Effects of Unconventional Additives in Gasoline on the Performance of a Vehicle
Abstract
:1. Introduction
2. Experimental Device, Method and Fuel
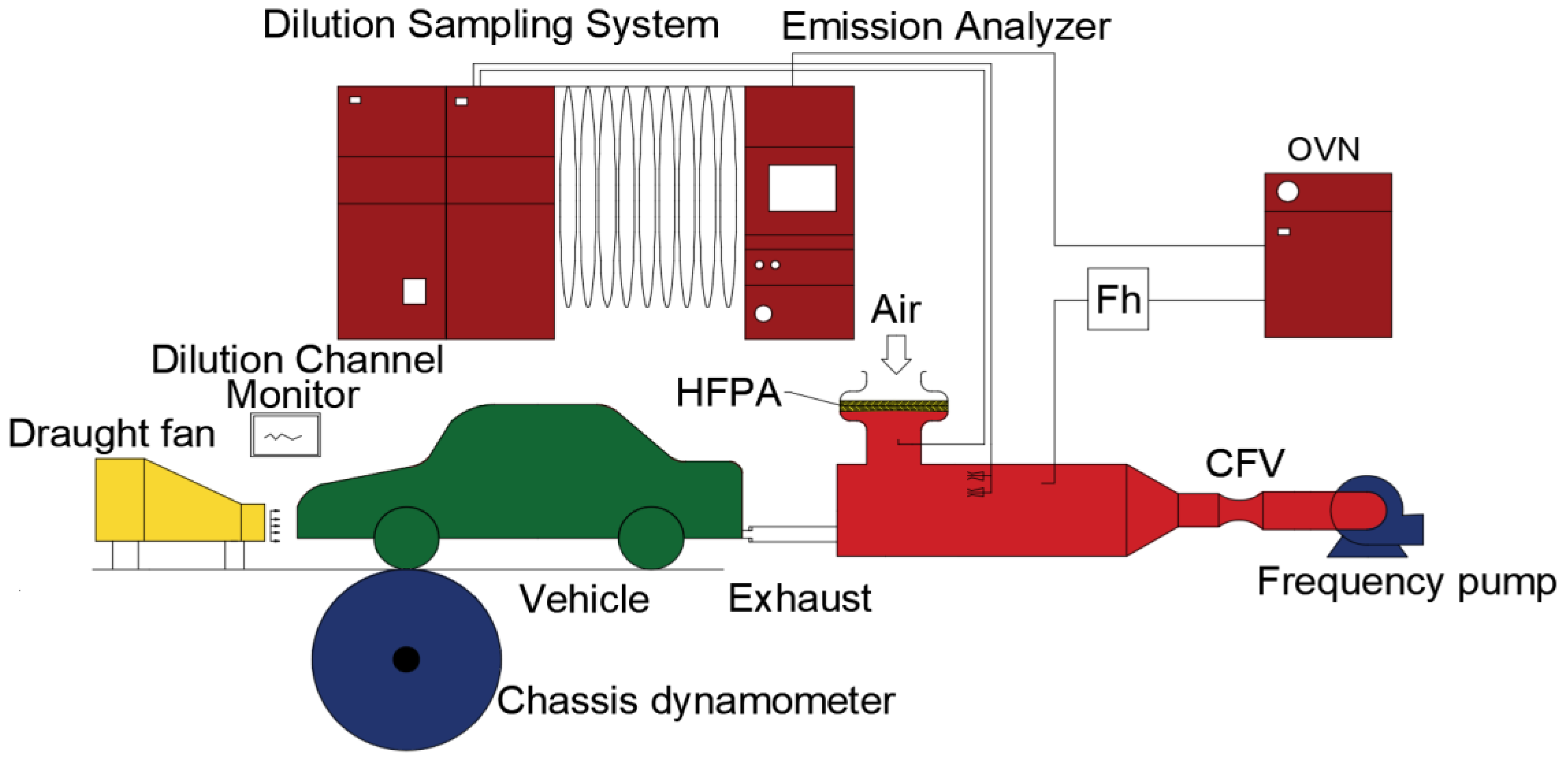
2.1. Experimental Device
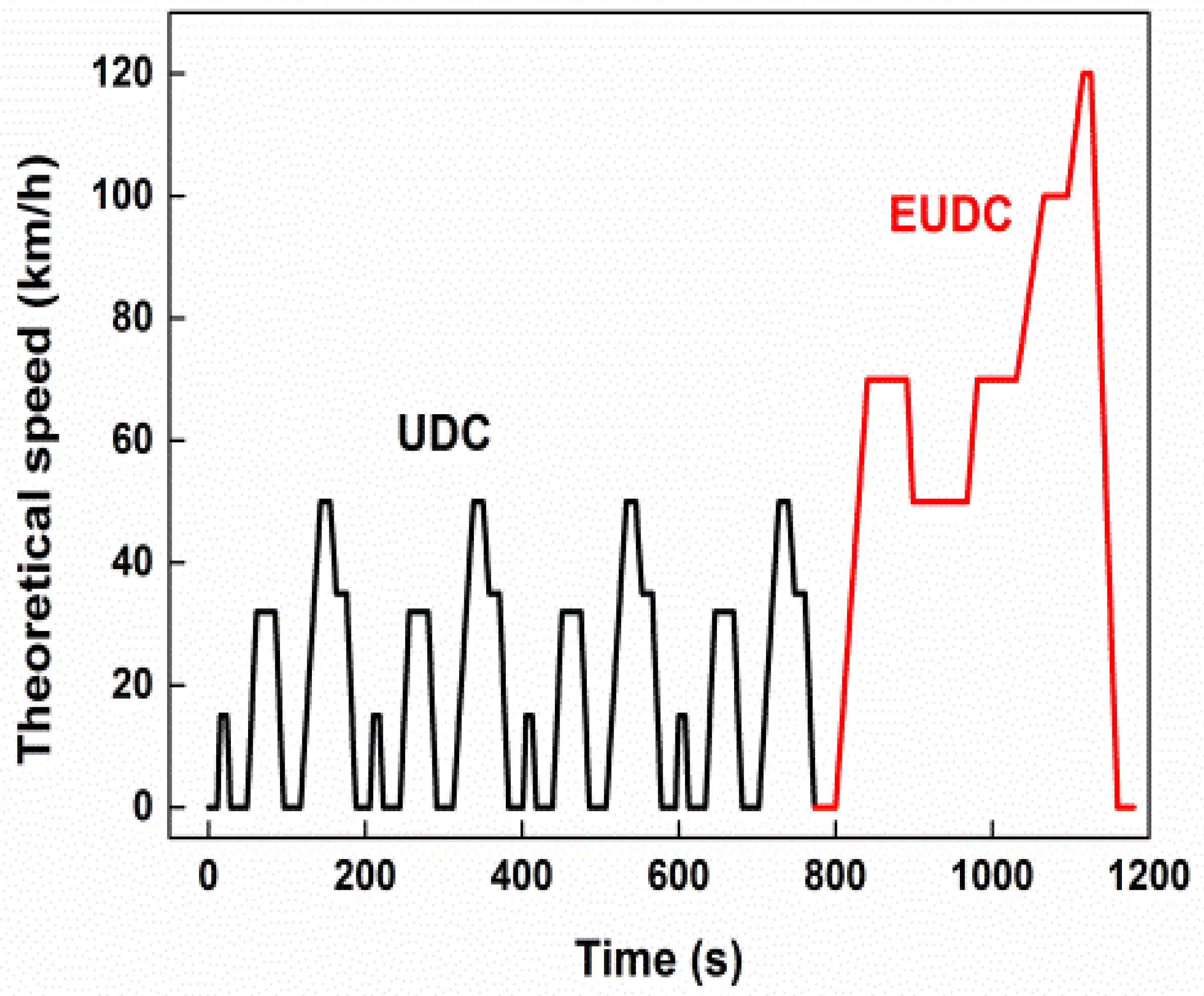
2.2. Experimental Method
2.3. Experimental Fuels
3. Results and Discussions
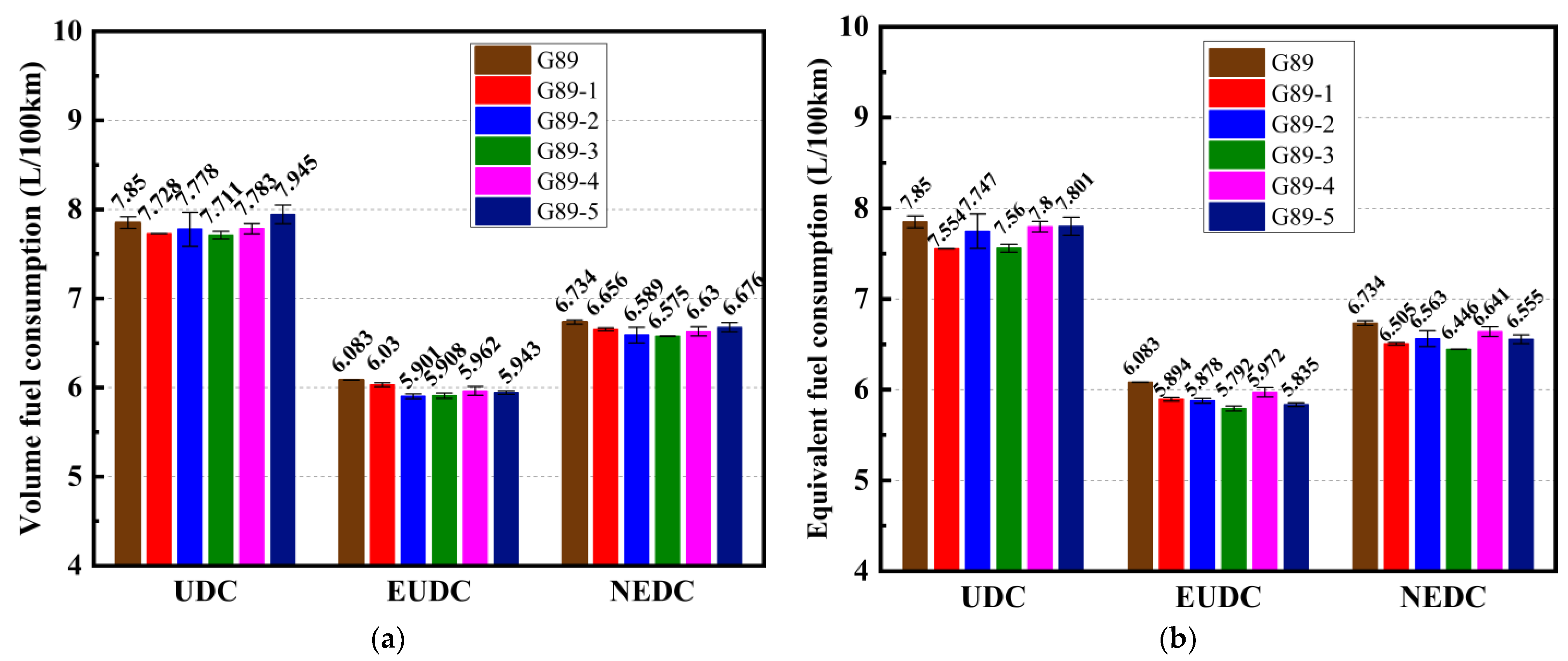
3.1. Effects of Different Unconventional Additives on Fuel Consumption
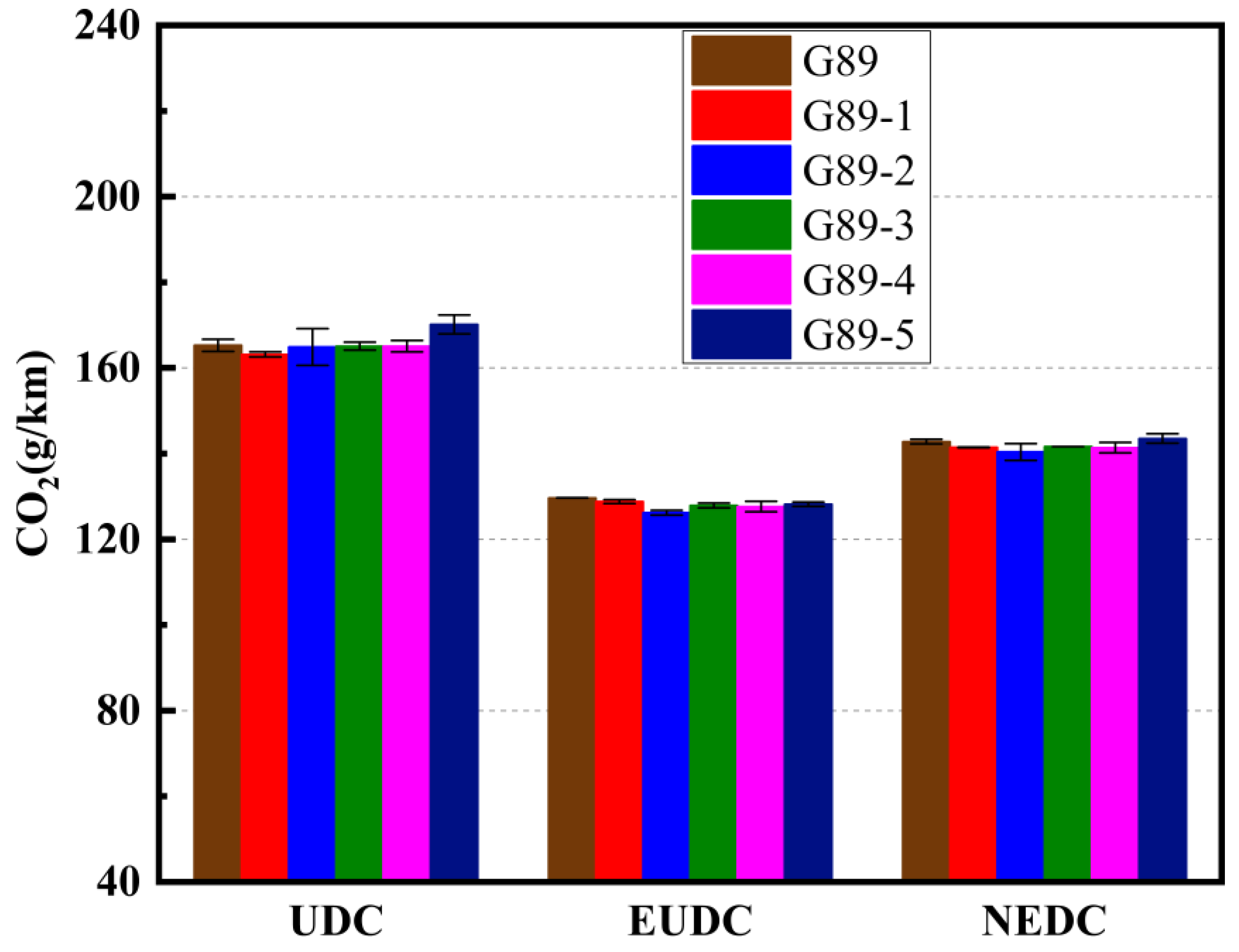
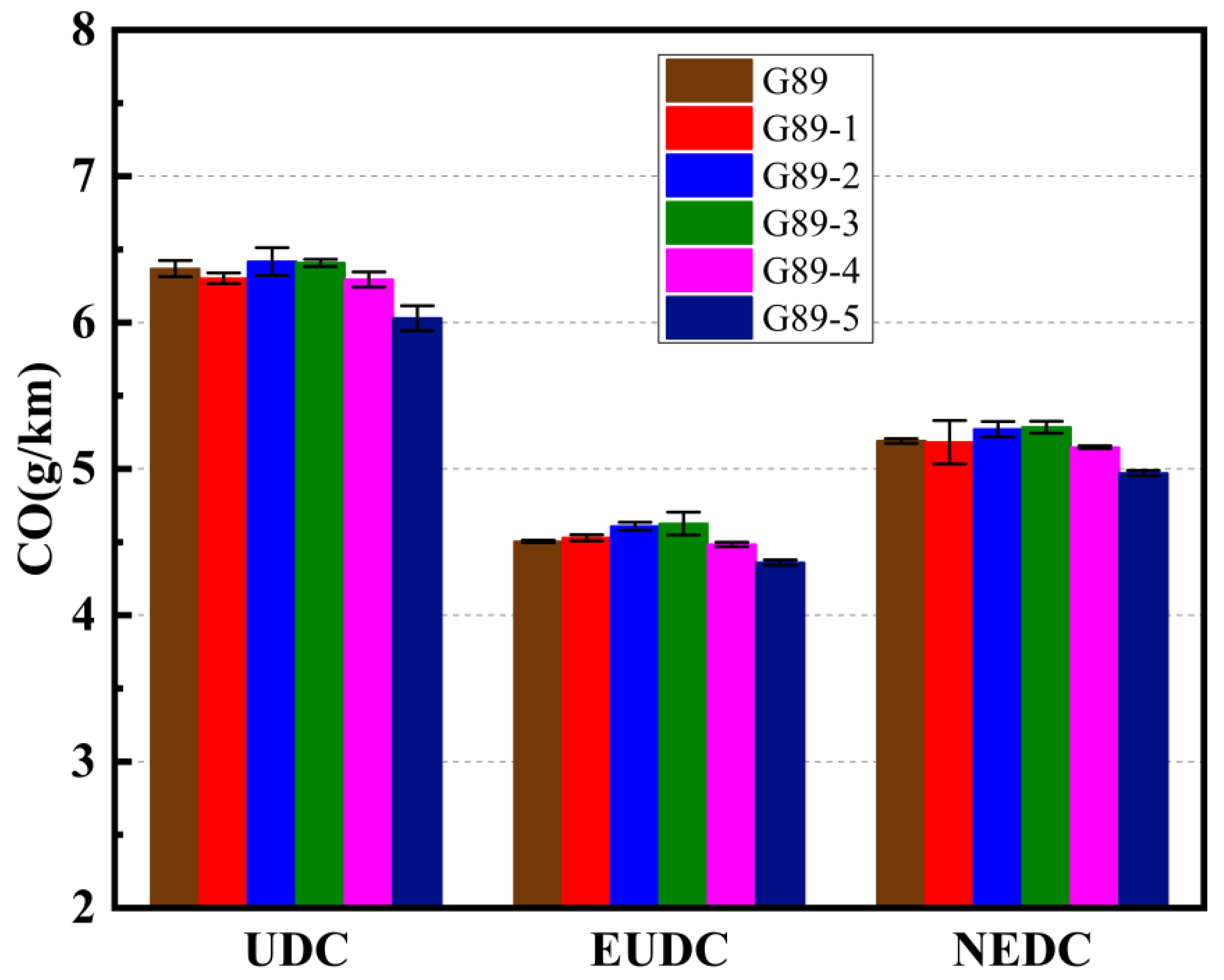
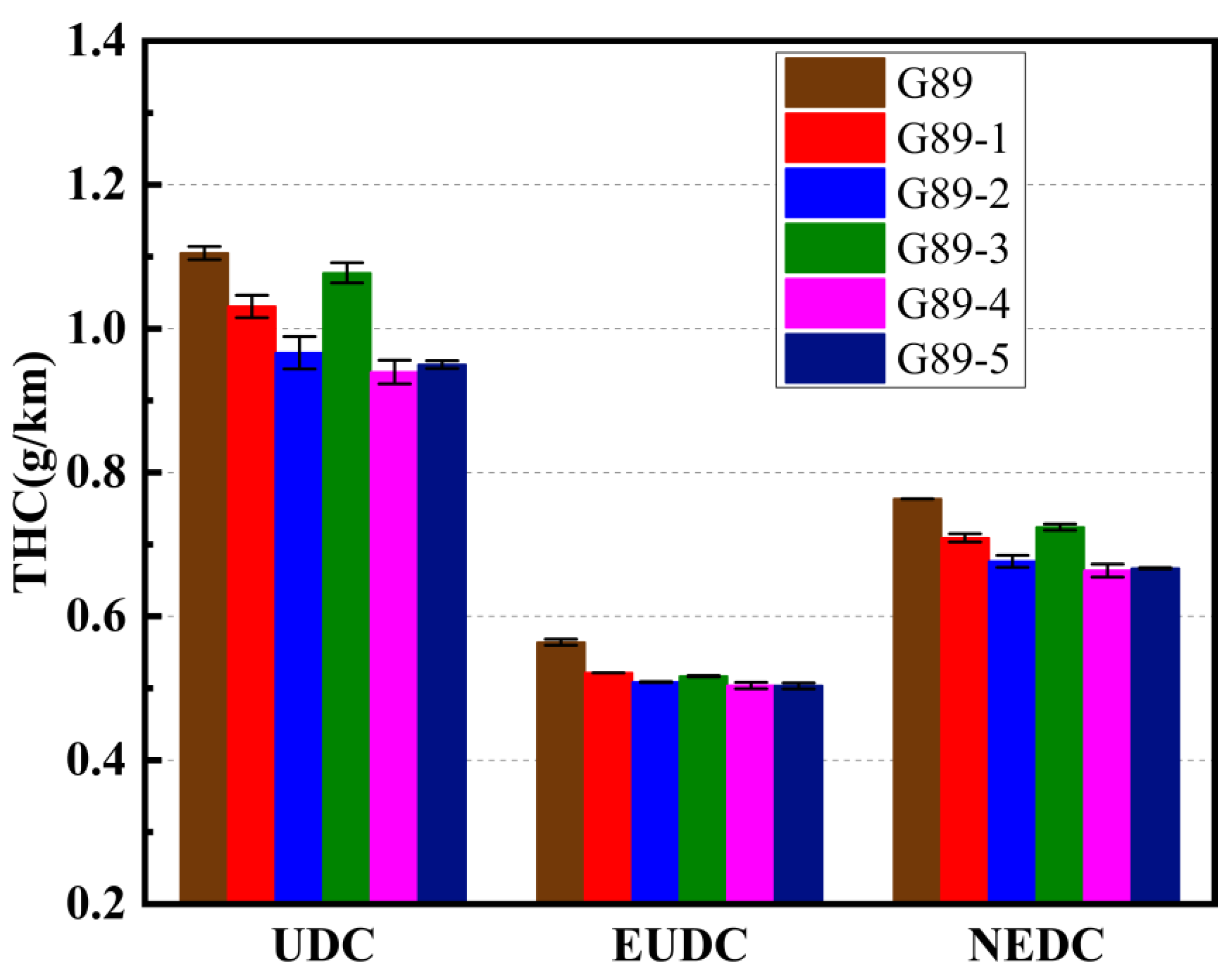
3.2. Effects of Different Unconventional Additives on Gaseous Emissions
3.3. Effects of Different Unconventional Additives on Acceleration Performance of the PFI Vehicle
4. Conclusions
- (1)
- In terms of fuel consumption over the entire NEDC cycle, the five additives all decreased the volume fuel consumption and equivalent fuel consumption to varying degrees. The decrease in volume fuel consumption from highest to lowest was G89-3, G89-2, G89-4, G89-1 and G89-5, while the reduction in equivalent fuel consumption from highest to lowest was G89-3, G89-1, G89-5, G89-2 and G89-4. On the whole, blending sec-butyl acetate (G89-3) showed the best improvement in fuel consumption;
- (2)
- In terms of gaseous emissions over the entire NEDC cycle: G89-1 decreased the CO2 and THC emissions but increased the CO and NOx emissions; G89-2 and G89-3 both decreased the CO2, THC and NOx emissions but increased the CO emissions; G89-4 could decrease all four gaseous emissions; G89-5 decreased the CO, THC and NOx emissions but increased the CO2 emissions. On the whole, G89-4, with only 1.5% of p-methylphenol in the fuel volume, had the best performance in decreasing the gaseous emissions, which indicates that blending p-methylphenol in gasoline showed better potential for helping vehicles to achieve cleaner emissions;
- (3)
- In terms of acceleration performance, the five additives all shortened the acceleration time and the acceleration time from highest to lowest was G89-3, G89-4, G89-5, G89-1 and G89-2. It should be noted that the effects of the different additives on shortening the acceleration time were basically consistent with the RON of the fuel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Dong, S.J.; Cheng, X.B.; Ou, B.; Liu, T.; Wang, Z. Experimental and numerical investigations on the cyclic variability of an ethanol/diesel dual-fuel engine. Fuel 2016, 186, 665–673. [Google Scholar] [CrossRef]
- Kalghatgi, G.T. Developments in internal combustion engines and implications for combustion science and future transport fuels. Proc. Combust. Inst. 2015, 35, 101–115. [Google Scholar] [CrossRef]
- Kalghatgi, G.T. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; United States Energy Information Administration: Washington, DC, USA, 2016.
- B.P. BP Statistical Review of World Energy 2019; Pureprint Group Limited: London, UK, 2019. [Google Scholar]
- Yamada, H.; Hayashi, R.; Tonokura, K. Simultaneous measurements of on-road/in-vehicle nanoparticles and NOx while driving: Actual situations, passenger exposure and secondary formations. Sci. Total Environ. 2016, 563–564, 944–955. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, R.; Qin, Y.; Peng, J.; Li, M.; Lei, J.; Wu, Y.; Hu, M.; Shuai, S. The impact of fuel compositions on the particulate emissions of direct injection gasoline engine. Fuel 2016, 166, 543–552. [Google Scholar]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Abdelkareem, M.A.; Kamil, M.; Olabi, A.G. Recent trends for introducing promising fuel components to enhance the anti-knock quality of gasoline: A systematic review. Fuel 2021, 291, 120112. [Google Scholar] [CrossRef]
- Goyal, H.; Kook, S.; Ikeda, Y. The influence of fuel ignition quality and first injection proportion on gasoline compression ignition (GCI) combustion in a small-bore engine. Fuel 2019, 235, 1207–1215. [Google Scholar] [CrossRef]
- Liu, H.-F.; Wen, M.-S.; Cui, Y.-Q.; Zhang, C.-Q.; Zheng, Z.-Q.; Yao, M.-F. Effect of Blending n-Butanol in Diesel on Flame Development and Spectrum. Spectrosc. Spectr. Anal. 2020, 40, 1998–2004. [Google Scholar]
- Zheng, Z.; Yue, L.; Liu, H.; Zhu, Y.; Zhong, X.; Yao, M. Effect of two-stage injection on combustion and emissions under high EGR rate on a diesel engine by fueling blends of diesel/gasoline, diesel/n-butanol, diesel/gasoline/n-butanol and pure diesel. Energy Convers. Manag. 2015, 90, 1–11. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Zhang, D.; Dong, F.; Liu, X.; Yang, Y.; Huang, H.; Wang, Y.; Wang, Q.; Zheng, Z. Investigation on Blending Effects of Gasoline Fuel with N-Butanol, DMF, and Ethanol on the Fuel Consumption and Harmful Emissions in a GDI Vehicle. Energies 2019, 12, 1845. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.; Zhang, C.; Yue, Z.; Liu, X.; Yang, Y.; Dong, F.; Liu, H.; Yao, M. Effects of Gasoline Octane Number on Fuel Consumption and Emissions in Two Vehicles Equipped with GDI and PFI Spark-Ignition Engine. J. Energy Eng. 2020, 146, 04020069. [Google Scholar] [CrossRef]
- de la Luz, A.P.; Iuga, C.; Vivier-Bunge, A. An effective force field to reproduce the solubility of MTBE in water. Fuel 2020, 264, 116761. [Google Scholar] [CrossRef]
- Topgül, T. The effects of MTBE blends on engine performance and exhaust emissions in a spark ignition engine. Fuel Process. Technol. 2015, 138, 483–489. [Google Scholar] [CrossRef]
- Poulopoulos, S.; Philippopoulos, C. Influence of MTBE addition into gasoline on automotive exhaust emissions. Atmos. Environ. 2000, 34, 4781–4786. [Google Scholar] [CrossRef]
- Franklin, P.; Koshland, C.; Lucas, D.; Sawyer, R.F. Evaluation of combustion by-products of MTBE as a component of reformulated gasoline. Chemosphere 2001, 42, 861–872. [Google Scholar] [CrossRef]
- Facetti, J.F.; Nunez, R.; Gomez, L.C.; Ojeda, J.; Bernal, C.; Leon-Ovelar, R.; Carvallo, F. Methyl tert-butyl ether (MtBE) in deep wells of the Patiño Aquifer, Paraguay: A preliminary characterization. Sci. Total Environ. 2019, 647, 1640–1650. [Google Scholar] [CrossRef]
- Rosell, M.; Lacorte, S.; Barceló, D. Simultaneous determination of methyl tert-butyl ether, its degradation products and other gasoline additives in soil samples by closed-system purge-and-trap gas chromatography—Mass spectrometry. J. Chromatogr. A 2006, 1132, 28–38. [Google Scholar] [CrossRef]
- Geo, V.E.; Godwin, D.J.; Thiyagarajana, S.; Saravanan, C.G.; Aloui, F. Effect of higher and lower order alcohol blending with gasoline on performance, emission and combustion characteristics of SI engine. Fuel 2019, 256, 115806. [Google Scholar]
- Wang, C.; Li, Y.; Xu, C.; Badawy, T.; Sahu, A.; Jiang, C. Methanol as an octane booster for gasoline fuels. Fuel 2019, 248, 76–84. [Google Scholar] [CrossRef]
- Waluyo, B.; Setiyo, M.; Wardana, I.N.G. Fuel performance for stable homogeneous gasoline-methanol-ethanol blends. Fuel 2021, 294, 120565. [Google Scholar] [CrossRef]
- Bilgin, A.; Sezer, I. Effects of methanol addition to gasoline on the performance and fuel cost of a spark ignition engine. Energy Fuels 2008, 22, 2782–2788. [Google Scholar] [CrossRef]
- Abu-Zaid, M.; Badran, O.; Yamin, J. Effect of Methanol Addition on the Performance of Spark Ignition Engines. Energy Fuels 2004, 18, 312–315. [Google Scholar] [CrossRef]
- Hao, H.; Liu, Z.; Zhao, F.; Du, J.; Chen, Y. Coal-derived alternative fuels for vehicle use in China: A review. J. Clean. Prod. 2017, 141, 774–790. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.L.; Asa-Awuku, A.; Jung, H.; Johnson, K.C.; Durbin, T.D. The impact of ethanol and iso-butanol blends on gaseous and particulate emissions from two passenger cars equipped with spray-guided and wall-guided direct injection SI (spark ignition) engines. Energy 2015, 82, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Sun, P.; Du, Y.; Yu, X.; Dong, W.; Zhou, J. Improvement of combustion and emission by combined combustion of ethanol premix and gasoline direct injection in SI engine. Fuel 2021, 292, 120403. [Google Scholar] [CrossRef]
- da Silva, A., Jr.; Hauber, J.; Cancino, L.R.; Huber, K. The research octane numbers of ethanol-containing gasoline surrogates. Fuel 2019, 243, 306–313. [Google Scholar] [CrossRef]
- Ni, B.; Chen, Y. National Standard of the People’s Republic of China: Ethanol Gasoline for Vehicles (E10) (GB 18351-2010); China Standard Press: Beijing, China, 2010. [Google Scholar]
- Park, C.; Choi, Y.; Kim, C.; Oh, S.; Lim, G.; Moriyoshi, Y. Performance and exhaust emission characteristics of a spark ignition engine using ethanol and ethanol-reformed gas. Fuel 2010, 89, 2118–2125. [Google Scholar] [CrossRef]
- Turner, D.; Xu, H.; Cracknell, R.F.; Natarajan, V.; Chen, X. Combustion performance of bio-ethanol at various blend ratios in a gasoline direct injection engine. Fuel 2011, 90, 1999–2006. [Google Scholar] [CrossRef]
- Ceviz, M.A.; Yüksel, F. Effects of ethanol–unleaded gasoline blends on cyclic variability and emissions in an SI engine. Appl. Therm. Eng. 2005, 25, 917–925. [Google Scholar] [CrossRef]
- He, B.-Q.; Wang, J.-X.; Hao, J.-M.; Yan, X.-G.; Xiao, J.-H. A study on emission characteristics of an EFI engine with ethanol blended gasoline fuels. Atmos. Environ. 2003, 37, 949–957. [Google Scholar] [CrossRef]
- Balki, M.K.; Sayin, C.; Canakci, M. The effect of different alcohol fuels on the performance, emission and combustion characteristics of a gasoline engine. Fuel 2014, 115, 901–906. [Google Scholar] [CrossRef]
- Esan, A.O.; Adeyemi, A.D.; Ganesan, S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production. J. Clean. Prod. 2020, 257, 120561. [Google Scholar] [CrossRef]
- Kartikeya, S.; Vimal, C.S. Synthesis of organic carbonates from alcoholysis of urea: A review. Catal. Rev. 2017, 59, 1–43. [Google Scholar]
- Maier, T.; Härtl, H.; Jacob, E.; Wachtmeister, G. Dimethyl carbonate (DMC) and Methyl Formate (MeFo): Emission characteristics of novel, clean and potentially CO2-neutral fuels including PMP and sub-23 nm nanoparticle-emission characteristics on a spark-ignition DI-engine. Fuel 2019, 256, 115925. [Google Scholar] [CrossRef]
- Wen, L.-B.; Xin, C.-Y.; Yang, S.-C. The effect of adding dimethyl carbonate (DMC) and ethanol to unleaded gasoline on exhaust emission. Appl. Energy 2010, 87, 115–121. [Google Scholar] [CrossRef]
- Thornton, S.F.; Nicholls, H.C.G.; Rolfe, S.A.; Mallinson, H.E.H.; Spence, M.J. Biodegradation and fate of ethyl tert-butyl ether (ETBE) in soil and groundwater: A review. J. Hazard. Mater. 2020, 391, 122046. [Google Scholar] [CrossRef]
- Antonio, V.; Ouldouz, G.; David, V.; Estarellas, C.; López, M.; Gómez-Catalán, J.; Lavilla, R.; Delgado, J.; Luque, F.J. Holistic approach to anti-knock agents: A high-throughput screening of aniline-like compounds. Fuel 2021, 305, 121518. [Google Scholar]
- Liu, M.M. Synthesis process of Sec-butyl acetate. Petrochem. Technol. 2017, 24, 271. [Google Scholar]
- Chemical Dictionary. Available online: http://www.chemdict.com/datcdcfa7j/ (accessed on 28 January 2022).
- Xiao, H.; Guo, F.; Wang, R.; Yang, X.; Li, S.; Ruan, J. Combustion performance and emission characteristics of diesel engine fueled with iso-butanol/biodiesel blends. Fuel 2020, 268, 117387. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Balla, H.H.; Al-Dulaimi, Z.M.H.; Kareem, Z.S.; Al-Zuhairy, M.S. Effect of ethanol-gasoline blends on SI engine performance and emissions. Case Stud. Therm. Eng. 2021, 25, 100891. [Google Scholar] [CrossRef]
- Effect of Ethanol Gasoline Fuel Characteristics on Direct Injection Supercharged Passenger Vehicle Performance. 2020. Available online: https://d.wanfangdata.com.cn/periodical/nrjgc202005008 (accessed on 28 January 2022).
- Jin, D.; Choi, K.; Myung, C.-L.; Lim, Y.; Lee, J.; Park, S. The impact of various ethanol-gasoline blends on particulates and unregulated gaseous emissions characteristics from a spark ignition direct injection (SIDI) passenger vehicle. Fuel 2017, 209, 702–712. [Google Scholar] [CrossRef]
- Oda, K.; Hosono, K.; Isoda, T.; Aihara, H.; Kojima, K.; Shibata, G. Effect of Gasoline Composition on Engine Performance. SAE 1993, 930375. [Google Scholar]
- Aikawa, K.; Sakurai, T.; Jetter, J.J. Development of a predictive model for gasoline vehicle particulate matter emissions. SAE Int. J. Fuels Lubr. 2010, 3, 610–622. [Google Scholar] [CrossRef]
- Experimental Study on Vehicle and Emission Performance of Different Oxygen-Containing Blending Gasoline Fuel. Chin. Intern. Combust. 2019. Available online: https://d.wanfangdata.com.cn/periodical/nrjgc201906001 (accessed on 28 January 2022).
- Liu, H.F.; Wen, M.S.; Zhang, C.Q. Effects of blending ratio of methanol on performance and emissions of a passenger vehicle with GDI engine. In Proceedings of the 7th International Seminar on Motor Vehicle Emission Control and Supervision Technology, Wuhan, China, 17–19 July 2021. [Google Scholar]
- Dong, F.; Liu, H.F.; Yang, Y. Gasoline Application Technology Question and Answer; China Quality Supervision Press: Beijing, China, 2018; p. 44. [Google Scholar]
- Shen, Y.T.; Shuai, S.J.; Wang, J.X. Study on the effect of olefin on engine emission and combustion characteristics. Automot. Eng. 2008, 30, 644–647. [Google Scholar]
- Takei, Y.; Uehara, T.; Hoshi, H.; Sugiyama, S.; Okada, M. Effect of California Phase 2 Reformulated Gasoline Specifications on Exhaust Emission Reduction: Part 3; SAE International: Warrendale, PA, USA, 1997; p. 972851. [Google Scholar]
- Pechout, M.; Dittrich, A.; Vojtisek-Lom, M. Operation of an Ordinary PFI Engine on n-butanol and Iso-butanol and Their Blends with Gasoline; No.: 2014-01-2618; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]



| Parameter | Value |
|---|---|
| Injection system | PFI |
| Displacement (L) | 1.6 |
| Intake system | Naturally aspirated |
| Cylinder number | 4 |
| Maximum power (kW) | 90 |
| Rated speed (rpm) | 6000 |
| Maximum torque (N·m) | 154 |
| Compress ratio | 10.2 |
| Emission standard | China V |
| Cylinder diameter × stroke (mm) | 80.5 × 78.5 |
| Parameter | Model Specifications | Manufacturer |
|---|---|---|
| Chassis dynamometer | ROADSIM 48”compact | AVL, Austria |
| Emission analyzer | MEXA-7200H | Horiba, Japan |
| Dilute sampling system | CVS-7200T | Horiba, Japan |
| Dilution channel | DLS-7100E | Horiba, Japan |
| Equipment | Test Project | Measurement Errors |
|---|---|---|
| Emission analyzer | CO2 | ≤1% of full scale or 2% of measured value, whichever was the smallest |
| CO | ||
| THC | ||
| NOx | ||
| Chassis dynamometer | Constant speed difference | <0.05% of full scale |
| Time measurement tolerance | 0.00005% | |
| Constant traction tolerance | <0.2% of full scale |
| Fuel | G89 | G89-1 | G89-2 | G89-3 | G89-4 | G89-5 |
|---|---|---|---|---|---|---|
| Additives | — | ETBE | N-Methylaniline | Sec-butyl acetate | P-methylphenol | Isobutanol |
| Additives blending rate | — | 15% (5) | 1.5% (1) | 9% (3) | 1.5% (1) | 12% (4) |
| Additives molecular formula | — | C6H14O | C7H9N | C6H12O2 | C7H8O | C4H10O |
| Additives latent heat of vaporization (kJ/kg@20 °C) | — | 327.870 (2) | 516.952 (4) | 313.846 (1) | 431.024 (3) | 688.059 (5) |
| Boiling point/°C | 169.4 (1) | 172.7 (2) | 180.9 (5) | 173.8 (3) | 178.5 (4) | 184.6 (6) |
| RON (research octane number) | 89.0 (1) | 93.1 (5) | 94.8 (6) | 89.3 (2) | 89.5 (3) | 90.9 (4) |
| Density at 20 °C/(kg/m3) | 723.6 (1) | 724.9 (2) | 727.2 (4) | 735.8 (6) | 726.4 (3) | 730.4 (5) |
| Lower heating value (Mj/L) | 33.528 (5) | 32.771 (1) | 33.394 (4) | 32.871 (2) | 33.584 (6) | 32.919 (3) |
| T50/°C | 89.5 (3) | 78 (1) | 92.9 (4) | 99.4 (6) | 93.5 (5) | 88.5 (2) |
| T90/°C | 153.4 (4) | 151.4 (2) | 156.6 (6) | 151 (1) | 156.1 (5) | 151.8 (3) |
| Aromatics content (%) | 26.45 (4) | 22.13 (1) | 27.32 (5) | 23.4 (3) | 27.38 (6) | 22.71 (2) |
| Olefin content (%) | 6.19 (6) | 4.96 (1) | 6.11 (4) | 6.09 (3) | 6.13 (5) | 5.79 (2) |
| C9+ aromatics content (vol %) | 16.786 (6) | 14.268 (1) | 16.534 (4) | 15.275 (3) | 16.534 (4) | 14.772 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Zhang, X.; Wen, M.; Zhang, C.; Kong, X.; Jin, Z.; Zheng, Z.; Liu, H. Effects of Unconventional Additives in Gasoline on the Performance of a Vehicle. Energies 2022, 15, 1605. https://doi.org/10.3390/en15051605
Lin M, Zhang X, Wen M, Zhang C, Kong X, Jin Z, Zheng Z, Liu H. Effects of Unconventional Additives in Gasoline on the Performance of a Vehicle. Energies. 2022; 15(5):1605. https://doi.org/10.3390/en15051605
Chicago/Turabian StyleLin, Mao, Xiaoteng Zhang, Mingsheng Wen, Chuanqi Zhang, Xiangen Kong, Zhiyang Jin, Zunqing Zheng, and Haifeng Liu. 2022. "Effects of Unconventional Additives in Gasoline on the Performance of a Vehicle" Energies 15, no. 5: 1605. https://doi.org/10.3390/en15051605
APA StyleLin, M., Zhang, X., Wen, M., Zhang, C., Kong, X., Jin, Z., Zheng, Z., & Liu, H. (2022). Effects of Unconventional Additives in Gasoline on the Performance of a Vehicle. Energies, 15(5), 1605. https://doi.org/10.3390/en15051605








