Effect of Cellulose Material-Based Additives on Dispersibility of Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Mechanical Treatment of MWCNTs
2.2. Fabrication of MWCNT-Cellulose Nanofluids
3. Structural Analysis
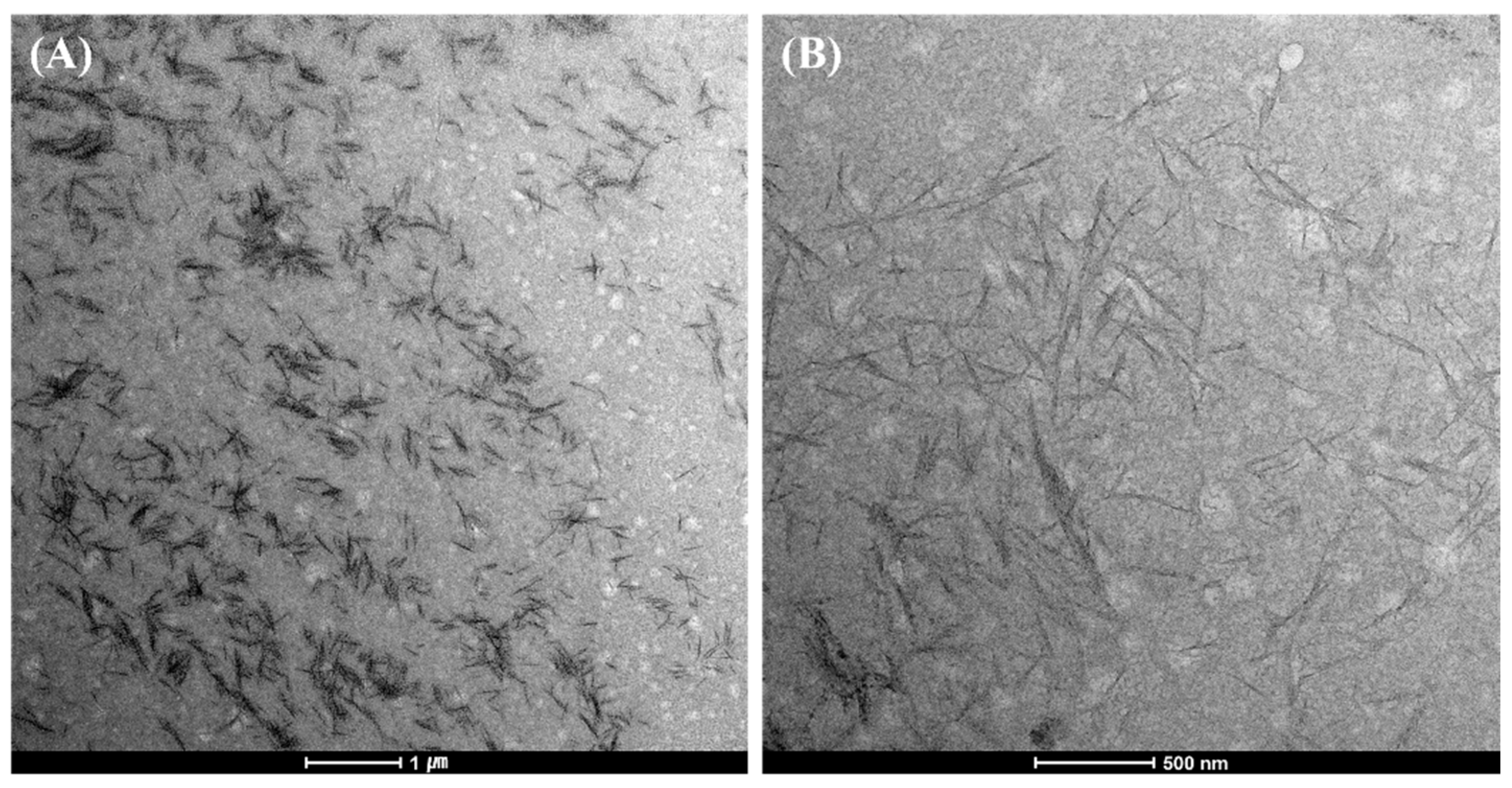
3.1. Morphological Observation and Analysis of CNC
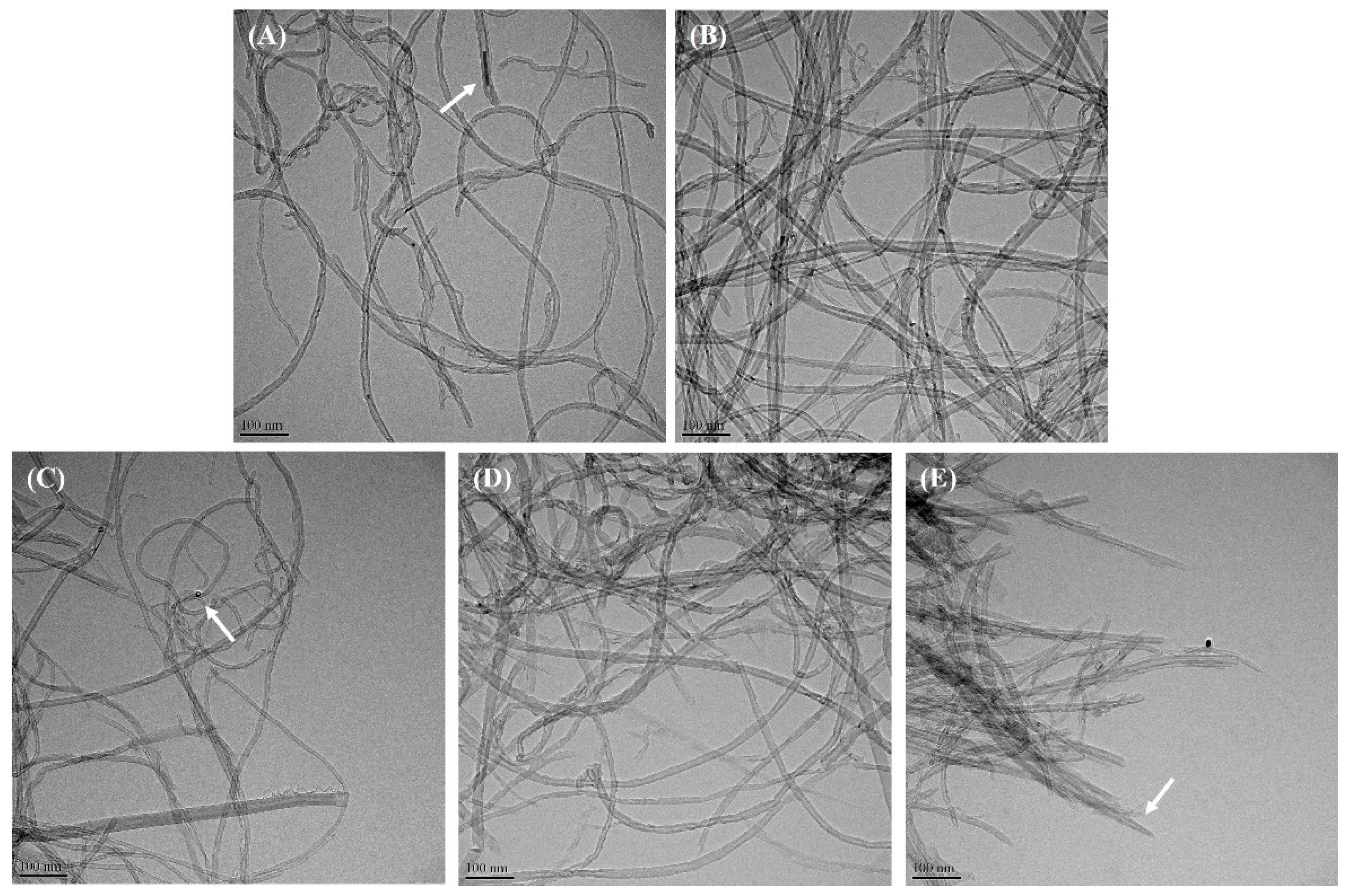
3.2. Morphological Observation and Analysis of MWCNTs
3.3. Morphological Observation and Analysis of the MWCNTs–CNC
4. Dispersion and Stability
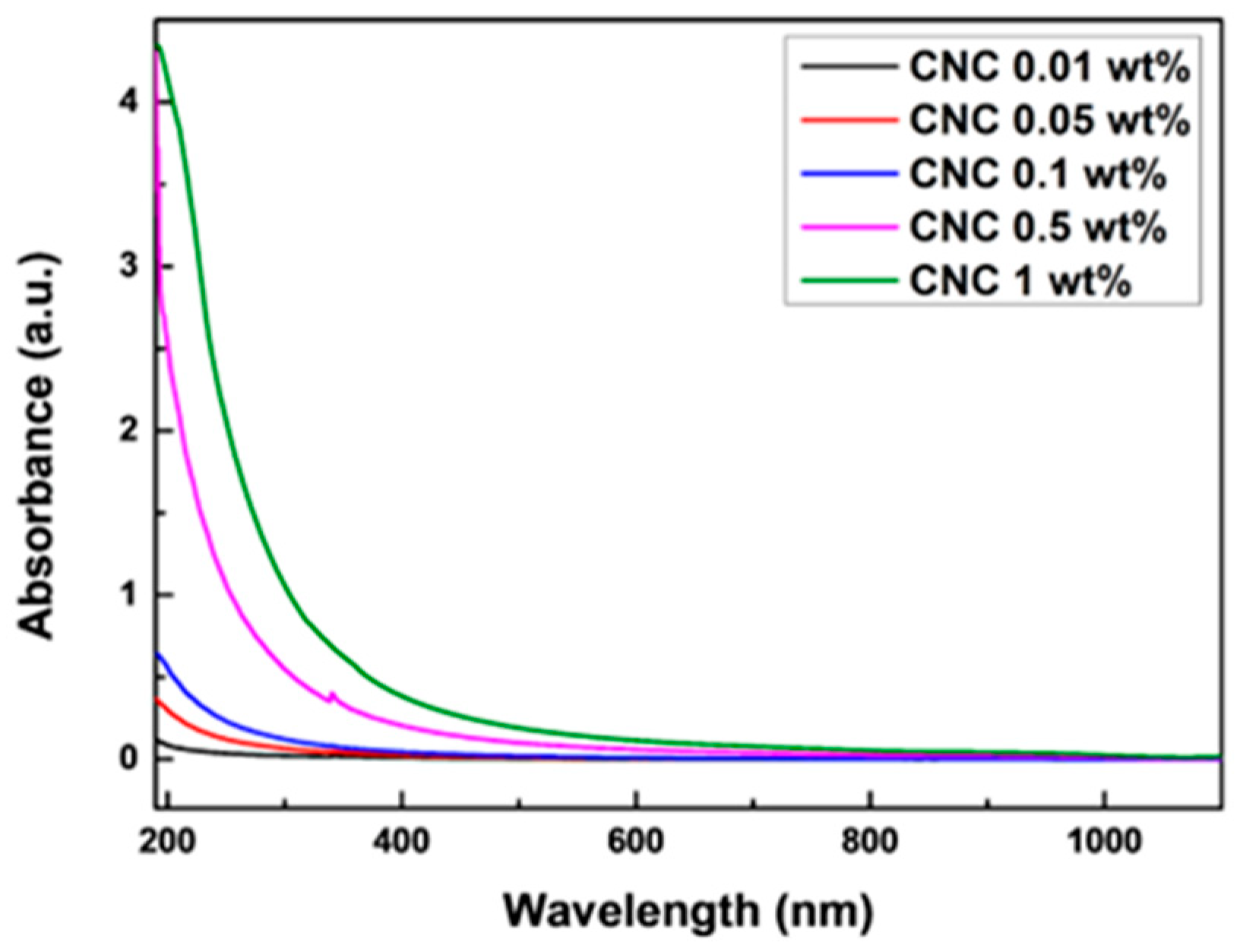
4.1. Dispersion and Stability Characteristics of CNC
4.2. Dispersion and Stability Characteristics of MWCNTs
4.3. Dispersion and Stability Characteristics of MWCNTs with CNC
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chakraborty, S.; Sarkar, I.; Ashok, A.; Sengupta, I.; Pal, S.K.; Chakraborty, S. Synthesis of Cu-Al LDH nanofluid and its application in spray cooling heat transfer of a hot steel plate. Powder Technol. 2018, 335, 285–300. [Google Scholar] [CrossRef]
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Kotsilkova, R.; Nesheva, D.; Nedkov, I.; Krusteva, E.; Stavrev, S. Rheological, electrical, and microwave properties of polymers with nanosized carbon particles. J. Appl. Polym. Sci. 2004, 92, 2220–2227. [Google Scholar] [CrossRef]
- Kaneto, K.; Tsuruta, M.; Sakai, G.; Cho, W.Y.; Ando, Y. Electrical conductivities of multi-wall carbon nano tubes. Synth. Met 1999, 103, 2543–2546. [Google Scholar] [CrossRef]
- Nazari, M.; Karami, M.; Ashouri, M. Comparing the thermal performance of water, Ethylene Glycol, Alumina and CNT nanofluids in CPU cooling: Experimental study. Exp. Therm. Fluid Sci. 2014, 57, 371–377. [Google Scholar] [CrossRef]
- Kang, W.; Shin, Y.; Cho, H. Experimental investigation on the heat transfer performance of evacuated tube solar collector using CuO nanofluid and water. J. Mech. Sci. Technol. 2019, 33, 1477–1485. [Google Scholar] [CrossRef]
- Yuan, C.; Hung, C.H.; Chen, K.C. Electrokinetic remediation of arsenate spiked soil assisted by CNT-Co barrier—The effect of barrier position and processing Fluid. J. Hazard. Mater. 2009, 171, 563–570. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Sulaiman, F.A.; Karaipekli, A.; Tyagi, V.V. Diatomite/CNTs/PEG composite PCMs with shape-stabilized and improved thermal conductivity: Preparation and thermal energy storage properties. Energy Build. 2018, 164, 166–175. [Google Scholar] [CrossRef]
- Shahsavar, A.; Godini, A.; Sardari, P.T.; Toghraie, D.; Salehipour, H. Impact of variable fluid properties on forced convection of Fe3O4/CNT/water hybrid nanofluid in a double-pipe mini-channel heat exchanger. J. Therm. Anal. Calorim. 2019, 137, 1031–1043. [Google Scholar] [CrossRef]
- Pramanik, C.; Gissinger, J.R.; Kumar, S.; Heinz, H. Carbon Nanotube Dispersion in Solvents and Polymer Solutions: Mechanisms, Assembly, and Preferences. ACS Nano 2017, 11, 12805–12816. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of Carbon Nanotubes: Mixing, Sonication, Stabilization, and Composite Properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Plyhm, T.; Barbezat, M.; Necola, A.; Terrasi, G.P. Carbon nanotube (CNT)–epoxy nanocomposites: A systematic investigation of CNT dispersion. J. Nanopart. Res. 2011, 13, 6493–6506. [Google Scholar] [CrossRef]
- Rahmam, S.; Mohamed, N.M.; Sufian, S. Effect of acid treatment on the multiwalled carbon nanotubes. Mater. Res. Innov. 2014, 18 (Suppl. 6), S6-196–S6-199. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Al-Tawbini, B.; Bukhari, A.A.; Abuilaiwi, F.A.; Fettouhi, M.B. Effect of Carboxylic Functional Group Functionalized on Carbon Nanotubes Surface on the Removal of Lead from Water. Bioinorg. Chem. Appl. 2010, 2010, 603978. [Google Scholar] [CrossRef]
- Deline, A.R.; Frank, B.P.; Smith, C.L.; Sigmon, L.R.; Wallace, A.N.; Gallagher, M.J.; Fairbrother, D.H. Influence of Oxygen-Containing Functional Groups on the Environmental Properties, Transformations, and Toxicity of Carbon Nanotubes. Chem. Rev. 2020, 120, 11651–11697. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Bae, J.; Hur, J.; Park, S.J.; Park, M.S.; Kim, I.T. Tailoring of Aqueous-Based Carbon Nanotube–Nanocellulose Films as Self-Standing Flexible Anodes for Lithium-Ion Storage. Nanomaterials 2019, 9, 655. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electrical Properties and Applications of Carbon Nanotube Structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, X.; Zhang, X. Mechanical properties of super honeycomb structures based on carbon nanotubes. Nanotechnology 2007, 18, 075711. [Google Scholar] [CrossRef]
- Nasrin, R.; Rahim, N.A.; Fayaz, H.; Hasanuzzaman, M. Water/MWCNT nanofluid based cooling system of PVT: Experimental and numerical research. Renew. Energy 2018, 121, 286–300. [Google Scholar] [CrossRef]
- Eltaweel, M.; Abdel-Rehim, A.A.; Attia, A.A. Energetic and Energetic Analysis of a Heat Pipe Evacuated Tube Solar Collector Using MWCNT/Water Nanofluid. Case Stud. Therm. Eng. 2020, 22, 100743. [Google Scholar] [CrossRef]
- Pal, A.; Sasmal, A.; Manoj, B.; Rao, D.P.; Haldar, A.K.; Sen, S. Enhancement in energy storage and piezoelectric performance of three phase (PZT/MWCNT/PVDF) composite. Mater. Chem. Phys. 2020, 244, 122639. [Google Scholar] [CrossRef]
- Cui, H.; Yan, X.; Monasterio, M.; Xing, F. Effects of Various Surfactants on the Dispersion of MWCNTs–OH in Aqueous Solution. Nanomaterials 2017, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Brozena, A.H.; Wang, Y. Double-walled carbon nanotubes: Challenges and opportunities. Nanoscale 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Shen, C.; Ma, D.; Meany, B.; Isaacs, L.; Wang, Y. Acyclic Cucurbit[n]uril Molecular Containers Selectively Solubilize Single-Walled Carbon Nanotubes in Water. J. Am. Chem. Soc. 2012, 134, 7254–7257. [Google Scholar] [CrossRef]
- Hou, P.-X.; Liu, C.; Cheng, H.-M. Purification of carbon nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kanyó, T.; Kónya, Z.; Kiricsi, I. Long-time low-impact ball milling of multi-wall carbon nanotubes. Carbon 2005, 43, 994–1000. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Chanda, S. Cellulose nanocrystal based composites: A review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Liang, T.; Wang, X.; Wu, J.; Hu, X.; Liang, J. Oxidation of multiwalled carbon nanotubes by air: Benefits for electric double layer capacitors. Powder Technol. 2004, 142, 175–179. [Google Scholar] [CrossRef]
- Vennerberg, D.C.; Quirino, R.L.; Jang, Y.; Kessler, M.R. Oxidation Behavior of Multiwalled Carbon Nanotubes Fluidized with Ozone. ACS Appl. Mater. Interfaces 2014, 6, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Bossa, N.; Qu, F.; Winglee, J.; Li, G.; Sun, K.; Wiesner, M.R. Comparison of Hydrophilicity and Mechanical Properties of Nanocomposite Membranes with Cellulose Nanocrystals and Carbon Nanotubes. Environ. Sci. Technol. 2016, 51, 253–262. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Zhao, C.; Yang, F. Improvement of antifouling performances for modified PVDF ultrafiltration membrane with hydrophilic cellulose nanocrystal. Appl. Surf. Sci. 2018, 440, 1091–1100. [Google Scholar] [CrossRef]
- Kangas, H.; Lahtinen, P.; Sneck, A.; Saariaho, A.M.; Laitinen, O.; Hellén, E. Characterization of fibrillated celluloses. A short review and evaluation of characteristics with a combination of methods. Nord. Pulp Pap. Res. J. 2014, 29, 129–143. [Google Scholar] [CrossRef]
- Lee, A.; Beak, S.; Lee, S.; Kim, G.; Lee, D.; Kim, S.; Jeong, H. Hydrophilic/Hydrophobic Characteristics on the Carbon Nanotube Buckypapers with various mechanical and chemical manufacture process. Diam. Relat. Mater. 2020, 110, 108152. [Google Scholar] [CrossRef]
- Xing, Y.; Li, L.; Chusuei, C.C.; Hull, R.V. Sonochemical Oxidation of Multiwalled Carbon Nanotubes. Langmuir 2005, 21, 4185–4190. [Google Scholar] [CrossRef]



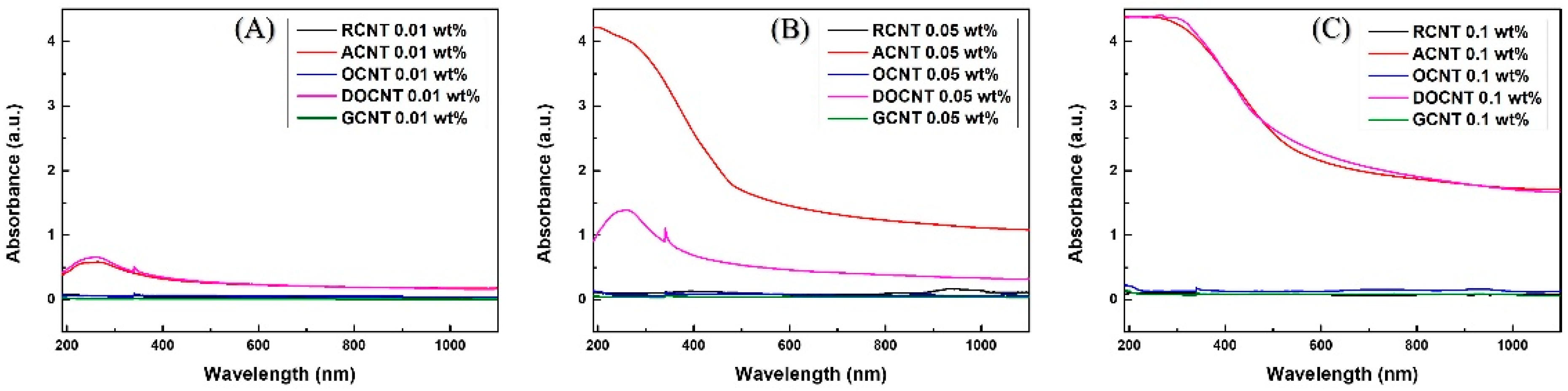
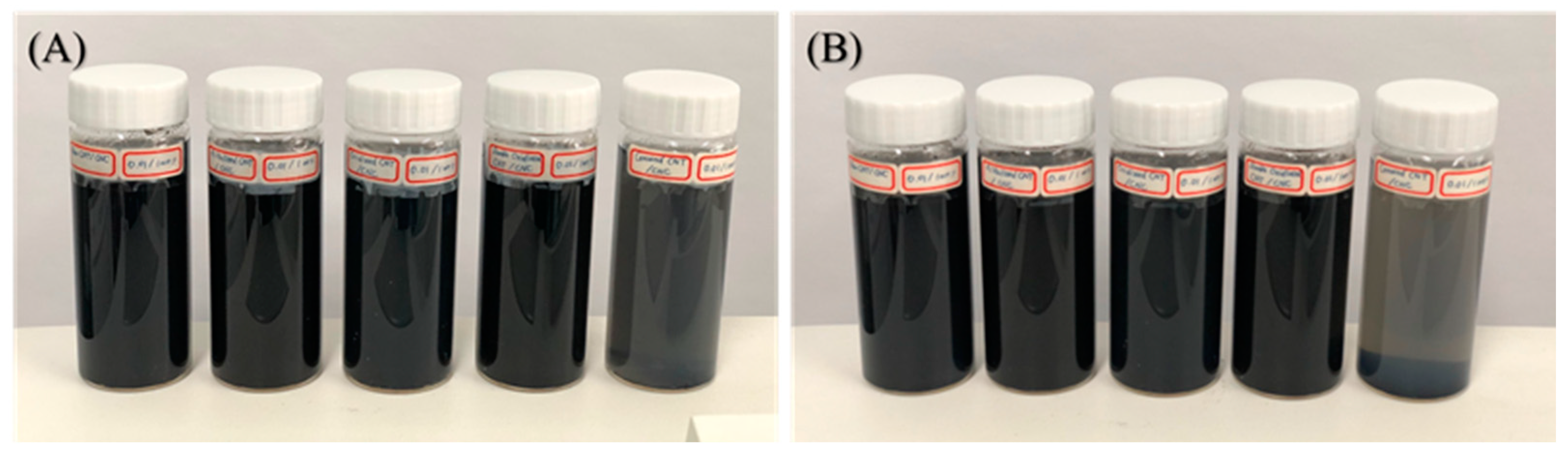
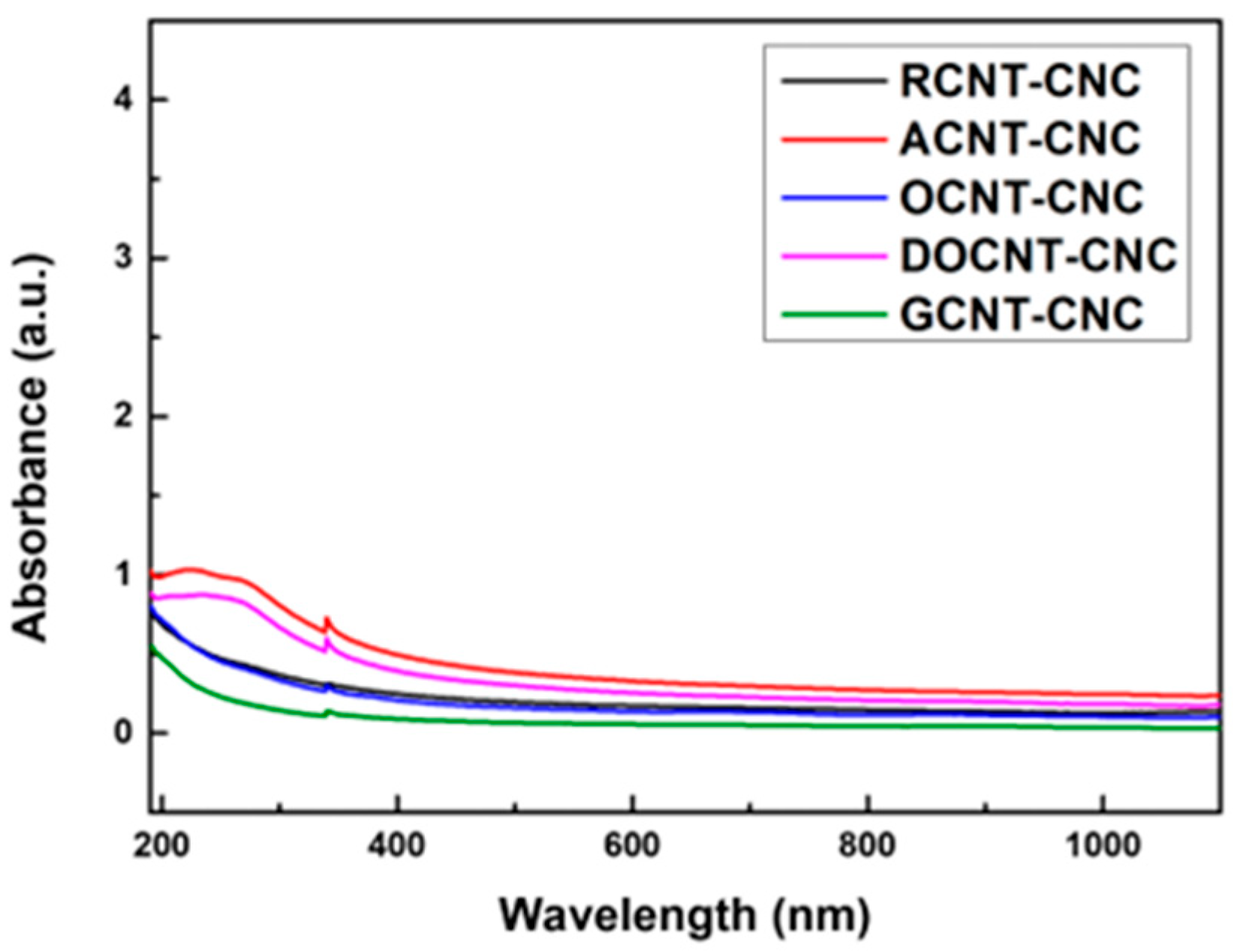
| Dispersion (Absorbance, at 250 nm) | ||||
|---|---|---|---|---|
| MWCNTs | MWCNTs with CNC | % | ||
| RCNTs | 0.062 | RCNTs–CNC | 0.47 | 86 |
| ACNTs | 0.575 | ACNTs–CNC | 0.988 | 41 |
| OCNTs | 0.06 | OCNTs–CNC | 0.452 | 86 |
| DOCNTs | 0.653 | DOCNTs–CNC | 0.859 | 23 |
| GCNTs | 0.011 | GCNTs–CNC | 0.23 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, A.; Baek, S.; Sung, Y.; Jeong, H. Effect of Cellulose Material-Based Additives on Dispersibility of Carbon Nanotubes. Energies 2022, 15, 8822. https://doi.org/10.3390/en15238822
Lee S, Lee A, Baek S, Sung Y, Jeong H. Effect of Cellulose Material-Based Additives on Dispersibility of Carbon Nanotubes. Energies. 2022; 15(23):8822. https://doi.org/10.3390/en15238822
Chicago/Turabian StyleLee, Seunghyeon, Ajeong Lee, Seungyeop Baek, Yonmo Sung, and Hyomin Jeong. 2022. "Effect of Cellulose Material-Based Additives on Dispersibility of Carbon Nanotubes" Energies 15, no. 23: 8822. https://doi.org/10.3390/en15238822
APA StyleLee, S., Lee, A., Baek, S., Sung, Y., & Jeong, H. (2022). Effect of Cellulose Material-Based Additives on Dispersibility of Carbon Nanotubes. Energies, 15(23), 8822. https://doi.org/10.3390/en15238822







