Application of Gas Dissolved in Fuel in the Aspect of a Hypocycloidal Pump Design
Abstract
1. Introduction
2. Methods
2.1. The Coefficient of Solubility and Compressibility of Exhaust Gas in Diesel Fuel
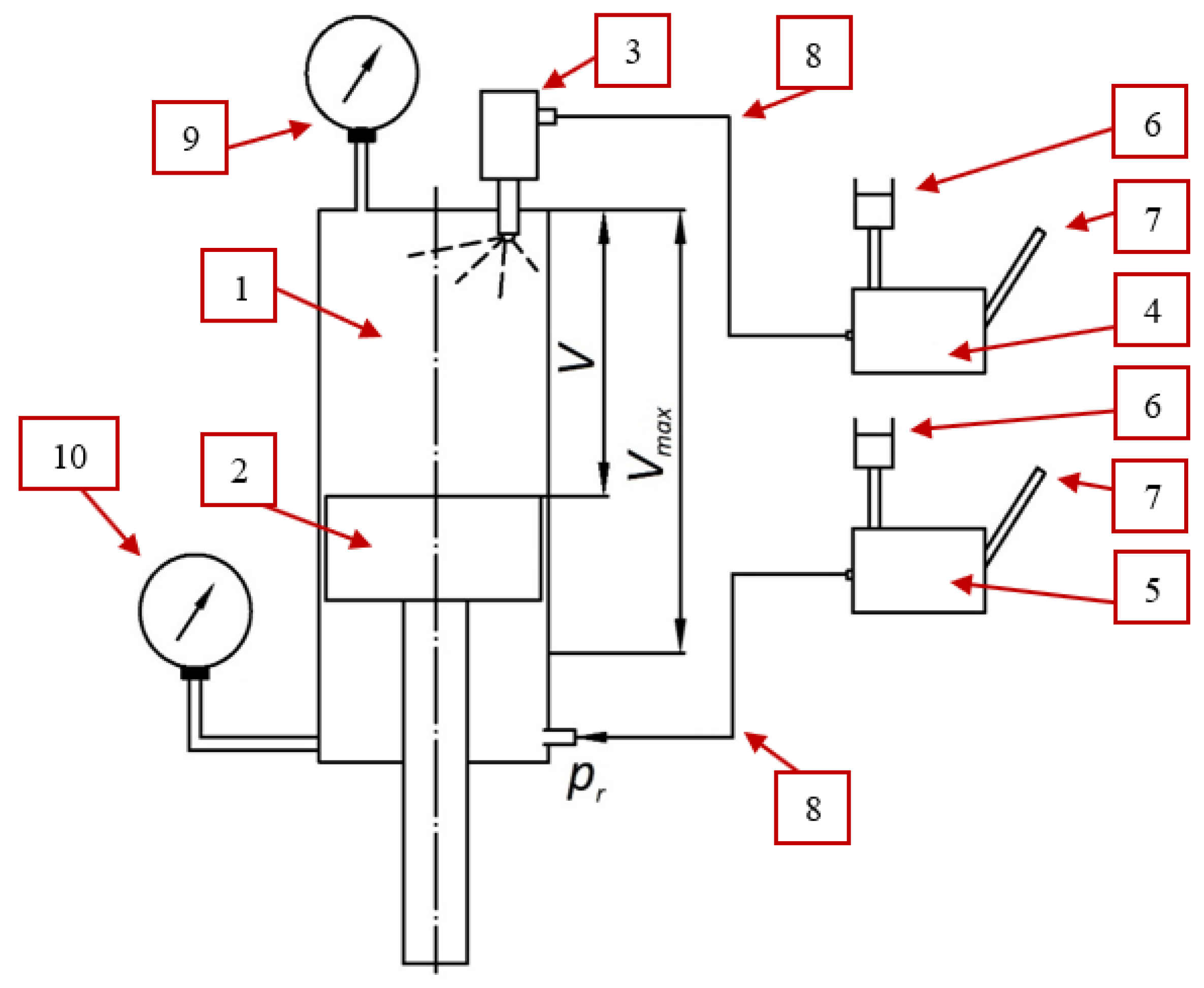
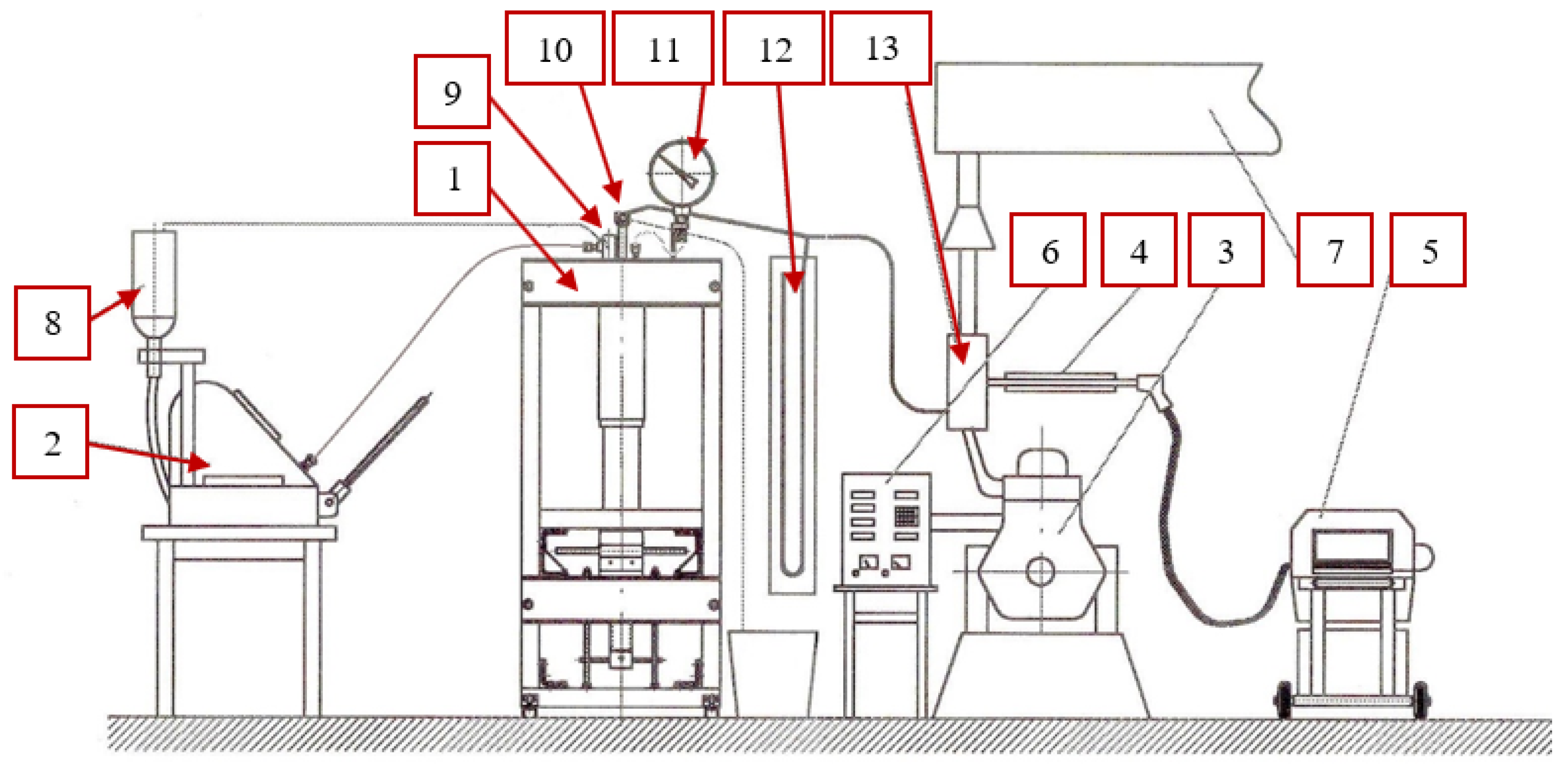
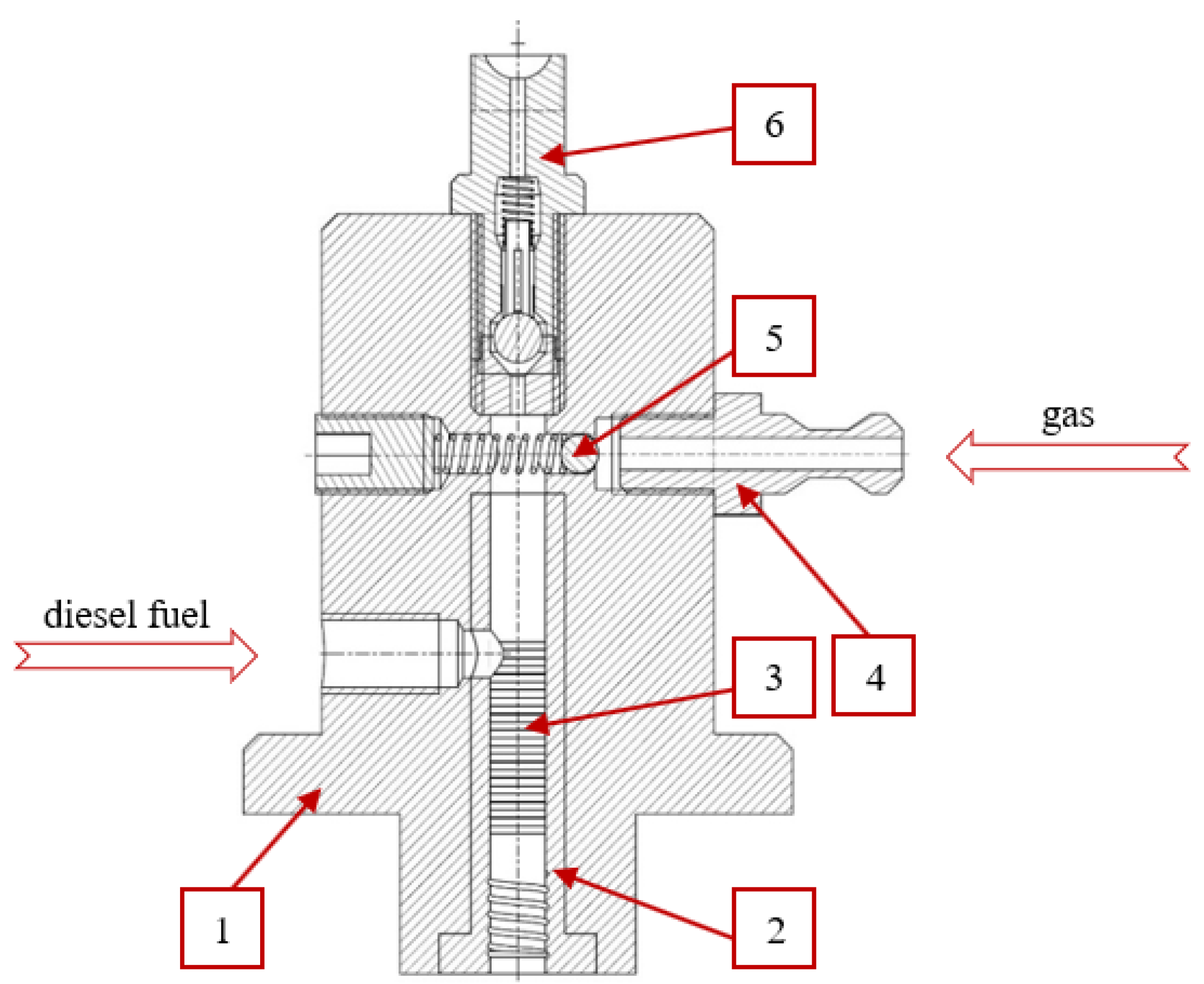
2.2. Research Stand
3. Results of the Measurements and Discussion
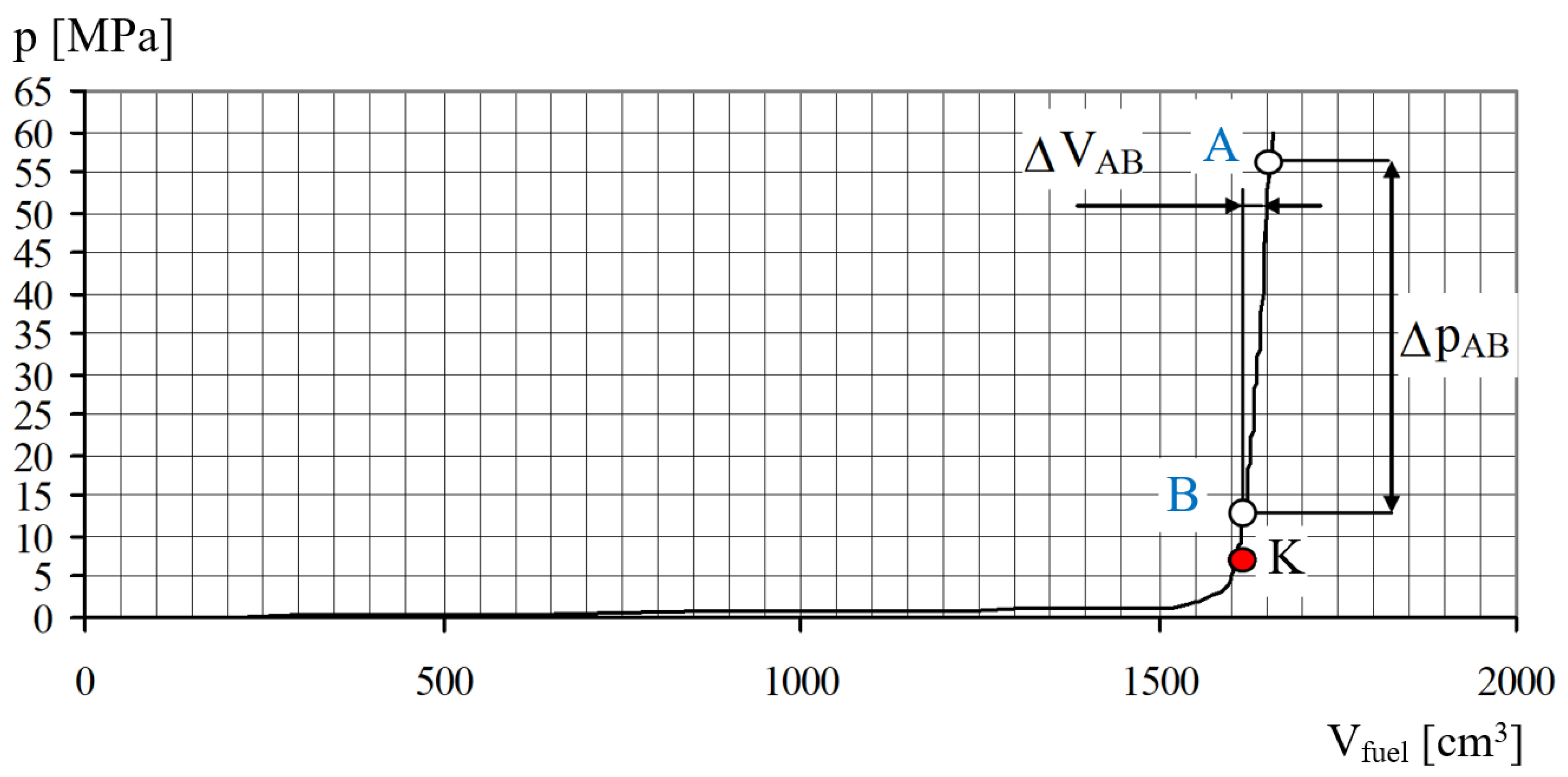
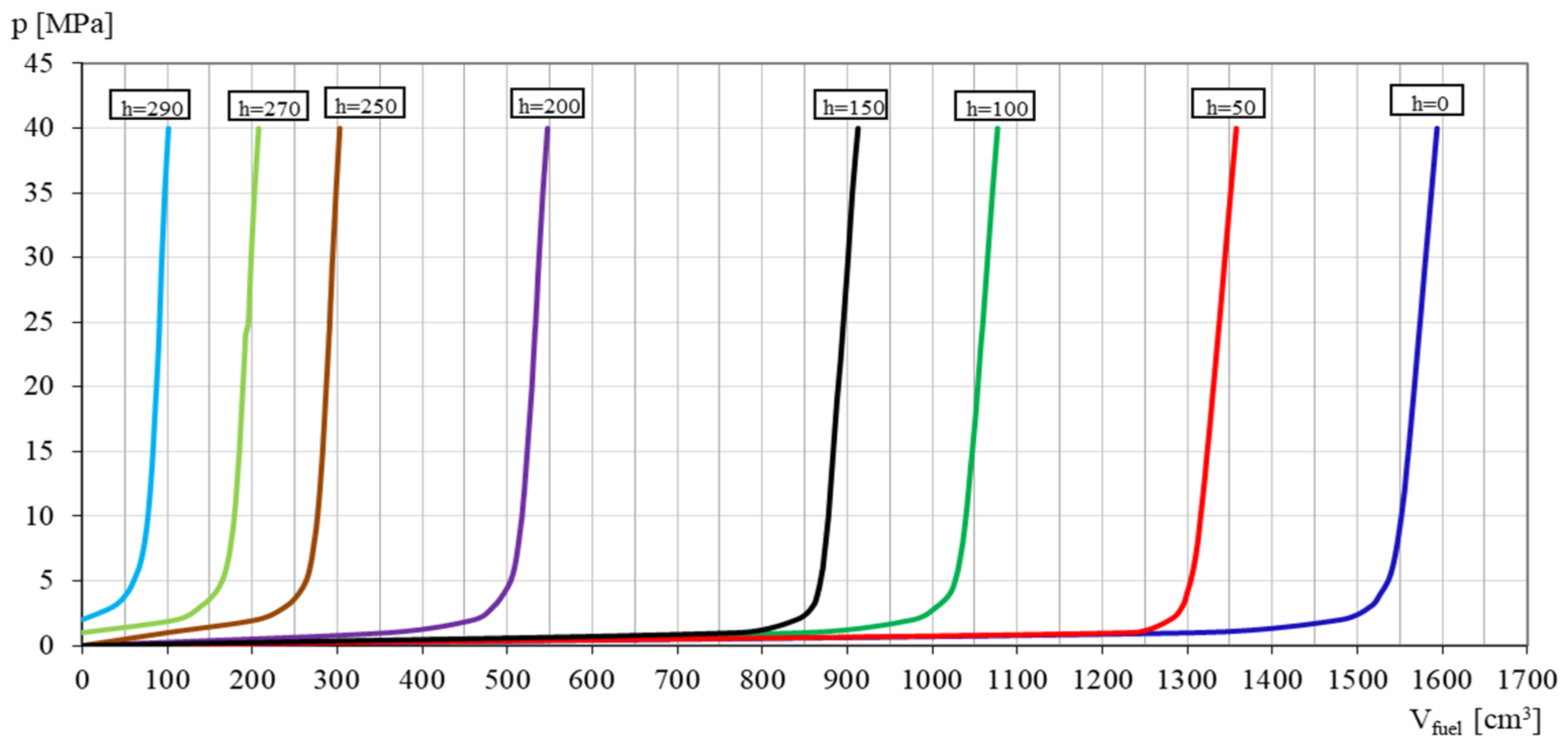
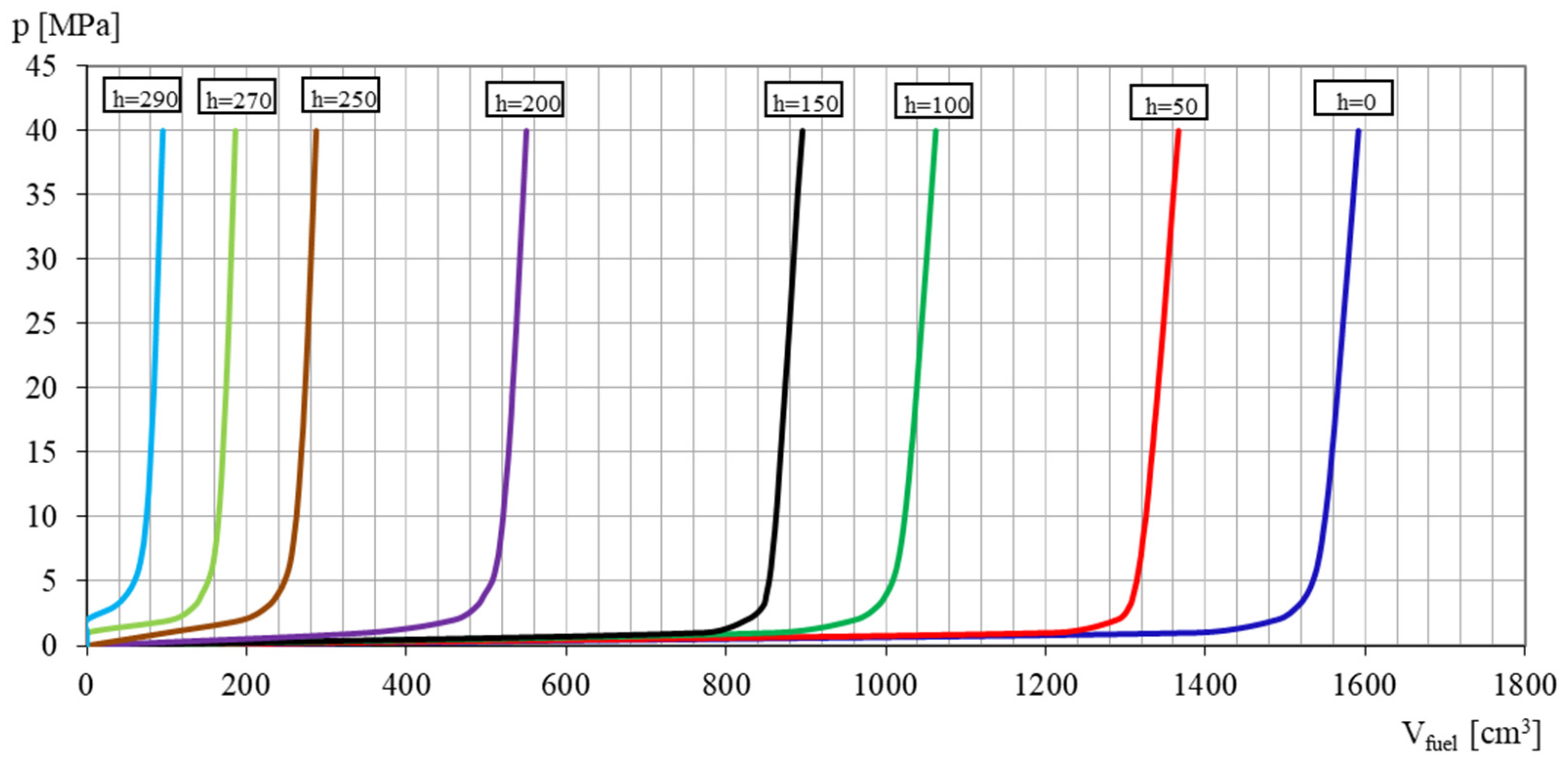
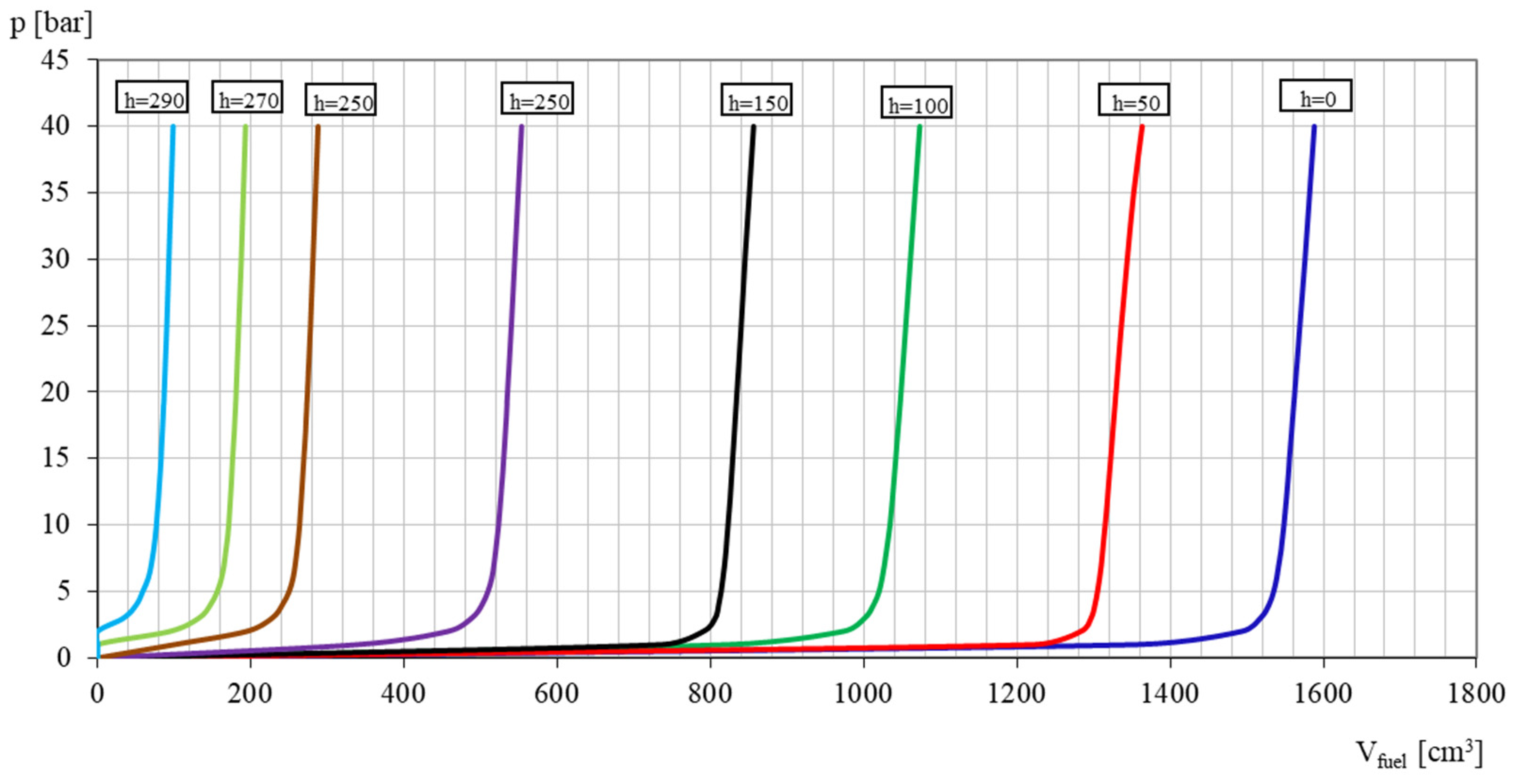
3.1. Coefficient of Solubility
3.2. Coefficient of Compressibility
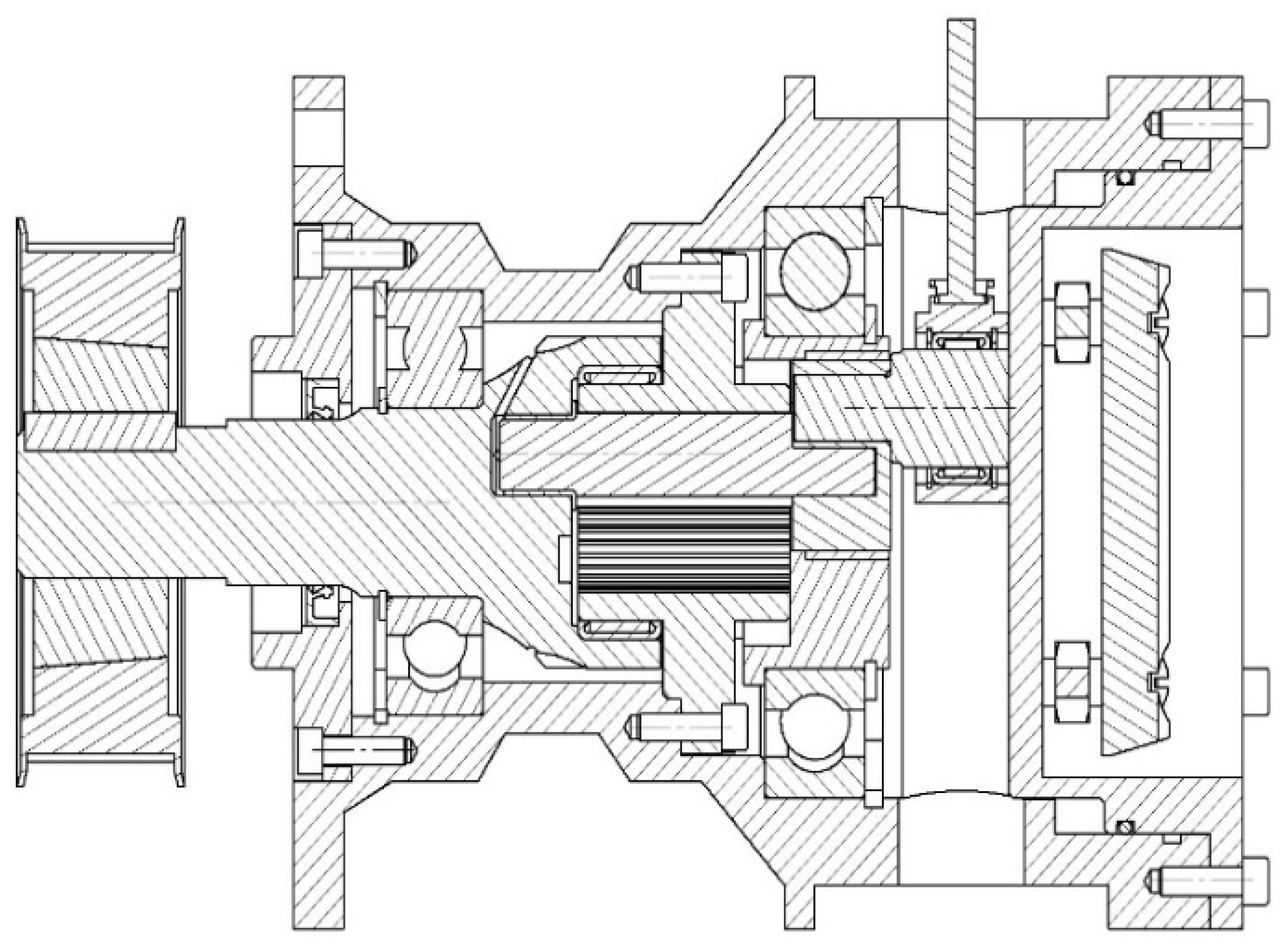
4. A Pump with a Hypocycloidal Drive
5. Conclusions and Trends in Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jasiński, R.; Galant-Gołębiewska, M.; Nowak, M.; Kurtyka, K.; Kurzawska, P.; Maciejewska, M.; Ginter, M. Emissions and Concentrations of Particulate Matter in Poznan Compared with Other Polish and European Cities. Atmosphere 2021, 12, 533. [Google Scholar] [CrossRef]
- Jasiński, R.; Galant-Gołębiewska, M.; Nowak, M.; Ginter, M.; Kurzawska, P.; Kurtyka, K.; Maciejewska, M. Case Study of Pollution with Particulate Matter in Selected Locations of Polish Cities. Energies 2021, 14, 2529. [Google Scholar] [CrossRef]
- Basińska, M.; Ratajczak, K.; Michałkiewicz, M.; Fuć, P.; Siedlecki, M. The Way of Usage and Location in a Big City Agglom-eration as Impact Factors of the Nurseries Indoor Air Quality. Energies 2021, 14, 7534. [Google Scholar] [CrossRef]
- Gawron, B.; Białecki, T.; Janicka, A.; Górniak, A.; Zawiślak, M. An innovative method for exhaust gases toxicity evaluation in the miniature turbojet engine. Aircr. Eng. Aerosp. Technol. 2017, 89, 757–763. [Google Scholar] [CrossRef]
- Feng, R.; Li, G.; Sun, Z.; Hu, X.; Deng, B.; Fu, J. Potential of emission reduction of a turbo-charged non-road diesel engine without aftertreatment under multiple operating scenarios. Energy 2023, 263, 125832. [Google Scholar] [CrossRef]
- Feng, R.; Hu, X.; Li, G.; Sun, Z.; Deng, B. A comparative investigation between particle oxidation catalyst (POC) and diesel particulate filter (DPF) coupling aftertreatment system on emission reduction of a non-road diesel engine. Ecotoxicol. Environ. Saf. 2022, 238, 113576. [Google Scholar] [CrossRef]
- Kozak, M.; Lijewski, P.; Waligórski, M. Exhaust Emissions from a Hybrid City Bus Fuelled by Conventional and Oxygenated Fuel. Energies 2022, 15, 1123. [Google Scholar] [CrossRef]
- Merkisz, J.; Siedlecki, M. Specific emissions analysis for a combustion engine in dynamometer operation in relation to the thermal state of the exhaust gas aftertreatment systems in a modified NRSC test. MATEC Web Conf. 2017, 118, 27. [Google Scholar] [CrossRef]
- Jasiński, R. Number and mass analysis of particles emitted by aircraft engine. MATEC Web Conf. 2017, 118, 23. [Google Scholar] [CrossRef]
- Sawczuk, W.; Merkisz-Guranowska, A.; Rilo Cañás, A.-M. Assessment of disc brake vibration in rail vehicle operation on the basis of brake stand. Eksploat. Niezawodn. 2021, 23, 221–230. [Google Scholar] [CrossRef]
- Sawczuk, W.; Ulbrich, D.; Kowalczyk, J.; Merkisz-Guranowska, A. Evaluation of Wear of Disc Brake Friction Linings and the Variability of the Friction Coefficient on the Basis of Vibroacoustic Signals. Sensors 2021, 21, 5927. [Google Scholar] [CrossRef] [PubMed]
- Kałużny, J.; Waligórski, M.; Szymański, G.M.; Merkisz, J.; Różański, J.; Nowicki, M.; Al Karawi, M.; Kempa, K. Reducing friction and engine vibrations with trace amounts of carbon nanotubes in the lubricating oil. Tribol. Int. 2020, 151, 1–27. [Google Scholar] [CrossRef]
- Pielecha, I. The influence of petrol injection parameters on the structure of geometry of fuel spray injected from outward-opening injectors. Fuel 2018, 222, 64–73. [Google Scholar] [CrossRef]
- Ye, P.; Sun, C.; Lapuerta, M.; Agudelo, J.; Wal, R.V.; Boehman, A.L.; Toops, T.; Daw, S. Impact of rail pressure and biodiesel fueling on the particulate morphology and soot nanostructures from a common-rail turbocharged direct injection diesel engine. Int. J. Engine Res. 2014, 17, 193–208. [Google Scholar] [CrossRef]
- Meek, G.A.; Williams, R.; Thornton, D.; Knapp, P.; Cosser, S. F2E—Ultra High Pressure Distributed Pump Common Rail System. SAE Tech. Pap. 2014, 1–8. [Google Scholar] [CrossRef]
- Wloka, J.; Pflaum, S.; Wachtmeister, G. Potential and Challenges of a 3000 Bar Common-Rail Injection System Considering Engine Behavior and Emission Level. SAE Int. J. Engines 2010, 3, 801–813. [Google Scholar] [CrossRef]
- Oki, M.; Matsumoto, S.; Toyoshima, Y.; Ishisaka, K.; Tsuzuki, N. 180MPa Piezo Common Rail System. SAE Tech. Pap. 2006, 1–10. [Google Scholar] [CrossRef]
- Petkar, R.M.; Kardile, C.A.; Deshpande, P.V.; Isenburg, R.; Soorajith, R. Influence of Increased Diesel Fuel Spray Velocities and Improved Spray Penetration in DI Engines. SAE Tech. Pap. Ser. 2006, 1–8. [Google Scholar] [CrossRef]
- Yue, Z.; Reitz, R.D. An equilibrium phase spray model for high-pressure fuel injection and engine combustion simulations. Int. J. Engine Res. 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Beck, J.C.; Watkins, A.P. The simulation of fuel sprays using the moments of the drop number size distribution. Int. J. Engine Res. 2004, 5, 1–21. [Google Scholar] [CrossRef]
- Kang, J.; Bae, C.; Lee, K.O. Initial development of non-evaporating diesel sprays in common-rail injection systems. Int. J. Engine Res. 2003, 4, 283–298. [Google Scholar] [CrossRef]
- Ferrari, A.; Vitali, R. Instantaneous torque, energy saving and flow rate ripple analysis of a common rail pump equipped with different delivery-pressure control systems. Int. J. Engine Res. 2017, 19, 1036–1047. [Google Scholar] [CrossRef]
- Magryta, P.; Geca, M. FEM analysis of piston for aircraft two stroke diesel engine. MATEC Web Conf. 2019, 252, 1–6. [Google Scholar] [CrossRef][Green Version]
- Bor, M.; Borowczyk, T.; Karpiuk, W.; Smolec, R. Determination of the response time of new generation electromagnetic injectors as a function of fuel pressure using the internal photoelectric effect. In Proceedings of the 2018 International Interdisciplinary PhD Workshop (IIPhDW), Swinoujscie, Poland, 9–12 May 2018; 2018; pp. 335–339. [Google Scholar] [CrossRef]
- Sanda, J.; Wada, Y.; Fujimoto, H. Improvement of combustion and emissions in diesel engines by means of enhanced mixture formation based on flash boiling of mixed fuel. Int. J. Engine Res. 2008, 9, 15–27. [Google Scholar] [CrossRef]
- Kwak, K.H.; Jung, D.; Borgnakke, C. Enhanced spray and evaporation model with multi-fuel mixtures for direct injection in-ternal combustion engines. Int. J. Engine Res. 2014, 15, 488–503. [Google Scholar] [CrossRef]
- Battino, R.; Clever, H.L. The Solubility of Gases in Liquids. Chem. Rev. 1966, 66, 395–463. [Google Scholar] [CrossRef]
- Merkisz, J.; Pielecha, J.; Pielecha, I. Road Test Emissions Using On-board Measuring Method for Light Duty Diesel Vehicles. Jordan J. Mech. Ind. Eng. 2011, 5, 89–96. [Google Scholar]
- Merkisz, J.; Bajerlein, M.; Kozak, W. Dissolving Oxygen in Diesel Fuel as a Way to Make Road Transport More Environ-mentally Friendly. WIT Trans. Built Environ. 2008, 101, 325–334. [Google Scholar] [CrossRef]
- Merkisz, J.; Bajerlein, M.; Kozak, W.; Markowski, J. The Influence of CNG Dissolved in the Diesel Fuel on the Combustion Process and Concentration of Toxic Compounds in Exhaust Gas. SAE Tech. Pap. 2008, 1–10. [Google Scholar] [CrossRef]
- Ma, X.; Abe, Y.; Kaneko, A.; Fujimoto, S.; Murakami, C. Study on Dissolution Process of Liquid CO2 into Water under High Pressure Condition for CCS. Energy Procedia 2017, 114, 5430–5437. [Google Scholar] [CrossRef]
- Kozak, W.; Bajerlein, M.; Markowski, J. Verification of the concept of spray mechanism assisted with air dissolved in diesel oil. Combust. Engines 2006, 124, 21–37. [Google Scholar] [CrossRef]
- Battino, R.; Rettich, T.R.; Wilhelm, E. Gas Solubilities in Liquid Water Near the Temperature of the Density Maximum, Tmax (H2O) = 277.13 K. Monatsh. Chem. 2018, 149, 219–230. [Google Scholar] [CrossRef]
- Bankowski, K.; Gajewska, I. (Edited by) Physicochemical Guide (Original: Poradnik Fizykochemiczny), 2nd ed.; WNT: Warsaw, Poland, 1974; p. 415. [Google Scholar]
- Kozak, W. Shaping Injection Parameters for Compression-Ignition Engines (Original: Ksztaltowanie Parametrow Wtrysku do Silnikow o Zapłonie Samoczynnym); No 427; Poznan Univeristy of Technology Publishing House: Poznan, Poland, 2008; pp. 43–67. [Google Scholar]
- Guillon, M. Theory and Calculation of Hydraulic Systems; WNT: Warsaw, Poland, 1966; p. 108. (In Polish) [Google Scholar]
- Atkins, P.; De Paula, J. Physical Chemistry, 9th ed.; W. H. Freeman and Company: New York, NY, USA, 2010; p. 718. [Google Scholar]
- Xu, X.; Xu, H.; Deng, H.; Gu, F.; Talbot, C. An Investigation of a Hypocycloid Mechanism Based Twin-Rotor Piston Engine. J. Mech. Eng. Sci. 2015, 229, 106–115. [Google Scholar] [CrossRef]
- Aziz, E.S. Enhanced Hypocycloid Gear Mechanism for Internal Combustion Engine Applications. J. Mech. Des. 2016, 138, 125002. [Google Scholar] [CrossRef]
- ElBahloul, M.A.; Aziz, E.S.; Chassapis, C. Mechanical Efficiency Prediction Methodology of the Hypocycloid Gear Mechanism for Internal Combustion Engine Application. Int. J. Interact. Des. Manuf. 2019, 13, 221–233. [Google Scholar] [CrossRef]
- Bajerlein, M.; Karpiuk, W.; Smolec, R. Use of Gas Desorption Effect in Injection Systems of Diesel Engines. Energies 2021, 14, 244. [Google Scholar] [CrossRef]
- Bajerlein, M.; Bor, M.; Karpiuk, W.; Smolec, R.; Spadło, M. Strength analysis of critical components of high-pressure fuel pump with hypocycloid drive. Bull. Pol. Acad. Sci. Tech. Sci. 2020, 68, 1341–1350. [Google Scholar] [CrossRef]
- Bor, M.; Borowczyk, T.; Idzior, M.; Karpiuk, W.; Smolec, R. Analysis of hypocycloid drive application in a high-pressure fuel pump. MATEC Web Conf. 2017, 118, 00020. [Google Scholar] [CrossRef]
- Karpiuk, W. Cavitating Fuel Flows in Hypocycloid Pump from the Perspective of Applying the Effect of Gas Desorption from The Solution with Nucleation of Gas Bubbles. Adv. Sci. Technol. Res. J. 2020, 14, 56–66. [Google Scholar] [CrossRef]
- Bor, M.; Borowczyk, T.; Karpiuk, W.; Spadło, M.; Smolec, R. Concept of a pump for diesel engines fuel supply using hypocycloid drive. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 421, p. 042034. [Google Scholar] [CrossRef]
- Bor, M.; Karpiuk, W.; Krakowiak, M.; Borowczyk, T.; Smolec, R. Assessment of the Possibility of Applying Ceramic Materials in Common Rail Injection Systems. Adv. Sci. Technol. Res. J. 2018, 12, 312–321. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajerlein, M.; Karpiuk, W.; Smolec, R. Application of Gas Dissolved in Fuel in the Aspect of a Hypocycloidal Pump Design. Energies 2022, 15, 9163. https://doi.org/10.3390/en15239163
Bajerlein M, Karpiuk W, Smolec R. Application of Gas Dissolved in Fuel in the Aspect of a Hypocycloidal Pump Design. Energies. 2022; 15(23):9163. https://doi.org/10.3390/en15239163
Chicago/Turabian StyleBajerlein, Maciej, Wojciech Karpiuk, and Rafał Smolec. 2022. "Application of Gas Dissolved in Fuel in the Aspect of a Hypocycloidal Pump Design" Energies 15, no. 23: 9163. https://doi.org/10.3390/en15239163
APA StyleBajerlein, M., Karpiuk, W., & Smolec, R. (2022). Application of Gas Dissolved in Fuel in the Aspect of a Hypocycloidal Pump Design. Energies, 15(23), 9163. https://doi.org/10.3390/en15239163







