1. Introduction
Approximately 86.4% of the energy resources required by man are supplied by fossil fuels, which has increased environmental and health problems worldwide [
1]. By 2030, the transport sector will consume the same amount of fossil fuels that all sectors consumed in 2003 worldwide, approximately 55,029 Mtoe [
2].
The heating process constitutes more than 35% of the energy consumed industrially in the world, commonly the temperature of the heat supply of industrial processes is below 400 °C, and approximately 80% of the energy used to produce it comes from natural gas and petroleum derivatives [
3]. Therefore, the consumption of renewable energies such as solar, wind, hydraulic, and biomass energy is very relevant. Solar energy has a great potential to produce clean and environmentally friendly heating.
Industry energy demand has been on the rise in recent decades; even as equipment specifications and process operation have improved, inefficiencies in the conversion and utilization of process energy remain a significant factor for the reduction in energy consumption [
4]. Exergy represents the amount of useful work that can be obtained from a system when it is brought into thermodynamic equilibrium with the environment. Exergy analysis of a process allows focusing on the thermodynamic losses and inefficiencies of each unit or block in a production process. However, the number of studies based on the exergy of industrial chemical processes is lower than in other research fields [
5]. The analysis of mass and energy flows in a system is fundamental for the reduction in energy consumption.
The use of renewable energies contributes to achieving the sustainability of the process. Biofuels are a renewable energy source that has been a viable alternative to face the growing energy demand due to their environmental advantages. However, its integration into the world energy market remains predominantly weak, being less than 13% worldwide [
6]. Biodiesel continues to be the highest production biofuel in the world, and the European Union is the largest producer, where 70% of biofuel is produced for the transport sector [
7]. Developing countries are highly dependent on imported fossil fuels such as diesel and simultaneously face the challenge of reducing their greenhouse gas emissions [
8].
Biodiesel is obtained by the transesterification of triglycerides with alcohol in the presence of a catalyst, biocatalyst, or without a catalyst [
9]. Conventional methods for biodiesel production are named according to the type of catalyst used and include acid, alkaline, and enzymatic, all in a homogeneous form. The use of catalysts seeks to reduce reaction times and achieve the desired reaction conditions [
10]. A widely used variant is the alkaline, which uses batch-type reactors with a reaction time of 1 h, 1 atm, 65 °C, and a typically molar ratio of alcohol to triglyceride of 6:1 [
11]. These conditions are achieved by adding KOH, NaOH, or CH
3ONa [
12]. However, this type of catalyst hinders the separation process, and if free fatty acid (FFA) content is not taken care of, the resulting reaction produces soap instead of biodiesel [
13]. Currently, heterogeneous catalysts have been studied for biodiesel production, mainly composed of oxides, metals, zeolites, or animal waste with high calcium content [
14,
15]. Once the biodiesel is obtained, these catalysts simplify the separation process at a lower cost than conventional chemicals. One of its main disadvantages is the reaction times, which are longer than those using homogeneous catalysts [
15].
The supercritical process allows using low-quality feedstocks for biodiesel production, e.g., oils with higher levels of FFA or moisture. The triglycerides are converted to biodiesel directly by applying this process. Neither FFA nor water harms the process. Several studies of the noncatalytic transesterification process with methanol and ethanol were carried out at temperatures ranging from 280 to 425 °C, pressures ranging from 8 to 43 MPa, a molar ratio of alcohol to oil ranging from 6:1 to 50:1, and reaction times ranging from 4 to 90 min depending on the type of oil and equipment used. These variables influence the process’s effectiveness and biodiesel yield [
16]. Studies were conducted on the integration of solar energy in the biodiesel production process. Most studies focused on a laboratory scale, integrating solar energy in the reaction stage, directly concentrating the solar radiation with a Scheffler reflector or Fresnel lenses [
17,
18,
19]. Solar energy integration in different areas of the biodiesel production process was marginally studied. If a greater scaling is considered, it is necessary to use linear parabolic trough collectors (PTC) to supply a large energy demand [
20]. PTC are the most appropriate technologies to supply solar thermal energy at medium temperature ranges due to their ability to maintain significant stability in the energy supply to a process [
21].
A significant amount of exergetic studies are focused on the analysis of combustion engines where biodiesel is used as fuel to study the behavior of the engines at different mixtures of diesel–biodiesel, which allows the identification of diverse operating parameters [
22,
23,
24]. In general, studies show that energy and exergetic efficiency is higher when using biodiesel as fuel in these engines [
25]. Exergy analysis of a transesterification process provides relevant information to reduce irreversibilities and optimize the process in terms of mass and energy.
Few exergetic studies analyze the integration of renewable energies in biodiesel production; most of them were performed comparing different production methods to identify the system irreversibilities and exergetic efficiencies, thus seeking to reduce the exergy destruction [
26]. Some studies show that the use of different catalysts has an effect on reducing exergy destruction [
27]. Reaction conditions effects on exergy were also carried out [
28]. Extensive exergy analysis was developed to study biodiesel production with different raw materials, such as waste vegetal oil (WVO) and rapeseed crops. The results show that the biodiesel produced from WVO consumes fewer resources than the production from rapeseed crops [
29]. Recent exergy analyses of a biodiesel production process with solar energy integration were performed for alkaline production and the cogeneration of electrical energy through an organic Rankine cycle [
30]. It highlights the potential of solar energy as an energy source for biodiesel production and the relevance of exergetic analysis in the development and implementation of new technologies.
With the development of different clean technologies and the growing need to obtain energy in a sustainable and economically viable way, research efforts were made to integrate unlike renewable energy sources to satisfy the energy needs of different processes [
31,
32]. Nevertheless, there are no reported works that extensively analyze the energy and exergetic behavior of biodiesel processes that integrate solar energy. Despite presenting significant advantages in biofuel purification, the exergetic analysis of supercritical processes has been less studied and contrasted with conventional ones. Therefore, the present work analyzed and compared two processes for biodiesel production that integrate solar energy to determine the process with the higher efficiency from an energy and exergy approach. The study included the structure, simulation, and comparison between biodiesel produced through alkaline catalysis and biodiesel obtained under supercritical conditions. The energy and exergetic efficiencies were evaluated for each process and stage, identifying the utmost inefficiencies and highlighting the advantages and disadvantages for each process.
3. Results and Discussion
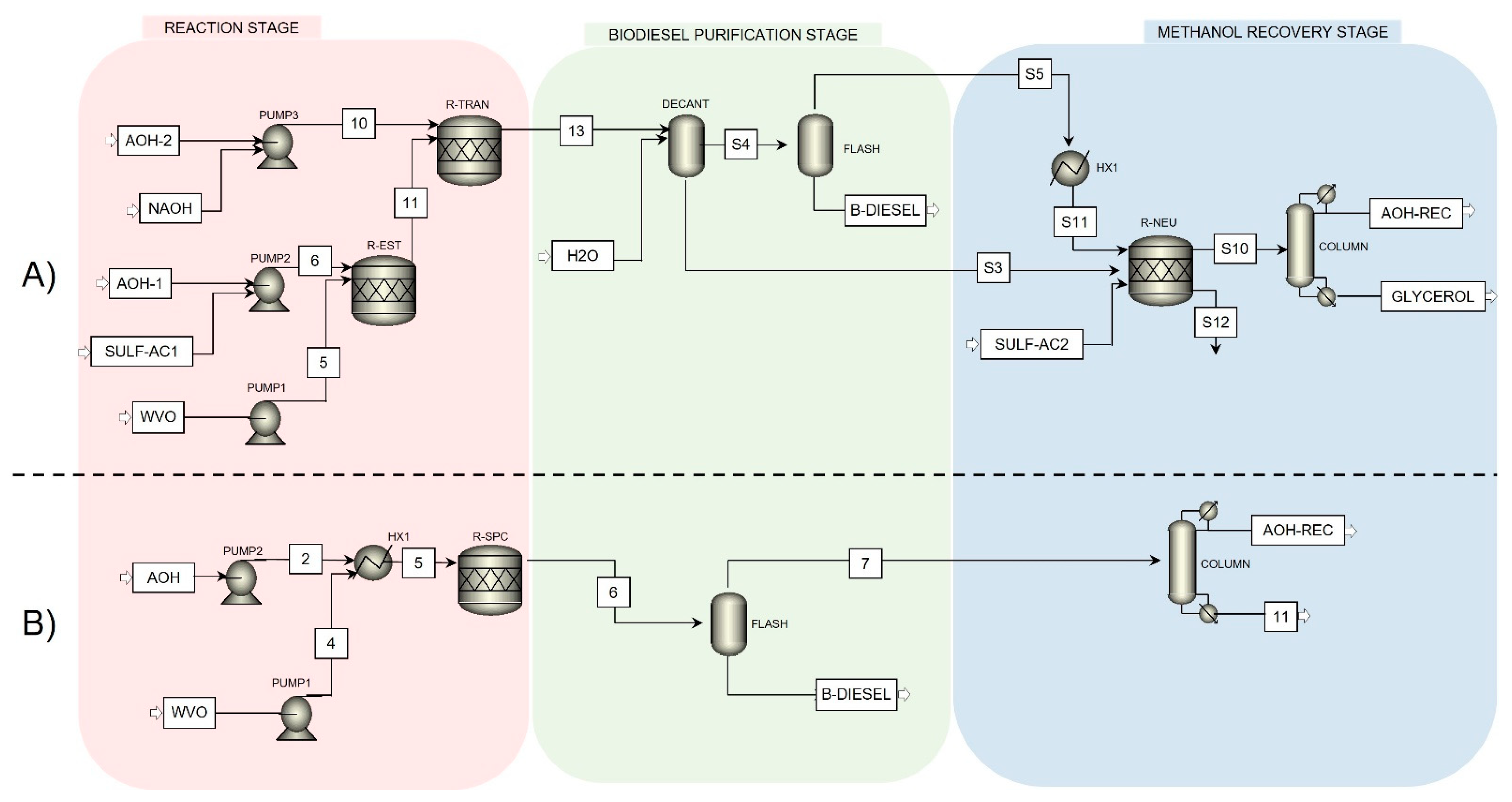
Both processes were designed to operate with a supply of 100 kg/h of WVO and have a partial source of energy through solar thermal energy. These two different reaction pathways for biodiesel production entail diverse challenges and implications that directly impact the design and implementation of the process, including the solar collection system. The simulation results for both process streams can be seen in
Table 2 and
Table 3.
The alkaline catalyst biodiesel production process reached a global yield of 91.3%, with a production of 101.85 kg/h of biodiesel. Although, the supercritical conditions process produces 102.5 kg/h of biodiesel with a global yield of 98.9%. The difference in the performance of both processes is due to the absence of catalysts in the supercritical process, which facilitates the separation of biodiesel from the reaction products, and the by-product 1,2,3 trimethyl glycerol triether is part of the biofuel.
An exhaustive bibliographic review was carried out and did not find studies that could be globally compared with the results obtained since there are no processes that fully fit the proposed work. A comparison was made between typical sections of the processes.
In order to validate the biodiesel production process, it was decided to evaluate the performance of the transesterification reactor. Although this unit is standard in biodiesel production processes, the specific data obtained at the outlet of the reactor under supercritical conditions were less reported.
In
Table 4, a validation of the biodiesel production results is presented, specifically at the output of the transesterification reactor of similar processes.
Different equipment and stages in biodiesel production require different amounts of mechanical and thermal energy, the latter for both cooling and heating. The alkaline biodiesel process, although it is one of the most widely used commercial methods, it implies more operations for the process and greater consumption of energy, based on the current work.
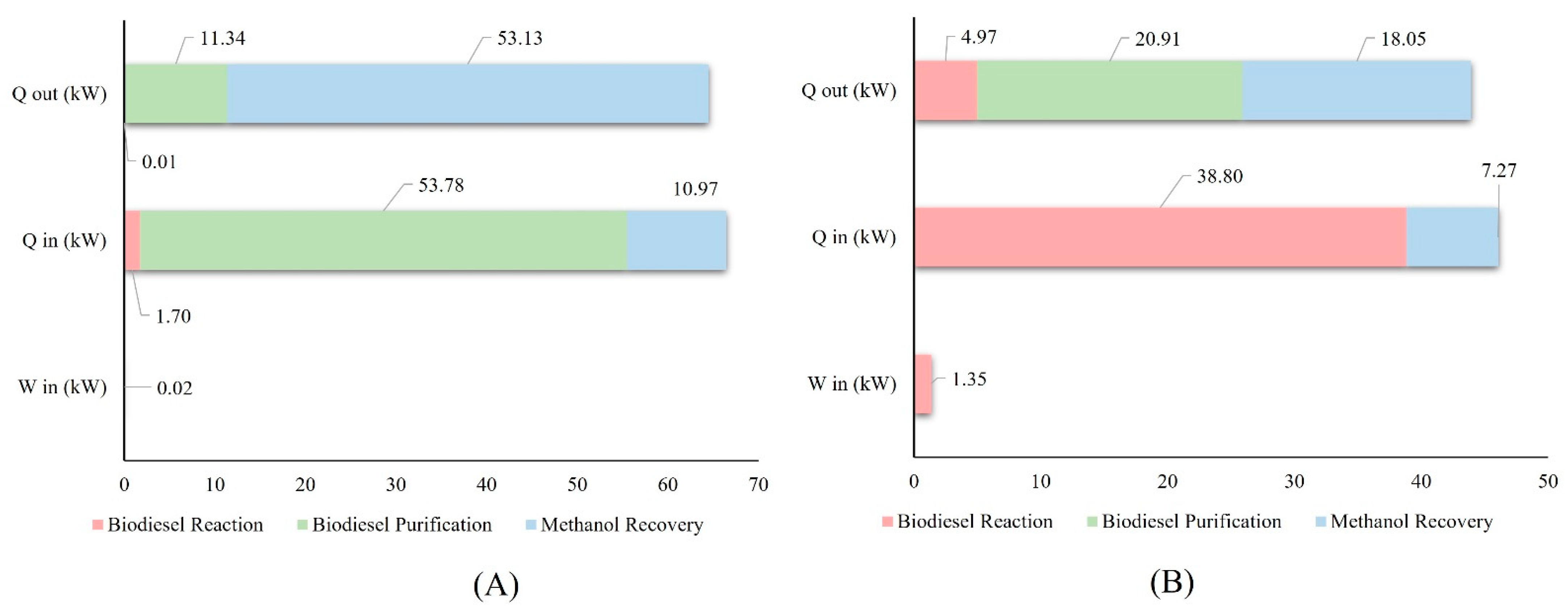
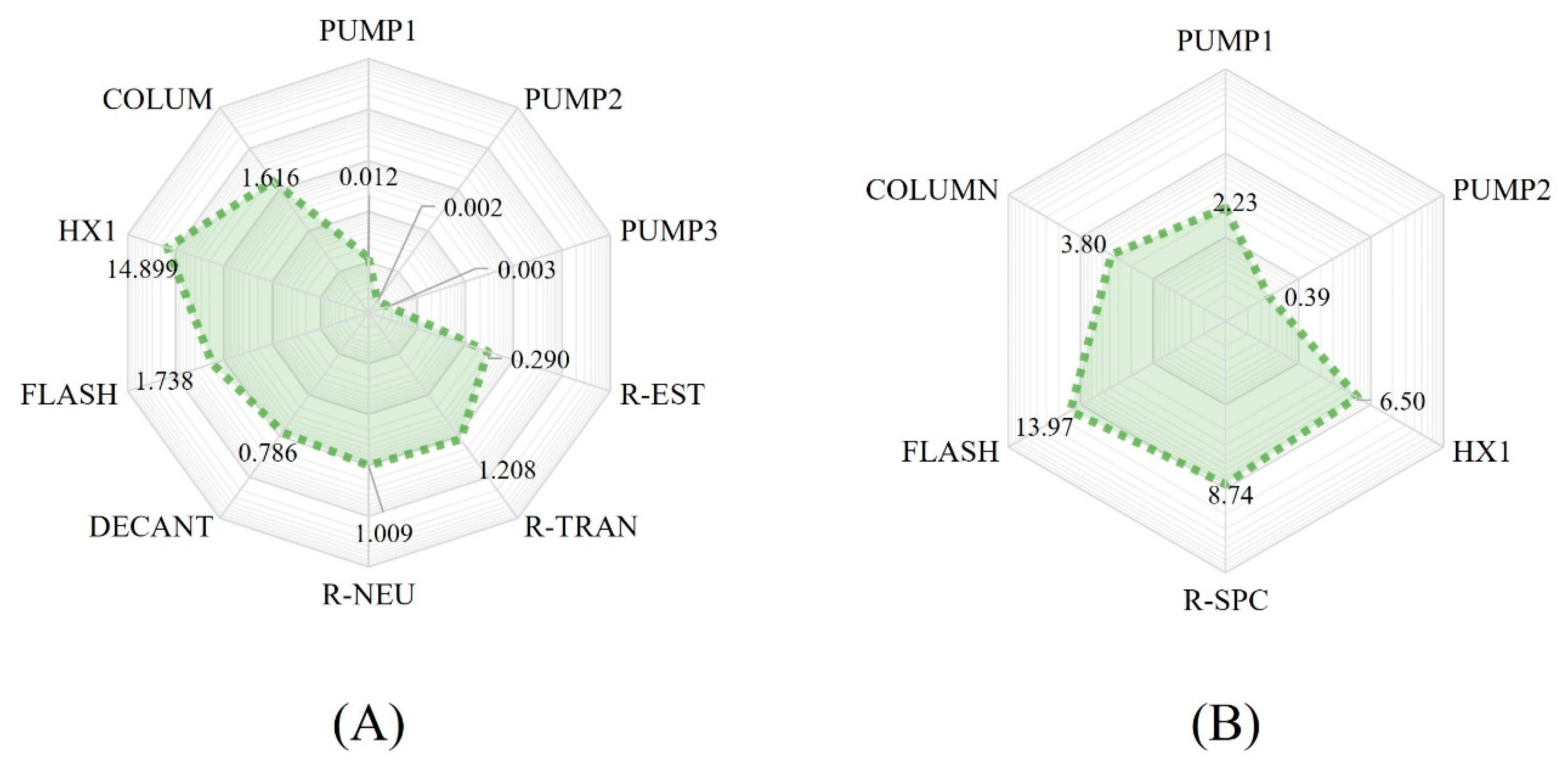
Figure 3 shows the energy loads required for both processes in the main stages.
The difference in the energy load required for each process lies in different stages. The alkaline process requires 53.78 kW of heating for the biodiesel purification stage, as shown in
Figure 3A. Meanwhile, the highest energy demand for the supercritical process is found in the reaction stage, specifically in the heating to reach the appropriate temperature conditions for the reaction, requiring 38.80 kW.
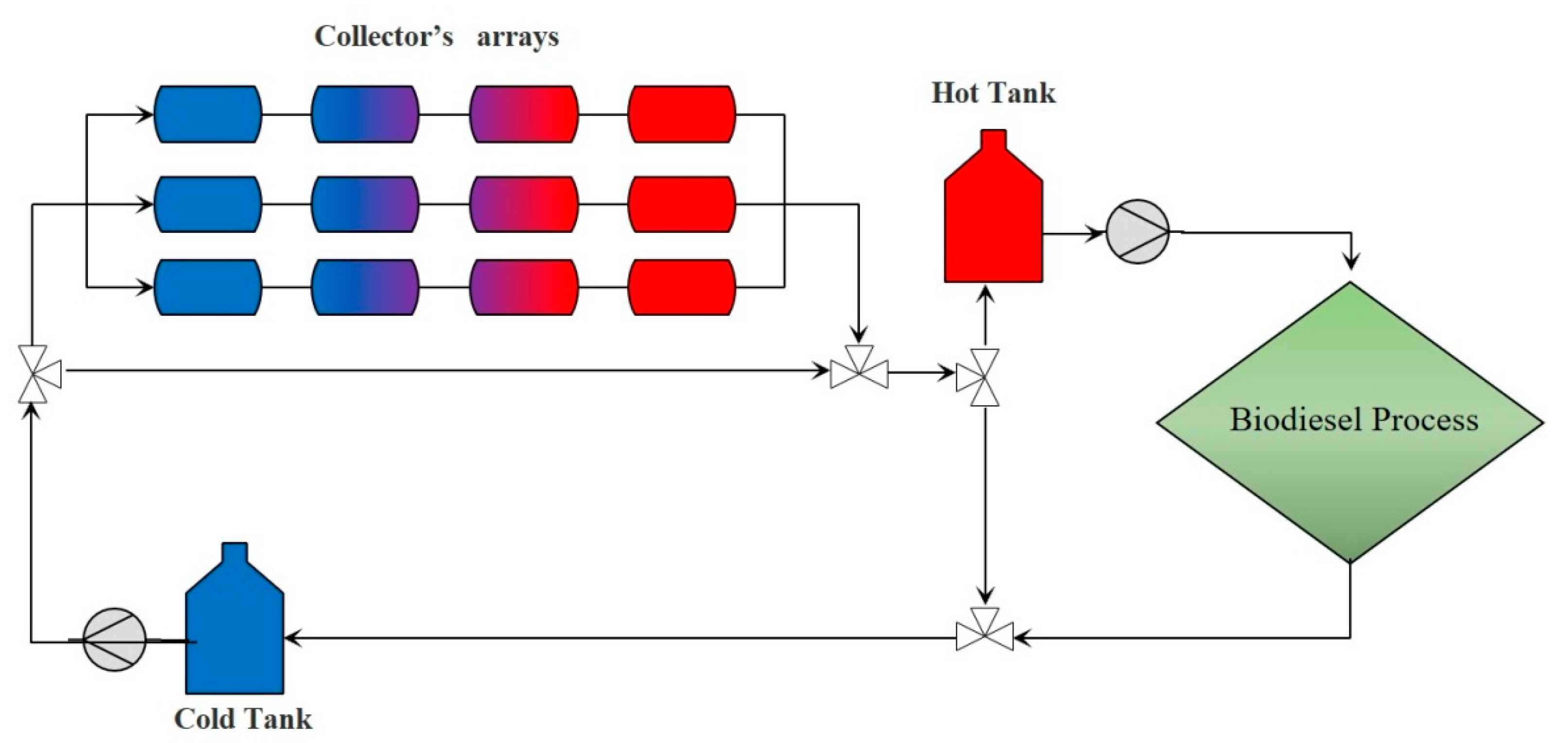
Based on the operating conditions and thermal energy demands of each process, a system of solar collectors was structured to supply solar thermal energy to fulfill the energy demands.
Table 5 presents the solar collection system required for each process.
According to a previous research [
34], the number of collectors in the array influences the overall energy efficiency of the process. However, at processes with temperature requirements below 400 °C and with an arrangement of more than 6 collectors in series and 13 in parallel, the increase in efficiency is marginal while reducing the economic viability of the project. The solar collector system structured for the alkaline biodiesel production process delivers only the thermal energy required by the Flash separation equipment, an essential part of biodiesel purification. The energy supplied by solar energy in this process is approximately 81%, while for the process under supercritical conditions, it is only possible to provide 74.5%. For the process under supercritical conditions, the energy is provided for preheating the reactants and as a support of the distillation reboiler column at the methanol recovery stage. The operating temperature of each process is the main reason for the difference in the total collection area for each one. The supercritical process operates at temperatures above 200 °C. Therefore, the number of solar collectors required in series and parallel increases to maintain a constant supply of HTF at a stable temperature, suitable for energy transfer to the process.
Although both processes have different equipment and energy loads, analyses of the first and second laws of thermodynamics were performed to optimize the use of energy. The biodiesel production process and the solar collection system were accounted as the same process for these analyses.
Considering that the first law analysis is an energy analysis of the process, the energy efficiency for each one was determined using Equation (15), as well as the energy invested per kilogram of biofuel produced according to Equation (16).
The first law energy efficiency was 91.64% for the process under supercritical conditions and 88.59% for the alkaline route. These results were because producing biodiesel by the alkaline route requires a higher amount of thermal energy to separate the biofuel. The energy of the raw material concerning the product is relatively the same for both processes. The transfer conditions for the thermal energy supply influence the efficiency with which these energies are used. The use of solar energy supposes energy savings because of the partial supply of energy. However, the process under supercritical conditions consumes more energy with 737.9 kJ per kg of biodiesel produced than the alkaline catalysis process with 454.5 kJ per kg of biodiesel.
Estimating the exergy destruction enables identifying the points in the process where the energy is not being used optimally or in general to identify which process has greater irreversibilities.
For the exergy balance, the chemical exergy of each stream was calculated from the chemical exergy of each species, multiplied for its mass fraction in the flow.
Table 6 shows the chemical exergies of the species involved in the processes. The chemical exergies of the species used in the simulations were similar to the values reported in the literature.
The physical exergy of each stream was obtained from Aspen Plus
®, and the exergetic balance was performed in Excel
®. For the global exergetic efficiency of each process, Equation (17) was used:
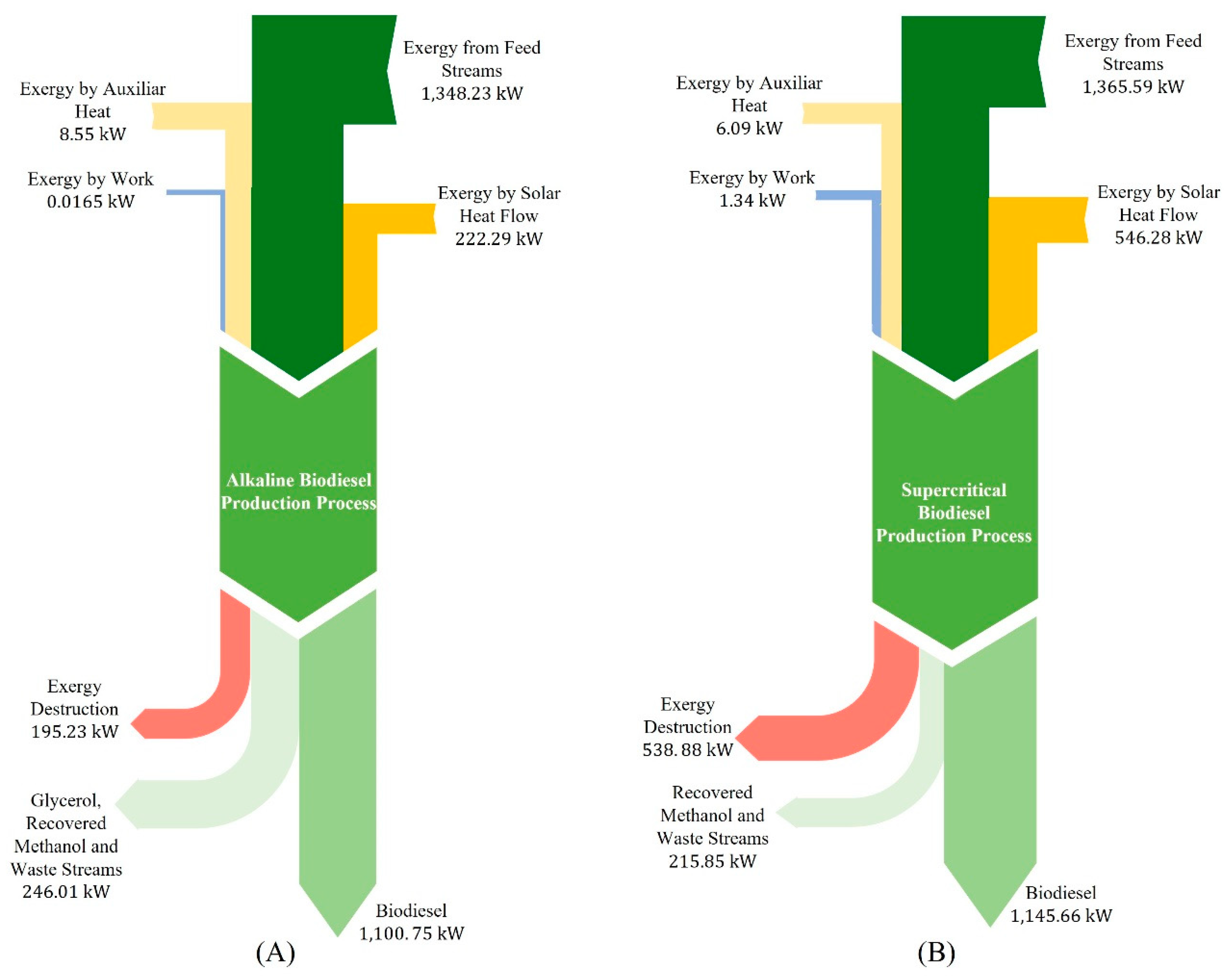
Figure 4 presents the global exergy balance for each process.
In
Table 7, a validation of the exergy analysis for the biodiesel production results is presented, compared to similar processes.
The processes contrasted with the present study imply different production capacities and process designs; for that reason, there are significant differences in the energy requirements, irreversibilities, and global exergetic efficiency of the process. However, the operating conditions and production method are equivalent to the simulated processes.
In the case of the process under supercritical conditions, few exergy studies were reported with the level of detail that allows a better comparison of the performed analysis. An important characteristic that influences exergetic efficiency is the process energy source. For the present study, solar energy as the foremost source represents the top influence on exergy destruction and exergetic efficiency.
The process at supercritical conditions exhibits high irreversibility with 538.88 kW of wasted useful energy and an overall exergetic efficiency of 70.9%. Meanwhile, the exergetic efficiency of the alkaline process was 85.2%, with only 195.23 kW of exergy destruction, thus having a more efficient use of the available exergy. Both processes present their highest exergy loss in the solar thermal energy collection system due to the large amount of solar energy that reaches the collectors, and it is not fully utilized. If the exergy losses contributed by the collector system are ignored, and only the exergy losses in the biodiesel production process are considered, it is possible to identify the stages with the highest irreversibilities.
Figure 5 illustrates the contribution of each operation to exergy destruction in each process.
The supercritical process continues to have greater irreversibility, considering only the exergy losses of the biodiesel production process. The stage with the highest exergy destruction in this process is the biodiesel reaction stage. It involves the transference of a large amount of thermal energy, representing 50.12% of the exergy destroyed in this stage, followed by the purification stage with 39%. Regarding the alkaline process, the greatest exergy destruction corresponds to the methanol recovery stage, contributing 81% of the irreversibilities. Although the highest energy load for this process occurs in the purification stage, the methanol recovery stage involves the condensation of a stream, which means the release of a significant amount of thermal energy.
Since solar energy is the main source of energy for these processes, the effect of the variation in the solar exergy was analyzed and compared with the exergy destruction and with the second law efficiency. The operation of the process on a typical summer day in Mexicali, Baja California, Mexico, is depicted in
Figure 6.
In both processes, the exergy provided by the solar resource varies depending on the radiation transition during the day. The destruction of exergy for both processes is mainly contributed by solar exergy. As the solar exergy increases, so does the destruction of the exergy, decreasing the efficiency of the second law, which depends on the input exergy.
4. Conclusions
In the present work, the biodiesel production process was studied from the perspective of the first and second thermodynamics laws, using two routes for transesterification of triglycerides and converting them into methyl esters. Both processes were supplied mainly with a renewable energy source such as solar energy through a parabolic trough collector’s system. The supercritical process has a higher biodiesel global yield than the alkaline process. However, the high operating temperatures of the supercritical process require a large solar collector system to have a stable temperature in the heat transfer fluid, even though the heating needs of the alkaline process are higher. The solar thermal energy collection system can supply 81% of the energy required by the alkali process and 74.5% of the supercritical process. From the energy and exergy analysis, it can be noted that the energy efficiency of the supercritical process is higher. Nevertheless, the exergetic efficiency of the alkaline process is higher than the supercritical one. The alkaline process has an exergetic efficiency of 85.2%. Irreversibilities in the supercritical process is higher and mainly presented as energy losses due to heat transfer. It is essential to identify that high operation temperatures favor this inefficiency, leading to exergy destruction. Solar collection systems contribute from 85% to 93% of the exergy destroyed by the global process for both cases. The solar resource is abundant, and it is one of the competitive advantages presented in the proposed processes. The irreversibilities reductions must be centralized in the biodiesel production process itself.
In the supercritical process, the focal source of exergy destruction is in the reaction stage due to the high operating temperatures. This characteristic of the process limits the alternatives to reduce the destroyed exergy in the reaction stage.
The alkaline process presents its greatest irreversibilities in the methanol recovery stage by destroying 14.8 kW of exergy in the condenser of the distillation column. This exergy could be exploited by using that stream as a preheating source for the same process, thus increasing the energy efficiency of the system. Based on the analyses results, the alkaline biodiesel production process has the highest advantages when using solar energy as the main source of energy, compared to a process in supercritical conditions that present bigger irreversibilities and require more infrastructure to collect the solar resource. Each solar collection system has its limitations due to the intermittency of the solar resource, especially in the hours of the day when solar radiation is not enough. However, using solar energy as the foremost energy source offers an alternative to fossil fuels and hence an environmental benefit concurrently with the use of biodiesel.