Abstract
The need for renewable and sustainable fuel and energy storage sources is pressing. Biohydrogen has the potential to be a storable energy carrier, a direct fuel and a diverse building block for various downstream products. Utilizing microbial electrolysis cells (MECs) to produce biohydrogen from residue streams, such as the organic fraction of municipal solid waste (OFMSW), agricultural residues and wastewater facilitate utilization and energy recovery from these streams, paving the path for a circular economy. The advantages of using hydrogen include high gravimetric energy density and, given the MEC pathway, the ability to capture heavy metals, ammonia and phosphates from waste streams, thereby allowing for multiple revenue streams emanating from MECs. A review of the MEC technology and its application was carried out to investigate the use of MEC in sustainable biohydrogen production. This review summarizes different MEC designs of varying scales, including anode materials, cathode materials, and configuration possibilities. This review highlights the accomplishments and challenges of small-scale to large-scale MECs. Suggestions for improving the successful upscaling of MECs are listed, thus emphasizing the areas for continued research.
1. Introduction
Energy demand has been increasing alongside a growing human population [1] and a growing number of consumables purchased, shipped, used and discharged [2]. The access and availability of products have led to an increase in both types of waste and the quantity generated. Waste generated by humans is the third largest source of methane emissions after industrial and agricultural sources. Although CO2 remains the most significant contributor to global warming, methane has a more considerable comparative impact due to its increased efficiency at trapping CO2 radiation, thus causing dire consequences for climate change [3,4]. As the industrial, agricultural and transportation sectors formulate and implement their respective strategies in combating climate change, the utilization of waste as a resource transcends sectoral boundaries. Reducing emissions is a retrogressive action that addresses the challenges cumulated due to inaction over the past decades [5]. Some contemporary waste streams create value using energy generation, and others might serve as product precursors.
1.1. Hydrogen as the Product
Hydrogen is a synthetic energy carrier, implying that it is a direct energy source and constitutes a possibility for energy storage in the form of chemical potential. These abilities make hydrogen a promising key player in the future of renewable energy systems [6].
The demand for hydrogen has continuously increased since 1975, with ammonia production accounting for the bulk of the demand. However, global hydrogen consumption is expected to increase drastically due to its properties, making it a central building block in mitigating fossil-derived energy sources used in hard-to-eliminate emission sectors [7].
Hydrogen can be produced by various methods, including electrolysis, thermal water splitting, gasification, dark fermentation, photoelectrochemical cells, fossil fuel reforming, artificial photosynthesis, aqueous phase reforming and conventional reforming methods [8,9,10]. These production techniques may be divided into categories based on their CO2 emissions and environmental impact. The four main sections in which hydrogen production has been categorized are brown, grey, blue and green [11].
These color codes are designated in terms of the degree of CO2 emissions and capture, as seen in Figure 1. The brown hydrogen category includes carbon and fossil-fuel production methods, such as gasification by coal, which emits the highest levels of CO2. The grey hydrogen category includes industrially produced hydrogen, e.g., through steam reforming using natural gas, but with no carbon capture and high CO2 emissions. Blue hydrogen is produced during the carbon capture process and is, therefore, the category that emits the least CO2. Green hydrogen is the ideal climate-neutral production method, i.e., it is produced through renewable electricity and electrolysis, emitting zero CO2 [8,12].
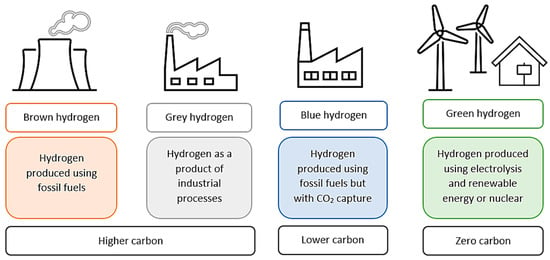
Figure 1.
Differences in hydrogen production methods and their carbon emission impact.
Green hydrogen possesses a higher purity than blue hydrogen, which needs further purification if it is to be implemented in a vehicle’s fuel cell. However, blue hydrogen is suitable for industrial use without purification, and the production of blue hydrogen eliminates the industry’s emissions by reducing CO2 on a large scale and with a low cost [13].
In 2019, hydrogen production was 75 million tons per year and was used primarily in refining and in the chemical industry [14]. It is estimated that, in 2070, hydrogen and hydrogen-based fuels will account for 13% of all energy needs, compared to 1% in 2019, with most of the hydrogen being used in the transportation and chemical industries [14]. In 2070, the transportation industry will account for 70% of hydrogen consumption, divided into shipping (52%), aviation (40%) and the remaining road transport [14]. In 2019, hydrogen production was responsible for greenhouse gas (GHG) emissions of more than 800 million tons of CO2, corresponding to around 2% of the global CO2 emissions from the energy sector [14]. Of the many developing strategies and projects working on a low-carbon hydrogen value chain, fuel cells in transportation and electrolysis production are among the more advanced methods. However, hydrogen-fueled engines for road transportation (mainly cars), ships and ammonia-fueled ships are currently only in the prototype stage [14,15].
1.1.1. Power-to-X and the Role of Hydrogen in Denmark
The use of hydrogen already extends to several parts of the global energy sector, and production is projected to grow six-fold from 2021 to 2050 to meet 10% of the total energy consumption [16]. As part of meeting the European Green Deal, renewable hydrogen production is expected to reach 10 million tons in Europe by 2030 [17]. The energy directive promotes using hydrogen to decarbonize hard-to-eliminate emission industries and heavy-duty and long-distance transport.
Power-to-X (PtX or P2X) is a collective term for renewable technologies in which electricity production is achieved through renewable resources, which convert into hydrogen, synthetic gases, fuels or chemicals. These conversion technologies allow for the storage and utilization of excess renewable energy via the production of green fuels and chemicals while also providing low capital-intensive decarbonization [13,16,18]. The benefits of implementing PtX technology in the energy sector include decentralized production, seasonal energy storage, decarbonization and grid stability, which become increasingly important as contributions from the wind and solar sectors steadily grow [13].
1.1.2. From Hydrogen to Ammonia
The future of hydrogen as an energy source seems promising; however, there are drawbacks to the technology. The low volumetric energy density of hydrogen, both in gaseous and liquid forms, makes hydrogen challenging to store, thus decreasing its accessibility by making it harder to deliver and distribute. The low energy density of hydrogen is potentially one of the most significant barriers to implementing hydrogen-fueled energy [19]. To utilize and exploit the full potential of hydrogen as an emission-free fuel source, the shipping and storing of hydrogen may lean on another often-used chemical asset—ammonia.
Ammonia has a high hydrogen density of 17% due to having three bound hydrogen atoms, enabling its use as a fuel for combustion systems directly or as a fuel in fuel cells [20]. Thus, ammonia has been gaining increased interest due to its potential as a carbon-free fuel for shipping because of its high liquefaction temperature and energy density compared with hydrogen [21]. The volumetric energy density of ammonia is 11.5 MJ · L−1 and is therefore superior to liquid hydrogen with 8.491 MJ · L−1 and compressed hydrogen with 4.5 MJ · L−1 at 690 bar and 25 °C [22]. Moreover, ammonia can be stored and transported at −33 °C compared with cryogenic hydrogen storage at −253 °C, significantly reducing the economic cost of being less energy intensive [23]. Additionally, ammonia allows for safer handling and distribution, as it is less hazardous than hydrogen. Although toxic, its smell can be detected even at safe concentration levels (<1 ppm). Ammonia has a narrower flammable range than hydrogen and is often considered nonflammable when transported, whereas hydrogen burns with an invisible flame [24]. Similar to hydrogen, ammonia is ultimately carbon free, although CO2 emissions during ammonia production depend on the energy source. Both hydrogen and ammonia utilize energy sources for production, such as fossil fuels, biomass, renewable electricity and nuclear and solar energy [25].
Exploring different alternatives to storing and shipping hydrogen has led to extensive research and numerous studies [26,27,28,29,30,31,32,33,34,35,36]. However, compared with much of the work performed, the key advantage of exploiting liquid ammonia is its ease of storage and distribution, thereby overcoming the issues of hydrogen infrastructure [33]. For hydrogen production to become sustainable, environmentally friendly and carbon neutral, emphasis must be given to production from renewable sources. This can be achieved by utilizing a microbial electrolysis cell (MEC) assisted with a renewable energy source for external power and waste to ensure and optimize a circular bioeconomy [37]. This paper investigates the current state-of-the-art MEC technology, including set-up configurations, the potential of varied feedstock utilization, the prospect of upscaling and current limitations.
2. Current State-of-the-Art for MEC Technology
2.1. Operating Principle
Microbial electrolysis cell (MEC) is an anaerobic biological process used to convert organic compounds into hydrogen or methane. MEC technology is related to traditional electrolysis, such as alkaline water electrolysis (AWE) or proton exchange membrane (PEM) electrolysis. The main difference is that the hydrogen evolution reaction (HER) is catalyzed by electroactive bacteria under anaerobic conditions. Moreover, MEC technology resembles another hydrogen production technology: the microbial fuel cell (MFC). The technologies differ, as MEC produces hydrogen from anaerobic electrogenic bacteria with an applied voltage, whereas MFC produces an electrical current from aerobic electrogenic bacteria [38,39,40,41]. As MEC and MFC are structurally similar, MFC is representable in many experimental studies and is therefore included in the coming sections.
An MEC consists of an anode and cathode coupled to a power source and submerged in a suitable electrolyte, facilitating electron transport via microorganisms pre-inoculated in the anaerobic container (cf. Figure 2). The two electrodes can be submerged into the electrolyte in the same container, or they may be separated by a membrane or placed in separate containers with different electrolytes, providing several configuration options [39]. MEC technology utilizes bacteria for the catalysis of the electrochemical oxidation or reduction reactions used to produce current [41].
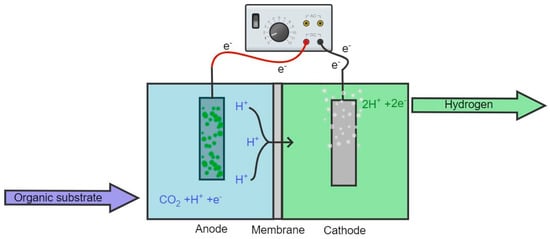
Figure 2.
Simple construction scheme of two-chamber MEC separated by a membrane. The green attachment on the anode represents the microbial presence.
The electrons and protons generated at the anode during oxidation are utilized at the cathode to produce biohydrogen but require additional voltage to trigger biohydrogen production [42]. The optimally applied voltage lies in the range of 0.5–1.0 V [43], which is lower than the voltage required for water electrolysis (1.23–1.8 V) [39], which indicates an advantage to MEC technology. In theory, small voltage can be supplied by renewable energy sources, such as wind or solar energy, during hours of low demand [44].
The mechanism in MEC, as seen in Figure 3, can be summarized as the breakdown of organic matter by exoelectrogenic bacteria into ions, which the bacteria can transport outside of their cell to the surface of the anode inside the MEC chamber. The exoelectrogenic bacteria are attached to the surface of the anode, where they oxidize the organic material into CO2, releasing protons to the solution and electrons to the anode. When exoelectrogenic or anode-respiring bacteria consume organic matter under anaerobic conditions, they donate their final electron as part of their electron transport chain to the terminal electron acceptor (TEA) at the electrode [10,39].
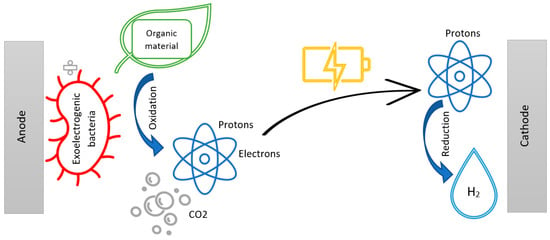
Figure 3.
An illustration of the mechanism in the MEC chamber during electron transfer.
The applied small current ensures the transfer of electrons from the anode to the cathode being fused to produce biohydrogen and reducing protons. The system should be kept under strictly anaerobic conditions at both the anode and cathode, since aerobic conditions at the cathode produce water and electricity, thus converting the setup into an MFC [37,39].
Electrogenic bacteria are bacteria capable of transferring electrical charges from or to an electrode [45]. Electrogenic bacteria possess the most critical role in hydrogen production through MEC technology. Thus, the choice and type of exoelectrogenic bacteria are essential to ensure ideal conditions for optimized hydrogen production. Several microbial species have been documented as electrogenic bacteria, which are useful in MEC systems. Mixed microbial communities are often utilized for MECs, since studies have proven that their electroactive efficiency compared with pure bacterial cultures is higher when used in mixed cultures [46,47].
Methanogens are another niche of microbes that are encountered and found in MECs with mixed cultures. Methanogens facilitate biofilm formation at the cathode and use the hydrogen and electrons to produce methane, decreasing the yield and contaminating the hydrogen. As the MEC is operated anaerobically, the presence of methanogens such as the Methanobrevibacter species is inevitable when using mixed cultures [39,48]. However, although methanogens are often found in MECs, especially during biofilm formation, electrogenic bacteria capable of producing current densities are more relevant and desired when aiming to produce hydrogen through MEC.
Table 1 shows and summarizes some commonly found electrogenic species in MEC systems. The table presents relevant information on the bacterial description and its function in MEC.

Table 1.
Overview of commonly found bacteria in MECs and MFCs, including several electrogenic bacteria, each with its biological description and function in MEC.
The most abundant bacteria observed in MEC are proteobacteria, firmicutes and bacteroides. The microbial analyses of several studies have shown that proteobacteria are abundant in 20% to 46% of the total microbial communities found in MFCs and MECs. One study investigated the distribution of different proteobacteria in MFC inoculated with a sediment–water slurry and found that the most abundant classes were the gamma- (62.4%), epsilon- (19.3%) and beta-proteobacteria (17.6%), whereas alpha- (0.65%) and delta-paroteobacteria (<0.1%) were also present in smaller degrees [75]. Another similar study observed that the distribution of proteobacteria consisted of gamma-proteobacteria (19%), epsilon-proteobacteria (2%), delta-proteobacteria (1%) and alpha-proteobacteria (0.9%) [76]. These findings indicate that gamma-proteobacteria are common electrogenic bacteria in MEC and MFC. Firmicutes have been observed in the range of 25% to 32%, whereas bacteroides have been observed in the range 17% to 36% in MFCs and MECs [49,50,51,52,53,54,55,56,59,60,63,65,70,72,74,75,77,78].
Electrogenic bacteria, such as Geobacter and Shewanella, are characterized as extracellular electron transferors (EETs), i.e., they are capable of donating or accepting electrons. This ability to transfer electrons is utilized in MEC for hydrogen production, as the electrons utilized in those reactions are delivered by electrogenic bacteria [79,80].
The chemical description of biohydrogen production at the electrodes includes the hydrogen evolution reaction (HER). The HER comprises multiple reactions in which the first step is the electrochemical Volmer reaction, followed by a chemical Tafel reaction or an electrochemical Heyrovský reaction [81]. The HER starts with the Volmer reaction, where protons are coupled to electrons on the electrode’s metal surface (MS), producing hydrogen ions bound to the surface.
The next step may involve either the Tafel reaction or the Heyrovský reaction. The Tafel reaction utilizes two different molecules of hydrogen bound to a metal surface, which are liberated into hydrogen gas (H2).
The alternative Heyrovský reaction utilizes protons soluble in the substrate and combines them with the hydrogen attached to the metal surface to produce hydrogen gas (H2) [82,83].
The productivity can be affected by numerous factors, including the MEC design; however, the choice and profile of the substrate and feedstock might be underestimated, since the type and accessibility of carbohydrates have proven essential to the efficiency and operation of a MEC [84,85].
2.2. Anode Material
The material used for the anode can heavily affect the MEC’s performance. MEC has three active materials responsible for converting feedstock to biohydrogen, i.e., the anode, cathode and membrane. These active materials can be configured to accommodate a wide range of feedstock and microbial cultures. Most research suggests that the limiting parameter is coupled to the bacterial activity at the anode [86] or the catalysis and availability of protons at the cathode [87].
The common factor of anodes is the coupling of the anode to a more conductive metal, thereby allowing a concentrated flow of electrons, ensuring bonding and thriving microbes. The bonding is facilitated when the right functional groups are attached to the anode [88]. Attachment to the electrode mainly occurs via the adsorption or entrapment of the microbial species. Both can be affected by the anode’s surface properties, roughness and porosity [89]. Furthermore, a successful anode requires a strong and resilient construction with a developed surface area, still allowing for substrate flow and high proton and electron productivity [90,91]. However, biocompatibility is vital for achieving the required microbial adhesion. Therefore, the presence of molecular affinity, chemical bonding or electrostatic forces is necessary [88].
Carbon and metal-based anodes are commonly used in MECs as an optional mix in which the metal is used to conduct the current [57,92,93]. Carbon-based anodes comprise carbon felt [94,95,96], carbon cloth [97,98], graphite (as felt, plate or fiber) [99,100,101], carbon fiber, carbon brushes, carbon mesh, carbon paper, activated carbon (granules), graphite granules, etc. (as seen in Table 2). When investigating the differences between carbon and graphite materials, it should be noted that some studies state that graphite materials are carbon, as it is an allotrope of carbon. Purely metal-based anodes consists of stainless steel (mesh [102], sheets [103] and scrubbers [104]), nickel [105], silver [106], titanium [107], copper [108], etc.

Table 2.
Pictures of most applied carbon-based materials used in MEC.
Carbon felt exhibits a high porous structure while being an excellent electric conductor. Carbon felt is also a low-cost anode with a sizeable flexible surface area when considering its flat-plate surface [109,110]. Carbon cloth possesses high mechanical strength, but as it is a dense structure, clogging is a potential hazard [111]. Carbon cloth is also an expensive carbon-based anode, especially for larger-scale MECs. A carbon brush is a carbon-based anode with a large surface area per unit of volume. These brushes are often based on a titanium wire, which increases the conductivity compared with other carbon materials and increases the overall price. The carbon brush has been proposed as a next-generation anode since its open structure allows for a high surface area with a lower chance of clogging [90,111]. Carbon mesh can be used as an alternative to carbon cloth, which can be an expensive anode. Carbon mesh has a low cost but possesses many of the same properties as a carbon cloth. Carbon mesh is less prone to clogging due to its more open structure [112]. Carbon paper has a paper-like structure, which offers a slightly porous structure, but it has not been assessed to be an optimal solution for larger-scale operations, as the material is fragile [113,114]. Activated carbon granules have a large surface area and porous structure, promoting biofilm formation. Carbon granules are also a low-cost material but have the potential to clog. The conductivity of carbon granules is often relatively low, as the granules have a poor connection to the current collector. However, the granules have another property that can be exploited, as the structure allows for easier entrapment of pollutants, such as heavy metals [115].
Stainless steel is another material used as an anode; however, the material is constricted, as the surface area is reduced compared to carbon-based anodes. Stainless steel can be used with other materials such as graphene to increase biofilm formation [116]. Stainless steel is one of the cheapest materials used for anodes and has excellent electric conduction, exhibiting strong mechanical strength and corrosion resistance and having a long lifetime. Other materials such as nickel [105], silver [106], titanium [117] and copper [118] have also been tested as anode materials, but in their pure forms, these materials lack the ability of microbial adhesion. However, most can use a combination of materials, such as a carbon-based anodes modified with a nickel coating [105]. Both copper and nickel ions can be poisonous for microbes and are undesirable when released into, e.g., wastewater, which makes these materials less ideal, as it constrains the environmental aspect of the MEC design.
Most bacteria’s outer membrane consists of proteins and amino acids which are negatively charged. Thus if the anode material can be configured to be more positively charged by embedding nitrogen molecules in the structure by nitrogen doping, biofilm formation can be enhanced and promoted [124]. The chemical bonding between C-, N-, O- and S-containing functional groups can be exploited when optimizing the anode.
Y. Zhau et al. (2018) [109] investigated the modification of carbon felt anodes by double oxidant HNO3/H2O2 and found it to decrease the inoculation period and to increase electron production for the anodes [109]. Another study by S. Rozenfeld et al. (2019) [86] investigated how to improve anode activity by utilizing two different anode materials: plasma-pretreated carbon cloth and stainless steel. The study found that plasma pretreated anodes, with the introduction of nitrogen doping atoms, increased -C-OH, -COOH, and =C=O groups, and this relationship was found to increase both biofilm formation and the current in biohydrogen production.
Already known surface modifications have proven to increase the productivity of electrolysis cells with methods as simple as utilizing the heating of the material with ammonia, giving currents of 2400 mW · m−2 [125] and hydrogen production of 7.4 L H2 · L−1 · day−1 [126].
When comparing anode materials with and without surface modification, increased productivity was found for surface-modified anodes, cf. Table 3. As surface modification substantially increases productivity, future studies should investigate optimal treatments allowing for high productivity for a long duration, which can feed into scalability for both the anodes and optimized surface modification.

Table 3.
Summary of anode materials, their advantages/disadvantages, their modifications and their performance in an MEC/MFC setting.
For MEC to become commercially successful, the anode material must have a low cost with stable operation over time. The anode must be environmentally friendly and have strong mechanical strength [90,115]. The most commonly used anode material capable of complying with the success criteria is the carbon-based anode, which has been used in many studies [90,115,127]. Carbon-based anodes have the advantage of good attachment for electrogenic microbial species, and some exhibit a large surface area in a small volume due to their 3D structure. However, this can also be a problem, as the pores can be affected by clogging, thereby restricting flow. This decreases electron transfer and biohydrogen production, resulting in the usage of 2D carbon anodes.
Anodes are typically defined as 2D or 3D structures depending on the surface. Three-dimensional structures have been preferred in a laboratory scale and have shown the highest biohydrogen production and current density, as seen in Table 3. However, a study conducted by E. Blanchet et al. (2016) [128] mimicked an industrial MEC operation based on food waste and wastewater, where 3D (carbon felt) and 2D (carbon cloth) anodes were used for comparison. The study found that the 3D structure performed worse than the 2D structure, as there was no clogging in the 2D structure. This contradicts previous studies, suggesting that 3D anodes are not always the preferred choice [128]. This could especially be relevant for the upscaling process, as larger facilities are able to implement 2D anodes with a faster flow, ultimately creating the potential for higher production of protons and electrons. Therefore, 2D structures show an interesting approach when dealing with larger particle substrates, as the chance of clogging decreases.
An investigation into 2D structured anodes was conducted by D. Pocaznoi et al. (2012) [129], comparing the following anode materials: stainless steel, flat-plate graphite and carbon cloth. The study found that carbon-based materials increased biofilm formation compared with stainless steel. Comparing the flat-plate graphite with stainless steel, no difference in biofilm formation was found, but the stainless steel had twice the current density. Comparing the stainless steel with the carbon cloth, a higher current density was exhibited by the carbon cloth. The study concluded that the topography of stainless steel prevented it from being the preferred anode material, but surface treatment can be used to increase the current density [129]. Therefore, combining the electric properties of stainless steel with a biofilm-promoting carbon material can provide anodes with higher hydrogen productivity.
No single anode material has been able to satisfy all criteria for an excellent industrial and environmentally friendly anode while still being economically favorable. Carbon-based materials with a metal current collector outperform their solely metal-based counterparts, but few have provided sufficient biohydrogen production. Almost all studies have used surface modification on their anode in one form or another, and it seems generally accepted that surface modifications increase the performance of almost all materials, as shown in Table 3 [90,112,115,130]. Surface treatments are worth exploring, where treatments with low environmental effects and prices can bring the anodes closer to a viable industrial MEC. Additionally, surface treatment and combinations with other anode materials could make the metals a viable candidate in the future, especially considering the costs and operation times of anodes.
2.3. Cathode Material
To drive the HER reaction in an MEC, a catalyst is needed, predominantly implemented as a catalyst coating/catalytic material or by using microbes to drive catalysis [39,43,144,145]. Like the anode, the cathode must exhibit different properties to be a viable candidate for an industrially applicable MEC. These properties include fast catalysis, low overpotential, a low cost and a long operation time [146].
Cathodes can be based on current-collecting materials or carbon-based materials, used as a basis for the structure and electron transportation. Materials used as an anode can be used as a base; the difference is denoted by incorporating a catalyst. Traditional catalysts, such as noble metals, e.g., platinum or palladium, incorporated as a coating can also be used due to stable catalytic activity. Platinum-based cathodes are well established and known for their good catalytic properties and low degree of overpotential [55,56]. However, using platinum has the drawbacks of a negative impact on the environment and a high cost; however, it is often used as a baseline for comparison [147]. Platinum can be a viable option for lab-scale MECs, but the use of complex substrates for larger operations would require protection from sulfide poisoning from platinum, which reduces its catalytic effectiveness [147,148]. Palladium-based catalysts have been explored as an alternative used as a coating, as palladium is more resilient and can be acquired at a lower cost [149].
Nickel has been proposed as an alternative to platinum, as it exhibits good catalytic activities and high corrosion resistance at a low cost [150,151]. Nickel is often used as an alloy, as it increases the active surface area and lowers the overpotential, as explained in a study by A. Jeremiasse et al. (2010) [150]. The study found that nickel foam decreased the overpotential and achieved production of 50 L H2 · L−1 · day−1 [150]. Nickel foam also showed potential as a cathode base utilized with a platinum coating [152]. Nickel foam can be used as a versatile cathode at a low cost and can be a cheap alternative to platinum cathodes as a baseline comparison for future studies. This was investigated by Z. Yan et al. (2012) [153], who combined a low concentration of platinum and nickel nanoparticles and compared the performance to a Pt/C catalyst. The study found that the new nickel alloy was stable and could produce current densities in the same range at a quarter of the price [153]. This tendency aligns with other findings [154,155], as shown in Table 4, but the toxicity of nickel ions should be investigated before upscaling. Nickel foam has the advantage of easier scalability, as the material can be found in nickel metal hydride (NIMH) batteries produced on a large scale, and the material is therefore readily available.
Many of the highest-performing MECs have been constructed based on expensive catalysts, which are rarely viable in an industrial setting. However, research with biotic cathodes has started to increase HER productivity and current density, enabling the use of microbial species instead of a catalyst [68,90]. Biotic cathodes are an inexpensive catalyst and can be used for industrial settings with a low maintenance cost, as the biofilm driving the biotic catalysis is replenished by itself. However, using a biotic cathode, especially in a one-chamber configuration, also creates the possibility of cultivating methanogens. Methanogens can be eliminated or decreased by utilizing some of the following tactics: using a pure culture, exposing electrodes to oxygen before each batch, temperature shock, the addition of beryllium sulfide or by modifying the MEC design [68]. Furthermore, biocathodes have thus far not been reviewed as the highest-producing MEC [147], and thus, a cost–benefit analysis should be implemented to ensure their viability.
Evaluating the optimal cathode material and catalyst is a complex decision, but it should focus on cost, efficiency and scalability. The basis of the cathode has not been proven to be the deciding factor and does not affect hydrogen production in the same manner as the catalyst. As the base material constitutes a large fraction of the total cathode, investigation into the structural effect when scaling from small MECs to larger systems should be investigated, as larger MECs would require a more robust system. The focus aims to find new catalysts or to optimize previous catalysts to decrease costs and improve catalytic properties.
If a biotic cathode is utilized, this enables the production of pure biohydrogen, where methanogens can also be cultivated, leading to hydrogen loss. The presence of methanogens can be prevented, and it should not be the deciding factor. Nickel alloys provide a cheaper alternative to platinum catalysts coupled with HER-catalyzing microbial species. Nickel alloys outperform platinum-based alternatives and are becoming well studied at the laboratory-scale, as seen in Table 4. Thus far, biotic cathodes have experienced lower hydrogen productivity than platinum-based cathodes, and methods enabling higher productivity, either through biofilms, cultures or material optimization, must be explored.

Table 4.
Summary of cathode materials, their advantages/disadvantages, catalytic loading and their performance in an MEC/MFC setting.
Table 4.
Summary of cathode materials, their advantages/disadvantages, catalytic loading and their performance in an MEC/MFC setting.
| Cathode Material | Catalyst Loading In mg · cm−2 | Advantages | Disadvantages | MEC/MFC Performance | Reference |
|---|---|---|---|---|---|
| Pt-Co/G (15 wt.% Pt) on carbon cloth | 2.5 | Less Pt needed compared with Pt/C coating | Pt is expensive and would need repleting | 1378 mW · m−2 | [153] |
| FePC-supported multiwalled carbon nanotubes on carbon cloth | 1 | Alternative to Pt, cheaper than Pt | Carbon nanotubes need further investigation to become a stable cathode | 601 mW · m−2 | [156] |
| MnOx on carbon paper | 0.1 | Alternative to Pt and cheaper than Pt, low catalyst loading needed | - | 772.8 mW · m−3 | [157] |
| Stainless steel 306 (12% Ni) | - | Ni incorporated into stainless steel enables the catalysis of HER | Investigated for water electrolysis | - | [158] |
| Ni/AC/PTFE-coated stainless steel mesh | 6.5 | Ni can substitute noble catalysts with high activity, high porosity | Ni ions can be poisonous | 1.88 L H2 · L−1 · day−1 | [151] |
| Ni2P/C coated on stainless steel mesh | 0.5 | Large chemical stability, HPR as high as Pt-based cathodes | 0.29 L H2 · L−1 · day−1 | [159] | |
| Pt-coated carbon cloth Pt-coated nickel foam | 0.5 0.5 | Reliable, efficient, long operation time Cheaper and higher HPR than similar Pt-coated carbon cloth, porous | Week base, expensive catalyst Not stable in the same duration as Pt-coated carbon cloth, relatively expensive | 0.67 L H2 · L−1 · day−1 0.71 L H2 · L−1 · day−1 | [152] |
| Nickel foam | - | High productivity, large surface area enables fast catalysis. | Problems connected to scaling, quick decrease in MEC performance | 50 L H2 · L−1 · day−1 | [150] |
| Biotic based on wastewater incorporated on stainless steel mesh | - | Cheap, environmentally friendly, long operation time | Not as effective as Pt or similar catalysts | 240 A · m−3 | [134] |
| Biotic based on urban wastewater and MFC inoculum incorporated on granular graphite | - | Cheap, environmentally friendly, long operation time, found to be as effective or more effective than carbon-based cathodes | Relatively low HPR | 0.9 L H2 · L−1 NCC · day−1 | [144] |
| Pd/GO-C incorporated on carbon paper | 0.25 | Cheap compared to Pt, efficient catalyst | Expensive compared to nickel or stainless steel | 901 mW · m−2 | [149] |
2.4. Membrane Configuration
Membranes are utilized to separate the anodic and cathodic compartments of a two-chamber system, eliminating the possibility of short-circuits while enabling proton transport. The main advantages of using a membrane are enabling individual choices of anolytes and catholytes and a higher hydrogen purity [160,161]. The separation between the two compartments further inhibits diffusion back across the membrane, limiting the amount of hydrogen returning to the bacterial cultures.
Cation exchange membranes (CEM) are one of the more commonly used membranes, often in the form of the Nafion® membrane. Initially used for the conduction of cations such as Na+, K+, Ca2+, Mg2+ and NH4+, CEM is also utilized for MEC studies and, therefore, is called a proton exchange membrane [161,162,163]. However, as cations are abundant in many MEC substrates, competition between protons and cation transport can decrease permeability. Nafion®’s use is further limited for larger MEC upscaling due to its cost, operation time, gas crossover and increased internal resistance. Therefore, efforts to investigate new alternatives and composites have been made [161,164,165].
A suggestion for an alternative membrane based on a study by T. Sleutels et al. (2009) includes anion exchange membranes (AEMs), which were found to decrease the overall internal resistance of two-chamber MECs together with a higher HPR [166]. Further extensive literature reviews on different membranes and their properties can be found in [161,164,167,168,169,170,171].
2.5. Type of Configuration
The composition of the MEC can be designed in various ways and by using many different materials. Different MEC configurations operating under the same conditions and using the same materials can provide different current densities and biohydrogen productivity. This is caused due to varying configurations in shape, electrode distancing, membranes and types of inoculum [172].
The two-chamber configuration most effectively inhibits methanogens, creating a more pure biohydrogen gas [39,92]. Utilizing a two-chamber MEC also creates the possibility of using different substrates for the anodic and cathodic chamber, which can increase biohydrogen productivity.
The catholyte can be designed differently regarding pH, ionic concentrations and buffers, improving biohydrogen productivity. This might invoke a potential pH gradient between the membrane, which increases the overpotential in the system. When utilizing a two-chamber configuration, distances between the electrodes are generally larger, thus decreasing the current density and affecting biohydrogen productivity [39].
Incorporating a membrane also increases the internal resistance, affecting the electron transfer from the anode to the cathode. In a study by A. Kadier et al. (2016) [173], the two-chamber MEC was found to be have a complex design while posing practical challenges when transitioning to scaling [173]. The two-chamber MEC can be designed into different architectures with the H-type [174], plate type [175] and tubular type [176], but the type of architecture has not been proven to be the decisive factor. This is caused by dependence on the setup, where simple cells often have been found to create lower biohydrogen production. A tubular reactor of 1 L constructed by K. Guo et al. (2017) [176] managed to create the highest biohydrogen productivity of a two-chamber MEC in that size for that time, reaching 7.1 L H2 · L−1 · day−1. The study suggested that the design and productivity were scalable without significant changes in internal resistance, indicating that the MEC can be viable for larger upscaling [176].
The construction of the one-chamber MEC is a response to many of the difficulties experienced by two-chamber MECs [92]. A one-chamber MEC likely has lower overpotential and internal resistance when removing the membrane, which can provide a foundation for increased biohydrogen productivity. One-chamber MECs can decrease the electrode distance significantly, thereby allowing for easy and fast electron transfer.
A study by Y. Fang et al. (2008) [177] investigated the internal resistances in a two-chambered MFC and found that up to 86% of the internal resistance could be attributed to the membrane [177]. One disadvantage of utilizing a one-chamber MEC is that the cultivation of methanogens is possible, which decreases the biohydrogen productivity, but different measures can be implemented to counteract this effect [68]. It is not always unwanted to convert biohydrogen into methane or to have a mixture of gases as a product. This can be the case in anaerobic digestion, where an MEC can be configured to produce methane, whereby methanogens pose as less of an issue [178].
Often, MEC is inoculated with either a single species or a complex microbial culture, which, for the latter, creates the possibility of cultivating methanogens for a one-chamber MEC. Each type of inoculum has its advantage, where a single species can ease monitoring the effect of any changes made to the system. It also eliminates methanogens consuming biohydrogen, decreasing the yield and purity. Complex microbial cultures increase diversity, which the MEC more robust, and it also allows for the degradation of multiple substrates while achieving higher biohydrogen productivity [47,95,134]. For larger-scale applications, complex cultures can decrease operational costs, as no sterilization of the substrate is required [179].
However, the MEC has proven to be difficult to upscale [167], especially when adapting to low-cost feedstock that is not chemically defined. Some of the problems linked to upscaling lie in the conductivity of the feedstock, electrode distances, biohydrogen loss, and the price of construction/running and maintaining a larger facility [81], all of which lead to decreased current densities and biohydrogen production. Therefore, an efficient way of predicting larger-scale MECs is needed if MECs are to be industrially applicable.
A study by L. Singh et al. (2021) [180] proved the scalability of a 0.15 L one-chamber MEC by an internal resistance analysis, in which they predicted the current density for a scaled MEC of 10 L. The scaled 10 L MECs were constructed in their laboratory, and they successfully proved that the design was scalable with a high current density and even suggested that they have potential for further upscaling [180]. The 10 L MEC provided biohydrogen productivity of 5.9 L H2 · L−1 · day−1 and a current density of 970 A · m−3, which, to the authors’ knowledge, was one of the highest hydrogen production rates (HPRs) achieved at that size, as shown in Table 5 [180].

Table 5.
Summary of some MEC configurations, sizes, anode/cathode materials, voltages and biohydrogen productivity.
3. Feedstocks for the Production of Biohydrogen
The MEC possesses the significant advantage of not being limited by the type of carbohydrates or substrate composition, which means, in theory, that almost any source of organic matter can be utilized for sustainable biohydrogen production. Most substrates are divided into three types, namely non-fermentable (acetic acid, glycerol, etc. [181]), fermentable (glucose, cellulose, lignocellulosic biomass, etc. [182]) and wastewater (domestic, sewage, landfill, etc. [183]) [146]. However, in most MEC studies experimenting at the laboratory scale, the leading substrate is often acetic acid because it is readily accessible [47,49,95,101,134,184].
Biohydrogen may be produced through dark fermentation, where carbohydrates, such as glucose, are converted into biohydrogen through two metabolic pathways depending on the bacteria metabolizing the carbohydrate. The production of only four moles of biohydrogen per one mole of glucose is common for both pathways [185].
Regarding stoichiometry, the fermentation pathway to biohydrogen is inferior to that of MEC. Utilizing fermentation is therefore rejected as a biohydrogen production method. However, acetic acid is produced through glucose fermentation, which is often used as a feedstock in MEC studies [47,49,95,101,134,184].
When acetic acid is used as feedstock in MEC, the oxidation reaction is governed by the exoelectrogenic bacteria attached to the anode. Therefore, the final biohydrogen is produced at the cathode [186].
Anode:
Cathode
Acetic acid is typically regarded as a waste product in the yeast fermentation of glucose, and instead of discarding the waste product, it can be utilized in biohydrogen production.
Aside from acetic acid, another used feedstock in MEC studies is glucose or glycerol as a byproduct from biodiesel fuel production. P.A. Selembo et al. (2009) [181] produced biohydrogen fed with glycerol and glucose as by-products from biodiesel fuel production. Glycerol is overproduced due to biodiesel production, with an annual production of 980 million liters of glycerol per year, compared with a world demand of 216 million liters per year in 2009 [181]. The study compared pure glycerol (P-glycerol), biodiesel byproduct glycerol (B-glycerol) and glucose fed to a one-chambered MEC inoculated with domestic wastewater. The best performing glycerol was pure glycerol, yielding a biohydrogen concentration of 3.9 mol H2 · mol−1 and a production rate of 2.0 L H2 · L−1 · day−1, whereas B-glycerol only yielded a biohydrogen production rate of 0.41 L H2 · L−1 · day−1. B-glycerol contains a mix of glycerol, soaps, methanol and water. The presence of methanol in the mix would explain the lower production rates compared to P-glycerol. Glucose yielded a biohydrogen concentration of 7.2 mol H2 · mol−1 and a production rate of 1.9 L H2 · L−1 · day−1. This means that glycerol byproducts can be used to produce biohydrogen at satisfying rates and without prior purification. Optimizing the B-glycerol mix by removing the methanol pre-MEC can increase the biohydrogen production concentration and rate [181].
Y. Feng et al. (2011) [93] investigated an MFC fed with biodiesel waste mainly containing glycerol. A total of 20 one-chambered MFCs were inoculated and fed with either biodiesel wastewater or glycerol containing 10% domestic wastewater. The results showed that both types of feedstock ensured power generation in the MFC, and biodiesel wastewater slightly outperformed the glycerol and wastewater mixture [93].
N. Montpart et al. (2015) [85] treated complex substrates in a one-chamber MEC and observed the potential of using dairy waste products as feedstock for biohydrogen production. The experimental setup consisted of an MFC and an MEC inoculated with effluent from anaerobic digestor sludge fed individually with glycerol, milk, potato starch and a mix of all three.
The results showed that the MFC had been able to produce satisfying current densities, whereas the MEC produced biohydrogen only when fed with milk and the mix. The wastewater of the dairy industry could be another potential waste that is ready to be utilized as a source of organic substrates for biohydrogen production through MEC. The article declares that neither the biodiesel industry’s wastewater nor the potato industry’s wastewater is a possible substrate source [85]. However, multiple other studies investigating and validating glycerol and biodiesel production waste streams have proven the substrate to be applicable for biohydrogen production through MEC [93,181].
S.B. Velasquez-Orta et al. (2011) [84] verified that simpler substrates perform better than complex substrates, such as starch. To prove this, six one-chambered MFCs were inoculated with municipal sewage effluent and were fed a synthetic medium containing either acetate, glucose or starch. As expected, the overall performance was better in the MFCs utilizing more simple substrates (acetate and glucose) than in the MFCs using complex substrates (starch), which might have needed a pre-processing step before being a viable substrate [84].
L. Lu et al. (2010) [163] investigated using proteins as the substrate for biohydrogen production in MECs. The degradation and conversion of proteins by exoelectrogenic bacteria is essential due to its large concentration in many wastewaters.
To investigate wastewater as a substrate, a one-chamber MEC and MFC and a two-chambered MEC were inoculated with domestic wastewater and were fed with bovine serum albumin (BSA) and a complex mixture of different proteins (peptone). The study showed that generating electricity and producing biohydrogen was possible when fed with BSA and peptone. The BSA-fed MEC performed better than the peptone-fed MEC in biohydrogen production [163].
3.1. Wastewater
Readily increasing amounts of waste have been and will continue to be a recurring problem for many years [187]. Therefore, utilizing waste as the substrate for biohydrogen production ensures a circular bio-economy while reducing the cost of wastewater treatment and sludge disposal.
The wastewater composition and source affect its potential as a substrate for both MFCs and MECs, since the percentage of organic feeding materials govern the biohydrogen production rate, biofilm formation and current density generation [146]. Industrial wastewaters contain many harmful chemicals, antibiotics, dyes and other contaminants, possibly affecting bacterial function [188]. Another contaminated wastewater-based feedstock is landfill leachate, which has been proven to be a potential substrate despite its high concentration of harmful components and heavy metals [189,190].
However, using the rich bacterial diversity often found in landfills leachates as an inoculum source often compensate for the presence of contaminants. Other less harmful feedstocks with potential include protein-based, cellulose-based and sugar-based wastewaters. Particularly, sugar-based wastewater has been proven to be one of the most promising substrates due to its large content of simple carbohydrates, which has proven to be effective in biohydrogen production in MECs [146].
R.D. Cusick et al. (2010) [191] investigated energy recovery from an MFC and MEC fed with winery watewater or domestic wastewater in a small-scale (0.03 L) one-chamber, yielding a maximum of 0.17 L H2 · L−1 · day−1 [191]. The success led R.D. Cusick and B. Logan (2011) [48] to upscale their theory and investigate the prospect of using wastewater in a pilot-scale continuous-flow one-chamber MEC fed with winery wastewater.
The pilot-scale reactor, measuring 1000 L, was packed with 144 electrodes of cathodes made of stainless steel grade 304 and anodes made of graphite fiber brushes. The hydrogen production rate reached a maximum of 0.19 L H2 · L−1 · day−1 with a current density generation of 7.4 A · m−3. However, although it was an overall success, the study had one major concern.
When scaled up from the laboratory scale to the pilot scale, the increased ratio of methane production through hydrogenotrophic methanogenesis converting the hydrogen into methane gave a ratio of 86% methane in the final gas solution [48].
An article by E.S. Heidrich et al. (2013) [192] investigated the production of biohydrogen based on domestic wastewater. The article utilized a 120 L MEC reactor incorporated in a wastewater treatment facility in England under poor conditions due to low-concentration feed, no heating, cheap materials and no additional supplement of acetate or buffer. However, the pilot-scale MEC produced almost pure hydrogen over a 3-month period, proving that MEC can produce biohydrogen from real domestic wastewater at the pilot-scale using low-cost materials at ambient temperatures [192].
B. Min et al. (2005) [193] used swine wastewater for MFC to produce electricity. The initial tests used a two-chambered MFC fed and inoculated with swine wastewater from a swine farm in Pennsylvania, USA. The two-chambered MFC showed a power generation of 45 mW · m−2. Additional tests with the one-chambered MFC involved inoculating and feeding with the same swine wastewater, which yielded six times more power, with 261 mW · m−2. These results demonstrate that animal wastewater is equally feasible in generating electricity using both one- and two-chambered MFCs [193].
A review article by K. Katuri et al. (2019) [44] investigated the integration challenges when utilizing urban wastewater for MEC by comparing existing work.
Five pilot-scale (120–1000 L) MECs treating domestic wastewater were presented, and their biohydrogen production rate ranged from 0.005 L H2 · L−1 · day−1 to 0.19 L H2 · L−1 · day−1. The study further investigated multiple waste treatment integration possibilities, such as side-stream urban wastewater treatment, fermentation for cellulose sludge treatment and anaerobic digestion, to treat waste-activated sludge. It was concluded that optimizing spacing between electrodes and increasing the surface-area-to-reactor-volume ratio may improve the prospect of utilizing MEC for urban wastewater treatment [44].
Another environmentally harmful waste type is leachate, which contains multiple soluble and toxic compounds, materials and microorganisms, which should be managed to protect the surrounding area from landfill. A master thesis by L. Damiano (2009) [183] investigated the use of leachate as a substrate and inoculum for MFCs to produce electricity and treat landfill leachate. Three one-chambered MFCs in different setups were inoculated and fed with landfill leachate from a facility in Holland. The three MFCs were operated successfully using landfill leachate as an inoculum and feedstock. Landfill leachate served as a prominent feedstock with a high amount of organics, conductivity and only minimal solids, as well as the advantage of utilizing the feedstock as an inoculum [183].
An article by A. Escapa et al. (2012) [194] studied the performance of a continuous-flow MEC fed with domestic wastewater. The continuous MEC design reduced up to 76% of the COD of domestic wastewater treatment, including a hydrogen production rate of 0.3 L · Lr−1 · day−1 [194]. In another study by A. Escapa et al. (2012) [195], a case study was performed on the investment cost of applying the MEC technology to wastewater treatment plants. The team estimated that an industrial-scale MEC operating at a current density of 5 A ma−2 (amperes per square meter of the anode) and an energy consumption of 0.9 kWh kg-COD−1 (kilowatt-hour per kg of removed chemical oxygen demand (COD)) with a total cost of €1220 ma−3 (euro per m3 of the anodic chamber) can be established as a target purchase cost, at which a break-even point can be established after approximately seven years. Such a research study investigating the inclusion of MEC technology is crucial in an upcoming market that is expanding more each year [195].
T. Fudge et al. (2021) utilized MEC technology in traditional wastewater treatment and focused on its implementation and adoption on an industrial-scale along with economic and market-specific aspects of such an upscale [196].
H. Guo et al. (2019) [197] investigated the application of a stacked multi-electrode MEC for rapid and low-sludge treatment of municipal wastewater. The design successfully enhanced the electric current generation, rapidly removed organics and proved to be capable of low-sludge wastewater treatment [197].
A. Kadier et al. (2020) [198] reviewed the biorefinery perspectives of MEC for hydrogen production through wastewater treatment. The findings studied the integration possibilities with other technologies, such as MEC–MFC coupled systems, submersible MEC (SMEC), solar-powered MEC and dark fermentation with MEC–MFC coupled systems. In summary, different integration technologies and the advantages of integrating MEC with different energy-generating processes might improve the rate of biorefinery growth toward sustainable development [198].
3.2. Solid Waste
Wastewater has been used as an inoculum and substrate in MECs and has proven to be effective and successful in small-scale and pilot-scale experimentation. This review expands to accommodate two more types of substrates to existing solid waste: municipal solid waste (MSW) and the organic fraction of municipal solid waste (OFMSW). The use of OFMSW as a substrate in MEC is more recent, and the studies utilizing it are significantly fewer, although the theory holds great potential [182,199,200,201].
Organic compounds can be electron donors for proton reduction in biological biohydrogen production. The primary biomass sources include plant residues, crops or grains, as well as organic waste generated from human activities, such as solid waste [81]. OFMSW can become a potential ideal feedstock because it is widely available and contains easily degradable organic compounds.
A. Karluvali et al. (2015) [200] investigated the use of OFMSW in MFC for electricity generation. MFC as a pretreatment method for OFMSW provided sustainable, renewable and clean energy from removing organic matter. A tubular MFC was inoculated with granular anaerobic sludge from a wastewater treatment facility, and OFMSW was used as the substrate from a compost and recovery facility in Turkey. The setup proved that bioelectricity generation based on OFMSW is possible [200]. B. Pendyala et al. (2016) [182] optimized the performance of MFC by feeding different types of OFMSW, or, more specifically, food waste (FW), paper–cardboard waste (PCW) and garden waste (GW). A one-chambered MFC was inoculated with anaerobic municipal wastewater and was fed with either FW, PCW or GW individually or as a mix. The maximum power generation exceeded that of other reports utilizing wastewater as a substrate. However, the power that was produced was unpredictable due to variations in the feedstock’s organic loading rate and chemical composition. The MFC fed with the individual feedstock showed that the individual feedstock did not produce a higher power concentration than that of the mixed feedstock. However, it was proved that mixed anaerobic cultures are suitable and able to break down complex substrates for electricity generation [182].
The major disadvantage of using OFMSW as a substrate for bacterial consumption is the lack of uniformity in substrate composition, which is required by the bacteria [146]. OFMSW contains mainly carbohydrates, proteins and fats, which all may influence the efficiency of MECs and MFCs. If OFMSW is to be considered a feedstock for MEC, the impact of compositional variations on bacterial function and subsequent hydrogen productivity must be further studied.
Aside from biohydrogen production, MEC holds great potential in the recovery and production of value-added bio-products. A. Kadier et al. [81] investigated innovative possibilities of utilizing MEC and MFC to convert biomass into bioenergy. Among those discussed in the review is ammonium recovery from human urine, which can produce ammonium in wastewater treatment, thereby reducing the downstream costs of wastewater treatment facilities. In addition, MEC has been proposed to recover metals from wastes through energy-efficient means, recovering various metals such as Cu, Cd, Ag, Ni and Fe [81,202].
4. Current Limitations and Improvement to MECs
MEC technology has been validated on a laboratory scale, as proven by most of the articles summarized above. This section highlights some of the most critical limitations of the MEC before upscaling. Some studies have investigated the upscaling of MEC technology and design for the pilot scale, often operated in volumes of 10–1000 L under multiple research objectives and with varying degrees of success [48,192,203,204,205].
A successful MEC upscale is measurable when the bottlenecks from the laboratory experiments have been addressed prior to industrial applications. Four studies by L. Singh et al. (2021) [180], P. Dange et al. (2021) [146], Kadier et al. (2016) [173] and K. Guo et al. (2017) [176] all proved that upscaling from smaller MECs into larger ranges of 1–10 L was possible, thereby creating a foundation for pilot-scale MEC. However, successful laboratory-scale results are often inconsistent with those reported in larger-scale systems, as the current density, production rate and efficiency often decrease as the dimensions of the system increase [206].
The MECs found to be scalable are far from industrially applicable, as 10 L setups still need significant upscaling. Models have proved to be reliable in predicting upscaled current density, voltage and hydrogen productivity. However, it should be noted that the model is based on chemically defined media; therefore, it is not assured that the model’s predictability can be extended to utilize complex feed streams. Thus, reliable models enabling precise predictions are needed to decrease the cost of developing larger-scale MECs, which has not been conducted yet.
The main problems associated with scaling and biohydrogen productivity are biohydrogen loss due to methanogens, the operational and design configuration and a lack of understanding of microbial constraints, which need to be solved before further upscaling. These problems are not necessarily found in small-scale MECs, but they are profound when graduating to the pilot scale, where the scaling factors increase the risks. Methanogens could prove to be one of the current limitations, as methanogens were found in many of the pilot-scale MECs, cf. Table 6. These included MECs separated by a membrane, which were found to provide the highest purity of hydrogen on a laboratory scale. However, in a pilot-scale MEC, the architectures and increased complexity lead to methane formation at the cathodes.

Table 6.
Summary of some pilot-scale MEC configurations, their anode/cathode materials, the feedstock used, their performance, their limitations and their problems connected with scaling. COD: chemical oxygen demand; TOC: total organic carbon.
Other problems include electrode distances and optimal architecture of the MEC, the utilization of heterogeneous feedstock [207], leading to decreased feedstock conductivity, and biohydrogen loss while running and maintaining a larger facility [81], all of which lead to decreased current densities and biohydrogen production. The ideal spacing between electrodes and increasing the surface-area-to-reactor-volume ratio can be solved by implementing a simpler design architecture that is more susceptible to proportional scaling. A more straightforward design could decrease the cost of manufacturing due to non-expensive materials and primitive framework, allowing for easier maintenance [204]. As reviewed earlier, the one-chamber configuration is thought to have the best possibility for industrial scaling, allowing easier configuration for continuous production and lowering the cost.
Connecting multiple MEC in a series seems to provide the highest hydrogen productivity, although some setups still encounter problems with internal resistances, cf. Table 6. Thus, an exploration into reducing internal resistances by modifying architectural MEC designs could lead to hydrogen productivity in ranges closer to the theoretical maximal. Industrial-sized upscaling is a continuous debate between optimal and high-performing solutions and low-cost operations. The cost may be reduced by using low-cost alternative catalysts, such as implementing biocathodes, which can replace platinum as the typical expensive cathode catalyst. The cost of MEC biohydrogen production is nearly ten times higher compared to alkaline water electrolysis, which may be the main reason for retaining the technology as an industrial application [206]. The extensive cost is mainly due to lower biohydrogen production rates, which are still below many accepted hydrogen production techniques [206].
As the MEC is operated anaerobically, the presence of methanogens such as the Methanobrevibacter species is inevitable when using mixed cultures, especially in industrial settings, as the sterilization of the feed stream would further decrease economic viability [39,48]. It is commonly seen that scaling from a small laboratory scale to the pilot scale increases the ratio of methane production through hydrogenotrophic methanogenesis [48]. Several strategies have been employed to inhibit methanogenesis in MECs, including physical, chemical and biological methods, such as temperature shock, low pH, carbonate limitation, methanogenic inhibitors and UV–IR radiation [206]. Adding chemical inhibitors may be effective, but it increases operational costs and could result in contamination. A study by J. A. Baeza (2017) [205] used a methanogenic inhibitor, sodium 2-bromoethanesulfonate, to chemically prevent methanogenic growth and activity, which proved to be successful [205].
Utilizing a membrane for separation of the anode/cathode and only utilizing microbial species in the anode were found by P. Clauwaert et al. (2009) [100] to eliminate methane production, but they have proven to be difficult in larger MECs. Thus, a one-chamber design can be modified to isolate the cathode from the mixed cultures, or methanogenic inhibitors can be added to the media to prevent hydrogenotrophic methanogenesis.
Some high-productivity two-chamber MEC designs have been constructed on an intermediate scale (see Table 5) but are confined to using platinum, and they could likely be improved if changed to a one-chamber setup. It is unlikely that a common architecture could apply to all different MEC applications, substrates and cultures, thus justifying that adjustments and testing are crucial for upscaling, leading to the importance of verifying methods of testing small-scale MECs. The inability to proportionally upscale from the laboratory to the pilot scale supports the idea of converting MEC by models into an estimation, where the behavior in large-scale applications is tested before implementation [180]. Therefore, several optimal conditions need to be identified to investigate the possibilities of further upscaling beyond 1000 L. Moreover, the importance of a well-defined feedstock may contribute to maintaining biohydrogen productivity during upscaling, as this parameter has already been identified as a cause for decreased productivity in pilot-scale MECs, cf. Table 6.
Economic Viability and Life Cycle Assessment
To assess the prospects and commercialization potential, economic viability has been evaluated and reviewed through techno-economic assessments (TEAs) along with Life Cycle Assessments (LCAs) [115,209]. Observing the MECs based on their techno-economic possibilities, two critical challenges have been identified: the materials of the electrode and the reactor design [210,211]. The main capital and operating costs were found to be the anode material cost (59%) and the electricity consumption (up to 69%), respectively [212]. Similarly, the exceeding material cost of the cathode catalyst has been evaluated, and studies have found that the biocathode may be a promising alternative to noble metals. This is due to its low cost, excellent stability and ecologically sound qualities [209,211,213]. Similarly, using nickel powder as a cathode catalyst allows for more cost-effective cathode components [214,215]. Other studies have concluded that, in double-chamber configurations, the membrane is the primary cost-sensitive component [82,115,214].
Thus, the significant capital costs associated with MECs are the main barrier to commercialization. In addition, hydrogen by itself can prove to be an essential technical barrier; if hydrogen is not well managed on the cathode side, it can give rise to undesired microorganisms that might limit the overall performance of the reactor [115,209].
In the future, cost-related optimization should be aimed toward developing low-cost reactors capable of competing with various energy-generating and wastewater-treatment technologies [211].
The reviewed LCA studies showed contradicting results, where some deduced that the environmental impacts from the construction phase of MEC were negligible, and others inferred that the construction phase championed GHG emissions [213,216,217].
MECs and MFCs are considered sustainable platforms for converting waste into energy due to their ability to eliminate organic matter from wastewater with simultaneous power or hydrogen generation [217,218,219]. The economic balance emphasizes that it is preferable to aim for high biogas productivity, since the gain from the biogas is ten-fold higher than that from hydrogen due to the high volume of produced biogas [209]. An environmental disadvantage to MECs is the global warming potential and smog creation potential elevated from electricity usage, inorganic chemical and glassware reactor production [220].
Additionally, if the carbon dioxide produced in the MEC is not utilized locally, it can be transferred into the national gas grid and transported over long distances to a different end location. This can reduce congestion in the electricity transportation grid by providing an interface between the gas and electricity grids. In turn, MECs could benefit from the lower energy price produced during low-demand periods. Nevertheless, as both energy production from renewable sources and the production and composition of WW are unpredictable, suitable control systems and strategies are indispensable if the two systems are to be combined efficiently [115,209].
Furthermore, given the amount of research in this scientific area, the technology is expected to soon develop cheaper materials and better microorganisms. As it is impractical to store or transport hydrogen at atmospheric pressure due to its low volumetric energy density, the energy required to compress and store the hydrogen should be incorporated into future LCAs and TEAs [115,214,217,219]. The evaluations presented herein are a small sample from a larger assessment pool that is investigated and broadened each year due to the dynamic nature of the bioelectrochemistry field.
5. Summary and Prospects
MEC technology provides solutions to some challenging problems today, including recovering energy from waste such as OFMSW, creating sustainable zero-emission fuel, and energy storage while purifying contaminated substrates, e.g., heavy metals, ammonia, and phosphate. Additionally, excess energy from renewable energy resources during low demand can be converted by MECs to biohydrogen, thereby preventing surplus energy from being unutilized.
The potential of biohydrogen produced is, to a great extent, governed by the feedstock used as the substrate. Most of the literature suggests that a low cost and ease of availability are the two critical factors that can influence the upscaling potential of MECs. Some waste streams might need to be purified in order to achieve a high HPR, which can potentially increase the operational cost of an MEC. OFMSW and wastewater are two of the most investigated and viable substrates utilized in MECs, contributing to the EU’s ambitions of achieving a circular bioeconomy. Both substrates exhibit variations in their composition, affecting the HPR potential and thus must be addressed in the design.
In addition to feedstock, the design parameters significantly impact the viability of MECs. Both anode/cathode materials and configurations have been identified as vital parameters for the high performance of an MEC. A large surface area and microbial stability are crucial for the anode in high-performing MECs. A metal current collector is often incorporated at the anode to enable good conductivity. Although carbon-based materials of 3D anodes are preferred on a laboratory scale, 3D anodes in larger MECs with substrates such as wastewater and OFMSW often clog, thus increasing the need for maintenance and reducing the life of anodes.
A novel design for 2D anodes remains a better choice on a large scale. A low-cost anode with surface treatment could be viable in viable large-scale MEC. Stainless steel has also been proposed as an anode and cathode due to its high conductivity and low price; however, the surface area should be enlarged to accommodate better bacterial adhesion.
Cathode materials exhibiting high catalytic properties are needed to ensure fast catalysis when upscaling an MEC. Platinum seems to be the obvious choice as a coating, but its high cost, low lifetime and environmental unfriendliness make it an unattractive option. Therefore, the search for an alternative with similar properties is necessary. Biocatalysts offer an exciting possibility; however, extensive research is needed to find cultures and materials for adhesion. Findings from the literature suggest the need for favorable conditions for fast catalysis, stable output, durable cathodes and fast biofilm formation. Nickel alloys are also a low-cost material used as cathode. Both nickel alloys and biocatalysts have been found to match the platinum-based counterpart in their performance and can potentially be expanded with further research.
Methanogens can be inhibited by utilizing a two-chamber configuration, but this does impose larger overpotential in the system and does make the configuration more costly, which is not preferred for upscaling. One-chamber configurations were reviewed to be the most viable configuration for industrial applications; however, they have the drawback of less customizability and the chance of cultivating methanogens, thus contaminating biohydrogen with methane.
Microbial species also affect the overall success of the MEC. Many studies could benefit from better control of microbial species, especially when using mixed cultures originating from, e.g., wastewater or similar sources. Most studies reported preferring mixed cultures found naturally occurring in the chosen substrate. They resulted in similar or higher HPR, but it could also lead to methanogen production in one-chamber MECs. Some studies reported decreasing the initial waiting period by acclimatizing the electrodes with a known electrogenic bacteria, which improved suitability for large-scale MECs.
Some problems associated with upscaling include biohydrogen loss, the presence of methanogens, operation and design cost, microbial constraints, electrode distances, optimal architecture and low-cost feedstock. The one-chamber configuration was the most viable larger-scale MEC with the potential for further expansion. However, some design features, such as optimal electrode spacing, electrode surface-area-to-reactor-volume ratio and the cultures’ adaption towards an industrial feedstock, such as OFMSW or wastewater, need to be optimized before MEC can gain a foothold. Furthermore, reliable tools for the predictive modeling of MEC can provide the intermediate step between laboratory-scale and industrial-scale designs.
Hydrogen as an energy carrier has gained impetus driven by sustainable ambitions and directives from policymakers. MEC could become a key player in the green energy transition and has the potential to change the way the transportation industry operates. As things stand, MEC technology is expensive compared to other types of hydrogen production, such as conventional water hydrolysis. Focused research is needed to understand the ever-changing dynamics of mixed microbial cultures and their interactions with surfaces, along with the search for novel materials for anode/cathode driven by the intent to commercialize and upscale. Addressing the discussed limitations could be the key to implementing viable MEC technology in industrial settings.
In summary, MEC holds potential as a wastewater treatment process while simultaneously producing sustainable biohydrogen. MEC can utilize differing feedstock only limited by complex substrates, such as starch, which may need further research. MEC has been operated successfully both on a laboratory scale and pilot scale and needs to advance.
Furthermore, for its technological evolution, the design and architecture of the anode/cathode materials in the MEC and their placement in the MEC chamber remain critical for obtaining optimal biohydrogen production.
Author Contributions
C.K., L.S.J., T.C., N.B.J. and M.H.T. conceptualized and planned the structure of the manuscript. C.K. and L.S.J. performed the literature search and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the Flexi Green Fuels Project of the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 101007130.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, Y. How Population Growth Impacts Energy Consumption in Guangdong in China; Research Features: Stonehouse, UK, 2019. [Google Scholar]
- Hopfenberg, R.; Pimentel, D. Human Population Numbers as a Function of Food Supply. Environ. Dev. Sustain. 2001, 3, 1–15. [Google Scholar] [CrossRef]
- Shindell, D. Global Methane Assessment: Benefits and Costs of Mitigating Methane Emissions; Climate & Clean Air Coalition: Paris, France, 2021. [Google Scholar]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.M.B.; Miller, H.L., Jr. Climate Change the Physical Science Basis; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colón, J.; Ponsá, S.; Al-Mansour, F.; et al. Municipal Solid Waste Management and Waste-to-Energy in the Context of a Circular Economy and Energy Recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. 4.24 Hydrogen Energy Conversion Systems. In Comprehensive Energy Systems; Elsevier: Oxford, UK, 2018; Volume 4, pp. 947–984. ISBN 978-0-12-814925-6. [Google Scholar]
- International Energy Agency. The Future of Hydrogen; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A Critical Review on Catalyst Design for Aqueous Phase Reforming. Int. J. Hydrogen Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Tak, S.S.; Shetye, O.; Muley, O.; Jaiswal, H.; Malik, S.N. Emerging Technologies for Hydrogen Production from Wastewater. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Brown, F.; Roberts, D. Green, Blue, Brown: The Colours of Hydrogen Explained. Available online: https://blog.csiro.au/green-blue-brown-hydrogen-explained/ (accessed on 5 October 2021).
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Daiyan, R.; MacGill, I.; Amal, R. Opportunities and Challenges for Renewable Power-to-X. ACS Energy Lett. 2020, 5, 3843–3847. [Google Scholar] [CrossRef]
- International Energy Agency. Energy Technology Perspectivies 2020; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Lindstrand, N. Unlocking Ammonia’s Potential for Shipping. Available online: https://www.man-es.com/discover/two-stroke-ammonia-engine (accessed on 5 October 2021).
- International Energy Agency. Global Hydrogen Review 2021; International Energy Agency: Paris, France, 2021. [Google Scholar]
- European Union 2030 Climate Target Plan. Available online: https://ec.europa.eu/clima/eu-action/european-green-deal/2030-climate-target-plan_en (accessed on 7 October 2021).
- de Vasconcelos, B.; Lavoie, J.-M. Recent Advances in Power-to-X Technology for the Production of Fuels and Chemicals. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Thomas, G.; Parks, G. Potential Roles of Ammonia in a Hydrogen Economy; Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, 2006. [Google Scholar]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Amar, I.A.; Lan, R.; Petit, C.T.G.; Tao, S. Solid-State Electrochemical Synthesis of Ammonia: A Review. J. Solid State Electrochem. 2011, 15, 1845. [Google Scholar] [CrossRef]
- Tullo, A.H. Is Ammonia the Fuel of the Future? Chem. Eng. News 2021, 99, 20–22. [Google Scholar] [CrossRef]
- Minic, D.C.E.-D. Ammonia as a Hydrogen Source for Fuel Cells: A Review; IntechOpen: Rijeka, Croatia, 2012; p. 13. [Google Scholar]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and Related Chemicals as Potential Indirect Hydrogen Storage Materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chemie Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards Meeting Hydrogen-Powered Vehicle Requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef]
- Sartbaeva, A.; Kuznetsov, V.L.; Wells, S.A.; Edwards, P.P. Hydrogen Nexus in a Sustainable Energy Future. Energy Environ. Sci. 2008, 1, 79–85. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel Hydrogen Storage Materials: A Review of Lightweight Complex Hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zhang, L. Hydrogen Storage in Metal–Organic Frameworks. Adv. Mater. 2010, 22, E117–E130. [Google Scholar] [CrossRef]
- Graetz, J. New Approaches to Hydrogen Storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible Ammonia-Based and Liquid Organic Hydrogen Carriers for High-Density Hydrogen Storage: Recent Progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using Ammonia as a Sustainable Fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an Ammonia-Mediated Hydrogen Economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Ammonia as a Green Fuel and Hydrogen Source for Vehicular Applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Cotterill, S.; Heidrich, E.; Curtis, T. Microbial electrolysis cells for hydrogen production. Microb. Electrochem. Fuel Cells 2016, 287–319. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.-E. Microbial Fuel Cell as New Technology for Bioelectricity Generation: A Review. Alexandria Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alexandria Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Kadier, A.; Yuliasni, R.; Sapuan, S.; Ilyas, R.A.; Rai, P.K.; Ma, P.-C.; Rajasekar, A.; Alabbosh, K.; Hamid, A.; Abu Hasan, H. The Role of Microbial Electrolysis Cell in Bioenergy Production: Current Applications and Pilot Plant Experiences. In Bioelectrochemical Systems; Springer: Singapore, 2021; pp. 323–342. [Google Scholar] [CrossRef]
- Adekunle, A.; Raghavan, V.; Tartakovsky, B. A Comparison of Microbial Fuel Cell and Microbial Electrolysis Cell Biosensors for Real-Time Environmental Monitoring. Bioelectrochemistry 2019, 126, 105–112. [Google Scholar] [CrossRef]
- Kadier, A.; Chaurasia, A.K.; Sapuan, S.M.; Ilyas, R.A.; Ma, P.C.; Alabbosh, K.F.S.; Rai, P.K.; Logroño, W.; Hamid, A.A.; Abu Hasan, H. Essential Factors for Performance Improvement and the Implementation of Microbial Electrolysis Cells (MECs). In Bioelectrochemical Systems; Springer: Singapore, 2021; pp. 139–168. ISBN 978-981-15-6871-8. [Google Scholar]
- Lim, S.S.; Fontmorin, J.-M.; Izadi, P.; Wan Daud, W.R.; Scott, K.; Yu, E.H. Impact of Applied Cell Voltage on the Performance of a Microbial Electrolysis Cell Fully Catalysed by Microorganisms. Int. J. Hydrogen Energy 2020, 45, 2557–2568. [Google Scholar] [CrossRef]
- Katuri, K.P.; Ali, M.; Saikaly, P.E. The Role of Microbial Electrolysis Cell in Urban Wastewater Treatment: Integration Options, Challenges, and Prospects. Curr. Opin. Biotechnol. 2019, 57, 101–110. [Google Scholar] [CrossRef]
- Sacco, N.J.; Bonetto, M.C.; Cortón, E. Isolation and Characterization of a Novel Electrogenic Bacterium, Dietzia Sp. RNV-4. PLoS ONE 2017, 12, e0169955. [Google Scholar] [CrossRef]
- Chabert, N.; Amin Ali, O.; Achouak, W. All Ecosystems Potentially Host Electrogenic Bacteria. Bioelectrochemistry 2015, 106, 88–96. [Google Scholar] [CrossRef]
- Call, D.F.; Wagner, R.C.; Logan, B.E. Hydrogen Production by Geobacter Species and a Mixed Consortium in a Microbial Electrolysis Cell. Appl. Environ. Microbiol. 2009, 75, 7579–7587. [Google Scholar] [CrossRef]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a Pilot-Scale Continuous Flow Microbial Electrolysis Cell Fed Winery Wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Almatouq, A.; Babatunde, A.O.; Khajah, M.; Webster, G.; Alfodari, M. Microbial Community Structure of Anode Electrodes in Microbial Fuel Cells and Microbial Electrolysis Cells. J. Water Process Eng. 2020, 34, 101140. [Google Scholar] [CrossRef]
- UniProt Proteomes-Geobacter Sp. (Strain M18). Available online: https://www.uniprot.org/proteomes/UP000001442 (accessed on 8 March 2021).
- Guo, Y.; Wang, J.; Shinde, S.; Wang, X.; Li, Y.; Dai, Y.; Ren, J.; Zhang, P.; Liu, X. Simultaneous Wastewater Treatment and Energy Harvesting in Microbial Fuel Cells: An Update on the Biocatalysts. RSC Adv. 2020, 10, 25874–25887. [Google Scholar] [CrossRef]
- Babauta, J.; Renslow, R.; Lewandowski, Z.; Beyenal, H. Electrochemically Active Biofilms: Facts and Fiction. A Review. Biofouling 2012, 28, 789–812. [Google Scholar] [CrossRef]
- Hari, A.R.; Venkidusamy, K.; Katuri, K.P.; Bagchi, S.; Saikaly, P.E. Temporal Microbial Community Dynamics in Microbial Electrolysis Cells–Influence of Acetate and Propionate Concentration. Front. Microbiol. 2017, 8, 1371. [Google Scholar] [CrossRef]
- Proteomes-Shewanella Sp. (Strain ANA-3). Available online: https://www.uniprot.org/proteomes/UP000002589 (accessed on 8 March 2021).
- Hodgson, D.M.; Smith, A.; Dahale, S.; Stratford, J.P.; Li, J.V.; Grüning, A.; Bushell, M.E.; Marchesi, J.R.; Avignone Rossa, C. Segregation of the Anodic Microbial Communities in a Microbial Fuel Cell Cascade. Front. Microbiol. 2016, 7, 699. [Google Scholar] [CrossRef]
- Tahernia, M.; Plotkin-Kaye, E.; Mohammadifar, M.; Gao, Y.; Oefelein, M.R.; Cook, L.C.; Choi, S. Characterization of Electrogenic Gut Bacteria. ACS Omega 2020, 5, 29439–29446. [Google Scholar] [CrossRef]
- Croese, E.; Jeremiasse, A.W.; Marshall, I.P.G.; Spormann, A.M.; Euverink, G.-J.W.; Geelhoed, J.S.; Stams, A.J.M.; Plugge, C.M. Influence of Setup and Carbon Source on the Bacterial Community of Biocathodes in Microbial Electrolysis Cells. Enzym. Microb. Technol. 2014, 61–62, 67–75. [Google Scholar] [CrossRef]
- Walker, C.B.; He, Z.; Yang, Z.K.; Ringbauer, J.A.; He, Q.; Zhou, J.; Voordouw, G.; Wall, J.D.; Arkin, A.P.; Hazen, T.C.; et al. The Electron Transfer System of Syntrophically Grown Desulfovibrio vulgaris. J. Bacteriol. 2009, 191, 5793–5801. [Google Scholar] [CrossRef]
- Figueiredo, G.G.O.; Lopes, V.R.; Romano, T.; Camara, M.C. Clostridium. In Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020; pp. 477–491. ISBN 978-0-12-823414-3. [Google Scholar]
- Juang, D.F.; Yang, P.C.; Lee, C.H.; Hsueh, S.C.; Kuo, T.H. Electrogenic Capabilities of Gram Negative and Gram Positive Bacteria in Microbial Fuel Cell Combined with Biological Wastewater Treatment. Int. J. Environ. Sci. Technol. 2011, 8, 781–792. [Google Scholar] [CrossRef]
- Milner, D.A.; Pecora, N.; Solomon, I.; Soong, T.R. (Eds.) Bacillus Species Infections. In Diagnostic Pathology: Infectious Diseases; Elsevier: Philadelphia, PA, USA, 2015; pp. II-2-2–II-2-5. ISBN 978-0-323-37677-8. [Google Scholar]
- Kamaraj, Y. Electrogenicity Assessment of Bacillus Subtilis and Bacillus Megaterium Using Microbial Fuel Cell Technology. Int. J. Appl. Res. 2015, 1, 435–438. [Google Scholar]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Pham, T.H.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Vanhaecke, L.; De Maeyer, K.; Höfte, M.; Verstraete, W.; Rabaey, K. Metabolites Produced by Pseudomonas Sp. Enable a Gram-Positive Bacterium to Achieve Extracellular Electron Transfer. Appl. Microbiol. Biotechnol. 2008, 77, 1119–1129. [Google Scholar] [CrossRef]
- Gatidou, G.; Samanides, C.G.; Fountoulakis, M.S.; Vyrides, I. Microbial Electrolysis Cell Coupled with Anaerobic Granular Sludge: A Novel Technology for Real Bilge Water Treatment. Chemosphere 2022, 296, 133988. [Google Scholar] [CrossRef]
- Zecchin, S.; Mueller, R.C.; Seifert, J.; Stingl, U.; Anantharaman, K.; von Bergen, M.; Cavalca, L.; Pester, M. Rice Paddy Nitrospirae Carry and Express Genes Related to Sulfate Respiration: Proposal of the New Genus “Candidatus Sulfobium”. Appl. Environ. Microbiol. 2018, 84, e02224-17. [Google Scholar] [CrossRef]
- Gobalakrishnan, R.; Bhuvaneswari, R. Microbial fuel cells potential of marine actinobacteria Actinoalloteichus sp. MHA15 from the Havelock island of the Andamans, India. Biotechnol. Res. Innov. 2019, 3, 144–158. [Google Scholar] [CrossRef]
- Hasany, M.; Mardanpour, M.M.; Yaghmaei, S. Biocatalysts in Microbial Electrolysis Cells: A Review. Int. J. Hydrogen Energy 2016, 41, 1477–1493. [Google Scholar] [CrossRef]
- Ye, J.-Y.; Pan, Y.; Wang, Y.; Wang, Y.-C. Enhanced Hydrogen Production of Rhodobacter Sphaeroides Promoted by Extracellular H+ of Halobacterium Salinarum. Ann. Microbiol. 2021, 71, 15. [Google Scholar] [CrossRef]
- Salar-Garcia, M.J.; Obata, O.; Kurt, H.; Chandran, K.; Greenman, J.; Ieropoulos, I.A. Impact of Inoculum Type on the Microbial Community and Power Performance of Urine-Fed Microbial Fuel Cells. Microorganisms 2020, 8, 1921. [Google Scholar] [CrossRef]
- Cadirci, B.H. An electricity production study by Rhodobacter sphaeroides. Int. J. Hydrogen Energy 2018, 43, 18001–18006. [Google Scholar] [CrossRef]
- Cho, Y.K.; Donohue, T.J.; Tejedor, I.; Anderson, M.A.; McMahon, K.D.; Noguera, D.R. Development of a Solar-Powered Microbial Fuel Cell. J. Appl. Microbiol. 2008, 104, 640–650. [Google Scholar] [CrossRef]
- Holmes, D.E.; Nevin, K.P.; Lovley, D.R. Comparison of 16S RRNA, NifD, RecA, GyrB, RpoB and FusA Genes within the Family Geobacteraceae Fam. Nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1591–1599. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Ren, N. Pyrosequencing Reveals Highly Diverse Microbial Communities in Microbial Electrolysis Cells Involved in Enhanced H2 Production from Waste Activated Sludge. Water Res. 2012, 46, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Uria, N.; Ferrera, I.; Mas, J. Electrochemical Performance and Microbial Community Profiles in Microbial Fuel Cells in Relation to Electron Transfer Mechanisms. BMC Microbiol. 2017, 17, 208. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the Anode of Microbial Fuel Cells: Pure Cultures versus Mixed Communities. Microb. Cell Fact. 2019, 18, 39. [Google Scholar] [CrossRef]
- Xie, X.; Yu, G.; Liu, N.; Bao, Z.; Criddle, C.S.; Cui, Y. Graphene–Sponges as High-Performance Low-Cost Anodes for Microbial Fuel Cells. Energy Environ. Sci. 2012, 5, 6862–6866. [Google Scholar] [CrossRef]
- Khilyas, I.V.; Sorokin, A.A.; Kiseleva, L.; Simpson, D.J.W.; Fedorovich, V.; Sharipova, M.R.; Kainuma, M.; Cohen, M.F.; Goryanin, I. Comparative Metagenomic Analysis of Electrogenic Microbial Communities in Differentially Inoculated Swine Wastewater-Fed Microbial Fuel Cells. Scientifica (Cairo) 2017, 2017, 7616359. [Google Scholar] [CrossRef]
- Yasri, N.; Roberts, E.P.L.; Gunasekaran, S. The Electrochemical Perspective of Bioelectrocatalytic Activities in Microbial Electrolysis and Microbial Fuel Cells. Energy Rep. 2019, 5, 1116–1136. [Google Scholar] [CrossRef]
- Aiyer, K.S. How Does Electron Transfer Occur in Microbial Fuel Cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef]
- Kadier, A.; Al-Shorgani, N.K.N.; Jadhav, D.A.; Sonawane, J.M.; Mathuriya, A.S.; Kalil, M.S.; Hasan, H.A.; Alabbosh, K.F.S. Microbial Electrolysis Cell (MEC): An Innovative Waste to Bioenergy and Value-Added By-product Technology. In Bioelectrosynthesis: Principles and Technologies for Value-Added Products; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 95–128. [Google Scholar] [CrossRef]
- Bajracharya, S.; ElMekawy, A.; Srikanth, S.; Pant, D. Cathodes for Microbial Fuel Cells. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing: Boston, MA, USA, 2016; pp. 179–213. ISBN 978-1-78242-375-1. [Google Scholar]
- Jeremiasse, A.W. Cathode Innovations for Enhanced H2 Production through Microbial Electrolysis; Wageningen University: Wageningen, The Netherlands, 2011. [Google Scholar]
- Velasquez-Orta, S.B.; Yu, E.; Katuri, K.P.; Head, I.M.; Curtis, T.P.; Scott, K. Evaluation of Hydrolysis and Fermentation Rates in Microbial Fuel Cells. Appl. Microbiol. Biotechnol. 2011, 90, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Montpart, N.; Baeza, M.; Baeza, J.A.; Guisasola, A. Low-Cost Fuel-Cell Based Sensor of Hydrogen Production in Lab Scale Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2016, 41, 20465–20472. [Google Scholar] [CrossRef]
- Rozenfeld, S.; Hirsch, L.O.; Gandu, B.; Farber, R.; Schechter, A.; Cahan, R. Improvement of Microbial Electrolysis Cell Activity by Using Anode Based on Combined Plasma-Pretreated Carbon Cloth and Stainless Steel. Energies 2019, 12, 1968. [Google Scholar] [CrossRef]
- Borole, A.P.; Lewis, A.J. Proton Transfer in Microbial Electrolysis Cells. Sustain. Energy Fuels 2017, 1, 725–736. [Google Scholar] [CrossRef]
- Li, C.; Cheng, S. Functional Group Surface Modifications for Enhancing the Formation and Performance of Exoelectrogenic Biofilms on the Anode of a Bioelectrochemical System. Crit. Rev. Biotechnol. 2019, 39, 1015–1030. [Google Scholar] [CrossRef]
- Böl, M.; Ehret, A.E.; Bolea Albero, A.; Hellriegel, J.; Krull, R. Recent Advances in Mechanical Characterisation of Biofilm and Their Significance for Material Modelling. Crit. Rev. Biotechnol. 2013, 33, 145–171. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering Materials and Biology to Boost Performance of Microbial Fuel Cells: A Critical Review. Energy Environ. Sci. 2008, 1, 417–429. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kumar, G.; Bakonyi, P.; Xu, K.; Zhao, Y. Microbial Electrolysis Cell Platform for Simultaneous Waste Biorefinery and Clean Electrofuels Generation: Current Situation, Challenges and Future Perspectives. Prog. Energy Combust. Sci. 2017, 63, 119–145. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Liu, Y.; Lee, H.; Ren, N. Treatment of Biodiesel Production Wastes with Simultaneous Electricity Generation Using a Single-Chamber Microbial Fuel Cell. Bioresour. Technol. 2011, 102, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Gu, W.; Zhou, Q.; Zhang, L.; Wei, C.; Yang, Q.; Li, D. Ammonia Oxidation and Denitrification in a Bio-Anode Single-Chambered Microbial Electrolysis Cell. Bioresour. Technol. 2020, 310, 123466. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Manuel, M.-F.; Wang, H.; Guiot, S.R. High Rate Membrane-Less Microbial Electrolysis Cell for Continuous Hydrogen Production. Int. J. Hydrogen Energy 2009, 34, 672–677. [Google Scholar] [CrossRef]
- Spiess, S.; Kucera, J.; Seelajaroen, H.; Sasiain, A.; Thallner, S.; Kremser, K.; Novak, D.; Guebitz, G.; Haberbauer, M. Impact of Carbon Felt Electrode Pretreatment on Anodic Biofilm Composition in Microbial Electrolysis Cells. Biosensors 2021, 11, 170. [Google Scholar] [CrossRef]
- Gandu, B.; Rozenfeld, S.; Ouaknin Hirsch, L.; Schechter, A.; Cahan, R. Immobilization of Bacterial Cells on Carbon-Cloth Anode Using Alginate for Hydrogen Generation in a Microbial Electrolysis Cell. J. Power Sources 2020, 455, 227986. [Google Scholar] [CrossRef]
- Chang, S.-H.; Huang, B.-Y.; Wan, T.-H.; Chen, J.-Z.; Chen, B.-Y. Surface Modification of Carbon Cloth Anodes for Microbial Fuel Cells Using Atmospheric-Pressure Plasma Jet Processed Reduced Graphene Oxides. RSC Adv. 2017, 7, 56433–56439. [Google Scholar] [CrossRef]
- Roubaud, E.; Lacroix, R.; Da Silva, S.; Etcheverry, L.; Bergel, A.; Basséguy, R.; Erable, B. Benchmarking of Industrial Synthetic Graphite Grades, Carbon Felt, and Carbon Cloth as Cost-Efficient Bioanode Materials for Domestic Wastewater Fed Microbial Electrolysis Cells. Front. Energy Res. 2019, 7, 106. [Google Scholar] [CrossRef]
- Clauwaert, P.; Verstraete, W. Methanogenesis in Membraneless Microbial Electrolysis Cells. Appl. Microbiol. Biotechnol. 2009, 82, 829–836. [Google Scholar] [CrossRef]
- Croese, E.; Keesman, K.J.; Widjaja-Greefkes, H.C.A.; Geelhoed, J.S.; Plugge, C.M.; Sleutels, T.H.J.A.; Stams, A.J.M.; Euverink, G.-J.W. Relating MEC Population Dynamics to Anode Performance from DGGE and Electrical Data. Syst. Appl. Microbiol. 2013, 36, 408–416. [Google Scholar] [CrossRef]
- Masoudi, M.; Rahimnejad, M.; Mashkour, M. Fabrication of Anode Electrode by a Novel Acrylic Based Graphite Paint on Stainless Steel Mesh and Investigating Biofilm Effect on Electrochemical Behavior of Anode in a Single Chamber Microbial Fuel Cell. Electrochim. Acta 2020, 344, 136168. [Google Scholar] [CrossRef]
- Todoroki, N.; Wadayama, T. Electrochemical Stability of Stainless-Steel-Made Anode for Alkaline Water Electrolysis: Surface Catalyst Nanostructures and Oxygen Evolution Overpotentials under Applying Potential Cycle Loading. Electrochem. Commun. 2021, 122, 106902. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Patil, S.A.; Ghosh, P.C.; Adeloju, S.B. Low-Cost Stainless-Steel Wool Anodes Modified with Polyaniline and Polypyrrole for High-Performance Microbial Fuel Cells. J. Power Sources 2018, 379, 103–114. [Google Scholar] [CrossRef]
- Tahir, K.; Miran, W.; Jang, J.; Maile, N.; Shahzad, A.; Moztahida, M.; Ghani, A.A.; Kim, B.; Jeon, H.; Lim, S.-R.; et al. Nickel Ferrite/MXene-Coated Carbon Felt Anodes for Enhanced Microbial Fuel Cell Performance. Chemosphere 2021, 268, 128784. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Barua, S.; Sharaf, A.; Liu, Y.; Dhar, B.R. Impact of Antimicrobial Silver Nanoparticles on Anode Respiring Bacteria in a Microbial Electrolysis Cell. Chemosphere 2018, 213, 259–267. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Mustafa, M. Review on Material and Design of Anode for Microbial Fuel Cell. Energies 2022, 15, 2283. [Google Scholar] [CrossRef]
- Zhu, X.; Logan, B.E. Copper anode corrosion affects power generation in microbial fuel cells. J. Chem. Technol. Biotechnol. 2013, 89, 471–474. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, T.; Dong, Z.; Wang, Y. Modification of Carbon Felt Anodes Using Double-Oxidant HNO3/H2O2 for Application in Microbial Fuel Cells. RSC Adv. 2018, 8, 2059–2064. [Google Scholar] [CrossRef]
- Hidalgo, D.; Tommasi, T.; Bocchini, S.; Chiolerio, A.; Chiodoni, A.; Mazzarino, I.; Ruggeri, B. Surface Modification of Commercial Carbon Felt Used as Anode for Microbial Fuel Cells. Energy 2016, 99, 193–201. [Google Scholar] [CrossRef]
- Baek, G.; Saikaly, P.E.; Logan, B.E. Addition of a Carbon Fiber Brush Improves Anaerobic Digestion Compared to External Voltage Application. Water Res. 2021, 188, 116575. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of Carbon Mesh Anodes and the Effect of Different Pretreatment Methods on Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6870–6874. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-F.; Zhang, F.; Huang, B.-C.; Yu, H.-Q. Enhancing Electricity Generation of Microbial Fuel Cell for Wastewater Treatment Using Nitrogen-Doped Carbon Dots-Supported Carbon Paper Anode. J. Clean. Prod. 2019, 229, 412–419. [Google Scholar] [CrossRef]
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The Effects of Carbon Electrode Surface Properties on Bacteria Attachment and Start up Time of Microbial Fuel Cells. Carbon N. Y. 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial Electrolysis Cells: An Emerging Technology for Wastewater Treatment and Energy Recovery. From Laboratory to Pilot Plant and Beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Yaashikaa, P.R.; Jeevanantham, S.; Gayathri, B. Microbial Electrolysis Cells and Microbial Fuel Cells for Biohydrogen Production: Current Advances and Emerging Challenges. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Michaelidou, U.; ter Heijne, A.; Euverink, G.J.W.; Hamelers, H.V.M.; Stams, A.J.M.; Geelhoed, J.S. Microbial Communities and Electrochemical Performance of Titanium-Based Anodic Electrodes in a Microbial Fuel Cell. Appl. Environ. Microbiol. 2011, 77, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Kim, J.H.; Koo, K.; Kim, S. A Photo-Assisted Microbial Electrolysis Cell for the Exclusive Biohydrogen Production Using a MoS2-Coated p-Type Copper Oxide. J. Power Sources 2018, 373, 79–84. [Google Scholar] [CrossRef]
- Purushotham, K.G.; Sendilvelan, S. Analysis of Microbial Fuel Cell for Energy Harvesting with Waste Water and Molasses. Int. J. Pharma Bio Sci. 2017, 8, 63–69. [Google Scholar]
- Fuel Cell Store. Available online: https://www.fuelcellstore.com/isomolded-plate-024 (accessed on 7 October 2021).
- Delord, B.; Neri, W.; Bertaux, K.; Derre, A.; Ly, I.; Mano, N.; Poulin, P. Carbon Nanotube Fiber Mats for Microbial Fuel Cell Electrodes. Bioresour. Technol. 2017, 243, 1227–1231. [Google Scholar] [CrossRef]
- Amazon Microbial Fuel Cell Carbon Brush. Available online: https://www.amazon.com/Microbial-carbon-brush-conductive-5cm10cm15cm(LHW)/dp/B08154Z4C4 (accessed on 7 October 2021).
- Wei, J.; Liang, P.; Huang, X. Recent Progress in Electrodes for Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Wu, X.; Qiao, Y.; Guo, C.; Shi, Z.; Li, C.M. Nitrogen Doping to Atomically Match Reaction Sites in Microbial Fuel Cells. Commun. Chem. 2020, 3, 68. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. High Hydrogen Production Rate of Microbial Electrolysis Cell (MEC) with Reduced Electrode Spacing. Bioresour. Technol. 2011, 102, 3571–3574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.G.; Sarathi, V.G.S.; Nahm, K.S. Recent Advances and Challenges in the Anode Architecture and Their Modifications for the Applications of Microbial Fuel Cells. Biosens. Bioelectron. 2013, 43, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, E.; Erable, B.; De Solan, M.-L.; Bergel, A. Two-Dimensional Carbon Cloth and Three-Dimensional Carbon Felt Perform Similarly to Form Bioanode Fed with Food Waste. Electrochem. Commun. 2016, 66, 38–41. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless Steel Is a Promising Electrode Material for Anodes of Microbial Fuel Cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Ammonia Treatment of Carbon Cloth Anodes to Enhance Power Generation of Microbial Fuel Cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Hu, H.; Fan, Y.; Liu, H. Hydrogen Production Using Single-Chamber Membrane-Free Microbial Electrolysis Cells. Water Res. 2008, 42, 4172–4178. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial Electrolysis Cell with a Microbial Biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Carmona-Martínez, A.A.; Bakar, M.H.A.; Jahim, J.M.; Ismail, M. A Comprehensive Study on Development of a Biocathode for Cleaner Production of Hydrogen in a Microbial Electrolysis Cell. J. Clean. Prod. 2017, 164, 1135–1144. [Google Scholar] [CrossRef]
- Call, D.; Logan, B. A Method for High Throughput Bioelectrochemical Research Based on Small Scale Microbial Electrolysis Cells. Biosens. Bioelectron. 2011, 26, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, A.; Witarsa, F.; Guo, X.; Yong, L.; Lansing, S.; Qiu, L. Next Generation Digestion: Complementing Anaerobic Digestion (AD) with a Novel Microbial Electrolysis Cell (MEC) Design. Int. J. Hydrogen Energy 2017, 42, 28681–28689. [Google Scholar] [CrossRef]
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Jain, A.; Aguilera, A.; He, Z. Effective Control of Biohythane Composition through Operational Strategies in an Innovative Microbial Electrolysis Cell. Appl. Energy 2017, 206, 879–886. [Google Scholar] [CrossRef]
- Lanas, V.; Ahn, Y.; Logan, B.E. Effects of Carbon Brush Anode Size and Loading on Microbial Fuel Cell Performance in Batch and Continuous Mode. J. Power Sources 2014, 247, 228–234. [Google Scholar] [CrossRef]
- Li, L.; Jiang, B.; Tang, D.; Zhang, X.; Yuan, K.; Zhang, Q. Alkaline Treatment of Used Carbon-Brush Anodes for Restoring Power Generation of Microbial Fuel Cells. RSC Adv. 2018, 8, 36754–36760. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Sayed, E.T.; Obaid, M.; Choi, Y.-J.; Park, S.-G.; Al-Qaradawi, S.; Chae, K.-J. Transition Metal Nanoparticles Doped Carbon Paper as a Cost-Effective Anode in a Microbial Fuel Cell Powered by Pure and Mixed Biocatalyst Cultures. Int. J. Hydrogen Energy 2018, 43, 21560–21571. [Google Scholar] [CrossRef]
- Jiang, D.; Li, B. Granular Activated Carbon Single-Chamber Microbial Fuel Cells (GAC-SCMFCs): A Design Suitable for Large-Scale Wastewater Treatment Processes. Biochem. Eng. J. 2009, 47, 31–37. [Google Scholar] [CrossRef]
- Mu, Y.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Decolorization of Azo Dyes in Bioelectrochemical Systems. Environ. Sci. Technol. 2009, 43, 5137–5143. [Google Scholar] [CrossRef]
- Abbas, A.A.; Farrag, H.H.; El-Sawy, E.; Allam, N.K. Microbial Fuel Cells with Enhanced Bacterial Catalytic Activity and Stability Using 3D Nanoporous Stainless Steel Anode. J. Clean. Prod. 2021, 285, 124816. [Google Scholar] [CrossRef]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Vilajeliu-Pons, A.; Bañeras, L.; Balaguer, M.D.; Colprim, J. Assessment of Biotic and Abiotic Graphite Cathodes for Hydrogen Production in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2014, 39, 1297–1305. [Google Scholar] [CrossRef]
- Croese, E.; Pereira, M.A.; Euverink, G.-J.W.; Stams, A.J.M.; Geelhoed, J.S. Analysis of the Microbial Community of the Biocathode of a Hydrogen-Producing Microbial Electrolysis Cell. Appl. Microbiol. Biotechnol. 2011, 92, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Dange, P.; Pandit, S.; Jadhav, D.; Shanmugam, P.; Gupta, P.K.; Kumar, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K. Recent Developments in Microbial Electrolysis Cell-Based Biohydrogen Production Utilizing Wastewater as a Feedstock. Sustainability 2021, 13, 8796. [Google Scholar] [CrossRef]
- Tang, J.; Bian, Y.; Jin, S.; Sun, D.; Ren, Z.J. Cathode Material Development in the Past Decade for H2 Production from Microbial Electrolysis Cells. ACS Environ. Au 2022, 2, 20–29. [Google Scholar] [CrossRef]
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An Overview of Cathode Material and Catalysts Suitable for Generating Hydrogen in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757. [Google Scholar] [CrossRef]
- Wang, J.; Mu, K.; Zhao, X.; Luo, D.; Yu, X.; Li, W.; Chu, J.; Yang, J.; Yang, Q. Uniform Distribution of Pd on GO-C Catalysts for Enhancing the Performance of Air Cathode Microbial Fuel Cell. Catalysts 2021, 11, 888. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Saakes, M.; Buisman, C.J.N. Ni Foam Cathode Enables High Volumetric H2 Production in a Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2010, 35, 12716–12723. [Google Scholar] [CrossRef]
- Son, S.; Koo, B.; Chai, H.; Tran, H.V.H.; Pandit, S.; Jung, S.P. Comparison of Hydrogen Production and System Performance in a Microbial Electrolysis Cell Containing Cathodes Made of Non-Platinum Catalysts and Binders. J. Water Process Eng. 2021, 40, 101844. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; He, Z.; Guo, Z.; Zhou, A.; Wang, A. Cathodic Hydrogen Recovery and Methane Conversion Using Pt Coating 3D Nickel Foam Instead of Pt-Carbon Cloth in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2017, 42, 19604–19610. [Google Scholar] [CrossRef]
- Yan, Z.H.; Wang, M.; Huang, B.X.; Zhao, J.S.; Liu, R.M. Carboxyl Multi-Wall Carbon Nanotubes Supported Pt-Ni Alloy Nanoparticles as Cathode Catalyst for Microbial Fuel Cells. Int. J. Electrochem. Sci. 2012, 7, 10825–10834. [Google Scholar]
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High Power Density Microbial Fuel Cell with Flexible 3D Graphene–Nickel Foam as Anode. Nanoscale 2013, 5, 10283–10290. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Krishnaraj, N.; Selvam, A.; Wong, J.W.-C.; Lee, P.K.H.; Leung, M.K.; Berchmans, S. Effect of composites based nickel foam anode in microbial fuel cell using Acetobacter aceti and Gluconobacter roseus as a biocatalysts. Bioresour. Technol. 2016, 217, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, B.; Jeon, Y.; Zhong, S.; Zhou, S.; Kim, S. Iron Phthalocyanine Supported on Amino-Functionalized Multi-Walled Carbon Nanotube as an Alternative Cathodic Oxygen Catalyst in Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 5849–5854. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-W.; Sun, X.-F.; Huang, Y.-X.; Sheng, G.-P.; Zhou, K.; Zeng, R.J.; Dong, F.; Wang, S.-G.; Xu, A.-W.; Tong, Z.-H.; et al. Nano-Structured Manganese Oxide as a Cathodic Catalyst for Enhanced Oxygen Reduction in a Microbial Fuel Cell Fed with a Synthetic Wastewater. Water Res. 2010, 44, 5298–5305. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Ramírez, J.M.; Campos-Cornelio, M.L.; Uribe Godínez, J.; Borja-Arco, E.; Castellanos, R.H. Studies on the Hydrogen Evolution Reaction on Different Stainless Steels. Int. J. Hydrogen Energy 2007, 32, 3170–3173. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Habas, S.E.; Schaidle, J.A.; Logan, B.E. Application of Phase-Pure Nickel Phosphide Nanoparticles as Cathode Catalysts for Hydrogen Production in Microbial Electrolysis Cells. Bioresour. Technol. 2019, 293, 122067. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.F.; Chang, I.-S.; Kim, I.S. Selective Inhibition of Methanogens for the Improvement of Biohydrogen Production in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2010, 35, 13379–13386. [Google Scholar] [CrossRef]
- Park, S.-G.; Chae, K.-J.; Lee, M. A Sulfonated Poly(Arylene Ether Sulfone)/Polyimide Nanofiber Composite Proton Exchange Membrane for Microbial Electrolysis Cell Application under the Coexistence of Diverse Competitive Cations and Protons. J. Memb. Sci. 2017, 540, 165–173. [Google Scholar] [CrossRef]
- Zamora, P.; Georgieva, T.; Ter Heijne, A.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Saakes, M.; Buisman, C.J.N.; Kuntke, P. Ammonia Recovery from Urine in a Scaled-up Microbial Electrolysis Cell. J. Power Sources 2017, 356, 491–499. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Xie, T.; Ren, N.; Logan, B.E. Hydrogen Production from Proteins via Electrohydrogenesis in Microbial Electrolysis Cells. Biosens. Bioelectron. 2010, 25, 2690–2695. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lin, Y.; Ma, H.; Jia, H.; Liu, X.; Lin, J. Preparation of Sulfonated Poly(Ether Ether Ketone) (SPEEK) Membrane Using Ethanol/Water Mixed Solvent. Mater. Lett. 2016, 169, 69–72. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Hamelers, H.V.M.; Rozendal, R.A.; Buisman, C.J.N. Ion Transport Resistance in Microbial Electrolysis Cells with Anion and Cation Exchange Membranes. Int. J. Hydrogen Energy 2009, 34, 3612–3620. [Google Scholar] [CrossRef]
- Radhika, D.; Shivakumar, A.; Kasai, D.; Koutavarapu, R.; Peera, G. Microbial Electrolysis Cell as a Diverse Technology: Overview of Prospective Applications, Advancements, and Challenges. Energies 2022, 15, 2611. [Google Scholar] [CrossRef]
- Zuo, Y.; Cheng, S.; Logan, B. Ion Exchange Membrane Cathodes for Scalable Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 6967–6972. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Z.; Ren, S.; Han, D.; Xiao, M.; Wang, S.; Meng, Y. A Robust Composite Proton Exchange Membrane of Sulfonated Poly (Fluorenyl Ether Ketone) with an Electrospun Polyimide Mat for Direct Methanol Fuel Cells Application. Polymers 2021, 13, 523. [Google Scholar] [CrossRef]
- Amrut Pawar, A.; Karthic, A.; Lee, S.; Pandit, S.; Jung, S.P. Microbial Electrolysis Cells for Electromethanogenesis: Materials, Configurations and Operations. Environ. Eng. Res. 2022, 27, 200480–200484. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effect of the Type of Ion Exchange Membrane on Performance, Ion Transport, and PH in Biocatalyzed Electrolysis of Wastewater. Water Sci. Technol. 2008, 57, 1757–1762. [Google Scholar] [CrossRef]
- Zhao, W.; Ci, S. Nanomaterials As Electrode Materials of Microbial Electrolysis Cells for Hydrogen Generation. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–242. ISBN 978-0-12-814837-2. [Google Scholar]
- Kadier, A.; Kalil, M.; Abdeshahian, P.; Kuppam, C.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A. Recent Advances and Emerging Challenges in Microbial Electrolysis Cells (MECs) for Microbial Production of Hydrogen and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Liu, B.; Ren, N. Enhanced Hydrogen Production from Waste Activated Sludge by Cascade Utilization of Organic Matter in Microbial Electrolysis Cells. Water Res. 2012, 46, 1015–1026. [Google Scholar] [CrossRef]
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-Stage Conversion of Crude Glycerol to Energy Using Dark Fermentation Linked with Microbial Fuel Cell or Microbial Electrolysis Cell. N. Biotechnol. 2014, 31, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Prévoteau, A.; Rabaey, K. A Novel Tubular Microbial Electrolysis Cell for High Rate Hydrogen Production. J. Power Sources 2017, 356, 484–490. [Google Scholar] [CrossRef]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the Internal Resistance Distribution of Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Anaerobic Digestion and Electromethanogenic Microbial Electrolysis Cell Integrated System: Increased Stability and Recovery of Ammonia and Methane. Renew. Energy 2018, 120, 178–189. [Google Scholar] [CrossRef]
- Ferraren-De Cagalitan, D.D.T.; Abundo, M.L.S. A Review of Biohydrogen Production Technology for Application towards Hydrogen Fuel Cells. Renew. Sustain. Energy Rev. 2021, 151, 111413. [Google Scholar] [CrossRef]
- Singh, L.; Miller, A.G.; Wang, L.; Liu, H. Scaling-up up-Flow Microbial Electrolysis Cells with a Compact Electrode Configuration for Continuous Hydrogen Production. Bioresour. Technol. 2021, 331, 125030. [Google Scholar] [CrossRef]
- Selembo, P.A.; Perez, J.M.; Lloyd, W.A.; Logan, B.E. High Hydrogen Production from Glycerol or Glucose by Electrohydrogenesis Using Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2009, 34, 5373–5381. [Google Scholar] [CrossRef]
- Pendyala, B.; Chaganti, S.R.; Lalman, J.A.; Heath, D.D. Optimizing the Performance of Microbial Fuel Cells Fed a Combination of Different Synthetic Organic Fractions in Municipal Solid Waste. Waste Manag. 2016, 49, 73–82. [Google Scholar] [CrossRef]
- Damiano, L. Electricity Production from the Management of Municipal Solid Waste Leachate with Microbial Fuel Cells. Master’s Thesis, University of New Hampshire, Durham, NH, USA, 2009. [Google Scholar]
- Wang, W.; Zhang, B.; He, Z. Bioelectrochemical Deposition of Palladium Nanoparticles as Catalysts by Shewanella Oneidensis MR-1 towards Enhanced Hydrogen Production in Microbial Electrolysis Cells. Electrochim. Acta 2019, 318, 794–800. [Google Scholar] [CrossRef]
- Patil, K.; Kulkarni, B. Review of Recovery Methods for Acetic Acid from Industrial Waste Streams by Reactive Distillation. J. Water Pollut. Purif. Res. 2014, 1, 1–6. [Google Scholar]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Chavez, D. Solid Waste Management; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Li, S.; Gang, C. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries 2018, 4, 34. [Google Scholar] [CrossRef]
- Hassan, M.; Fernandez, A.S.; Martin, I.S.; Xie, B.; Moran, A. Hydrogen evolution in microbial electrolysis cells treating landfill leachate: Dynamics of anodic biofilm. Int. J. Hydrogen Energy 2018, 43, 13051–13063. [Google Scholar] [CrossRef]
- Sonawane, J.; Adeloju, S.B.; Ghosh, P.C. Landfill leachate: A promising substrate for microbial fuel cells. Int. J. Hydrogen Energy 2017, 42, 23794–23798. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A Monetary Comparison of Energy Recovered from Microbial Fuel Cells and Microbial Electrolysis Cells Fed Winery or Domestic Wastewaters. Int. J. Hydrogen Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of Hydrogen from Domestic Wastewater in a Pilot-Scale Microbial Electrolysis Cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity Generation from Swine Wastewater Using Microbial Fuel Cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef]
- Escapa, A.; Gil-Carrera, L.; García, V.; Morán, A. Performance of a Continuous Flow Microbial Electrolysis Cell (MEC) Fed with Domestic Wastewater. Bioresour. Technol. 2012, 117, 55–62. [Google Scholar] [CrossRef]
- Escapa, A.; Gómez, X.; Tartakovsky, B.; Morán, A. Estimating Microbial Electrolysis Cell (MEC) Investment Costs in Wastewater Treatment Plants: Case Study. Int. J. Hydrogen Energy 2012, 37, 18641–18653. [Google Scholar] [CrossRef]
- Fudge, T.; Bulmer, I.; Bowman, K.; Pathmakanthan, S.; Gambier, W.; Dehouche, Z.; Al-Salem, S.M.; Constantinou, A. Microbial Electrolysis Cells for Decentralised Wastewater Treatment: The Next Steps. Water 2021, 13, 445. [Google Scholar] [CrossRef]
- Guo, H.; Kim, Y. Stacked multi-electrode design of microbial electrolysis cells for rapid and low-sludge treatment of municipal wastewater. Biotechnol. Biofuels 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Jain, P.; Lai, B.; Kalil, M.S.; Kondaveeti, S.; Alabbosh, K.F.S.; Abu-Reesh, I.M.; Mohanakrishna, G. Biorefinery Perspectives of Microbial Electrolysis Cells (MECs) for Hydrogen and Valuable Chemicals Production through Wastewater Treatment. Biofuel Res. J. 2020, 7, 1128–1142. [Google Scholar] [CrossRef]
- Gurjar, R.; Behera, M. Treatment of Organic Fraction of Municipal Solid Waste in Bioelectrochemical Systems: A Review. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020018. [Google Scholar] [CrossRef]
- Karluvalı, A.; Köroğlu, E.O.; Manav, N.; Çetinkaya, A.Y.; Özkaya, B. Electricity Generation from Organic Fraction of Municipal Solid Wastes in Tubular Microbial Fuel Cell. Sep. Purif. Technol. 2015, 156, 502–511. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Mathur, S.; Balan, V.; Liao, W.; Pareek, N.; Vivekanand, V. Organic Fraction of Municipal Solid Waste: Overview of Treatment Methodologies to Enhance Anaerobic Biodegradability. Front. Energy Res. 2018, 6, 75. [Google Scholar] [CrossRef]
- Yu, E.H. Resource Recovery with Microbial Electrochemical Systems. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing: Boston, MA, USA, 2016; pp. 321–339. ISBN 978-1-78242-375-1. [Google Scholar]
- Cotterill, S.E.; Dolfing, J.; Jones, C.; Curtis, T.P.; Heidrich, E.S. Low Temperature Domestic Wastewater Treatment in a Microbial Electrolysis Cell with 1 M2 Anodes: Towards System Scale-Up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef]
- Isabel San-Martín, M.; Mateos, R.; Carracedo, B.; Escapa, A.; Morán, A. Pilot-Scale Bioelectrochemical System for Simultaneous Nitrogen and Carbon Removal in Urban Wastewater Treatment Plants. J. Biosci. Bioeng. 2018, 126, 758–763. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical Hydrogen Production from Urban Wastewater on a Pilot Scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.J. Microbial Electrolysis Cells for Waste Biorefinery: A State of the Art Review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef]
- Rousseau, R.; Ketep, S.F.; Etcheverry, L.; Délia, M.-L.; Bergel, A. Microbial Electrolysis Cell (MEC): A Step Ahead towards Hydrogen-Evolving Cathode Operated at High Current Density. Bioresour. Technol. Reports 2020, 9, 100399. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Sotres, A.; Alonso, R.M.; Díaz-Marcos, J.; Morán, A.; Escapa, A. Assessing Anodic Microbial Populations and Membrane Ageing in a Pilot Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2019, 44, 17304–17315. [Google Scholar] [CrossRef]
- Segundo-Aguilar, A.; González-Gutiérrez, L.V.; Payá, V.C.; Feliu, J.; Buitrón, G.; Cercado, B. Energy and Economic Advantages of Simultaneous Hydrogen and Biogas Production in Microbial Electrolysis Cells as a Function of the Applied Voltage and Biomass Content. Sustain. Energy Fuels 2021, 5, 2003–2017. [Google Scholar] [CrossRef]
- Koul, Y.; Devda, V.; Varjani, S.; Guo, W.; Ngo, H.H.; Taherzadeh, M.J.; Chang, J.-S.; Wong, J.W.C.; Bilal, M.; Kim, S.-H.; et al. Microbial Electrolysis: A Promising Approach for Treatment and Resource Recovery from Industrial Wastewater. Bioengineered 2022, 13, 8115–8134. [Google Scholar] [CrossRef]
- Stoll, Z.A.; Ma, Z.; Trivedi, C.B.; Spear, J.R.; Xu, P. Sacrificing Power for More Cost-Effective Treatment: A Techno-Economic Approach for Engineering Microbial Fuel Cells. Chemosphere 2016, 161, 10–18. [Google Scholar] [CrossRef]
- Jourdin, L.; Sousa, J.; van Stralen, N.; Strik, D.P.B.T.B. Techno-Economic Assessment of Microbial Electrosynthesis from CO2 and/or Organics: An Interdisciplinary Roadmap towards Future Research and Application. Appl. Energy 2020, 279, 115775. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wu, X.; E, J.; Lu, N.; Wang, T.; Zuo, H. System Development and Environmental Performance Analysis of a Pilot Scale Microbial Electrolysis Cell for Hydrogen Production Using Urban Wastewater. Energy Convers. Manag. 2019, 193, 52–63. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Gallego, Y.A.; Diels, L.; Vanbroekhoven, K. An Introduction to the Life Cycle Assessment (LCA) of Bioelectrochemical Systems (BES) for Sustainable Energy and Product Generation: Relevance and Key Aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. Hydrogen Production with Nickel Powder Cathode Catalysts in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2010, 35, 428–437. [Google Scholar] [CrossRef]
- Shemfe, M.; Gadkari, S.; Yu, E.; Rasul, S.; Scott, K.; Head, I.M.; Gu, S.; Sadhukhan, J. Life Cycle, Techno-Economic and Dynamic Simulation Assessment of Bioelectrochemical Systems: A Case of Formic Acid Synthesis. Bioresour. Technol. 2018, 255, 39–49. [Google Scholar] [CrossRef]
- Foley, J.; Rozendal, R.; Hertle, C.; Lant, P.; Rabaey, K. Life Cycle Assessment of High-Rate Anaerobic Treatment, Microbial Fuel Cells, and Microbial Electrolysis Cells. Environ. Sci. Technol. 2010, 44, 3629–3637. [Google Scholar] [CrossRef]
- Savla, N.; Suman; Pandit, S.; Verma, J.P.; Awasthi, A.K.; Sana, S.S.; Prasad, R. Techno-Economical Evaluation and Life Cycle Assessment of Microbial Electrochemical Systems: A Review. Curr. Res. Green Sustain. Chem. 2021, 4, 100111. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Abu-Reesh, I.M.; He, Z.; Yuan, C. Life Cycle Environmental Impact Comparison of Bioelectrochemical Systems for Wastewater Treatment. Procedia CIRP 2019, 80, 382–388. [Google Scholar] [CrossRef]
- Öksüz, S. Life Cycle Assessment of Microbial Electrolysis Cells for Hydrogen Generation Using TRACI Methodology. Sak. Univ. J. Sci. 2022, 26, 620–632. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).







