Novel Nanomaterials for Hydrogen Production and Storage: Evaluating the Futurity of Graphene/Graphene Composites in Hydrogen Energy
Abstract
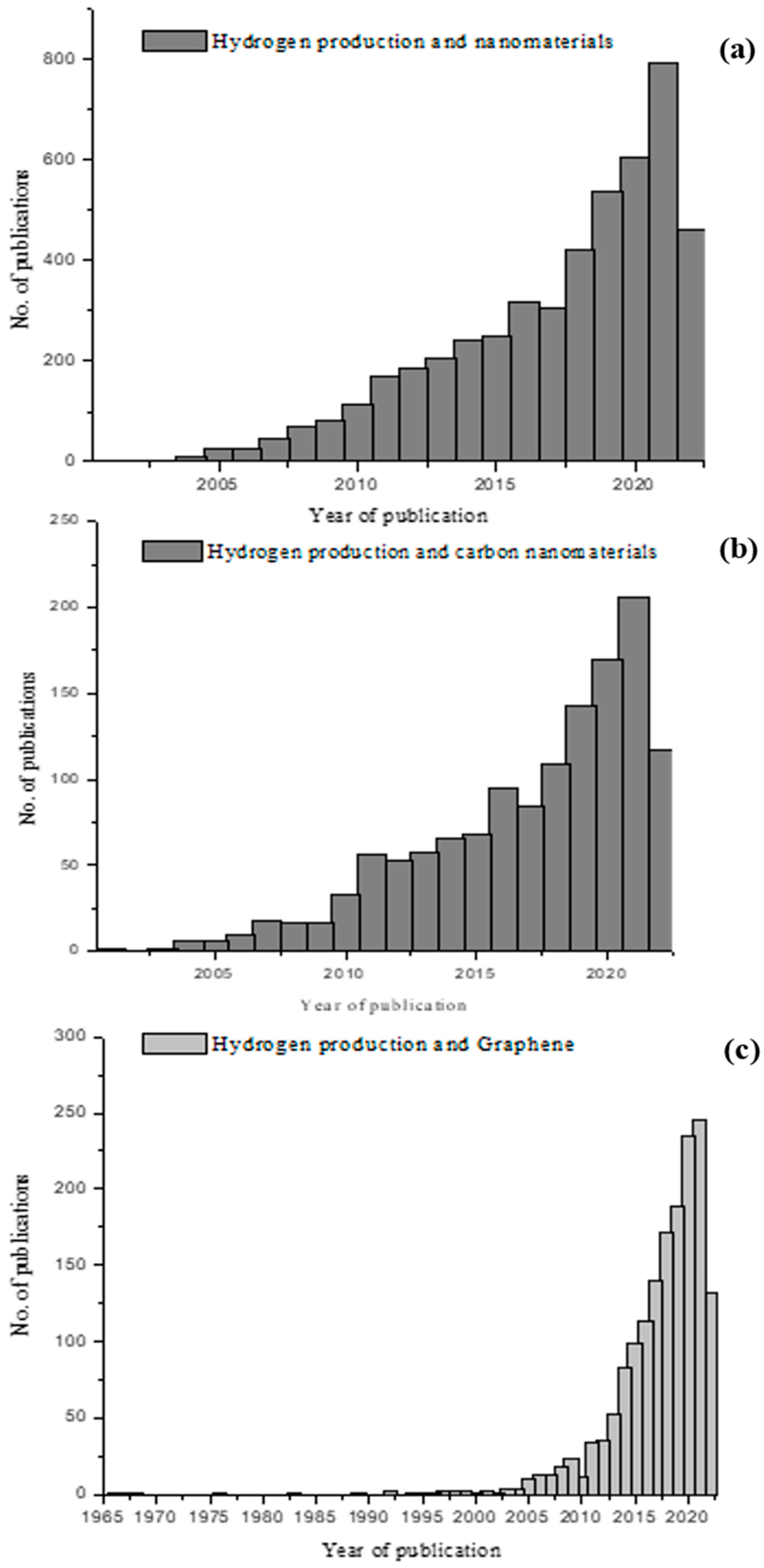
1. Introduction
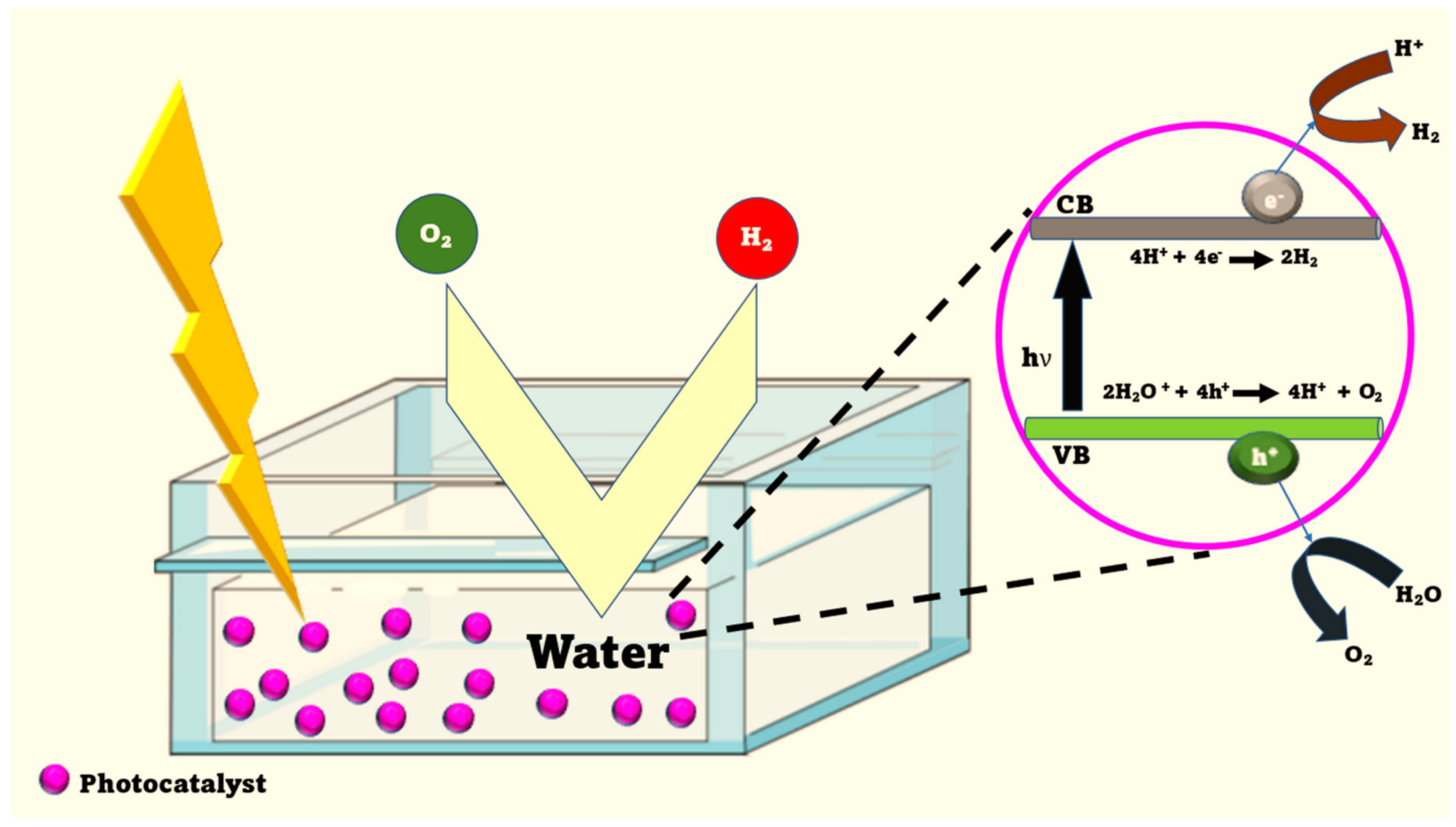
2. Nanomaterials Used in Hydrogen Production
3. Nanomaterials Used in Hydrogen Storage
4. Graphene-Based Nanocomposites for Hydrogen Energy Applications
5. Future Perspectives
6. Concluding Remarks
Funding
Conflicts of Interest
References
- Dudley, B. BP Statistical Review of World Energy; BP Statistical Review: London, UK, 2018; p. 00116. [Google Scholar]
- D’Amato, M.; Cecchi, L.; Annesi-Maesano, I. Climate change and respiratory diseases. Eur. Respir. Rev. 2014, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, T.; Abbasi, S.A. Renewable Energy Sources: Their Impact on Global Warming and Pollution; PHI Learning Pvt. Ltd.: Delhi, India, 2011. [Google Scholar]
- Kaygusuz, K. Energy for sustainable development: A case of developing countries. Renew. Sustain. Energy Rev. 2012, 16, 1116–1126. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z. A review on fundamentals for designing oxygen evolution electrocatalysts. J. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Dincer, I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Revankar, S.T. Chapter four-nuclear hydrogen production. In Storage and Hybridization of Nuclear Energy; Bindra, H., Revankar, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 49–117. [Google Scholar]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Jones, R.H.; Thomas, G.J. Materials for the Hydrogen Economy; CRC Press: Boca Raton, FL, USA, 2007; Catalog no.5024. [Google Scholar]
- Basic Research Needs for hydrogen Economy. In Report on the Basic Energy Sciences Workshop on Hydrogen Production, Storage, and Use; Argonne National Laboratory: Lemont, IL, USA, 2003. Available online: https://www.hydrogen.energy.gov/pdfs/nhe_rpt.pdf (accessed on 14 October 2022).
- Schlapbach, L. Hydrogen as a fuel and its storage for mobilityand transport. MRS Bull. 2002, 27, 675–676. [Google Scholar] [CrossRef]
- Read, C.; Thomas, G.; Ordaz, C.; Satyapal, S.U.S. Department of Energy’s system targets for on-board vehicular hydrogen storage. Mater. Matters 2007, 2, 3. [Google Scholar]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Chandra, D.; Reilly, J.J.; Chellappa, R. Metal hydrides for vehicular applications: The state of the art. JOM 2006, 58, 26–32. [Google Scholar] [CrossRef]
- Seayad, A.M.; Antonell, D.M. Recent advances in hydro gen storage in metal-containing inorganic nanostructures and related materials. Adv. Mater. 2004, 16, 765–777. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Wicke, B.G. Bottling the hydrogen genie. Ind. Phys. 2004, 10, 20–23. [Google Scholar]
- Schuth, F. Technology: Hydrogen and hydrates. Nature 2005, 434, 712–713. [Google Scholar] [CrossRef]
- Schüth, F.; Bogdanović, B.; Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2004, 10, 2249–2258. [Google Scholar] [CrossRef]
- McKeown, N.B.; Makhseed, S.; Msayib, K.J.; Ooi, L.-L.; Helliwell, M.; Warren, J.E. A phthalocyanine clathrate of cubic symmetry containing interconnected solvent-filled voids of nanometer dimensions. Angew. Chem. Int. 2005, 44, 7546–7549. [Google Scholar] [CrossRef]
- Fichtner, M. Nanotechnological aspects in materials for hydrogen storage. Adv. Eng. Mater. 2005, 7, 443–455. [Google Scholar] [CrossRef]
- Sahoo, M.; Panigrahi, C.; Vishwakarma, S.; Kumar, J. A Review on Nanotechnology: Applications in Food Industry, Future Opportunities, Challenges and Potential Risks. J. Nanotechnol. Nanomater. 2022, 3, 28–33. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at asemiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review andrecent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B., Jr. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Roy, S.C.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Kim, S.; Yoriya, S.; LaTempa, T.J.; Grimes, C.A. Synthesis and applicationsof electrochemically self-assembled titania nanotube arrays. Phys. Chem. Chem. Phys. 2010, 12, 2780–2800. [Google Scholar] [CrossRef]
- Mor, G.K.; Prakasam, H.E.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Vertically oriented Ti–Fe–O nanotube array films:toward a useful material architecture for solar spectrum waterphotoelectrolysis. Nano Lett. 2007, 7, 2356–2364. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced photocleavage of water using titania nanotubearrays. Nano Lett. 2005, 5, 191–195. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; Shankar, K.; Mor, G.K.; Grimes, C.A. Water-photolysis properties of micron-length highly-ordered titaniananotube-arrays. J. Nanosci. Nanotechnol. 2005, 5, 1158–1165. [Google Scholar] [CrossRef]
- Shankar, K.; Mor, G.K.; Prakasam, H.E.; Yoriya, S.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Highly-ordered TiO2 nanotubearrays up to 220 mm in length: Use in water photoelectrolysis and dye-sensitized solar cells. Nanotechnology 2007, 18, 065707. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Yoriya, S.; Prakasam, H.E.; Varghese, O.K.; Mor, G.K.; Latempa, T.A.; Fitzgerald, A.; Grimes, C.A. Enhancedphotocleavage of water using titania nanotube arrays. J. Phys. Chem. B 2006, 110, 16179–16184. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Wilke, R.H.T.; Sharma, S.; Shankar, K.; Latempa, T.J.; Choi, K.S.; Grimes, C.A. p-Type Cu-Ti-O nanotube arrays and their use in self-biased heterojunction photoelectrochemical diodes for hydrogen generation. Nano Lett. 2008, 8, 1906–1911. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Kapilashrami, M.; Bakke, J.R.; Bent, S.F.; Chuang, C.-H.; Pong, W.-F.; Guo, J.; Vayssieres, L.; Mao, S.S. TiO2–SnO2: F interfacial electronic structure investigated by softx-ray absorption spectroscopy. Phys. Rev. B 2012, 85, 125109. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor nanowires for energyconversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef]
- Lin, Y.J.; Yuan, G.B.; Liu, R.; Zhou, S.; Sheehan, S.W.; Wang, D.W. Semiconductor nanostructure-based photoelectrochemical watersplitting: A brief review. Chem. Phys. Lett. 2011, 507, 209–215. [Google Scholar] [CrossRef]
- Yang, X.; Wolcott, A.; Wang, G.; Sobo, A.; Fitzmorris, R.C.; Qian, F.; Zhang, J.Z.; Li, Y. Nitrogen-doped ZnO nanowire arrays forphotoelectrochemical water splitting. Nano Lett. 2009, 9, 2331–2336. [Google Scholar] [CrossRef]
- Shet, S.; Ahn, K.S.; Nuggehalli, R.; Yan, Y.F.; Turner, J.; Al-Jassim, M. Phase separation in Ga and N co-incorporated ZnO films and itseffects on photo-response in photoelectrochemical water splitting. Thin Solid Film. 2011, 519, 5983–5987. [Google Scholar] [CrossRef]
- Yousefi, M.; Amiri, M.; Azimirad, R.; Moshfegh, A.Z. Enhanced photoelectrochemical activity of Ce doped ZnO nanocomposite thin films under visible light. J. Electroanal. Chem. 2011, 661, 106–112. [Google Scholar] [CrossRef]
- Kim, H.; Seol, M.; Lee, J.; Yong, K. Highly Efficient Photoelectrochemical Hydrogen Generation Using Hierarchical ZnO/WOx Nanowires Cosensitized with CdSe/CdS. J. Phys. Chem. C 2011, 115, 25429–25436. [Google Scholar] [CrossRef]
- Wang, G.; Yang, X.; Qian, F.; Zhang, J.Z.; Li, Y. Double-sided CdS and CdSe quantum dot co-sensitized ZnO nanowire arrays for photoelectrochemical hydrogen generation. Nano Lett. 2010, 10, 1088–1092. [Google Scholar] [CrossRef]
- Chouhan, N.; Yeh, C.L.; Hu, S.-F.; Liu, R.-S.; Chang, W.-S.; Chen, K.-H. Photocatalytic CdSe QDs-decorated ZnO nanotubes: Aneffective photoelectrode for splitting water. Chem. Commun. 2011, 47, 3493–3495. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Ma, Z.; Liu, D.; Mao, S.S.; Antoun, B.R. Engineering impurity distributions in photoelectrodes for solar water oxidation. Adv. Energy Mater. 2012, 2, 52–57. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Vayssieres, L.; Shen, S.; Guo, L.; Wheeler, D.A.; Zhang, J.Z.; Antoun, B.R.; Mao, S.S. A perspective on solar-driven water splitting with all-oxide hetero-nanostructures. Energy Environ. Sci. 2011, 4, 3889–3899. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Morphology, structure and photocatalytic performance of ZnIn2S4 synthesized via a solvothermal/hydrothermal route in different solvents. J. Phys. Chem. Solids 2008, 69, 2426–2432. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Cetyltrimethylammonium bromide (CTAB)-assisted hydrothermal synthesis of ZnIn2S4 as an efficient visible-light-driven photocatalyst for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 4501–4510. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guo, L. Crystallite, optical and photocatalytic properties of visible-light-driven ZnIn2S4 photocatalysts synthesized via a surfactant-assisted hydrothermal method. Mater. Res. Bull. 2009, 44, 100–105. [Google Scholar] [CrossRef]
- Shen, S.; Mao, S.S. Nanostructure designs for effective solar to hydrogen conversion. Nanophotonics 2012, 1, 31–50. [Google Scholar] [CrossRef]
- Shen, S.; Shi, J.; Guo, P.; Guo, L. Visible-light-driven photocatalytic water splitting on nanostructured semiconducting materials. Int. J. Nanotechnol. 2011, 8, 523. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recentprogress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Jang, J.W.; Kim, H.G.; Joshi, U.A.; Lee, J.S. Fabrication of CdS nanowires decorated with TiO2 nanoparticles for photocatalytic hydrogen production under visible light irradiation. Int. J. Hydrogen Energy 2008, 33, 5975–5980. [Google Scholar] [CrossRef]
- Sebastian, P.J.; Castaneda, R.; Ixtlilco, L.; Mejia, R.; Pantojaand, J.; Olea, A. Synthesis and characterization of nanostructured semiconductors for photovoltaic and photoelectrochemical cell applications. Proc. SPIE 2008, 7044, 704405. [Google Scholar] [CrossRef]
- Silva, L.A.; Ryu, S.Y.; Choi, J.; Choi, W.; Hoffmann, M.R. Photocatalytic Hydrogen Production with Visible Light over Pt-Interlinked Hybrid Composites of Cubic-Phase and Hexagonal-Phase CdS. J. Phys. Chem. C 2008, 112, 12069. [Google Scholar] [CrossRef]
- Chen, X.; Yu, T.; Fan, X.; Zhang, H.; Li, Z.; Ye, J.; Zou, Z.; Chen, X.; Yu, T.; Fan, X.; et al. Enhanced activity of mesoporous Nb2O5 for photocatalytic hydrogen production. Appl. Surf. Sci. 2007, 253, 8500. [Google Scholar] [CrossRef]
- Takahara, Y.; Konde, J.; Takata, N.T.; Lu, D.; Domen, K. Mesoporous tantalum oxide-characterization and photocatalytic activity for the overall water decomposition. Chem. Mater. 2001, 13, 1194. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jang, J.W.; Youn, D.H.; Magesh, G.; Lee, J.S. A Stable and Efficient Hematite Photoanode in a Neutral Electrolyte for Solar Water Splitting: Towards Stability Engineering. Adv. Energy Mater. 2014, 4, 1400476. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- Hitoki, G.; Takata, T.; Kondo, J.N.; Hara, M.; Domen, H.K.K. An oxynitride, TaON, as an efficient water oxidation photocatalyst under visible light irradiation (λ ≤ 500 nm). Chem. Commun. 2002, 16, 1698–1699. [Google Scholar] [CrossRef]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H.J. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Wang, Q. Nanostructure-based WO3 photoanodes for photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2012, 14, 7894–7911. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, E.; Diner, I.; Naterer, G.F. Radiative heat transfer and catalyst performance in a large-scale continuous flow photoreactor for hydrogen production. Chem. Eng. Sci. 2012, 84, 638–645. [Google Scholar] [CrossRef]
- Wu, N.L.; Lee, M.S. Enhanced TiO2 photocatalysis by Cu in hydrogen production from aqueous methanol solution. Int. J. Hydrogen Energy 2004, 29, 1601. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, W. Dual Photocatalytic Pathways of Trichloro acetate Degradation on TiO2: Effects of Nanosized Platinum Deposits on Kinetics and Mechanism. J. Phys. Chem. B 2002, 106, 13311–13317. [Google Scholar] [CrossRef]
- Jin, S.; Shiraishi, F. Photocatalytic activities enhanced for decompositions of organic compounds over metal-photo depositing titanium dioxide. Chem. Eng. J. 2004, 97, 203–211. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Wu, J.C.S.; Chou, H.-Y. Effects of sol–gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction. J. Catal. 2004, 221, 432–440. [Google Scholar] [CrossRef]
- Liu, S.; Qu, Z.; Han, X.; Sun, C.L. A mechanism for enhanced photocatalytic activity of silver-loaded titanium dioxide. Catal. Today 2004, 93–95, 877–884. [Google Scholar] [CrossRef]
- Kamat, P.V.; Flumiani, M.; Dawson, A. Metal–metal and metal–semiconductor composite nanoclusters. Colloids Surf. A 2002, 202, 269–279. [Google Scholar] [CrossRef]
- Bardos, E.S.; Czili, H.; Horvath, A. Photocatalytic Degradation of Anionic Surfactant in Titanium Dioxide Suspension. J. Photochem. Photobiol. A 2003, 154, 195. [Google Scholar]
- Shen, S.; Guo, L.; Chen, X.; Ren, F.; Mao, S.S. Effect of Ag2S onsolar-driven photocatalytic hydrogen evolution of nanostructured CDs. Int. J. Hydrogen Energy 2010, 35, 7110–7115. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Ren, F.; Kronawitter, C.X.; Mao, S.S.; Guo, L. Solar light-driven photocatalytic hydrogen evolution overZnIn2S4 loaded with transition-metal sulfides. Nanoscale Res. Lett. 2011, 6, 290. [Google Scholar] [CrossRef]
- Mao, S.S.; Shen, S.; Guo, L. Liejin Guob Nanomaterials for renewable hydrogen production, storage and utilization. Prog. Nat. Sci. Mater. Int. 2012, 22, 522–534. [Google Scholar] [CrossRef]
- Singh, R.; Altaee, A.; Gautam, S. Nanomaterials in the advancement of hydrogen energy storage. Heliyon 2020, 6, e04487. [Google Scholar] [CrossRef]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. ExceptionalH2 saturation uptake in microporous metal-organic frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar] [CrossRef]
- Report of the Basic Energy Science Workshop on Hydrogen Production, Storage and Use; Argonne National Laboratory: Lemont, IL, USA, 2003.
- Grochala, W.; Edwards, P.P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Sherif, S.A.; Barbir, F.; Vieziroglu, T.N.; Mahishi, M.; Srinivasan, S.S. Hydrogen Energy Technologies. In Handbook of Energy Efficiency and Renewable Energy; Kreith, F., Goswami, D.Y., Eds.; Chapter 27; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bogdanovic, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Jensen, C.M.; Zidan, R.A. Hydrogen Storage Materials and Method of Making by Dry Homogenation. US Patent 6471935, 2002. [Google Scholar]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Ruckenstein, E. H2 Storage in Li3N. Temperature-Programmed Hydrogenation and Dehydrogenation. Ind. Eng. Chem. Res. 2003, 42, 5135–5139. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Au, M. Destabilized and Catalyzed Alkali Metal Borohydrides for Hydrogen Storage with Good Reversibility. US Patent Appl. Publ. 0194695, 2006. [Google Scholar]
- Srinivasan, S.S.; Escobar, D.; Jurczyk, M.; Goswami, Y.; Stefanakos, E.K. Nanocatalyst doping of Zn(BH4)2 for on-boardhydrogen storage. J. Alloys Compd. 2008, 462, 294–302. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Siegel, D.J.; Halliday, D.; Drews, A.; Carter, R.O., III; Wolverton, C.; Lewis, G.J.; Sachtler, J.W.A.; Low, J.J.; et al. Hydrogen storage properties of 2LiNH2 + LiBH4 + MgH2. J. Alloys Compd. 2007, 446–447, 345–349. [Google Scholar] [CrossRef]
- Lewis, G.J.; Sachtler, J.W.A.; Low, J.J.; Lesch, D.A.; Faheem, S.A.; Dosek, P.M.; Knight, L.M.; Halloran, L.; Jensen, C.M.; Yang, J.; et al. High throughput screening of the ternary LiNH2-MgH2-LiBH4 phase diagram. J. Alloys Compd. 2007, 446–447, 355–359. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rçnnebro, E.J. Hydrogen Storage Properties of Nanosized MgH2−0.1TiH2 Prepared by Ultrahigh-Energy−High-Pressure Milling. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar] [CrossRef]
- Gutowska, A.; Li, L.Y.; Shin, Y.S.; Wang, C.M.M.; Li, X.H.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chem. 2005, 117, 3644–3648. [Google Scholar] [CrossRef]
- Chaise, A.; de Rango, P.; Marty, P.; Fruchart, D. Experimental and numerical study of a magnesium hydride tank. Int. J. Hydrogen Energy 2010, 35, 6311–6322. [Google Scholar] [CrossRef]
- Chaise, A.; de Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S.; Olives, R.; Garrier, S. Enhancement of hydrogen sorption in magnesium hydride using expanded natural graphite. Int. J. Hydrogen Energy 2009, 34, 8589–8596. [Google Scholar] [CrossRef]
- Pourpoint, T.L.; Sisto, A.; Smith, K.C.; Voskuilen, T.G.; Visaria, M.K.; Zheng, Y.; Fisher, T.S. Fisher, HT2008. In Proceedings of the ASME Summer Heat Transfer Conference, Jacksonville, FL, USA, 10–14 August 2008; Volume 1, pp. 37–46. [Google Scholar]
- Sudan, P.; Züttel, A.; Mauron, P.; Emmenegger, C.; Wenger, P.; Schlapbach, L. Physisorption of hydrogen in single walled carbon nanotubes. Carbon 2003, 41, 2377–2383. [Google Scholar] [CrossRef]
- Viswanathan, B.; Sankaran, M.; Schibioh, M.A. Carbonnanomaterials: Are they appropriate candidates for hydrogenstorage? Bull. Catal. Soc. India 2003, 2, 12–32. [Google Scholar]
- Nijkamp, M.G.; Raaymakers, J.E.M.J.; van Dillen, A.J.; de Jong, K.P. Hydrogen storage using physisorption—Materials demands. Appl. Phys. A 2001, 72, 619–623. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Costa, P.M.F.J.; Coleman, K.S.; Green, M.L.H. Influence of catalyst metal particles on the hydrogen sorption of single walled carbon nanotube materials. Nanotechnology 2005, 16, 512–517. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; Brien, S.C.O.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y. IR characterizations of lithium imideand amide. J. Alloys Compd. 2005, 395, 236–239. [Google Scholar] [CrossRef]
- Salehabadi, A.; Umar, M.F.; Ahmad, A.; Ahmad, M.I.; Ismail, N.; Rafatullah, M. Carbon-based nanocomposites in solid-state hydrogen storage technology: An overview. Int. J. Energy Res. 2020, 44, 11044–11058. [Google Scholar] [CrossRef]
- Poirier, E.; Chahine, R.; Be´nard, P.; Dorval-Douville, G.; Lafi, L.; Chandonia, P.A. Hydrogen Adsorption Measurements and Modeling on Metal-Organic Frameworks and Single-Walled Carbon Nanotubes. Langmuir 2006, 22, 8784. [Google Scholar] [CrossRef]
- Ding, R.G.; Finnerty, J.J.; Zhu, Z.H.; Yan, Z.F.; Lu, G.Q. Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Valencia, CA, USA, 2004; Volume X, pp. 1–21. [Google Scholar]
- Ströbel, R.; Garche, J.; Moseley, P.T.; Jörissen, L.; Wolf, G. Hydrogen storage by carbon materials. J. Power Sources 2006, 159, 781. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, Y.-H.; Dillon, A.C.; Heben, M.J.; Zhang, S.B. Hydrogen Storage in Novel Organometallic Buckyballs. Phys. Rev. Lett. 2005, 94, 155504. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.; Heben, M. Hydrogen storage using carbon adsorbents: Past, present and future. Appl. Phys. A 2001, 72, 133–142. [Google Scholar] [CrossRef]
- Chahine, R.; Bose, T.K. Low-pressure adsorption storage of hydrogen. Int. J. Hydrogen Energy 1994, 19, 161–164. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Romo, F.J.U.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Côté, A.P.; Bennin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Kaufmann, A.; Mukasyan, A.; Varma, A. Single and multi-wall carbon nanotubes produced using the floating catalyst method: Synthesis, purification and hydrogen up take. Carbon 2006, 44, 2160–2170. [Google Scholar] [CrossRef]
- Panella, B.; Kossykh, L.; Dettlaff-Weglikowska, U.; Hirscher, M.; Zerbi, G.; Roth, S. Volumetric measurement of hydrogen storage in HCl-treated polyaniline and polypyrrole. Synth. Met. 2005, 151, 208–210. [Google Scholar] [CrossRef]
- Deng, F.; Zou, J.; Zhao, L.; Zhou, G.; Luo, X.; Luo, S. Chapter 3 Nanomaterial-Based Photocatalytic Hydrogen Production. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–82. [Google Scholar]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Bolotin, K.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Observation of electron-hole puddles in graphene using a scan ning single electron transistor. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; He, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photo cacatalyticc splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Xie, H.; Hou, C.; Wang, H.; Zhang, Q.; Li, Y. S, N co-doped graphene quantumdot/TiO2 composites for efficient photocatalytic hydrogen generation. Nano. Res. Let. 2017, 12, 400–408. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and Reduced Graphene Oxide as Efficient Photocatalysts for Hydrogen Evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Hao, X.; Jin, Z.; Xu, J.; Min, S.; Lu, G. Functionalization of TiO2 with graphene quantum dots for efficient photocatalytic hydrogen evolution. Superlattices Microstruct. 2016, 94, 237–244. [Google Scholar] [CrossRef]
- Fan, X.; Chen, X.; Dai, L. 3D graphene based materials for energy storage. Curr. Opin. Colloid Interface Sci. 2015, 20, 429–438. [Google Scholar] [CrossRef]
- Ohashi, T. Carbon nanotubes in carbon nanomaterials foradvanced energy systems. In Advances in Materials Synthesis and Device Applications; Lu, W., Baek, J.-B., Dai, L., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Masjedi-Arani, M.; Salavati-Niasari, M. Cd2SiO4/Graphene nanocomposite: Ultrasonic assisted synthesis, characterization and electrochemical hydrogen storage application. Ultrason. Sonochemistry 2018, 43, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ngqalakwezi, A.; Nkazi, D.; Seifert, G.; Ntho, T. Effects of reduction of graphene oxide on the hydrogen storage capacities of metal graphene nanocomposite. Catal. Today 2019, 358, 338–344. [Google Scholar] [CrossRef]
- Cho, E.S.; Ruminski, A.M.; Aloni, S.; Liu, Y.-S.; Guo, Y.-S.L.J.; Urban, J.J. Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage. Nat. Commun. 2016, 7, 10804. [Google Scholar] [CrossRef] [PubMed]
- Nivetha, R.; Grace, A.N. Manganese and zinc ferrite-based graphene nanocomposites for electrochemical hydrogen evolution reaction. J. Alloys Compd. 2019, 796, 185–195. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Mozhzhuhin, S.A.; Volodin, A.; Fursikov, P.V.; Lototskyy, M.; Yartys, V.A. Hydrogen storage behavior of magnesium catalyzed by nickel-graphene nanocomposites. Int. J. Hydrogen Energy 2019, 44, 29212–29223. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M.; Sabet, M. Novel green synthesis of ZnAl2O4 and ZnAl2O4/graphene nanocomposite and comparison of electrochemical hydrogen storage and Coulombic efficiency. J. Clean. Prod. 2018, 178, 14–21. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hu, R. Synthesis of three-dimensional hierarchically porous reduced graphene oxide–TiO2 nanocomposite for enhanced hydrogen storage. Ceram. Int. 2018, 44, 12458–12465. [Google Scholar] [CrossRef]
- Du, J.; Lan, Z.; Zhang, H.; Lü, S.; Liu, H.; Guo, J. Catalytic enhanced hydrogen storage properties of Mg-based alloy by the addition of reduced graphene oxide supported V2O3 nanocomposite. J. Alloys Compd. 2019, 802, 660–667. [Google Scholar] [CrossRef]
- Jain, V.; Kandasubramanian, B. Functionalized graphene materials for hydrogen storage. J. Mater. Sci. 2019, 55, 1865–1903. [Google Scholar] [CrossRef]
- Ataca, C.; Akturk, E.; Ciraci, S.; Ustunel, H. High-capacity hydrogen storage by metallized graphene. Appl. Phys. Lett. 2008, 93, 043123. [Google Scholar] [CrossRef]
- Ataca, C.; Akturk, E.; Ciraci, S. Hydrogen storage of calcium atoms adsorbed on graphene: First-principles plane wave calculations. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 79, 041406. [Google Scholar] [CrossRef]
- Wang, L.; Lee, K.; Sun, Y.-Y.; Lucking, M.; Chen, Z.; Zhao, J.J.; Zhang, S.B. Graphene Oxide as an Ideal Substrate for Hydrogen Storage. ACS Nano 2009, 3, 2995–3000. [Google Scholar] [CrossRef]
- Ao, Z.; Jiang, Q.; Zhang, R.; Tan, T.; Li, S. Al doped graphene: A promising material for hydrogen storage at room temperature. J. Appl. Phys. 2009, 105, 074307. [Google Scholar] [CrossRef]
- Er, S.; de Wijs, G.A.; Brocks, G. DFT Study of Planar Boron Sheets: A New Template for Hydrogen Storage. J. Phys. Chem. C 2009, 113, 18962–18967. [Google Scholar] [CrossRef]
- Li, D.; Ouyang, Y.; Li, J.; Sun, Y.; Chen, L. Hydrogen storage of beryllium adsorbed on graphene doping with boron: First-principles calculations. Solid State Commun. 2011, 152, 422–425. [Google Scholar] [CrossRef]
- Ao, Z.; Hernandez-Nieves, A.; Peeters, F.; Li, S. Electric Field Activated Hydrogen Dissociative Adsorption to Nitrogen-Doped Graphene. Phys. Chem. Chem. Phys. 2012, 14, 1463–1467. [Google Scholar] [CrossRef]
- Ozturk, Z.; Baykasoglu, C.; Kirca, M. Sandwiched graphene-fullerene composite: A novel 3-D nanostructured material for hydrogen storage. Int. J. Hydrog. Energy 2016, 41, 6403–6411. [Google Scholar] [CrossRef]
- Srinivas, G.; Zhu, Y.; Piner, R.; Skipper, N.; Ellerby, M.; Ruoff, R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon 2010, 48, 630–635. [Google Scholar] [CrossRef]
- Guo, C.X.; Wang, Y.; Li, C.M. Hierarchical Graphene-Based Material for Over 4.0 wt.% Physisorption Hydrogen Storage Capacity. ACS Sustain. Chem. Eng. 2012, 1, 14–18. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Aminorroaya-Yamini, S.; Nevirkovets, I.; Konstantinov, K.; Liu, H.K. Enhanced Hydrogen Storage in Graphene Oxide-MWCNTs Composite at Room Temperature. Adv. Energy Mater. 2012, 2, 1439–1446. [Google Scholar] [CrossRef]
- Huang, C.-C.; Pu, N.-W.; Wang, C.-A.; Huang, J.-C.; Sung, Y.; Ger, M.-D. Hydrogen storage in graphene decorated with Pd and Pt nano-particles using an electroless deposition technique. Sep. Purif. Technol. 2011, 82, 210–215. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, C.X.; Wang, X.; Guan, C.; Yang, H.; Wang, K.; Li, C.M. Hydrogen storage in a Ni–B nanoalloy-doped three-dimensional graphene material. Energy Environ. Sci. 2010, 4, 195–200. [Google Scholar] [CrossRef]
- Liu, S.; Sun, L.; Xu, F.; Zhang, J.; Jiao, C.; Li, F.; Li, Z.; Wang, S.; Wang, Z.; Jiang, X.; et al. Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ. Sci. 2013, 6, 818. [Google Scholar] [CrossRef]
- Reguera, E. Materials for Hydrogen Storage in Nanocavities: Design criteria. Int. J. Hydrogen Energy 2009, 34, 9163–9167. [Google Scholar] [CrossRef]
- Reguera, L.; Balmaseda, J.; del Castillo, L.F. Hydrogen Storage in Porous Cyanometalates: Role of the Exchangeable Alkali Metal. J. Phys. Chem. C 2008, 112, 5589–5597. [Google Scholar] [CrossRef]
- Reguera, L.; Krap, C.P.; Balmaseda, J. Hydrogen Storage in Copper Prussian Blue Analogues: Evidence of H2 Coordination to the Copper Atom. J. Phys. Chem. C 2008, 112, 15893–15899. [Google Scholar] [CrossRef]
- Reguera, E. Hydrogen storage in nanocavities. Rev. Cuba. Física 2009, 26, 3–14. [Google Scholar]
- Niemann, M.U.; Srinivasan, S.S.; Phani, A.R.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K. Nanomaterials for Hydrogen Storage Applications: A Review. J. Nanomater. 2008, 2008, 950967. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.; Svec, F. Nanoporous polymers for hydrogen storage. Small 2009, 5, 1098–1111. [Google Scholar] [CrossRef]

| Graphene Type | Material Composition | Hydrogen Storage | Reference |
|---|---|---|---|
| Graphene nanocomposite | Graphene/Li | 12.8 (maximum gravimetric density, wt.%) | [141] |
| Graphene nanocomposite | Graphene/Ca | 8.4 (maximum gravimetric density, wt.%) | [142] |
| Graphene nanocomposite | Graphene oxide/Ti | 4.9 (maximum gravimetric density, wt.%) | [143] |
| Graphene nanocomposite | Graphene/Al | 5.13 (maximum gravimetric density, wt.%) | [144] |
| Graphene nanocomposite | Graphene/Li/B | 10.7 (maximum gravimetric density, wt.%) | [145] |
| Graphene nanocomposite | Be adsorbed on B-doped graphene | 15.1 (maximum gravimetric density, wt.%) | [146] |
| Graphene nanocomposite | Reduced graphene oxide/Mg | 6.5 (maximum gravimetric density, wt.%) | [134] |
| Graphene nanocomposite | N doped graphene | 7.23 (maximum gravimetric density, wt.%) | [147] |
| Graphene nanocomposite | Lithium-doped fullerene/graphene | 5 | [148] |
| Graphene nanosheets | Graphene nanosheets | 1.2 wt.% 77 K | [149] |
| Graphene | Hierarchical graphene | 4.01 wt.% 77 K | [150] |
| Graphene nanocomposite | Graphene oxide MWCNT | 2.6 wt.% 298 K/50 bar | [151] |
| Graphene nanocomposite | Pt/Pd/Graphene | 0.156 303 K/57 bar | [152] |
| Graphene nanocomposite | N-doped palladium-decorated graphene | 2.10 wt.% 298 K/20 bar | [153] |
| Graphene nanocomposite | Ni (0.83 wt.%) and B (1.09 wt.%) doped graphene | 4.4 wt.% 77 K/1.06 bar | [154] |
| Graphene nanocomposite | Cu-BTC with 9 wt.% graphene | 3.58 wt.% 77 K/43 atm | [155] |
| Graphene nanocomposite | Cd2SiO4/graphene | Not specified | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhari, A.H. Novel Nanomaterials for Hydrogen Production and Storage: Evaluating the Futurity of Graphene/Graphene Composites in Hydrogen Energy. Energies 2022, 15, 9085. https://doi.org/10.3390/en15239085
Jawhari AH. Novel Nanomaterials for Hydrogen Production and Storage: Evaluating the Futurity of Graphene/Graphene Composites in Hydrogen Energy. Energies. 2022; 15(23):9085. https://doi.org/10.3390/en15239085
Chicago/Turabian StyleJawhari, Ahmed Hussain. 2022. "Novel Nanomaterials for Hydrogen Production and Storage: Evaluating the Futurity of Graphene/Graphene Composites in Hydrogen Energy" Energies 15, no. 23: 9085. https://doi.org/10.3390/en15239085
APA StyleJawhari, A. H. (2022). Novel Nanomaterials for Hydrogen Production and Storage: Evaluating the Futurity of Graphene/Graphene Composites in Hydrogen Energy. Energies, 15(23), 9085. https://doi.org/10.3390/en15239085





