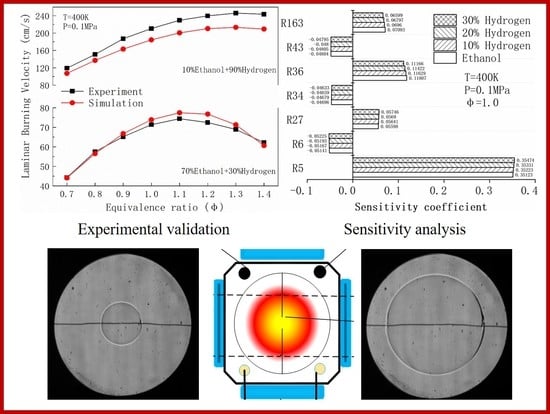
Experimental and Numerical Study on the Effect of Hydrogen Addition on Laminar Burning Velocity of Ethanol–Air Mixtures
Abstract
:1. Introduction
2. Study Methodology
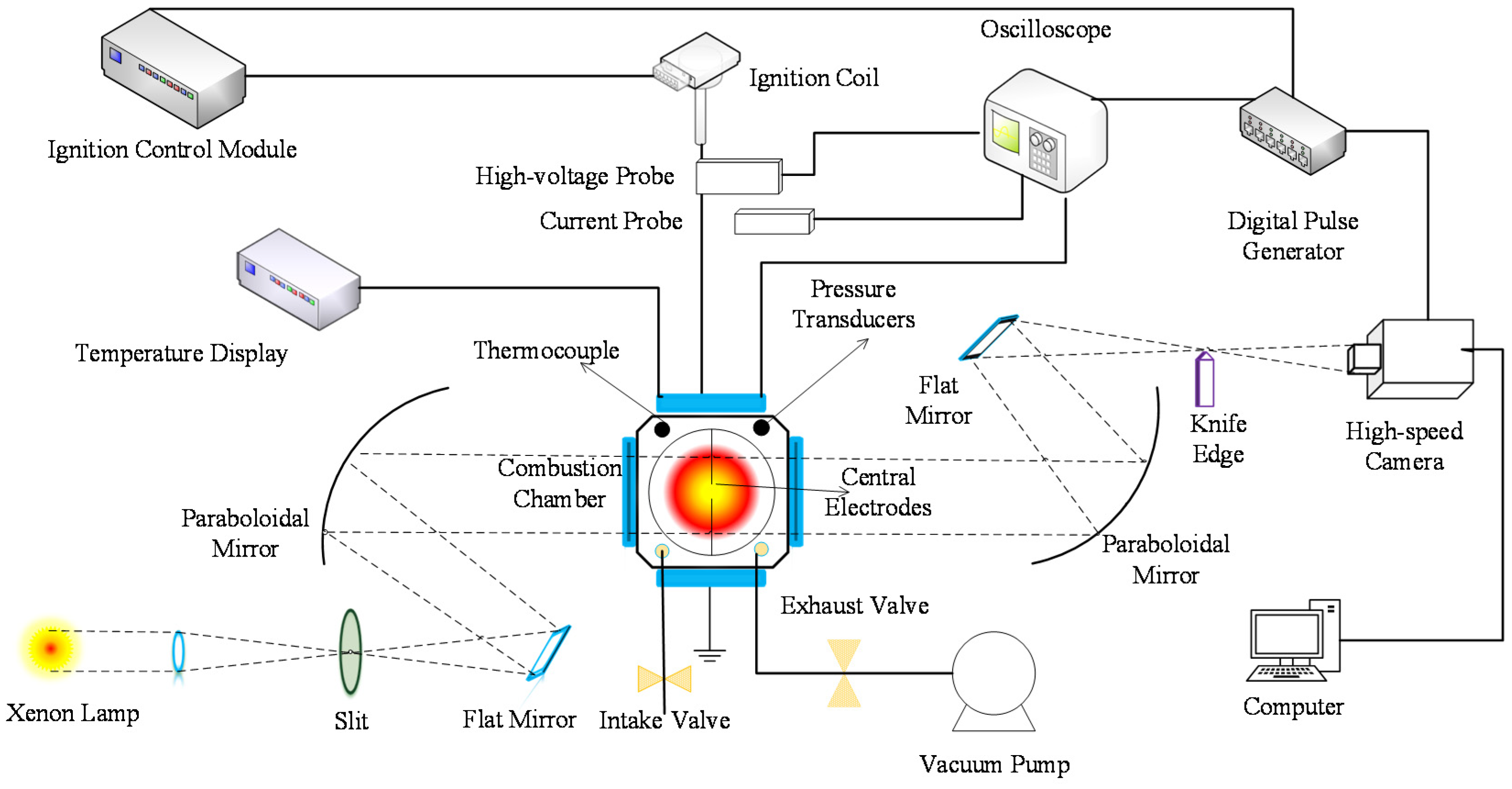
2.1. Experimental Setup
2.2. Extrapolation Methodology and Data Processing
2.3. Numerical Simulations
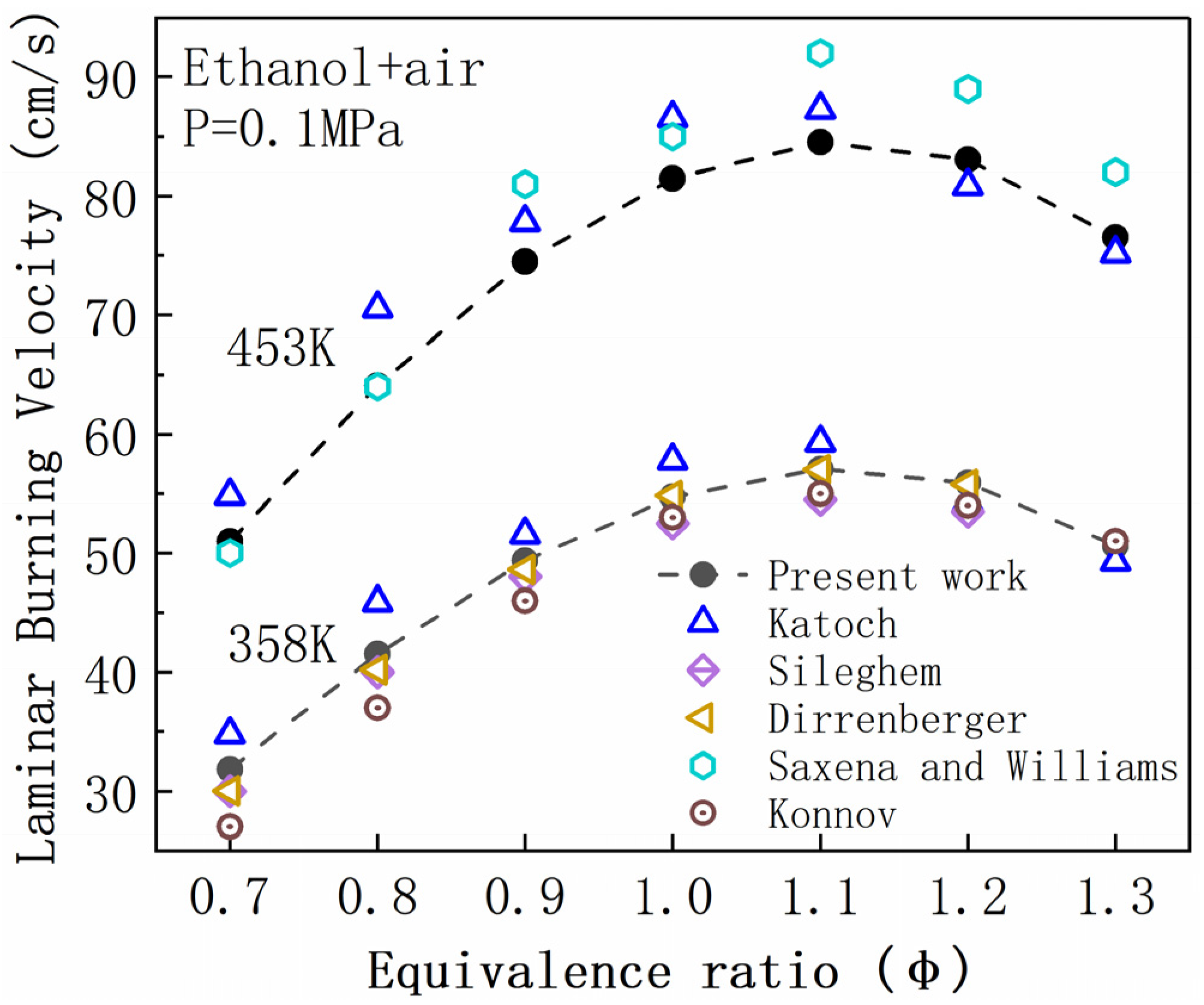
3. Validation of the LBV
4. Result and Discussion
4.1. Laminar Burning Velocity
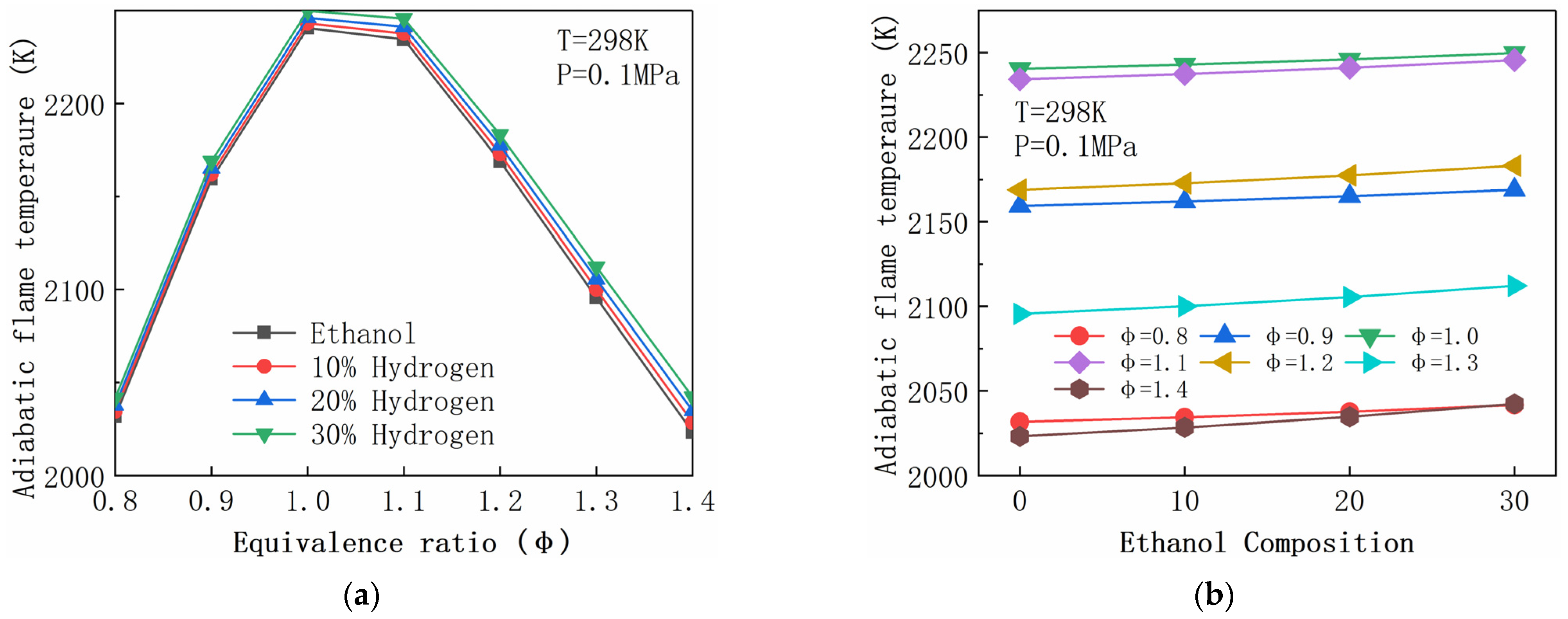
4.2. Adiabatic Flame Temperature
4.3. Heat Release
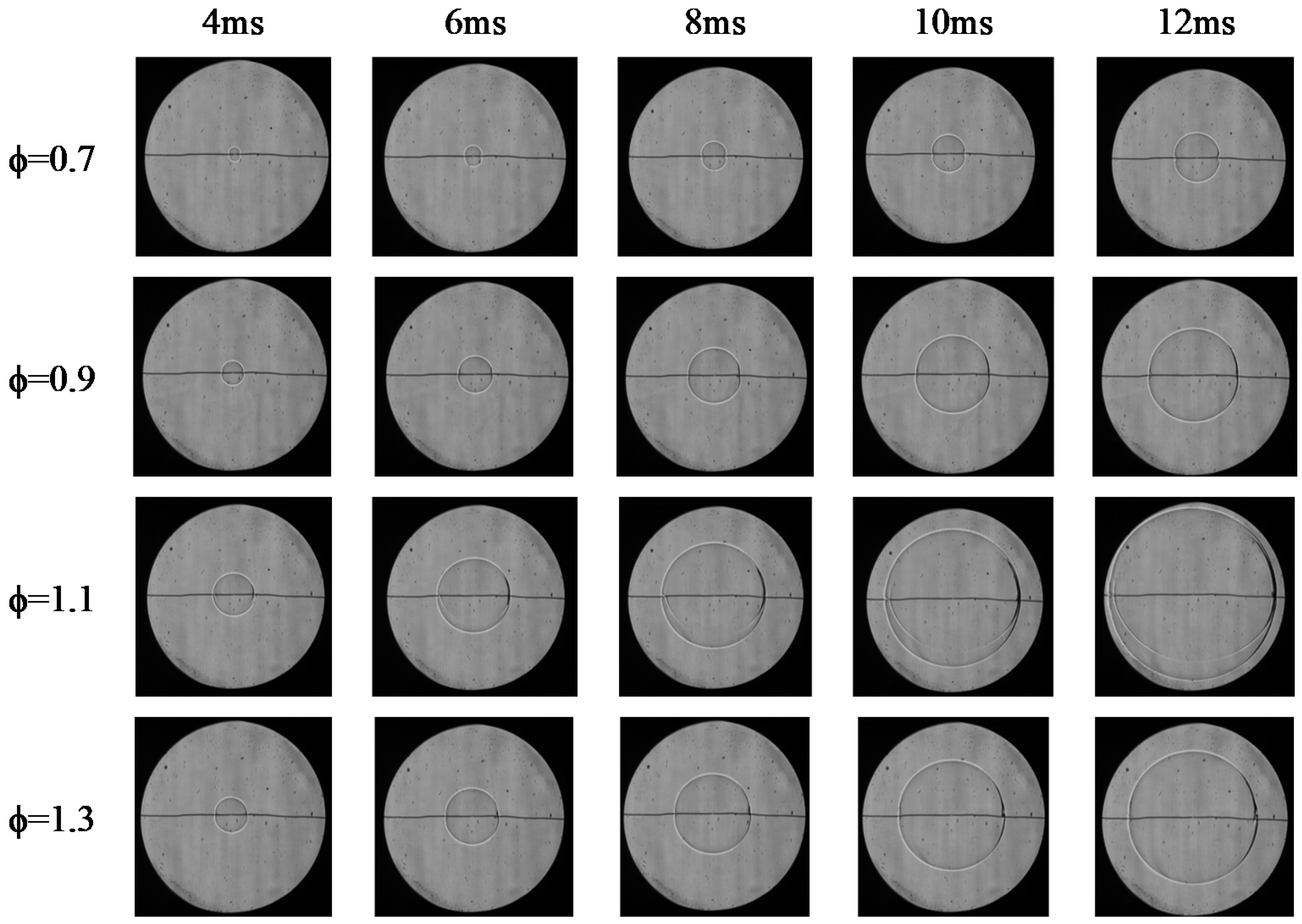
4.4. Flame Instability
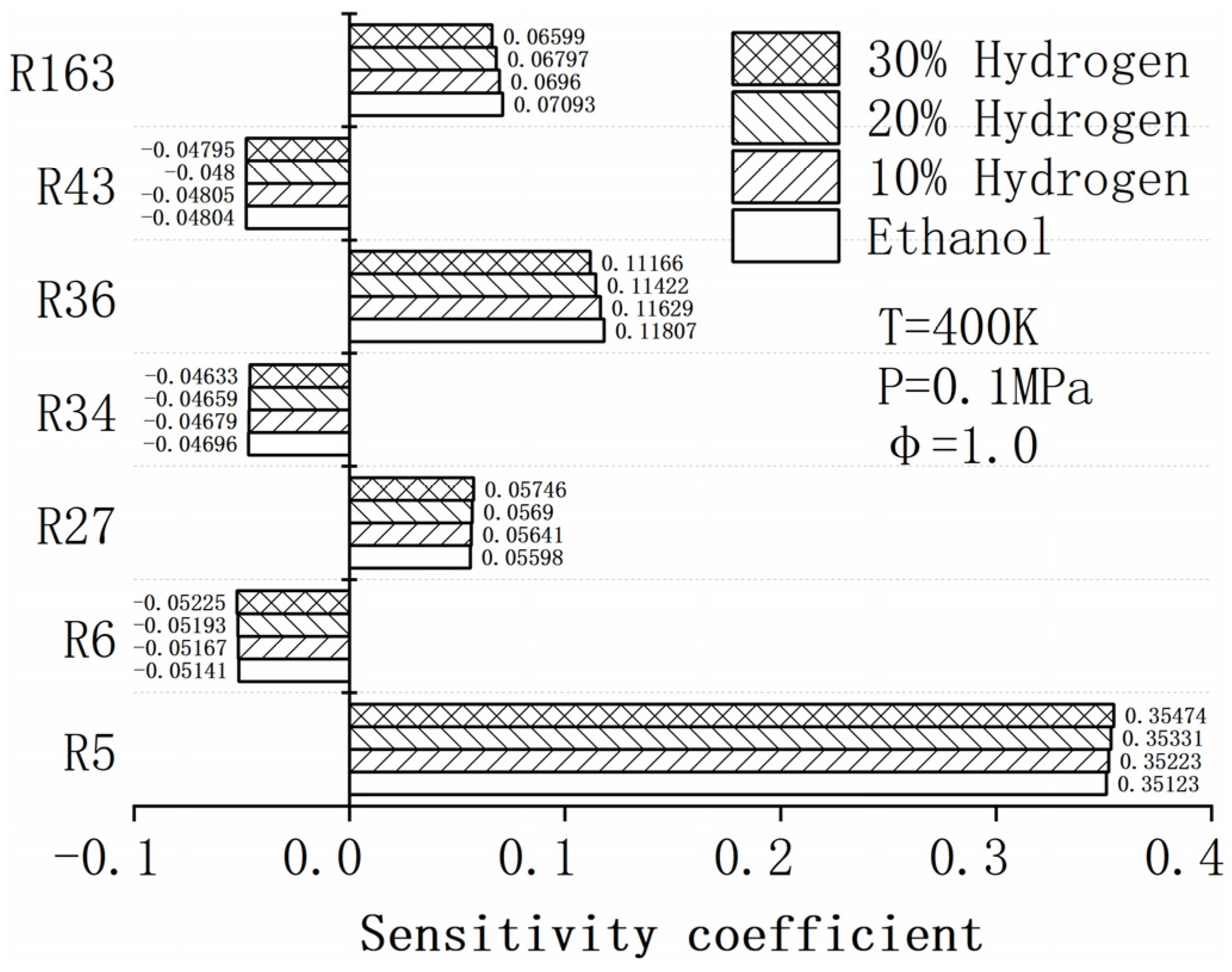
4.5. Sensitivity Analysis
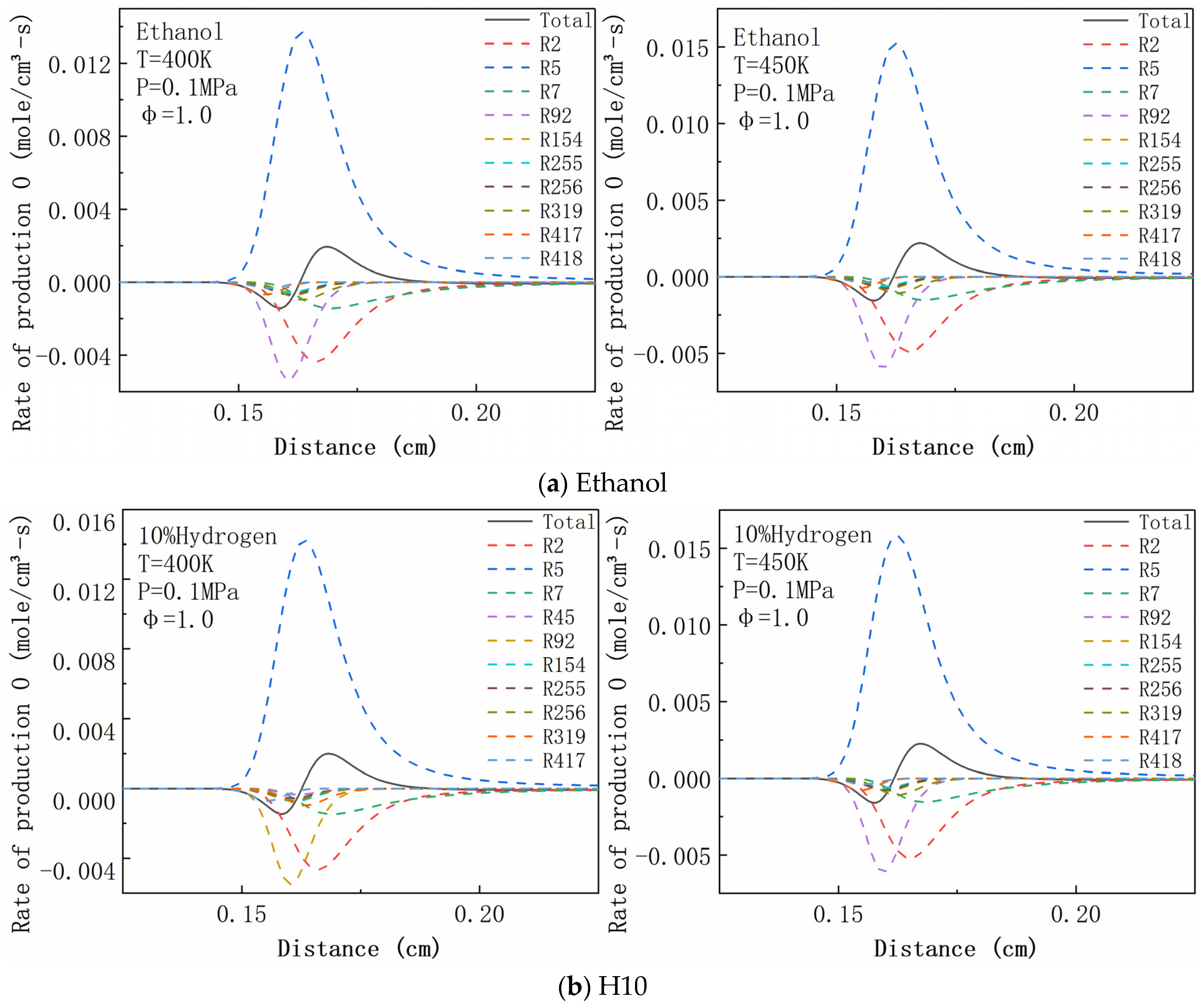
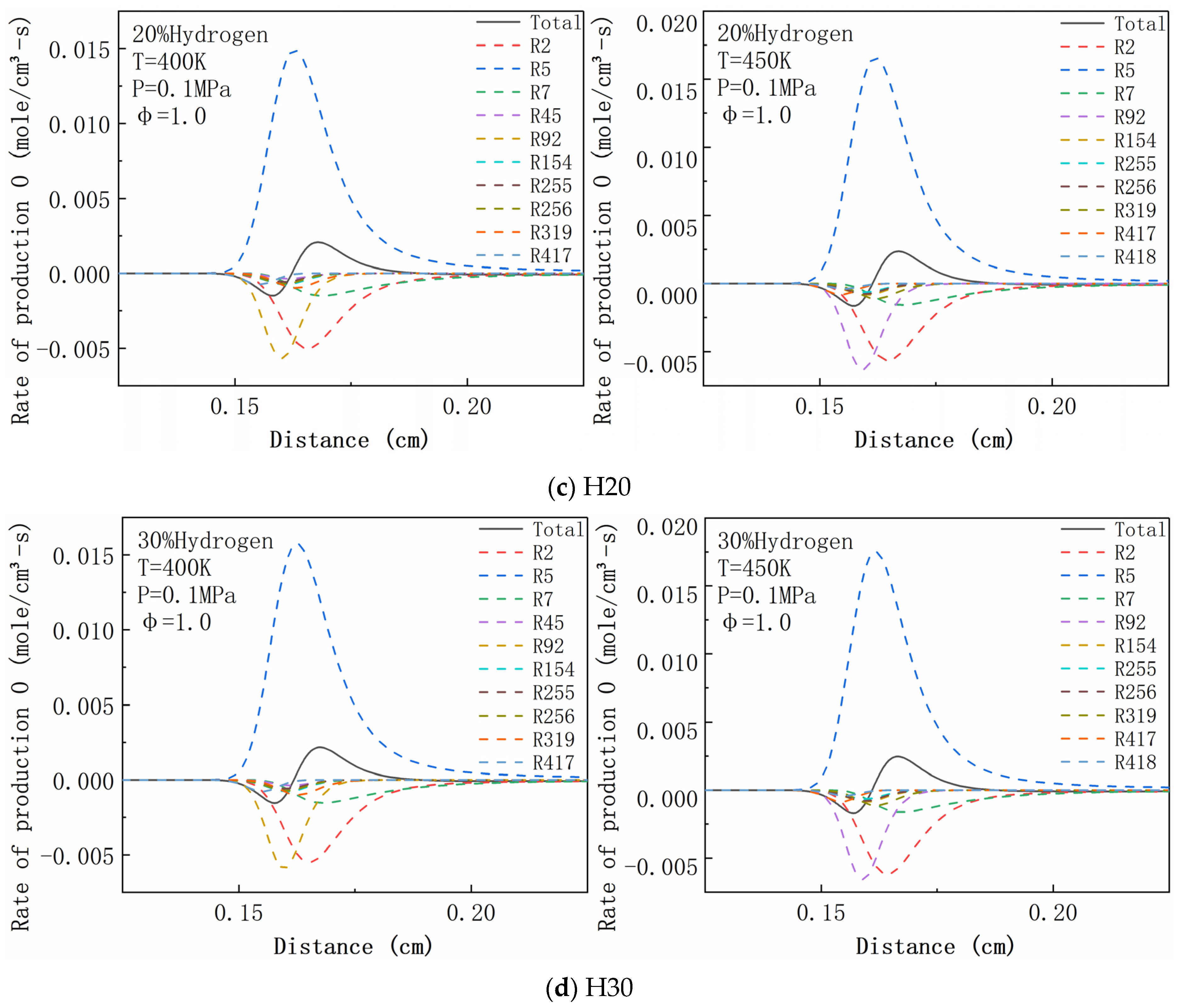
4.6. Rate of Production Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyriakides, A.; Dimas, V.; Lymperopoulou, E.; Karonis, D.; Lois, E. Evaluation of gasoline–ethanol–water ternary mixtures used as a fuel for an Otto engine. Fuel 2013, 108, 208–215. [Google Scholar] [CrossRef]
- Park, C.; Choi, Y.; Kim, C.; Oh, S.; Lim, G.; Moriyoshi, Y. Performance and exhaust emission characteristics of a spark ignition engine using ethanol and ethanol-reformed gas. Fuel 2010, 89, 2118–2125. [Google Scholar] [CrossRef]
- Sales, L.C.M.; Sodré, J.R. Cold start characteristics of an ethanol-fuelled engine with heated intake air and fuel. Appl. Therm. Eng. 2012, 40, 198–201. [Google Scholar] [CrossRef]
- Lowesmith, B.; Mumby, C.; Hankinson, G.; Puttock, J. Vented confined explosions involving methane/hydrogen mixtures. Int. J. Hydrogen Energy 2011, 36, 2337–2343. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Huang, Z.; Jin, C.; He, J.; Wang, J.; Wang, X.; Miao, H. Explosion characteristics of hydrogen–nitrogen–air mixtures at elevated pressures and temperatures. Int. J. Hydrogen Energy 2009, 34, 554–561. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhao, D.; Yin, J.; Liu, L.; Shu, C.-M. Experimental study on explosion characteristics of hydrogen–propane mixtures. Int. J. Hydrogen Energy 2019, 44, 22712–22718. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A. Laminar burning velocity of hydrogen–methane/air premixed flames. Int. J. Hydrogen Energy 2007, 32, 637–646. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A.; Long, E.; Hargrave, G. Time-Resolved Particle Image Velocimetry of dynamic interactions between hydrogen-enriched methane/air premixed flames and toroidal vortex structures. Int. J. Hydrogen Energy 2012, 37, 16201–16213. [Google Scholar] [CrossRef] [Green Version]
- Di Sarli, V.; Di Benedetto, A. Effects of non-equidiffusion on unsteady propagation of hydrogen-enriched methane/air premixed flames. Int. J. Hydrogen Energy 2013, 38, 7510–7518. [Google Scholar] [CrossRef]
- Di Sarli, V. Stability and Emissions of a Lean Pre-Mixed Combustor with Rich Catalytic/Lean-burn Pilot. Int. J. Chem. React. Eng. 2014, 12, 77–89. [Google Scholar] [CrossRef]
- Garcia-Agreda, A.; Di Sarli, V.; Di Benedetto, A. Bifurcation analysis of the effect of hydrogen addition on the dynamic behavior of lean premixed pre-vaporized ethanol combustion. Int. J. Hydrogen Energy 2012, 37, 6922–6932. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Yamin, J. The effect of hydrogen addition on premixed laminar acetylene–hydrogen–air and ethanol–hydrogen–air flames. Int. J. Hydrogen Energy 2013, 38, 7499–7509. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Liang, J.; Dong, F.; Li, Y.; Gao, X. Effects of hydrogen addition on the premixed laminar-flames of ethanol–air gaseous mixtures: An experimental study. Int. J. Hydrogen Energy 2012, 37, 4490–4501. [Google Scholar] [CrossRef]
- Ji, C.; Liu, X.; Wang, S.; Gao, B.; Yang, J. A laminar burning velocity correlation for combustion simulation of hydrogen-enriched ethanol engines. Fuel 2014, 133, 139–142. [Google Scholar] [CrossRef]
- Cammarota, F.; Benedetto, A.D.; Sarli, V.D.; Salzano, E. The effect of hydrogen addition on the explosion of ethanol/air mixtures. Chem. Eng. Trans. 2012, 26, 405–410. [Google Scholar]
- Zhang, Z.; Li, G.; Ouyang, L.; Pan, Z.; You, F.; Gao, X. Experimental determination of laminar burning velocities and Markstein lengths for 75% hydrous-ethanol, hydrogen and air gaseous mixtures. Int. J. Hydrogen Energy 2011, 36, 13194–13206. [Google Scholar] [CrossRef]
- Xu, C.; Zhong, A.; Wang, H.; Jiang, C.; Sahu, A.; Zhou, W.; Wang, C. Laminar burning velocity of 2-methylfuran-air mixtures at elevated pressures and temperatures: Experimental and modeling studies. Fuel 2018, 231, 215–223. [Google Scholar] [CrossRef]
- Xu, C.; Fang, D.; Luo, Q.; Ma, J.; Xie, Y. A comparative study of laser ignition and spark ignition with gasoline–air mixtures. Opt. Laser Technol. 2014, 64, 343–351. [Google Scholar] [CrossRef]
- Xu, C.; Hu, Y.; Li, X.; Zhou, X.; Zhong, A. Comparative experimental study of ethanol-air premixed laminar combustion characteristics by laser induced spark and electric spark ignition. Korean J. Chem. Eng. 2017, 34, 574–579. [Google Scholar] [CrossRef]
- Galmiche, B.; Halter, F.; Foucher, F. Effects of high pressure, high temperature and dilution on laminar burning velocities and Markstein lengths of iso-octane/air mixtures. Combust. Flame 2012, 159, 3286–3299. [Google Scholar] [CrossRef]
- Zhou, J.; Cordier, M.; Mounaïm-Rousselle, C.; Foucher, F. Experimental estimate of the laminar burning velocity of iso-octane in oxygen-enriched and CO2-diluted air. Combust. Flame 2011, 158, 2375–2383. [Google Scholar] [CrossRef]
- Baloo, M.; Dariani, B.M.; Akhlaghi, M.; AghaMirsalim, M. Effects of pressure and temperature on laminar burning velocity and flame instability of iso-octane/methane fuel blend. Fuel 2016, 170, 235–244. [Google Scholar] [CrossRef]
- Cano, J.P.C.; Cabot, G.; Foucher, F.; de Persis, S. Effects of oxygen enrichment and water dilution on laminar methane flames at high pressure. Fuel 2018, 225, 499–508. [Google Scholar] [CrossRef]
- Zhang, Y.; El-Merhubi, H.; Lefort, B.; Le Moyne, L.; Curran, H.J.; Kéromnès, A. Probing the low-temperature chemistry of ethanol via the addition of dimethyl ether. Combust. Flame 2018, 190, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Zhou, J.; Long, J. Effect of high temperature and pressure on laminar burning velocity and reaction kinetics of methane/iso-octane mixtures. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–16. [Google Scholar] [CrossRef]
- Katoch, A.; Millán-Merino, A.; Kumar, S. Measurement of laminar burning velocity of ethanol-air mixtures at elevated temperatures. Fuel 2018, 231, 37–44. [Google Scholar] [CrossRef]
- Sileghem, L.; Alekseev, V.; Vancoillie, J.; Nilsson, E.; Verhelst, S.; Konnov, A. Laminar burning velocities of primary reference fuels and simple alcohols. Fuel 2013, 115, 32–40. [Google Scholar] [CrossRef]
- Dirrenberger, P.; Glaude, P.; Bounaceur, R.; Le Gall, H.; da Cruz, A.P.; Konnov, A.; Battin-Leclerc, F. Laminar burning velocity of gasolines with addition of ethanol. Fuel 2013, 115, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Konnov, A.; Meuwissen, R.; de Goey, L. The temperature dependence of the laminar burning velocity of ethanol flames. Proc. Combust. Inst. 2011, 33, 1011–1019. [Google Scholar] [CrossRef]
- Saxena, P.; Williams, F.A. Numerical and experimental studies of ethanol flames. Proc. Combust. Inst. 2007, 31, 1149–1156. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Han, X.; Chen, C.; He, Y.; Zhu, Y.; Cen, K. Experimental and numerical study of the effect of elevated pressure on laminar burning velocity of lean H2/CO/O2/diluents flames. Fuel 2020, 273, 117753. [Google Scholar] [CrossRef]
- Srinidhi, C.; Madhusudhan, A.; Channapattana, S.V.; Gawali, S.V. Comparitive investigation of performance and emission features of methanol, ethanol, DEE, and nanopartilces as fuel additives in diesel-biodiesel blends. Heat Transf. 2020, 50, 2624–2642. [Google Scholar] [CrossRef]
- Waluyo, B.; Setiyo, M.; Saifudin; Wardana, I. Fuel performance for stable homogeneous gasoline-methanol-ethanol blends. Fuel 2021, 294, 120565. [Google Scholar] [CrossRef]
- Gainey, B.; Yan, Z.; Lawler, B. Autoignition characterization of methanol, ethanol, propanol, and butanol over a wide range of operating conditions in LTC/HCCI. Fuel 2020, 287, 119495. [Google Scholar] [CrossRef]
- Nabi, M.N. Theoretical investigation of engine thermal efficiency, adiabatic flame temperature, NOx emission and combustion-related parameters for different oxygenated fuels. Appl. Therm. Eng. 2010, 30, 839–844. [Google Scholar] [CrossRef]
- Jarungthammachote, S. Simplified model for estimations of combustion products, adiabatic flame temperature and properties of burned gas. Therm. Sci. Eng. Prog. 2019, 17, 100393. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Li, L.; Qi, F. Experimental and theoretical investigation on cellular instability of methanol/air flames. Fuel 2018, 225, 95–103. [Google Scholar] [CrossRef]
- Mohammad, A.; Juhany, K.A. Laminar burning velocity and flame structure of DME/methane + air mixtures at elevated temperatures. Fuel 2019, 245, 105–114. [Google Scholar] [CrossRef]

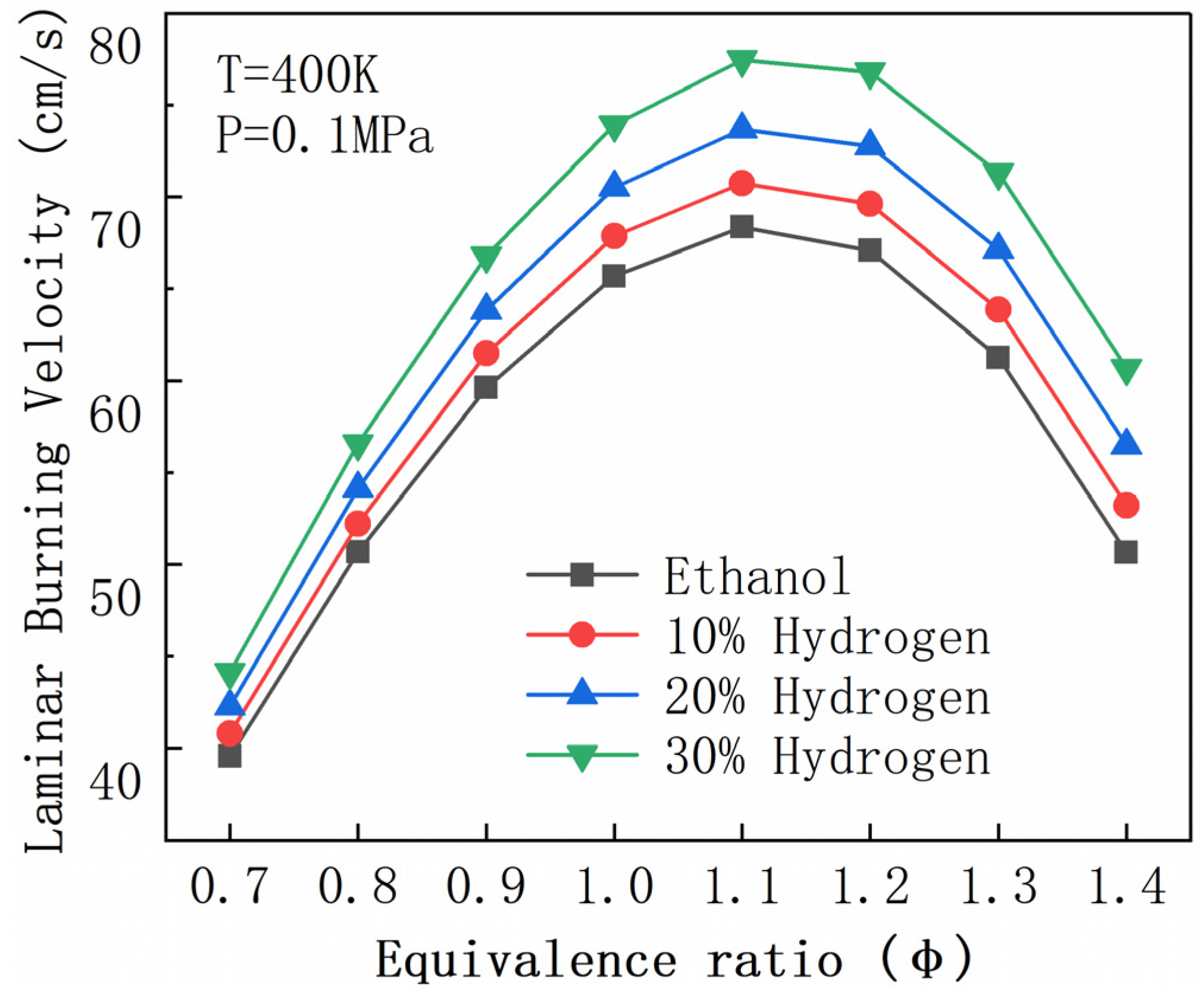
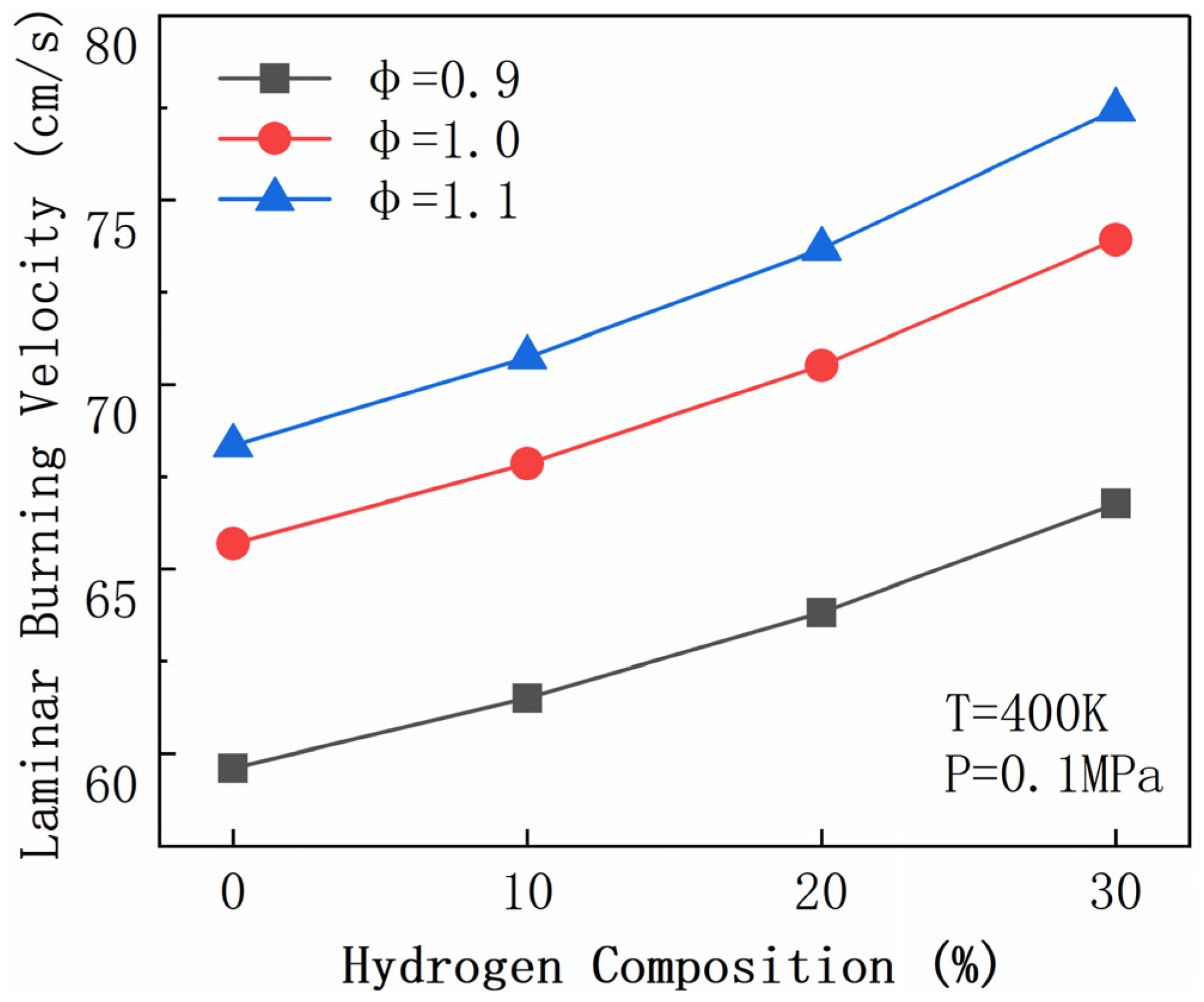
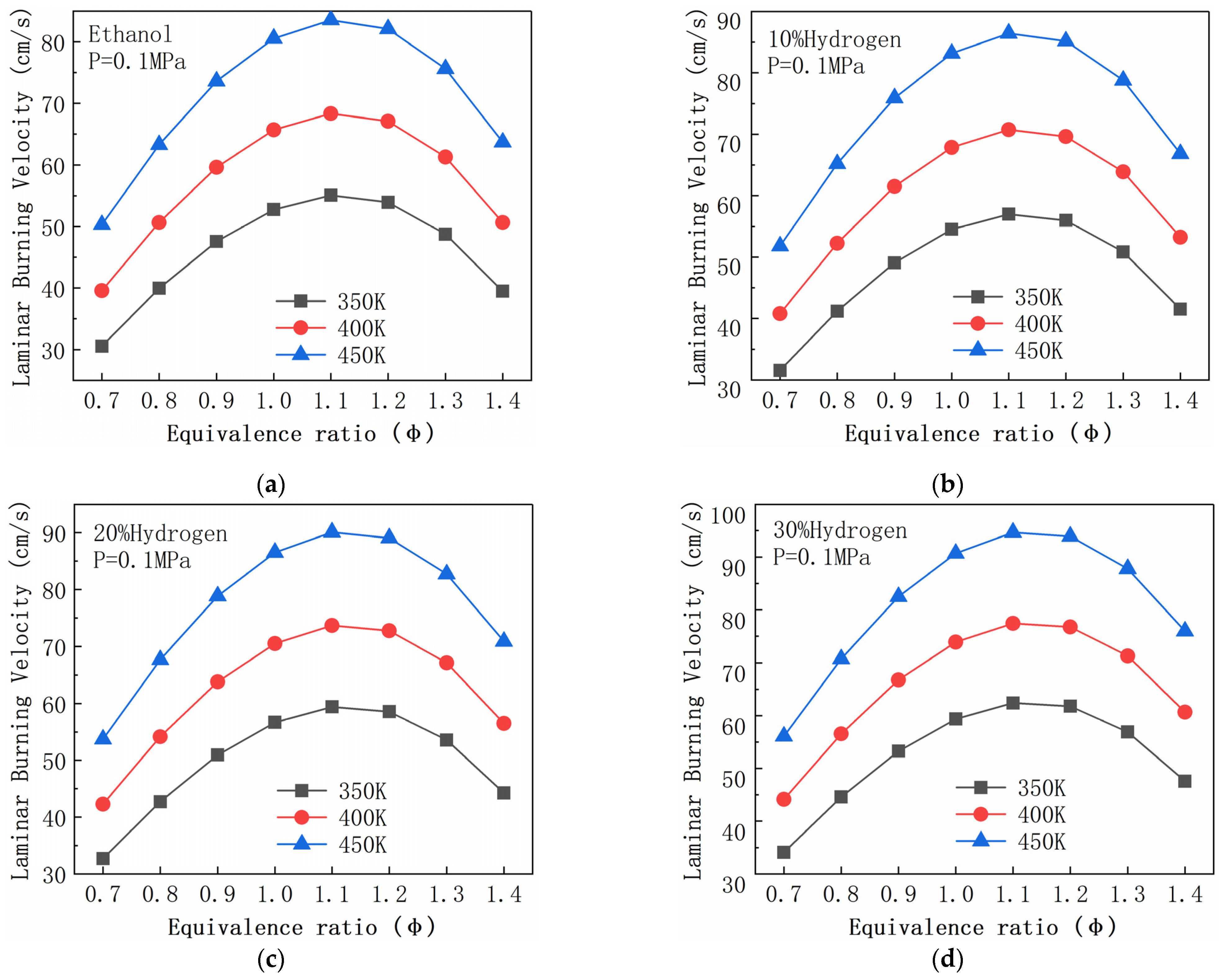
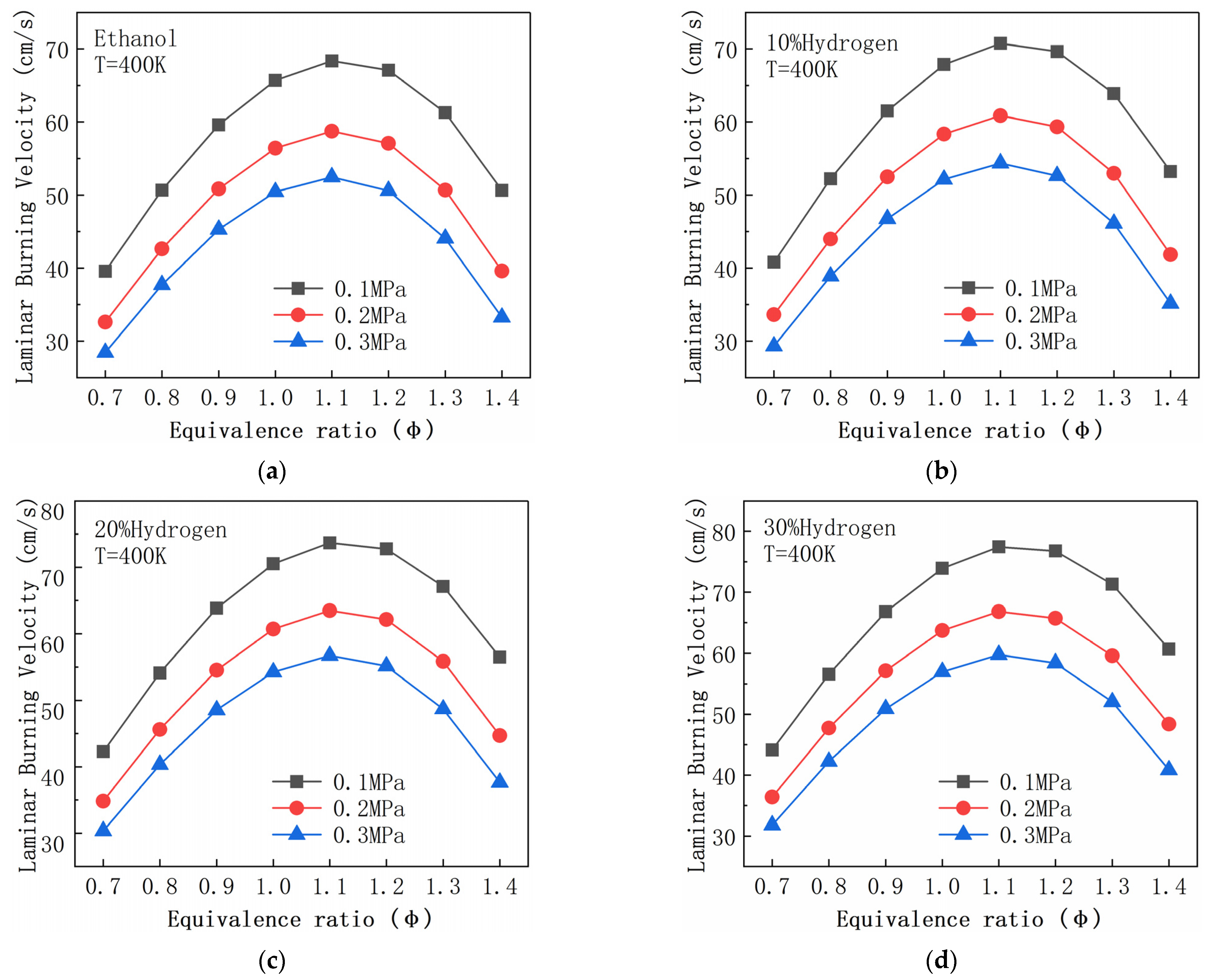



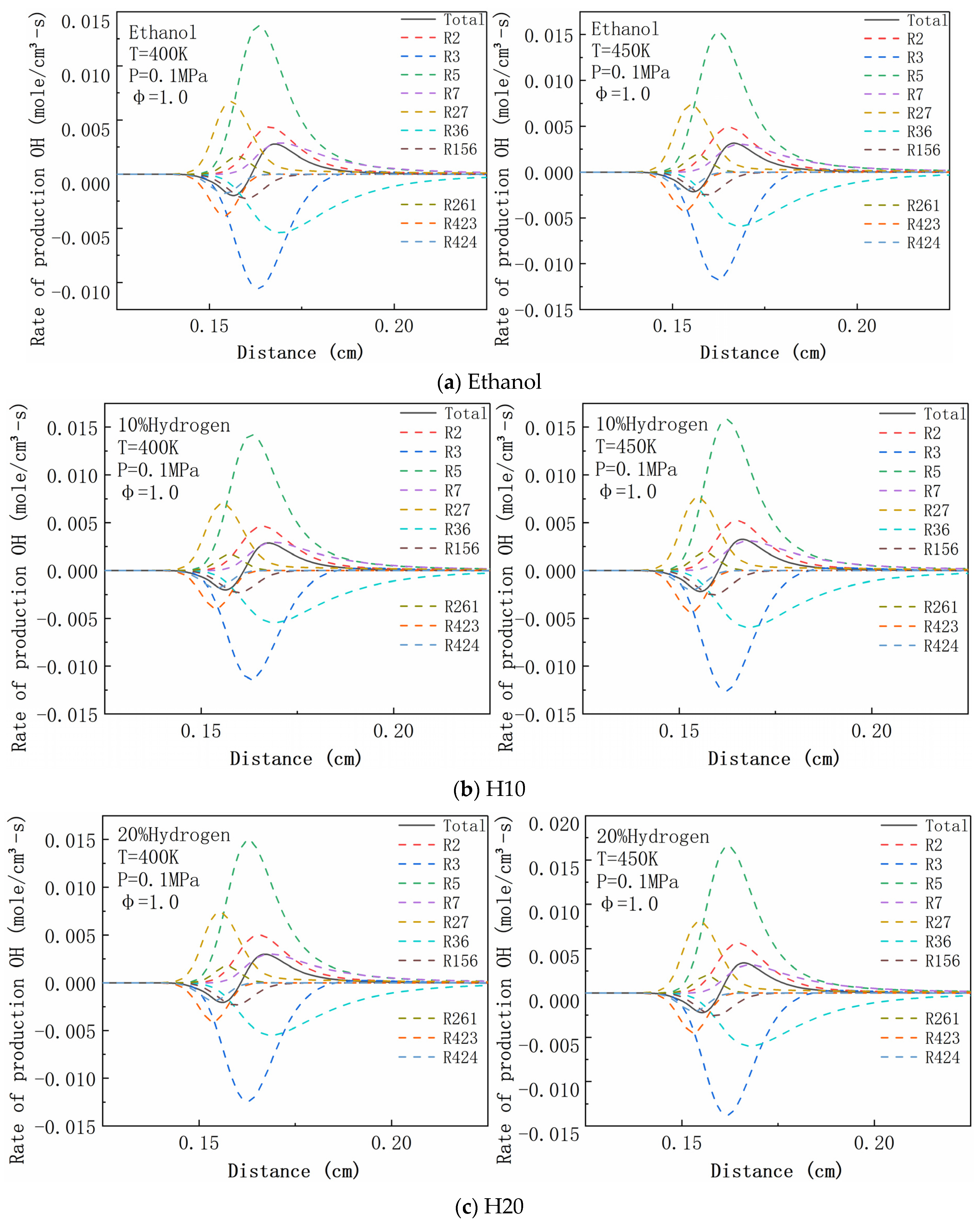
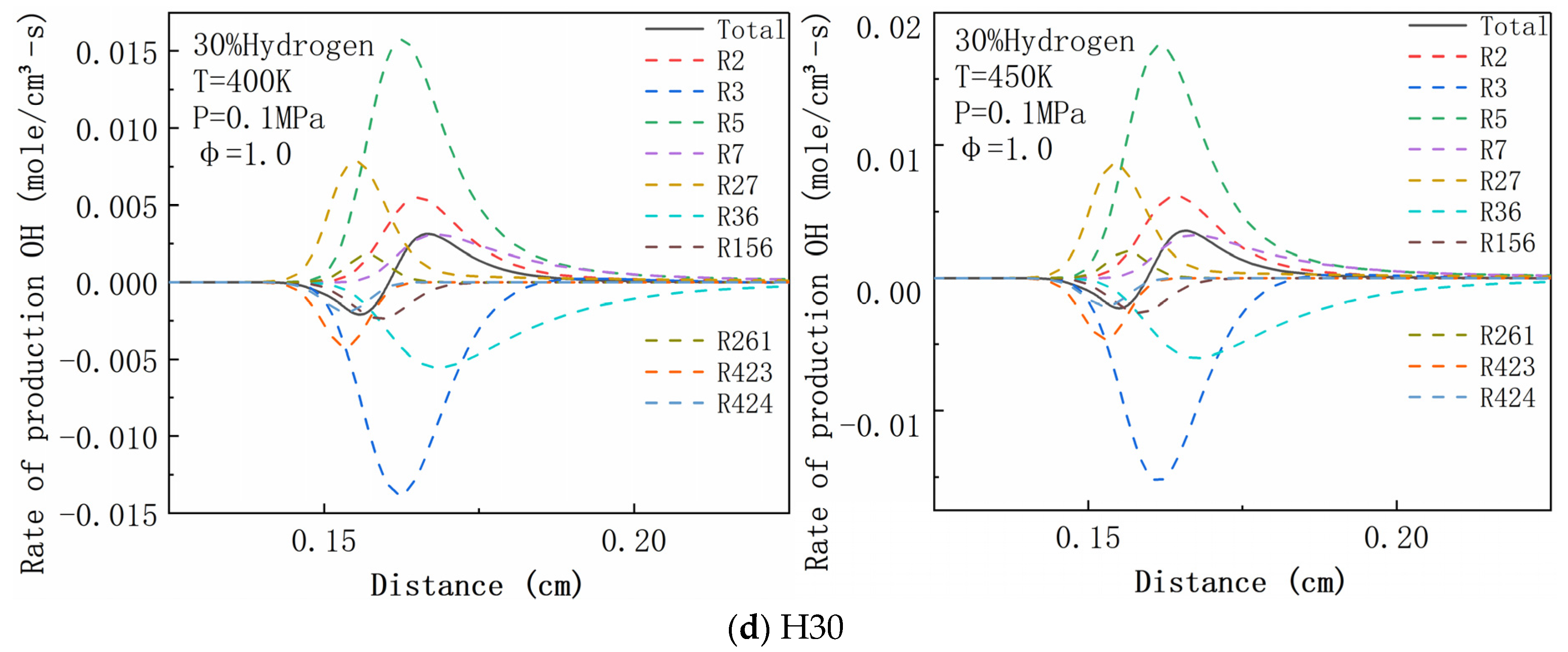
| R2 | H2 + O <=> H + OH | R156 | CH2O + OH <=> HCO + H2O |
| R3 | H2 + OH <=> H + H2O | R255 | C2H4 + O <=> CH3 + HCO |
| R5 | O2 + H <=> O + OH | R256 | C2H4 + O <=> CH2CHO + H |
| R7 | O + H2O <=> 2OH | R261 | C2H4 + OH <=> C2H3OH + H |
| R27 | HO2 + H <=> 2OH | R319 | C2H2 + O <=> HCCO + H |
| R36 | CO + OH <=> CO2 + H | R417 | C2H5OH + O <=> CH3CHOH + OH |
| R45 | CH4 + O <=> CH3 + OH | R418 | C2H5OH + O <=> CH2CH2OH + OH |
| R92 | CH3 + O <=> CH2O + H | R423 | C2H5OH + OH <=> CH3CHOH + H2O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Lu, C.; Xu, C.; Yu, Z. Experimental and Numerical Study on the Effect of Hydrogen Addition on Laminar Burning Velocity of Ethanol–Air Mixtures. Energies 2022, 15, 3114. https://doi.org/10.3390/en15093114
Zhou J, Lu C, Xu C, Yu Z. Experimental and Numerical Study on the Effect of Hydrogen Addition on Laminar Burning Velocity of Ethanol–Air Mixtures. Energies. 2022; 15(9):3114. https://doi.org/10.3390/en15093114
Chicago/Turabian StyleZhou, Jianxi, Chenyu Lu, Cangsu Xu, and Zitao Yu. 2022. "Experimental and Numerical Study on the Effect of Hydrogen Addition on Laminar Burning Velocity of Ethanol–Air Mixtures" Energies 15, no. 9: 3114. https://doi.org/10.3390/en15093114
APA StyleZhou, J., Lu, C., Xu, C., & Yu, Z. (2022). Experimental and Numerical Study on the Effect of Hydrogen Addition on Laminar Burning Velocity of Ethanol–Air Mixtures. Energies, 15(9), 3114. https://doi.org/10.3390/en15093114