Abstract
Bio-jet fuels prepared by the thermochemical conversion of triglyceride can be used as complete substitutes of jet fuels. A bio-jet fuel prepared as a substitute of the RP-3 jet fuel and the RP-3 jet fuel itself were, respectively, used to fuel a small aviation piston engine. The characteristic tests of the engine were carried out, and the performances of the power, economy, emissions, and heat release law of the engine fueled with the two fuels were analyzed. The feasibility of the bio-jet fuel as a substitute for the RP-3 jet fuel was proved by the experimental results, which show that when the engine is fueled with the bio-jet fuel, the power and economy performance do not deteriorate; however, the HC emissions increase at small and medium throttle openings, while at large throttle openings, the performances of power and economy decreases, the emissions of HC and NOx increase, and the CO emission decreases. The bio-jet fuel is more prone to spontaneous combustion than the RP-3 jet fuel, so knock combustion would be more likely to occur at large throttle openings, and large cooling air flux is required to cool the cylinder because spontaneous combustion would increase heat release.
1. Introduction
Converting waste bio-oils into liquid biofuels has been gaining attention from all over the world. On one hand, biofuel is environmentally friendly, because it is potentially carbon neutral. The CO2 released from burning biofuel would be recycled and reused by existing plants which could be used to prepare biofuels, while the CO2 released from burning fossil fuels would be directly released to the atmosphere [1]. On the other hand, the stock of fossil fuels is limited, while the raw material of preparing biofuels is sustainable. Therefore, the liquid biofuel is an effective way to achieve carbon neutrality and solve the energy crisis.
Studies about liquid biofuels at present mainly focus on biodiesels. Many studies have proved that fueling internal combustion engines with a mixture of biodiesel and fossil fuel could help to stabilize combustion, decreasing the ignition delay time and reducing the emissions of hydrocarbon and soot [2,3,4,5]. Fueling with biodiesel may also lead to higher fuel consumption, higher emissions of nitrogen oxide (NOx), and lower thermal efficiency and power output [5,6,7], which could be improved by adjusting the portion of biodiesel in the mixture, using new types of biodiesel and developing preparation technology [8,9,10]. Biodiesel has been widely used, and related industrial devices have been established in Europe, America, and other regions [11,12].
However, biodiesel is mainly applied in vehicle engines and other non-aviation gas turbines at present because it has not met the standard of jet fuel [13]. Jet fuels have stricter requirements for physicochemical properties than vehicle fuels due to the strict security requirements of aircrafts. The main composition of biodiesels is fatty acid methyl esters, which is quite different from fossil jet fuels, whose main component is hydrocarbon, including chain hydrocarbons (alkanes, alkenes), cyclanes, and arenes with carbon molecular weight between C9 and C15. The content and the molecular structure of the components of jet fuels have significant influence on a fuel’s physicochemical properties, for example, straight-chain hydrocarbons affect almost all kinds of physical properties, and cyclanes and arenes affect density, heating value, freezing point, and aniline point [14]. In addition, the components of jet fuels also affect the operational performance of an aircraft. For example, alkanes and alkenes help to improve engine starting performance, and arenes increases the sealability of fuel systems. Therefore, the jet fuels have stringent requirements for the molecular structure and the content of their components.
The two main methods of preparing bio-jet fuels are high-pressure hydrogenation of bio-oils and chemical transformation of wood fiber. The first method takes bio-oils as raw material. It firstly removes the carboxyl from the raw oil to form chain alkanes, then separates the chain alkanes by distillation and rectification, and finally synthesizes straight-chain alkanes whose chain lengths match the requirements for jet fuel [15,16]. The main defect of high-pressure hydrogenation is that the production contains no aromatic hydrocarbons or cycloalkanes, so biofuels prepared by this method can only partly substitute jet fuels. The second method takes wood fiber as raw material. It firstly degrades the wood fiber to sugar, then translates the monosaccharide derivative to organic synthetic intermediate and increases the length of the carbon chains by chemical recombination, finally removing the oxygen by high-pressure hydrogenation to obtain straight-chain alkanes [17]. A technology frequently used in this method is the Fischer–Tropsch synthesis [18]. The main defect of the chemical transformation of wood fiber is that there are key technical issues and application issues still unsolved because of the big difference between the structures of the wood fiber and those of fossil fuel.
Engine tests of fueling with bio-jet fuels prepared by the two methods have been carried out. For example, Bester et al. [19], Bulzan et al. [20], and Klingshirm et al. [21] compared the performances of aviation gas turbines fueled with Jet A/JP-8, bio-jet fuels prepared via the Fischer-Tropsch synthesis, bio-jet fuels prepared via the hydrogenation method, and blends of JP-A/JP-8 and bio-jet fuels. The results show that the emission performance is improved by the bio-jet fuel, while the engine operation performance is influenced slightly. The bio-jet fuels they used have few aromatics, which is thought to be responsible for the emission improvement. Similar results were obtained by Khandelwal et al. [22] and Badami et al. [23]; although, they used bio-jet fuels whose aromatics contents were about half and two thirds, respectively, of Jet A.
The studies above reflect two things, one is that the bio-jet fuels studied have a lack of arenes, and the other is that the application objects are mainly gas turbines. Studies about applying bio-jet fuels that can completely substitute fossil jet fuels on small aviation piston engines are relatively rare. Small aviation piston engines are mainly used to drive unmanned aerial vehicles, whose application scenarios frequently include forests, reef islands, and oceans. These places are generally in remote areas and lack transportation, so fuel supply is a problem, which could be resolved by preparing bio-jet fuels through daily waste oils.
Xu’s team proposed a method to prepare bio-jet fuels via the thermochemical conversion of triglyceride [24,25,26]. In this method, the bio-oil is pyrolyzed and distilled to obtain straight-chain hydrocarbons with chain lengths similar to those of jet fuels. Then, parts of the straight-chain hydrocarbons are turned to arenes using molecular sieve catalysts, and parts of the arenes are further turned into cyclanes. The method is highly efficient, and the production contains all the key components of jet fuels, including alkanes, arenes, and cyclanes, which potentially makes it able to completely substitute fossil jet fuels. Li [26] prepared a bio-jet fuel to substitute RP-3 jet fuel using the thermochemical conversion method, and the bio-jet fuel was proved to be able to completely substitute RP-3 jet fuel through an analysis of the components and physicochemical properties of the jet fuel.
Based on the works of Xu and Li, this study carried out an experimental investigation to figure out the feasibility of the bio-jet fuel prepared by Xu and Li as a complete substitute of RP-3 jet fuel to fuel a small aviation piston engine. In the experiment, the bio-jet fuel and the RP-3 jet fuel were, respectively, used to fuel a small, two-stroke, spark-ignition (SI) aviation piston engine. The characteristic tests of the engine, equipped with propellers, were carried out, and the performances of power, economy, and emission, and the heat release law of the engine fueled with the two fuels at different throttle openings, were analyzed.
2. Experimental Methods
2.1. Bio-Jet Fuel Preparation
The soybean was chosen as the raw material to prepare the bio-jet fuel. The composition of soybean is relatively simple, so the bio-jet fuel transferred from soybean was relatively easy to analyze, and relatively fewer interference factors were introduced into the experiment from the preparation process. This does not mean that waste oil is not able to be used as a raw material for bio-jet fuel preparation. In fact, transferring waste oils into bio-jet fuels was an important motivation for developing the thermochemical conversion method, and the feasibility and applicability of transferring waste triglyceride into jet fuels via the thermochemical conversion method have been proved in Ref. [27].
Figure 1 shows the main steps of the preparation technology route, as follows:
- Hyperthermia catalytical pyrolysis:
Mix the soybean oil with the catalyst and then pyrolyze the mixture at a high temperature to produce pyrolysis oil, whose composition is similar to the RP-3 jet fuel.
- 2.
- Distillation:
Distill the pyrolysis oil to separate it into light oil and heavy oil. The light oil mainly contains alkanes and alkenes, whose carbon chains are mainly C9–C15, like the RP-3 jet fuel.
- 3.
- Molecule structure adjustment:
Turn part of the alkanes and the alkenes of the light oil into arenes by HZSM-5-type molecular sieve catalysts, and then hydrogenate part of the arenes under high pressures to produce cyclanes; therefore, a bio-jet fuel containing alkanes, arenes, and cyclanes can be obtained.
The detailed preparation process can be found in Li’s work [26].
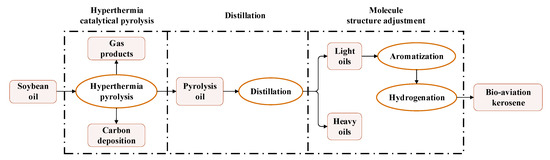
Figure 1.
Technology route of the bio-jet fuel preparation.
The key physical properties for jet fuels are measured at 20 °C. Table 1 compares the properties of the bio-jet fuel with those of RP-3 jet fuel. It shows that the properties of the bio-jet fuel meet the standards of RP-3 jet fuel. Notice that the viscosity of the bio-jet fuel is almost twice the standard limit of RP-3 jet fuel. The viscosity has great influence on the fuel atomization performance. In our previous experiment, the particle Sauter mean diameters of the two fuels, atomized by a centrifugal nozzle at different atomization pressures, were measured, and the probability density distribution of the particle diameters at atomization pressure of 4 bar are shown in Figure 2 [28]. The figure shows that the bio-jet fuel forms much larger particles than the RP-3 jet fuel.

Table 1.
Physical properties of fuels at 20 °C.
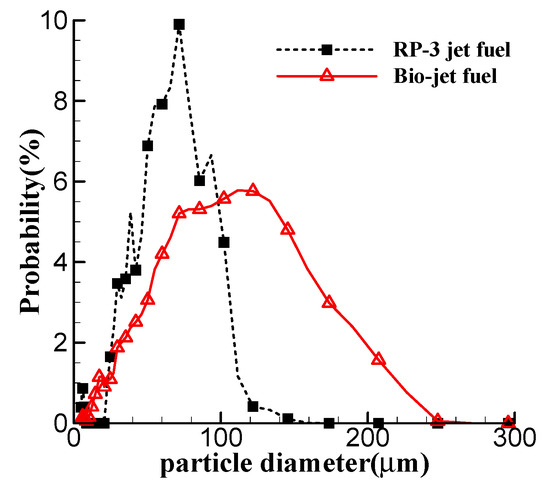
Figure 2.
Probability distribution of atomized particle diameter.
2.2. Experimental Details
The small, two-stroke, SI aviation piston engine is shown in Figure 3. The engine has two horizontally opposed cylinders and uses a leaf valve to control inflow air. The engine is able to be fueled by a variety of fuels by adopting the port fuel injection strategy and crankcase preheating technology. Listed in Table 2 are the main performance parameters of the engine.
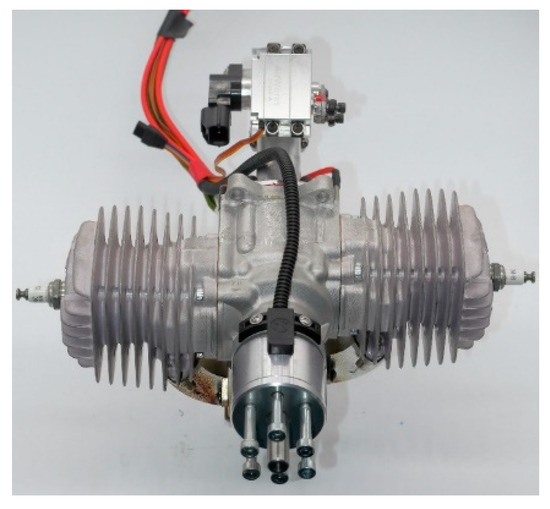
Figure 3.
The two-stroke, SI aviation piston engine.

Table 2.
Engine performance parameters.
The tests were carried out on our homemade PTT30 propeller test bench, as shown in Figure 4. The test bench is mainly composed of a bearing housing and a rigid connecting support, attached with the bearing shaft. The engine is fixed on the rigid connecting support and the propeller is installed on the engine output shaft. When the engine drives the propeller, the torque is transferred via the rigid support to a torque sensor that is installed on the bearing shaft. An encoder used to measure the crankshaft position was attached on the engine output shaft through a synchronous drive belt. A Kistler 6052C pressure sensor was plugged into the cylinder to measure the cylinder pressure, and a Kistler SPC 2852A signal conditioning system was used to amplify the cylinder pressure signal. An NHA-506 exhaust gas analyzer was used to measure and analyze the compositions of exhaust gas. A UEGO sensor was plugged into the exhaust manifold to monitor the excess air coefficient. A fuel consumption meter was connected with the fuel tank to measure the fuel consumption. When the engine worked, an ECU was used to adjust the fuel injection to control the excess air coefficient, a servo was installed on the intake valve of the engine to adjust the throttle opening, and a capacitor discharge ignition system was used to ignite the engine.
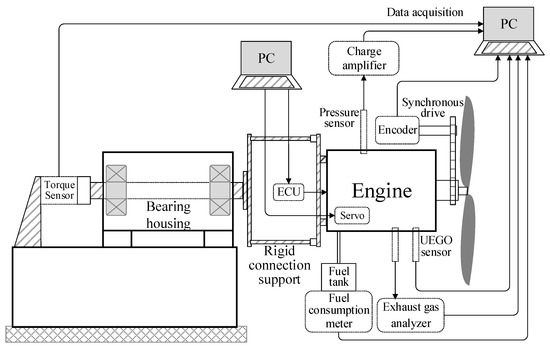
Figure 4.
Schematic diagram of test bench.
All tests were carried out at normal temperature and pressure. The temperature, pressure, and humidity of the environment were 7 °C~10 °C, 102 kPa~103 kPa, and 56~65%, respectively. The excess air coefficient of the fuel–air mixture was controlled at around 1.0. The throttle openings were from 30% to 75%, which corresponds to speeds from 4500 rpm to 6500 rpm. The load of the engine was to drive the propeller, so the engine speed increases as the throttle opening increases, and the spark timing was adjusted correspondingly to maintain stable operation of the engine. The relationship between the spark timing and the engine speed was pre-set, as shown in Figure 5. BTDC in the figure means before top dead center.
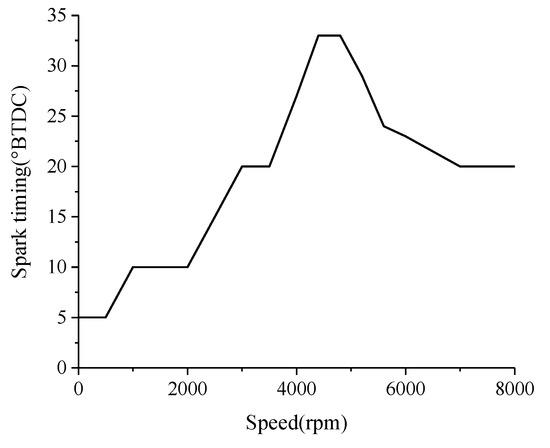
Figure 5.
Relationship between spark timing and speed.
The main test procedures were as follows:
- Preheat the cylinder to 70 °C.
- Start the engine and run the engine at idle speed until the cylinder temperature reaches 135 °C.
- Adjust control parameters to make the engine work on the specified working conditions.
- Measure the data when the cylinder temperature stabilizes at 170 °C and the rotating speed is steady.
In the tests, the temperatures of the two cylinders were maintained automatically at 170 °C by using a cooling fan and a frequency converter to avoid damaging the engine. To avoid mutual mixture of the bio-jet fuel and the RP-3 jet fuel, the entire oil circuit was replaced with a new one before changing the fuel.
3. Results
3.1. Performances of Power and Economy
The brake powers and speeds of the engine fueled with the RP-3 jet fuel and the bio-jet fuel at different throttle openings are presented in Figure 6. The figure shows that the brake power and speed increase as the throttle opening increases. At small throttle openings, the brake power and speed of the bio-jet fuel were very close to those of RP-3 jet fuel, and became slightly higher than those of RP-3 jet fuel at medium throttle openings. As the throttle opening increased to over 60%, the brake power and speed of the bio-jet fuel became smaller than those of RP-3 jet fuel. At 75% throttle opening, the brake power and speed of the bio-jet fuel were smaller than those of RP-3 jet fuel by about 5% and 1.7%, respectively.
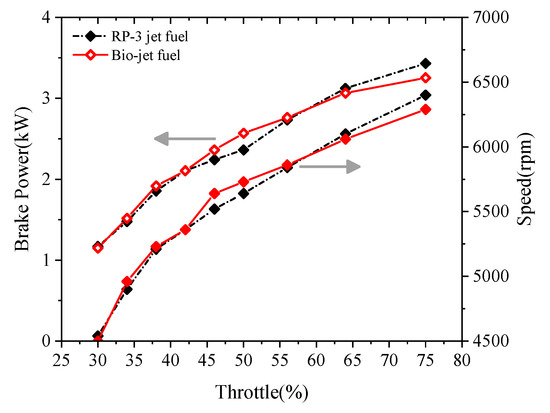
Figure 6.
Variation of brake power and speed against throttle opening.
Figure 7 shows the variation of the brake specific fuel consumption (BSFC) of the engine against the throttle opening. The curves show that the BSFC first decreased and then increased, and it reached a low plateau from throttle opening of about 35% to 60%. The load of the engine is to drive the propellor, so the speed increased as the throttle opening increased. Large throttle opening leads to small pumping loss, while at the same time, large speed leads to large friction loss and large scavenging loss, caused by the short-circuiting of the scavenge duct and the exhaust duct. Under the combined effects of these factors, the BSFC presented a trend of decreasing first and then increasing as the throttle opening increased. The throttle opening of 35–60% corresponds to the most economic working condition of the engine. The range of the economic condition of the engine remained consistent when the fuel was changed from RP-3 jet fuel to the bio-jet fuel, and the BSFC of the bio-jet fuel was comparable to that of RP-3 jet fuel under the economic condition. When the throttle opening increased to over 55%, the BSFC of the bio-jet fuel became higher than RP-3 jet fuel, and it was about 10% higher than RP-3 jet fuel at 75% throttle opening.
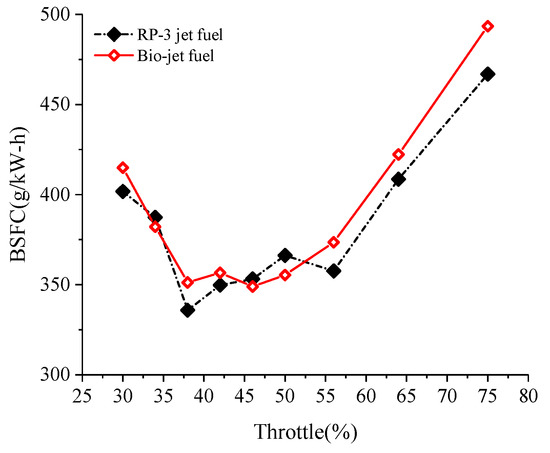
Figure 7.
Variation of BSFC against throttle opening.
In terms of power performance, the bio-jet fuel is comparable to RP-3 jet fuel. The bio-jet fuel was better at medium throttle openings and worse at large throttle openings than RP-3 jet fuel, but the differences between them were quite small, less than 5%. In terms of economy performance, the bio-jet fuel was a little worse than RP-3 jet fuel. The differences between them occurred mainly at large throttle openings, where the BSFC of the bio-jet fuel was about 10% higher than RP-3 jet fuel.
3.2. Heat Release Law
Figure 6 and Figure 7 show that there were some differences between the brake power and BSFC of the engine fueled with the bio-jet fuel and RP-3 jet fuel at medium and large throttle openings. The cylinder pressure and the apparent heat release rate (AHRR) at throttle openings of 46% and 64% are plotted in Figure 8 to figure out the reasons for these differences. The crank angle of 0° in the figures is the top dead center (TDC). The cylinder pressure drawn in Figure 8 is an average of 200 cycles of cylinder pressures. The AHRR is the heat released at each crank angle and is calculated using the cylinder pressure data according to the following equation, via the software GT-Power:
where the subscript means the total quantities of the burned and unburned gases in the cylinder; is time; is the cylinder pressure; is the cylinder volume; is the heat; is the total mass of gases in the cylinder; is the sensible energy of the gases in the cylinder. The three terms on the right side of Equation (1) mean the energy change induced by pressure, combustion, and the state of the gases itself, respectively.
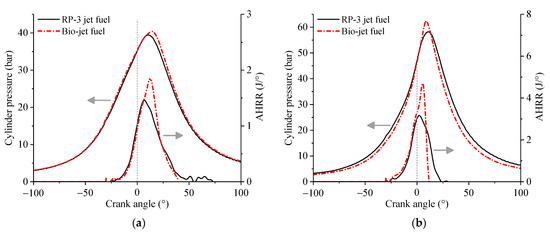
Figure 8.
Variation of cylinder pressure and AHRR against crank angle at (a) 46% throttle opening and (b) 64% throttle opening.
The cylinder pressure curves in Figure 8 show that the bio-jet fuel has larger maximum cylinder pressure than RP-3 jet fuel. At the 46% throttle opening, the crank angles of maximum cylinder pressure of the bio-jet fuel and RP-3 jet fuel were about 14° and 10.9° after the TDC, respectively. As the throttle opening increased to 64%, the crank angle of the maximum cylinder pressure of the bio-jet fuel moved forward to about 8.8°, while that of RP-3 jet fuel remained almost unchanged. The AHRR curves show that the bio-jet fuel had larger maximum AHRR than RP-3 jet fuel. From the spark time to the time when the AHRR of RP-3 jet fuel reached the maximum, the AHRR developments of the bio-jet fuel and RP-3 jet fuel are basically the same. When the AHRR of RP-3 jet fuel began to drop from the maximum, the AHRR of the bio-jet fuel maintained a rapid upward trend until it reached the maximum, and then it declined with a much greater rate than RP-3 jet fuel.
Statistic calculations were made to find the reason for the difference between the cylinder pressure and AHRR of the two fuels. According to the calculation results, the probability of knock combustion occurrence of the engine fueled with the bio-jet fuel was about 72% at the 64% throttle opening, while the corresponding probability of RP-3 jet fuel was only about 4%. Figure 9 shows the cylinder pressure and the corresponding AHRR of the bio-jet fuel of one cycle at the 64% throttle opening. It can be seen in the figure that the cylinder pressure presented high-frequency vibrations near the peak point, and the AHRR curve also shows a significant spike at the corresponding time, which suggests that knock combustion occurred. It can be noticed that the vibration amplitude of the cylinder pressure was not large, which means the knock combustion was not intense enough to damage the engine. In the experiment, it was found that, once the throttle opening increased to over 75%, the knock intensity would increase significantly, which would make the cylinder temperature rise sharply, and the engine would be damaged.
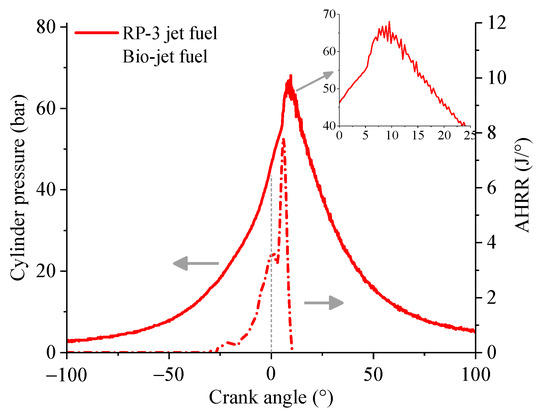
Figure 9.
Variation of cylinder pressure and AHRR of one cycle for the bio-jet fuel at 64% throttle opening.
Knock combustion is a strong, spontaneous combustion. It indicates that the difference between RP-3 jet fuel and the bio-jet fuel should be attributed to the fact that the bio-jet fuel has a higher tendency to spontaneously combust. The velocity variation of the cylinder cooling air could also prove the occurrence of spontaneous combustion. Figure 10 shows the cooling air velocity. At conditions of throttle opening less than 40%, the velocity of cooling air of the bio-jet fuel was close to that of RP-3 jet fuel. At throttle openings over 40%, the cooling air velocity of the bio-jet fuel became larger than the RP-3 jet fuel, and the difference between them rose with the increased throttle opening. This was because, as the throttle opening increased and the cylinder pressure and cylinder temperature increased, so did the possibility of spontaneous combustion. When the throttle opening increased to over 40%, spontaneous combustion of the mixture of bio-jet fuel and air occurred, so the cylinder temperature rose, and the cooling air flux was turned up to prevent the cylinder from being damaged. As the throttle opening continued to increase, the intensity of spontaneous combustion increased, and knock combustion was triggered, which made the cylinder temperature rise sharply and therefore the cooling air velocity increased rapidly.
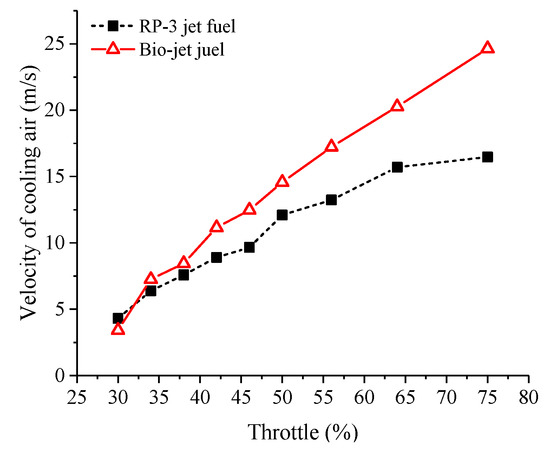
Figure 10.
Variation of cooling air velocity against throttle opening.
The distributions of the cylinder pressure, the AHRR and the cooling air velocity indicate that the bio-jet fuel has higher tendency of spontaneous combustion and knock combustion than the RP-3 jet fuel. According to the preparation technology of the bio-jet fuel [24,26], the bio-jet fuel contains no branched alkanes, and its alkanes are mainly in the form of straight-chain alkanes, while about 20% of RP-3 jet fuel’s composition is branched alkanes [29]. The straight-chain alkanes are more active than the branched alkanes [30], so the bio-jet fuel is more prone to spontaneous combustion. The poor atomization performance of the bio-jet fuel is another important reason for the high tendency of spontaneous combustion. The experiment about piston engine fueled by jet fuels proved that, as the excess air coefficient of the fuel–air mixture becomes larger than 1.0, the possibility of knock combustion increases, and the highest knock intensity occurs at an excess air coefficient of 1.2 [31]. According to Figure 2, the bio-jet fuel would distribute more unevenly in the cylinder than the RP-3 jet fuel, and there are more areas where the excess air coefficient is over 1.0, which increases the tendency of spontaneous combustion.
Theoretically, spontaneous combustion increases the burning rate, and rapid combustion would increase the maximum cylinder pressure and the power, as shown by the case of 46% throttle opening. Meanwhile, too rapid combustion would release heat too fast, and the energy would largely be dissipated through the cylinder wall instead of being transferred into the kinetic energy of the piston, so the power would decrease, as shown by the case of 64% throttle opening.
3.3. Emission Performance
Figure 11 compares the volume fractions of NOx and unburned intermediate reactive products CO and HC in the exhaust gas. It shows that the engine exhaust emitted more HC when burning the bio-jet fuel than when burning RP-3 jet fuel under all test conditions, and the difference between them increased from about 30% to 100% as the throttle opening rose from 30% to 75%. For throttle openings smaller than 50%, the volume fractions of NOx and CO of the bio-jet fuel were close to those of RP-3 jet fuel, while for throttle openings larger than 50%, the volume fractions of NOx and CO of the bio-jet fuel became larger and smaller, respectively, than those of RP-3 jet fuel. At 75% throttle opening, the NOx volume fraction of the bio-jet fuel was almost 3.53 times larger than that of RP-3 jet fuel, and the CO volume fraction of the bio-jet fuel was about 63% of that of the RP-3 jet fuel.
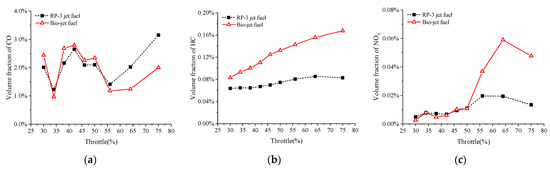
Figure 11.
Volume fractions of the (a) CO, (b) HC, and (c) NOx in the exhaust gas against throttle opening.
Compared with the RP-3 jet fuel, the bio-jet fuel had higher HC emissions at all throttle openings, and higher NOx emissions and lower CO emissions at throttle openings over 50%. The large HC emission is due to the fact that the bio-jet fuel is not burned as fully as RP-3 jet fuel, because of the poor fuel atomization performance. At large throttle openings, the fuel injection quantity increased, so the bio-jet fuel could not be atomized uniformly and there were both high fuel concentration and low fuel concentration areas in the cylinder, which resulted in local rich fuel combustion and knock combustion. The local rich fuel combustion led to higher HC emissions, and accordingly, lower CO emissions. Local rich fuel combustion would form local high-temperature zones, and the occurrence of knock combustion would also significantly increase the cylinder temperature, which creates conditions for the production of NOx.
4. Discussion and Conclusions
To study the feasibility of the bio-jet fuel prepared via the thermochemical conversion of triglyceride as a complete substitute of RP-3 jet fuel, characteristic tests of a small aviation piston engine, fueled, respectively, with bio-jet fuel and RP-3 jet fuel, were carried out. Conclusions below were obtained based on the experimental results:
- When the cooling requirement of the cylinder was satisfied, the performances of the power and the economy of the engine were not degraded by burning the bio-jet fuel at small and medium throttle openings, and the performance even increased slightly at medium throttle openings. For throttle openings larger than 60%, the performances of power and economy degraded. At 75% throttle opening, the brake power decreased by about 5%, and the BSFC increased by about 10% when the engine was fueled with the bio-jet fuel.
- The bio-jet fuel is more prone to spontaneous combustion than RP-3 jet fuel. Spontaneous combustion helped to increase the maximum cylinder pressure and the power at medium throttle openings but led to knock combustion and thus decreased the performances of power and economy at large throttle openings. Spontaneous combustion also increased heat release and then increased the cylinder temperature, so larger cooling air flux was required when the engine was fueled with the bio-jet fuel.
- Burning the bio-jet fuel leads to higher HC emissions than burning the RP-3 jet fuel. The emissions of CO and NOx from burning the bio-jet fuel were close to those from burning RP-3 jet fuel at small and medium throttle openings, but for throttle openings larger than 50%, burning the bio-jet fuel significantly increased the NOx emissions and decreased the CO emissions.
According to the conclusions, it is feasible to fuel a small aviation piston engine with bio-jet fuel as a complete substitute of RP-3 jet fuel; however, cooling requirements should be enhanced for medium and large throttle openings, and the load boundary should be limited to avoid the occurrence of knock combustion.
The differences between the engine performances from burning the bio-jet fuel and burning the RP-3 jet fuel should be attributed to the fact that the bio-jet fuel is more prone to spontaneous combustion than RP-3 jet fuel. One reason is that the alkanes in the bio-jet fuel are mainly straight-chain alkanes, while the alkanes in RP-3 jet fuel are mainly branched alkanes. Another reason is the bio-jet fuel is more viscous than RP-3 jet fuel, which results in poor performance of fuel atomization. Therefore, to completely substitute RP-3 jet fuel with bio-jet fuel, it is not enough to adjust the contents of alkanes, arenes, and cyclanes; it is also necessary to adjust the contents of straight-chain alkanes and branched alkanes. In addition, the atomization performance of a bio-jet fuel should be improved. In our future research, we are going to study the influence of alkane structure on the combustion performance of bio-jet fuels, and we will aim to improve the preparation technology, to prepare bio-jet fuels with better physical properties.
Furthermore, according to Ref. [26], there are some differences between the content ratios of compositions, with different carbon molecular weights between bio-jet fuel and RP-3 jet fuel. For example, RP-3 jet fuel has higher content of carbon chains with molecular weights between C10 and C12 compared with bio-jet fuel. This may be a potential reason for the above differences, on which our planned future works will focus.
Author Contributions
Investigation, C.Z.; data curation, C.Z. and L.L.; formal analysis, C.Z., L.L. and F.Y.; resources, W.C., G.L. and J.X.; writing—original draft preparation, C.Z. and L.L.; writing—review and editing, L.L., W.C. and J.X.; funding acquisition, W.C. and J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 31770612).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the support of the National Science Foundation of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chiong, M.C.; Chong, C.T.; Ng, J.; Lam, S.S.; Tran, M.; Chong, W.W.F.; Jaafar, M.N.M.; Valera-Medina, A. Liquid biofuels production and emissions performance in gas turbines: A review. Energy Convers. Manag. 2018, 173, 640–658. [Google Scholar] [CrossRef] [Green Version]
- Nam, V.D.; Lim, M.T.; Lim, O. Study on auto-ignition characteristics of gasoline-biodiesel blend fuel in a rapid compression expansion machine. Energy Procedia 2017, 105, 1789–1795. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. A study on combustion and emission of GCI engines fueled with gasoline-biodiesel blends. Fuel 2017, 189, 141–154. [Google Scholar] [CrossRef]
- Adams, C.A.; Loeper, P.; Krieger, R.; Andrie, M.J.; Foster, D.E. Effects of biodiesel–gasoline blends on gasoline direct-injection compression ignition (GCI) combustion. Fuel 2013, 111, 784–790. [Google Scholar] [CrossRef]
- Subramaniam, M.; Solomon, J.M.; Nadanakumar, V.; Anaimuthu, S.; Sathyamurthy, R. Experimental investigation on performance, combustion and emission characteristics of DI diesel engine using algae as a biodiesel. Energy Rep. 2020, 6, 1382–1392. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B.K. Numerical prediction of the performance, combustion and emission characteristics of a CI engine using different biodiesels. Clean. Technol. Environ. 2018, 8, 1773–1790. [Google Scholar] [CrossRef]
- Muralidharan, K.; Vasudevan, D. Performance, emission and combustion characteristics of a variable compression ratio engine using methyl esters of waste cooking oil and diesel blends. Appl. Energy 2011, 88, 3959–3968. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Agudelo, J.R. Diesel particulate emissions from used cooking oil biodiesel. Bioresour. Technol. 2008, 99, 731–740. [Google Scholar] [CrossRef]
- Xuan, T.; Cao, J.; He, Z.; Wang, Q.; Zhong, W.; Leng, X.; Li, D.; Shang, W. A study of soot quantification in diesel flame with hydrogenated catalytic biodiesel in a constant volume combustion chamber. Energy 2018, 145, 691–699. [Google Scholar] [CrossRef]
- Derick, A.; Mei, D.; Zuo, L.; Zhang, Q.; Wang, J. A review on partial hydrogenation of biodiesel and its influence on fuel property. Fuel 2019, 251, 660–668. [Google Scholar]
- Ilya, G.; Ritvik, S.; Xuesong, Z.; César, I.R.; L, G.K.; Philip, R.G. Sustainable bioenergy production from marginal lands in the US Midwest. Nature 2013, 493, 514–517. [Google Scholar]
- Zhan, L. Optimization Study of Combustion and Emission Characteristics for a Compression Ignition Engine Fueled by Gasoline/Hydrogenated Catalytic Biodiesel Blends. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2020. [Google Scholar]
- Cremonez, P.A.; Feroldi, M.; de Araújo, A.V.; Borges, M.N.; Meier, T.W.; Feiden, A.; Teleken, J.G. Biofuels in Brazilian aviation: Current scenario and prospects. Renew. Sustain. Energy Rev. 2015, 43, 1063–1072. [Google Scholar] [CrossRef]
- Liu, G.; Shen, H.; Qu, H.; Zhang, X.; Mi, Z. Chemical composition-property relation of jet fuels. J. Fuel Chem. Technol. 2007, 6, 737–742. [Google Scholar]
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Q.; Guan, Q.; He, L.; Li, W. Bio-aviation fuel production from hydroprocessing castor oil promoted by the nickel-based bifunctional catalysts. Bioresour. Technol. 2015, 183, 93–100. [Google Scholar] [CrossRef]
- Wang, T.; Li, K.; Liu, Q.; Zhang, Q.; Qiu, S.; Long, J.; Chen, L.; Ma, L.; Zhang, Q. Aviation fuel synthesis by catalytic conversion of biomass hydrolysate in aqueous phase. Appl. Energy 2014, 136, 775–780. [Google Scholar]
- Zhang, C.; Hui, X.; Lin, Y.; Sung, C. Recent development in studies of alternative jet fuel combustion: Progress, challenges, and opportunities. Renew. Sustain. Energy Rev. 2016, 54, 120–138. [Google Scholar] [CrossRef] [Green Version]
- Bester, N.; Yates, A. Assessment of the operational performance of Fischer-Tropsch synthetic-paraffinic kerosene in a T63 gas turbine compared to conventional jet A-1 fuel. In Proceedings of the ASME Turbo Expo 2009: Power for Land, Sea and Air, Orlando, FL, USA, 8–12 June 2009. [Google Scholar]
- Bulzan, D.; Anderson, B.; Wey, C.; Howard, R.; Winstead, E.; Beyersdorf, A.; Et, A. Gaseous and particulate emissions results of the NASA Alternative Aviation Fuel Experiment (AAFEX). In Proceedings of the ASME Turbo Expo 2010: Power for Land, Sea and Air, Glasgow, Scotland, 14–18 June 2010. [Google Scholar]
- Klingshirn, C.D.; Dewitt, M.J.; Striebich, R.; Anneken, D.; Brigalli, D. Hydroprocessed renewable jet fuel evaluation, performance, and emissions in a T63 turbine engine. In Proceedings of the ASME Turbo Expo 2011: Turbine Technical Conference & Exposition, Vancouver, BC, Canada, 6–10 June 2011. [Google Scholar]
- Khandelwal, B.; Roy, S.; Lord, C.; Blakey, S. Comparison of vibrations and emissions of conventional jet fuel with stressed 100% SPK and fully formulated synthetic jet fuel. Aerospace 2014, 1, 52–66. [Google Scholar] [CrossRef]
- Badami, M.; Nuccio, P.; Pastrone, D.; Signoretto, A. Performance of a small-scale turbojet engine fed with traditional and alternative fuels. Energy Convers. Manag. 2014, 82, 219–228. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Jiang, J.; Liu, P.; Zhai, Q.; Wang, F. A Method of Preparing Bio-Jet Fuel via Thermochemical Conversion of Triglyceride. China Patent CN201711052081.X, 27 March 2018. [Google Scholar]
- Li, F.; Jiang, J.; Liu, P.; Zhai, Q.; Wang, F.; Hse, C.; Xu, J. Catalytic cracking of triglycerides with a base catalyst and modification of pyrolytic oils for production of aviation fuels. Sustain. Energy Fuels 2018, 2, 1206–1215. [Google Scholar] [CrossRef]
- Li, F. Study on the Preparation of Aviation Hydrocarbon Fuel via Thermochemical Conversion of Triglycerides. Master’s Thesis, Chinese Academy of Forestry, Nanjing, China, 2018. [Google Scholar]
- Xu, J.; Long, F.; Jiang, J.; Li, F.; Zhai, Q.; Wang, F.; Liu, P.; Li, J. Integrated catalytic conversion of waste triglycerides to liquid hydrocarbons for aviation biofuels. J. Clean. Prod. 2019, 222, 784–792. [Google Scholar] [CrossRef]
- Yang, F. Study on the Basic Characteristics of Bio-Aviation Kerosene. Master’s Thesis, Nanjing University of Aeronautics and Astronautics, Nanjing, China, 2019. [Google Scholar]
- Guo, L.; Hao, K.; Wen, Y.; Wang, D.; Fu, Z.; Xu, Z. Chemical composition analysis of commercial jet fuel. Chin. J. Anal. Lab. 2021, 3, 330–335. [Google Scholar]
- Zhang, L. Study on the Quantitative Mapping Relationship from Fuel Structure Molecular to Combustion Characteristic Parameters. Master’s Thesis, Jilin University, Changchun, China, 2019. [Google Scholar]
- Rui, L.; Sheng, J.; Ma, J.; Yang, G.; Dong, X.; Liang, Y. Knock combustion investigation on a two-stroke spark ignition UAV engine burning RP-3 kerosene fuel. Aircr. Eng. Aerosp. Technol. 2019, 10, 1278–1284. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).