Hydrogen Production as a Clean Energy Carrier through Heterojunction Semiconductors for Environmental Remediation
Abstract
:1. Introduction
1.1. Hydrogen as a Clean Energy Carrier
1.2. Hydrogen from Semiconductor-Based Photocatalysis
2. Materials and Methods
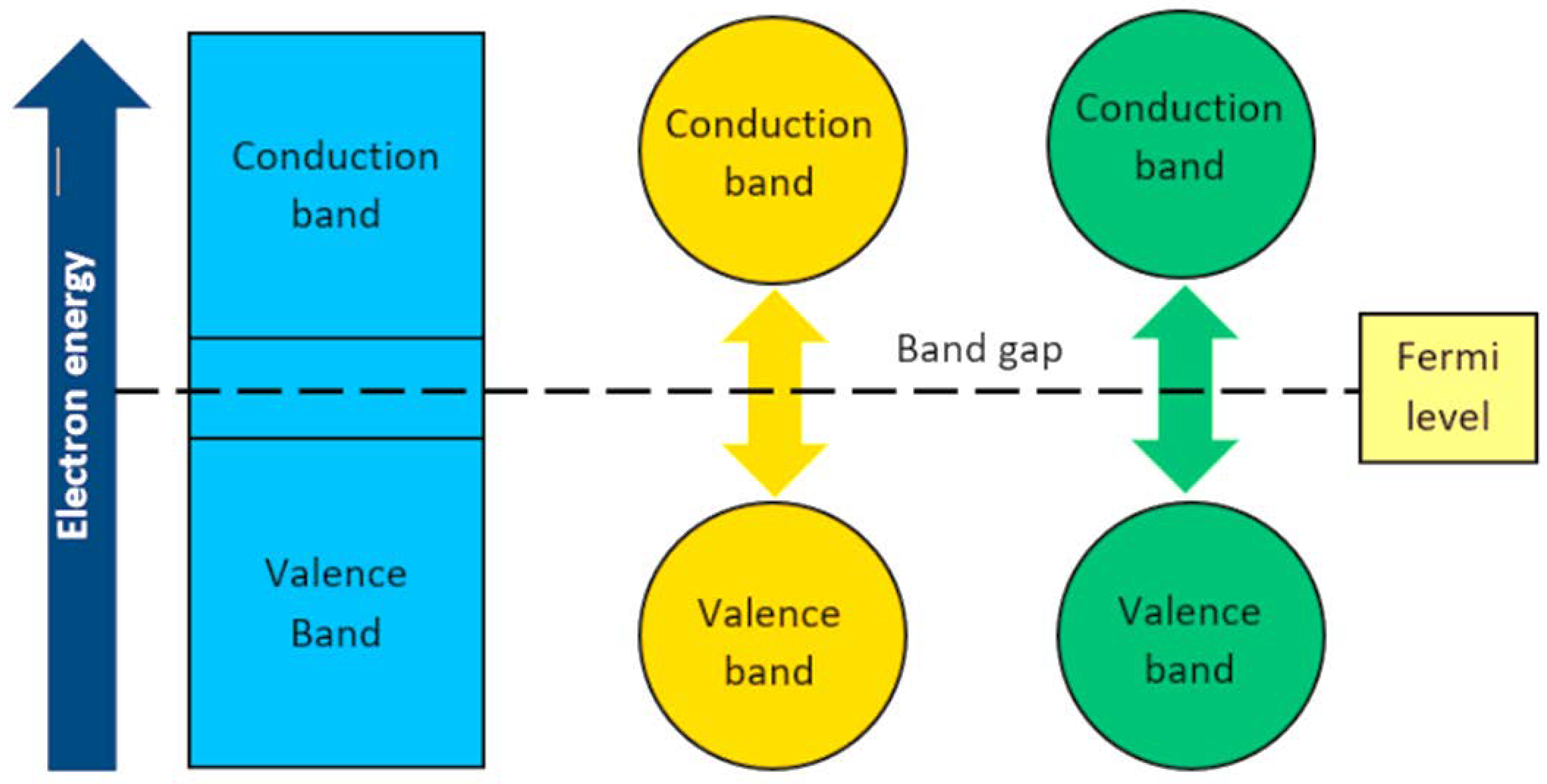
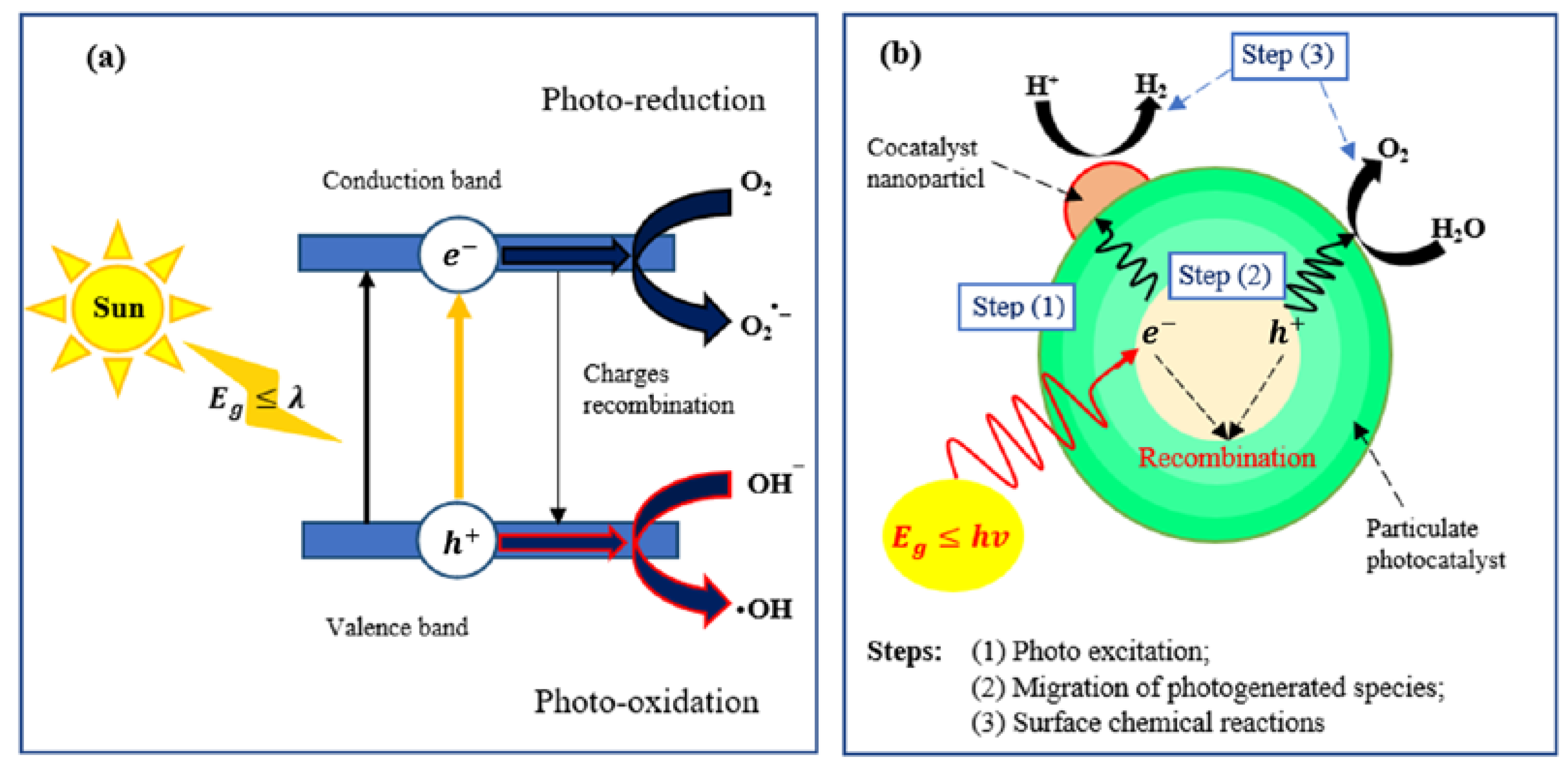
2.1. Semiconductor Photocatalysis
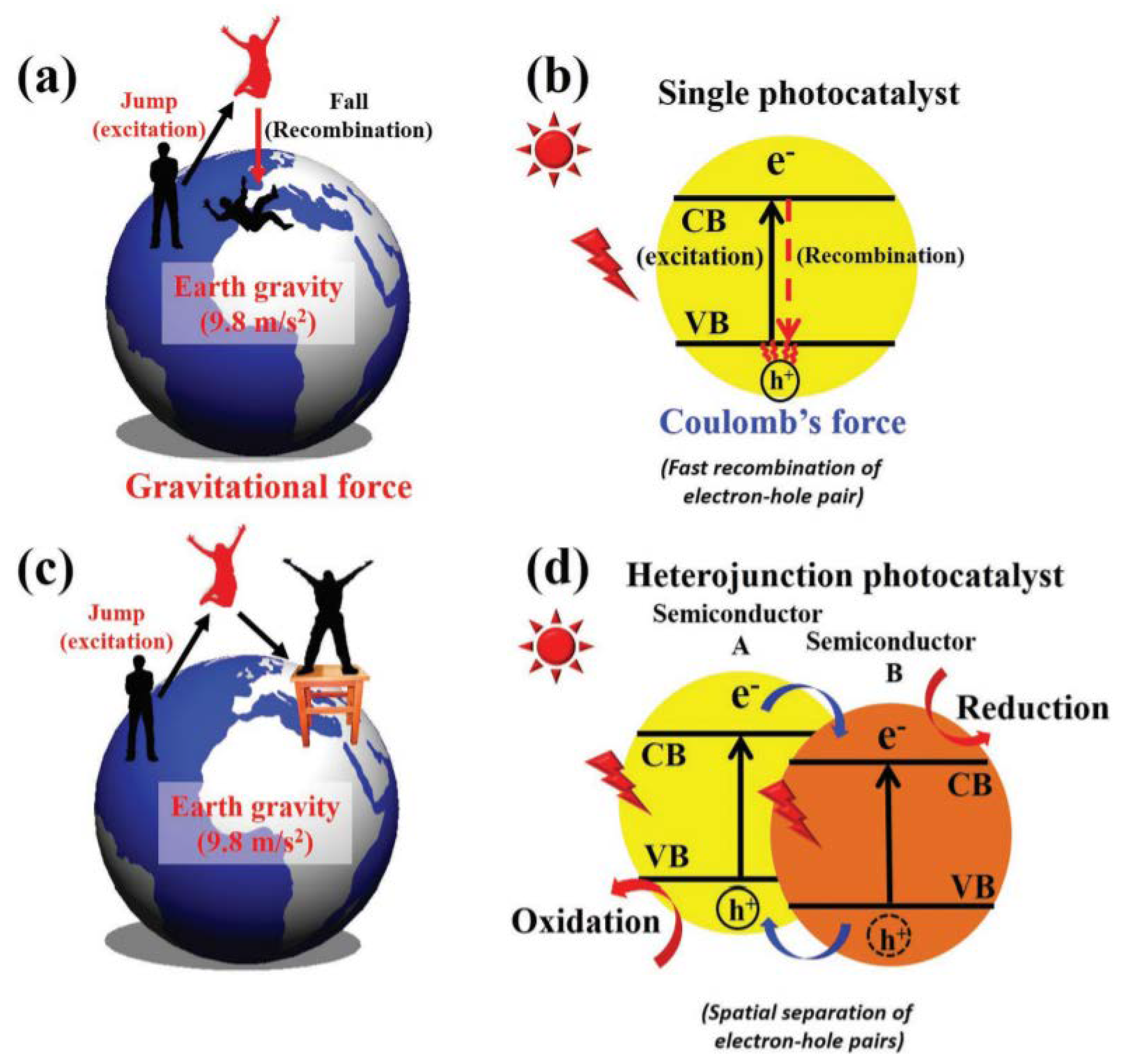
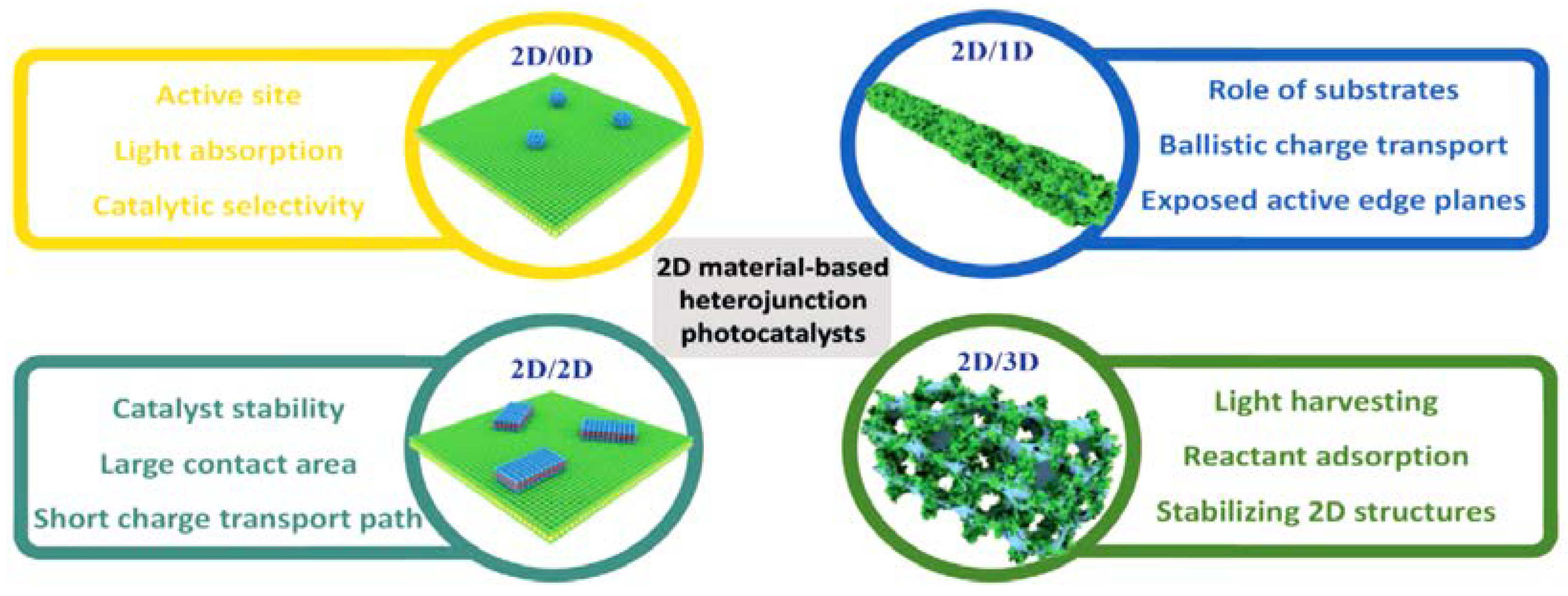
2.2. Semiconductor Heterojunctions
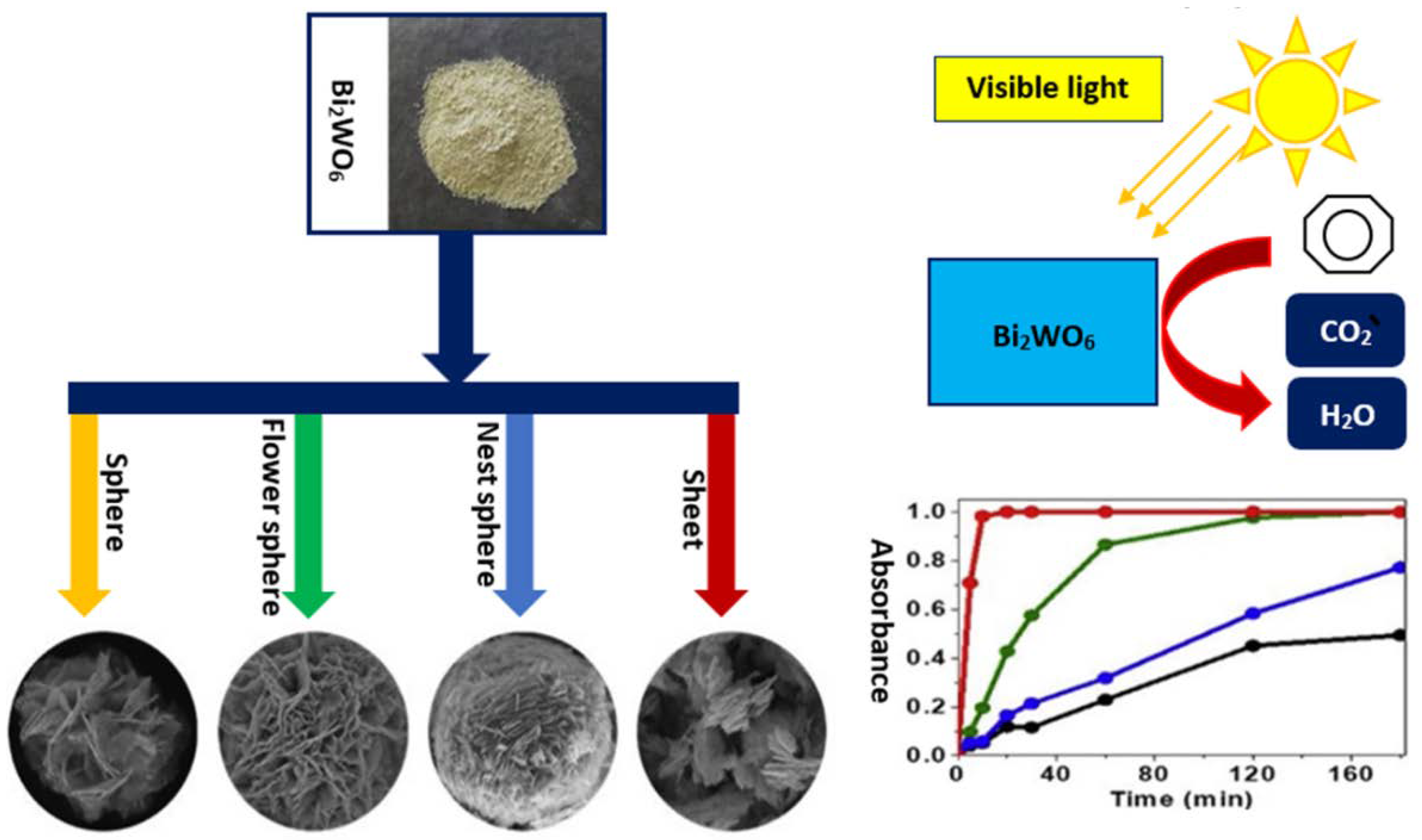
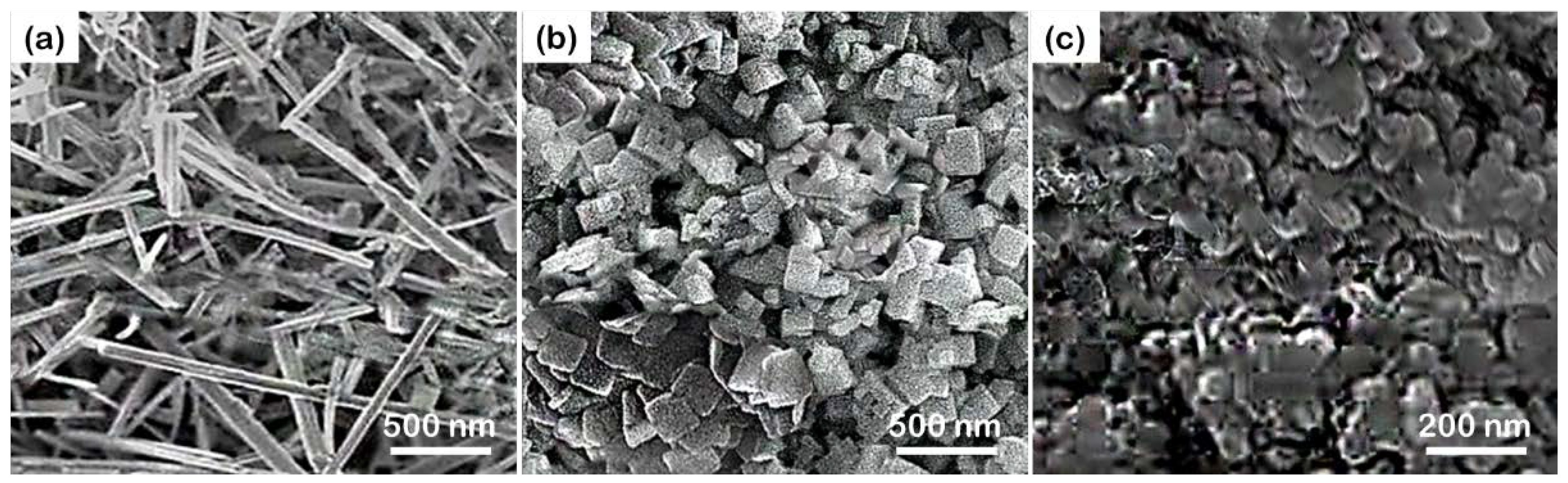
The Effect of Morphology in Heterojunctions
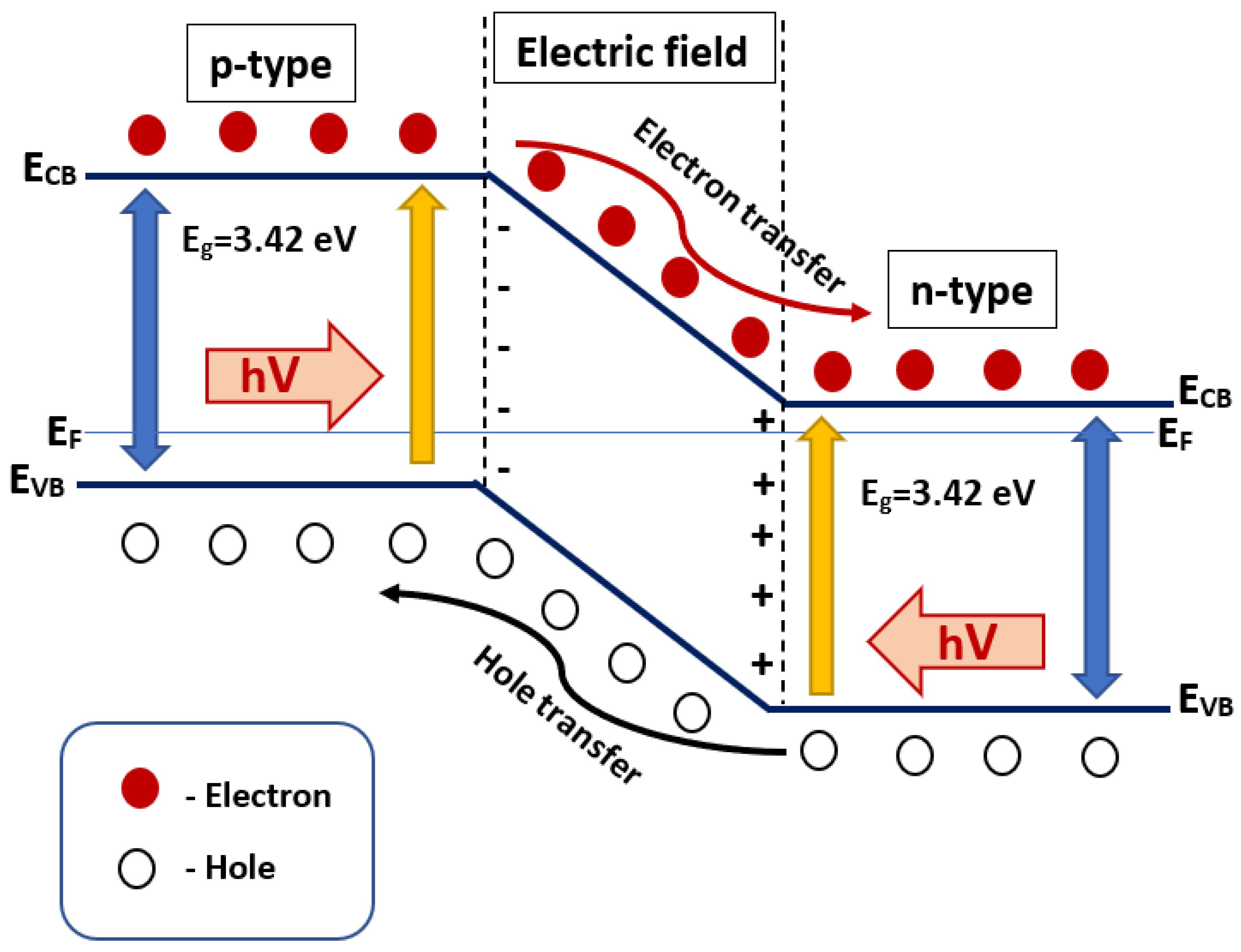
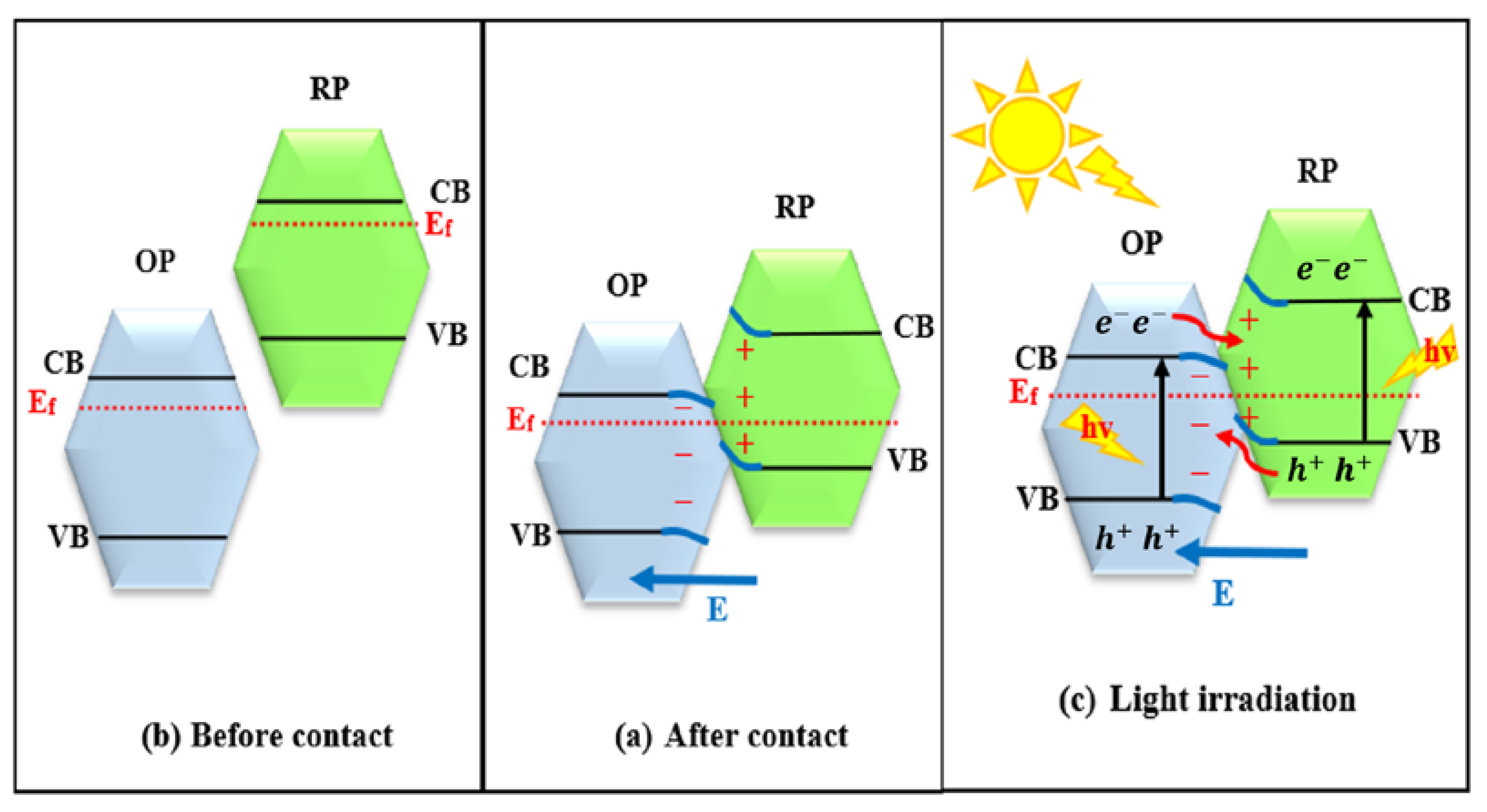
2.3. p-n Heterojunctions
2.4. Non p-n Heterojunctions
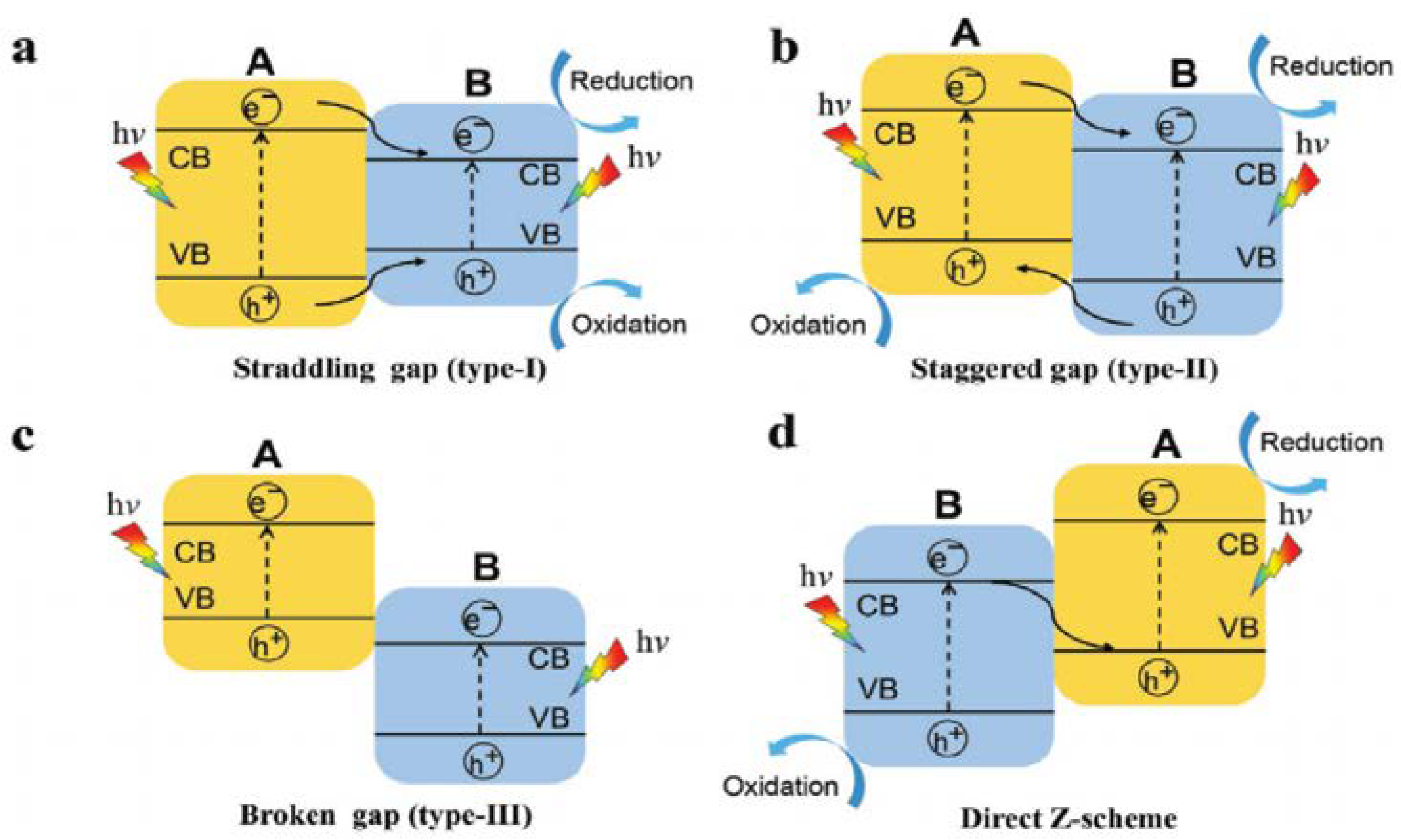
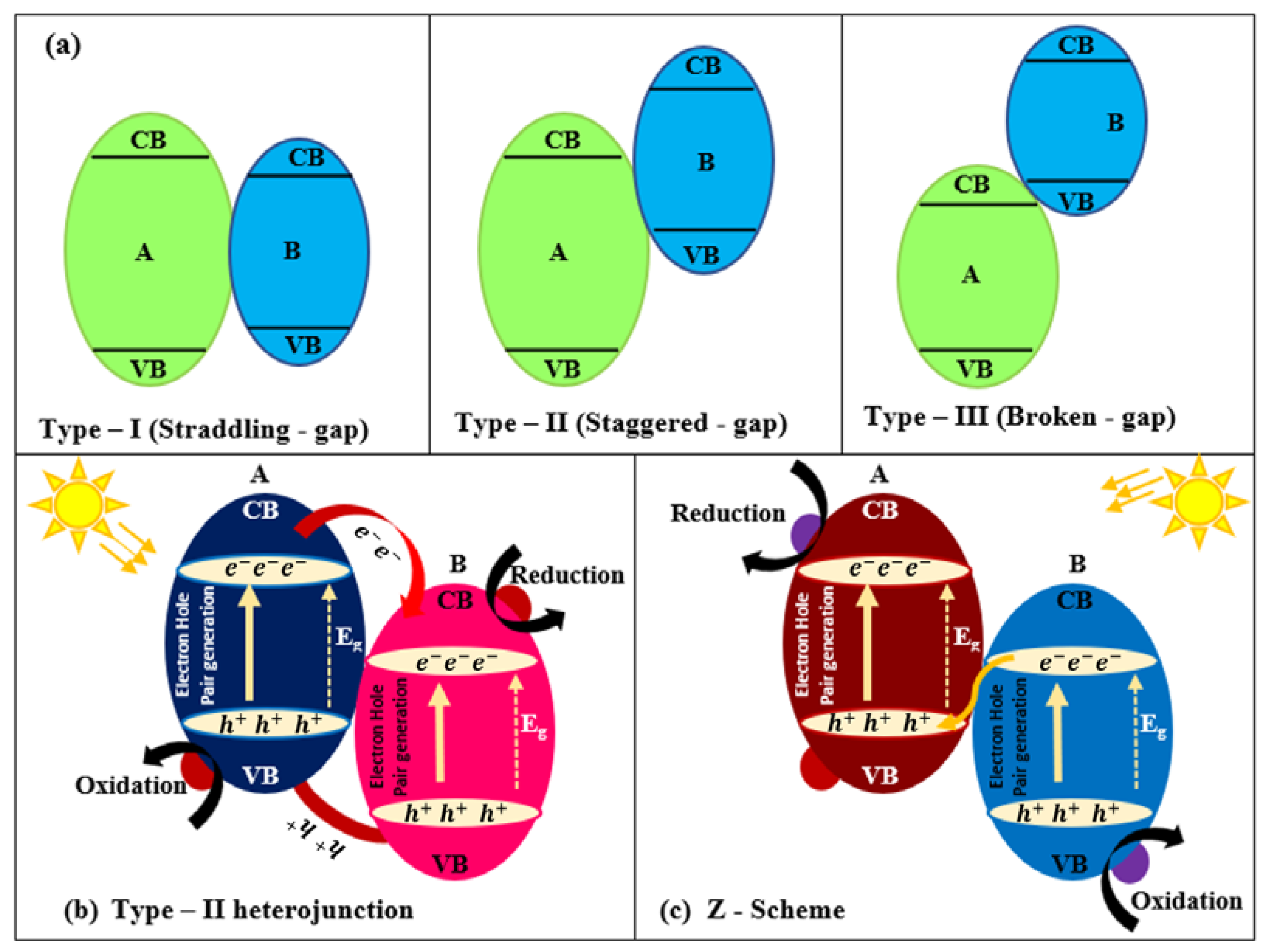
2.4.1. Type I: Heterojunctions Made by Visible-Light-Active and UV-Light-Active Components
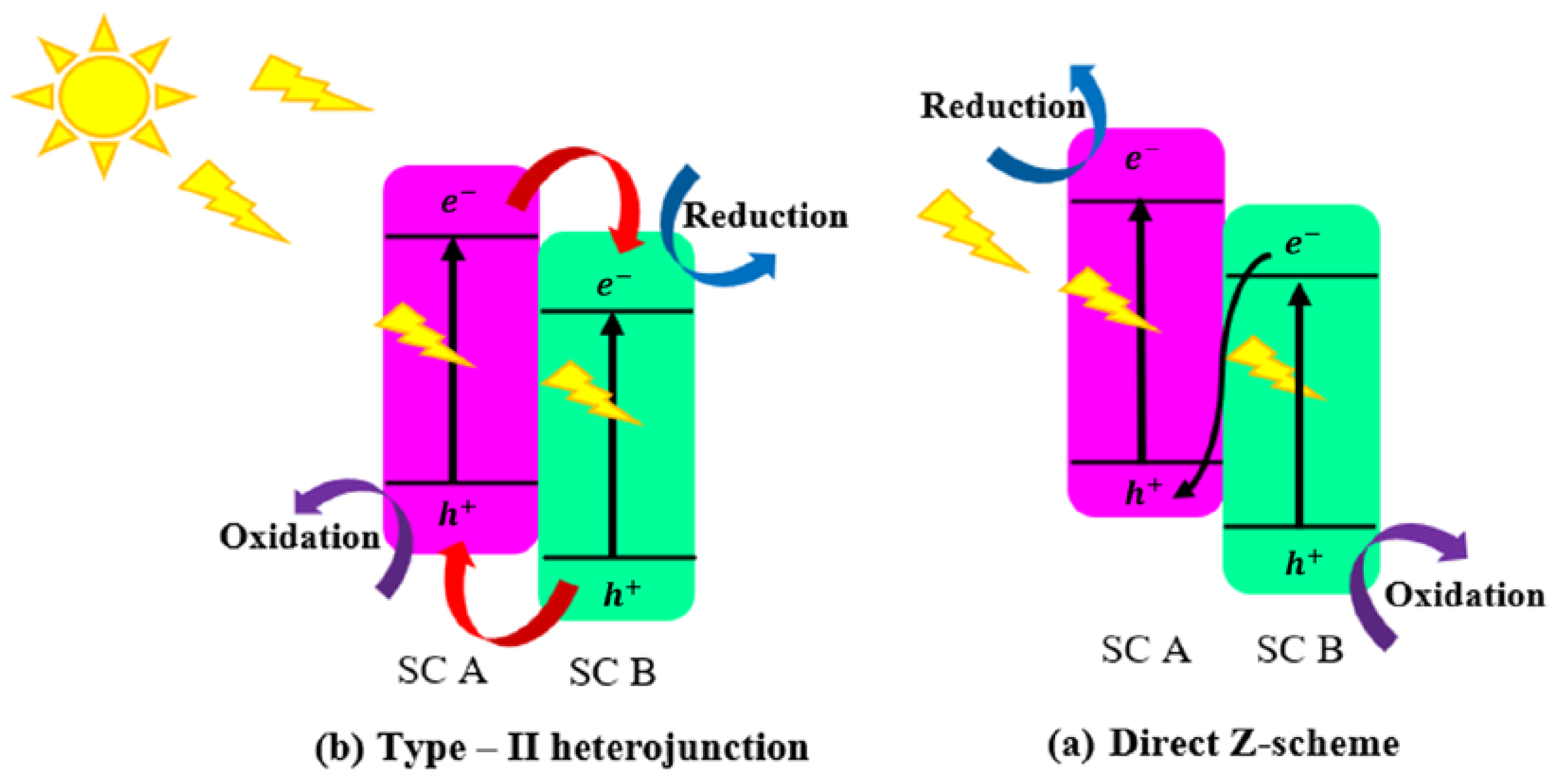
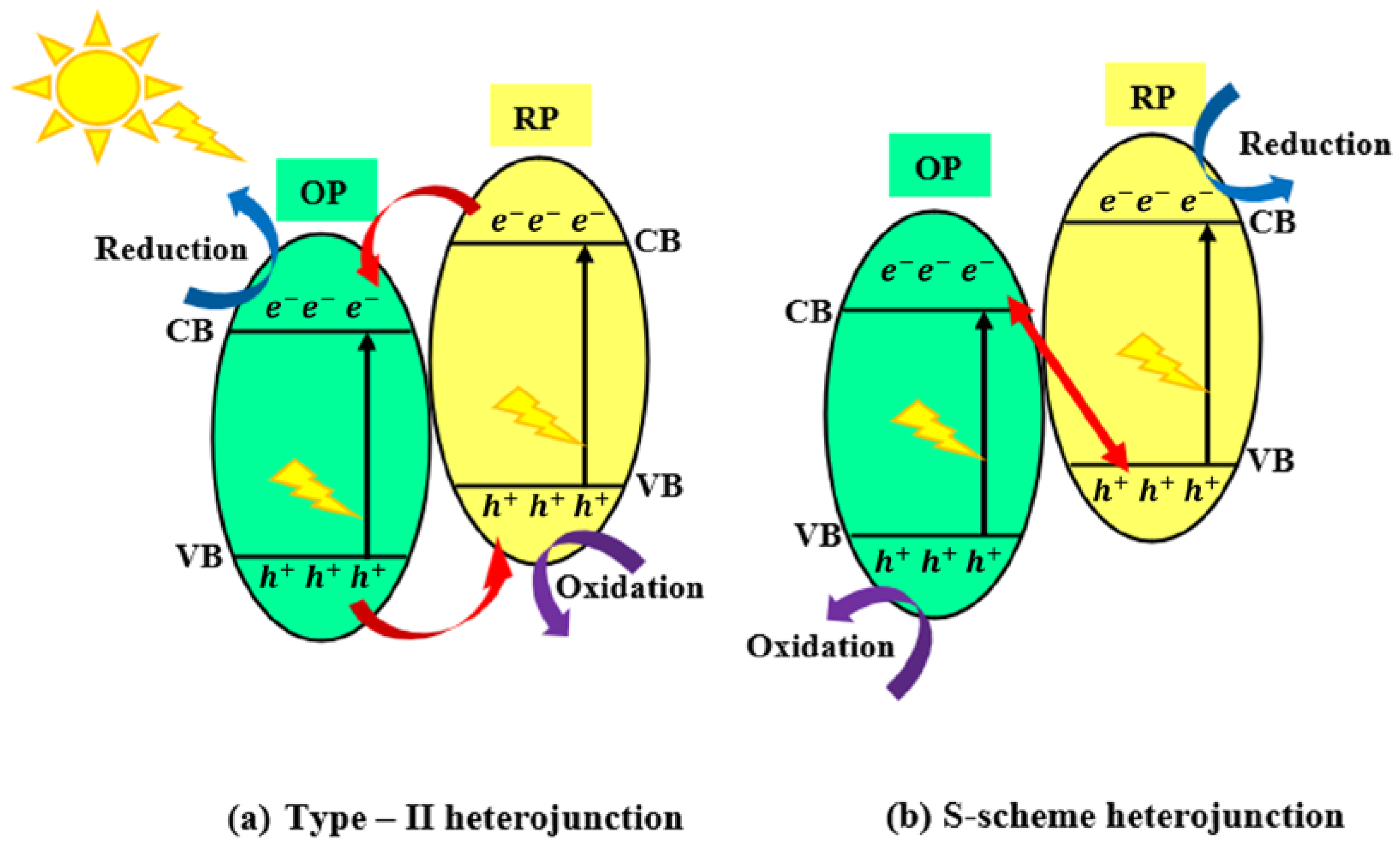
2.4.2. Type II: Heterojunctions Made by Two Visible-Light-Active Components
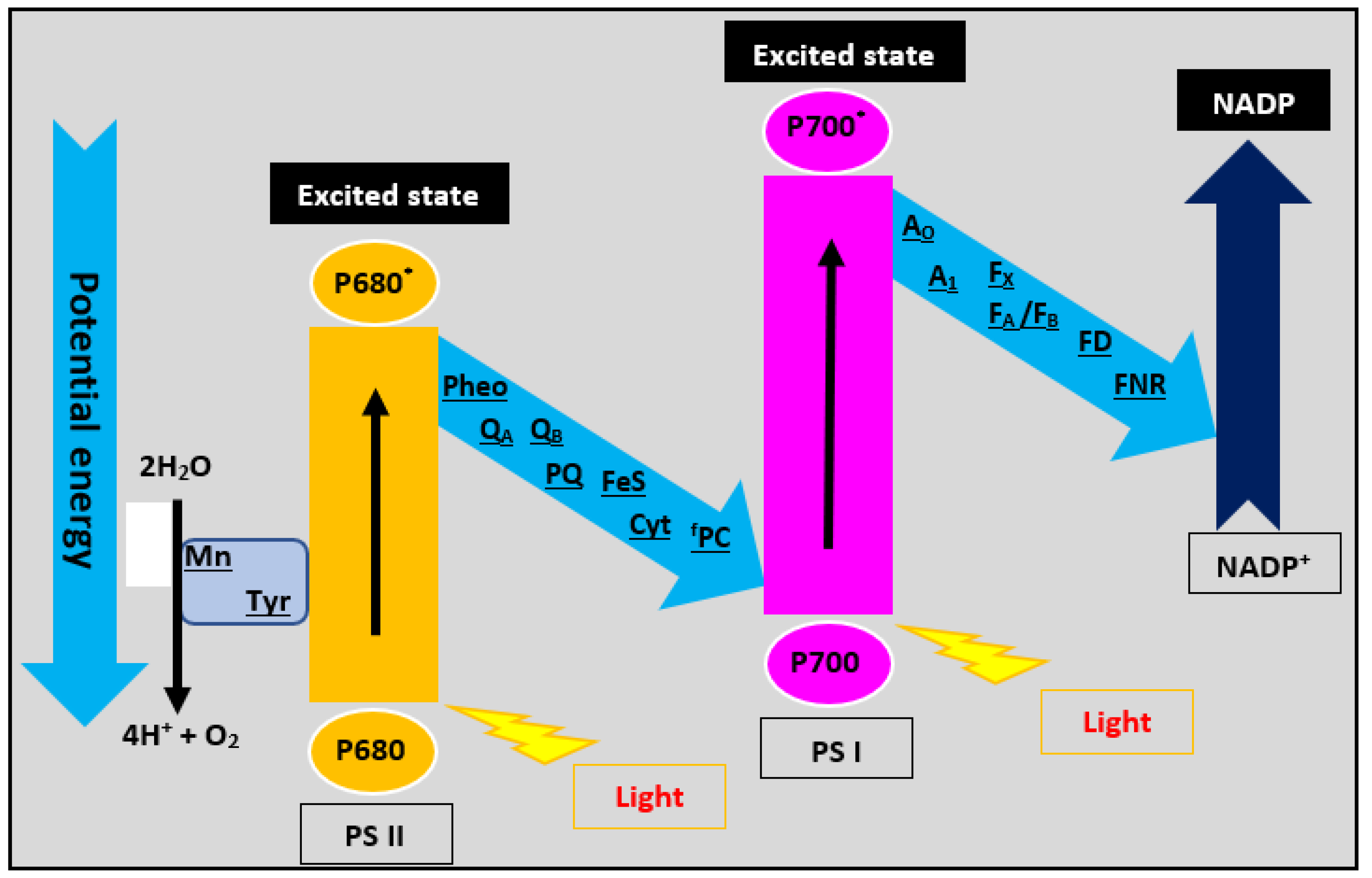
2.4.3. Type III: Heterojunctions with Z-Scheme Mechanism
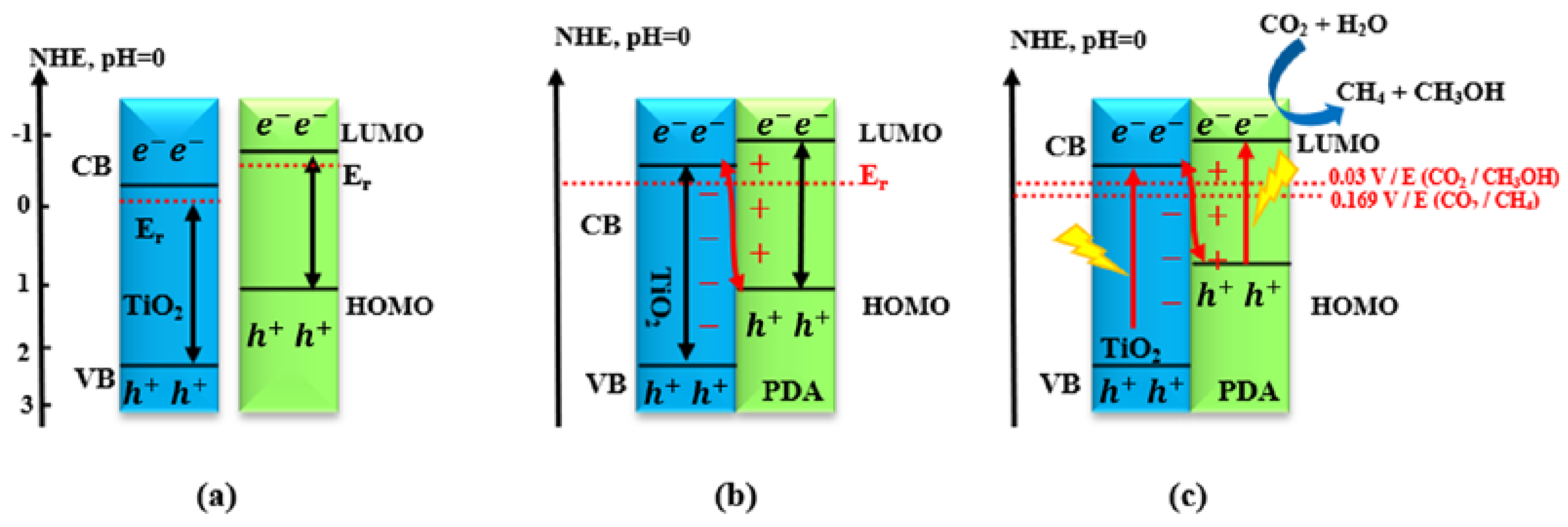
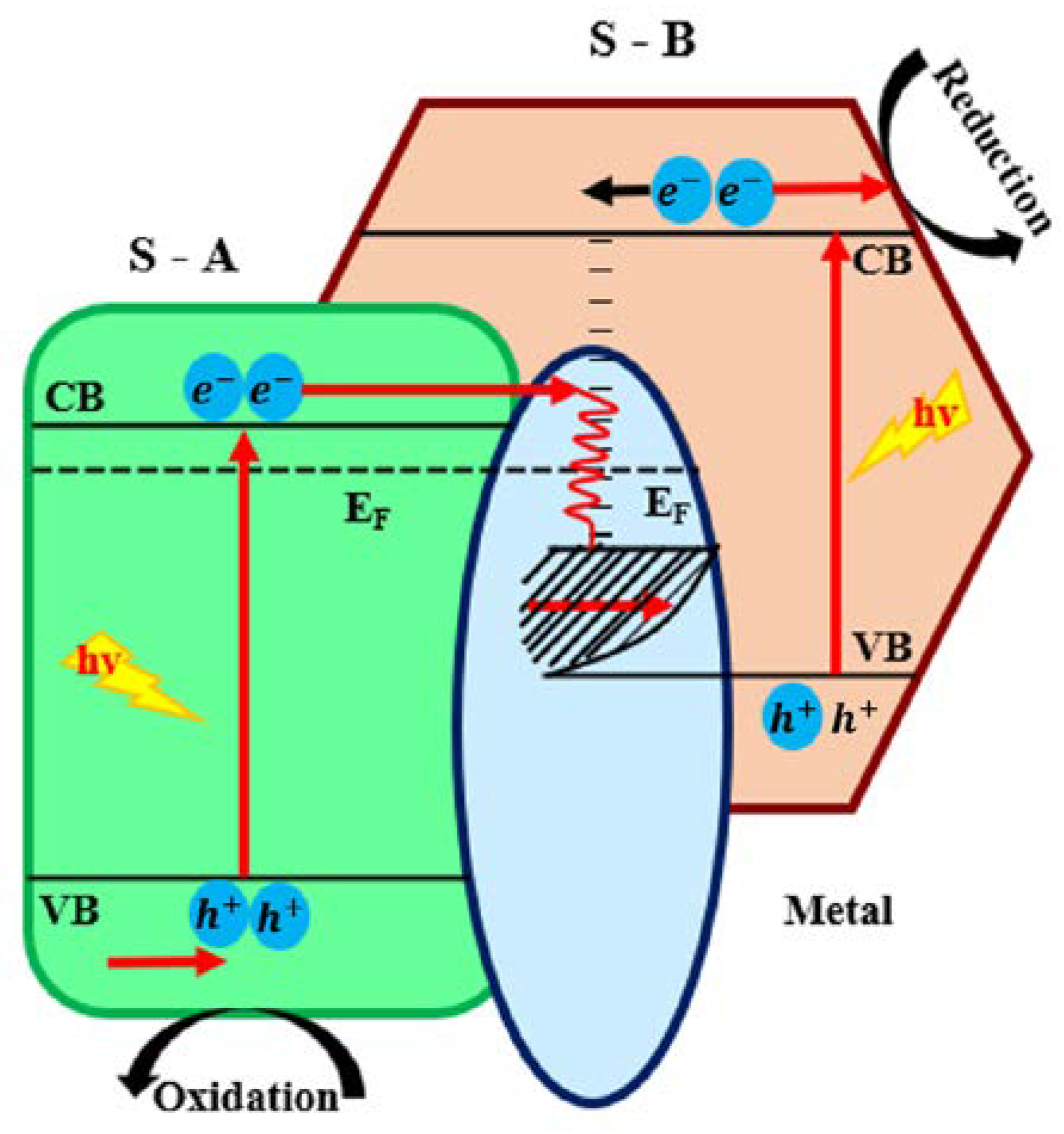
2.4.4. Type IV: Heterojunctions with S-Scheme Mechanism
2.5. Multi-Component, Hybrid, and Other Semiconductor Heterojunctions
3. Conclusions and Future Prospective
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Victoria, M.; Haegel, N.; Peters, I.M.; Sinton, R.; Jäger-Waldau, A.; del Cañizo, C.; Breyer, C.; Stocks, M.; Blakers, A.; Kaizuka, I.; et al. Solar photovoltaics is ready to power a sustainable future. Joule 2021, 5, 1041–1056. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Olabi, A.G.; Bahri, A.S.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrogen Energy 2021, 46, 23498–23528. [Google Scholar] [CrossRef]
- Li, S.; Kang, Q.; Baeyens, J.; Zhang, H.L.; Deng, Y.M. Hydrogen Production: State of Technology. IOP Conf. Ser. Earth Environ. Sci. 2020, 544, 012011. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Hong, S.; Kumar, D.P.; Reddy, D.A.; Choi, J.; Kim, T.K. Excellent photocatalytic hydrogen production over CdS nanorods via using noble metal-free copper molybdenum sulfide (Cu2MoS4) nanosheets as co-catalysts. Appl. Surf. Sci. 2017, 396, 421–429. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Li, Y.; Fan, J.; Lv, K. MXenes as noble-metal-alternative co-catalysts in photocatalysis. Chin. J. Catal. 2021, 42, 3–14. [Google Scholar] [CrossRef]
- Modak, B.; Ghosh, S.K. Improving visible light photocatalytic activity of KTaO3 using cation-anion dopant pair. Sol. Energy Mater. Sol. Cells 2017, 159, 590–598. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, T.; Liu, Q.; Zhang, Z.; Fang, X. Insight into the Enhanced Photocatalytic Activity of Potassium and Iodine Codoped Graphitic Carbon Nitride Photocatalysts. J. Phys. Chem. C 2016, 120, 25328–25337. [Google Scholar] [CrossRef]
- Li, A.; Chang, X.; Huang, Z.; Li, C.; Wei, Y.; Zhang, L.; Wang, T.; Gong, J. Thin Heterojunctions and Spatially Separated Cocatalysts To Simultaneously Reduce Bulk and Surface Recombination in Photocatalysts. Angew. Chemie Int. Ed. 2016, 55, 13734–13738. [Google Scholar] [CrossRef]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically Ordered Macro−Mesoporous TiO2−Graphene Composite Films: Improved Mass Transfer, Reduced Charge Recombination, and Their Enhanced Photocatalytic Activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef]
- Xing, X.; Tang, S.; Hong, H.; Jin, H. Concentrated solar photocatalysis for hydrogen generation from water by titania-containing gold nanoparticles. Int. J. Hydrogen Energy 2020, 45, 9612–9623. [Google Scholar] [CrossRef]
- Lavorato, C.; Primo, A.; Molinari, R.; Garcia, H. N-Doped Graphene Derived from Biomass as a Visible-Light Photocatalyst for Hydrogen Generation from Water/Methanol Mixtures. Chem. Eur. J. 2014, 20, 187–194. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Overview of Photocatalytic Membrane Reactors in Organic Synthesis, Energy Storage and Environmental Applications. Catalysts 2019, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Ayodhya, D.; Veerabhadram, G. A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy 2018, 9, 83–113. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Philippe, L. Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater. Catalysts 2019, 9, 974. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Shaeri, M.H.; Gode, C.; Armoon, H.; Shamsborhan, M. The synergistic effect of dilute alloying and nanostructuring of copper on the improvement of mechanical and tribological response. Compos. Part B Eng. 2019, 164, 508–516. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Shamsborhan, M. Monotonic and dynamic mechanical properties of PTCAE aluminum. J. Alloys Compd. 2017, 705, 28–37. [Google Scholar] [CrossRef]
- Ichikawa, S.; Doi, R. Hydrogen production from water and conversion of carbon dioxide to useful chemicals by room temperature photoelectrocatalysis. Catal. Today 1996, 27, 271–277. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Chen, Y.; Zhuang, Z.; Chen, F.-F.; Zhu, Y.-J.; Yu, Y. Recycling heavy metals from wastewater for photocatalytic CO2 reduction. Chem. Eng. J. 2020, 402, 125922. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, Y.; Ke, X.; Tong, X.; Sun, Z.; Liang, M.; Xue, S. Photocatalytic reduction of carbon dioxide to formic acid, formaldehyde, and methanol using dye-sensitized TiO2 film. Appl. Catal. B Environ. 2013, 129, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Pietro, A.; Cristina, L.; Raffaele, M. Hydrogen Production and Organic Synthesis in Photocatalytic Membrane Reactors: A Review. Int. J. Membr. Sci. Technol. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-Light Photocatalysts and Their Perspectives for Building Photocatalytic Membrane Reactors for Various Liquid Phase Chemical Conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Nie, J.; Dewil, R.; Baeyens, J.; Deng, Y. The Direct Reduction of Iron Ore with Hydrogen. Sustainability 2021, 13, 8866. [Google Scholar] [CrossRef]
- Lu, N.; Jing, X.; Zhang, J.; Zhang, P.; Qiao, Q.; Zhang, Z. Photo-assisted self-assembly synthesis of all 2D-layered heterojunction photocatalysts with long-range spatial separation of charge-carriers toward photocatalytic redox reactions. Chem. Eng. J. 2022, 431, 134001. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Huang, H. Junction Engineering for Photocatalytic and Photoelectrocatalytic CO2 Reduction. Sol. RRL 2021, 5, 2000430. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Xing, D.; Wang, J.; Zheng, L.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; et al. 2D/2D heterostructure of ultrathin BiVO4/Ti3C2 nanosheets for photocatalytic overall Water splitting. Appl. Catal. B Environ. 2021, 285, 119855. [Google Scholar] [CrossRef]
- Tang, M.; Ao, Y.; Wang, C.; Wang, P. Rationally constructing of a novel dual Z-scheme composite photocatalyst with significantly enhanced performance for neonicotinoid degradation under visible light irradiation. Appl. Catal. B Environ. 2020, 270, 118918. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Zhang, Y.; Tian, N.; Ma, T.; Huang, H. Inside-and-Out Semiconductor Engineering for CO2 Photoreduction: From Recent Advances to New Trends. Small Struct. 2021, 2, 2000061. [Google Scholar] [CrossRef]
- Djavanroodi, F.; Ebrahimi, M.; Nayfeh, J.F. Tribological and mechanical investigation of multi-directional forged nickel. Sci. Rep. 2019, 9, 241. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Y.; Zhu, X.; Fang, J.; Xu, W.; Hu, X.; Li, R.; Yao, L.; Qin, J.; Fang, Z. Semiconductor heterojunctions for photocatalytic hydrogen production and Cr(VI) Reduction: A review. Mater. Res. Bull. 2022, 147, 111636. [Google Scholar] [CrossRef]
- Peroulis, D.; Waghmare, P.R.; Mitra, S.K.; Manakasettharn, S.; Taylor, J.A.; Krupenkin, T.N.; Zhu, W.; Nessim, G.D.; Marano, F.; Guadagnini, R.; et al. Conduction Mechanisms in Organic Semiconductors. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2012; pp. 493–500. [Google Scholar]
- Ali, R.H. New Trends for Removal of Water Pollutants. In Prime Archives in Material Science; Heimann, R.B., Ed.; Vide Leaf, Hyderabad: Hyderabad, India, 2020. [Google Scholar]
- Kisch, H. Semiconductor Photocatalysis-Mechanistic and Synthetic Aspects. Angew. Chemie Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tadé, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef]
- Böer, K.W. Semiconductor Heterojunctions. In Survey of Semiconductor Physics; Springer Netherlands: Dordrecht, The Netherlands, 1992; pp. 676–700. [Google Scholar]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Su, Q.; Li, Y.; Hu, R.; Song, F.; Liu, S.; Guo, C.; Zhu, S.; Liu, W.; Pan, J. Heterojunction Photocatalysts Based on 2D Materials: The Role of Configuration. Adv. Sustain. Syst. 2020, 4, 2000130. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, Y.; Cao, H.; Sun, J.; Xu, Q.; Wang, L. Insight into the influence of morphology of Bi2WO6 for photocatalytic degradation of VOCs under visible light. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 568, 327–333. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Hu, Y.; Chen, Y.; Liu, W.; Yuan, R.; Li, Z. A Templated Method to Bi2WO6 Hollow Microspheres and Their Conversion to Double-Shell Bi2O3/Bi2WO6 Hollow Microspheres with Improved Photocatalytic Performance. Inorg. Chem. 2012, 51, 6245–6250. [Google Scholar] [CrossRef]
- Farhadian, M.; Sangpour, P.; Hosseinzadeh, G. Morphology dependent photocatalytic activity of WO3 nanostructures. J. Energy Chem. 2015, 24, 171–177. [Google Scholar] [CrossRef]
- Mou, H.; Song, C.; Zhou, Y.; Zhang, B.; Wang, D. Design and synthesis of porous Ag/ZnO nanosheets assemblies as super photocatalysts for enhanced visible-light degradation of 4-nitrophenol and hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 565–573. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Shaeri, M.H.; Naseri, R.; Gode, C. Equal channel angular extrusion for tube configuration of Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2018, 731, 569–576. [Google Scholar] [CrossRef]
- Jing, X.; Lu, N.; Huang, J.; Zhang, P.; Zhang, Z. One-step hydrothermal synthesis of S-defect-controlled ZnIn2S4 microflowers with improved kinetics process of charge-carriers for photocatalytic H2 evolution. J. Energy Chem. 2021, 58, 397–407. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Pirhashemi, M.; Ghosh, S. ZnO/ZnBi2O4 nanocomposites with p-n heterojunction as durable visible-light-activated photocatalysts for efficient removal of organic pollutants. J. Alloys Compd. 2020, 826, 154229. [Google Scholar] [CrossRef]
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020, 8, 822. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Zhang, Y.; Tian, N. Novel BiIO4/BiVO4 composite photocatalyst with highly improved visible-light-induced photocatalytic performance for rhodamine B degradation and photocurrent generation. RSC Adv. 2015, 5, 1161–1167. [Google Scholar] [CrossRef]
- Dong, D.; Yan, C.; Huang, J.; Lu, N.; Wu, P.; Wang, J.; Zhang, Z. An electron-donating strategy to guide the construction of MOF photocatalysts toward co-catalyst-free highly efficient photocatalytic H2 evolution. J. Mater. Chem. A 2019, 7, 24180–24185. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Jiang, X.; Meng, S.; Fu, X. Fabrication and characterization of novel Z-scheme photocatalyst WO3/g-C3N4 with high efficient visible light photocatalytic activity. Mater. Chem. Phys. 2015, 149–150, 512–521. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Wang, X.; Wu, Y.; Lin, H.; Zhao, L.; Weng, W.; Wan, H.; Fan, M. Enhanced photodegradation activity of methyl orange over Z-scheme type MoO3–g-C3N4 composite under visible light irradiation. RSC Adv. 2014, 4, 13610–13619. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Katsumata, H.; Tachi, Y.; Suzuki, T.; Kaneco, S. Z-scheme photocatalytic hydrogen production over WO3/g-C3N4 composite photocatalysts. RSC Adv. 2014, 4, 21405–21409. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Ji, L.; Jiang, X.; Fu, X. Preparation and characterization of direct Z-scheme photocatalyst Bi2O3/NaNbO3 and its reaction mechanism. Appl. Surf. Sci. 2014, 292, 357–366. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; He, Y.; Guo, Y.; Zhang, T.; Zhang, Y. Mediator-free direct Z-scheme photocatalytic system: BiVO4/g-C3N4 organic–inorganic hybrid photocatalyst with highly efficient visible-light-induced photocatalytic activity. Dalt. Trans. 2015, 44, 4297–4307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, D.; Xing, C.; Chen, L.; Chen, M.; He, M. Novel AgI-decorated β-Bi2O3 nanosheet heterostructured Z-scheme photocatalysts for efficient degradation of organic pollutants with enhanced performance. Dalt. Trans. 2015, 44, 11582–11591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wen, Z.; Liu, J.; Ke, J.; Duan, X.; Wang, S. Z-scheme plasmonic Ag decorated WO3/Bi2WO6 hybrids for enhanced photocatalytic abatement of chlorinated-VOCs under solar light irradiation. Appl. Catal. B Environ. 2019, 242, 76–84. [Google Scholar] [CrossRef]
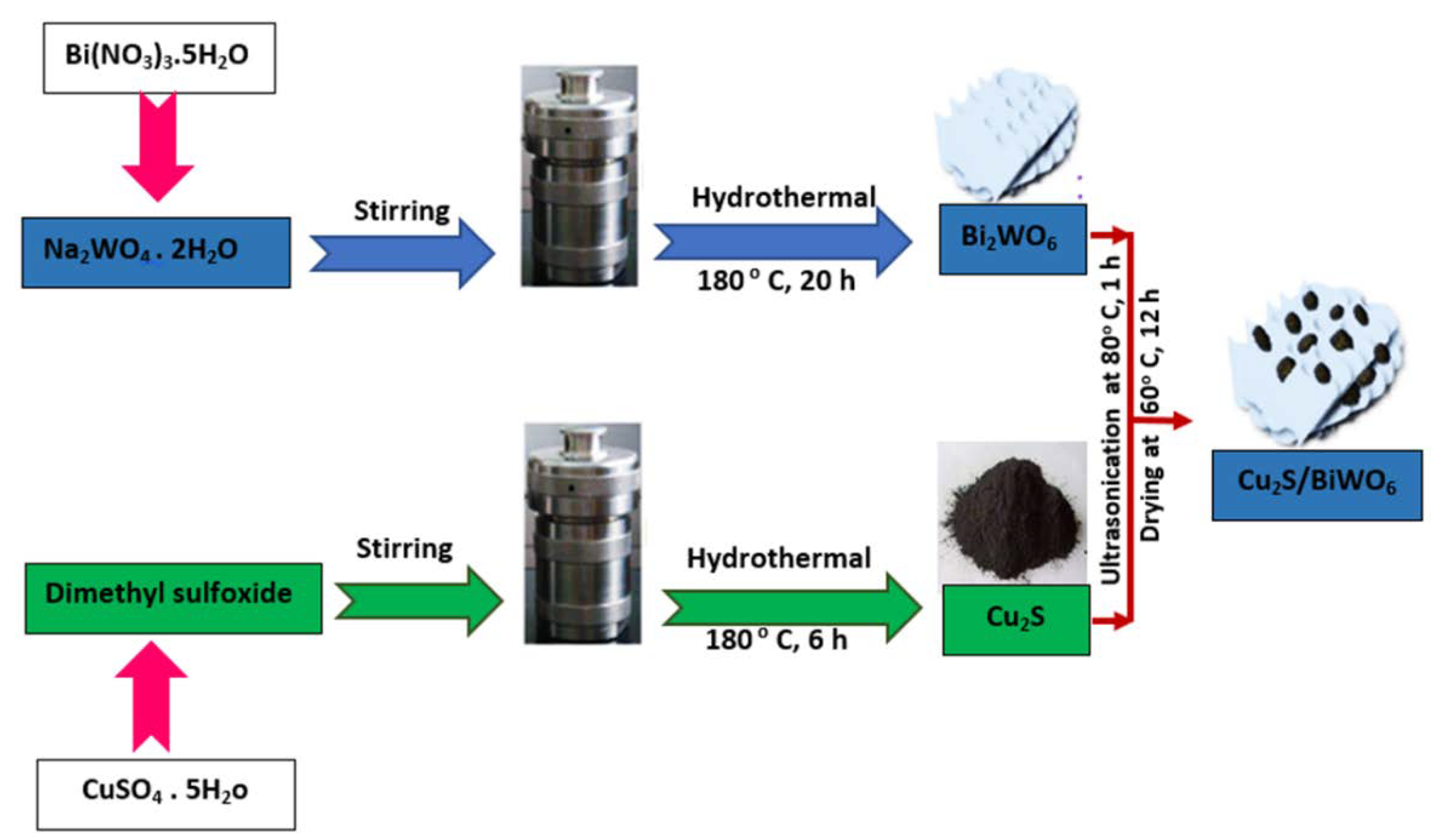
- Tang, Q.-Y.; Chen, W.-F.; Lv, Y.-R.; Yang, S.-Y.; Xu, Y.-H. Z-scheme hierarchical Cu2S/Bi2WO6 composites for improved photocatalytic activity of glyphosate degradation under visible light irradiation. Sep. Purif. Technol. 2020, 236, 116243. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Zhang, Q.; Tao, D. Mechanochemical synthesis of a Z-scheme Bi2WO6/CuBi2O4 heterojunction and its visible-light photocatalytic degradation of ciprofloxacin. J. Alloys Compd. 2020, 845, 156291. [Google Scholar] [CrossRef]
- Li, Q.; Lu, M.; Wang, W.; Zhao, W.; Chen, G.; Shi, H. Fabrication of 2D/2D g-C3N4/Au/Bi2WO6 Z-scheme photocatalyst with enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2020, 508, 144182. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Deng, F.; Luo, X.; Dionysiou, D.D. Rapid toxicity elimination of organic pollutants by the photocatalysis of environment-friendly and magnetically recoverable step-scheme SnFe2O4/ZnFe2O4 nano-heterojunctions. Chem. Eng. J. 2020, 379, 122264. [Google Scholar] [CrossRef]
- Mei, F.; Dai, K.; Zhang, J.; Li, W.; Liang, C. Construction of Ag SPR-promoted step-scheme porous g-C3N4/Ag3VO4 heterojunction for improving photocatalytic activity. Appl. Surf. Sci. 2019, 488, 151–160. [Google Scholar] [CrossRef]
- Jia, X.; Han, Q.; Zheng, M.; Bi, H. One pot milling route to fabricate step-scheme AgI/I-BiOAc photocatalyst: Energy band structure optimized by the formation of solid solution. Appl. Surf. Sci. 2019, 489, 409–419. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Zhang, W.; Liu, Q.; Zhang, M.; Zulfiqar; Tang, H. Build-in electric field induced step-scheme TiO2/W18O49 heterojunction for enhanced photocatalytic activity under visible-light irradiation. Ceram. Int. 2020, 46, 23–30. [Google Scholar] [CrossRef]
- Hu, T.; Dai, K.; Zhang, J.; Zhu, G.; Liang, C. One-pot synthesis of step-scheme Bi2S3/porous g-C3N4 heterostructure for enhanced photocatalytic performance. Mater. Lett. 2019, 257, 126740. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2-reduction selectivity. Appl. Catal. B Environ. 2021, 289, 120039. [Google Scholar] [CrossRef]
- Kumar, R.; Raizada, P.; Verma, N.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Van Le, Q.; Nguyen, V.-H.; Selvasembian, R.; Singh, P. Recent advances on water disinfection using bismuth based modified photocatalysts: Strategies and challenges. J. Clean. Prod. 2021, 297, 126617. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L. Photocatalytic Activity of S-Scheme Heterostructure for Hydrogen Production and Organic Pollutant Removal: A Mini-Review. Nanomaterials 2021, 11, 871. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A. Photocatalytic reduction and removal of mercury ions over mesoporous CuO/ZnO S-scheme heterojunction photocatalyst. Ceram. Int. 2021, 47, 9659–9667. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, L.; Wang, G.; Li, Y.; Wang, Y. Graphdiyne formed a novel CuI-GD/g-C3N4 S-scheme heterojunction composite for efficient photocatalytic hydrogen evolution. Sustain. Energy Fuels 2020, 4, 5088–5101. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0D/2D S-Scheme Heterojunction over Polymeric Carbon Nitride for Visible-Light Photocatalytic Inactivation of Bacteria. Angew. Chemie Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Meng, A.; Cheng, B.; Ho, W.; Yu, J. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Deng, H.; Fei, X.; Yang, Y.; Fan, J.; Yu, J.; Cheng, B.; Zhang, L. S-scheme heterojunction based on p-type ZnMn2O4 and n-type ZnO with improved photocatalytic CO2 reduction activity. Chem. Eng. J. 2021, 409, 127377. [Google Scholar] [CrossRef]
- Brabec, C.J.; Heeney, M.; McCulloch, I.; Nelson, J. Influence of blend microstructure on bulk heterojunction organic photovoltaic performance. Chem. Soc. Rev. 2011, 40, 1185–1199. [Google Scholar] [CrossRef] [Green Version]
- Vandewal, K.; Himmelberger, S.; Salleo, A. Structural Factors That Affect the Performance of Organic Bulk Heterojunction Solar Cells. Macromolecules 2013, 46, 6379–6387. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, X.; Zhang, X.; Chai, B.; Peng, T. Efficient photocatalytic hydrogen production over Ni@C/TiO2 nanocomposite under visible light irradiation. Chem. Phys. Lett. 2011, 503, 262–265. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Zeng, P.; Mao, J. Synthesis of floriated In2S3 decorated with TiO2 nanoparticles for efficient photocatalytic hydrogen production under visible light. J. Mater. Chem. 2011, 21, 14587. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting Efficient Charge Separation and Unprecedented Activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Martha, S.; Das, D.P.; Biswal, N.; Parida, K.M. Facile synthesis of visible light responsive V2O5/N,S–TiO2 composite photocatalyst: Enhanced hydrogen production and phenol degradation. J. Mater. Chem. 2012, 22, 10695. [Google Scholar] [CrossRef]
- Jang, J.S.; Hong, S.J.; Kim, J.Y.; Lee, J.S. Heterojunction photocatalyst TiO2/AgGaS2 for hydrogen production from water under visible light. Chem. Phys. Lett. 2009, 475, 78–81. [Google Scholar] [CrossRef]
- Zhu, S.; Yao, F.; Yin, C.; Li, Y.; Peng, W.; Ma, J.; Zhang, D. Fe2O3/TiO2 photocatalyst of hierarchical structure for H2 production from water under visible light irradiation. Microporous Mesoporous Mater. 2014, 190, 10–16. [Google Scholar] [CrossRef]
- Amirav, L.; Alivisatos, A.P. Photocatalytic Hydrogen Production with Tunable Nanorod Heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Lu, X.-H.; Xie, S.-L.; Zhai, T.; Zhao, Y.-F.; Zhang, P.; Zhang, Y.-L.; Tong, Y.-X. Monodisperse CeO2/CdS heterostructured spheres: One-pot synthesis and enhanced photocatalytic hydrogen activity. RSC Adv. 2011, 1, 1207. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, G.; Wang, Q.; Yu, Y.; Zhou, C.; Wang, Y. Sonochemistry synthesis and enhanced photocatalytic H2-production activity of nanocrystals embedded in CdS/ZnS/In2S3 microspheres. Nanoscale 2012, 4, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/Graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Dai, K.; Zhang, J.; Chen, S. Noble-metal-free Ni2P modified step-scheme SnNb2O6/CdS-diethylenetriamine for photocatalytic hydrogen production under broadband light irradiation. Appl. Catal. B Environ. 2020, 269, 118844. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Zhuang, C.; Peng, T.; Li, R.; Li, X. Highly Asymmetric Phthalocyanine as a Sensitizer of Graphitic Carbon Nitride for Extremely Efficient Photocatalytic H2 Production under Near-Infrared Light. ACS Catal. 2014, 4, 162–170. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Guo, P.; Wu, P.; Guo, L. Spatial engineering of photo-active sites on g-C3N4 for efficient solar hydrogen generation. J. Mater. Chem. A 2014, 2, 4605. [Google Scholar] [CrossRef]
- Kang, H.W.; Lim, S.N.; Song, D.; Park, S. Bin Organic-inorganic composite of g-C3N4–SrTiO3:Rh photocatalyst for improved H2 evolution under visible light irradiation. Int. J. Hydrogen Energy 2012, 37, 11602–11610. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Mao, J.; Li, K.; Zan, L. Graphitic carbon nitride (g-C3N4)–Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys. 2012, 14, 16745. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, T.W.; Kim, I.Y.; Hwang, S.-J. Cocatalyst-Free Photocatalysts for Efficient Visible-Light-Induced H2 Production: Porous Assemblies of CdS Quantum Dots and Layered Titanate Nanosheets. Adv. Funct. Mater. 2011, 21, 3111–3118. [Google Scholar] [CrossRef]
- Oskoui, M.S.; Khatamian, M.; Haghighi, M.; Yavari, A. Photocatalytic hydrogen evolution from water over chromosilicate-based catalysts. RSC Adv. 2014, 4, 19569. [Google Scholar] [CrossRef]
- Fang, J.; Xu, L.; Zhang, Z.; Yuan, Y.; Cao, S.; Wang, Z.; Yin, L.; Liao, Y.; Xue, C. Au@TiO2–CdS Ternary Nanostructures for Efficient Visible-Light-Driven Hydrogen Generation. ACS Appl. Mater. Interfaces 2013, 5, 8088–8092. [Google Scholar] [CrossRef]
- Bahadoran, A.; Masudy-Panah, S.; De Lile, J.R.; Li, J.; Gu, J.; Sadeghi, B.; Ramakrishna, S.; Liu, Q. Novel 0D/1D ZnBi2O4/ZnO S-scheme photocatalyst for hydrogen production and BPA removal. Int. J. Hydrogen Energy 2021, 46, 24094–24106. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, S.; Wang, D.; Zhang, C.; Sun, D. Ultrasound-assisted synthesis of high-efficiency Ag3PO4/CeO2 heterojunction photocatalyst. Ceram. Int. 2015, 41, 8956–8963. [Google Scholar] [CrossRef]
- Tho, N.T.M.; Khanh, D.N.N.; Thang, N.Q.; Lee, Y.-I.; Phuong, N.T.K. Novel reduced graphene oxide/ZnBi2O4 hybrid photocatalyst for visible light degradation of 2,4-dichlorophenoxyacetic acid. Environ. Sci. Pollut. Res. 2020, 27, 11127–11137. [Google Scholar] [CrossRef]
- Selvi, S.; Rajendran, R.; Jayamani, N. Hydrothermal fabrication and characterization of novel CeO2/PbWO4 nanocomposite for enhanced visible-light photocatalytic performance. Appl. Water Sci. 2021, 11, 93. [Google Scholar] [CrossRef]
- Li, X.; Yao, C.; Lu, X.; Hu, Z.; Yin, Y.; Ni, C. Halloysite–CeO2–AgBr nanocomposite for solar light photodegradation of methyl orange. Appl. Clay Sci. 2015, 104, 74–80. [Google Scholar] [CrossRef]
- Zhakeyev, A.; Wang, P.; Zhang, L.; Shu, W.; Wang, H.; Xuan, J. Additive Manufacturing: Unlocking the Evolution of Energy Materials. Adv. Sci. 2017, 4, 1700187. [Google Scholar] [CrossRef] [Green Version]
- Attarilar, S.; Djavanroodi, F.; Ebrahimi, M.; Al-Fadhalah, K.J.; Wang, L.; Mozafari, M. Hierarchical Microstructure Tailoring of Pure Titanium for Enhancing Cellular Response at Tissue-Implant Interface. J. Biomed. Nanotechnol. 2021, 17, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Attarilar, S.; Wang, L.; Lu, W.; Yang, J.; Fu, Y. A Review on Design and Mechanical Properties of Additively Manufactured NiTi Implants for Orthopedic Applications. Int. J. Bioprint. 2021, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhuang, Y.; Yuan, Y.; Zhan, W.; Wang, X.; Han, X.; Zhao, Y. Nitrogen-Doped Carbon-Coated CuO-In2O3 p–n Heterojunction for Remarkable Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2019, 9, 1902839. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Mousavi, M.; Ghasemi, J.B.; Van Le, Q.; Delbari, S.A.; Sabahi Namini, A.; Shahedi Asl, M.; Shokouhimehr, M.; Mohammadi, M. Novel p–n Heterojunction Nanocomposite: TiO2 QDs/ZnBi2O4 Photocatalyst with Considerably Enhanced Photocatalytic Activity under Visible-Light Irradiation. J. Phys. Chem. C 2020, 124, 27519–27528. [Google Scholar] [CrossRef]
- Attarilar, S.; Ebrahimi, M.; Djavanroodi, F.; Fu, Y.; Wang, L.; Yang, J. 3D Printing Technologies in Metallic Implants: A Thematic Review on the Techniques and Procedures. Int. J. Bioprint. 2021, 7, 306. [Google Scholar] [CrossRef]
- Gellé, A.; Jin, T.; de la Garza, L.; Price, G.D.; Besteiro, L.V.; Moores, A. Applications of Plasmon-Enhanced Nanocatalysis to Organic Transformations. Chem. Rev. 2020, 120, 986–1041. [Google Scholar] [CrossRef]
- Linic, S.; Chavez, S.; Elias, R. Flow and extraction of energy and charge carriers in hybrid plasmonic nanostructures. Nat. Mater. 2021, 20, 916–924. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Bi, Z.; Ni, W.; Gurzadyan, G.; Zhu, Y.; Zhang, Z. Plasmonic Active “Hot Spots”-Confined Photocatalytic CO2 Reduction with High Selectivity for CH4 Production. Adv. Mater. 2022, 34, 2109330. [Google Scholar] [CrossRef]


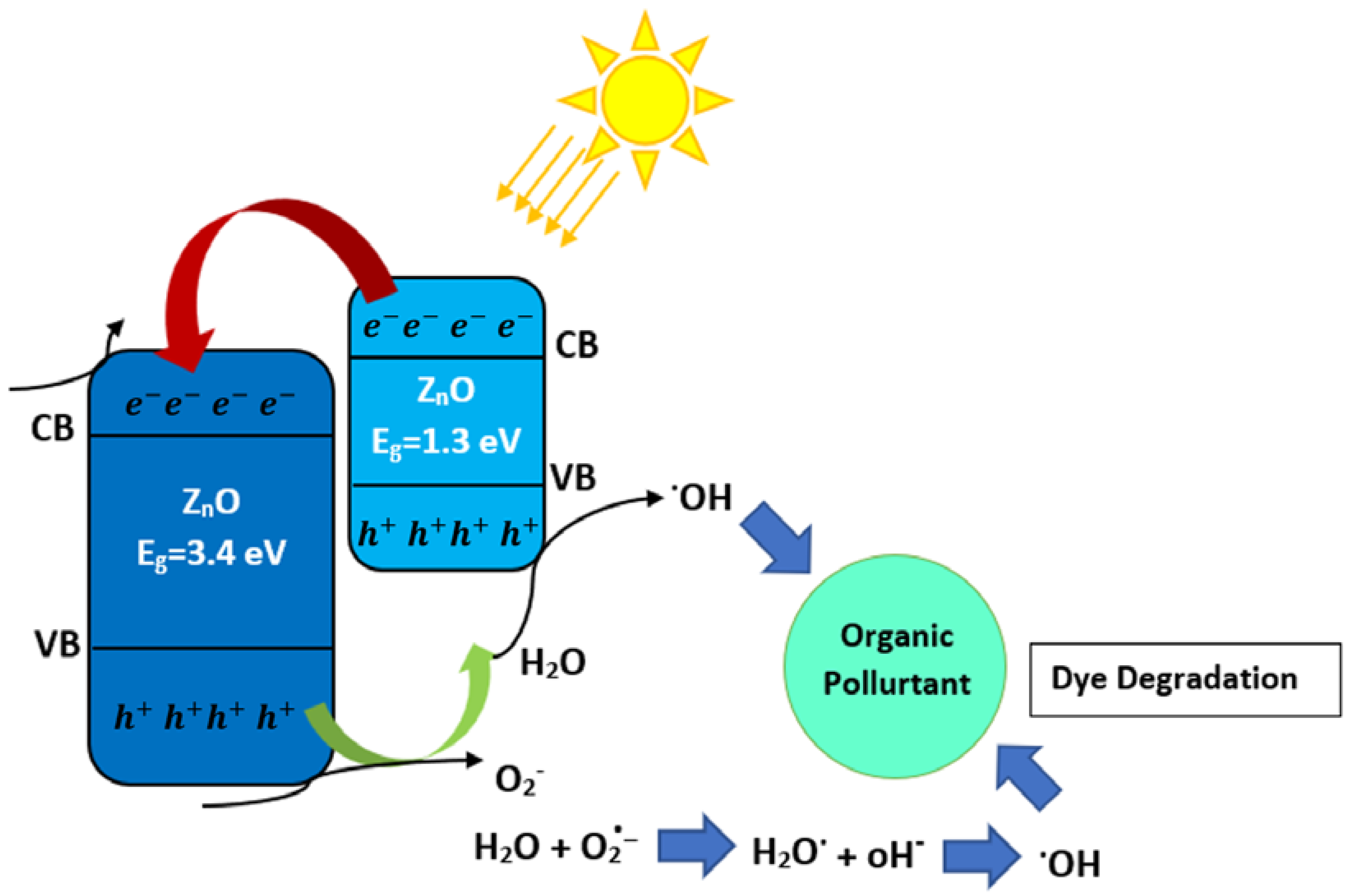
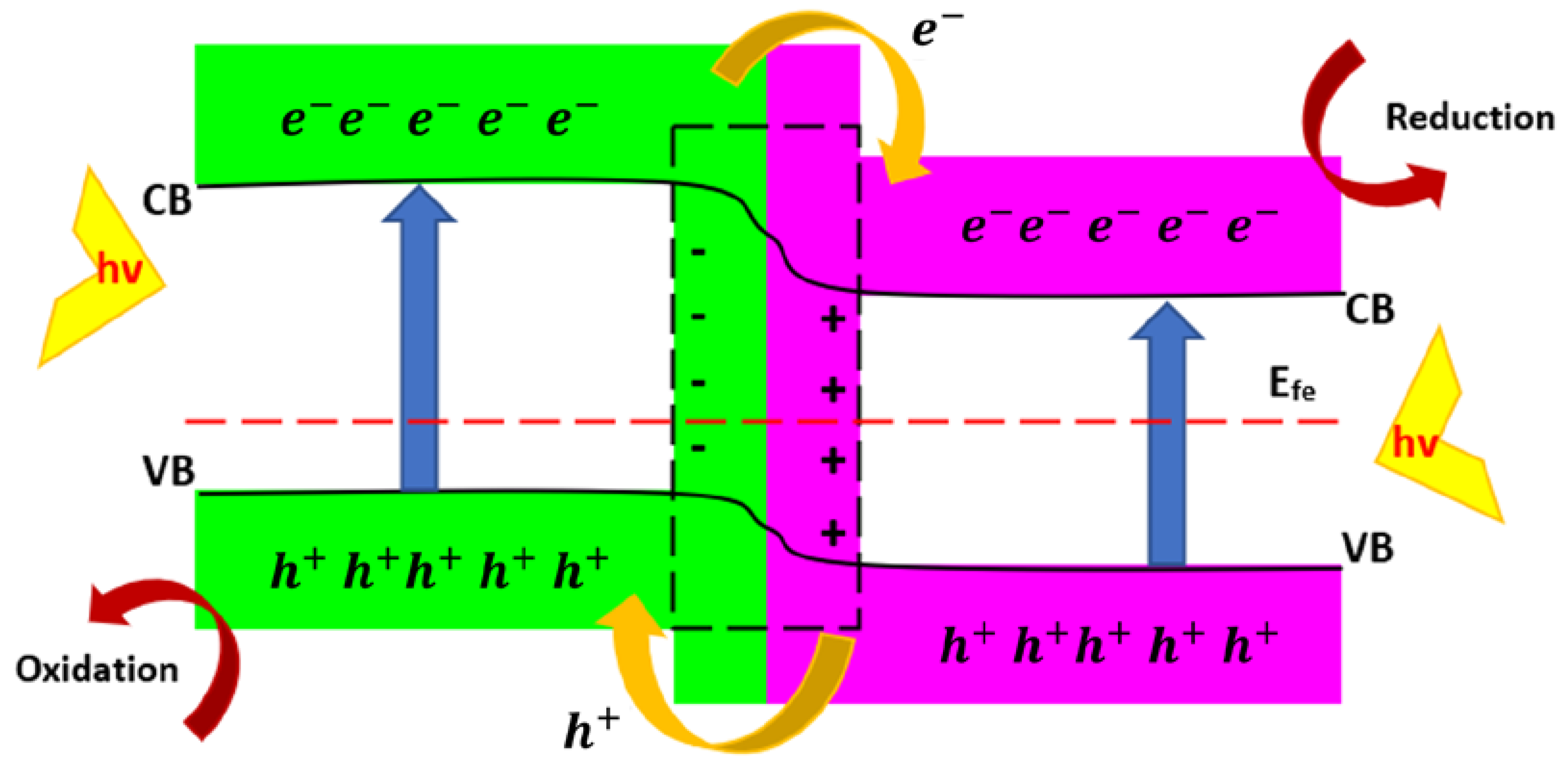
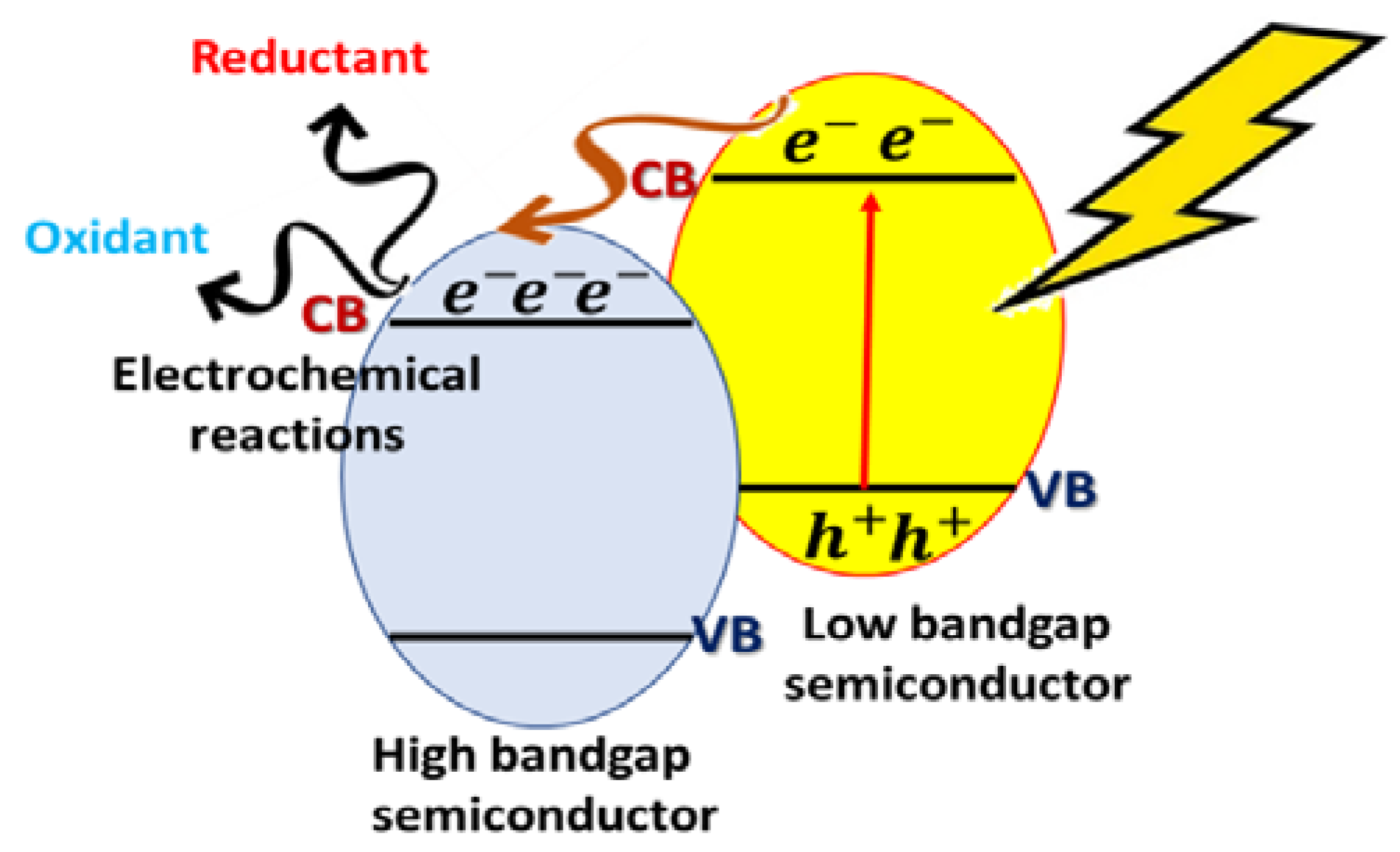
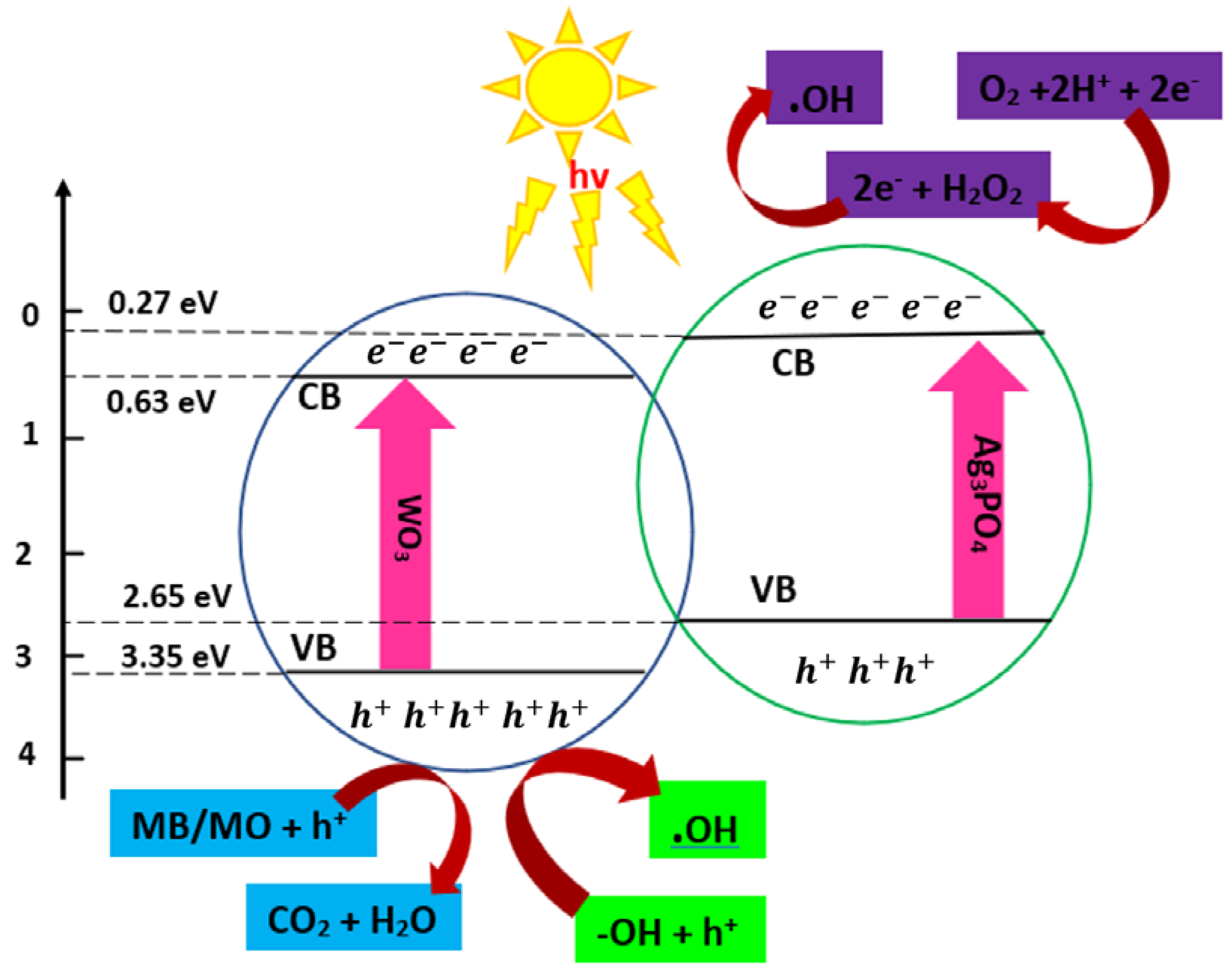
| Semiconductor | Materials Involved in Heterojunction | Application/Result | Hydrogen Production Rate (μmol h−1 g−1) | Ref |
|---|---|---|---|---|
| TiO2-based | Carbon coated Ni/TiO2 | Photostability in an aqueous suspension | 2000 | [89] |
| graphene/TiO2 | H2-production activity in aqueous solutions containing methanol | 736 | [90] | |
| In2S3/(Pt-TiO2) | Photocatalytic H2 production | 1350 | [91] | |
| Au/TiO2 | Excellent photoelectrochemical response | 0.5 | [92] | |
| V2O5/N,S–TiO2 | Improved H2 production and phenol degradation | 2966 | [93] | |
| TiO2/AgGaS2 | Improved H2 production rate | 4200 | [94] | |
| Fe2O3/TiO2 | Improved H2 production rate | 7253 | [95] | |
| Pt@TiO2@In2O3@MnOx | High photocatalytic activity toward water and alcohol oxidation. | - | [10] | |
| WO3/TiO2 with graphene modification | Promoted photocatalytic H2 production activity | 245.8 | [85] | |
| Macro−Mesoporous TiO2−Graphene | Enhanced capacity for rapid adsorption and photodegradation of organic dyes | - | [11] | |
| Au/TiO2 | Light intensity plays a key role in photocatalysis | 7–9 kW/m2 | [12] | |
| TiO2/C3N4/Ti3C2 | Improved CO2 reduction activity | CO yield: 3.14 | [77] | |
| TiO2/polydopamine | Improved CO2 reduction activity and selectivity | Methane yield: 1.50 | [78] | |
| CdS-based | CdS nanorods/CdSe | Improved H2 production rate | 40,500 | [96] |
| CeO2/CdS | Promoted photocatalytic hydrogen activity | 223 | [97] | |
| CdS/ZnS/In2S3 | Promoted photocatalytic hydrogen activity | 8100 | [98] | |
| MoS2/G-CdS | Improved H2 production rate | 9000 | [99] | |
| CdS nanorods/Cu2MoS4 | Superior photocatalytic activity for H2 evolution | 15,560 | [6] | |
| CdS-based MOFs | Without co-catalyst material for highly efficient photocatalytic H2 evolution | 26,100 | [54] | |
| SnNb2O6/CdS-diethylenetriamine | Excellent photocatalytic hydrogen production. | 7808 | [100] | |
| Graphitic carbon nitride-based | g-C3N4/zinc phthalocyanine | Sensitizer of Graphitic Carbon Nitride | 12,500 | [101] |
| WO3/g-C3N4 | Enhanced photocatalytic activity | 110 | [63] | |
| ZnFe2O4/g-C3N4 | Efficient solar H2 generation | 200.77 | [102] | |
| g-C3N4–SrTiO3:Rh | Enhanced H2 evolution | 2223 | [103] | |
| (g-C3N4)–Pt-TiO2 | Photocatalytic H2 production | 1780 | [104] | |
| K–I co-doped g-C3N4 | Enhanced charge separation efficiency | 41.23 | [9] | |
| g-C3N4/Au/Bi2WO6 | Promoted visible-light-driven photocatalytic activity | - | [70] | |
| WO3/g-C3N4 | Excellent photocatalytic H2 generation activity | 982 | [81] | |
| g-C3N4/WO3/AgI | Enhanced performance for neonicotinoid degradation | - | [28] | |
| SnS2/RGO/g-C3N4 | Enhanced photocatalytic activity for H2 generation | 2050 | [25] | |
| CuI-GD/g-C3N4 | Efficient photocatalytic H2 evolution | 43 | [83] | |
| g-C3N4–TiO2 | Photocatalytic degradation of formaldehyde in air | - | [61] | |
| WO3/g-C3N4 | Enhanced photocatalytic activity with high dependency to WO3 content | 110 | [63] | |
| CdS and TiO2 -based | sub-nanometer thick layered TiO2 nanosheet/ CdS (QDs) | Efficient solar H2 generation | 1000 | [105] |
| Chromosilicate decorated with (CdS/C or CdS-TiO2/C) | Improved H2 production rate | 2580 | [106] | |
| Au@TiO2–CdS | Efficient solar H2 generation | 1970 | [107] | |
| Other | KTaO3 | Reduction in band gap leading to enhanced absorption of visible light | - | [8] |
| N-doped graphene ((N)G) | High efficiency for the photocatalytic generation of H2 from water/methanol mixtures | 336–4999 | [13] | |
| ZnO@ZnS micro/nanoferns | Excellent photocatalytic performance to decontaminate inorganic-organic biopollutant systems | - | [16] | |
| Calcium silicate hydrate (CSH) nanosheets | Harmful heavy metals in polluted water are removed and collected by adsorbents | CO yield: 17,100 | [20] | |
| BiVO4/Ti3C2 nanosheets | Improved photocatalytic activity | Up to 1800 | [27] | |
| Ag/LaVO4/BiVO4 | Enhanced photocatalytic H2 evolution | 3720 | [33] | |
| AgI-decorated β-Bi2O3 | Efficient degradation of organic pollutants | - | [66] | |
| Ag/ZnO nanosheets | Enhanced visible-light degradation | 443.6 | [44] | |
| ZnIn2S4 microflowers | Improved kinetics process of charge-carriers for photocatalytic H2 evolution | 2400 | [47] | |
| ZnO/ZnBi2O4 | Durable visible-light-activated photocatalysts for efficient removal of organic pollutants | - | [50] | |
| ZnBi2O4/ZnO | Enhanced H2 production and BPA degradation | 3440 | [108] | |
| BiIO4/BiVO4 | Promoted visible-light-induced photocatalytic performance for rhodamine B degradation and photocurrent evolution | - | [53] | |
| Bi2O3/NaNbO3 | Highly active photocatalytic performance | - | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahadoran, A.; Liu, Q.; Ramakrishna, S.; Sadeghi, B.; De Castro, M.M.; Cavaliere, P.D. Hydrogen Production as a Clean Energy Carrier through Heterojunction Semiconductors for Environmental Remediation. Energies 2022, 15, 3222. https://doi.org/10.3390/en15093222
Bahadoran A, Liu Q, Ramakrishna S, Sadeghi B, De Castro MM, Cavaliere PD. Hydrogen Production as a Clean Energy Carrier through Heterojunction Semiconductors for Environmental Remediation. Energies. 2022; 15(9):3222. https://doi.org/10.3390/en15093222
Chicago/Turabian StyleBahadoran, Ashkan, Qinglei Liu, Seeram Ramakrishna, Behzad Sadeghi, Moara Marques De Castro, and Pasquale Daniele Cavaliere. 2022. "Hydrogen Production as a Clean Energy Carrier through Heterojunction Semiconductors for Environmental Remediation" Energies 15, no. 9: 3222. https://doi.org/10.3390/en15093222
APA StyleBahadoran, A., Liu, Q., Ramakrishna, S., Sadeghi, B., De Castro, M. M., & Cavaliere, P. D. (2022). Hydrogen Production as a Clean Energy Carrier through Heterojunction Semiconductors for Environmental Remediation. Energies, 15(9), 3222. https://doi.org/10.3390/en15093222









