Effect of Ignition Energy and Hydrogen Addition on Laminar Flame Speed, Ignition Delay Time, and Flame Rising Time of Lean Methane/Air Mixtures
Abstract
:1. Introduction
2. Methodology
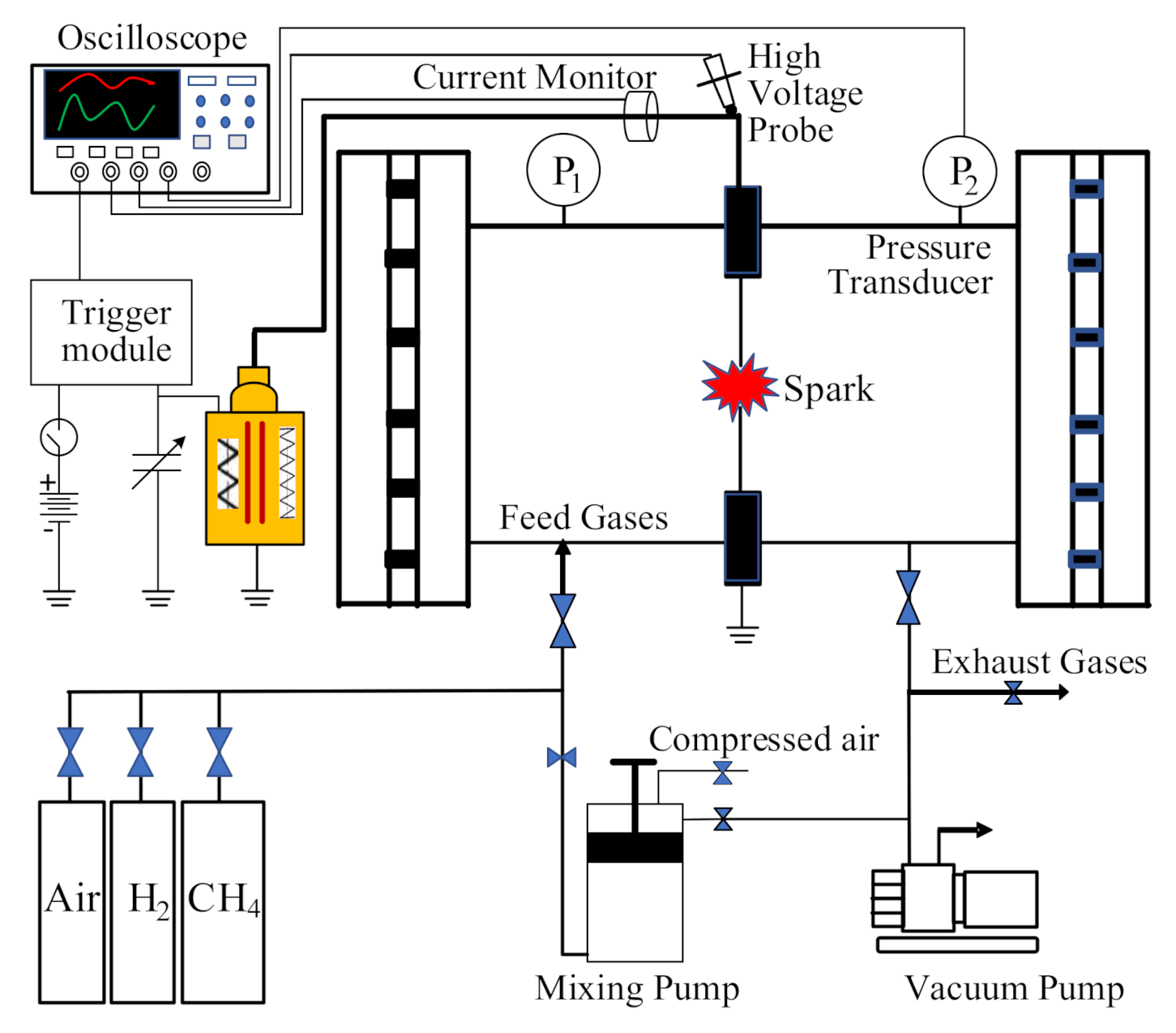
2.1. Experimental Setup, Procedure, and Conditions
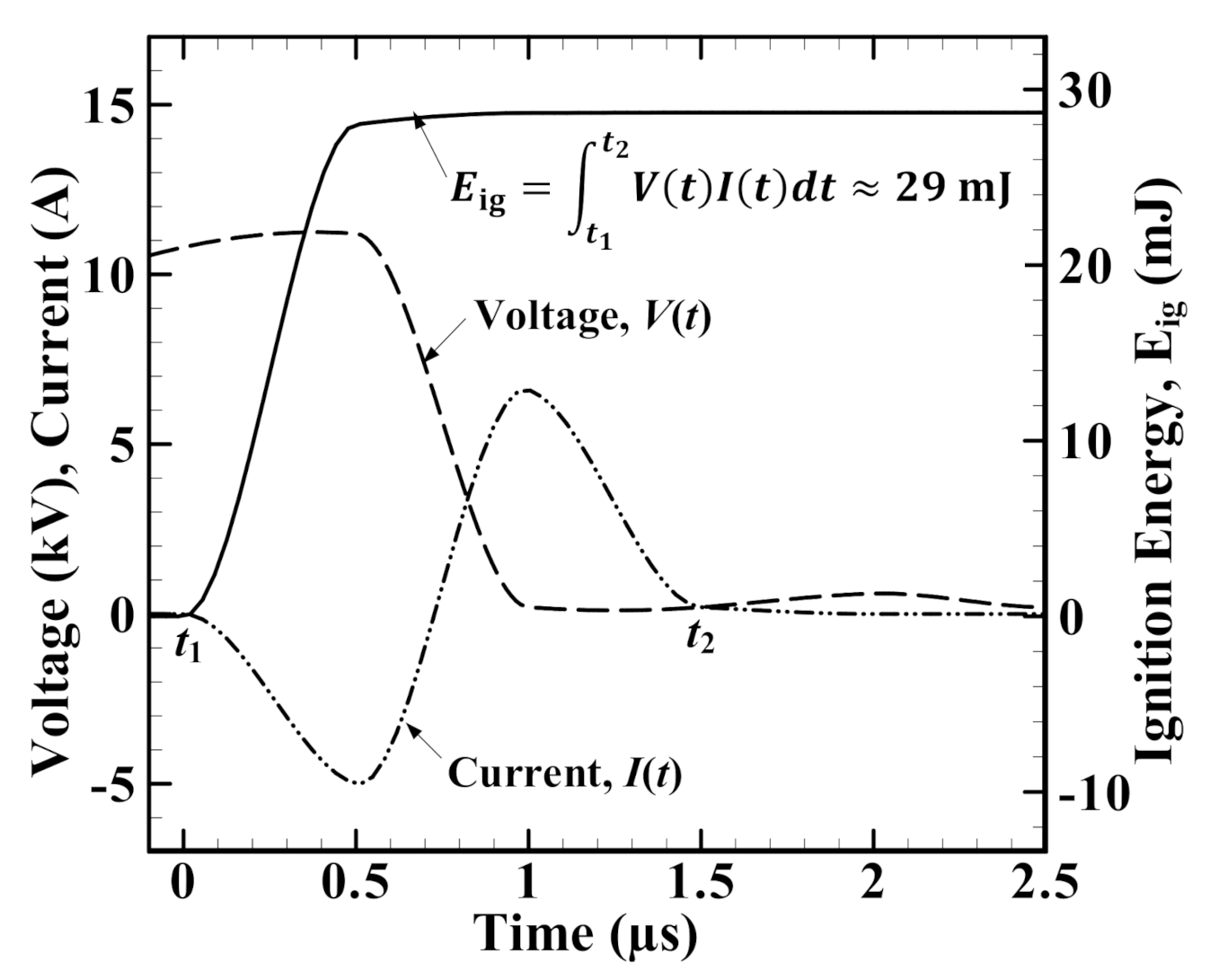
2.2. Data Processing and Parameter Definition
3. Results and Discussion
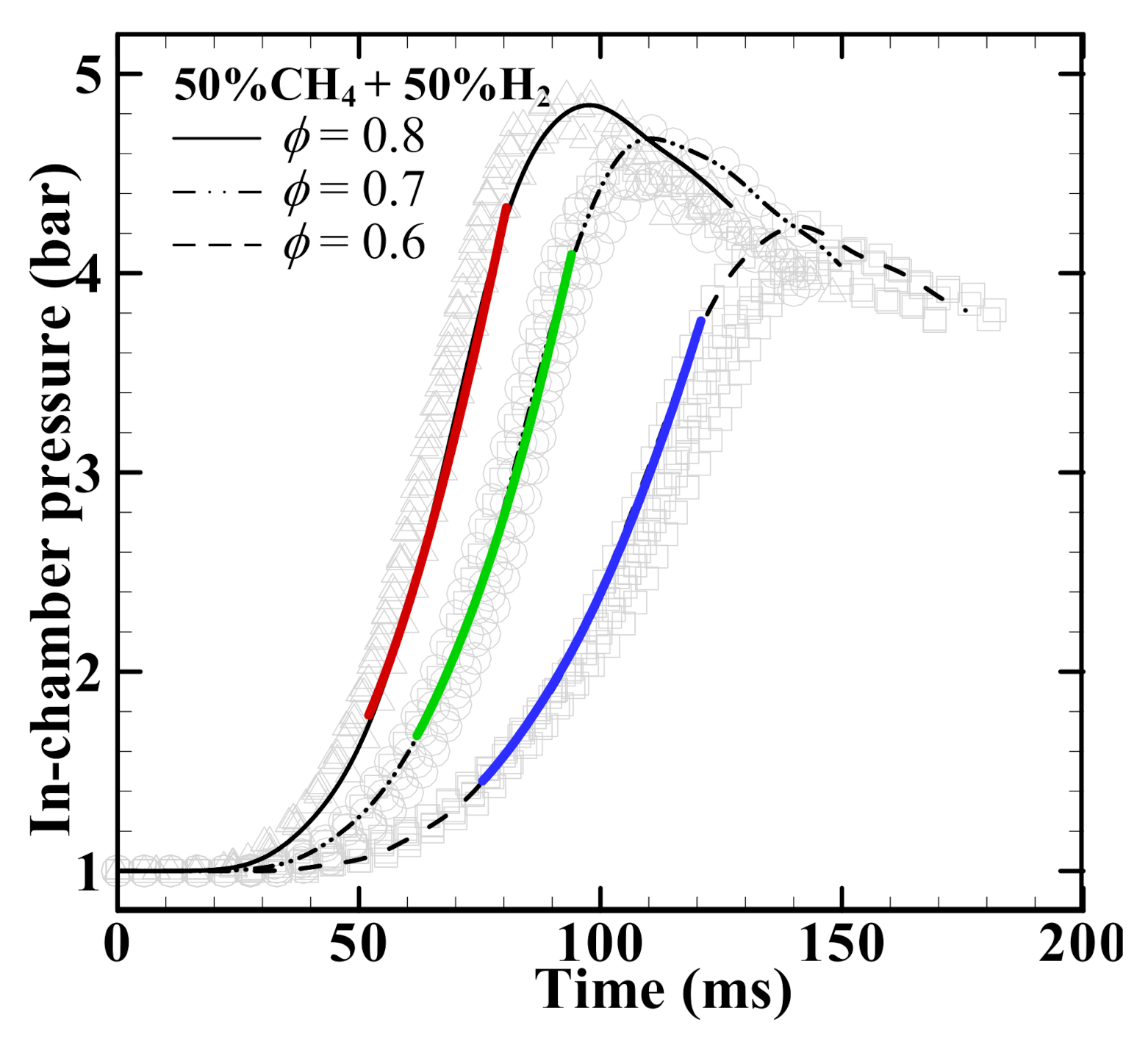
3.1. In-Chamber Pressure Profile and Laminar Burning Velocity Su0
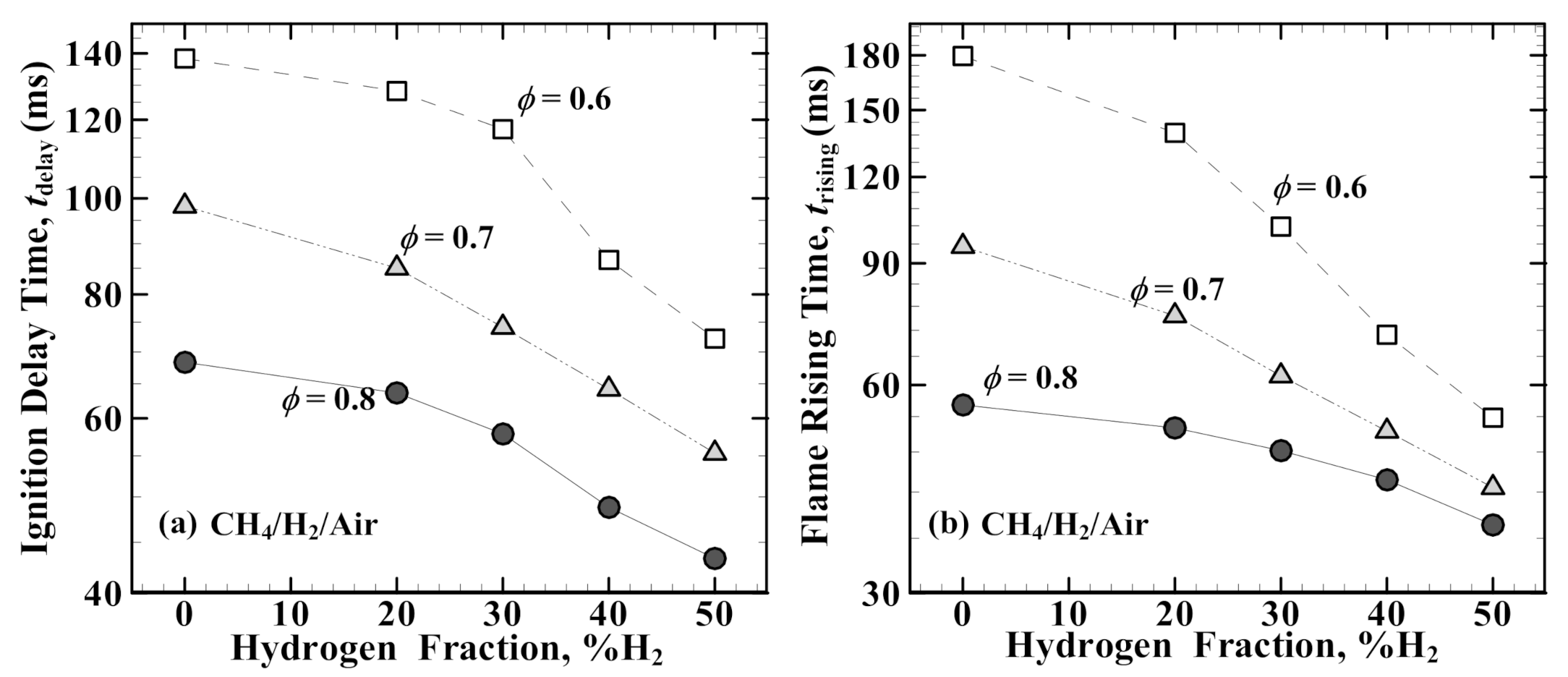
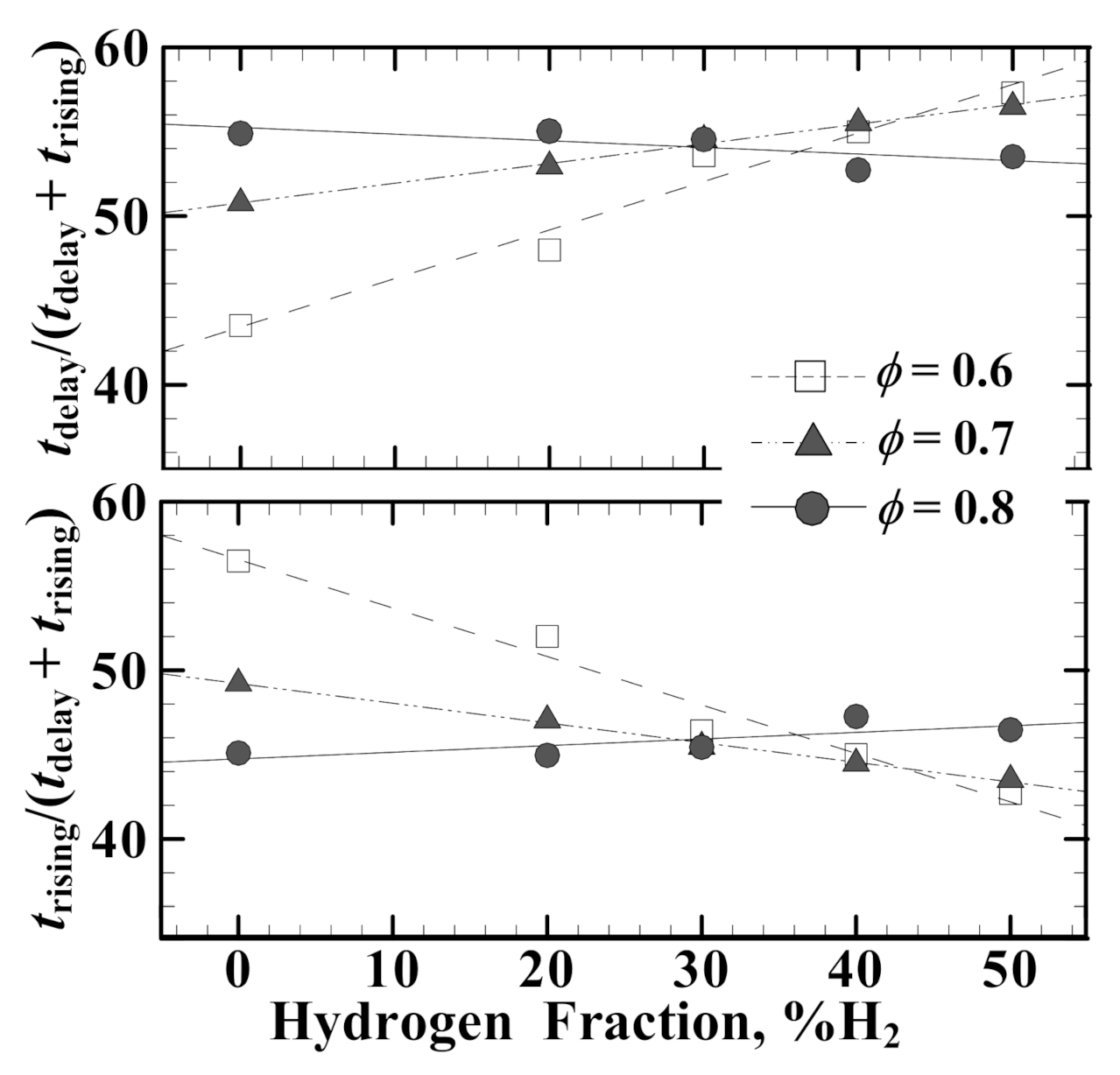
3.2. Ignition Delay Time (tdelay) and Flame Rising Time (trising)
4. Conclusions
- (1)
- The peak of the in-chamber pressure profile increases when increasing hydrogen volume fraction at three equivalence ratios of 0.6, 0.7, and 0.8, suggesting a higher heat release.
- (2)
- The leftward moving of peak pressure is observed when increasing hydrogen volume fraction, equivalence ratio, and ignition energy, suggesting a faster flame propagation and/or shorter explosion duration time.
- (3)
- Su0 increases non-linearly with increasing hydrogen volume fraction for all equivalence ratios (ϕ = 0.6, 0.7, and 0.8). Su0 gradually increases as the hydrogen fraction increases from 0 to 20% and is rather rapid when the hydrogen fraction is greater than 20%.
- (4)
- There is a tdelay transition at a critical value ignition energy (i.e., Eig,critical ∼24 mJ for 50% CH4/50% H2/air mixture at ϕ = 0.8) in which tdelay drastically drops in the pre-transition and gradually decreases in the post-transition. The existence of Eig,critical could partly explain why very high Eig >> Eig,critical has less of a contribution to the improvement of SI engine performance and emissions.
- (5)
- Both tdelay and trising decrease with increasing hydrogen fraction in the lean methane/air mixtures. For the leaner mixtures (ϕ = 0.6 and 0.7), the dropping slope of trising is much steeper than that of tdelay, while the reverse trend is true at ϕ = 0.8.
Author Contributions
Funding
Conflicts of Interest
References
- Okafor, E.; Hayakawa, A.; Nagano, Y.; Kitagawa, T. Effects of hydrogen concentration on premixed laminar flames of hydrogen–methane–air. Int. J. Hydrogen Energy 2014, 39, 2409. [Google Scholar] [CrossRef]
- Benaissa, S.; Adouane, B.; Ali, S.M.; Mohammad, A. Effect of hydrogen addition on the combustion characteristics of premixed biogas/hydrogen-air mixtures. Int. J. Hydrogen Energy 2021, 46, 18661–18677. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- El-Kassaby, M.M.; Eldrainy, Y.A.; Khidr, M.E.; Khidr, K.I. Effect of hydroxy (hho) gas addition on gasoline engine performance and emissions. Alex. Eng. J. 2016, 55, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Nakata, K.; Nogawa, S.; Takahashi, D.; Yoshihara, Y.; Kumagai, A.; Suzuki, T. Engine technologies for achieving 45% thermal efficiency of s.I. Engine. SAE Int. J. Engines 2015, 9, 179–192. [Google Scholar] [CrossRef]
- Rapp, V.H.; DeFilippo, A.; Saxena, S.; Chen, J.-Y.; Dibble, R.W.; Nishiyama, A.; Moon, A.; Ikeda, Y. Extending lean operating limit and reducing emissions of methane spark-ignited engines using a microwave-assisted spark plug. J. Combust. 2012, 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, N.; Sugiura, A.; Abe, Y.; Suzuki, K. Development of ignition technology for dilute combustion engines. SAE Int. J. Engines 2017, 10, 984–995. [Google Scholar] [CrossRef]
- Tsuboi, S.; Miyokawa, S.; Matsuda, M.; Yokomori, T.; Iida, N. Influence of spark discharge characteristics on ignition and combustion process and the lean operation limit in a spark ignition engine. Appl. Energy 2019, 250, 617–632. [Google Scholar] [CrossRef]
- Aleiferis, P.G.; Taylor, A.M.K.P.; Ishii, K.; Urata, Y. The nature of early flame development in a lean-burn stratified-charge spark-ignition engine. Combust. Flame 2004, 136, 283–302. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, M. Future gasoline engine ignition: A review on advanced concepts. Int. J. Engine Res. 2020, 22, 1743–1775. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Y.; Liu, H.; Li, Y.; Wang, J.; Zhao, S. Experimental study on thermal efficiency and emission characteristics of a lean burn hydrogen enriched natural gas engine. Int. J. Hydrogen Energy 2007, 32, 5067–5075. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Y. Study on the extension of lean operation limit through hydrogen enrichment in a natural gas spark-ignition engine. Int. J. Hydrogen Energy 2008, 33, 1416–1424. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.G.; Lei, Y.; Bai, X.L.; Sun, X.N.; Wang, D.J.; Yao, B.F. Effects of engine operating parameters on lean combustion limit of hydrogen enhanced natural gas engine. Adv. Mater. Res. 2011, 383–390, 6116–6121. [Google Scholar] [CrossRef]
- Karim, G.A.; Wierzba, I.; Al-Alousi, Y. Methane-hydrogen mixtures as fuels. Int. J. Hydrogen Energy 1996, 21, 625–631. [Google Scholar] [CrossRef]
- Akansu, S.; Kahraman, N.; Ceper, B. Experimental study on a spark ignition engine fuelled by methane–hydrogen mixtures. Int. J. Hydrogen Energ 2007, 32, 4279–4284. [Google Scholar] [CrossRef]
- Ilbas, M.; Crayford, A.; Yilmaz, I.; Bowen, P.; Syred, N. Laminar-burning velocities of hydrogen–air and hydrogen–methane–air mixtures: An experimental study. Int. J. Hydrogen Energy 2006, 31, 1768–1779. [Google Scholar] [CrossRef]
- Chen, Z. Effects of hydrogen addition on the propagation of spherical methane/air flames: A computational study. Int. J. Hydrogen Energy 2009, 34, 6558–6567. [Google Scholar] [CrossRef]
- Di Sarli, V.; Benedetto, A.D. Laminar burning velocity of hydrogen–methane/air premixed flames. Int. J. Hydrogen Energy 2007, 32, 637–646. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Jin, C.; Zheng, J. Experimental and numerical study on laminar burning characteristics of premixed methane–hydrogen–air flames. Int. J. Hydrogen Energy 2009, 34, 4876–4888. [Google Scholar] [CrossRef]
- Donohoe, N.; Heufer, A.; Metcalfe, W.K.; Curran, H.J.; Davis, M.L.; Mathieu, O.; Plichta, D.; Morones, A.; Petersen, E.L.; Güthe, F. Ignition delay times, laminar flame speeds, and mechanism validation for natural gas/hydrogen blends at elevated pressures. Combust. Flame 2014, 161, 1432–1443. [Google Scholar] [CrossRef] [Green Version]
- Di Sarli, V.; Di Benedetto, A.; Long, E.J.; Hargrave, G.K. Time-resolved particle image velocimetry of dynamic interactions between hydrogen-enriched methane/air premixed flames and toroidal vortex structures. Int. J. Hydrogen Energy 2012, 37, 16201–16213. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.-Y. Turbulent explosion characteristics of stoichiometric syngas. Int. J. Energy Res. 2018, 42, 1225–1236. [Google Scholar] [CrossRef]
- Salzano, E.; Cammarota, F.; Di Benedetto, A.; Di Sarli, V. Explosion behavior of hydrogen–methane/air mixtures. J. Loss Prevent. Process Ind. 2012, 25, 443–447. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Fang, Y.; Liu, B.; Zeng, K.; Miao, H.; Jiang, D. Combustion behaviors of a direct-injection engine operating on various fractions of natural gas–hydrogen blends. Int. J. Hydrogen Energy 2007, 32, 3555–3564. [Google Scholar] [CrossRef]
- Petrukhin, N.V.; Grishin, N.N.; Sergeev, S.M. Ignition delay time—An important fuel property. Chem. Technol. Fuels Oils 2016, 51, 581–584. [Google Scholar] [CrossRef]
- Hwang, J.; Bae, C.; Park, J.; Choe, W.; Cha, J.; Woo, S. Microwave-assisted plasma ignition in a constant volume combustion chamber. Combust. Flame 2016, 167, 86–96. [Google Scholar] [CrossRef]
- Jung, D.; Sasaki, K.; Iida, N. Effects of increased spark discharge energy and enhanced in-cylinder turbulence level on lean limits and cycle-to-cycle variations of combustion for si engine operation. Appl. Energy 2017, 205, 1467–1477. [Google Scholar] [CrossRef]
- Kelley, A.P.; Jomaas, G.; Law, C.K. Critical radius for sustained propagation of spark-ignited spherical flames. Combust. Flame 2009, 156, 1006–1013. [Google Scholar] [CrossRef]
- Chen, Z.; Burke, M.P.; Ju, Y. Effects of lewis number and ignition energy on the determination of laminar flame speed using propagating spherical flames. Proc. Combust. Inst. 2009, 32, 1253–1260. [Google Scholar] [CrossRef]
- Chen, Z.; Burke, M.P.; Ju, Y. On the critical flame radius and minimum ignition energy for spherical flame initiation. Proc. Combust. Inst. 2011, 33, 1219–1226. [Google Scholar] [CrossRef]
- Han, J.; Yamashita, H.; Hayashi, N. Numerical study on the spark ignition characteristics of a methane–air mixture using detailed chemical kinetics: Effect of equivalence ratio, electrode gap distance, and electrode radius on mie, quenching distance, and ignition delay. Combust. Flame 2010, 157, 1414–1421. [Google Scholar] [CrossRef]
- Kravchik, T.; Sher, E.; Heywood, J.B. From spark ignition to flame initiation. Combust. Sci. Technol. 1995, 108, 1–30. [Google Scholar] [CrossRef]
- Zhou, M.; Li, G.; Liang, J.; Ding, H.; Zhang, Z. Effect of ignition energy on the uncertainty in the determination of laminar flame speed using outwardly propagating spherical flames. Proc. Combust. Inst. 2019, 37, 1615–1622. [Google Scholar] [CrossRef]
- Matsugi, A.; Shiina, H.; Takahashi, A.; Tsuchiya, K.; Miyoshi, A. Burning velocities and kinetics of h2/nf3/n2, ch4/nf3/n2, and c3h8/nf3/n2 flames. Combust. Flame 2014, 161, 1425–1431. [Google Scholar] [CrossRef]
- Chen, R.; Okazumi, R.; Nishida, K.; Ogata, Y. Effect of ethanol ratio on ignition and combustion of ethanol-gasoline blend spray in disi engine-like condition. SAE Int. J. Fuels Lubr. 2015, 8, 264–276. [Google Scholar] [CrossRef]
- Saito, N.; Minamoto, Y.; Yenerdag, B.; Shimura, M.; Tanahashi, M. Effects of turbulence on ignition of methane–air and n-heptane–air fully premixed mixtures. Combust. Sci. Technol. 2017, 190, 452–470. [Google Scholar] [CrossRef]
- Bradley, D.; Lung, F.K.K. Spark ignition and the early stages of turbulent flame propagation. Combust. Flame 1987, 69, 71–93. [Google Scholar] [CrossRef]
- Castela, M.; Stepanyan, S.; Fiorina, B.; Coussement, A.; Gicquel, O.; Darabiha, N.; Laux, C.O. A 3-d dns and experimental study of the effect of the recirculating flow pattern inside a reactive kernel produced by nanosecond plasma discharges in a methane-air mixture. Proc. Combust. Inst. 2017, 36, 4095–4103. [Google Scholar] [CrossRef] [Green Version]
- Bane, S.P.M.; Ziegler, J.L.; Shepherd, J.E. Investigation of the effect of electrode geometry on spark ignition. Combust. Flame 2015, 162, 462–469. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.T.; Luong, V.V.; Pham, Q.T.; Phung, M.T.; Do, P.N. Effect of Ignition Energy and Hydrogen Addition on Laminar Flame Speed, Ignition Delay Time, and Flame Rising Time of Lean Methane/Air Mixtures. Energies 2022, 15, 1940. https://doi.org/10.3390/en15051940
Nguyen MT, Luong VV, Pham QT, Phung MT, Do PN. Effect of Ignition Energy and Hydrogen Addition on Laminar Flame Speed, Ignition Delay Time, and Flame Rising Time of Lean Methane/Air Mixtures. Energies. 2022; 15(5):1940. https://doi.org/10.3390/en15051940
Chicago/Turabian StyleNguyen, Minh Tien, Van Van Luong, Quoc Thai Pham, Minh Tung Phung, and Phu Nguu Do. 2022. "Effect of Ignition Energy and Hydrogen Addition on Laminar Flame Speed, Ignition Delay Time, and Flame Rising Time of Lean Methane/Air Mixtures" Energies 15, no. 5: 1940. https://doi.org/10.3390/en15051940
APA StyleNguyen, M. T., Luong, V. V., Pham, Q. T., Phung, M. T., & Do, P. N. (2022). Effect of Ignition Energy and Hydrogen Addition on Laminar Flame Speed, Ignition Delay Time, and Flame Rising Time of Lean Methane/Air Mixtures. Energies, 15(5), 1940. https://doi.org/10.3390/en15051940





