Meranti (Shorea sp.) Biochar Application Method on the Growth of Sengon (Falcataria moluccana) as a Solution of Phosphorus Crisis
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Study
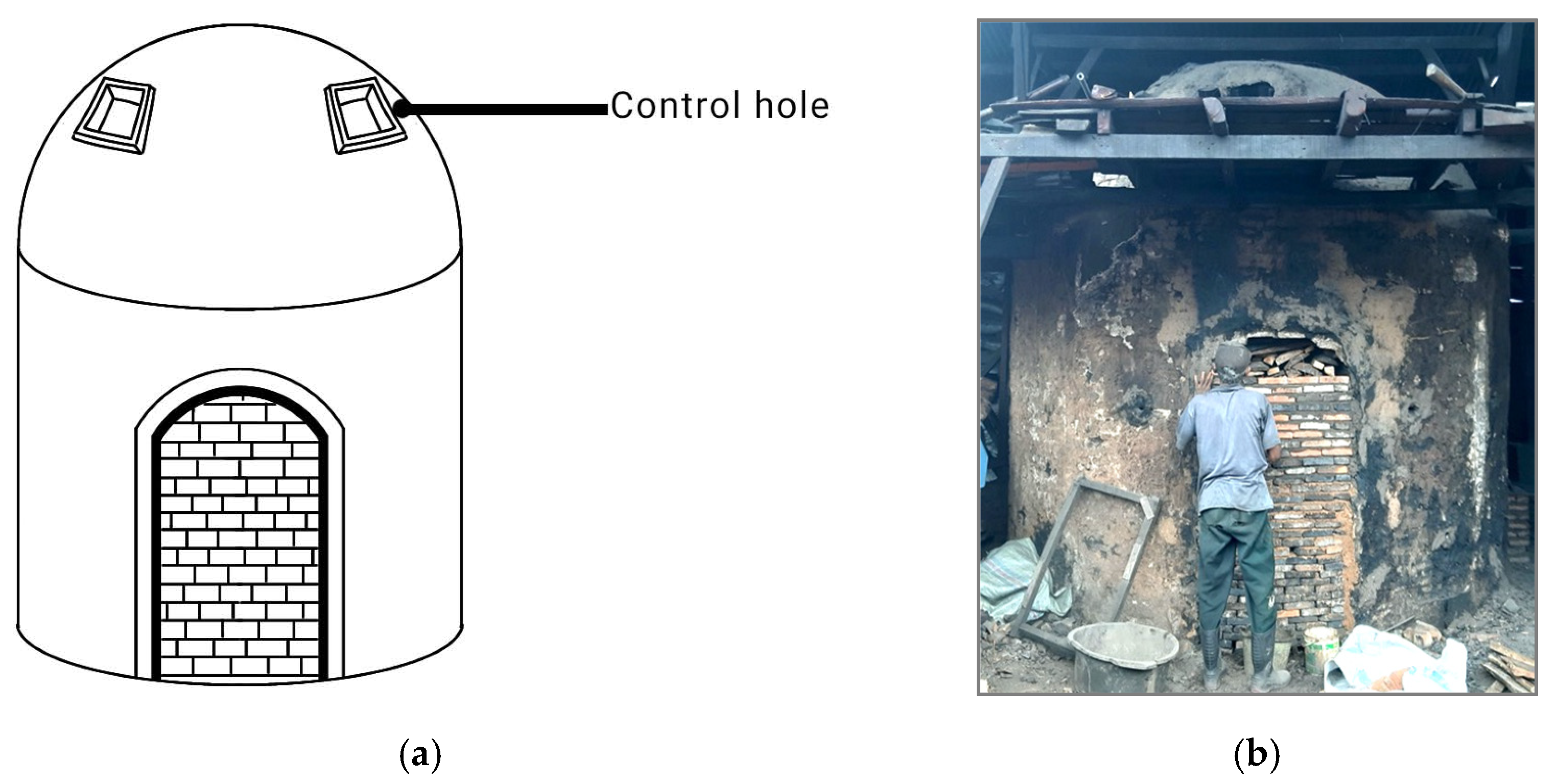
2.2. Biochar Production
2.3. Experiment Design
2.4. Biochar Application
2.5. P-Uptake on Leaves
2.6. Total-P, Organic-C, pH, and CEC Analysis
2.7. Statical Analysis
3. Results
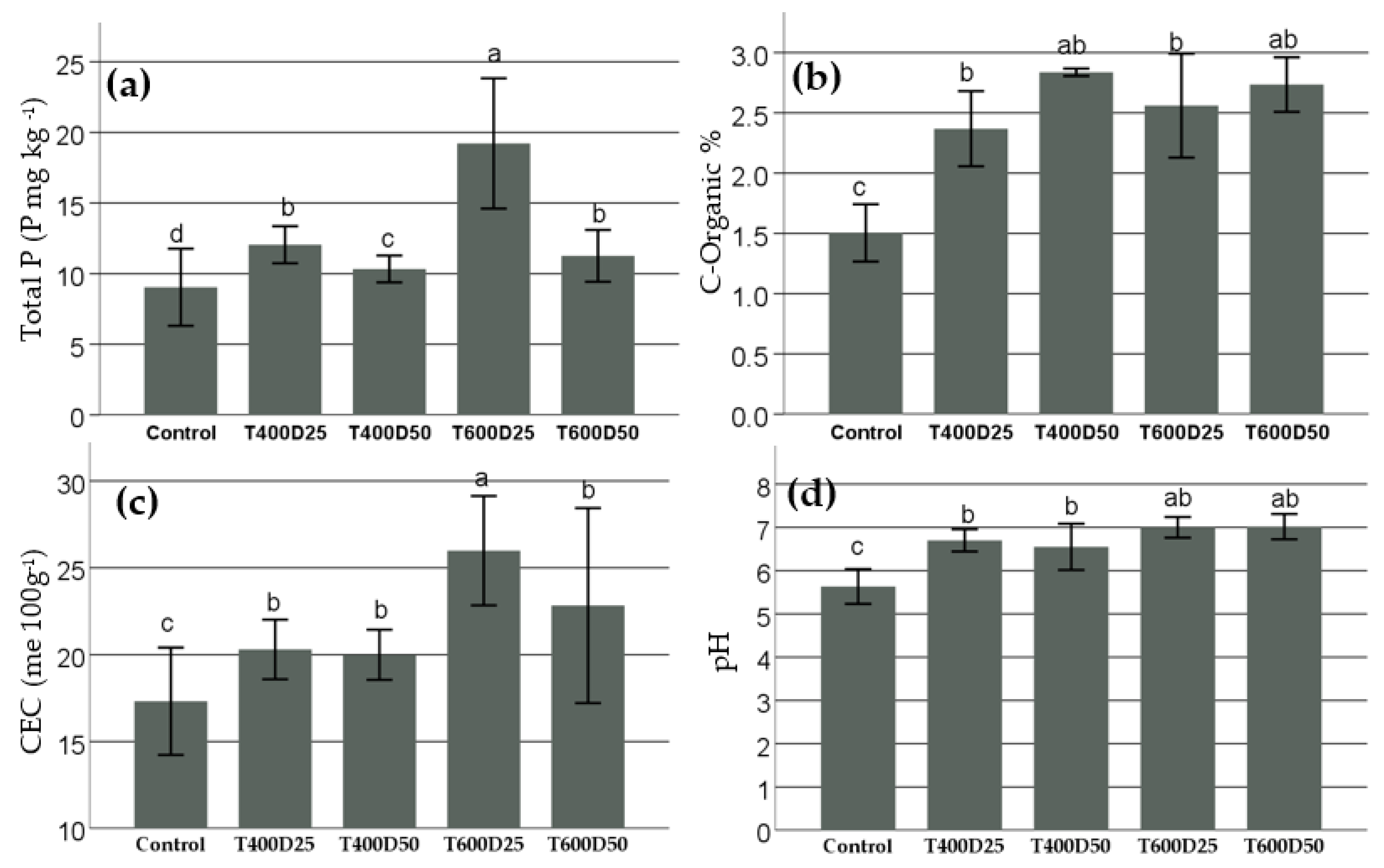
3.1. Biochar Effect on Total P, Organic-C, CEC, and pH
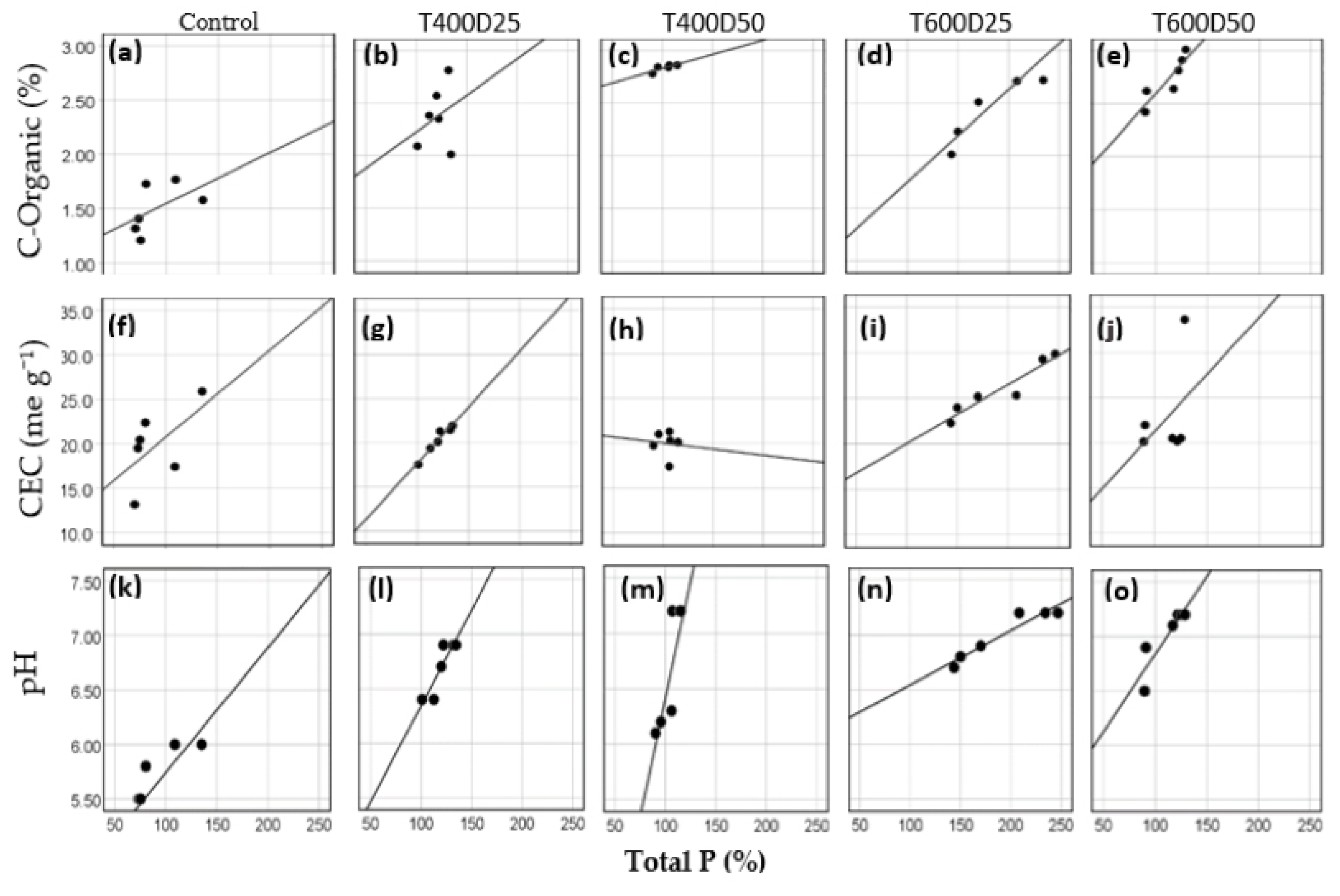
3.2. Correlation of Total P with Organic-C, pH, and CEC
3.3. P-Uptake on Leaf
3.4. Plant Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werner, E.; Ami, N. The phosphorus cycle. Aquat. Ecol. 2014, 6, 347–363. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Mirmanto, J.; Proctor, J.; Green, J.; Nagy, J. Suriantata Effects of nitrogen and phosphorus fertilization in lowland evergreen rainforest. R. Soc. 2018, 388, 539–547. [Google Scholar]
- Riniarti, M.; Setiawan, A. Soil fertility on two type of land cover in kesatuan pengelolaan hutan lindung (KPHL) batutegi lampung. J. Sylva Lestari 2014, 2, 99. [Google Scholar] [CrossRef]
- Giesler, R.; Ilvesniemi, H.; Nyberg, L.; Van Hees, P.; Starr, M.; Bishop, K.; Kareinen, T.; Lundström, U.S. Distribution and mobilization of Al, Fe and Si in three podzolic soil profiles in relation to the humus layer. Geoderma 2000, 94, 249–263. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Tiessen, H. Phosphorus in the global environment. In The Ecophysiology of Plant-Phosphorus Interactions; Business Media: Berlin, Germany, 2008; pp. 1–7. [Google Scholar]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Kavanová, M.; Lattanzi, F.A.; Grimoldi, A.A.; Schnyder, H. Phosphorus deficiency decreases cell division and elongation in grass leaves. Plant Physiol. 2006, 141, 766–775. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Dai, A.; Liu, L.; Li, Z. Root and shoot traits responses to phosphorus deficiency and QTL analysis at seedling stage using introgression lines of rice. J. Genet. Genom. 2009, 36, 173–183. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphorus and plant nutrition: An overview. In Phosphorus: Agriculture and the Environment; Cabrera, M., Ed.; Purdue University: West Lafayette, IN, USA, 2015; pp. 355–378. ISBN 9780891182696. [Google Scholar]
- Harper, D. Eutrophication of Freshwaters, 1st ed.; Springer Netherlands: Dordrecht, The Netherlands, 1992; Volume 1, ISBN 978-94-010-5366-2. [Google Scholar]
- Rast, W.; Thornton, J.A. Trends in eutrophication research and control. Hydrol. Process. 1996, 10, 295–313. [Google Scholar] [CrossRef]
- Xie, Z.; Li, S.; Tang, S.; Huang, L.; Wang, G.; Sun, X.; Hu, Z. Phosphorus Leaching from Soil Profiles in Agricultural and Forest Lands Measured by a Cascade Extraction Method. J. Environ. Qual. 2019, 48, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; He, W.; Yin, G.; Xu, S. Comparison of bio-chars formation derived from fast and slow pyrolysis of walnut shell. Fuel 2020, 261, 116450. [Google Scholar] [CrossRef]
- Koufopanos, C.A.; Lucchesi, A.; Maschio, G. Kinetic modelling of the pyrolysis of biomass and biomass components. Can. J. Chem. Eng. 1989, 67, 75–84. [Google Scholar] [CrossRef]
- Hidayat, W.; Qi, Y.; Jang, J.H.; Febrianto, F.; Lee, S.H.; Chae, H.M.; Kondo, T.; Kim, N.H. Carbonization characteristics of juvenile woods from some tropical trees planted in Indonesia. J. Fac. Agric. Kyushu Univ. 2017, 62, 145–152. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Yu, P.; Huang, L.; Li, Q.; Lima, I.M.; White, P.M.; Gu, M. Effects of mixed hardwood and sugarcane biochar as bark-based substrate substitutes on container plants production and nutrient leaching. Agronomy 2020, 10, 156. [Google Scholar] [CrossRef]
- Mašek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 2013, 103, 151–155. [Google Scholar] [CrossRef]
- Hidayat, W.; Kim, Y.K.; Jeon, W.S.; Lee, J.A.; Kim, A.R.; Park, S.H.; Maail, R.S.; Kim, N.H. Qualitative and quantitative anatomical characteristics of four tropical wood species from moluccas, indonesia. J. Korean Wood Sci. Technol. 2017, 42, 369–381. [Google Scholar]
- McBeath, A.V.; Smernik, R.J. Variation in the degree of aromatic condensation of chars. Org. Geochem. 2009, 40, 1161–1168. [Google Scholar] [CrossRef]
- Mahmoud, E.; El-Beshbeshy, T.; El-Kader, N.A.; El Shal, R.; Khalafallah, N. Impacts of biochar application on soil fertility, plant nutrients uptake and maize (Zea mays L.) yield in saline sodic soil. Arab. J. Geosci. 2019, 12, 719. [Google Scholar] [CrossRef]
- Criscuoli, I.; Ventura, M.; Wiedner, K.; Glaser, B.; Panzacchi, P.; Ceccon, C.; Loesch, M.; Raifer, B.; Tonon, G. Stability of woodchips biochar and impact on soil carbon stocks: Results from a two-year field experiment. Forests 2021, 12, 1350. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Smebye, A.; Alling, V.; Vogt, R.D.; Gadmar, T.C.; Mulder, J.; Cornelissen, G.; Hale, S.E. Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 2016, 142, 100–105. [Google Scholar] [CrossRef]
- Alexander, M. Biochemical Ecology of Soil Microorganisms. Annu. Rev. Microbiol. 1964, 18, 217–250. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Wu, H.; Lai, C.; Zeng, G.; Liang, J.; Chen, J.; Xu, J.; Dai, J.; Li, X.; Liu, J.; Chen, M.; et al. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: A review. Crit. Rev. Biotechnol. 2017, 37, 754–764. [Google Scholar] [CrossRef]
- Stotzky, G. Influence of Clay Minerals on Microorganisms. Can. J. Microbiol. 1966, 12, 1235–1246. [Google Scholar] [CrossRef]
- Wijaya, B.A.; Riniarti, M.; Prasetia, H.; Hidayat, W.; Niswati, A.; Hasanudin, U.; Banuwa, I.S. The interaction of dose treatment and pyrolysis temperature of meranti wood biochar (Shorea spp.) affects the growth rate of sengon (Paraserianthes moluccana). ULIN J. Hutan Trop. 2021, 5, 78. [Google Scholar] [CrossRef]
- Mazlan, M.A.F.; Uemura, Y.; Osman, N.B.; Yusup, S. Characterizations of bio-char from fast pyrolysis of Meranti wood sawdust. J. Phys. Conf. Ser. 2015, 622, 012054. [Google Scholar] [CrossRef]
- Gupta, G.K.; Gupta, P.K.; Mondal, M.K. Experimental process parameters optimization and in-depth product characterizations for teak sawdust pyrolysis. Waste Manag. 2019, 87, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, M.A.F.; Uemura, Y.; Yusup, S. Fast pyrolysis of rubber wood sawdust via a fluidized bed pyrolyzer: The effect of fluidization gas velocity. Sindh Univ. Res. J. 2016, 48, 9–15. [Google Scholar]
- Central Bureau of Statistics of the Republic of Indonesia. Available online: https://www.bps.go.id/indicator/60/502/1/produksi-kayu-bulat-perusahaan-hak-pengusahaan-hutan-hph-menurut-jenis-kayu.html (accessed on 28 December 2021).
- Susanto, D.; Widyarko, W. Sustainable Material: Used Wood As Building Material. Insist 2017, 2, 14. [Google Scholar] [CrossRef][Green Version]
- Sari, D.R. Ariyanto The potential of woody waste biomass from the logging activity at the natural forest of Berau District, East Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2018, 144, 012061. [Google Scholar] [CrossRef]
- Haryanto, A.; Hidayat, W.; Hasanudin, U.; Iryani, D.A.; Kim, S.; Lee, S.; Yoo, J. Valorization of indonesian wood wastes through pyrolysis: A review. Energies 2021, 14, 1407. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management, 1st ed.; Routledge: London, UK, 2009; ISBN 9781849770552. [Google Scholar]
- Li, S.; Zhang, Y.; Yan, W.; Shangguan, Z. Effect of biochar application method on nitrogen leaching and hydraulic conductivity in a silty clay soil. Soil Tillage Res. 2018, 183, 100–108. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Xu, Y.; Lu, X. Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 2020, 244, 125471. [Google Scholar] [CrossRef]
- Hughes, R.; Johnson, M.; Uowolo, A. The Invasive Alien Tree Falcataria moluccana: Its Impacts and Management. In Proceedings of the XIII International Symposium on Biological Control of Weeds, Waikoloa, HI, USA, 11–16 September 2011; pp. 218–223. [Google Scholar]
- Ridjayanti, S.M.; Bazenet, R.A.; Hidayat, W.; Banuwa, I.S.; Riniarti, M. The influence of adhesive content variation on the characteristics of sengon (Falcatataria moluccana) wood charcoal briquettes. Perennial 2021, 17, 5–11. [Google Scholar]
- Meteorology Climatology and Geophysics Council (BMKG), Data Online Pusat Database. Available online: https://dataonline.bmkg.go.id/home (accessed on 31 December 2021).
- Muir, A. The Podzol and Podzolic Soils. Adv. Agron. 1961, 13, 1–56. [Google Scholar] [CrossRef]
- Lee, Y.; Eum, P.R.B.; Ryu, C.; Park, Y.K.; Jung, J.H.; Hyun, S. Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresour. Technol. 2013, 130, 345–350. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Hidayat, W.; Riniarti, M.; Prasetia, H.; Niswati, A. Characteristics of biochar produced from the harvesting wastes of meranti (Shorea sp.) and oil palm (Elaeis guineensis) empty fruit bunches. Characteristics of biochar produced from the harvesting wastes of meranti (Shorea sp.) and oil palm (Elaeis. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Flindt, M.; Lillebø, A. Determination of Total Nitrogen and Phosphorus in Leaf Litter\rMethods to Study Litter Decomposition. In Methods to Study Litter Decomposition: A Practical Guide; Springer: Copenhagen, Denmark, 2005; pp. 53–59. ISBN 978-1-4020-3466-4. [Google Scholar]
- Martin, M.; Celi, L.; Barberis, E. Determination of low concentrations of organic phosphorus in soil solution. Commun. Soil Sci. Plant Anal. 1999, 30, 1909–1917. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Aran, D.; Maul, A.; Masfaraud, J.F. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. Comptes Rendus-Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Oman, S.F.; Camões, M.F.; Powell, K.J.; Rajagopalan, R.; Spitzer, P. Guidelines for potentiometric measurements in suspensions part A. The suspension effect: IUPAC technical report. Pure Appl. Chem. 2007, 79, 67–79. [Google Scholar] [CrossRef]
- De Vaus, D. Analyzing Social Science Data: 50 Key Problems in Data Analysis, 1st ed.; Sage Publication: New Delhi, India, 2002; Volume 1. [Google Scholar]
- Shigaki, F.; Sharpley, A.; Prochnow, L.I. Rainfall intensity and phosphorus source effects on phosphorus transport in surface runoff from soil trays. Sci. Total Environ. 2007, 373, 334–343. [Google Scholar] [CrossRef]
- Föllmi, K.B. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Sci. Rev. 1996, 40, 55–124. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Li, Y.; Teng, W.; Wang, W.; Yang, T. Transformation of apatite phosphorus and non-apatite inorganic phosphorus during incineration of sewage sludge. Chemosphere 2015, 141, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, T.; Tsujimoto, Y.; Rinasoa, S.; Rakotoson, T.; Andriamananjara, A.; Razafimbelo, T. Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 2019, 435, 27–38. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.N.; Shao, H.B.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Baigorri, R.; Francisco, S.S.; Urrutia, Ó.; García-Mina, J.M. Biochar-Ca and biochar-Al/-Fe-mediated phosphate exchange capacity are main drivers of the different biochar effects on plants in acidic and alkaline soils. Agronomy 2020, 10, 968. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Chardon, W.J.; de Willigen, P.; van Riemsdijk, W.H. Phosphorus Desorption Dynamics in Soil and the Link to a Dynamic Concept of Bioavailability. J. Environ. Qual. 2004, 33, 1393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Lago, B.C.; Silva, C.A.; Melo, L.C.A.; de Morais, E.G. Predicting biochar cation exchange capacity using Fourier transform infrared spectroscopy combined with partial least square regression. Sci. Total Environ. 2021, 794, 148762. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef]
- Herviyanti, H.; Maulana, A.; Prima, S.; Aprisal, A.; Crisna, S.D.; Lita, A.L.; Herviyanti, H. Effect of biochar from young coconut waste to improve chemical properties of ultisols and growth coffee [Coffea arabica L.] plant seeds. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2020; Volume 497, p. 012038. [Google Scholar]
- Lee, J.W.; Kidder, M.; Evans, B.R.; Paik, S.; Buchanan, A.C.; Garten, C.T.; Brown, R.C. Characterization of biochars produced from cornstovers for soil amendment. Environ. Sci. Technol. 2010, 44, 7970–7974. [Google Scholar] [CrossRef]
- Veiga, T.R.L.A.; Lima, J.T.; de Abreu Dessimoni, A.L.; Pego, M.F.F.; Soares, J.R.; Trugilho, P.F. Different plant biomass characterization for biochar production. Cerne 2017, 23, 529–536. [Google Scholar] [CrossRef]
- Amery, F.; Smolders, E. Unlocking fixed soil phosphorus upon waterlogging can be promoted by increasing soil cation exchange capacity. Eur. J. Soil Sci. 2012, 63, 831–838. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil ph affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Miao, Y.; Mai, Y.; Li, H.; Chen, X.; Chen, J. A lignin-biochar with high oxygen-containing groups for adsorbing lead ion prepared by simultaneous oxidization and carbonization. Bioresour. Technol. 2020, 307, 123165. [Google Scholar] [CrossRef]
- Dewi, W.S.; Prijono, S. Effect of high doses of rice husk biochar on soil physical properties and growth of maize on a typic kanhapludult. J. Tanah Sumberd. Lahan 2019, 6, 1157–1163. [Google Scholar] [CrossRef][Green Version]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Chen, J.-H. Barber Division s-4-soil fertility & plant nutrition. Soil Sci. Am. 1990, 54, 1032–1036. [Google Scholar] [CrossRef]
- Bolan, N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Riniarti, M.; Wahyuni, A.E. Surnayanti Soil inoculum heating impact on the ectomycorrhizal colonization ability of Shorea javanica roots. EnviroScienteae 2017, 13, 54–61. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Seeling, B.; Zasoski, R.J. Microbial effects in maintaining organic and inorganic solution phosphorus concentrations in a grassland topsoil. Plant Soil 1993, 148, 277–284. [Google Scholar] [CrossRef]
- Leys, N.M.; Bastiaens, L.; Verstraete, W.; Springael, D. Influence of the carbon/nitrogen/phosphorus ratio on polycyclic aromatic hydrocarbon degradation by Mycobacterium and Sphingomonas in soil. Appl. Microbiol. Biotechnol. 2005, 66, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Dalai, R.C. Soil Organic Phosphorus. Adv. Agron. 1977, 29, 83–117. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Henderson, P.J.F. The 12-transmembrane helix transporters. Curr. Opin. Cell Biol. 1993, 5, 708–721. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M.; Oku, H.; Nahar, K.; Hawrylak-Nowak, B. Plant nutrients and abiotic stress tolerance. In Plant Nutrients and Abiotic Stress Tolerance; Springer Nature Singapore Pte: Singapore, 2018; pp. 171–190. ISBN 9789811090448. [Google Scholar]
- Seigneuret, M.; Devaux, P.F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: Relation to shape changes. Proc. Natl. Acad. Sci. USA 1984, 81, 3751–3755. [Google Scholar] [CrossRef] [PubMed]
- Stouthamer, A.H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek 1973, 39, 545–565. [Google Scholar] [CrossRef]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 1978–2021. [Google Scholar] [CrossRef] [PubMed]




| Properties | Pyrolysis Temperature | |
|---|---|---|
| 400 °C | 600 °C | |
| Ash content | 0.76 | 02.52 |
| Volatile matter | 42.62 | 31.11 |
| Fixed carbon | 56.62 | 66.35 |
| pH | 8.5 | 8.7 |
| Parameter | Total P | ||||
|---|---|---|---|---|---|
| Control | T400D25 | T400D50 | T600D25 | T600D50 | |
| Organic-C | 0.540 | 0.292 | 0.832 | 0.937 | 0.885 |
| pH | 0.777 | 0.896 | 0.775 | 0.954 | 0.894 |
| CEC | 0.589 | 0.969 | −0.088 | 0.948 | 0.412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, B.A.; Hidayat, W.; Riniarti, M.; Prasetia, H.; Niswati, A.; Hasanudin, U.; Banuwa, I.S.; Kim, S.; Lee, S.; Yoo, J. Meranti (Shorea sp.) Biochar Application Method on the Growth of Sengon (Falcataria moluccana) as a Solution of Phosphorus Crisis. Energies 2022, 15, 2110. https://doi.org/10.3390/en15062110
Wijaya BA, Hidayat W, Riniarti M, Prasetia H, Niswati A, Hasanudin U, Banuwa IS, Kim S, Lee S, Yoo J. Meranti (Shorea sp.) Biochar Application Method on the Growth of Sengon (Falcataria moluccana) as a Solution of Phosphorus Crisis. Energies. 2022; 15(6):2110. https://doi.org/10.3390/en15062110
Chicago/Turabian StyleWijaya, Bangun Adi, Wahyu Hidayat, Melya Riniarti, Hendra Prasetia, Ainin Niswati, Udin Hasanudin, Irwan Sukri Banuwa, Sangdo Kim, Sihyun Lee, and Jiho Yoo. 2022. "Meranti (Shorea sp.) Biochar Application Method on the Growth of Sengon (Falcataria moluccana) as a Solution of Phosphorus Crisis" Energies 15, no. 6: 2110. https://doi.org/10.3390/en15062110
APA StyleWijaya, B. A., Hidayat, W., Riniarti, M., Prasetia, H., Niswati, A., Hasanudin, U., Banuwa, I. S., Kim, S., Lee, S., & Yoo, J. (2022). Meranti (Shorea sp.) Biochar Application Method on the Growth of Sengon (Falcataria moluccana) as a Solution of Phosphorus Crisis. Energies, 15(6), 2110. https://doi.org/10.3390/en15062110









