Lignin-First Biorefinery for Converting Lignocellulosic Biomass into Fuels and Chemicals
Abstract
1. Introduction
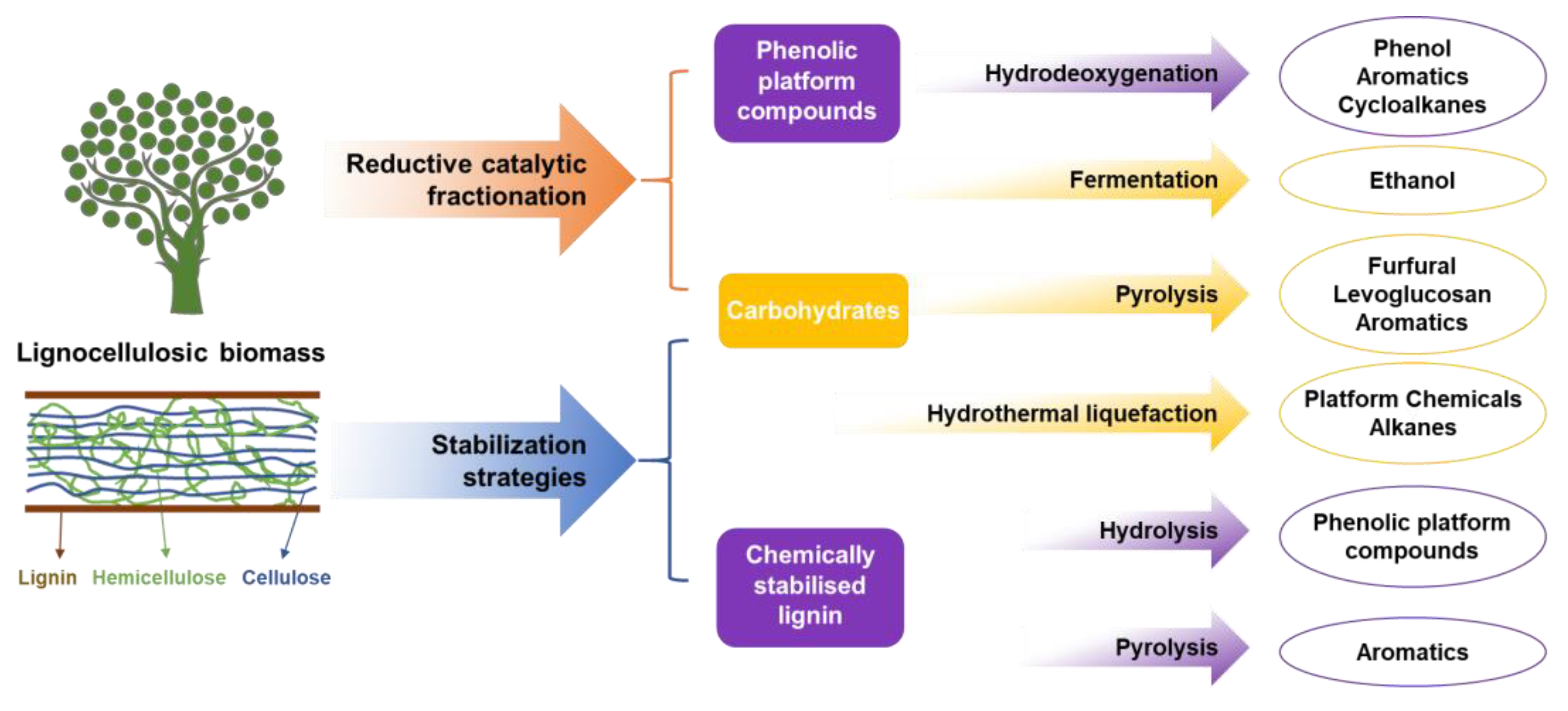
2. Overview of Lignin-First Biorefinery
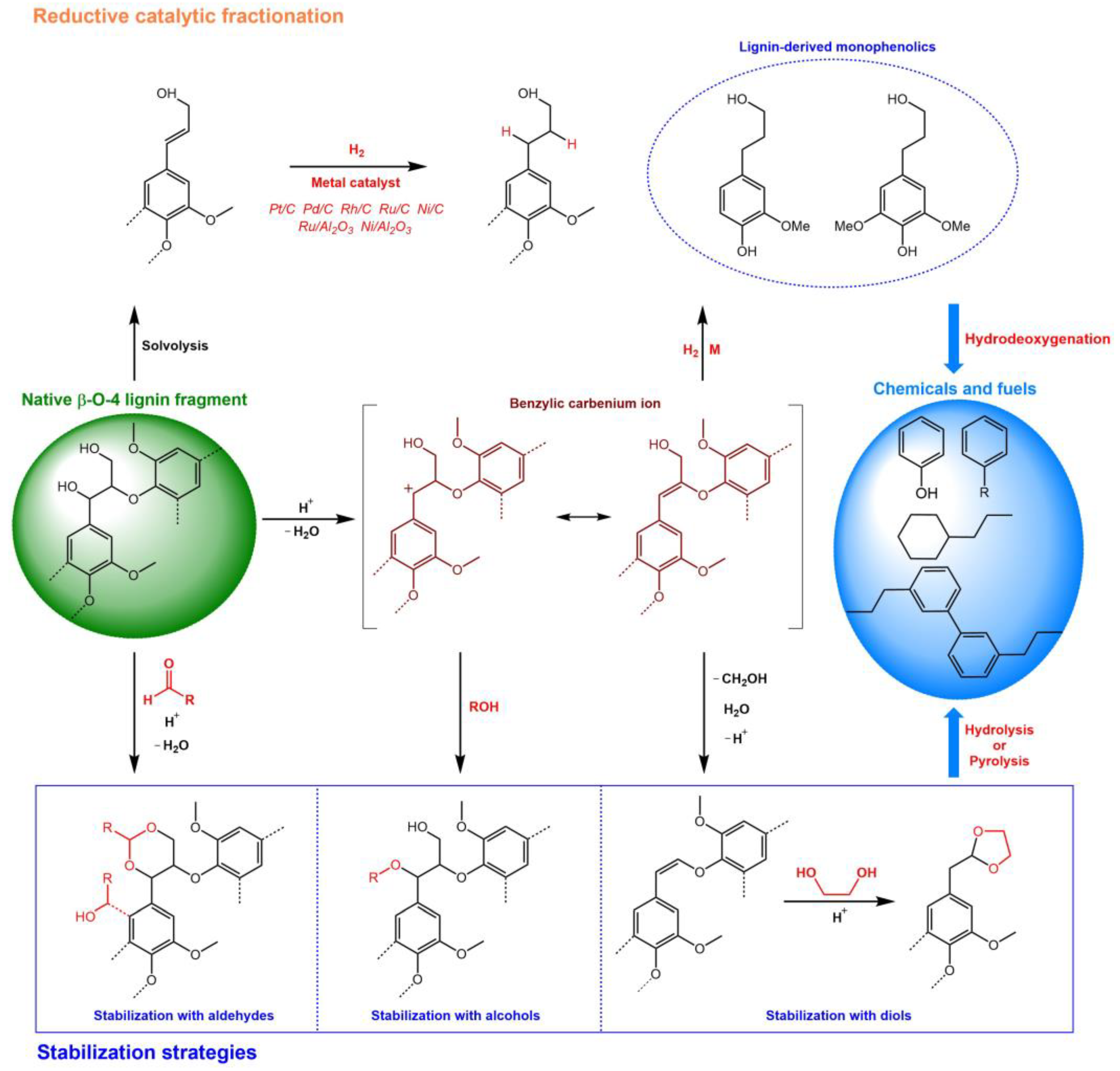
2.1. Reductive Catalytic Fractionation (RCF)
2.1.1. Role of the Catalyst Used
2.1.2. Influence of Solvents
2.1.3. Flow-Through Reactors
2.2. Stabilization Strategies
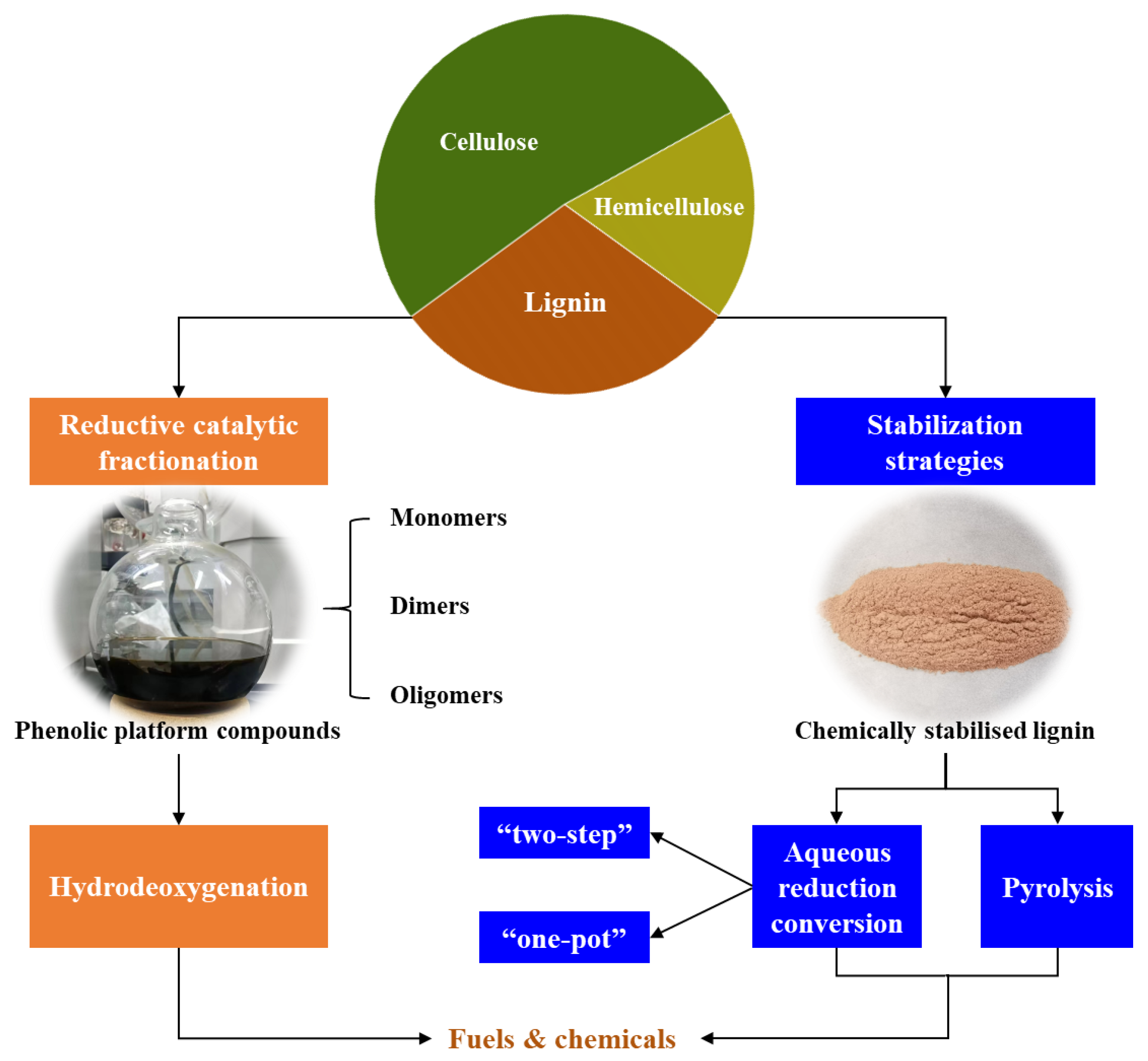
3. Downstream Value-Added Terminal Products
3.1. Lignin and Its Derivatives
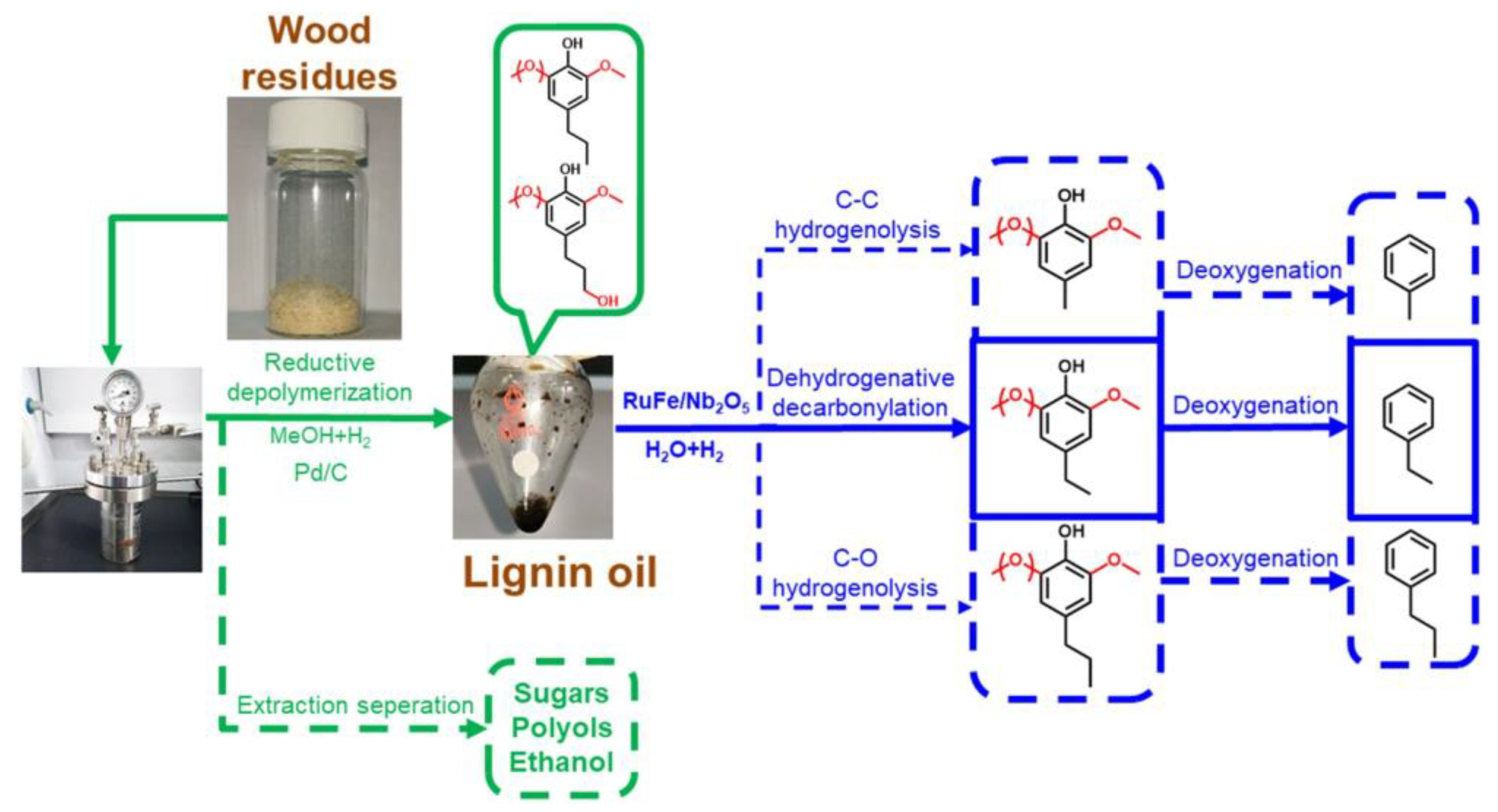
3.1.1. Phenolic Platform Compounds
3.1.2. Chemically Stabilized Lignin
3.2. Carbohydrate
3.2.1. Fermentation
3.2.2. Pyrolysis
3.2.3. Hydrothermal Liquefaction
4. Summary and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Cherubini, F.; Strømman, A.H. Chemicals from lignocellulosic biomass: Opportunities, perspectives, and potential of biorefinery systems. Biofuels Bioprod. Biorefin. 2011, 5, 548–561. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.V.; Samec, J.S. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; Peinder, P.d.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Wang, M.; Dewil, R.; Maniatis, K.; Wheeldon, J.; Tan, T.; Baeyens, J.; Fang, Y. Biomass-derived aviation fuels: Challenges and perspective. Prog. Energy Combust. Sci. 2019, 74, 31–49. [Google Scholar] [CrossRef]
- Shen, X.; Xin, Y.; Liu, H.; Han, B. Product-oriented Direct Cleavage of Chemical Linkages in Lignin. ChemSusChem 2020, 13, 4367–4381. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Koelewijn, S.-F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Hakim, S.H.; Zhou, S.; Won, W.; Hosseinaei, O.; Tao, J.; Garcia-Negron, V.; Motagamwala, A.H.; Mellmer, M.A.; Huang, K. Increasing the revenue from lignocellulosic biomass: Maximizing feedstock utilization. Sci. Adv. 2017, 3, e1603301. [Google Scholar] [CrossRef] [PubMed]
- Bartling, A.; Stone, M.L.; Hanes, R.J.; Bhatt, A.; Zhang, Y.; Biddy, M.J.; Davis, R.; Kruger, J.S.; Thornburg, N.E.; Luterbacher, J.; et al. Techno-economic analysis and life cycle assessment of a biorefinery utilizing reductive catalytic fractionation. Energy Environ. Sci. 2021, 14, 4147–4168. [Google Scholar] [CrossRef]
- Koranyi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules 2020, 25, 2815. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Kenny, J.K.; Brandner, D.G.; Neefe, S.R.; Michener, W.E.; Román-Leshkov, Y.; Beckham, G.T.; Medlin, J.W. Catalyst choice impacts aromatic monomer yields and selectivity in hydrogen-free reductive catalytic fractionation. React. Chem. Eng. 2022, 7, 2527–2533. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Kaiho, A.; Kogo, M.; Sakai, R.; Saito, K.; Watanabe, T. In situ trapping of enol intermediates with alcohol during acid-catalysed de-polymerisation of lignin in a nonpolar solvent. Green Chem. 2015, 17, 2780–2783. [Google Scholar] [CrossRef]
- Deuss, P.J.; Scott, M.; Tran, F.; Westwood, N.J.; de Vries, J.G.; Barta, K. Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J. Am. Chem. Soc. 2015, 137, 7456–7467. [Google Scholar] [CrossRef] [PubMed]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Li, L.; Dong, L.; Liu, X.; Guo, Y.; Wang, Y. Selective production of ethylbenzene from lignin oil over FeOx modified Ru/Nb2O5 catalyst. Appl. Catal. B Environ. 2020, 260, 118143. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Matsakas, L.; Lappas, A.A.; Rova, U.; Christakopoulos, P. Aromatics from Beechwood Organosolv Lignin through Thermal and Catalytic Pyrolysis. Energies 2019, 12, 1606. [Google Scholar] [CrossRef]
- Li, S.; Luo, Z.; Wang, W.; Sun, H.; Xie, J.; Liang, X. Catalytic fast pyrolysis of enzymatic hydrolysis lignin over Lewis-acid catalyst niobium pentoxide and mechanism study. Bioresour. Technol. 2020, 316, 123853. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, K.; Xiao, L.P.; Sun, R.C.; Song, G. Total utilization of lignin and carbohydrates in Eucalyptus grandis: An integrated biorefinery strategy towards phenolics, levulinic acid, and furfural. Biotechnol. Biofuels 2020, 13, 2. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.F.; Van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Questell-Santiago, Y.M.; Galkin, M.V.; Barta, K.; Luterbacher, J.S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 2020, 4, 311–330. [Google Scholar] [CrossRef]
- Sun, Z.; Cheng, J.; Wang, D.; Yuan, T.Q.; Song, G.; Barta, K. Downstream Processing Strategies for Lignin-First Biorefinery. ChemSusChem 2020, 13, 5199–5212. [Google Scholar] [CrossRef] [PubMed]
- Paone, E.; Tabanelli, T.; Mauriello, F. The rise of lignin biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Ferrini, P.; Rezende, C.A.; Rinaldi, R. Catalytic Upstream Biorefining through Hydrogen Transfer Reactions: Understanding the Process from the Pulp Perspective. ChemSusChem 2016, 9, 3171–3180. [Google Scholar] [CrossRef]
- Rinaldi, R.; Woodward, R.; Ferrini, P.; Rivera, H. Lignin-First Biorefining of Lignocellulose: The Impact of Process Severity on the Uniformity of Lignin Oil Composition. J. Braz. Chem. Soc. 2018, 30, 479–491. [Google Scholar] [CrossRef]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates, Using Earth Abundant Nickel Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive Catalytic Fractionation of Corn Stover Lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Mikhlin, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2020, 11, 42. [Google Scholar] [CrossRef]
- Taran, O.P.; Miroshnikova, A.V.; Baryshnikov, S.V.; Kazachenko, A.S.; Skripnikov, A.M.; Sychev, V.V.; Malyar, Y.N.; Kuznetsov, B.N. Reductive Catalytic Fractionation of Spruce Wood over Ru/C Bifunctional Catalyst in the Medium of Ethanol and Molecular Hydrogen. Catalysts 2022, 12, 1384. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Xiao, L.P.; Wang, B.; Sun, R.C.; Song, G. Sequential utilization of bamboo biomass through reductive catalytic fractionation of lignin. Bioresour. Technol. 2019, 285, 121335. [Google Scholar] [CrossRef] [PubMed]
- Renders, T.; Van den Bosch, S.; Vangeel, T.; Ennaert, T.; Koelewijn, S.-F.; Van den Bossche, G.; Courtin, C.M.; Schutyser, W.; Sels, B.F. Synergetic Effects of Alcohol/Water Mixing on the Catalytic Reductive Fractionation of Poplar Wood. ACS Sustain. Chem. Eng. 2016, 4, 6894–6904. [Google Scholar] [CrossRef]
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.I.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. (Camb) 2015, 51, 13158–13161. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Van den Bosch, S.; Renders, T.; De Boe, T.; Koelewijn, S.F.; Dewaele, A.; Ennaert, T.; Verkinderen, O.; Goderis, B.; Courtin, C.M.; et al. Influence of bio-based solvents on the catalytic reductive fractionation of birch wood. Green Chem. 2015, 17, 5035–5045. [Google Scholar] [CrossRef]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.; Samec, J.S. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef]
- Kumaniaev, I.; Subbotina, E.; Sävmarker, J.; Larhed, M.; Galkin, M.V.; Samec, J.S.M. Lignin depolymerization to monophenolic compounds in a flow-through system. Green Chem. 2017, 19, 5767–5771. [Google Scholar] [CrossRef]
- Renders, T.; Cooreman, E.; Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Vangeel, T.; Deneyer, A.; Van den Bossche, G.; Courtin, C.M.; Sels, B.F. Catalytic lignocellulose biorefining in n-butanol/water: A one-pot approach toward phenolics, polyols, and cellulose. Green Chem. 2018, 20, 4607–4619. [Google Scholar] [CrossRef]
- Parsell, T.H.; Owen, B.C.; Klein, I.; Jarrell, T.M.; Marcum, C.L.; Haupert, L.J.; Amundson, L.M.; Kenttämaa, H.I.; Ribeiro, F.; Miller, J.T. Cleavage and hydrodeoxygenation (HDO) of C–O bonds relevant to lignin conversion using Pd/Zn synergistic catalysis. Chem. Sci. 2013, 4, 806–813. [Google Scholar] [CrossRef]
- Klein, I.; Marcum, C.; Kenttämaa, H.; Abu-Omar, M.M. Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin. Green Chem. 2016, 18, 2399–2405. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Li, C.; Zheng, M.; Wang, A.; Zhang, T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: Simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012, 5, 6383–6390. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic Solvent Effects in Biomass Conversion Reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A Promising Technology for Conversion of Lignocellulose and Platform Chemicals. ChemSusChem 2017, 10, 2547–2559. [Google Scholar] [CrossRef]
- Chen, J.; Lu, F.; Si, X.; Nie, X.; Chen, J.; Lu, R.; Xu, J. High Yield Production of Natural Phenolic Alcohols from Woody Biomass Using a Nickel-Based Catalyst. ChemSusChem 2016, 9, 3353–3360. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Xiao, L.-P.; Guo, X.; Fang, Y.; Sun, R.-C.; Song, G. Fragmentation of Woody Lignocellulose into Primary Monolignols and Their Derivatives. ACS Sustain. Chem. Eng. 2019, 7, 4666–4674. [Google Scholar] [CrossRef]
- Chen, H.; Fu, Y.; Wang, Z.; Qin, M. Degradation and redeposition of the chemical components of aspen wood during hot water extraction. BioResources 2015, 10, 3005–3016. [Google Scholar] [CrossRef]
- Anderson, E.M.; Stone, M.L.; Katahira, R.; Reed, M.; Beckham, G.T.; Román-Leshkov, Y. Flowthrough Reductive Catalytic Fractionation of Biomass. Joule 2017, 1, 613–622. [Google Scholar] [CrossRef]
- Anderson, E.M.; Stone, M.L.; Hülsey, M.J.; Beckham, G.T.; Román-Leshkov, Y. Kinetic Studies of Lignin Solvolysis and Reduction by Reductive Catalytic Fractionation Decoupled in Flow-Through Reactors. ACS Sustain. Chem. Eng. 2018, 6, 7951–7959. [Google Scholar] [CrossRef]
- Cooreman, E.; Vangeel, T.; Van Aelst, K.; Van Aelst, J.; Lauwaert, J.; Thybaut, J.W.; Van den Bosch, S.; Sels, B.F. Perspective on Overcoming Scale-Up Hurdles for the Reductive Catalytic Fractionation of Lignocellulose Biomass. Ind. Eng. Chem. Res. 2020, 59, 17035–17045. [Google Scholar] [CrossRef]
- Brandner, D.; Kruger, J.S.; Thornburg, N.E.; Facas, G.G.; Kenny, J.K.; Dreiling, R.J.; Morais, A.R.C.; Renders, T.; Cleveland, N.S.; Happs, R.M.; et al. Flow-through solvolysis enables production of native-like lignin from biomass. Green Chem. 2021, 23, 5437–5441. [Google Scholar] [CrossRef]
- Jang, J.H.; Brandner, D.G.; Dreiling, R.J.; Ringsby, A.J.; Bussard, J.R.; Stanley, L.M.; Happs, R.M.; Kovvali, A.S.; Cutler, J.I.; Renders, T.; et al. Multi-pass flow-through reductive catalytic fractionation. Joule 2022, 6, 1859–1875. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products-strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Zhou, J.; Sun, G. Structural changes of poplar wood lignin after supercritical pretreatment using carbon dioxide and ethanol–water as co-solvents. RSC Adv. 2017, 7, 8314–8322. [Google Scholar] [CrossRef]
- Yadav, P.; Athanassiadis, D.; Antonopoulou, I.; Rova, U.; Christakopoulos, P.; Tysklind, M.; Matsakas, L. Environmental impact and cost assessment of a novel lignin production method. J. Clean. Prod. 2021, 279, 123515. [Google Scholar] [CrossRef]
- Gandolfi, S.; Ottolina, G.; Consonni, R.; Riva, S.; Patel, I. Fractionation of hemp hurds by organosolv pretreatment and its effect on production of lignin and sugars. ChemSusChem 2014, 7, 1991–1999. [Google Scholar] [CrossRef]
- Hu, G.; Cateto, C.; Pu, Y.; Samuel, R.; Ragauskas, A.J. Structural characterization of switchgrass lignin after ethanol organosolv pretreatment. Energy Fuels 2012, 26, 740–745. [Google Scholar] [CrossRef]
- Luo, H.; Abu-Omar, M.M. Lignin extraction and catalytic upgrading from genetically modified poplar. Green Chem. 2018, 20, 745–753. [Google Scholar] [CrossRef]
- Nishide, R.N.; Truong, J.H.; Abu-Omar, M.M. Organosolv Fractionation of Walnut Shell Biomass to Isolate Lignocellulosic Components for Chemical Upgrading of Lignin to Aromatics. ACS Omega 2021, 6, 8142–8150. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, D.S.; de Korte, J.; de Vries, E.P.C.; Hameleers, L.; Wilbers, E.; Jurak, E.; Deuss, P.J. Highly Efficient Semi-Continuous Extraction and In-Line Purification of High beta-O-4 Butanosolv Lignin. Front. Chem. 2021, 9, 655983. [Google Scholar] [CrossRef] [PubMed]
- Talebi Amiri, M.; Dick, G.R.; Questell-Santiago, Y.M.; Luterbacher, J.S. Fractionation of lignocellulosic biomass to produce uncondensed aldehyde-stabilized lignin. Nat. Protoc. 2019, 14, 921–954. [Google Scholar] [CrossRef] [PubMed]
- Lancefield, C.S.; Panovic, I.; Deuss, P.J.; Barta, K.; Westwood, N.J. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: Towards complete biomass valorisation. Green Chem. 2017, 19, 202–214. [Google Scholar] [CrossRef]
- Zhu, G.; Qiu, X.; Zhao, Y.; Qian, Y.; Pang, Y.; Ouyang, X. Depolymerization of lignin by microwave-assisted methylation of benzylic alcohols. Bioresour. Technol. 2016, 218, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Deuss, P.J.; Lancefield, C.S.; Narani, A.; de Vries, J.G.; Westwood, N.J.; Barta, K. Phenolic acetals from lignins of varying compositions via iron(iii) triflate catalysed depolymerisation. Green Chem. 2017, 19, 2774–2782. [Google Scholar] [CrossRef]
- De Santi, A.; Galkin, M.V.; Lahive, C.W.; Deuss, P.J.; Barta, K. Lignin-First Fractionation of Softwood Lignocellulose Using a Mild Dimethyl Carbonate and Ethylene Glycol Organosolv Process. ChemSusChem 2020, 13, 4468–4477. [Google Scholar] [CrossRef]
- Lan, W.; Du, Y.P.; Sun, S.; Behaghel de Bueren, J.; Héroguel, F.; Luterbacher, J.S. Continuous hydrogenolysis of acetal-stabilized lignin in flow. Green Chem. 2021, 23, 320–327. [Google Scholar] [CrossRef]
- Zhang, J.; Lombardo, L.; Gözaydın, G.; Dyson, P.J.; Yan, N. Single-step conversion of lignin monomers to phenol: Bridging the gap between lignin and high-value chemicals. Chin. J. Catal. 2018, 39, 1445–1452. [Google Scholar] [CrossRef]
- Stone, M.L.; Webber, M.S.; Mounfield, W.P.; Bell, D.C.; Christensen, E.; Morais, A.R.C.; Li, Y.; Anderson, E.M.; Heyne, J.S.; Beckham, G.T.; et al. Continuous hydrodeoxygenation of lignin to jet-range aromatic hydrocarbons. Joule 2022, 6, 2324–2337. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Hendriks, B.M.S.; Boot, M.D.; Hensen, E.J.M. Coupling organosolv fractionation and reductive depolymerization of woody biomass in a two-step catalytic process. Green Chem. 2018, 20, 2308–2319. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef] [PubMed]
- Op de Beeck, B.; Dusselier, M.; Geboers, J.; Holsbeek, J.; Morré, E.; Oswald, S.; Giebeler, L.; Sels, B.F. Direct catalytic conversion of cellulose to liquid straight-chain alkanes. Energy Environ. Sci. 2015, 8, 230–240. [Google Scholar] [CrossRef]
- Chernova, N.I.; Grigorenko, A.V.; Kiseleva, S.V.; Larina, O.M.; Kumar, V.; Vlaskin, M.S. Comparative Evaluation of Pyrolysis and Hydrothermal Liquefaction for Obtaining Biofuel from a Sustainable Consortium of Microalgae Arthrospira platensis with Heterotrophic Bacteria. Processes 2022, 10, 2202. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: Effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Li, T.; Miao, K.; Zhao, Z.; Li, Y.; Wang, H.; Watanabe, A.; Teramae, N.; Wang, K. Understanding cellulose pyrolysis under hydrogen atmosphere. Energy Convers. Manag. 2022, 254, 115195. [Google Scholar] [CrossRef]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Matsakas, L.; Aspden, J.; Lappas, A.A.; Rova, U.; Christakopoulos, P. Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation. Molecules 2018, 23, 1647. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, A.; Sharma, P.; Singh, S.; Das, D.; Chawla, G.; Singha, A.; Nain, L. Valorization of jute (Corchorus sp.) biomass for bioethanol production. Biomass Convers. Biorefin. 2020, 12, 5209–5220. [Google Scholar] [CrossRef]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Anderson, E.M.; Stone, M.L.; Katahira, R.; Reed, M.; Muchero, W.; Ramirez, K.J.; Beckham, G.T.; Roman-Leshkov, Y. Differences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. Nat. Commun. 2019, 10, 2033. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ludenhoff, J.M.; Dirks, M.; Ouyang, X.; Boot, M.D.; Hensen, E.J.M. Selective Production of Biobased Phenol from Lignocellulose-Derived Alkylmethoxyphenols. ACS Catal. 2018, 8, 11184–11190. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Liao, Y.; Schutyser, W.; Sels, B.F. Alkylphenols to phenol and olefins by zeolite catalysis: A pathway to valorize raw and fossilized lignocellulose. Green Chem. 2016, 18, 297–306. [Google Scholar] [CrossRef]
- Li, S.; Liu, B.; Truong, J.; Luo, Z.; Ford, P.C.; Abu-Omar, M.M. One-pot hydrodeoxygenation (HDO) of lignin monomers to C9 hydrocarbons co-catalysed by Ru/C and Nb2O5. Green Chem. 2020, 22, 7406–7416. [Google Scholar] [CrossRef]
- Saidi, M.; Moradi, P. Catalytic hydrotreatment of lignin-derived pyrolysis bio-oils using Cu/γ-Al2O3 catalyst: Reaction network development and kinetic study of anisole upgrading. Int. J. Energy Res. 2021, 45, 8267–8284. [Google Scholar] [CrossRef]
- Olcese, R.N.; Bettahar, M.; Petitjean, D.; Malaman, B.; Giovanella, F.; Dufour, A. Gas-phase hydrodeoxygenation of guaiacol over Fe/SiO2 catalyst. Appl. Catal. B Environ. 2012, 115–116, 63–73. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, N.; Gao, S.; Wang, J.; Meng, T.; Du, C.; Shen, T.; Huang, J.; Wu, Q.; Wang, H.; et al. Atomically Dispersed Co Catalyst for Efficient Hydrodeoxygenation of Lignin-Derived Species and Hydrogenation of Nitroaromatics. ACS Catal. 2020, 10, 8672–8682. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J. Effects of indium on Ni/SiO2 catalytic performance in hydrodeoxygenation of anisole as model bio-oil compound: Suppression of benzene ring hydrogenation and C–C bond hydrogenolysis. Chin. J. Catal. 2017, 38, 1818–1830. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-first biorefinery: A reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Leal, G.F.; Lima, S.; Graça, I.; Carrer, H.; Barrett, D.H.; Teixeira-Neto, E.; Curvelo, A.A.S.; Rodella, C.B.; Rinaldi, R. Design of nickel supported on water-tolerant Nb2O5 catalysts for the hydrotreating of lignin streams obtained from lignin-first biorefining. Iscience 2019, 15, 467–488. [Google Scholar] [CrossRef]
- Cao, Z.; Dierks, M.; Clough, M.T.; Daltro de Castro, I.B.; Rinaldi, R. A Convergent Approach for a Deep Converting Lignin-First Biorefinery Rendering High-Energy-Density Drop-in Fuels. Joule 2018, 2, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Hydrodeoxygenation of bio-derived phenols to hydrocarbons using RANEY Ni and Nafion/SiO2 catalysts. Chem. Commun. 2010, 46, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, J.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Aqueous-phase hydrodeoxygenation of bio-derived phenols to cycloalkanes. J. Catal. 2011, 280, 8–16. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Liu, R.; Wang, S.; Chen, L.; Li, K. Hydrodeoxygenation of Lignin-Derived Phenolic Monomers and Dimers to Alkane Fuels over Bifunctional Zeolite-Supported Metal Catalysts. ACS Sustain. Chem. Eng. 2014, 2, 683–691. [Google Scholar] [CrossRef]
- Jin, X.; Tsukimura, R.; Aihara, T.; Miura, H.; Shishido, T.; Nozaki, K. Metal–support cooperation in Al(PO3)3-supported platinum nanoparticles for the selective hydrogenolysis of phenols to arenes. Nat. Catal. 2021, 4, 312–321. [Google Scholar] [CrossRef]
- Wang, X.; Rinaldi, R. A route for lignin and bio-oil conversion: Dehydroxylation of phenols into arenes by catalytic tandem reactions. Angew. Chem. 2013, 52, 11499–11503. [Google Scholar] [CrossRef]
- Kong, J.; He, M.; Lercher, J.A.; Zhao, C. Direct production of naphthenes and paraffins from lignin. Chem. Commun. 2015, 51, 17580–17583. [Google Scholar] [CrossRef]
- Wang, X.; Rinaldi, R. Bifunctional Ni catalysts for the one-pot conversion of Organosolv lignin into cycloalkanes. Catal. Today 2016, 269, 48–55. [Google Scholar] [CrossRef]
- Ji, N.; Diao, X.; Li, X.; Jia, Z.; Zhao, Y.; Lu, X.; Song, C.; Liu, Q.; Li, C. Toward Alkylphenols Production: Lignin Depolymerization Coupling with Methoxy Removal over Supported MoS2 Catalyst. Ind. Eng. Chem. Res. 2020, 59, 17287–17299. [Google Scholar] [CrossRef]
- Luo, Z.; Qin, S.; Chen, S.; Hui, Y.; Zhao, C. Selective conversion of lignin to ethylbenzene. Green Chem. 2020, 22, 1842–1850. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Pan, X.; Ji, J.; Ren, Y.; Wang, H.; Ji, N.; Liu, Q.; Li, C. One-Pot Conversion of Lignin into Naphthenes Catalyzed by a Heterogeneous Rhenium Oxide-Modified Iridium Compound. ChemSusChem 2020, 13, 4409–4419. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, P.; Yu, W.; Shen, D.; Gu, S. Catalytic hydrogenolysis of lignin in ethanol/isopropanol over an activated carbon supported nickel-copper catalyst. Bioresour. Technol. 2021, 319, 124238. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, L.; Gu, J.; Gou, L.; Xie, L.; Wang, Y.; Dai, L. Catalytic hydrotreatment of kraft lignin into aromatic alcohols over nickel-rhenium supported on niobium oxide catalyst. Bioresour. Technol. 2020, 299, 122582. [Google Scholar] [CrossRef]
- Wang, H.; Ruan, H.; Pei, H.; Wang, H.; Chen, X.; Tucker, M.P.; Cort, J.R.; Yang, B. Biomass-derived lignin to jet fuel range hydrocarbons via aqueous phase hydrodeoxygenation. Green Chem. 2015, 17, 5131–5135. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Catalytic pyrolysis-GC/MS of lignin from several sources. Fuel Process. Technol. 2010, 91, 1446–1458. [Google Scholar] [CrossRef]
- Pattiya, A.; Titiloye, J.O.; Bridgwater, A.V. Fast pyrolysis of cassava rhizome in the presence of catalysts. J. Anal. Appl. Pyrolysis 2008, 81, 72–79. [Google Scholar] [CrossRef]
- Neumann, G.T.; Hicks, J.C. Novel Hierarchical Cerium-Incorporated MFI Zeolite Catalysts for the Catalytic Fast Pyrolysis of Lignocellulosic Biomass. ACS Catal. 2012, 2, 642–646. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Tang, Z.; Li, W.-z.; Zhu, X.-f. Catalytic upgrading of biomass fast pyrolysis vapors with titania and zirconia/titania based catalysts. Fuel 2010, 89, 2096–2103. [Google Scholar] [CrossRef]
- Ma, Z.; Ghosh, A.; Asthana, N.; van Bokhoven, J. Visualization of Structural Changes During Deactivation and Regeneration of FAU Zeolite for Catalytic Fast Pyrolysis of Lignin Using NMR and Electron Microscopy Techniques. ChemCatChem 2018, 10, 4431–4437. [Google Scholar] [CrossRef]
- Hemberger, P.; Custodis, V.B.F.; Bodi, A.; Gerber, T.; van Bokhoven, J.A. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat. Commun. 2017, 8, 15946. [Google Scholar] [CrossRef]
- Ma, Z.; van Bokhoven, J.A. Deactivation and Regeneration of H-USY Zeolite during Lignin Catalytic Fast Pyrolysis. ChemCatChem 2012, 4, 2036–2044. [Google Scholar] [CrossRef]
- Ma, Z.; Troussard, E.; van Bokhoven, J.A. Controlling the selectivity to chemicals from lignin via catalytic fast pyrolysis. Appl. Catal. A Gen. 2012, 423–424, 130–136. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Z.; Li, S.; Xue, S.; Sun, H. Novel Micro-Mesoporous Composite ZSM-5 Catalyst for Aromatics Production by Catalytic Fast Pyrolysis of Lignin Residues. Catalysts 2020, 10, 378. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Schüβler, F.; D’Amico, A.; Danilina, N.; Van Bokhoven, J.A.; Lercher, J.A.; Jones, C.W.; Sievers, C. Stability of zeolites in hot liquid water. J. Phys. Chem. C 2010, 114, 19582–19595. [Google Scholar] [CrossRef]
- Han, T.; Ding, S.; Yang, W.; Jönsson, P. Catalytic pyrolysis of lignin using low-cost materials with different acidities and textural properties as catalysts. Chem. Eng. J. 2019, 373, 846–856. [Google Scholar] [CrossRef]
- Deuss, P.J.; Kugge, C. “Lignin-first” catalytic valorization for generating higher value from lignin. Chem. Catal. 2021, 1, 8–11. [Google Scholar] [CrossRef]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X. Efficient sugar production from plant biomass: Current status, challenges, and future directions. Renew. Sustain. Energy Rev. 2022, 164, 112583. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Q.; You, T.; Zhang, X.; Xu, F.; Wu, Y. Short-time deep eutectic solvent pretreatment for enhanced enzymatic saccharification and lignin valorization. Green Chem. 2019, 21, 3099–3108. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Gao, X.; Guo, X.; Wang, S.; Fang, Y.; Song, G. Rational highly dispersed ruthenium for reductive catalytic fractionation of lignocellulose. Nat. Commun. 2022, 13, 4716. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.A.; Zhou, H.; Aui, A.; Wright, M.M.; Wen, Z.; Brown, R.C. A lignin-first strategy to recover hydroxycinnamic acids and improve cellulosic ethanol production from corn stover. Biomass Bioenergy 2020, 138, 105579. [Google Scholar] [CrossRef]
- Gong, X.; Sun, J.; Xu, X.; Wang, B.; Li, H.; Peng, F. Molybdenum-catalyzed hydrogenolysis of herbaceous biomass: A procedure integrated lignin fragmentation and components fractionation. Bioresour. Technol. 2021, 333, 124977. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Li, Y.; Wang, B.; Sun, F.; Xu, F.; Zhang, X. Mild fractionation of poplar into reactive lignin via lignin-first strategy and its enhancement on cellulose saccharification. Bioresour. Technol. 2022, 343, 126122. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Kou, L.; Zhang, X.; Tan, T. Bioethanol production from cellulose obtained from the catalytic hydro-deoxygenation (lignin-first refined to aviation fuel) of apple wood. Fuel 2019, 250, 245–253. [Google Scholar] [CrossRef]
- Ferrini, P.; Rinaldi, R. Catalytic biorefining of plant biomass to non-pyrolytic lignin bio-oil and carbohydrates through hydrogen transfer reactions. Angew. Chem. Int. Ed. Engl. 2014, 53, 8634–8639. [Google Scholar] [CrossRef]
- Bridgwater, A. Fast pyrolysis of biomass for the production of liquids. In Biomass Combustion Science, Technology and Engineering; Woodhead Publishing: Sawston, UK, 2013; pp. 130–171. [Google Scholar]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Zhang, Z.; Wang, Z.; Yang, S.; Yang, Y.; Wang, X.; Liu, S.; Zhang, Q.; Lei, T. Fast corn stalk pyrolysis and the influence of catalysts on product distribution. Bioresour. Technol. 2020, 301, 122739. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Wang, H.; Li, H.; Han, X.; Zeng, Y.; Xu, C.C. A review of bio-oil upgrading by catalytic hydrotreatment: Advances, challenges, and prospects. Mol. Catal. 2021, 504, 111438. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Santiviago, C.; Bilbao, J.; Gayubo, A.G. Upgrading of Bio-Oil in a Continuous Process with Dolomite Catalyst. Energy Fuels 2014, 28, 6419–6428. [Google Scholar] [CrossRef]
- Hu, Y.; Oduro, I.N.; Huang, Y.; Fang, Y. Structural characterization and pyrolysis behavior of holocellulose obtained from lignin-first biorefinery. J. Anal. Appl. Pyrolysis 2016, 120, 416–422. [Google Scholar] [CrossRef]
- Dickerson, T.; Soria, J. Catalytic Fast Pyrolysis: A Review. Energies 2013, 6, 514–538. [Google Scholar] [CrossRef]
- Lu, Q.; Ye, X.N.; Zhang, Z.B.; Dong, C.Q.; Zhang, Y. Catalytic fast pyrolysis of cellulose and biomass to produce levoglucosenone using magnetic SO4(2-)/TiO2-Fe3O4. Bioresour. Technol. 2014, 171, 10–15. [Google Scholar] [CrossRef]
- Liu, D.; Yu, Y.; Hayashi, J.-i.; Moghtaderi, B.; Wu, H. Contribution of dehydration and depolymerization reactions during the fast pyrolysis of various salt-loaded celluloses at low temperatures. Fuel 2014, 136, 62–68. [Google Scholar] [CrossRef]
- Fabbri, D.; Torri, C.; Baravelli, V. Effect of zeolites and nanopowder metal oxides on the distribution of chiral anhydrosugars evolved from pyrolysis of cellulose: An analytical study. J. Anal. Appl. Pyrolysis 2007, 80, 24–29. [Google Scholar] [CrossRef]
- Resende, F.L.P. Recent advances on fast hydropyrolysis of biomass. Catal. Today 2016, 269, 148–155. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A Critical Review on Hemicellulose Pyrolysis. Energy Technol. 2016, 5, 52–79. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Li, W.; Li, R.; Wu, Y. Unveiling the Pyrolysis Mechanisms of Hemicellulose: Experimental and Theoretical Studies. Energy Fuels 2019, 33, 4352–4360. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Brown, R.C.; Shanks, B.H. Product distribution from the fast pyrolysis of hemicellulose. ChemSusChem 2011, 4, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Demirbaş, A. Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers. Manag. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Huang, H.-j.; Yuan, X.-z. Recent progress in the direct liquefaction of typical biomass. Prog. Energy Combust. Sci. 2015, 49, 59–80. [Google Scholar] [CrossRef]
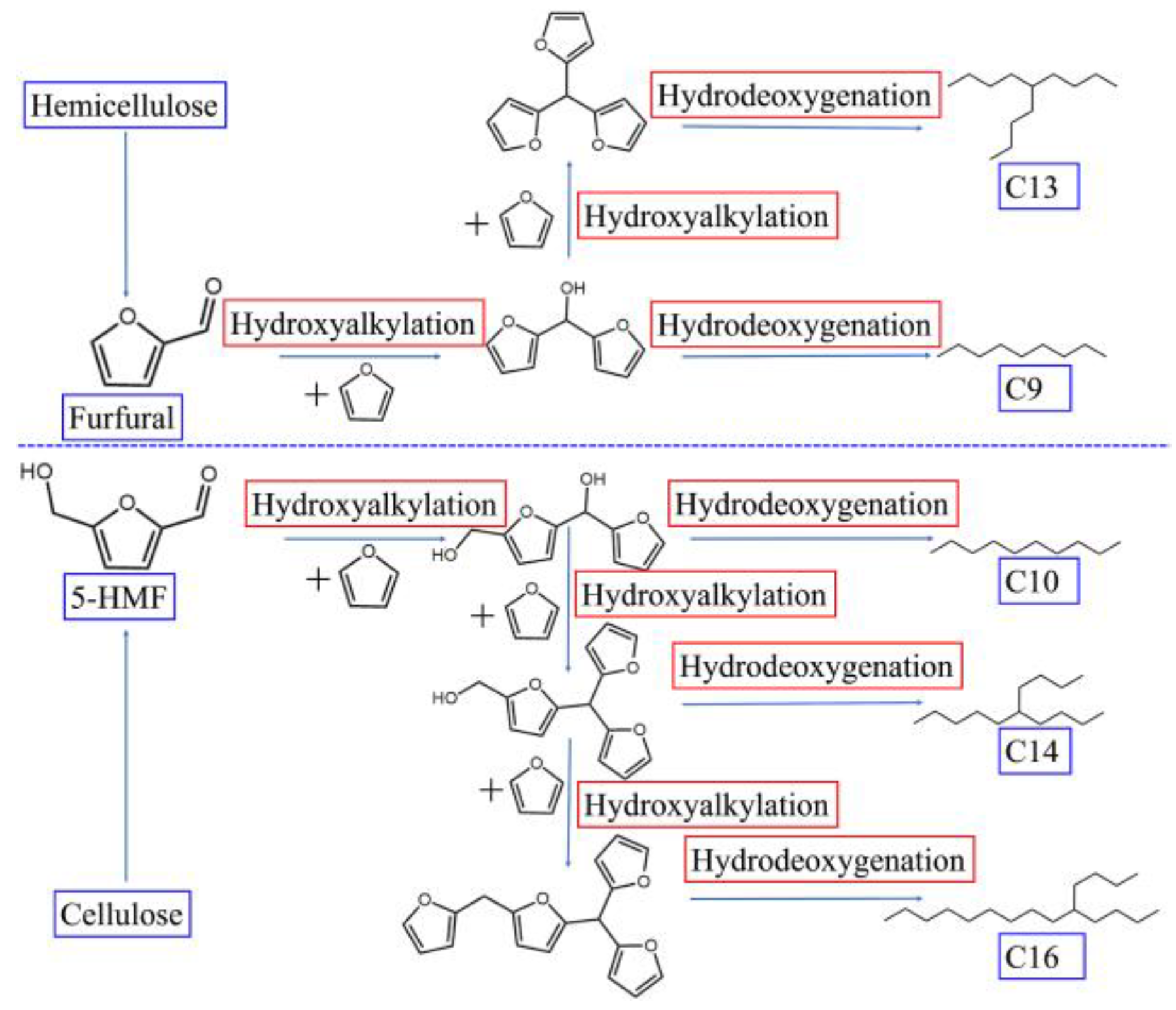
- Guo, T.; Li, X.; Liu, X.; Guo, Y.; Wang, Y. Catalytic Transformation of Lignocellulosic Biomass into Arenes, 5-Hydroxymethylfurfural, and Furfural. ChemSusChem 2018, 11, 2758–2765. [Google Scholar] [CrossRef]
- Osaka, Y.; Ikeda, Y.; Hashizume, D.; Iwamoto, M. Direct hydrodeoxygenation of cellulose and xylan to lower alkanes on ruthenium catalysts in subcritical water. Biomass Bioenergy 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Wang, H.; Yang, B.; Zhang, Q.; Zhu, W. Catalytic routes for the conversion of lignocellulosic biomass to aviation fuel range hydrocarbons. Renew. Sustain. Energy Rev. 2020, 120, 109612. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Chen, L.; Zhang, X.; Zhang, Q.; Wang, T.; Qiu, S.; Tan, J.; Li, K.; Wang, C.; et al. Production of bio-jet fuel from corncob by hydrothermal decomposition and catalytic hydrogenation: Lab analysis of process and techno-economics of a pilot-scale facility. Appl. Energy 2018, 227, 128–136. [Google Scholar] [CrossRef]
- Xu, W.; Xia, Q.; Zhang, Y.; Guo, Y.; Wang, Y.; Lu, G. Effective production of octane from biomass derivatives under mild conditions. ChemSusChem 2011, 4, 1758–1761. [Google Scholar] [CrossRef]



| Year | Key Focus | Reference |
|---|---|---|
| 2017 | Fractionation methods that implement active stabilization mechanisms; techno-economic considerations. | [17] |
| 2019 | Elementary reductive catalytic fractionation steps; recent innovations such as flow-through operation and synergy with feedstock engineering. | [31] |
| 2020 | The kinetics of lignin and polysaccharide depolymerization; the strategies for chemical functionalization. | [32] |
| 2020 | Chronological overview of the development of the “lignin-first” approach with the inclusion of reductive catalytic depolymerization of all lignocellulosic components. | [15] |
| 2020 | Downstream processing strategies of lignin monomers; methods of separation of aromatic monomers from lignin-first biorefinery. | [33] |
| 2020 | The fundamental catalytic reactions relevant to lignin-first biorefinery approach; the further transformations of lignin-derived monolignols and phenolics into value-added products. | [34] |
| 2021 | A set of guidelines for analyzing critical data from lignin-first approaches, including feedstock preparation and characterization, reactor design, catalyst efficiency, mass balances, and product yields. | [16] |
| Newest | The effects of catalyst, solvent, reactor configurations and functional group protection reagents on intermediate products; downstream processing strategies for lignin as well as carbohydrate fractions. | - |
| Feedstock | Catalyst | Solvent | Monomer Yield | Sugar Retention | Year Ref |
|---|---|---|---|---|---|
| Miscanthus | Ni/C | Methanol | 68 wt% | 86 wt% | 2016 [38] |
| Corn Stover | Ni/C | Methanol | 24.5 wt% | 76 wt% | 2016 [39] |
| Flax Shive | Ru/C | Ethanol | 9.5 wt% | Glucan 67.2 wt% | 2020 [40] |
| Spruce | Ru/C | Ethanol | 30 wt% | Glucan 84.4 wt% | 2022 [41] |
| Bamboo | Pd/C | Methanol | 32.2 wt% | Glucan 73.4 wt% Xylan 57.4 wt% | 2019 [42] |
| Eucalyptus | Pd/C | Methanol | 49.8 wt% | Glucan 82.5 wt% Xylan 67.8 wt% | 2020 [29] |
| Poplar | Pd/C | Methanol/ H2O (7:3) | 43.5 wt% | 66.7 wt% | 2016 [43] |
| Zn/Pd/C | Methanol | 54 wt% | 79 wt% | 2015 [44] | |
| Birch | Ru/C | Methanol | 51.5% (C-Yield) | 81% (C-Yield) | 2015 [35] |
| Pd/C | Methanol | 49.3% (C-Yield) | 89% (C-Yield) | 2015 [45] | |
| Pd/C | Water | 43.8 wt% | 55 wt% | 2016 [46] | |
| Pd/C | Ethanol/ H2O (1:1) | 36% (C-Yield) | 84.4 wt% | 2016 [47] | |
| Ni/Al2O3 a | Methanol | 36 wt% | 84.9 wt% | 2017 [30] | |
| Pd/C+H3PO4 b | Methanol/ H2O (7:3) | 37 wt% | 56 wt% | 2017 [48] |
| Feedstock | Conditions | Organic Media | Isolated Lignin a | Year Ref |
|---|---|---|---|---|
| Hemp Hurds | 165 °C 20 min | Methanol H2SO4 aqueous solution | 75 wt% | 2014 [68] |
| Switchgrass | 180 °C 60 min | Ethanol H2SO4 aqueous solution | 60.5 wt% | 2012 [69] |
| Poplar | 160 °C 30 min | Methanol H2SO4 aqueous solution Formaldehyde | 64 wt% | 2018 [70] |
| Walnut | 170 °C 30 min | Methanol H2SO4 aqueous solution Formaldehyde | 50 wt% | 2021 [71] |
| 120 °C 150 min | 1-butanol H2SO4 aqueous solution | 85 wt% | 2021 [72] | |
| Birch | 85 °C 180 min | Formaldehyde 1,4-dioxane Hydrochloric acid | 116 wt% | 2019 [73] |
| 95 °C 210 min | Propionaldehyde 1,4-dioxane Hydrochloric acid | 89 wt% | 2019 [73] |
| Pathways | Description | Feedstock | Conditions | Key Products | Ref |
|---|---|---|---|---|---|
| Hydrothermal liquefaction | The reaction of biomass in hot-compressed or sub-/supercritical water or solvent. | 2-methoxy-4-propylphenol | •Pt/C 400 °C •H-ZSM-5 350 °C | Phenol~60% | [79] |
| 3-(4-hydroxyphenyl)propanol | RuFe/Nb2O5 250 °C | Ethylbenzene~78.5% | [25] | ||
| RCF lignin oil | Mo2C 350–375 °C | C9-C12~56% C14-C20~11.9% | [80] | ||
| Organosolv oak lignin | Pd/C 180 °C | 4-n-propyl syringol /guaiacol~25% | [81] | ||
| Birch lignin | Ru/Nb2O5 250 °C | C7–C9 hydrocarbons~ 35.5% | [82] | ||
| Carbohydrates | FeCl3 200 °C | Furfural~55% Levulinic acid~76% | [38] | ||
| Microcrystalline cellulose | •Tungstosilicic acid •Ru/C 210 °C | C5-C6 alkane~60% | [83] | ||
| Microalgae | 330 °C | Bio-oil~45.7% | [84] | ||
| Pyrolysis | The light, small molecules are converted to oily products through homogeneous reactions in the gas phase. | Organosolv poplar lignin | HZSM-5 600 °C | Aromatic hydrocarbons ~3.57% | [85] |
| Enzymatic hydrolysis lignin | Nb2O5 650 °C | Aromatic hydrocarbons ~11.2% | [27] | ||
| Microcrystalline cellulose | 500 °C | Hydrocarbons~6.5% | [86] | ||
| Nannochloropsis sp. | HZSM-5 400 °C | Aromatic hydrocarbons ~48.60% (32.7 MJ/kg) | [87] | ||
| Microalgae | 600 °C | Bio-oil~21.9% | [84] | ||
| Fermentation | The heterogeneous biochemical process which is catalyzed by enzymes. | RCF pulp | •Accelerase trio enzyme mixture •GSE16-T18-HAA1 * yeast suspension | Ethanol~73% of the maximum theoretical yield | [30] |
| Organosolv beech pulp | •Commercial enzyme solution Cellic® CTec2 •Saccharomyces cerevisiae strain Ethanol Red® | Ethanol~83% of the maximum theoretical yield | [88] | ||
| Jute | •Commercial Cellulase •Beta-glucosidase enzymes •Saccharomyces cerevisiae JRC6 | Ethanol~77.73% | [89] | ||
| Microalgae | •Endoglucanase •β-glucosidase •Amylases | Ethanol~87.6% of the theoretical yield | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Qian, Q.; Sun, H.; Wei, Q.; Zhou, J.; Wang, K. Lignin-First Biorefinery for Converting Lignocellulosic Biomass into Fuels and Chemicals. Energies 2023, 16, 125. https://doi.org/10.3390/en16010125
Luo Z, Qian Q, Sun H, Wei Q, Zhou J, Wang K. Lignin-First Biorefinery for Converting Lignocellulosic Biomass into Fuels and Chemicals. Energies. 2023; 16(1):125. https://doi.org/10.3390/en16010125
Chicago/Turabian StyleLuo, Zhongyang, Qian Qian, Haoran Sun, Qi Wei, Jinsong Zhou, and Kaige Wang. 2023. "Lignin-First Biorefinery for Converting Lignocellulosic Biomass into Fuels and Chemicals" Energies 16, no. 1: 125. https://doi.org/10.3390/en16010125
APA StyleLuo, Z., Qian, Q., Sun, H., Wei, Q., Zhou, J., & Wang, K. (2023). Lignin-First Biorefinery for Converting Lignocellulosic Biomass into Fuels and Chemicals. Energies, 16(1), 125. https://doi.org/10.3390/en16010125








