Abstract
This paper presents the results of detonation and explosion characteristics for ammonium nitrate, aluminium, and aluminium-magnesium alloy powder mixtures. The following parameters were determined: detonation velocities, blast waves overpressure and their specific impulses. The emission of radiation was recorded, and the history of temperature of the detonation products cloud was established. It was shown that addition of aluminium-magnesium alloy powder increases blast waves’ characteristics. Moreover, it increases the time of the afterburning reaction after detonation and increases the temperature of detonation products.
1. Introduction
Ammonium nitrate explosives belong to high-energy mixtures, the essential ingredient of which is ammonium nitrate complemented by other organic or inorganic substances. Depending on the degree of surface development of its grains, ammonium nitrate (AN) can be an inertial fertilizer or an explosive. It is commonly used in the following forms:
- a comminuted form (e.g., powdered explosives);
- granulated in ammonium nitrate and fuel oil (ANFO);
- saturated (in suspension explosives);
- supersaturated aqueous solutions (e.g., emulsion explosives).
It was used as a replacement for high-energy individual explosives during World Wars I and II.
Ammonium nitrate is a substance with poorly indicated explosive properties, but its detonation parameters can be modified by introducing various additives, changing the grain shape or water content. Of the various substances increasing the detonation capacity of AN, the best turned out to be aluminium, a small content of which effectively increases the detonation parameters and the detonation capacity of ammonals. Aluminium–magnesium powder can also be a potentially effective additive.
Even though ammonium nitrate has been used in mining explosives for about 150 years, the research on detonation parameters and functional properties of ammonium nitrate explosives is still being carried out, mainly concerning ANFO [1,2,3,4,5,6] and emulsion explosives [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27], but also loose explosives [28,29,30,31].
The presented results of the research on ammonium detonation parameters, knowledge of AN decomposition conditions, and the factors determining the ignition and combustion of aluminium dust form the basis for determining the potential detonation mechanism. Three determining processes dominate the detonation process in ammonium: ammonium nitrate decomposition, ignition, and combustion of aluminium dust in oxidant decomposition products. In the first phase of the process, an exothermic decomposition of ammonium nitrate takes place—the products heated to high temperatures diffuse to the surface of flakes or granules of aluminium powder. A necessary condition to initiate ignition of aluminium is discontinuity of the outer oxide layer. This layer may be due to the effect of thermal stresses, as Al2O3 has a lower thermal expansion than aluminium. Another explanation may be the impact of the unload wave, which is generated after the shock wave passes. It can damage the oxide layer and thus accelerate the ignition of aluminium powder. The high-temperature characteristic for the explosive transformation reduces the ignition induction time.
The last but not less important step in the ammonium detonation process is the burning of aluminium in the stream of AN decomposition products, which may proceed according to the following reactions:
Under normal conditions, the process of aluminium burning is diffusive and occurs in the gas phase near the metal surface. On the other hand, under the pressure prevailing in the detonation wave, the process of aluminium burning probably occurs according to the mechanism typical for metals with a very high boiling point at the solid–liquid interface, ignoring the gas phase of the metal. As a result of smoking, mainly aluminium(III) oxide is formed, as shown by the above reactions (1)–(3). In the case of mixtures containing large amounts of other aluminium compounds, aluminium(I) oxide or aluminium nitride may be formed. Al2O and AlN have a much lower combining heat than Al2O3. Therefore, their appearance is energetically unfavourable [32].
2. Materials and Methods
2.1. Materials
2.1.1. Ammonium Nitrate
The mixtures used ground ammonium nitrate obtained by grinding PULAN N34-type ammonium nitrate produced by GRUPA AZOTY PUŁAWY. Sieve analysis was performed on a Retsch As 200 shaker to determine its fragmentation. The percentage content of individual fractions is shown in Figure 1.
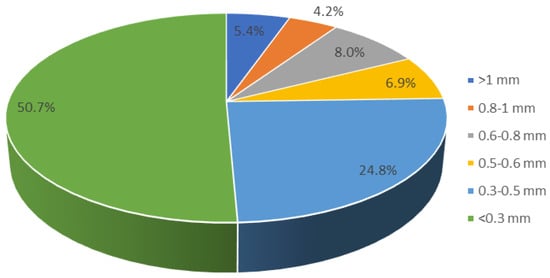
Figure 1.
The AN sieve analysis results.
2.1.2. Metal Powders
The tested explosive mixtures contained two types of metal powders: flaked aluminium (Alf) and aluminium-magnesium alloy powder (PAM). The flaked aluminium was powdered BLITZ Aluminium DEPUVAL 3083 produced by Benda-Lutz from aluminium with a minimum purity of 99.7%, a water coverage of 29,000 cm2/g, a residue on a 45 μm sieve (~325 mesh) of max. 0.8%, medium-sized flakes of 12 μm, and a bulk density of 0.4 kg/dm3. For AN/Al mixtures modification, powder PAM was used. Benda-Lutz produces an AlMg 50/75 powder containing Al and Mg, with approximately 50% of both. The PAM powder has a residue on a 75 μm sieve (~200 mesh) of max. 10%.
The structures of the powders used in this research are shown in Figure 2a,b. Figure 2a shows thin aluminium flakes surrounded by a small amount of inert matrix. The shape of aluminium particles allows good contact with ammonium nitrate. On the other hand, the matrix made of inert material protects flaked aluminium against dusting. A homogeneous mixture is obtained by mixing ammonium nitrate and aluminium powder. Figure 2b shows powder particles of the aluminium–magnesium alloy. The powder particles are of various sizes and irregular shapes. The larger particles have sharp edges, while the smaller ones mostly have oval shapes. The presence of small PAM powder particles may allow the oxidation reaction of Al and Mg to be accelerated.
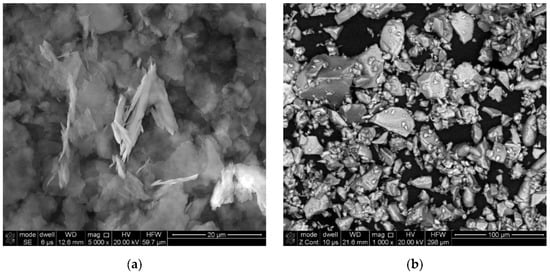
Figure 2.
SEM images of the powders: (a) Alf; (b) PAM.
2.2. Methods
2.2.1. Preparation of the Tested Explosives
The mixtures were prepared in two stages. In the first stage, Alf was mixed with the PAM powder, and the obtained mixture was added to the previously weighed AN and mixed until a homogenous mass was obtained. After mixing, the material was manually loaded onto PVC pipes. The height of the charges was adjusted to each mixture so that its weight was 400 g. The material was added in small portions and lightly tamped to obtain an even density of the charges. Small differences in height with a constant diameter do not significantly affect the measurement results.
Two ammonals with 3% and 7% Alf modified with the addition of the PAM powder were selected for the tests. The compositions of the tested mixtures are presented in Table 1.

Table 1.
Names and compositions of the tested explosives and detonation velocity results.
2.2.2. Detonation Velocity Measurement
Assessment of detonation velocities was performed utilizing the short circuit method. Shells of the studied explosive materials were made of polyvinyl chloride (PVC) pipes with an inner diameter and a wall thickness of 46 and 1.8 mm, respectively. Four short circuit sensors were located in the shells at a distance of 40 mm (from one another) and 15 mm from the last sensor to the bottom of the charge. At least two tests were performed for each investigated mixture.
2.2.3. Measurement of Air Blast Waves Overpressure
Blast waves overpressure values were measured utilizing piezoelectric sensors provided by PCB Piezotronics Europe GMBH (Huckelhoven, Germany). The measuring station included four 137A series sensors connected to an oscilloscope with a signal conditioner. Location of the sensors assured 2 and 2.5 m distance between the charge and the sensors’ pairs.
It should be noted that the measurement results obtained utilizing this approach are influenced by disturbances that may originate from the measuring system’s electrical disruptions, electrical network or mechanical interferences originating from vibrations of the pressure sensors’ mounting system. To normalise blast wave pressure histories and minimise any disturbances and incidental disruptions on the measured characteristics of blast waves, the overpressure histories obtained were approximated with a modified Friedlander Equation (4):
where stands for the peak overpressure immediately after the primary shock wave, corresponds to the time after the arrival of the primary shock at the gauge location, and and are constants.
2.2.4. Measurement of the Temperature of a Cloud of Detonation Products
A cloud of detonation products formed after the explosion oxidises in the presence of atmospheric oxygen, which results in the release of large amounts of thermal energy. Finally, the fireball spectrum was recorded by an Ocean Optics Inc. (Ostfildern, Germany) USB2000+ spectrometer following the same procedure and experimental conditions described by Maiz et al. [33,34].
Fireball temperatures were determined from the recorded fireball spectra using linear polychromatic fitting of the continuous part by Wien’s relation. The method was described in detail by Maiz et al. [33].
Experiments were performed in a semi-closed bunker. A scheme presenting the locations of the charges, piezoelectric sensors, and optic fibre is shown in Figure 3. The bunker was about 40 m3 in volume and had four small 0.05 m2 openings and a frontage-opened door with a surface of ca. 1.3 m2. The studied charges were placed in the bunker at a height of 1.7 m (above the ground) and the optic fibre was guided to the centre of the charge. A standard electrical detonator was utilized to initiate detonation.
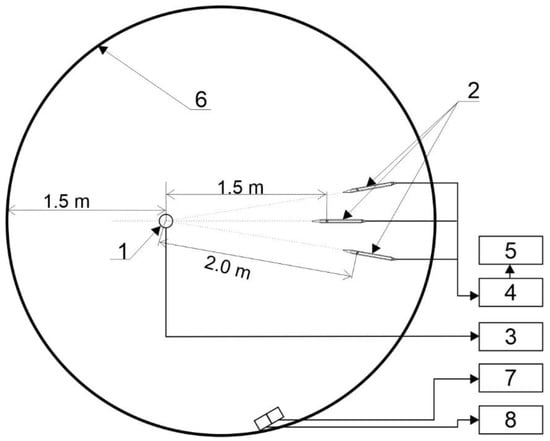
Figure 3.
Diagram of the system for air blast waves overpressure and temperature measurement: 1—explosive charge, 2—pencil probes, 3—time meter, 4—signal conditioner, 5—oscilloscope, 6—the wall of the bunker, 7—spectrometer, 8—signal generator.
3. Results
3.1. Results of the Detonation Velocity Measurements
The results of the detonation velocity measurements with the mean deviation are presented in Table 1. The table also includes the designations and compositions of the tested explosives. For the A7-25 composition, no detonation was observed and neither the detonation velocity nor the blast wave characteristics were measured.
3.2. Blast Wave Characteristics
For each test, four blast waves overpressure values were obtained. Two histories are for the sensors located at distances of 2 and 2.5 m. Figure 4 illustrates exemplary blast waves overpressure histories obtained for the A3-0 and A3-20 compositions.
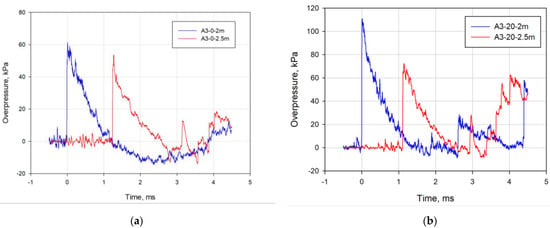
Figure 4.
Blast waves overpressure for compositions (a) A3-0 and (b) A3-20.
The obtained overpressure histories were approximated using a modified Friedlander equation. The obtained maximum overpressure and positive phase impulse values were averaged. Measurements were performed for all the tested materials. Table 2 and Table 3 present the determined characteristics of the air blast wave.

Table 2.
Results of the measurements of blast wave characteristics for ammonals with 3% Alf.

Table 3.
Results of the measurements of blast wave characteristics for ammonals with 7% Alf.
3.3. Detonation Products Cloud Spectra Analysis
Figure 5 shows typical light-intensity spectra from an explosion in the bunker recorded using a spectrometer. As seen in the figure, the spectra from the explosion of the tested A3-25 material in the bunker exhibit several broad emission lines with minuscule intensities and sharp intense lines at 589, 766, and 769 nm. The broad emission lines might be considered the sum of the contribution of several species. The sharp lines at 589, 766, and 769 nm are assigned to sodium and potassium impurities, respectively. Furthermore, a few more atomic lines are observed—a single band line at 670 nm characteristic of double-ionised aluminium. A few low-intensity lines characteristic of single-ionised chlorine at 618 and 619 nm and single-ionised magnesium at 518 nm are observed in the spectrum.
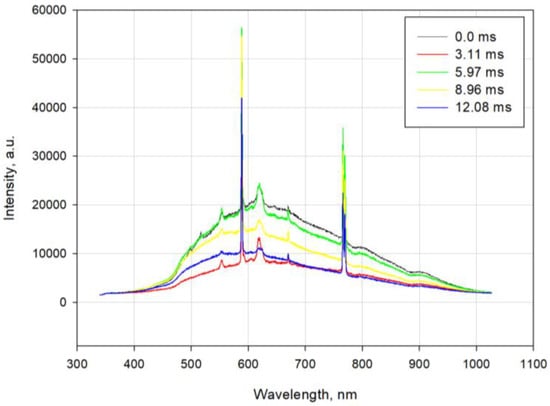
Figure 5.
Spectrometer-collected exemplary light-intensity spectra for the tested A3-25 explosive mixture.
For the analysis of experimental spectra, recordings were linear polychromatic fitting for temperature estimation [32]. This method is based on fitting spectral radiance variation with the wavelength using Wien’s relation. Spectral radiance can be expressed as follows:
where is the spectral radiance, is a constant, is the wavelength, , and is the temperature.
Equation (5) is equivalent to Equation (6):
Figure 6 shows the experimental variation of in the function of 1⁄λ for the A3-25 tested explosive.
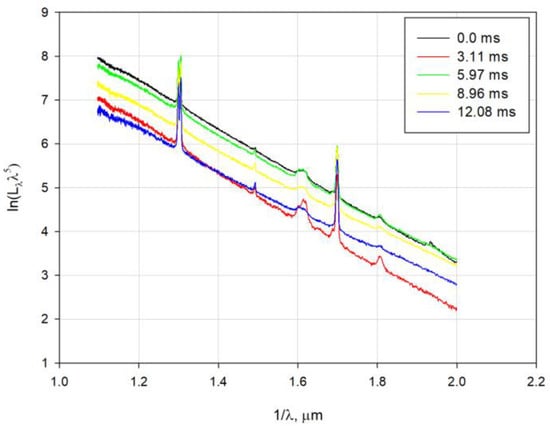
Figure 6.
Linear variation of in the function of for the A3-25 explosive.
Removing the intensive spectral line was necessary to determine temperature using linear fitting of the spectra recordings.
4. Discussion
4.1. Detonation Velocity
From the obtained results, it can be seen that the introduction of aluminium-magnesium alloy powder in place of ammonium nitrate in ammonal containing 7% flaked aluminium powder causes a continuous decrease in the detonation velocity. However, for the ammonal containing 3% flaked aluminium powder, the detonation velocity firstly increases, and after exceeding the 10% PAM addition, decreases proportionally to the content of PAM in the tested mixture. Figure 7 depicts the results of detonation velocity measurements for the explosive mixtures tested.
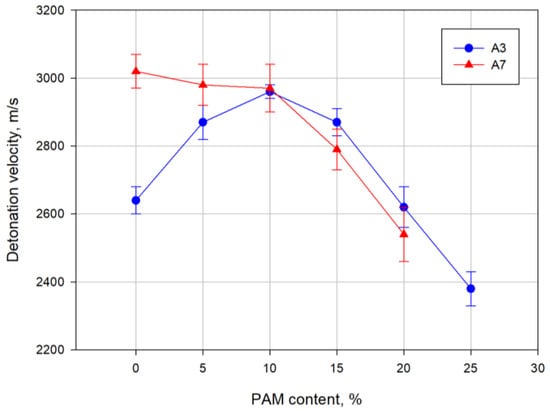
Figure 7.
Experimental detonation velocity of the tested explosives.
The curves of detonation velocity as a function of the aluminium-magnesium alloy powder content of the two tested ammonals are similar and typical for nonideal explosives. However, for the ammonal containing 7% flaked aluminium dust, the maximum detonation velocity is shifted towards the lower PAM powder content compared to the ammonal with a lower flaked aluminium content, which is related to the different amounts of Alf powder addition.
Such changes in detonation velocity as a function of PAM powder content are typical for mixtures of ammonium nitrate with metal powders. Initially, addition of a metal powder positively affects the increase in the heat of the detonation of the mixture through additional oxidation reactions in the chemical reaction zone of the detonation wave. An increase in the heat of the detonation causes an increase in the velocity of the detonation. By increasing the amount of the additive further, this positive effect is limited by the heat absorbed by the additional powder. In this case, adding a metal powder has an inert effect in the chemical reaction zone and reduces the detonation velocity.
The inert effect of metal powder addition is achieved earlier for the A7 mixture with a higher content of flaked Al. Therefore, the maximum value of the detonation velocity moves towards lower PAM contents.
4.2. Blast Waves Characteristics
The maximum overpressure results are shown in Figure 8. The results show that the addition of aluminium-magnesium alloy powder increases the air blast wave parameters proportionally to its content in the ammonal. The highest overpressure was obtained at a distance of 2 m for the ammonal containing 3% Alf and 25% PAM.
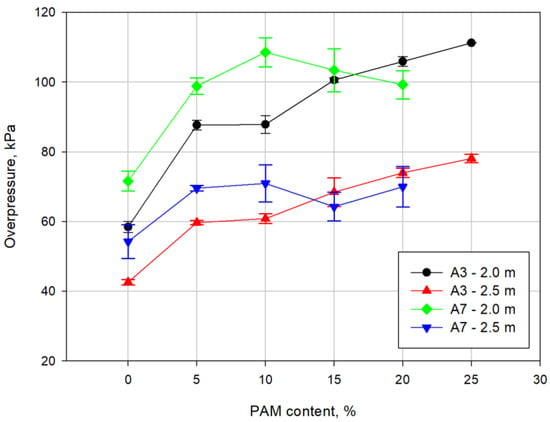
Figure 8.
Curves of blast waves overpressure as a function of the PAM powder content.
In addition, the maximum overpressure of the air blast wave at a distance of 2 m first increases with an increasing PAM content to reach the maximum, then decreases slightly and maintains a stable pressure value in the range of 15% to 20% of the additive’s content. Similar behaviour is observed for the overpressure at a distance of 2.5 m.
Changes in the blast waves overpressure have a different character for both mixtures. For the A3 mixture, a slow increase in overpressure is visible at a distance of 2 m and 2.5 m. The fine Alf powder reacts in the chemical reaction zone and releases enough heat energy to heat the PAM powder. Unreacted Alf and PAM react behind the chemical reactions zone of the detonation wave, affecting the maximum overpressure of the blast wave.
In the case of the A7 mixture, due to the constant decrease in detonation parameters (Figure 7), after the initial increase in the maximum overpressure after the addition of 5% PAM, slight changes are observed.
Figure 9 shows the curves of the positive phase impulse as a function of the PAM powder content. The impulse of the ammonal containing 3% Alf increases with the increasing PAM content, but for a distance of 2.5 m, a stable value of the impulse is noticeable in the range of 10% to 20% of the additive’s content. In contrast, the impulse value for the ammonal containing 7% of Alf initially increases until the first maximum is reached, and then a slight decrease from the 15% PAM content is observed.
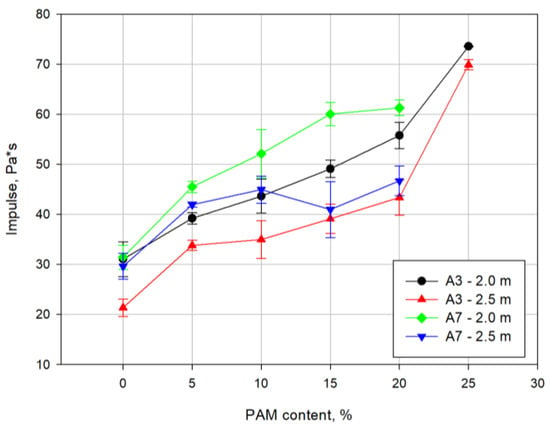
Figure 9.
Curves of the specific impulse as a function of the PAM powder content.
This steady increase in the blast wave pulse with increasing PAM content may result from the oxidation reaction of unreacted hot particles of the Alf and PAM powders. Heated metal powders release additional heat energy as a result of these reactions. The additional heat energy released behind the reaction zone of the detonation wave increases the blast wave impulse.
In order to confirm the further course of maximum overpressure and air blast wave impulse, additional tests need to be carried out on mixtures containing a higher PAM powder content to observe what maximum values for these parameters could be obtained. However, the decrease in detonation velocity at 25% PAM content indicates that a further increase of the PAM powder content related to the reduction of AN content may result in the inability to detonate.
4.3. Results of the Detonation Products Cloud Temperature
Changes in the temperature of the detonation products cloud as a function of time are presented in Figure 10. For the mixtures with the addition of Alf powder (A3-0 and A7-0) and the mixtures with 5% PAM powder, it was not possible to record irradiation spectra of the clouds of explosion products. For these mixtures, too low clouds radiation power was observed. Only one spectral measurement was obtained for the mixtures with the addition of 10% and 15% PAM. For other mixtures, it was possible to record 2 or 3 spectra and calculate the average temperature of the outer layers of the cloud as a function of time.
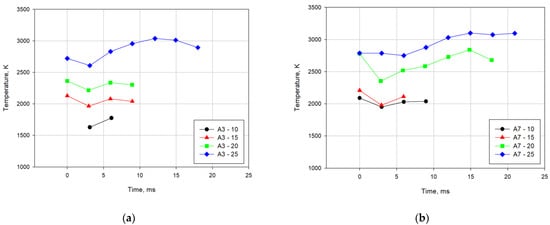
Figure 10.
Changes of temperatures of the detonation products clouds for the explosives containing (a) 3% Alf; (b) 7% Alf.
As can be seen, as the content of the PAM powder additive increases, the glow time of the explosion products and the average temperature of the outer layers of the product cloud increase for successive spectra measurement times.
For the temperature changes for the A7-25 mixture, the spectrum of explosion products was recorded in the absence of detonation of the mixture. It can be assumed that initiation of the explosion and extinction of the process took place, going from explosive to rapid combustion.
5. Conclusions
Mixtures containing different amounts of aluminium-magnesium alloy powder were investigated in this study. The results showed that addition of up to 10% of the tested additive to ammonal containing 3% flaked aluminium causes an increase in the detonation velocity, while contents above 10% result in a proportional decrease of the detonation velocity. It should be highlighted that a continuous decrease of detonation velocity was observed for the ammonal containing 7% flaked aluminium.
Introduction of PAM increases the air blast wave parameters. The highest results were obtained for the ammonal containing 25% of aluminium–magnesium powder. The tested mixtures exhibited a slight increase in density, even though the addition of 5% PAM caused a slight decrease in density compared to the initial ammonals.
It can be concluded that according to the test results, ammonals modified with PAM powders can be used for explosive welding of metals, blasting in rocks of low compactness, or demolition works. In addition, it seems promising to conduct a study of the effect of the content of aluminium–magnesium powder addition on the detonation parameters of ammonals containing less than 3% flaked aluminium or to continue research of the mixtures studied in this paper with a higher PAM content. However, increasing the amount of alloy powder in mixtures may result in the disappearance of detonation and the highest value of overpressure and pulse of the air blast wave, which may be indicative of doping of the alloy powder additive outside the chemical reaction zone of the detonation wave.
As the PAM powder content exceeded 10%, an increase in the glow time and average temperature of the outer layers of the explosion products cloud was observed.
Tests of mixtures of AN with Alf powder and the addition of various amounts of PAM powder allow the following conclusions to be drawn:
- -
- Introduction of more than 10% aluminium–magnesium powder into mixtures of ammonium nitrate and flaked aluminium decreases the detonation velocity.
- -
- Introduction of less than 10% PAM into the tested ammonal mixtures with 3% aluminium powder increases the detonation velocity.
- -
- Addition of PAM powder to ammonals increases the parameters of the air blast wave, indicating that the powder burns outside the chemical reaction zone of the detonation wave.
- -
- Addition of PAM powder to the tested ammonal mixtures increases the glow time of the explosion products cloud by at least 2.5 times.
- -
- Addition of PAM powder to the tested ammonal mixtures causes an increase in the average temperature of the explosion products cloud.
Interesting results for mixtures of ammonium nitrate with the addition of flaked aluminium and PAM powder allow the assumption that hybrid materials can be widely used in technical works. Therefore, additional tests of such mixtures are planned to determine other detonation parameters, such as the calorimetric heat of the explosion and detonation pressure. Due to the high fuel content in the form of metal powders, it is worth measuring the quasistatic pressure of the explosion in a closed chamber. It is also necessary to perform a series of thermochemical calculations to determine the theoretical parameters of detonation.
Author Contributions
Conceptualization, J.P., A.M., B.K. and P.P.; methodology, J.P.; validation, P.P., formal analysis, A.M.; writing—original draft preparation, J.P.; writing—review and editing, B.K.; visualization, J.P., P.P., A.M. and B.K.; supervision, J.P., P.P., A.M. and B.K.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Military University of Technology under research project UGB 22-795/2022, and this research was supported by the Polish Ministry of Education and Science.
Data Availability Statement
Not applicable.
Conflicts of Interest
Authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dobrilovič, M.; Bohenek, V.; Škrelec, V. Influence of the initiation energy on the velocity of detonation of ANFO explosive. Cent. Eur. J. Energ. Mater. 2013, 10, 555–568. [Google Scholar]
- Dobrilovič, M.; Bohenek, V.; Žaganec, S. Influence of explosive charge temperature on the velocity of detonation of ANFO explosives. Cent. Eur. J. Energ. Mater. 2014, 11, 191–197. [Google Scholar]
- Sitkiewicz-Wołodko, R.; Maranda, A.; Paszula, J.M. Modification of ANFO Detonation Parameters by Addition of Ground of Ammonium Nitrate(V) and Aluminium Powder. Cent. Eur. J. Energ. Mater. 2019, 16, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Žaganec, S.; Dobrilovič, M.; Bohenek, V. Borehole velocity of Detonation of ANFO and Heavy ANFO Explosives. In Proceedings of the 17th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 9–11 April 2014. [Google Scholar]
- Maranda, A.; Paszula, J.; Zawadzka-Małota, I.; Kuczyńska, B.; Witkowski, W.; Nikolczuk, K.; Wilk, Z. Aluminum Powder Influence on ANFO Detonation Parameters. In Proceedings of the 14th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 13–15 April 2011. [Google Scholar]
- Paszula, J.; Maranda, A.; Nikolczuk, K.; Giercuszkiewicz, A. Modification of the Detonation Parameters of Mining Explosives Containing Hydrogen Peroxide and Aluminium Powder. Cent. Eur. J. Energ. Mater. 2021, 18, 477–491. [Google Scholar] [CrossRef]
- Wang, L.; Fang, J. Rheological properties and water-in-oil structure stability of emulsion matrixes. Cent. Eur. J. Energ. Mater. 2013, 10, 87–102. [Google Scholar]
- Wang, L.Q.; Wang, N.F.; Fang, J. Rheology of typical emulsifiers and effects on stability of emulsion explosives. J. Beijing Inst. Technol. 2011, 20, 295–300. [Google Scholar]
- Ligiong, W.; Jie, F. Relationship between Rheological Properties and Stability of Emulsion Matrix. In Proceedings of the 15th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 18–20 April 2012. [Google Scholar]
- Zhou, H.S.; Xie, X.H.; Xu, K. Stability test of emulsion matrix in the emulsifier. Adv. Mater. Res. 2015, 1082, 26–29. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, D.; Hu, Y.; Ye, Z.; Wei, Y. Influence of iron ion on thermal behavior of ammonium nitrate and emulsion explosives. Cent. Eur. J. Energ. Mater. 2010, 7, 77–93. [Google Scholar]
- Maranda, A.; Paszula, J.; Drobysz, B. Badania parametrów detonacyjnych materiałów wybuchowych emulsyjnych o niskiej gęstości modyfikowanej mikrobalonami. Chemik 2014, 68, 17–22. [Google Scholar]
- Mendes, R.; Ribeiro, J.; Plaksin, I. Differences between the detonation behavior of emulsion explosives sensitized with glass or with polymeric microballoons. J. Phys. Conf. Ser. 2014, 500, 520–530. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Ma, H.H.; Shen, Z.W. Detonation characteristics of emulsion explosives sensitized by H2Mg. Combust. Explos. Shock Waves 2013, 49, 614–619. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Ma, H.H.; Liu, R.; Shen, Z.W. Pressure desensitization influential factors and mechanism of magnesium hydride sensitized emulsion explosives. Propellants Explos. Pyrotech. 2014, 39, 267–274. [Google Scholar] [CrossRef]
- Mendes, R.; Ribeiro, J.; Plaksin, I.; Campos, J. Non ideal detonation of emulsion explosives mixed with metal particles. AIP Conf. Proc. 2012, 1426, 267–270. [Google Scholar]
- Wang, L.; Wang, N.; Zhang, L. Study on key factors affecting energy output of emulsion explosives in underwater explosion. Propellants Explos. Pyrotech. 2012, 37, 83–92. [Google Scholar] [CrossRef]
- Hagfors, M.; Saavalainen, J. Underwater Explosions-Particle size Effect of Al Powder to the Energy Content of Emulsion Explosives. In Proceedings of the 36th Annual Conference on Explosives and Blasting Technique, Orlando, FL, USA, 7–10 February 2010. [Google Scholar]
- Mendes, R.; Ribeiro, J.; Plaksin, I.; Compos, J.; Farinha, R. Non Monotonic Detonation Velocity and Pressure Behaviour of Emulsion Explosive. In Proceedings of the 13th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 21–23 April 1010. [Google Scholar]
- Němec, O.; Jungová, M.; Zeman, S. Fortification of W/O Emulsions by Demilitarized Explosives. Cent. Eur. J. Energ. Mater. 2011, 8, 193–209. [Google Scholar]
- Němec, O.; Jungová, M.; Zeman, S. Modification of W/O emulsions by demilitarized Composition B. Propellants Explos. Pyrotech. 2013, 38, 142–146. [Google Scholar] [CrossRef]
- Němec, O.; Novotnŷ, M.; Jungová, M.; Zeman, S. Preliminary Verification of Fortification of W/O Type Emulsions with Demilitarized Explosives Based on TNT. In Proceedings of the 14th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 13–15 April 2011. [Google Scholar]
- Němec, O.; Jungová, M.; Pelikan, V.; Zeman, S. Note One Determination of Relative Explosive Strength of Fortified W/O Emulsions by Means of Ballistic Mortar. In Proceedings of the 15th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 18–20 April 2012. [Google Scholar]
- Maranda, A.; Paszula, J.; Nikolczuk, K.; Wilk, Z. Materiały wybuchowe emulsyjne zawierające chlorek sodu uczulane mikrobalonami. Chem. Ind. 2011, 90, 1254–1259. [Google Scholar]
- Maranda, A.; Czerwińska, A.; Paszula, J.; Jóźwik, P. Modyfikacja parametrów detonacyjnych materiałów wybuchowych emulsyjnych przez dodatek układu utleniacz-składnik palny. Przemysł Chem. 2020, 99, 40–45. [Google Scholar] [CrossRef]
- Huang, W.; Wu, H.; Yan, S. Relationship research between crystallization quantity of emulsion explosive and desensitization degree under dynamic pressure. Adv. Mater. Res. 2012, 393, 1389–1393. [Google Scholar] [CrossRef]
- Maranda, A.; Sitkiewicz-Wołodko, R.; Florczak, B.; Bajdor, K. Wodorek magnezu. Nowy sensybilizator emulsyjnych materiałów wybuchowych. Chem. Ind. 2016, 95, 2222–2447. [Google Scholar]
- Maranda, A.; Szymański, R. Badania średnicy krytycznej i prędkości detonacji mieszanin azotanu(V) amonu z wybranymi substancjami organicznymi. Chemik 2013, 47, 13–15. [Google Scholar]
- Maranda, A.; Nastała, A.; Buczkowski, D.; Witkowski, W. Badanie wpływu pestycydów na parametry materiałów wybuchowych typu saletrole i amonale. Chemik 2014, 48, 23–25. [Google Scholar]
- Maranda, A.; Papliński, A. Investigation of Sodium Azide Influence on Detonation Parameters of Aluminized Explosives. In Proceedings of the 18th Seminar on New Trends in Research of Energetic Materials, part II, Pardubice, Czech Republic, 15–17 April 2015. [Google Scholar]
- Paszula, J.; Trzciński, W.; Sprzątczak, K. Detonation Performance of Aluminium—Ammonium. Cent. Eur. J. Energ. Mater. 2008, 5, 6–11. [Google Scholar]
- Maranda, A. Przemysłowe Materiały Wybuchowe, Wyd; Wojskowa Akademia Techniczna: Warszawa, Poland, 2010. [Google Scholar]
- Maiz, L.; Trzciński, W.A.; Paszula, J. Optical Spectroscopy to Investigate Explosions of Homogeneous and Composite Explosives. Opt. Laser Eng. 2017, 88, 111–119. [Google Scholar] [CrossRef]
- Maiz, L.; Trzciński, W.A.; Paszula, J. Investigation of Fireball Temperatures in Confined Thermobaric Explosions. Propellants Explos. Pyrotech. 2017, 42, 142–148. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).