CO2 Emission Factors and Carbon Losses for Off-Road Mining Trucks
Abstract
:1. Introduction
- a.
- ISO 14064-1: This section presents the details of the principles and requirements for designing, developing, managing, and reporting greenhouse gas inventories. It also includes the procedures that are used to determine GHG emission limits, quantify, reduce, and improve GHG emissions management. Guidance on GHG inventory quality, internal auditing, and organizational responsibilities for verification activities are also part of this section.
- b.
- ISO 14064-2: This section focuses on projects that aim to reduce greenhouse gas emissions and improve their removal. It details the principles that are used to establish project baselines and quantify and report project performance.
- c.
- ISO 14064-3: This section provides the principles that are used to verify inventories and project performances.
2. Procedure
2.1. Emission Measurements
2.2. Diesel Formulation
2.3. Fuel Losses to Particles
2.4. Fuel Losses to Carbon Monoxide and Unburned Hydrocarbons
x1 CO + x2 CO2 + x3 SO2 + x4 N2 + x5 NO + x6 CmHn + x7 O2 + x8 H2O + Particles.
3. Results and Discussion
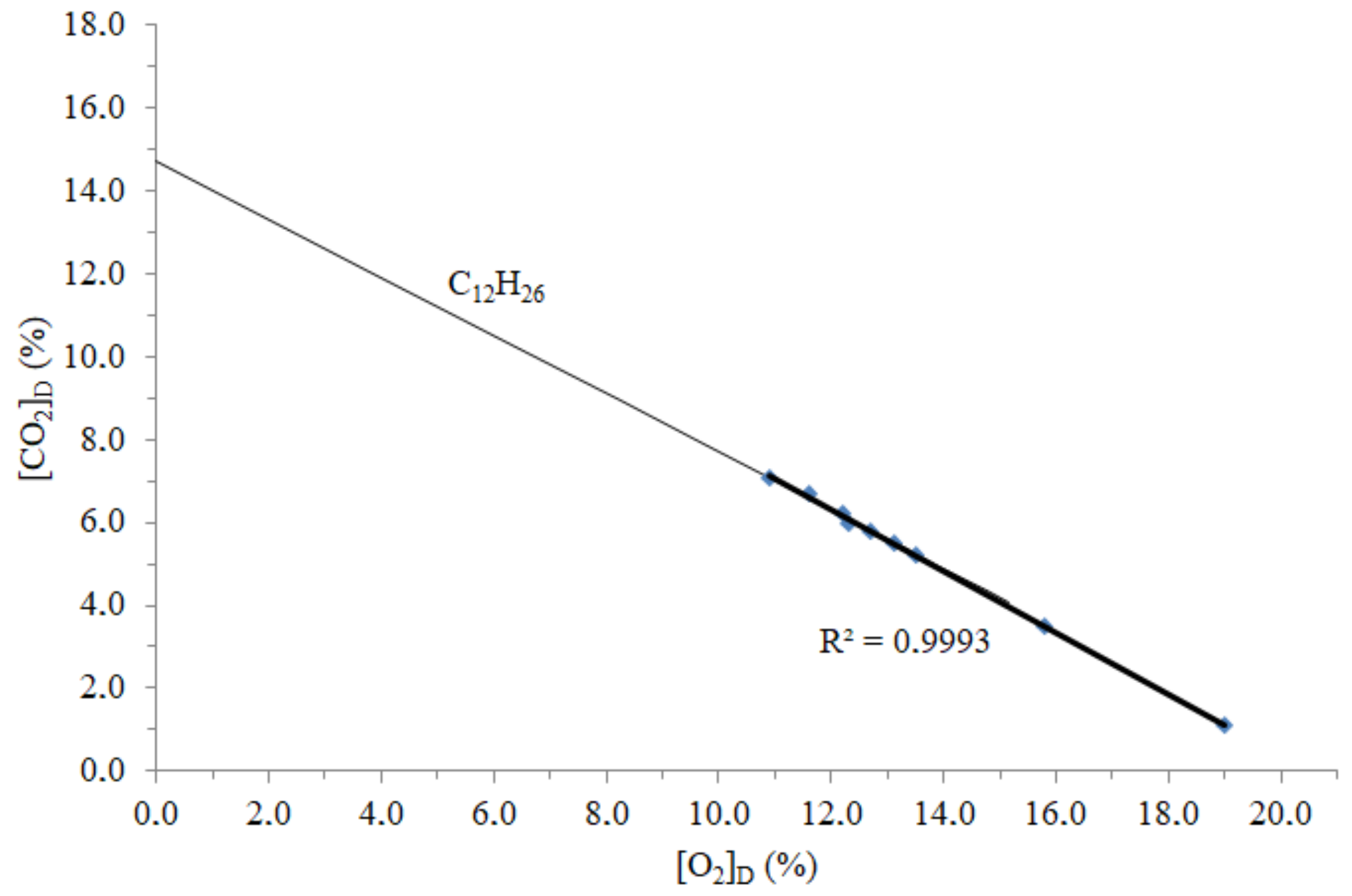
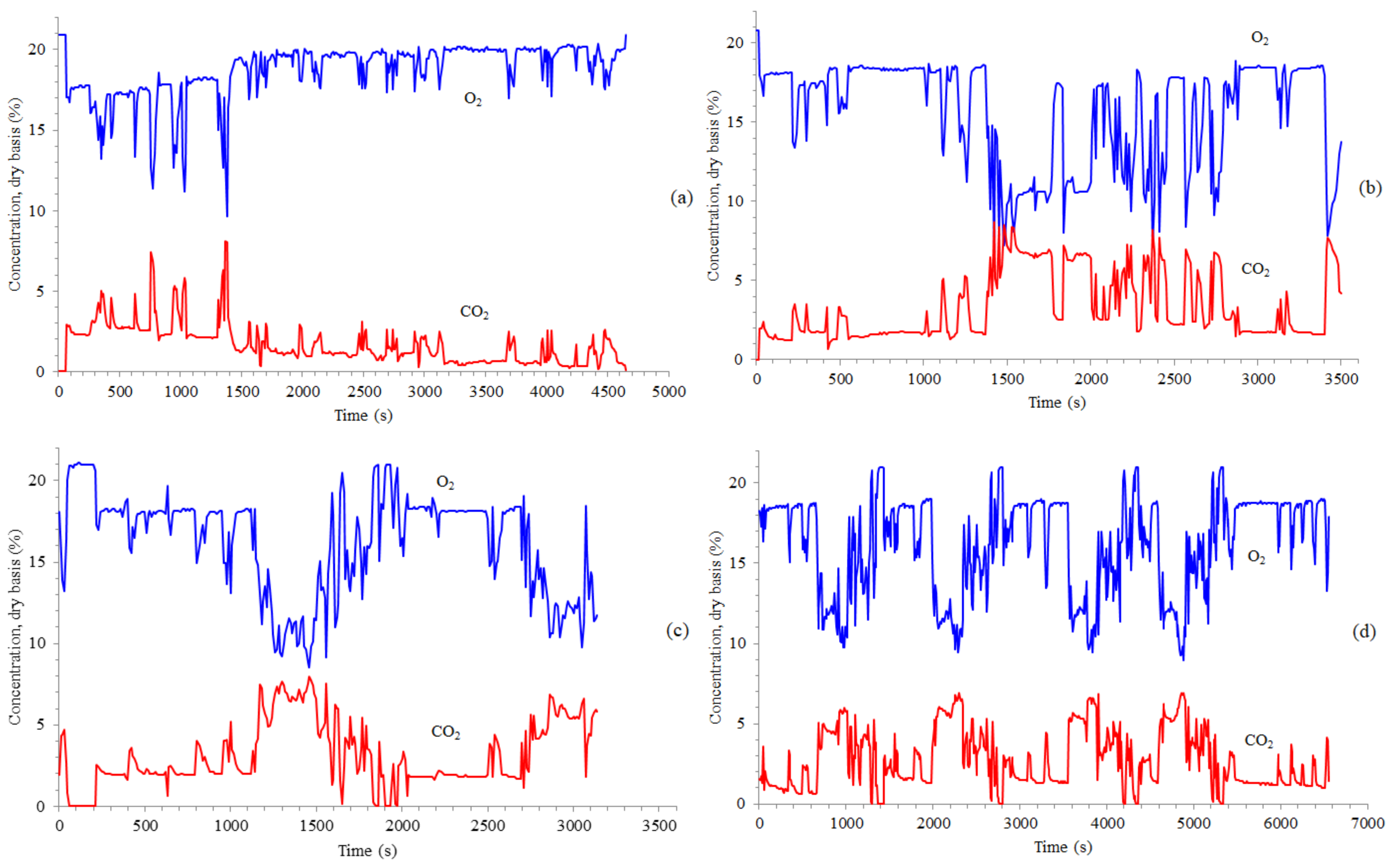
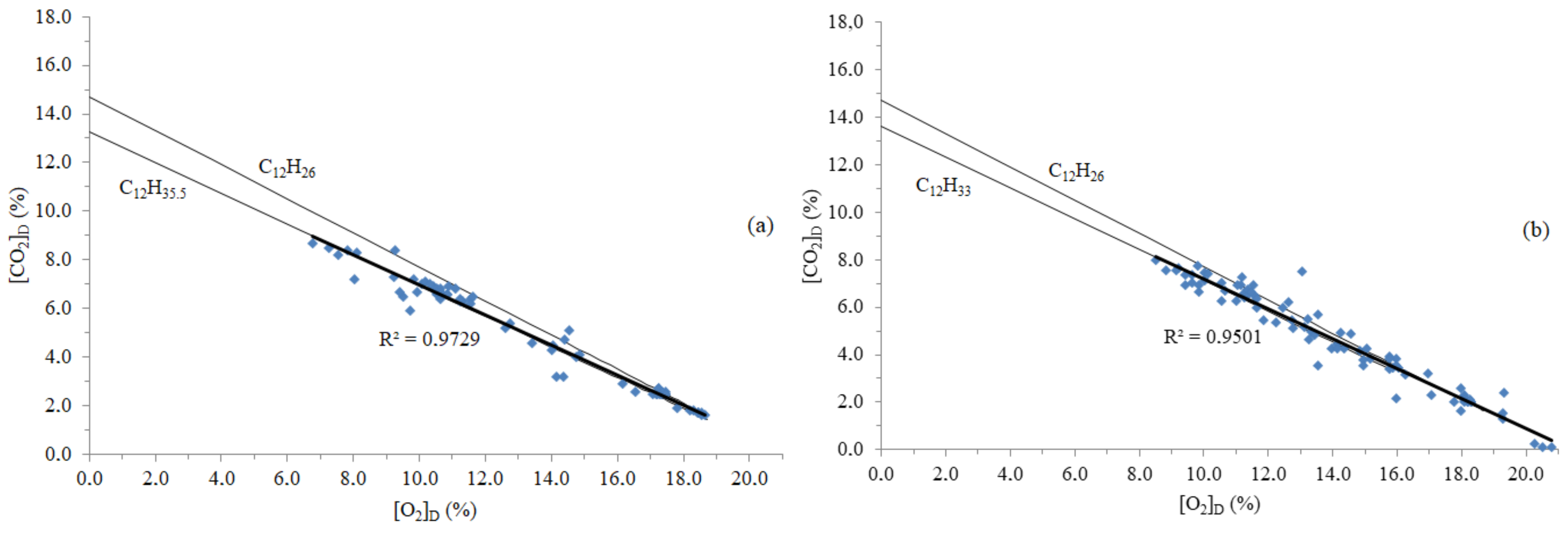
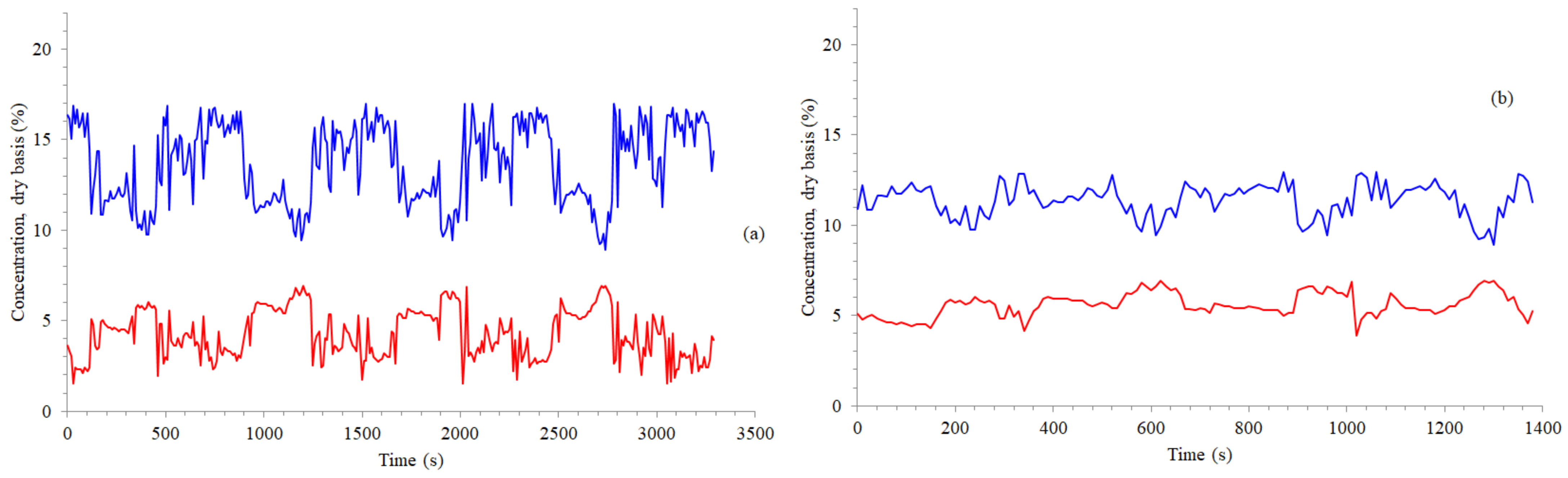
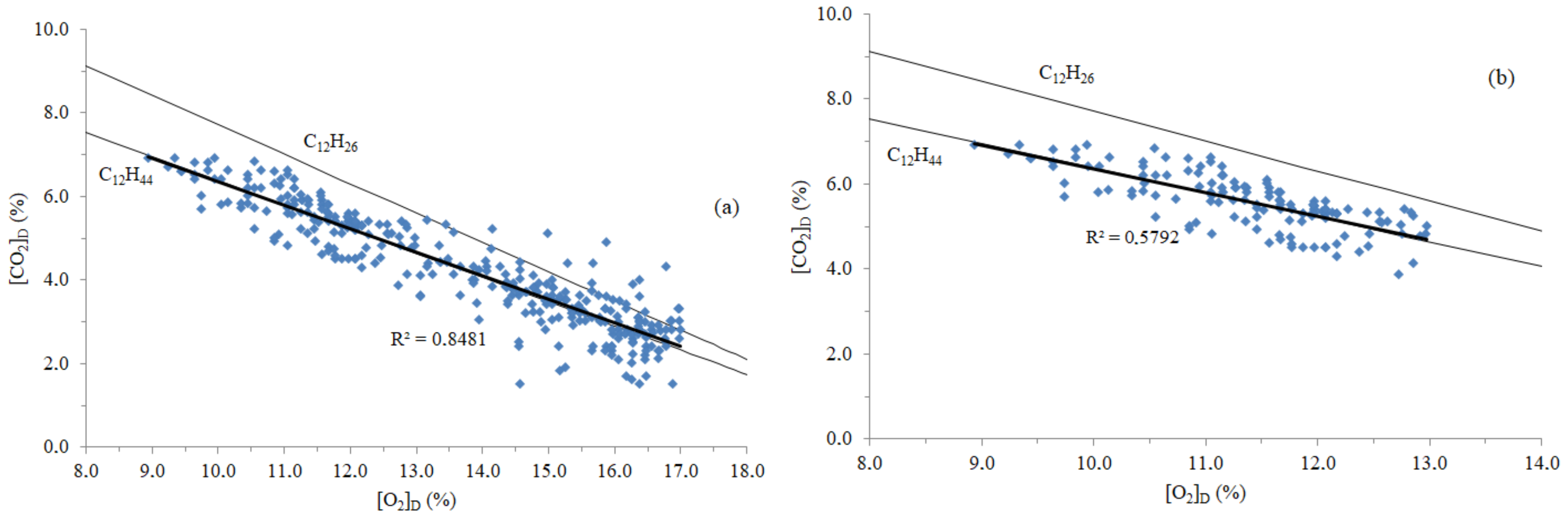
3.1. Time Concentrations, Carbon and Energy Losses, and Emission Factors
| Source | Emission Factor (kg CO2/kg Diesel) | % Default Value (IPCC [17]) |
|---|---|---|
| Laboratory [42] | 3.197 | 100.3% |
| Laboratory [40] | 3.106 | 97.5% |
| Truck #1 | 3.106 | 97.5% |
| Truck #2 | 2.298 | 72.1% |
| Truck #3 | 2.298 | 72.1% |
| Truck #4 | 2.065 | 64.8% |
| Li et al. (Truck) [35] | 3.157 | 99.1% |
| Li et al. (Truck) [35] | 3.183 | 99.9% |
| ECC Canada [44] | 3.212 a | 100.8% |
| IPCC [17] | 3.186 b | 100.0% |
- Truck #2: the original 350 points, corresponding to 0–3490 s, were substituted by 86 points, corresponding to 1260–2110 s;
- Truck #3: the original 460 points, corresponding to 0–4590 s, were substituted by 85 points, corresponding to 990–1830 s.
- Truck #2: average EF of 2.298 kg CO2/kg diesel becomes 2.401 kg CO2/kg diesel (4.5% change, 75.4% of IPCC default value); average carbon loss of 26.0% becomes 22.7% (3.3% difference);
- Truck #3: average EF of 2.298 kg CO2/kg diesel becomes 2.547 kg CO2/kg diesel (10.8% change, 79.9% of IPCC default value); average carbon loss of 26.0% becomes 18.0% (8.0% difference).
- Truck #4: the original 655 points were substituted by 329 points, corresponding to operation under [O2]D < 17%;
- Truck #4: the original 655 points were substituted by 138 points, corresponding to operation under [O2]D < 13%.
3.2. Error Estimates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| α | Air in excess factor |
| a | Hypothetical number of atoms of hydrogen in diesel’s empirical molecule |
| b | Hypothetical number of atoms of hydrogen in diesel’s empirical molecule |
| CH4 | Methane |
| CO2 | Carbon dioxide |
| CO | Carbon monoxide |
| CO2eq | Carbon dioxide equivalent emissions |
| COG | Coke oven gas |
| CmHn | Unburned hydrocarbon with m carbon and n hydrogen atoms |
| H2O | Water |
| ṁC,xi | Mass flow rate of carbon related to ith species |
| N2O | Nitrous oxide |
| N2 | Nitrogen |
| NO | Nitrogen monoxide |
| O2 | Oxygen |
| SO2 | Sulphur dioxide |
| x | Number of atoms of hydrogen in diesel’s empirical molecule |
| xi | Number of moles of ith species in combustion products |
| y | Number of mols of oxygen in reactants, mol |
| YC,fuel | Mass fraction of carbon in the fuel |
| [CO2]D | Molar concentration of CO2 in dry basis |
| [O2]D | Molar concentration of O2 in dry basis |
| [X]i,D | Molar concentration of ith species in combustion products |
Abbreviations
| ECC | Environment and Climate Change |
| EF | Emission factor |
| GHG | Greenhouse gases |
| IET | International Emissions Trading |
| IPCC | Intergovernmental Panel for Climate Change |
| NDIR | Infrared Non-dispersive System |
| PEMS | Portable Emissions Measurement Systems |
| UHC | Unburned hydrocarbons |
References
- Barrett, S. Political economy of the kyoto protocol. Clim. Chang. 2017, 14, 465–484. [Google Scholar] [CrossRef]
- Springer, U. The market for tradable GHG permits under the Kyoto Protocol: A survey of model studies. Energy Econ. 2003, 25, 527–551. [Google Scholar] [CrossRef]
- Johnson, E.; Heinen, R. Carbon trading: Time for industry involvement. Environ. Int. 2004, 30, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Karpf, A.; Mandel, A.; Battiston, S. Price and network dynamics in the European carbon market. J. Econ. Behav. Organ. 2018, 153, 103–122. [Google Scholar] [CrossRef] [Green Version]
- Watson, F. Global Carbon Market Grows 20% to $272 Billion in 2020: Refinitiv; S&P Glob Patts: London, UK, 2021. [Google Scholar]
- Aldhous, P. China’s Burning Ambition. Nature 2005, 435, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Hopke, P.K. Contemporary threats and air pollution. Atmos. Environ. 2009, 43, 87–93. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Xiao, L.; Liu, J.; Kao, W. Path towards achieving of China’s 2020 carbon emission reduction target-A discussion of low-carbon energy policies at province level. Energy Policy 2011, 39, 2740–2747. [Google Scholar] [CrossRef]
- Auffhammer, M.; Carson, R.T. Forecasting the path of China’s CO2 emissions using province-level information. J. Environ. Econ. Manag. 2008, 55, 229–247. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Fang, X.; Cao, Y.; Sun, C. Factors behind CO2 emission reduction in Chinese heavy industries: Do environmental regulations matter? Energy Policy 2020, 145, 111765. [Google Scholar] [CrossRef]
- Gerlagh, R.; Lise, W. Carbon taxes: A drop in the ocean, or a drop that erodes the stone? The effect of carbon taxes on technological change. Ecol. Econ. 2005, 54, 241–260. [Google Scholar] [CrossRef]
- Michaelowa, A.; Stronzik, M.; Eckermann, F.; Hunt, A. Transaction costs of the kyoto mechanisms. Clim. Policy 2003, 3, 261–278. [Google Scholar] [CrossRef] [Green Version]
- Nagase, K. “Carbon-Money Exchange” to contain global warming and deforestation. Energy Policy 2005, 33, 1233–1238. [Google Scholar] [CrossRef]
- ISO 14064-1:2018; Greenhouse Gases—Part 1: Specification with Guidance at the Organization Level for Quantification and Reporting of Greenhouse Gas Emissions and Removals. ISO: Geneva, Switzerland, 2019.
- Friedl, B.; Getzner, M. Determinants of CO2 emissions in a small open economy. Ecol. Econ. 2003, 45, 133–148. [Google Scholar] [CrossRef]
- Katta, A.K.; Davis, M.; Kumar, A. Assessment of greenhouse gas mitigation options for the iron, gold, and potash mining sectors. J. Clean. Prod. 2020, 245, 118718. [Google Scholar] [CrossRef]
- Inventories TF on NGG. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories—General Guidance and Reporting; IPCC, United Nations: Geneva, Switzerland, 2019; Volume 1. [Google Scholar]
- Benajes, J.; García, A.; Monsalve-Serrano, J.; Martínez-Boggio, S. Potential of using OMEx as substitute of diesel in the dual-fuel combustion mode to reduce the global CO2 emissions. Transp. Eng. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Song, H.; Ou, X.; Yuan, J.; Yu, M.; Wang, C. Energy consumption and greenhouse gas emissions of diesel/LNG heavy-duty vehicle fleets in China based on a bottom-up model analysis. Energy 2017, 140, 966–978. [Google Scholar] [CrossRef]
- Lao, J.; Song, H.; Wang, C.; Zhou, Y.; Wang, J. Beijing- Reducing atmospheric pollutant and greenhouse gas emissions of heavy duty trucks by substituting diesel with hydrogen in Tianjin-Hebei-Shandong region, China. Int. J. Hydrogen Energy 2021, 46, 18137–18152. [Google Scholar] [CrossRef]
- Xing, Y.; Song, H.; Yu, M.; Wang, C.; Zhou, Y.; Liu, G.; Du, L. The Characteristics of Greenhouse Gas Emissions from Heavy-Duty Trucks in the Beijing-Tianjin-Hebei (BTH) Region in China. Atmosphere 2016, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- González, R.M.; Marrero, G.; Rodriguez-López, J.; Marrero, A.S. Analyzing CO2 emissions from passenger cars in Europe: A dynamic panel data approach. Energy Policy 2019, 129, 1271–1281. [Google Scholar] [CrossRef]
- Li, X.; Yu, B. Peaking CO2 emissions for China’s urban passenger transport sector. Energy Policy 2019, 133, 110913. [Google Scholar] [CrossRef]
- Breed, A.; Speth, D.; Plötz, P. CO2 fleet regulation and the future market diffusion of zero-emission trucks in Europe. Energy Policy 2021, 159, 112640. [Google Scholar] [CrossRef]
- Anderhofstadt, B.; Spinler, S. Preferences for autonomous and alternative fuel-powered heavy-duty trucks in Germany. Transp. Res. Part D Transp. Environ. 2020, 79, 102232. [Google Scholar] [CrossRef]
- Quiros, D.; Smith, J.; Thiruvengadam, A.; Huai, T.; Hu, S. Greenhouse gas emissions from heavy-duty natural gas, hybrid, and conventional diesel on-road trucks during freight transport. Atmos. Environ. 2017, 168, 36–45. [Google Scholar] [CrossRef]
- Tietge, U.; Mock, P.; Franco, V.; Zacharof, N. From laboratory to road: Modeling the divergence between official and real-world fuel consumption and CO2 emission values in the German passenger car market for the years 2001–2014. Energy Policy 2017, 103, 212–232. [Google Scholar] [CrossRef]
- Brizuela, E. A novel presentation of Ostwald´s combustion. Ind. Comb. J. Int. Flame Res. Found. 2015. Available online: https://ifrf.net/research/archive/a-novel-presentation-of-ostwalds-combustion/ (accessed on 15 January 2022).
- Clairotte, M.; Suarez-Bertoa, R.; Zardini, A.; Giechaskiel, B.; Pavlovic, J.; Valverde, V.; Ciuffo, B.; Astorga, C. Exhaust emissions factors of greenhouse gases (GHGs) from European road vehicles. Environ. Sci. Eur. 2020, 32, 125–145. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Zhang, K.; Zhang, S.; Baldauf, R.; Snow, R.; Deshmukh, P.; Zheng, X.; He, L.; Hao, J. Evaluating mobile monitoring of on-road emission factors by comparing concurrent PEMS measurements. Sci. Total Environ. 2020, 736, 139507–139517 . [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J. Green traffic-oriented heavy-duty vehicle emissions characteristics of China VI based on portable emission measurement systems. IEEE Access 2020, 8, 106639–106647 . [Google Scholar] [CrossRef]
- He, L.; Zhang, S.; Hu, J.; Li, Z.; Zheng, X.; Cao, Y.; Xu, G.; Yan, M.; Wu, Y. On-road emission measurements of reactive nitrogen compounds from heavy-duty diesel trucks in China. Environ. Pollut. 2020, 262, 114280–114290. [Google Scholar] [CrossRef]
- Anable, J.; Brand, C.; Tran, M.; Eyre, N. Modelling transport energy demand: A socio-technical approach. Energy Policy 2012, 41, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Linton, C.; Grant-Muller, S.; Gale, W. Approaches and techniques for modelling CO2 emissions from road transport. Transp. Rev. 2015, 35, 533–553. [Google Scholar] [CrossRef]
- Li, X.; Ai, Y.; Ge, Y.; Qi, J.; Feng, Q.; Hu, J.; Porter, W.; Miao, Y.; Mao, H.; Jin, T. Integrated effects of SCR, velocity, and air-fuel ratio on gaseous pollutants and CO2 emissions from China V and VI heavy–duty diesel vehicles. Sci. Total Environ. 2022, 811, 152311–152319. [Google Scholar] [CrossRef]
- Nguyen, X.; Hoang, A.; Olçer, A.; Huynh, T. Record decline in global CO2 emissions prompted by COVID-19 pandemic and its implications on future climate changes policies. Energy Sources Part A 2021. [Google Scholar] [CrossRef]
- Hoang, T.; Nizetic, S.; Olcer, A.; Ong, H.; Chen, W.; Chong, C.; Thomas, S.; Bandh, S.; Nguyen, X. Impacts of COVID-19 on the global energy system and shift progress to renewable energy: Opportunities, challenges and policy implications. Energy Policy 2021, 154, 112322. [Google Scholar] [CrossRef]
- Nguyen, H.; Hoang, A.; Nizetic, S.; Nguyen, X.; Le, A.; Luong, C.; Chi, V.; Pham, V. The electric propulsion system as a green solution for management strategy of CO2 emissions in ocean shipping: A comprehensive review. Int. Trans. Electr. Energy Syst. 2021, 31, e12580. [Google Scholar] [CrossRef]
- Hoang, A.; Pham, V.; Nguyen, X. Integrating renewable sources into energy system for smart city as a sagacious strategy towards clean and sustainable process. J. Clean. Prod. 2021, 305, 127161. [Google Scholar] [CrossRef]
- Portela, P.; Coelho, F.; Vale Mining Company. Personal Communication, 2007.
- Borman, G.; Ragland, K. Combustion Engineering; McGraw-Hill: New York, NY, USA, 1998; pp. 125–126. [Google Scholar]
- Paz, E. Substitution of Diesel Fuel by Ethyl Alcohol in Industrial Burners. Ph.D. Thesis, Universidade Estadual Paulista Julio de Mesquita Filho, Guartinguetá, Brazil, 2007. (In Portuguese). [Google Scholar]
- Garcia, R. Fuels and Industrial Combustion. Interciência: Rio de Janeiro, Brazil, 2002. (In Portuguese) [Google Scholar]
- ECC Canada. Environment and Climate Change Canada. Greenhouse Gas Emissions—Canadian Environmental Sustainability Indicators; ECC Canada: Gatineau, QC, Canada, 2020; Volume 4. [Google Scholar]
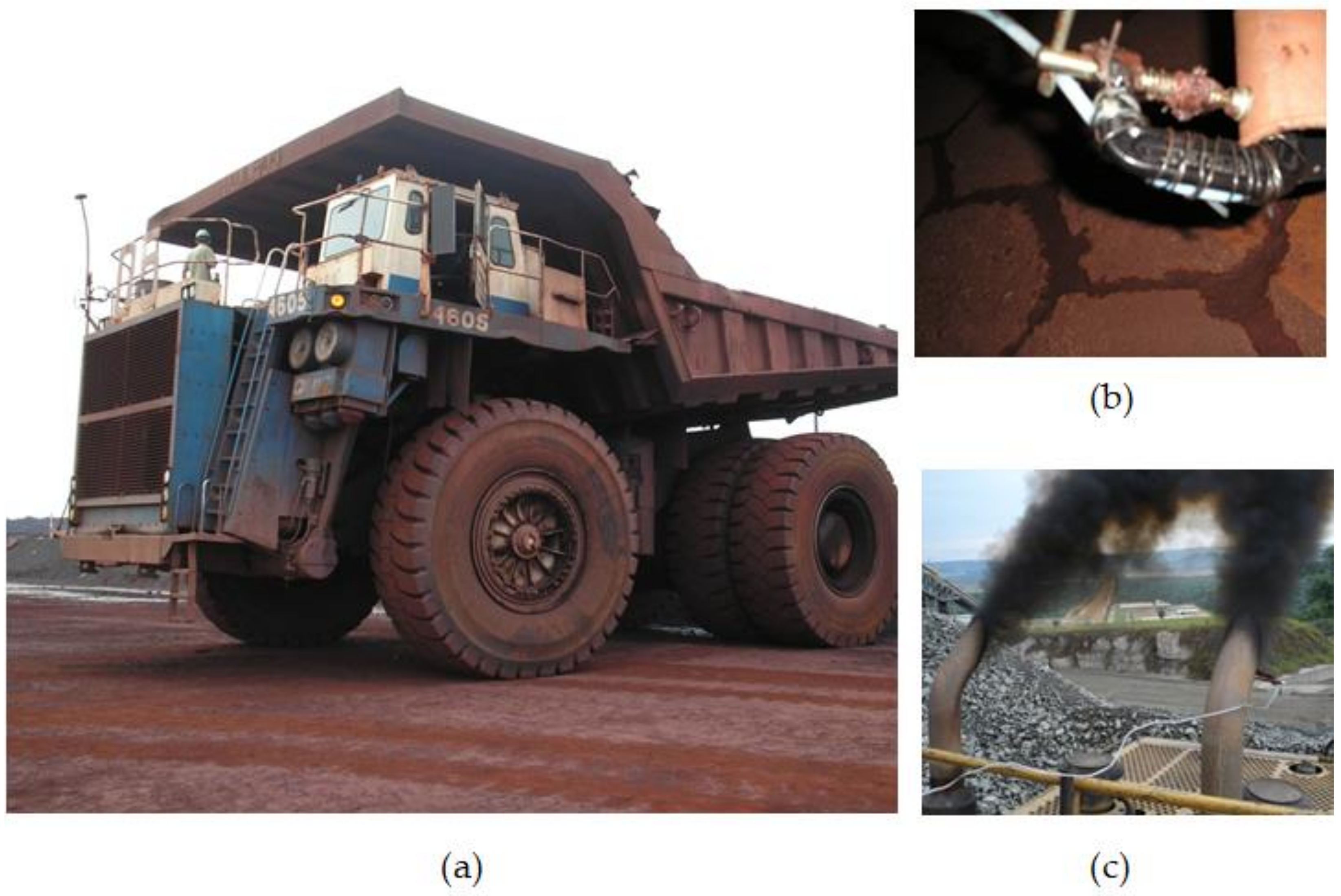

| Parameter | Sensor Type | Limits | Resolution | Maximum Response Time (s) |
|---|---|---|---|---|
| O2 | Electrochemical | 0–25.0% | 0.1% | 20 |
| CO | Electrochemical | 0–20,000 ppm | 1 ppm | 40 |
| CO2 | NDIR | 0–20.00% | 0.01% | 15 |
| NO | Electrochemical | 0–4000 ppm | 1 ppm | 40 |
| CxHy | NDIR | 0–50,000 ppm | 1 ppm | 15 |
| Tamb | Pt100 | −10–100 °C | 0.1 °C | |
| Tcomb gases | Thermocouple | 0–1000 °C | 0.1 °C | |
| Pressure | Bridge | ±150.00 hPa | 0.01 hPa |
| Truck #1 | Truck #2 | Truck #3 | Truck #4 | Total | % of Total | |
|---|---|---|---|---|---|---|
| # of points | 457 | 347 | 417 | 635 | 1856 | 100.0% |
| Below 3% | 456 | 347 | 412 | 622 | 1837 | 99.0% |
| Below 2% | 452 | 346 | 397 | 618 | 1813 | 97.7% |
| Below 1% | 431 | 329 | 305 | 509 | 1574 | 84.8% |
| [CO2] (%) | [O2] (%) | [CO] (%) | [SO2] (ppm) | [NOx] (p.p.m) | [CO2]corr (%) | [O2]corr (%) |
|---|---|---|---|---|---|---|
| 7.0 | 11.0 | 0.08 | 15.8 | 447 | 7.1 | 10.9 |
| 6.6 | 11.7 | 0.05 | 18.5 | 487 | 6.7 | 11.6 |
| 6.1 | 12.3 | 0.04 | 24.8 | 478 | 6.2 | 12.2 |
| 6.0 | 12.4 | 0.02 | 27.8 | 408 | 6.0 | 12.3 |
| 5.8 | 12.8 | 0.03 | 21.8 | 443 | 5.8 | 12.7 |
| 5.5 | 13.2 | 0.03 | 10.8 | 432 | 5.5 | 13.1 |
| 5.2 | 13.5 | 0.01 | 22.0 | 257 | 5.2 | 13.5 |
| 3.5 | 15.8 | 0.01 | 13.5 | 133 | 3.5 | 15.8 |
| 1.1 | 19.0 | 0.01 | 12.5 | 44 | 1.1 | 19.0 |
| Carbon Loss | Energy Loss | |
|---|---|---|
| Truck #1 | 0.0% | 0.0% |
| Truck #2 | 26.0% | 19.8% |
| Truck #3 | 26.0% | 19.8% |
| Truck #4 | 33.5% | 25.5% |
| Truck | Action | Fuel Formula | Emission Factor Error a |
|---|---|---|---|
| #1 | + + | C12H24.3 | 5.9% |
| + − | C12H24.3 | 5.9% | |
| − − | C12H26.1 | −0.3% | |
| − + | C12H26.1 | −0.3% | |
| #2 | + + | C12H36.4 | 2.4% |
| + − | C12H36.4 | 2.4% | |
| − − | C12H37.8 | −0.6% | |
| − + | C12H37.5 | 0.0% | |
| #3 | + + | C12H35.9 | 3.5% |
| + − | C12H36.0 | 3.3% | |
| − − | C12H37.6 | −0.2% | |
| − + | C12H37.6 | −0.2% | |
| #4 | + + | C12H43.2 | −0.4% |
| + − | C12H43.1 | −0.2% | |
| − − | C12H43 | 0.0% | |
| − + | C12H43.1 | −0.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, J.A., Jr.; de Castro, A.; Brasil, G.H.; de Souza, P.A., Jr.; Mendiburu, A.Z. CO2 Emission Factors and Carbon Losses for Off-Road Mining Trucks. Energies 2022, 15, 2659. https://doi.org/10.3390/en15072659
de Carvalho JA Jr., de Castro A, Brasil GH, de Souza PA Jr., Mendiburu AZ. CO2 Emission Factors and Carbon Losses for Off-Road Mining Trucks. Energies. 2022; 15(7):2659. https://doi.org/10.3390/en15072659
Chicago/Turabian Stylede Carvalho, João Andrade, Jr., André de Castro, Gutemberg Hespanha Brasil, Paulo Antonio de Souza, Jr., and Andrés Z. Mendiburu. 2022. "CO2 Emission Factors and Carbon Losses for Off-Road Mining Trucks" Energies 15, no. 7: 2659. https://doi.org/10.3390/en15072659
APA Stylede Carvalho, J. A., Jr., de Castro, A., Brasil, G. H., de Souza, P. A., Jr., & Mendiburu, A. Z. (2022). CO2 Emission Factors and Carbon Losses for Off-Road Mining Trucks. Energies, 15(7), 2659. https://doi.org/10.3390/en15072659







