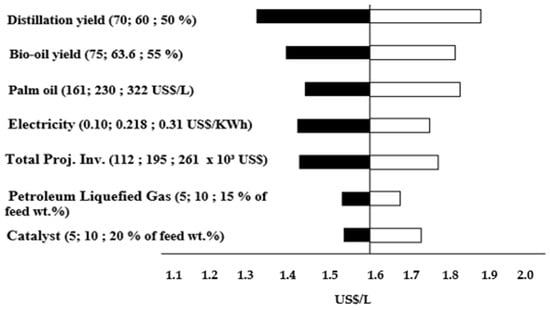
Figure 3.
Sensitivity analysis for 900 L/day to reach the baseline transportation fuel MFSP of 1.59 USD/L, the 10% facility of IRR is assumed.
Figure 4A illustrates the MFSP as a function of bio-oil yield by sensitivity analysis for a 900 L/day production. Figure 4B shows the MFSP as a function of distillation yield by sensitivity analysis for a 900 L/day production. Both graphics show that the increase in yield results in reduction in MFSP. Figure 4C illustrates that the increase in raw material cost (palm oil) results in an increase in the MFSP (the same behavior occurs in the Figure 4D).
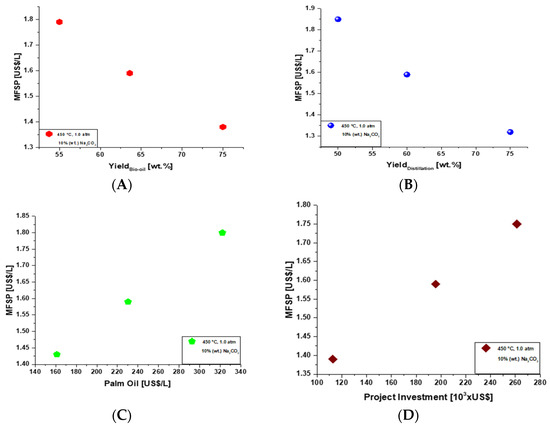
Figure 4.
(A)—MFSP as a function of bio-oil yield by sensitivity analysis for a 900 L/day production. (B)—MFSP as a function of distillation yield by sensitivity analysis for a 900 L/day production. (C)—MFSP as a function of cost of crude palm oil by sensitivity analysis for a 900 L/day production. (D)—MFSP as a function of project investment by sensitivity analysis for a 900 L/day production.
The breakeven point (BEP) is the point at which total cost and total revenue are equal, which means there is a balance between profit and loss [47,48]. The value of the breakeven point obtained was 1.58 USD/L.
3.2. Palm Oil Neutralization Sludge
The results presented is this work were from the pyrolysis of the palm oil neutralization sludge with 15% (wt.) of catalyst (Na2CO3) at 450 °C. The results presented by [40,41] of yields related to thermal–catalytic cracking and distillation are shown in Supplementary Table S7. One important point to consider is that a thermal–catalytic cracking availability of 56.25% is low. This may occur because of the high reaction time of the reactor, resulting in a low feed rate.
Supplementary Table S8 presents the physicochemical properties of organic liquid product (bio-oil) of palm oil neutralization sludge after the thermal–catalytic cracking process. The results are compared with the National Agency of Petroleum, Biofuels and Natural Gas of Brazil (ANP).
According to the results presented in Supplementary Table S8, one observes that density- and viscosity-measured values were close to those established by the ANP standard (values below the norm may be due to the presence of smaller hydrocarbon chains, which means light compounds). The carbon residue value was above the specified value; however, the increase in this value is expected since the bio-oil has not only hydrocarbon compounds, but also fatty materials, catalyst residue and unsaponifiables from the raw material, which contribute to increasing the value of the carbon residue of these samples. The corrosivity values for the copper sheet were in accordance with the ANP standard, characterizing the bio-oil with low capacity to cause corrosion in metallic parts [40,41].
Supplementary Table S9 presents the chemical composition of hydrocarbons and oxygenated compounds of the bio-oil of Experiment 5 [40,41]. The chemical analysis identified a high concentration of hydrocarbons with 91.22% (area), as well as a low concentration of oxygenated compounds with 8.78% (area). The main hydrocarbons present in the petroleum diesel are alkanes, olefins, naphthenics and aromatics [43,44].
The distillation of the organic liquid product from Experiment 5 was carried out at pilot scale using a distillation column in order to obtain the cutoff fractions of biofuels [36,37]. The physicochemical characteristics of the biofuels obtained after distillation at pilot scale are present in Supplementary Table S10.
The results obtained for the acidity index in Supplementary Table S10 also demonstrated relatively low values when compared to the distilled fractions of organic liquid products (bio-oils) from oilseeds. The corrosivity values to the copper sheet of these fractions were considered as having low corrosive capacity in metallic parts. The results of density and viscosity parameters for biogasoline and biokerosene were lower than the S10 diesel specified by ANP N° 65. This behavior occurs due to the composition of these fractions being formed from smaller hydrocarbon chains (approximate chains of C4–C12). The results of the sulfur content of the bio gasoline fraction had a content level close to the that of mineral diesel S10. However, when comparing it with the sulfur content of common Type A biogasoline established by ANP N° 57, whose value is 800 ppm, a low sulfur content of the bio gasoline fraction was found in relation to commercial Type A gasoline [40,41].
Supplementary Table S11 presents the percentage composition in hydrocarbons in the range of heavy diesel (175–235 °C) obtained from distillation at bench scale of the bio-oil, Experiment 5. The results demonstrate high content of hydrocarbons with 96.5% (area) and low content of other compounds with 3.5% (area).
Supplementary Table S12 presents the percentage composition of hydrocarbons in the range of heavy diesel (305–400 °C) obtained from the distillation at bench scale of the bio-oil [36,37]. The results demonstrate high content of hydrocarbons with 93.21% (area) and low content of oxygenated compounds with 6.79% (area).
Supplementary Table S13 presents the percentage composition in hydrocarbons in the range of light diesel (235–305 °C) obtained from the distillation in bench scale of the bio-oil [40,41]. The results demonstrate high content of hydrocarbons with 98.76% (area) and low content of oxygenated compounds with 1.24% (area).
Supplementary Table S14 shows the percentage of hydrocarbon composition in gasoline (40–175 °C) obtained at the pilot scale distillation of the organic liquid product (bio-oil) from Experiment 5 (15% Na2CO3 at 440 °C) [40,41]. According to the results, all components of this biogasoline were hydrocarbons constituting mainly olefins with 51.09% (area) and paraffins with 34.64% (area). Aromatic and naphthenic hydrocarbons showed low percentages. The composition of aromatic hydrocarbons in this fraction was found in accordance with the specification of ANP N° 57 (2011) for type C gasoline derived from petroleum, which establishes a maximum percentage of 45% (vol.) for aromatics; however, the content of olefins showed a result above the stipulated maximum of 30% (vol). It is suitable to highlight that this percentage value of the maximum content of aromatic and olefin hydrocarbons must be met after adding anhydrous ethanol to automotive gasoline, as recommended by ANP N° 57 (2011).
Table 10 demonstrates a total revenue, total expense and an annual profit of USD 59,278.0 (fifty-nine thousand two hundred eight dollars) per year. The MFSP obtained in this work for biofuels was 1.34 USD/L. The literature in Amaral et al. [30] demonstrated values of 0.68 up to 1.34 USD/L.

Table 10.
Revenues and expenses using palm oil neutralization sludge as raw material.
Table 11 presents the cash flow with the simple payback criterion. It can be observed that in the fourth year, the total investment is recovered, totaling USD 41,352.10 (forty-one thousand three hundred and fifty-two dollars and ten cents). This way, the project is considered economically feasible within an analysis horizon of 5 years.

Table 11.
Annual cash flow for palm oil neutralization sludge (Elaeis guineensis, Jacq) and simple payback analysis.
Table 12 presents the cash flow considering the discounted payback criterion. It can be observed that in the fifth year, the investment is totally recovered. This way, considering a 5-year analysis horizon, the project is considered economically feasible. The cash flow discount rate was 10% per year.

Table 12.
Annual cash flow for palm oil neutralization sludge (Elaeis guineensis, Jacq) and discounted payback analysis, profitability index analysis, internal rate of return (IRR) and net present value (NPV) analysis.
Table 12 presents the cash flow considering the net present value (NPV) criterion. It can be concluded that in the fifth year, there is a capital increase of USD 28,950.31 (twenty-eight thousand nine hundred and fifty dollars and thirty-one cents). This way, the project is considered economically feasible due to the NPV being positive. The cash flow discount rate was 10% per year.
Table 12 shows the cash flow considering the IRR criterion. It can be observed that in the fourth to fifth years inversion of signal occurs, which represents the IRR of the project as 10% per year. This means that the project is economically feasible. Thilakaratne et al. [46] obtained a minimum fuel selling price (MFSP) of USD 3.69 per gallon (0.98 USD/L) and Amaral et al. [30] of 1.34 USD/L considering 10% of IRR.
Table 12 demonstrates the cash flow with profitability index analysis based on results of the profitability index, equal to 1.15. According to the criteria of this index, the project is considered economically feasible.
Figure 5 is the sensitivity analysis for 450 L/day to reach an MFSP of 1.34 USD/L and 10% IRR. The results indicate that the distillation yield and bio-oil yield are the most significative variable that affect the MFSP. These results are in agreement with Brown et al. [47] and Amaral et al. [30].
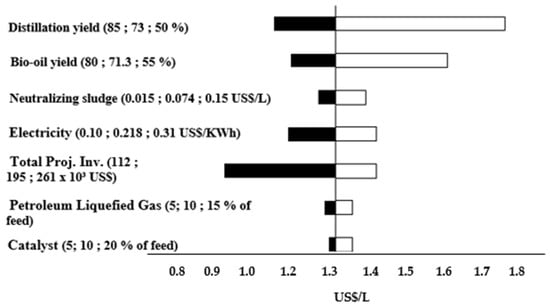
Figure 5.
Sensitivity analysis for 450 L/day to reach the baseline transportation fuel MFSP of 1.34 USD/L, the 10% facility IRR is assumed.
Figure 6A illustrates the MFSP as a function of bio-oil yield by sensitivity analysis for a 450 L/day production. Figure 6B shows the MFSP as a function of distillation yield by sensitivity analysis for a 450 L/day production. Both graphics show that the increase in yield results in a reduction in MFSP. Figure 6A illustrates that for 75% (wt.) of bio-oil yield the MFSP decreases to 1.19 USD/L. Figure 6B shows that when the distillation yield achieves 75% (wt.), the MFSP reduces to 1.15 USD/L. Figure 6C represents the MFSP as function of the palm oil neutralizing sludge cost, as well as Figure 6D demonstrating the MFSP as a function of the total capital investment (this latest graphic shows that if the project starts with approximately USD 120,000.00 (one hundred and twenty-thousand dollars), the MFSP may be reduced to 1 USD/L).
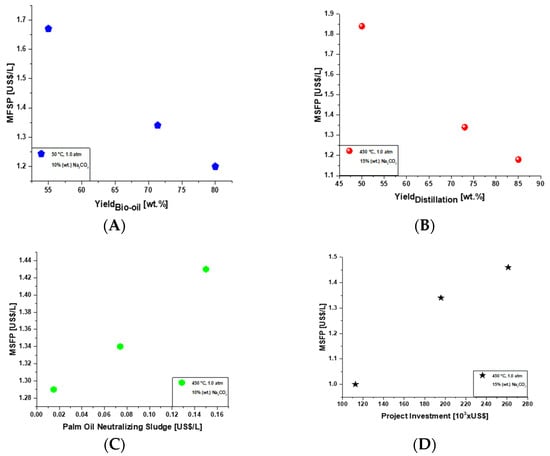
Figure 6.
(A)—MFSP as a function of bio-oil yield by sensitivity analysis for a 450 L/day production. (B)—MFSP as a function of distillation yield by sensitivity analysis for a 450 L/day production. (C)—MFSP as a function of cost of crude palm oil by sensitivity analysis for a 450 L/day production. (D)—MFSP as a function of project investment by sensitivity analysis for a 450 L/day production.
The breakeven point (BEP) is the point at which total cost and total revenue are equal, which means there is a balance between profit and loss [47,48]. The value of the breakeven point obtained was of 1.24 USD/L.
4. Conclusions
Starting from the feasibility project criteria indicators, it is possible to affirm that the thermal–catalytic cracking process of crude palm oil to the production of biofuels, coke and methane gas is feasible. This conclusion comes from the results of 4 years for simple payback, 5 years for discounted payback (considering an analysis horizon of 5 years). Additionally, a positive net present value (NPV of USD 3256.57) was obtained, as well as a profitability index higher than 1 (result of 1.02).
Regarding the feasibility assessment for the production of biofuels, coke and methane gas using palm oil neutralization sludge, the same indicators confirm the viability of the project. The simple payback criterion obtained was of 4 years, for discounted payback 5 years, the NPV was USD 28,950.31 and the profitability index was 1.15 (for each dollar invested in the project, a return of 1.15 dollars will occur, within an analysis horizon of 5 years).
The availability used for the project evaluation criteria with crude palm oil and palm oil neutralization sludge was 75% and 56.25%, respectively. This means that the time used to operate the equipment (procedures of load and unload) can be improved. This way, the results of the project´s evaluation indicators can be all improved, starting from the optimization of the pilot plant’s availability (such as use of semi-continuous process, optimization of the time reaction).
The MFSP obtained for the process using palm oil was 1.59 USD/L. The literature reported in this work shows values from 0.68 up to 1.34 USD/L. The main negative aspect that effected this result was the palm oil market price, which affected the costs. The MPSP obtained using palm oil neutralization sludge was 1.34 USD/L; although more stages are necessary in this process, the low estimation of the feedstock contributed to this result.
The internal rate of return (IRR) obtained in both projects was 10% per year, which means that the project is economically feasible. Amaral et al. [30] presented value a of 10% of IRR.
Sensibility analysis shows that the pyrolysis yield and distillation yield are the parameters that most affect the MFSP in both projects. It also demonstrated that a reduction in electrical energy could reduce the MFSP due to the high consumption of energy in the distillation stage. One alternative is the use of photovoltaic energy.
Another assumption used in this analysis was that only 10% of the total generated gas can provide revenues, which means that this estimation can be improved (such as the use of cogeneration). Other alternatives for producing biofuels, biogas and biochar can also be studied in the group (biomass such as seeds of Amazon fruits, bovine tallow, several vegetable oils of low cost in the Amazon region).
It is important to highlight the fact that several studies in the literature also present applications for biochar products, such as adsorbents, soil improvement and carbon sequestration, as mentioned by Santana Junior et al. [49].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16010492/s1. Supplementary Tables S1–S14. Table S1. Yields of thermal catalytic cracking of crude palm oil (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 10% (wt.) Na2CO3 as catalyst, and distillation of organic liquid products (OLP) [26,27,28,29,30,31]. Table S2. Physicochemical properties of crude palm oil used as raw by thermal catalytic cracking at 450 °C, 1.0 atmosphere, and 10% (wt.) Na2CO3 as catalyst [26,27,28,29,30,31]. Table S3. Chemical compositional of distillation fraction within the temperature range 40–175 °C (biogasoline), described in terms of oxygenated and hydrocarbons, of OLP obtained by thermal catalytic cracking at 450 °C, 1.0 atmosphere, and 10% (wt.) Na2CO3 as catalyst [26,27,28,29,30,31]. Table S4. Chemical compositional of distillation fraction within the temperature range 175–235 °C (biokerosene), described in terms of oxygenated and hydrocarbons, of OLP obtained by thermal catalytic cracking soap crude palm oil at 450 °C, 1.0 atmosphere, and 10% (wt.) Na2CO3 as catalyst [26,27,28,29,30,31]. Table S5. Chemical compositional of distillation fraction within the temperature range 235–305 °C (light green diesel), described in terms of oxygenated and hydrocarbons, of OLP obtained by thermal catalytic cracking of at 450 °C, 1.0 atmosphere, and 10% (wt.) Na2CO3 as catalyst [26,27,28,29,30,31]. Table S6. Chemical compositional of distillation fraction within the temperature range 305–400 °C (heavy green diesel), described in terms of oxygenated and hydrocarbons, of OLP obtained by thermal catalytic cracking of at 450 °C, 1.0 atmosphere, and 10% (wt.) Na2CO3 as catalyst [26,27,28,29,30,31]. Table S7. Yields of thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst, and distillation of organic liquid products (OLP) [34,35]. Table S8. Physicochemical properties of organic liquid product (bio-oil), of experiment 5, thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst [34,35]. Table S9. Chemicasl composition of organic liquid product (experiment 5), described in terms of oxygenated and hydrocarbons, obtained by thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst [34,35]. Table S10. The physicochemical properties of distillation fractions of biogasoline (40–175 °C) and biokerosene (175–235 °C), of OLP (experiment 5) obtained by thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst [34,35]. Table S11. Chemical compositional of distillation fraction within the temperature range 175–235 °C (heavy green diesel), described in terms of oxygenated and hydrocarbons, of OLP ob-tained by thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst [34,35]. Table S12. Chemical compositional of distillation fraction within the temperature range 305–400 °C (heavy green diesel), described in terms of oxygenated and hydrocarbons, of OLP obtained by thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst [34,35]. Table S13. Chemical compositional of distillation fraction within the temperature range 235–305 °C (light green diesel), described in terms of oxygenated and hydrocarbons, of OLP obtained by thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst [34,35]. Table S14. Chemical compositional of distillation fraction within the temperature range 40–175 °C (biogasoline), described in terms of oxygenated and hydrocarbons, of OLP obtained by thermal catalytic cracking of palm oil neutralizing sludge (Elaeis guineensis, Jacq) at 450 °C, 1.0 atmosphere, and 15% (wt.) Na2CO3 as catalyst [34,35].
Author Contributions
The individual contributions of all the coauthors are provided as follows: A.R.A. contributed to the formal analysis and writing—original draft preparation, investigation and methodology; W.G.D.S. contributed to the investigation and methodology; L.P.B. contributed to the investigation and methodology; C.C.F. contributed to the investigation and methodology; A.M.P. contributed to the investigation and methodology; L.M.P. contributed to the investigation and methodology; M.C.S. contributed to the investigation and methodology; F.P.d.C.A. contributed to the investigation and methodology; N.M.M. contributed to the investigation and methodology; J.A.R.P. contributed resources and chemical analysis; A.d.A.M. contributed to the investigation and methodology; S.A.P.d.M. contributed to the investigation and methodology; D.A.R.d.C. contributed to the investigation, methodology and co-supervision; S.D.J. contributed resources and chemical analysis; L.E.P.B. contributed to the investigation, methodology and resources; and N.T.M. contributed supervision, conceptualization and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
I would like to acknowledge and dedicate this research in memory of Hélio da Silva Almeida; he used to work at the Faculty of Sanitary and Environmental Engineering/UFPa and passed away on 13 March 2021. His contagious joy, dedication, intelligence, honesty, seriousness and kindness will always be remembered in our hearts.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biomassa e Bioenergia. Energias Renováveis Empregam 9.8 Milhões de Pessoas no Mundo. 2023. Available online: https://www.biomassabioenergia.com.br/imprensa/energias-renovaveis-empregam-81-milhoes-de-pessoas-no-mundo/20160530-095945-G045 (accessed on 11 December 2022).
- Novacana. Trabalhadores. 2015. Available online: https://www.novacana.com/n/cana/trabalhadores/biocombustiveis-empregos-mundo-metade-brasil-010615 (accessed on 11 December 2022).
- Matriz Energética, E.; ELÉTRICA. Empresa de Pesquisa Energética. 2018. Available online: https://www.epe.gov.br/pt/abcdenergia/matriz-energetica-e-eletrica (accessed on 21 December 2022).
- Suarez, P.A.Z.; Santos, A.L.F.; Rodrigues, J.P.; Alves, M.B. Biocombustíveis a partir de óleos e gorduras: Desafios tecnológicos para viabilizá-los. Quim. Nova 2009, 32, 768–775. [Google Scholar] [CrossRef]
- Ministério de Minas e Energia. Produção e Fornecimento de Biocombustíveis. 2020. Available online: https://www.gov.br/anp/pt-br/assuntos/producao-e-fornecimento-de-biocombustiveis (accessed on 11 December 2022).
- Ministério de Minas e Energia. Últimas Notícias. 2020. Available online: https://www.gov.br/anp/pt-br/canais_atendimento/imprensa/noticias-comunicados/mistura-de-biodiesel-ao-diesel-passa-a-ser-de-13-a-partir-de-hoje-1-3 (accessed on 11 December 2022).
- Abnisa, F.; Wan Daud, W.M.A.; Husin, W.N.W.; Sahu, J.N. Utilization possibilities of palm shell as a source of biomass energy in Malaysia by producing bio-oil in pyrolysis process. Biomass Bioenergy 2011, 35, 1863–1872. [Google Scholar] [CrossRef]
- Abnisa, F.; Arami-Niya, A.; Wan Daud, W.M.A.; Sahu, J.N.; Noor, I.M. Utilization of oil palm tree residues to produce bio-oil and bio-char via pyrolysis. Energy Convers. Manag. 2013, 76, 1073–1082. [Google Scholar] [CrossRef]
- Do, T.X.; Lim, Y.; Yeo, H. Techno-economic analysis of biooil production process from palm empty fruit bunches. Energy Convers. Manag. 2014, 80, 525–534. [Google Scholar] [CrossRef]
- Moncada, J.; Tamayo, J.; Cardona, C.A. Evolution from biofuels to integrated biorefineries: Techno-economic and environmental assessment of oil palm in Colombia. J. Clean. Prod. 2014, 81, 51–59. [Google Scholar] [CrossRef]
- Peryoga, Y.; Solikhah, M.D.; Raksodewanto, A.A. Production Cost Assessment of Palm Empty Fruit Bunch Conversion to Bi Oil via Fast Pyrolysis. Int. J. Adv. Sci. Eng. Inf. Technol. 2014, 4, 6. [Google Scholar] [CrossRef]
- Mabrouki, J.; Abbassi, M.A.; Guedri, K.; Omri, A.; Jeguirim, M. Simulation of biofuel production via fast pyrolysis of palm oil residues. Fuel 2015, 159, 819–827. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Al-Nadheri, W.M.A.; Jegarajan, D.; Sahu, J.N.; Mubarak, N.M.; Nizamuddin, S. Utilization of palm oil sludge through pyrolysis for bio-oil and bio-char production. Bioresour. Technol. 2015, 178, 65–69. [Google Scholar] [CrossRef]
- Lee, X.J.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Ng, H.K. Biochar potential evaluation of palm oil wastes through slow pyrolysis: Thermochemical characterization and pyrolytic kinetic studies. Bioresour. Technol. 2017, 236, 155–163. [Google Scholar] [CrossRef]
- Li, W.; Dang, Q.; Brown, R.C.; Laird, D.; Wright, M.M. The impacts of biomass properties on pyrolysis yields, economic and environmental performance of the pyrolysis-bioenergy-biochar platform to carbon negative energy. Bioresour. Technol. 2017, 241, 959–968. [Google Scholar] [CrossRef]
- Shemfe, M.; Gu, S.; Fidalgo, B. Techno-economic analysis of biofuel production via bio-oil zeolite upgrading: An evaluation of two catalyst regeneration systems. Biomass Bioenergy 2017, 98, 182–193. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Rasit, N.; Ooi, C.K.; Ma, N.L.; Ng, J.H.; Lam, W.H.; Chong, C.T.; Chase, H.A. Pyrolysis production of fruit peel biochar for potential use in treatment of palm oil mill effluent. J. Environ. Manag. 2018, 213, 400–408. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Ajumobi, O.; Dzidzienyo, P. Pyrolysis of date palm waste to biochar using concentrated solar thermal energy: Economic and sustainability implications. Waste Manag. 2019, 93, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lama, S.S.; Maharib, W.A.W.; Okc, Y.S.; Penga, W.; Chongd, C.T.; Mae, N.L.; Chasef, H.A.; Liewg, Z.; Yusuph, S.; Kwoni, E.E.; et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sustain. Energy Rev. 2019, 115, 109359. [Google Scholar] [CrossRef]
- Batlle, E.A.O.; Santiago, Y.C.; Venturini, O.J.; Palacio, J.C.E.; Lora, E.E.S.; Maya, D.M.Y.; Arrieta, A.R.A. Thermodynamic and environmental assessment of different scenarios for the insertion of pyrolysis technology in palm oil biorefineries. J. Clean. Prod. 2020, 250, 119544. [Google Scholar] [CrossRef]
- Vasu, H.; Wong, C.F.; Vijiaretnam, N.R.; Chong, Y.Y.; Thangalazhy-Gopakumar, S.; Gan, S.; Lee, L.Y.; Ng, H.K. Insight into Co-pyrolysis of Palm Kernel Shell (PKS) with Palm Oil Sludge (POS): Efect on Bio-oil Yield and Properties. Waste Biomass Valorization 2020, 11, 5877–5889. [Google Scholar] [CrossRef]
- Yeo, J.Y.J.; How, B.S.; Teng, S.Y.; Leong, W.D.; Ng, W.P.Q.; Lim, C.H.; Ngan, S.L.; Sunarso, J.; Lam, H.L. Synthesis of Sustainable Circular Economy in Palm Oil Industry Using Graph-Theoretic Method. Sustainability 2020, 12, 8081. [Google Scholar] [CrossRef]
- Yahya, S.A.; Iqbal, T.; Omar, M.M.; Ahmad, M. Techno-Economic Analysis of Fast Pyrolysis of Date Palm Waste for Adoption in Saudi Arabia. Energies 2021, 14, 6048. [Google Scholar] [CrossRef]
- Kaniapan, S.; Hassan, S.; Ya, H.; Nesan, K.P.; Azeem, M. The Utilisation of Palm Oil and Oil Palm Residues and the Relate Challenges as a Sustainable Alternative in Biofuel, Bioenergy, and Transportation Sector: A Review. Sustainability 2021, 13, 3110. [Google Scholar] [CrossRef]
- Terry, L.M.; Li, C.; Chew, J.J.; Aqsha, A.; How, B.S.; Loy, A.C.M.; Chin, B.L.F.; Khaerudini, D.S.; Hameed, N.; Guan, G.; et al. Bio-oil production from pyrolysis of oil palm biomass and the upgrading technologies: A review. Carbon Resour. Convers. 2021, 4, 239–250. [Google Scholar] [CrossRef]
- Pires, A.P.P.; Martinez-Valencia, L.; Tanzil, A.H.; Garcia-Perez, M.; García-Ojeda, J.C.; Bertok, B.; Heckl, I.; Argoti, A.; Friedler, F. Synthesis and Techno-Economic Analysis of Pyrolysis-Oil-Based Biorefineries Using P-Graph. Energy Fuels 2021, 35, 13159–13169. [Google Scholar] [CrossRef]
- Detchusananard, T.; Wuttipisan, N.; Limleamthong, P.; Prasertcharoensuk, P.; Maŕechal, F.; Arpornwichanop, A. Pyrolysis and gasification integrated process of empty fruit bunch for multi-biofuels production: Technical and economic analyses. Energy Convers. Manag. 2022, 258, 115465. [Google Scholar] [CrossRef]
- Attasophonwattana, P.; Sitthichirachat, P.; Siripaiboon, C.; Khaobang, T.K.C.; Panichnumsin, P.; Dinge, L.; Areeprasert, C. Evolving circular economy in a palm oil factory: Integration of pilot-scale hydrotherml carbonization, gasification, and anaerobic digestion for valorization of empty fruit bunch. Appl. Energy 2022, 324, 119766. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Alherbawi, M.; Shahbaz, M.; Mackey, H.R.; McKay, G.; Al-Ansari, T. Conversion of oil palm waste into value-added products through pyrolysis: A sensitivity and techno-economic investigation. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–21. [Google Scholar] [CrossRef]
- Amaral, A.R.; Bernar, L.P.; Ferreira, C.C.; de Oliveira, R.M.; Pereira, A.M.; Pereira, L.M.; Santos, M.C.; da Costa Assunção, F.P.; Bezerra, K.C.A.; Almeida, H.S.; et al. Economic feasibility assessment of the thermal catalytic process of wastes: Açaí seeds (Euterpe Oleracea) and Scum from grease traps. Energies 2022, 15, 7718. [Google Scholar] [CrossRef]
- Mota, S.A.P. Craqueamento Termo-Catalítico de Óleos Vegetais em Diferentes Escalas de Produção [Tese] (Doutorado em Engenharia de Recursos Naturais); Universidade de Federal do Pará: Belém, Brazil, 2013. [Google Scholar]
- Barnwal, B.K.; Sharma, M.P. Optimization of Biodiesel Production by Sunflower Oil Transesterification. Bioresour. Tecnol. 2005, 9, 363–378. [Google Scholar]
- da Mota, S.A.P.; Mancio, A.A.; Lhamas, D.E.L.; de Abreu, D.H.; da Silva, M.S.; dos Santos, W.G.; de Castro, D.A.R.; de Oliveira, R.M.; Araújo, M.E.; Borges Luiz, E.P.; et al. Production of green diesel by thermal catalytic cracking of crude palm oil (Elaeis guineensis Jacq) in a pilot plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]
- Mancio, A.A.; da Costa, K.M.B.; Ferreiraa, C.C.; Santos, M.C.; Lhamas, D.E.L.; da Mota, S.A.P.; Leão, R.A.C.; de Souza, R.O.M.A.; Araújo, M.E.; Borges, L.E.P.; et al. Thermal catalytic cracking of crude palm oil at pilot scale: Effect of the percentage of Na2CO3 on the quality of biofuels. Ind. Crops Prod. 2016, 91, 32–43. [Google Scholar] [CrossRef]
- Mâncio, A.A.; da Costa, K.M.B.; Ferreira, C.C.; Santos, M.C.; Lhamas, D.E.L.; da Mota, S.A.P.; Leão, R.A.C.; de Souza, R.O.M.A.; Araújo, M.E.; Borges, L.E.P.; et al. Process analysis of physicochemical properties and chemical composition of organic liquid products obtained by thermochemical conversion of palm oil. J. Anal. Appl. Pyrolysis 2017, 123, 284–295. [Google Scholar] [CrossRef]
- Ferreira, C.C.; Costa, E.C.; de Castro, D.A.R.; Pereira, M.S.; Mâncio, A.A.; Santos, M.C.; Lhamasd, D.E.L.; da Mota, S.A.P.; Leão, A.C.; Duvoisin, S., Jr.; et al. Deacidification of organic liquid products by fractional distillation in laboratory and pilot scales. J. Anal. Appl. Pyrolysis 2017, 127, 468–489. [Google Scholar] [CrossRef]
- da Mota, S.A.P.; da Mota, A.A.M.; Machado, N.T. Influence of fractional distillation on the yield and quality of biofuels obtained through thermal catalytic cracking of crude palm oil. Rev. DYNA 2021, 88, 62–71. [Google Scholar] [CrossRef]
- Mancio, A.A.; da Mota, S.A.P.; Ferreiraa, C.C.; Carvalho, T.U.S.; Netod, O.S.; Zamiand, J.R.; Araújo, M.E.; Borges, L.E.P.; Machado, N.T. Separation and characterization of biofuels in the jet fuel and diesel fuel ranges by fractional distillation of organic liquid products. Fuel 2018, 215, 212–225. [Google Scholar] [CrossRef]
- Natali, A.A. Dendê: Nascido para dar muito óleo. In Agrianual 1996: Anuário Estatístico da Agricultura Brasileira.; FNP: São Paulo, Brazil, 1996; pp. 219–226. [Google Scholar]
- Santos, M.C. Estudo do Processo de Craqueamento Termo-Catalítico da Borra de Neutralização do óleo de Palma pa Produção de Biocombustível [Tese] (Doutorado em Engenharia de Recursos Naturais); Universidade de Federal do Pará: Belém-Pará, Brazil, 2015. [Google Scholar]
- Santos, M.C.; Lourençoa, R.M.; de Abreua, D.H.; Pereira, A.M.; de Castro, D.A.R.; Pereira, M.S.; Almeida, H.S.; Mâncio, A.A.; Lhamas, D.E.L.; da Mota, S.A.P.; et al. Gasoline-like hydrocarbons by catalytic cracking of soap phase residue of neutralization process of palm oil (Elaeis guineensis Jacq). J. Taiwan Inst. Chem. Eng. 2017, 71, 106–119. [Google Scholar] [CrossRef]
- Haas, M.J. Improving the economics of biodiesel production through the use of low value lipids as feedstocks: Vegetable oil soapstock. Fuel Process. Technol. 2005, 86, 1087–1096. [Google Scholar] [CrossRef]
- 43. Szklo, A.S.; Uller, V.C.; Bonfá, M.H.P. Fundamentos do refino de petróleo: Tecnologia e economia; Interciência: Rio de Janeiro, Brazil, 2008; p. 200. [Google Scholar]
- Farah, M.A. Petróleo e Seus Derivados: Definição, Constituição, Aplicação, Especificações, Características de Qualidade; LTC: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Spath, P.L.; Dayton, D.C. Preliminary Screening—Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass—Derived Syngas; National Renewable Energy Laboratory, U.S. Department of Energy Laboratory: Golden, CO, USA, 2003. [Google Scholar] [CrossRef]
- Thilakaratne, R.; Brown, T.; Li, Y.; Hu, G.; Brown, R. Mild catalytic pyrolysis of biomass for production of transportation fuel: A techno-economic analysis. Green Chem. 2014, 16, 627. [Google Scholar] [CrossRef]
- Brown, T.R.; Thilakaratne, R.; Brown, R.C.; Hu, G. Techno-economy analysis of biomass to transportation fuels and 742 electricity via fast pyrolysis and hydroprocessing. Fuel 2013, 106, 463–469. [Google Scholar] [CrossRef]
- Jaroenkhasemmeesuk, C.; Tippayawong, N. Technical and Economic Analysis of a Biomass Pyrolysis Plant. Energy Procedia 2015, 79, 950–955. [Google Scholar] [CrossRef]
- Santana Junior, C.C.; Brito, M.R.; Barbosa, L.N.; Jaconi, A.; Rambo, M.K.D.; Rambo, M.C.D. Environmental-economic assessment of lignocellulosic residual from the Legal Amazon for conversion in biochars and bioproducts for biorefineries. Int. J. Adv. Eng. Res. Sci. (IJAERS) 2020, 7, 8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).