Abstract
Low global warming potential (GWP) refrigerants for the next-generation air conditioning systems have been investigated with target domestic applications. High-GWP refrigerants are mostly used in climate control applications such as heating, ventilation and air conditioning (HVAC) and refrigeration systems. The majority of refrigerants are responsible for significant environmental issues such as ozone layer depletion and global warming. The Montreal Protocol and the Kyoto Protocol have been implemented to address such issues. In the meantime, authorities in many countries have taken the initiative to phase out the usage of environmentally harmful refrigerants in vapor compression refrigeration systems. Following the global warming mitigation scheme by many signatory countries, research interest has been focused on finding alternative refrigerants with low or ultra-low GWP. This study considered the research trend and development of low-GWP refrigerants while examining system performance, safety issues, and the equivalent environmental impact as the critical assessment parameters. Here, the focus is primarily set on the potential of refrigerant blends (HFCs + HFOs) where the GWP value of 300 is set as the threshold value. Targeted for domestic heat pump systems, the performance of such systems using various refrigerant blends is collated and discussed. Many blends offer innovative drop-in replacements for R410A-conforming F-gas regulations. The technical difficulties and realistic remedies for the existing refrigerants are also discussed.
1. Introduction
Refrigerants as working substances considerably influence the performance of air conditioning and refrigeration systems [1]. The thermal and physical properties of such refrigerants starting from the PVT relationships are crucial in designing efficient yet cost-effective heat pump systems. Apart from the excellent performance requirement, other characteristics such as flammability, toxicity, and, most importantly, environmental friendliness must be accounted for in ranking refrigerants. Most of the early-generation refrigerants, such as CFCs and HCFCs, offer favorable flammability and toxicity but unfavorable ozone depletion potential (ODP) and GWP characteristics. Despite excellent technological advancement in pushing the COP of the mechanical vapor compression cycle closer to the Carnot limit, the adverse impacts of refrigerants on the environment, especially the GWP, remain a huge concern. Following the Montreal Protocol (signed in 1987), a successive phase-out of refrigerants that are responsible for depleting the ozone layer has been carried out: they have been replaced with a new type of ozone-friendly refrigerants [2,3]. During this time, air conditioning and refrigeration industries have undergone and overcome many complications in developing appropriate refrigerants to replace CFC and HCFC refrigerants [4,5,6,7,8,9]. Notably, the developed countries achieved the CFCs phase-out target in 1996, though the deadline for the developing countries was extended to 2010. On the other hand, the HCFCs phase-out will be accomplished by 2030 [10]. During the 28th meeting of the Montreal Protocol, known as the Kigali Amendment (MOP28-2016), the Parties agreed to work on phasing out HFCs based on the compromise consensus of MOP28 targeting to avoid 0.5 °C global warming by the end of the century.
R134a, R1234yf, R410A, R407C, and other refrigerants are currently the best alternatives to ozone-depleting refrigerants (CFCs and HCFCs), especially for household and car heat pump applications. Table 1 shows the most commonly used refrigerants in home and automotive applications together with their ODP and GWP values.

Table 1.
Commonly used refrigerants in residential and automobile applications.
It can be noticed from Table 1 that the global warming potential (GWP) of these refrigerants is considerably high. For instance, hydrofluorocarbon refrigerants R410A and R134a have no ODP but high GWP values of 1300 and 1923, respectively. That is why their environmental effect is significant in case of leakage during maintenance. Though the GWP of R410A is high, it has good thermophysical properties, which are favorable for air conditioning applications. The Kyoto Protocol (signed in 1997, COP3) [33] implements the objective of the United Nations Framework Convention on Climate Change (UNFCCC 1992). In the first commitment period (2008 to 2012), the state Parties assured to decrease the global warming gas emission to the environment by at least 5% below 1990 levels. In 2012, most developed countries, including the European Union, approved the Doha amendment to the Kyoto Protocol to reduce greenhouse gas emissions by 18% by 2020. The European Environment Agency [34] reported that the emission of fluorinated gases, including HFCs, PFCs, and SF6, will decrease by two-thirds of its 2014 value by 2030. The European Union Mobile Air Conditioning Directive [35] inhibits fluorinated gases with the GWP greater than 150 in all new passenger cars and light commercial vehicles manufactured in 2017 and later. New vehicles using these gases will not be registered, sold, or entered into service in the EU region. The US Environmental Protection Agency (EPA) is implementing the Clean Air Act, which is similar to the Montreal Protocol. This legislation requires common sense when managing refrigerants, implying that refrigerants cannot be released into the atmosphere during equipment installation, service, or retirement. The EPA [36] planned to phase out R134a in newly factory-made light-duty vehicles in model year 2021, but it will no longer be accepted in the MVAC system after model year 2025. However, the commercial production of R134a for servicing older vehicles would not be stopped. The Australian government has committed to reduce the import of greenhouse gases by 85% between the years 2018 and 2036. It has already imposed a $50/kg carbon tax for R410A [37]. The Paris Agreement (2015) also wishes to keep the global average temperature well below 2 °C above the preindustrial levels by reducing greenhouse emissions while pursuing efforts to limit the temperature increase to 1.5 °C [38]. The plan for phasing out high-GWP gases in Japan and other countries is listed in Table 2.

Table 2.
Current GWP and target low-GWP refrigerants implementation plan [34,36,37,39,40].
The search for environment-friendly refrigerants with higher energy efficiency has emerged as a critical task. The overall impact of a refrigerant on our environment includes both direct and indirect emissions. Direct emissions include the refrigerant amount in the system and emissions due to leakages, servicing, and disposal, and indirect emissions are related to operational time and hardware manufacturing, emissions during electricity production, i.e., kg CO2-equivalent emissions generated per unit of electricity produced. Recognizing the importance of environmental commitments as well as the need to comply with international rules and regulations, the relevant industry and research community began looking for low-GWP refrigerants around 2011. We have a few low-GWP refrigerants in the market, but they are not suitable for replacing the existing halogenated refrigerants [41,42]. This study was concentrated on ultra-low and low-GWP refrigerants providing system performance comparable to or better than that of the existing refrigerants in residential air conditioning. GWP100 values are classified into five levels based on the RTOC 2014 taxonomy, which is shown in Table 3.

Table 3.
Classification of GWP100 levels [2].
2. Low-GWP Refrigerants (Pure and Blended)
2.1. Pure Refrigerants
2.1.1. R1234yf
The hydrofluoroolefin 2,3,3,3-tetrafluoro-1-propene (R1234yf; CF3CF=CH2) is an ultra-low-GWP refrigerant. It is mildly flammable (A2L) and low-toxic [43,44,45]. Compared to R134a and R744, it has zero ODP and excellent life cycle climate performance (LCCP) [46]. The refrigerant’s critical temperature and pressure are 94.7 °C and 3.38 MPa, respectively [47,48,49,50]. To address the safety and performance of R1234yf considering its application in mobile air conditioning, SAE International started the CRP 1234 program in 2007. It has received attention from the research community as a potential replacement candidate for R134a, the most commonly used refrigerant in vehicles [51,52,53,54,55]. The performance of R11234yf is found to be slightly lower than that of R134a [31,32,56,57]. General Motors introduced R1234yf for vehicles in 2012, and presently such vehicles have numbered more than 100,000 worldwide [30]. However, when researchers analyzed the performance of R1234yf to replace R410A, a widely used refrigerant in residential applications, they found the COP was lower and required larger unit bodies as compared to R410A [58,59]. These differences are mainly due to its smaller volumetric capacity because a greater volumetric flow is required to maintain a similar cooling/heating load, resulting in a higher irreversible loss. Barve [59] concluded that refrigerant R1234yf is unsuitable for direct drop-in replacement of R410A without modifications of commercial heat pump systems or charge management in a high-temperature test. However, regarding power consumption by the compressor, R1234yf shows a better performance due to low refrigerant flow rates and lower pressure ratios. Insufficient production capacity and cost are the main limitations to using this refrigerant on a larger scale.
2.1.2. R1234ze(E)
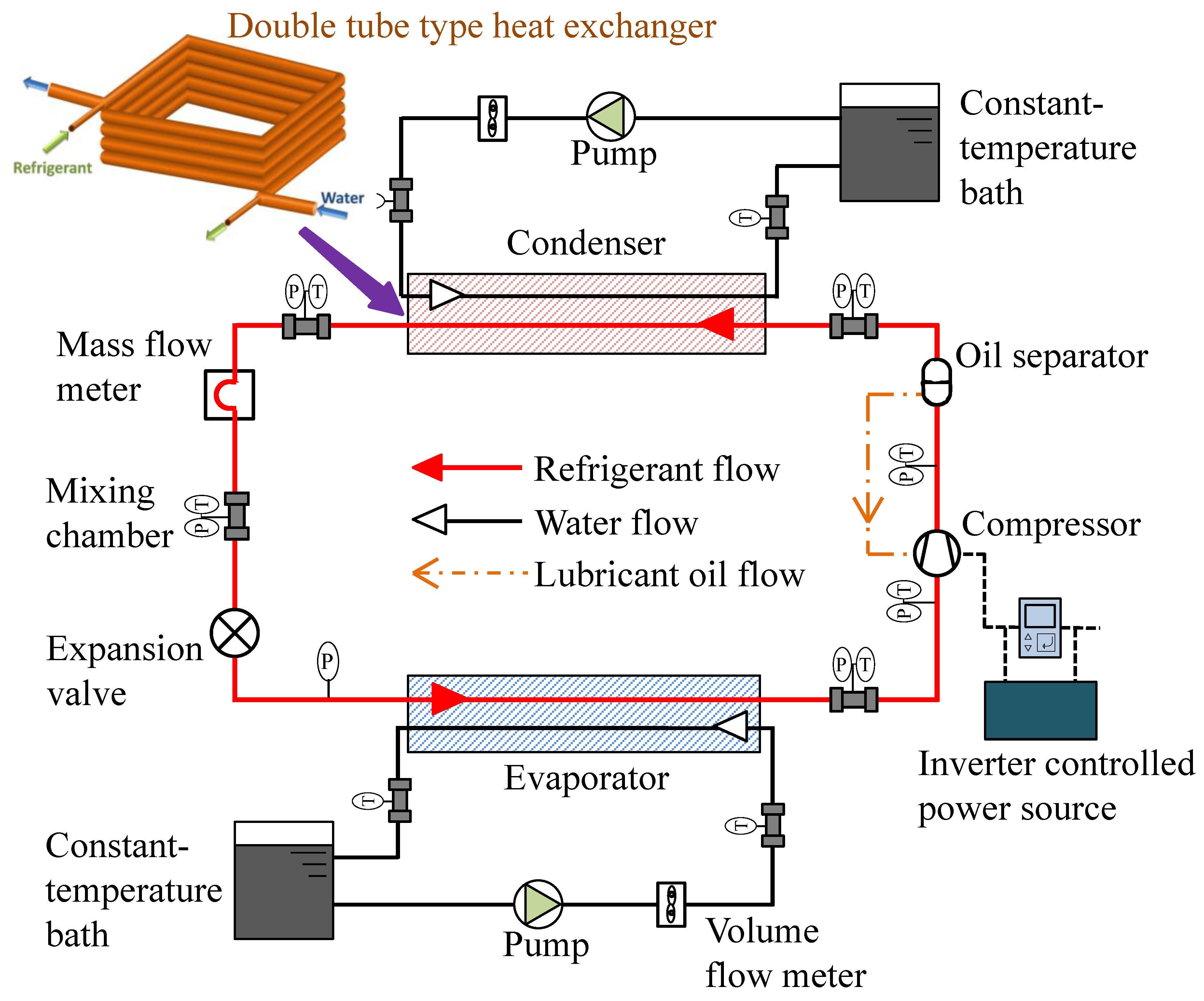
Because of its ultra-low GWP (<1), the hydrofluoroolefin 1,3,3,3-tetrafluoro-1-propene (R1234ze(E); CF3CH=CHF) has gained interest as a prospective refrigerant for industrial centrifugal chillers [60,61]. The critical pressure and temperature of the refrigerant are 3.63 MPa and 109.3 °C, respectively [62,63]. It is a promising refrigerant for high-temperature heat pumps that act as hot dryers and steam generators for industrial applications, including beverage concentration, sterilization of foods, drying lumber, solvent recovery, and distillation of petrochemical products. Fukuda et al. [64] experimentally studied the feasibility of R1234ze(E) and also R1234ze(Z) in a high-temperature heat pump system. In the same laboratory, Koyama et al. [65] investigated the compatibility of R1234ze(Z) with plastic, elastomers, metal, and its thermal stability. The authors found that the flammability of R1234ze(Z) is appropriate for its commercial use. Brown et al. and Li et al. [66,67] recommended R1234ze(Z) for high-temperature heat pump applications. The authors recommended an additional study to use it as an alternative to R114. To replace the widely used R410A with R1234ze(E), Koyama et al. noticed pure R1234ze(E) is not appropriate because of its limited volumetric capacity [68], but it is recommended for turbo refrigeration systems. In that case, the impeller size of the compressor is recommended to be enlarged as compared to that for R134a [69,70]. When compared to R134a, the refrigerant has a 9–15% energy-saving potential [70,71]. R1234ze(E) is also studied as a potential replacement of R134a [72,73]. Figure 1 shows the schematic layout of a water-cooled experimental setup used at the Koyama laboratory to investigate the performance of pure and blended refrigerants [74,75].
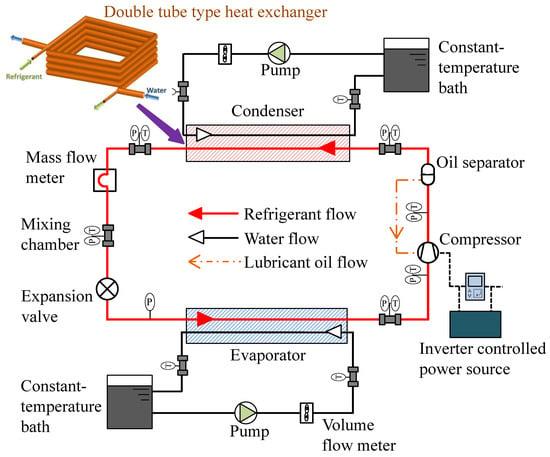
Figure 1.
Prototype of a next-generation refrigerant performance study [75].
Figure 2 shows the experimental COP of R1234ze(E) as compared to the widely used R410A and promising R32, where it is found that the COP of R1234ze(E) is lower in both the heating and cooling modes, notably in higher heating loads. The COP is considered for both the cycle (COPcycle) and the system (COPsystem) level following the equations below:
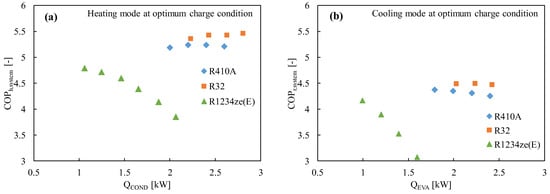
Figure 2.
The coefficient of performance of the R1234ze(E), R410A, and R32 refrigerants in a drop-in experiment in (a) the heating mode and (b) the cooling mode [75].
Irreversible loss is calculated using the following equations [58]
A double tube-type counter flow heat exchanger was used in the experiment. In the heating mode, the heat sink water temperatures at the inlet and the outlet of the condenser were set at 20 °C and 45 °C, respectively, and the corresponding inlet and outlet water temperatures in the evaporator were kept constant at 15 °C and 9 °C, respectively. In the cooling mode, the heat sink water temperature at the inlet and the outlet of the condenser was maintained at 30 °C and 45 °C, respectively, and the inlet and outlet water temperatures in the evaporator were maintained at 20 °C and 10 °C, respectively.
The heat transfer rate varied from 1.4 kW to 2.6 kW by adjusting the refrigerant flow, compressor rotational speed, and water flow. The degree of superheat was kept constant at 3–4 °C over the entire range of test conditions.
2.1.3. R152a
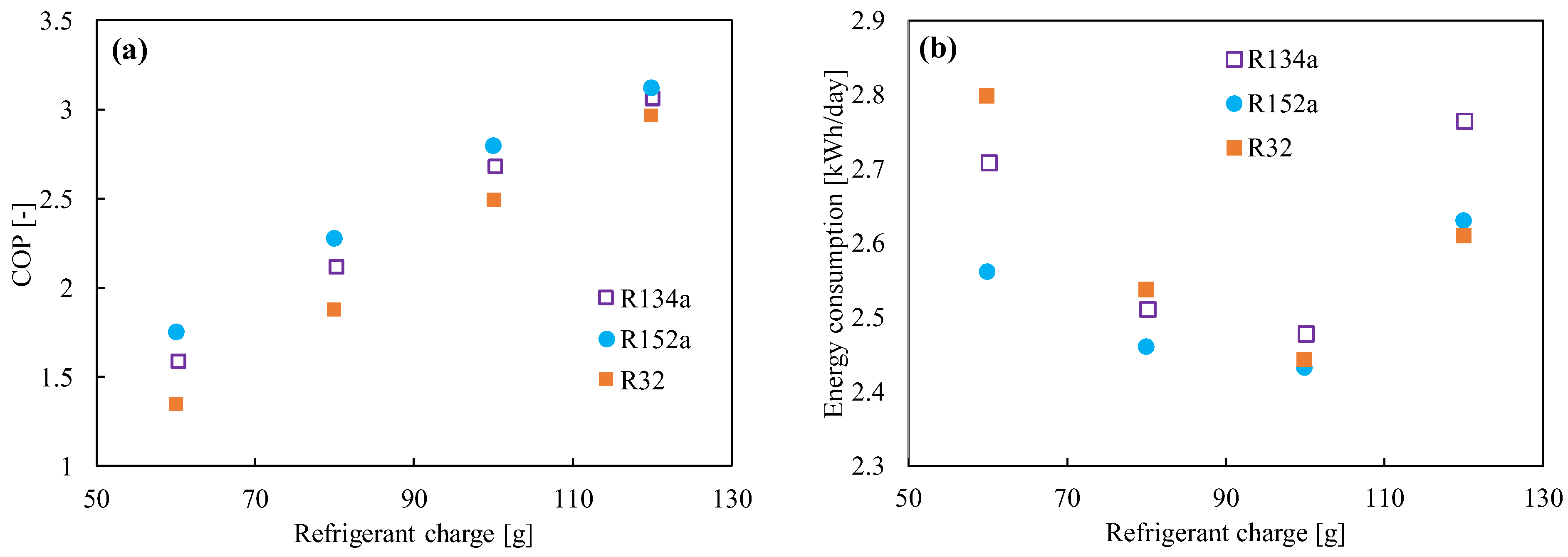
R152a (1,1 difluoroethane, C2H4F2) has been considered as a refrigerant blend for a long time, but not as a pure refrigerant. The refrigerant’s critical pressure and temperature are 4.52 MPa and 115 °C, respectively [76,77,78]. It does not have any ODP. Compared to the HFC and HFO refrigerants, R152a has a low global warming potential (138 [79]) and a lower price. The volumetric capacity and pressure of R152a are quite close to those of R134a. On the other hand, the energy efficiency, mass flow, and vapor density are much better than those of R134a [80]. The system performance in a vapor compression system was found to be higher than that of R134a [27,81]. Test results demonstrated a 4.7–13% improvement in the COP, with a decrease in the cooling capacity of about 0–10%. Figure 3 shows the comparison of the (a) COP and (b) energy consumption between R134a, R152a, and R32 in 120 L refrigerators for different charge amounts. It was found the COP increases with the increase in refrigerant amount, but the minimum power consumption reached 100 g. R152a is commonly used as an aerosol spray propellant and foam-blowing agent. It is also used as a component in a variety of refrigerant blends (R401A, R415A, R430A, R500, etc.) [82]. However, its flammability is rated as A2 by the ASHRAE [83], and hence flammable dangers may have been the primary factor preventing its use as a pure refrigerant up until now [81]. The minimum ignition energy for this refrigerant is 0.38 MJ, which is slightly higher than that for flammable hydrocarbons. Cabello et al. [84] also studied cascade refrigeration plants using R152a/R744 and R134a/R744 pairs for industrial and commercial applications. Though the comparative energy results did not show a great difference, its low GWP can be a great advantage from the environmental point of view.
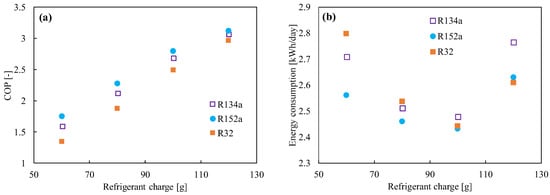
Figure 3.
(a) Coefficient of performance (COP) and (b) energy consumption as a function of the refrigerant’s charged amount [27].
2.1.4. R744
R744 (carbon dioxide, CO2) has been utilized in various types of vapor compressor systems for over 130 years [85,86,87,88]. It is a low-cost and natural refrigerant. However, it works at an exceptionally high-pressure level and operates in a transcritical cycle. The decline of using R744 started in the mid-1950s after the invention of high-performance CFC refrigerants. When the adverse effect of synthetic refrigerants was found in the late 1980s, there was a renewed interest in R744 as it is not toxic and flammable [86,89]. In the early 1990s, Gustav Lorentzen revived R744 as a refrigerant [90,91]. To assess the COP of R744 and R12, Lorentzen and Pettersen [92] designed a laboratory prototype considering its application in vehicle air conditioning systems. The authors speculated that the increased energy density might have a high cost but provide practical benefits due to their smaller size and weight. Koyama et al. [93] and Xue et al. [94] constructed a steady-state model where they assessed the heating and cooling performance of R744. Many researchers have studied theoretically and empirically the performance of R744. The performance has also been compared with other refrigerants as a feasible alternative to synthetic fluids [23,95,96,97,98,99]. Hwang et al. [100] tested the performance of several two-stage compressor R744 cycles and observed an 18–35% improvement in the COP over the basic cycles. Girotto et al. [96] calculated the COP of all-CO2 systems for a supermarket in northern Italy, estimated the annual energy consumption and the cost of CO2 installation. Even though the installation cost was 20% greater, the authors discovered a way to raise the efficiency of the CO2 system to match that of the R404A system. According to Maina and Huan [89] and Nekså [101], this refrigerant has been used for different purposes, including hot water generation, commercial refrigeration, heat pump dryers, etc.
2.1.5. R1336mzz(Z)
New refrigerant R1338mzz(Z) has been found very promising for high-temperature heat pump cycles. It is non-flammable, and the GWP of this refrigerant is only 2 [102,103,104]. The boiling and critical temperatures of R1336mzz-Z are 33.5 and 171.3 °C, respectively. Therefore, it can be potentially considered as an alternative to refrigerants HFC-245fa and HCFC-123. The pool boiling performance of R1336mzz(Z) on a flattened horizontal Turbo-ESP surface has been studied for air conditioning and industrial heat recovery applications [104]. The potential application of these refrigerants has also been studied for transcritical Rankine power cycles [105].
2.1.6. HC (Propane R290, N-Butane R600, Isobutane R600a)
In general, the natural refrigerant known as HC (hydrocarbon) has great efficiency, good miscibility with mineral oils, a lower compressor discharge pressure, and strong heat transfer properties. However, because of its significant flammability, this refrigerant cannot be used on a larger scale. Because many other nations have outlawed using flammable gases in the public, HCs are only used as refrigerants in Europe following some regulations. The theoretical and practical performance of R290 in contrast to R22 was examined by Lampugnani and Zgliczynski [106]. According to the experimental findings, R290 is a superb candidate to replace R22 from the thermodynamic standpoint. Granryd [107] discussed the advantages and disadvantages of employing HC as a refrigerant for refrigeration and heat pump applications. Numerous HCs have been discovered to exhibit advantageous properties as refrigerants from the thermodynamic and heat transport perspective. In their investigation, Halimic et al. [108] came to a conclusion that, after addressing technical operation and safety concerns, R290 is a desirable replacement for R12 in small residential refrigerators. Bjerre and Larsen [109] assessed the potential usage of R600 for residential applications and discovered that it performed 10% better than R134a. Palm and Somchai and Chimres [110,111] found the excellent performance of hydrocarbon compared with R134a, R22, and ammonia. According to the authors, the safety risk may be decreased by constructing systems hermetically with the least amount of connections and refrigerant charge or by using an indirect system. The accepted practice of employing HC in a vapor compression system was examined by Corberán et al. [112]. According to the IEC355.2.20 standard, up to 150 g of HC can be sealed in a regular refrigerator, and tiny freezers can be placed anywhere, regardless of the size of the room, with a few additional safety precautions. Some European refrigerator producers now have access to flammable HCs to make residential refrigerators. However, the failure rate of the compressor is increased with the vast commercialization of such refrigerants. Table 4 summarizes the flammability properties of pure refrigerants studied at many laboratories.

Table 4.
Flammability characteristics of some pure low-GWP refrigerants [46,61,107].
2.1.7. Other Natural Refrigerants
In home and automotive heat pump applications, water, ethanol, ammonia, and methanol, known as natural refrigerants, are receiving attention [87,113,114,115,116]. The thermodynamic properties of ammonia (R717) are excellent, and it is energy-efficient when applied in large industrial systems and commercial buildings, but its toxic behavior limits its usage as a refrigerant in domestic and automobile applications [117,118]. Due to their low volumetric efficiency, water (R718) and ethanol are not common refrigerants in conventional vapor compression systems, but they can be regarded as common refrigerants in sorption-based systems [119,120,121,122,123,124].
2.2. Refrigerant Mixtures
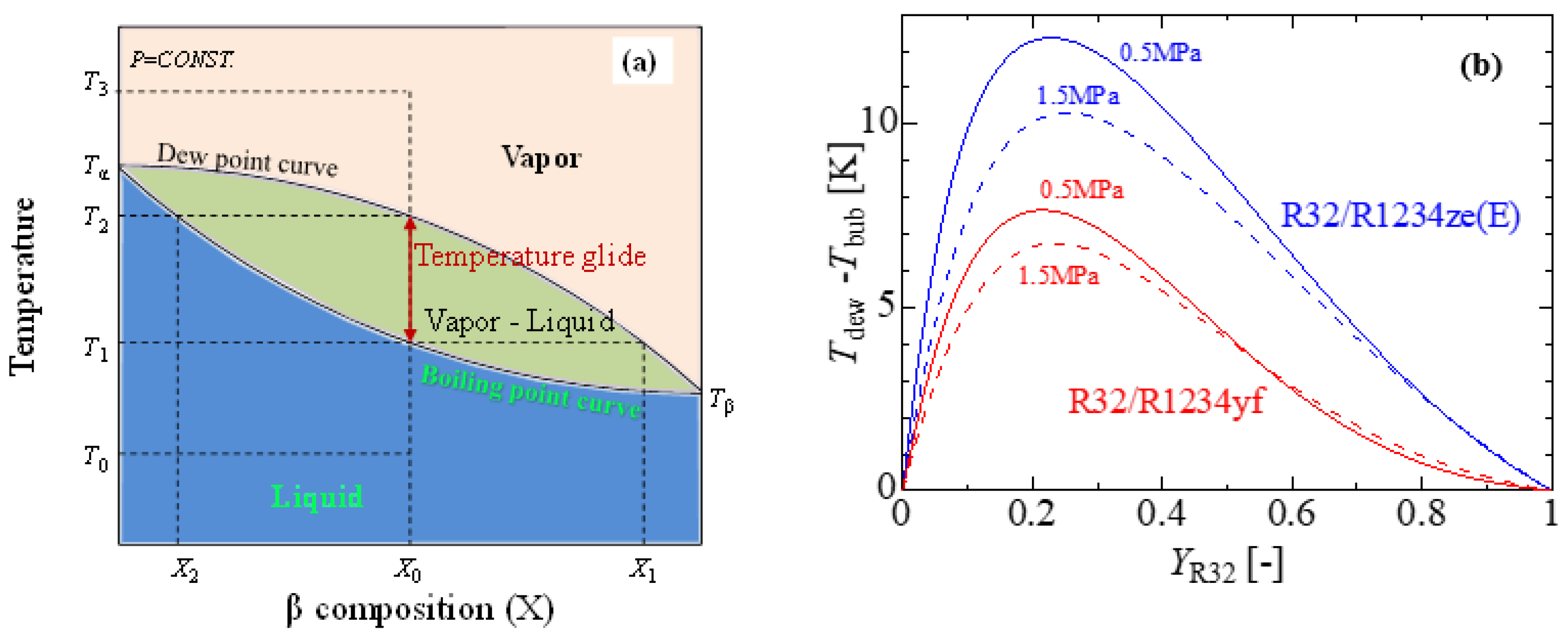
Due to their azeotropic character and generally well-developed thermophysical properties, pure refrigerants are a good choice for vapor compression systems. HFOs are reasonable alternatives regarding their toxicity, flammability, and ultra-low GWP. According to Bella and Kaemmer [125], pure HFOs are inefficient substitutes for R410A from the perspective of system performance. For drop-in replacement, rebuilding the system with a larger compressor, pipe, and heat exchangers is necessary. It has also been found that the volumetric capacity of the refrigerant is low, which leads to larger mass flow rates, larger pressure drops in the heat exchangers and connection pipes, and, ultimately, lower coefficients of performance (COP) [126,127]. The volumetric capacity as well as the COP can be improved by adding some other refrigerants with HFOs. In China, R32 has been investigated due to its lower cost as compared to R410A, while blends of R32 with HFOs have been studied in Japan and the USA [37]. A blend is often defined as a combination of two or more single-component refrigerants. Blends can be either azeotropic or zeotropic. Azeotropic blends act like pure refrigerants in such a way that they boil and condense at consistent temperatures at any given pressure. As opposed to azeotropic blends, zeotropic blends boil and condense at a variety of temperatures at a given pressure (see Figure 4a). This temperature range is known as temperature glide, which is essentially the difference between the refrigerant compound’s bubble point and dew point. Figure 4b shows the formation of temperate glides of the R32/R1234ze(E) and R32/R1234yf blends at a constant pressure. The maximum temperature glides (△T) of the R32/R1234yf and R32/R1234ze(E) mixtures at 0.5 MPa are 7.6 and 12.2 °C, respectively (source of thermophysical properties—REFPROP V9.1) [128]. Despite the vast possibilities of the mixing strategy, a few mixtures, such as binary and ternary mixtures, remain popular due to the possible complexity involved in the thermophysical properties of the mixtures.
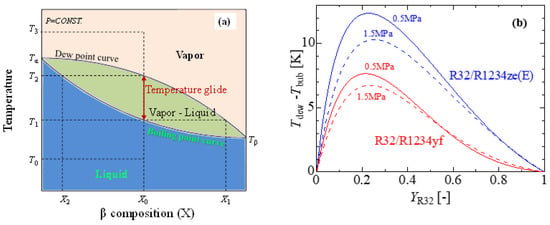
Figure 4.
Temperature glides of a binary mixture. (a) Temperature vs. mass fraction diagram of a zeotropic mixture, (b) temperate glides of the R32/R1234ze(E) and R32/R1234yf binary blends.
2.2.1. Binary Mixtures
R32/R1234ze
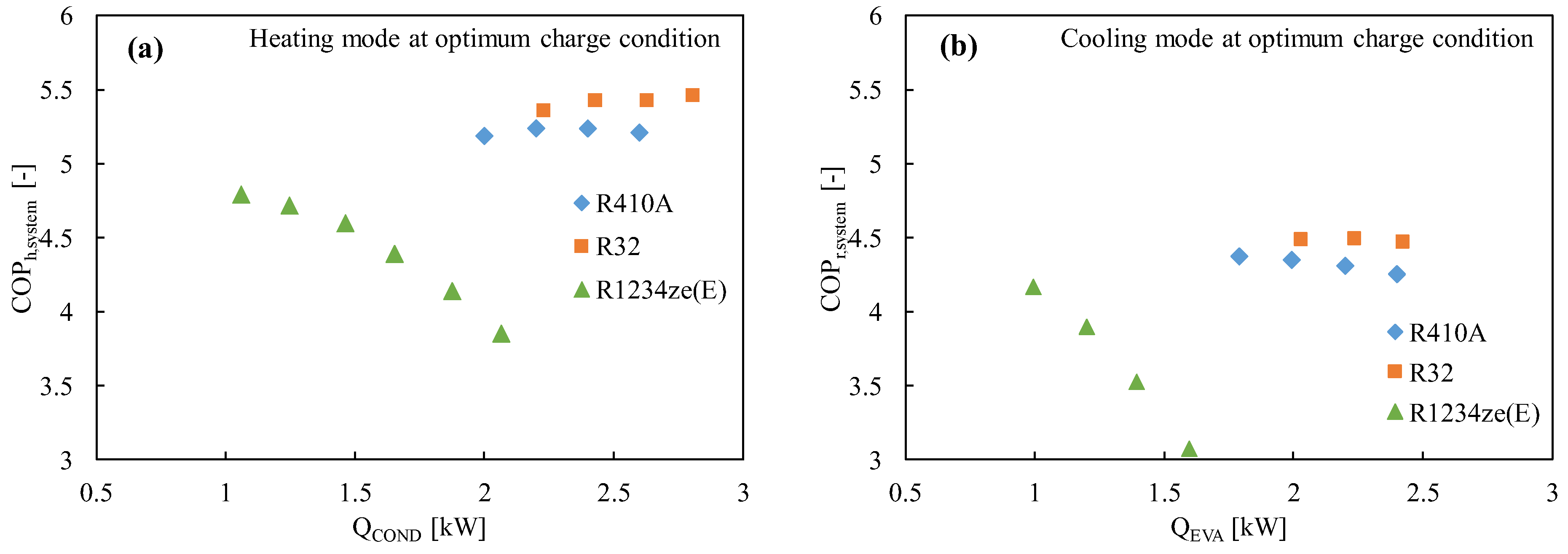
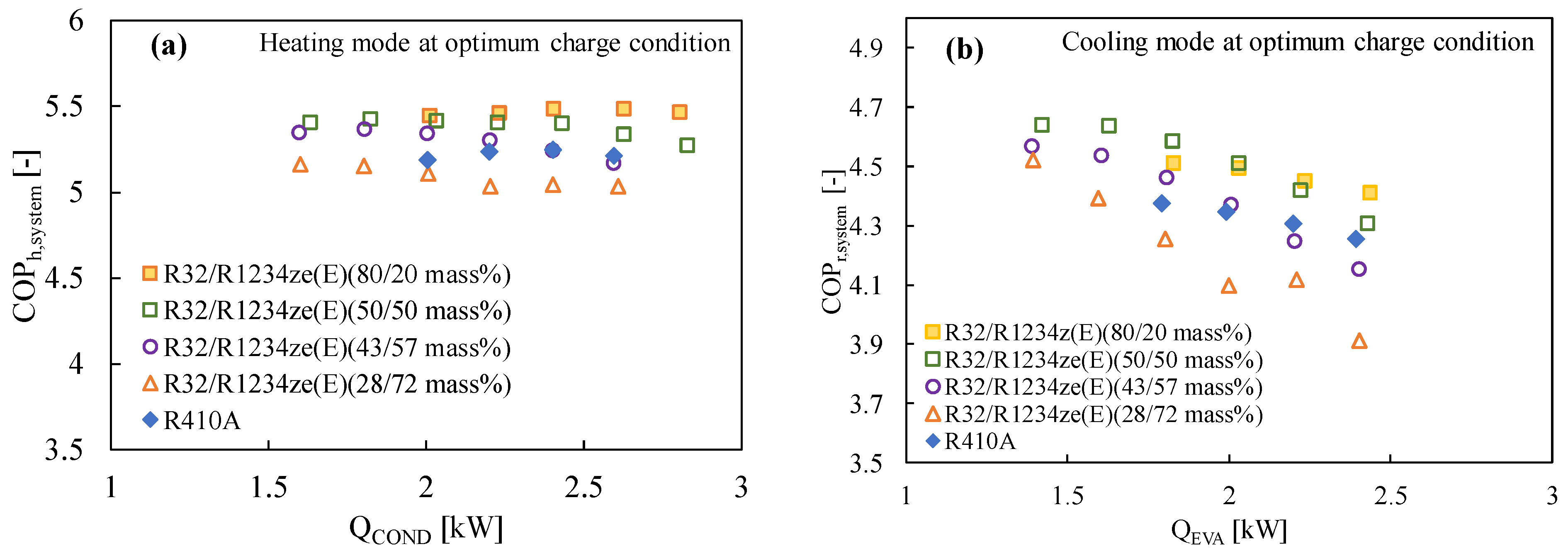
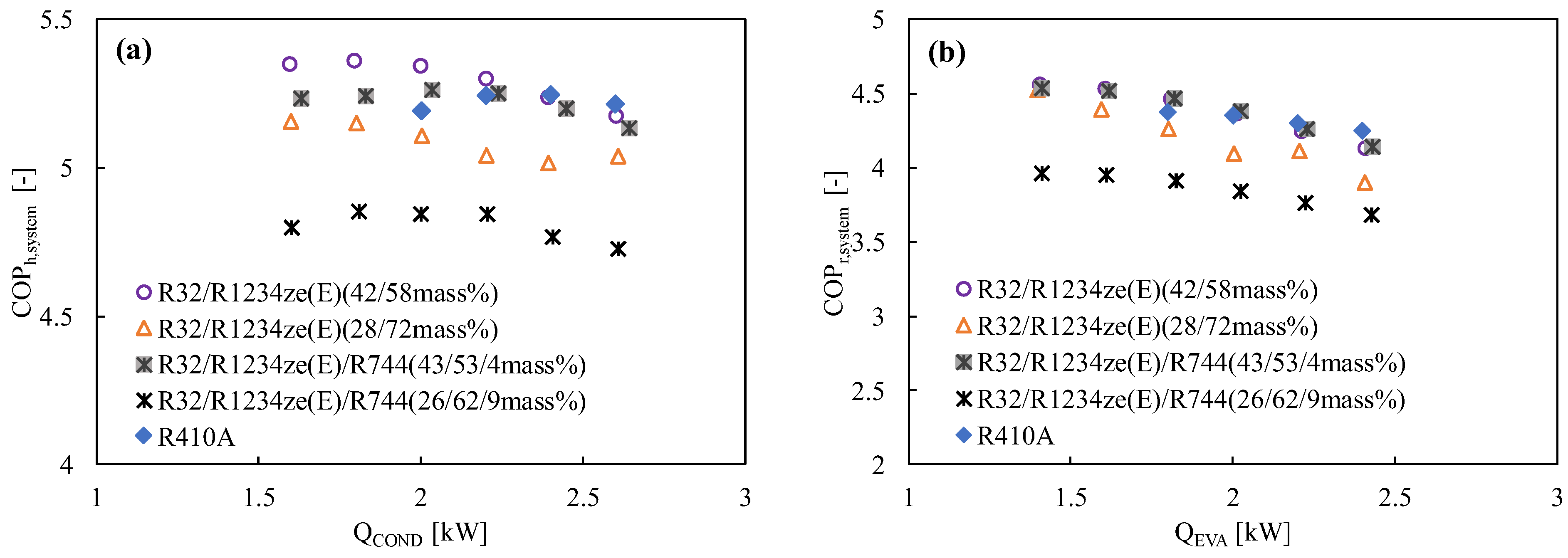
Numerous studies have demonstrated that R1234ze(E) has a poorer coefficient of performance (COP) than other widely used refrigerants like R410A or R134a, mostly because of its limited volumetric capacity and greater pressure drop [129]. R32 is typically blended in a variety of ratios to improve the performance of R1234ze(E) and the vapor density [69,130]. Table 5 lists the mixing techniques for various refrigerants and their corresponding GWP values, which are considered to be less than 300. The cycle performance analysis showed that the inclusion of R32 (50% mass) with R1234ze(E) boosted the volumetric capacity while maintaining the COP greater than that of R410A, suggesting this mixture is a strong contender to replace R410A [131]. The thermodynamic properties of the mixture developed by Akasaka [132] were used in the study. Kojima et al. [58] also studied the R32/R1234ze(E) mixture with the 42/58 and 28/72 (by mass) ratios keeping in mind the GWP values around 300 and 200, respectively. They found that these mixtures show comparable performance to that of R410A in the heating mode for the heat sink water temperature of 20→45 °C and heat source water temperature of 15→9 °C. The same authors reported that the COP of the R32/R1234ze(E) mixtures improved significantly for both modes of operation, which can be clearly found in Figure 2 and Figure 5. In Figure 5, it is further observed that the COP of the system improves with the increase in the R32 composition.

Table 5.
Literature review of pure/binary/ternary mixtures of low-GWP refrigerants.
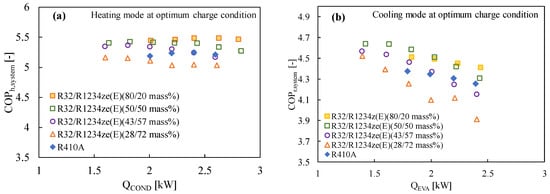
Figure 5.
Variation of the COP of the R32/R1234ze(E) mixtures with the heat transfer rate in the condenser and the evaporator in a drop-in experiment at the optimum charged condition in (a) the heating mode and (b) the cooling mode [75].
R32/R1234yf
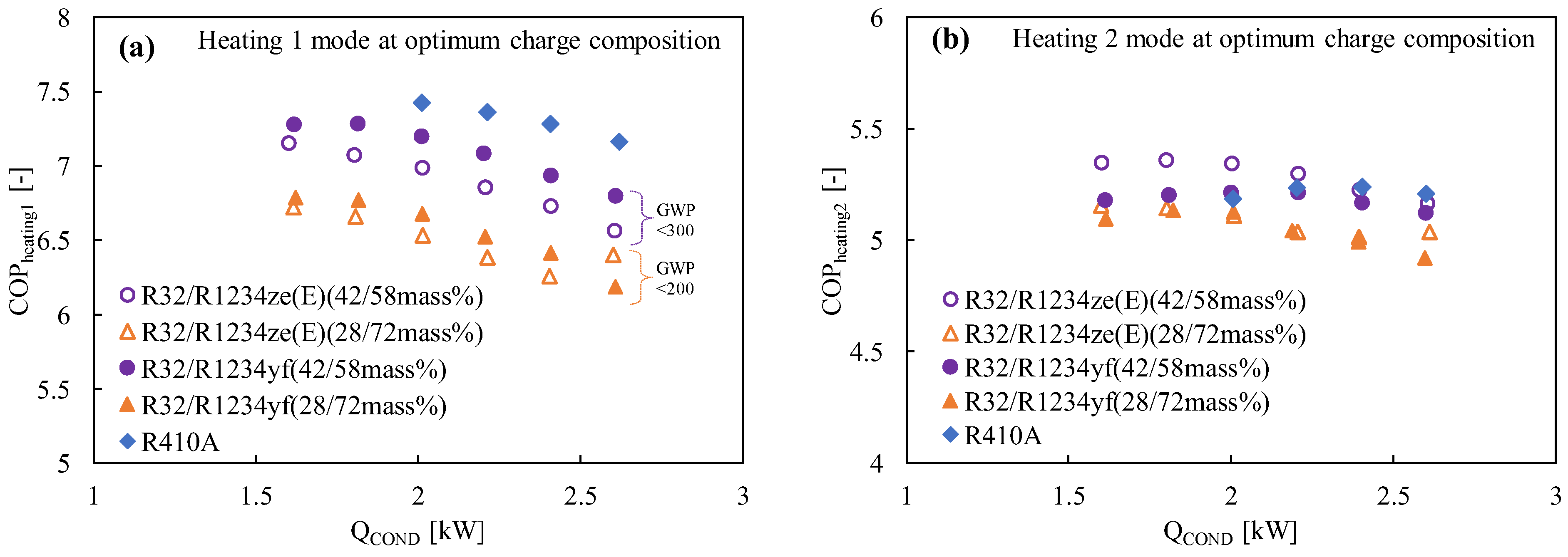
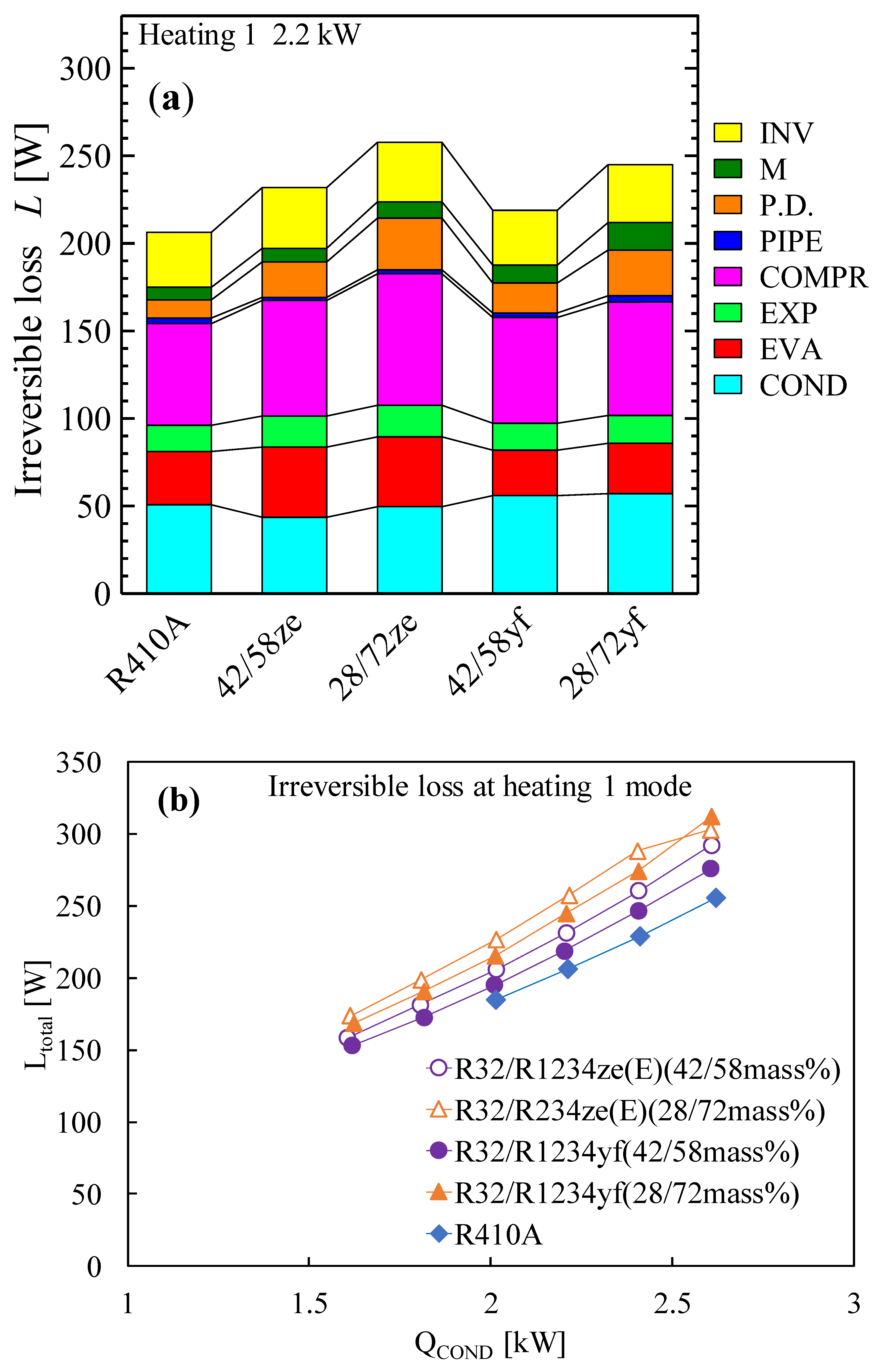
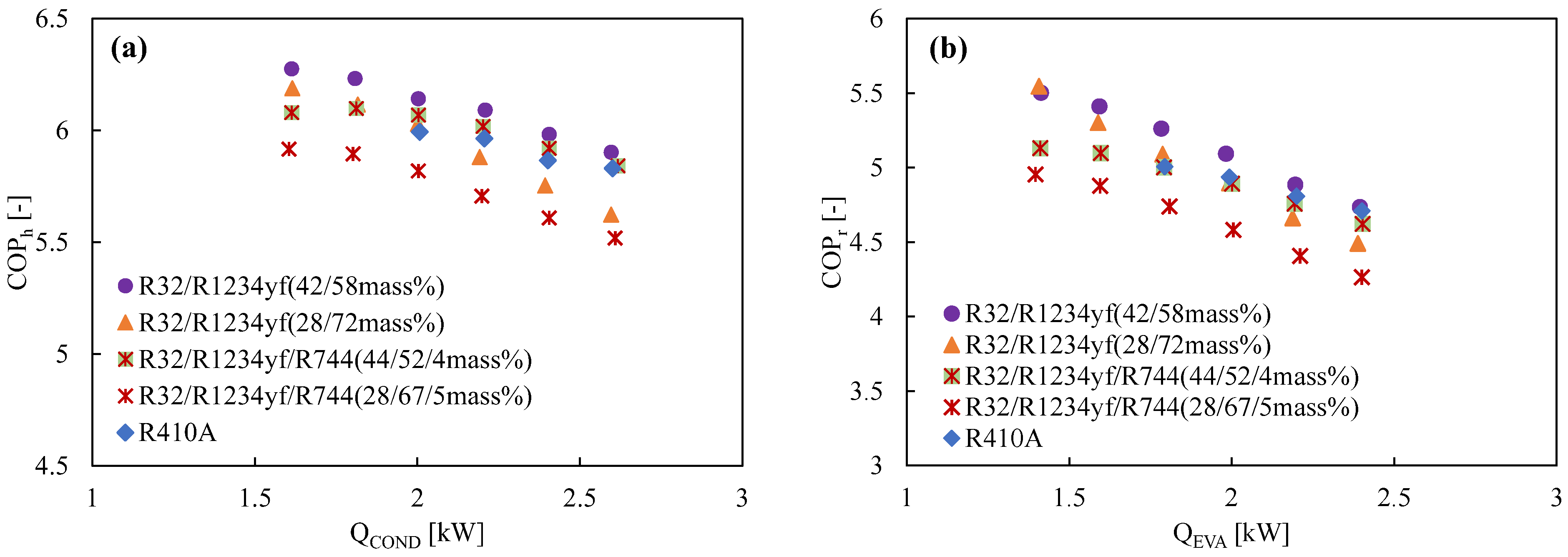
Due to its extremely low GWP and performance that is equivalent to that of R134a, refrigerant R1234yf can be regarded as a promising next-generation refrigerant. Prior to now, R1234yf has had a few drawbacks, most notably a volumetric capacity that was much smaller than that of R134a. However, pure R32 exhibits a very high temperature at the compressor output and a significantly greater GWP, making it a poor candidate for home air conditioning. In order to address their respective weaknesses, these two refrigerants are combined with different ratios. Akasaka [132,141] and Kamiaka et al. [126] developed the thermodynamic characteristics of the blend. Two blends, considering GWP 300 and 200, were studied in a drop-in experiment to replace R410A [133], and the performance of the R32/R1234yf binary mixture of 42/58 (by mass) was found to be considerably higher than that of R410A, whereas the 28/72 (by mass) mixture showed a lower performance due to the higher irreversible losses associated with pressure drop (Figure 6). Some similar mixtures with different mass percentage ratios are shown in Table 5, which have been studied under the AHRI research program [130], but the findings from their research are not available in the open literature. Figure 6 also shows the performance comparison in both the heating-1 and heating-2 conditions of the R32/R1234ze(E) and R32/R1234yf mixtures [58]. It was noted that both pairs showed a higher COP in heating 2, so it could be selected as an alternative to R410A under the heating-2 conditions. The irreversible loss properties for different mixtures are also compared with R410A in Figure 7. Figure 7a shows that the irreversible loss for the R32/R1234yf (42/58) mixture is minimum, whereas R32/R1234ze(E) (28/72) shows a higher irreversible loss among the studied pairs. Figure 7b shows that the total irreversible loss almost linearly increases with the heat load. The higher irreversible loss of a low-GWP mixture compared to R410A means the higher pressure drop caused by HFOs. Basically, the smaller irreversible loss indicates a higher COP.
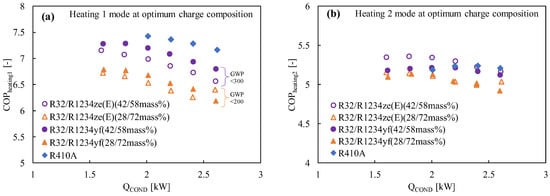
Figure 6.
Comparison of the COP with the heating load at the optimum charge condition in (a) heating mode 1, (b) heating mode 2 (for modes 1 and 2, the condenser water inlet–outlet temperatures were 20→30 and 20→45, respectively, while the evaporator water inlet–outlet temperatures were constant at 15→9) [58].
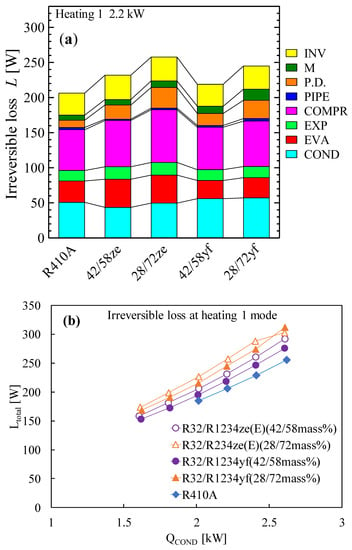
Figure 7.
Irreversible loss of R32/R1234ze(E) and R32/R1234yf mixtures in a drop-in experiment. (a) Irreversible loss in each component of the system. (b) Total irreversible loss for promising binary mixtures [58].
R32/R1123
AGC (Asahi Glass Co., Ltd., Japan) developed a novel refrigerant for air conditioning systems in 2014 that uses hydrofluoroolefin R1123 as its primary component and can cut GWP even further. Trifluoroethylene, or R1123, has a performance that is comparable to that of common refrigerants and a very low GWP (0.3) [142]. However, the R1123 refrigerant has disproportionate properties at a higher temperature. To make the system safe, the blend of R1123 and R32 was proposed to replace R410A. Higashi and Akasaka [143] measured the thermodynamic properties of the 60/40 and 40/60 R32/R1123 mixtures (by mass). When R410A is replaced in residential and commercial air conditioning systems, the new refrigerant blend, which is azeotropic, can provide good performance, according to AGC [39]. The performance of this mixture is being tested at many laboratories in Japan; however, cycle performance results are not available in the open literature.
HC Mixtures
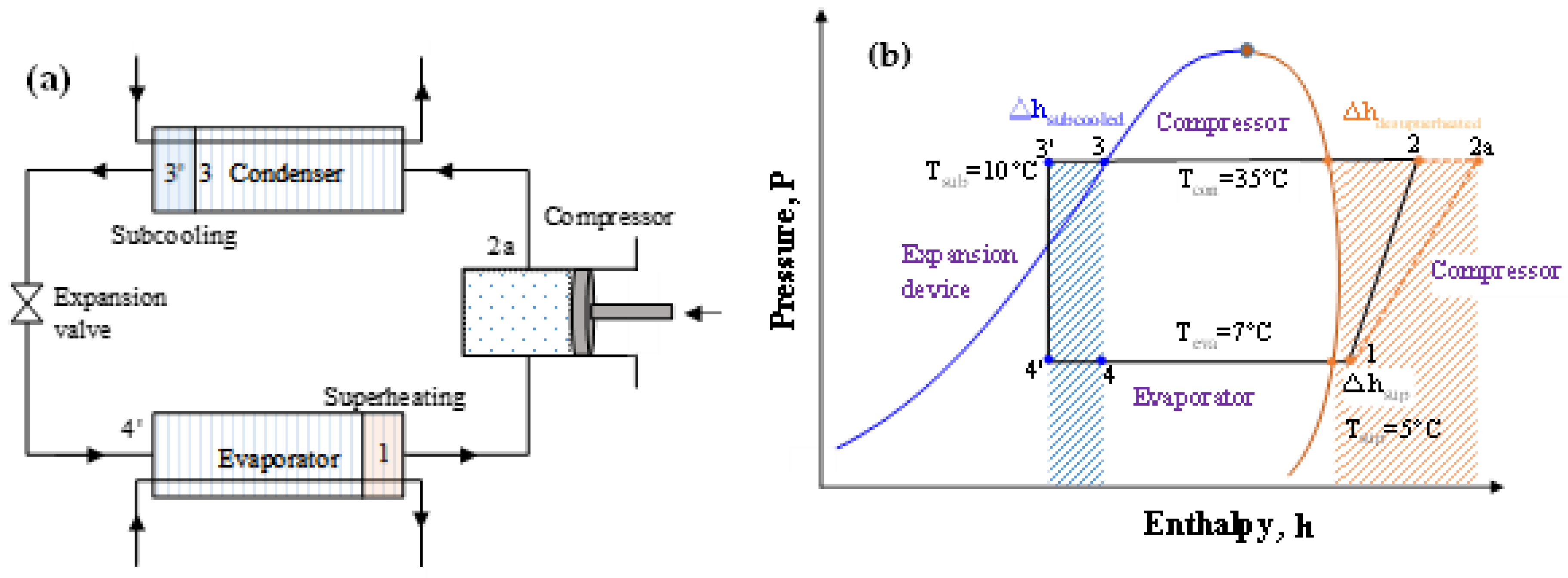
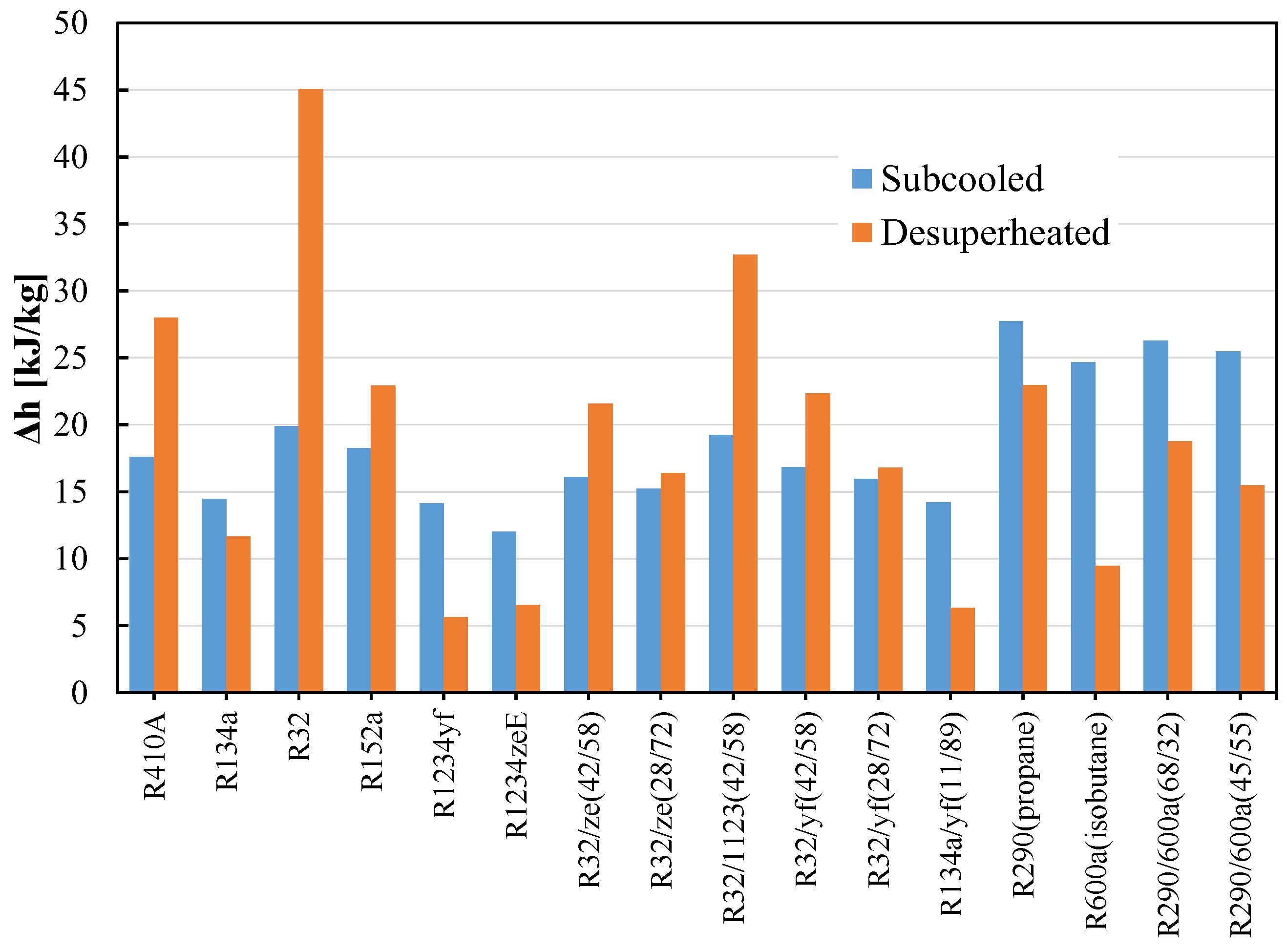
HC blends are ecofriendly refrigerants that may be employed in already-built systems. Due to their high flammability (A3), HC blends are recommended over halogenated refrigerants for usage in small systems with low charge amounts. Many studies investigated the R290/R600a combination as a replacement for R12 and discovered that it has a greater COP and refrigeration capacity than R12. R290/R600a mixtures have been investigated to replace R134a; it was found the mixtures are an appropriate alternative to R134a in terms of power consumption, charge amount, and cooling rate [111,137,138,140,144,145,146]. When the temperature glide matches the fluid temperature change, the 75/25 mixture (by mass) of R744/R290 increases the COP 12.8% more than R744 [147,148]. The performance of R32/R290 mixtures (68/32) as a drop-in replacement for R410A was found to be 6–7% lower than that of R410A, but the charge amount is reduced by 30–35%. In their assessment of the development of HC mixtures, Mohanraj et al. [149] found that few mixtures might work as viable replacements for halogen-based refrigerants. Figure 8a shows the schematic of the vapor compression cycle with the subcooling and superheating concept. Figure 8b shows the area of the subcooled and desuperheated enthalpy zone of an ideal thermodynamic cycle. The uncertainty of the measurements is not considered. The enthalpy change in this two-phase region for different pure and blended refrigerants are shown in Figure 9. It can be seen that the desuperheater heat for R32 and the R32/R1123 blend is higher; on the other hand, the subcooled heat for hydrocarbons and their blends are comparatively higher. The significant difference between these heats sometimes requires modification of the heat exchanger design for safe and efficient operation.
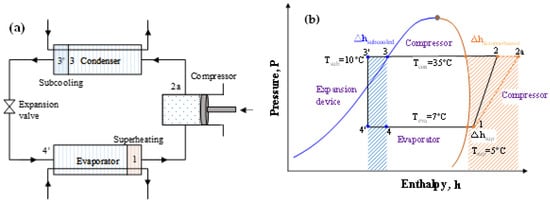
Figure 8.
(a) Schematic of the vapor compression cycle with subcooled heat and superheat. (b) Conceptual P–h diagram where the subcooled and desuperheated areas are textured.
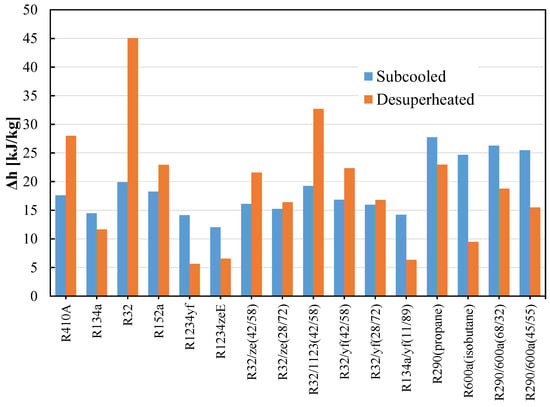
Figure 9.
Subcooled and desuperheated enthalpy of pure and blended refrigerants.
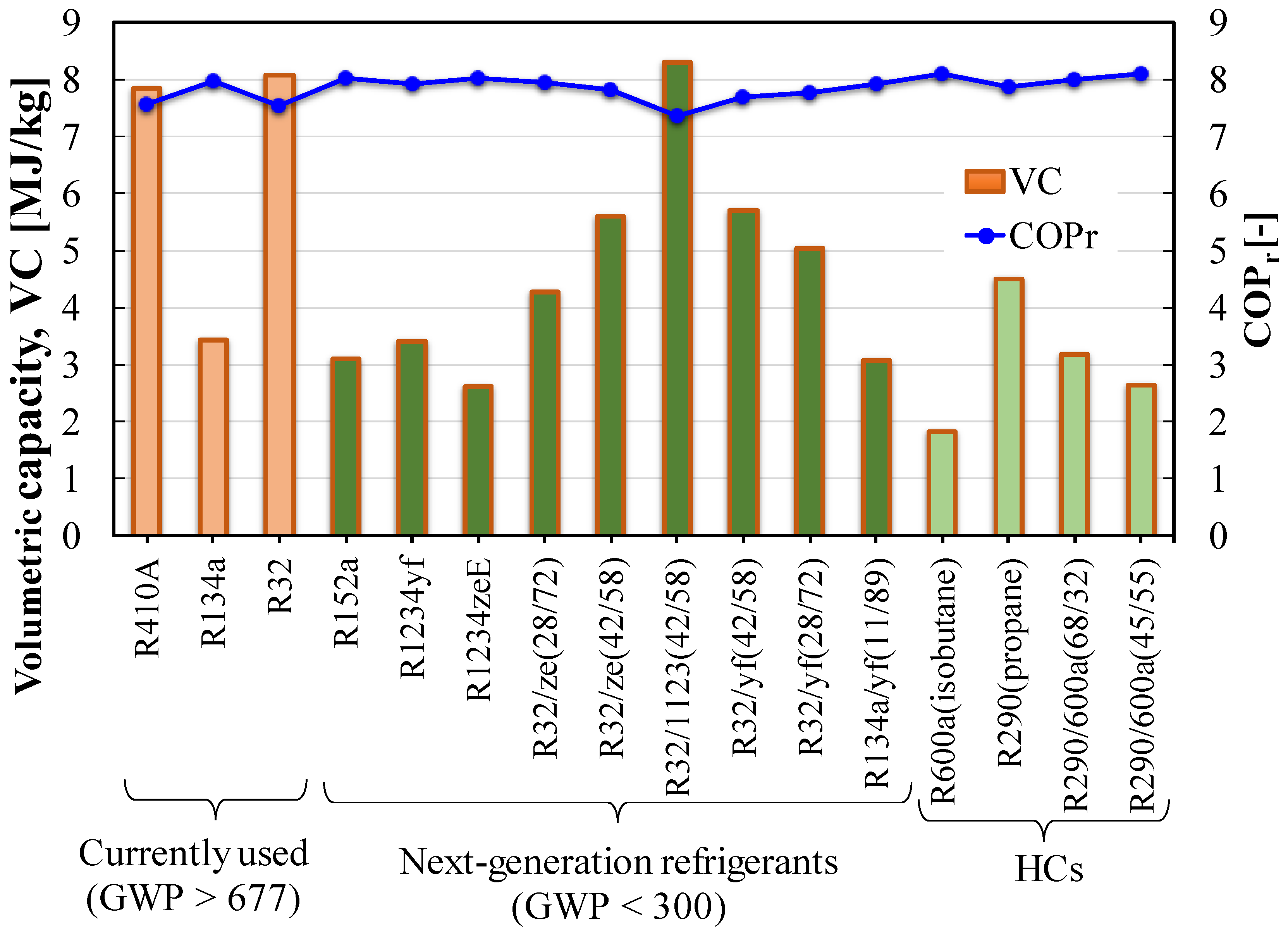
Figure 10 shows the COPs for the ideal cycle are almost steady for the studied pure and binary blends, whereas the volumetric capacities are significantly different. Formation of a blend improves the volumetric capacity. Moreover, the operation pressure for pure HFOs is considerably lower than that of R410A, which requires a new design of the compressor and the heat exchanger for drop-in replacement.
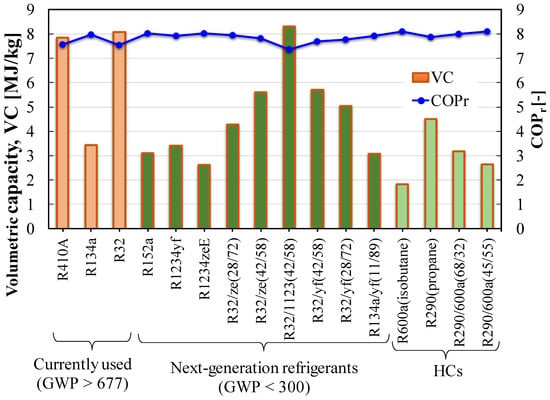
Figure 10.
COP and volumetric capacity of pure and blended refrigerants.
2.2.2. Ternary Mixtures
R744/R32/R1234ze(E)
For the case of binary blends of R32/R1234yf and R32/R1234ze(E), more than 50% R32 always shows a better performance than R410A. To maintain a GWP less than 300, the mass fraction of R32 must be below 50%, resulting in a lower COP, which is a consequence of limited volumetric capacity. To improve the volumetric capacity, Fukuda et al. [74,150] added R744 into the blend. In a drop-in experiment, the COP of the R744/R32/R1234ze(E) mixtures having GWP 300 was found comparable to that of R410A in both the heating and cooling modes (shown in Figure 11). However, the mixture with GWP 200 showed a lower performance than R410A in both modes due to higher irreversible losses strongly related to the volumetric capacity and temperature glide.
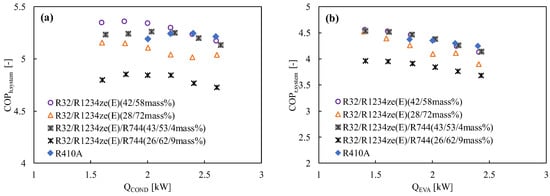
Figure 11.
Variation of the COP with the heating and cooling load for R1234ze(E)-based binary and ternary mixtures in a drop-in experiment in (a) the heating mode and (b) the cooling mode at the optimum charge condition [151].
R744/R32/R1234yf
Two ternary blends were formed by adding R744 into binary blend R32/R1234yf to enhance the volumetric capacity of the binary blend. The coefficient of performance of the ternary blends was experimentally studied, and their performance was compared with R410A [74]. For the blends, the authors considered the GWP value of 200 and 300. Temperature glide was a concern during the condensation and evaporation procedures since these combinations are zeotropic. The inclusion of R744 lowers the GWP but increases the temperature glide. The irreversible loss is minimized by matching the gliding temperature with the temperature changes in the heat sink and the heat source. Figure 12 shows the ternary blend with the GWP of nearly 300 performs very well in both the heating and cooling modes. This indicates the ternary 4/44/52 blend (by mass) can be used as a drop-in alternative to R410A [74]. In another study, Kondou et al. [152] showed that the boiling and condensation heat transfer coefficient decreased due to the mass transfer resistance in zeotropic binary and ternary mixtures. The AHRI research group performed their experimental study with a 7/30/63 ternary mixture (by mass) of R744/R32/R1234yf (GWP 206), but the results are not yet available in the open literature [130].
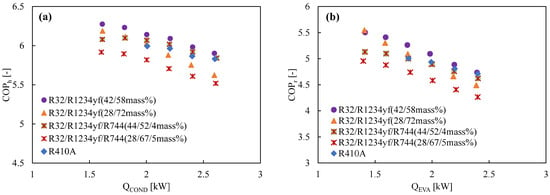
Figure 12.
Variation of the COP with heating and cooling load for R1234ze(E)-based binary and ternary mixtures in a drop-in experiment at the optimum charged condition in (a) the heating mode and (b) the cooling mode [133].
Other Ternary Mixtures
Ternary blend R744/R32/R134a (7/31/62) was studied theoretically and experimentally and compared with R32 [153]. This blend was found as a promising alternative to R22 as a drop-in replacement. It shows a 10% better COP. The blend is recommended for the use of low-temperature heat pumps because the condensing pressure is too high. The main problem with this blend is its GWP value (~1100). The performance of R410A was compared with R744/R32/propane (10/80/10, GWP ~540) [154], and the heating capacity of the blend was found to be higher than that of R410A. Two low-GWP refrigerant mixtures R32/R152a/R1234ze(E) (12/5/83) and R134a/R152a/R1234yf (7/11/82) have also been chosen as a promising alternative for next-generation heat pump systems [130].
3. Total Equivalent Warming Impact (TEWI) of a Refrigerant
The TEWI is related to the refrigerants used in refrigeration and air conditioning systems and comprises direct and indirect contributions to greenhouse gas emissions. The direct contribution is associated with the refrigerant’s GWP, charge amount, and leakage rates, including during servicing and decommissioning of the system. The indirect contribution accounts for the kg CO2-equivalent emissions generated during the production of the energy consumed by air conditioning and refrigeration systems. The TEWI also depends on the type of the system (e.g., domestic or automobile), its operating characteristics, and the system’s lifetime. TEWI calculation is sometimes simplified by neglecting the broader effects of manufacturing the refrigerant and equipment and of disposal of the refrigerant and equipment after decommissioning.
In this study, the TEWI was calculated as the sum of the kg CO2-equivalent emissions (direct effect, DE) due to refrigerant leakage into the atmosphere, disposal, and system lifetime and the CO2-equivalent emissions (indirect effect, IDE) during the production of the energy which is consumed by the system.
Here, a is the annual refrigerant leakage rate (%/year), b is the refrigerant recovery rate (%), M is the charge amount (kg), and Y is the system lifetime (years).
The indirect effect consists of the CO2-equivalent emissions due to the energy consumption of the system during the heating and cooling modes of operation.
Here, c is the carbon dioxide emission coefficient (kg CO2/kWh), HC—rated heating capacity (kW), CC is the rated cooling capacity (kW), tc is the annual use in the cooling mode (h/year), th is the annual use in the heating mode (h/year), and COP is the coefficient of performance in the heating or cooling mode.
Table 6 shows the operation condition used during the TEWI calculation for different refrigerants.

Table 6.
Operation condition considered for the TEWI calculation.
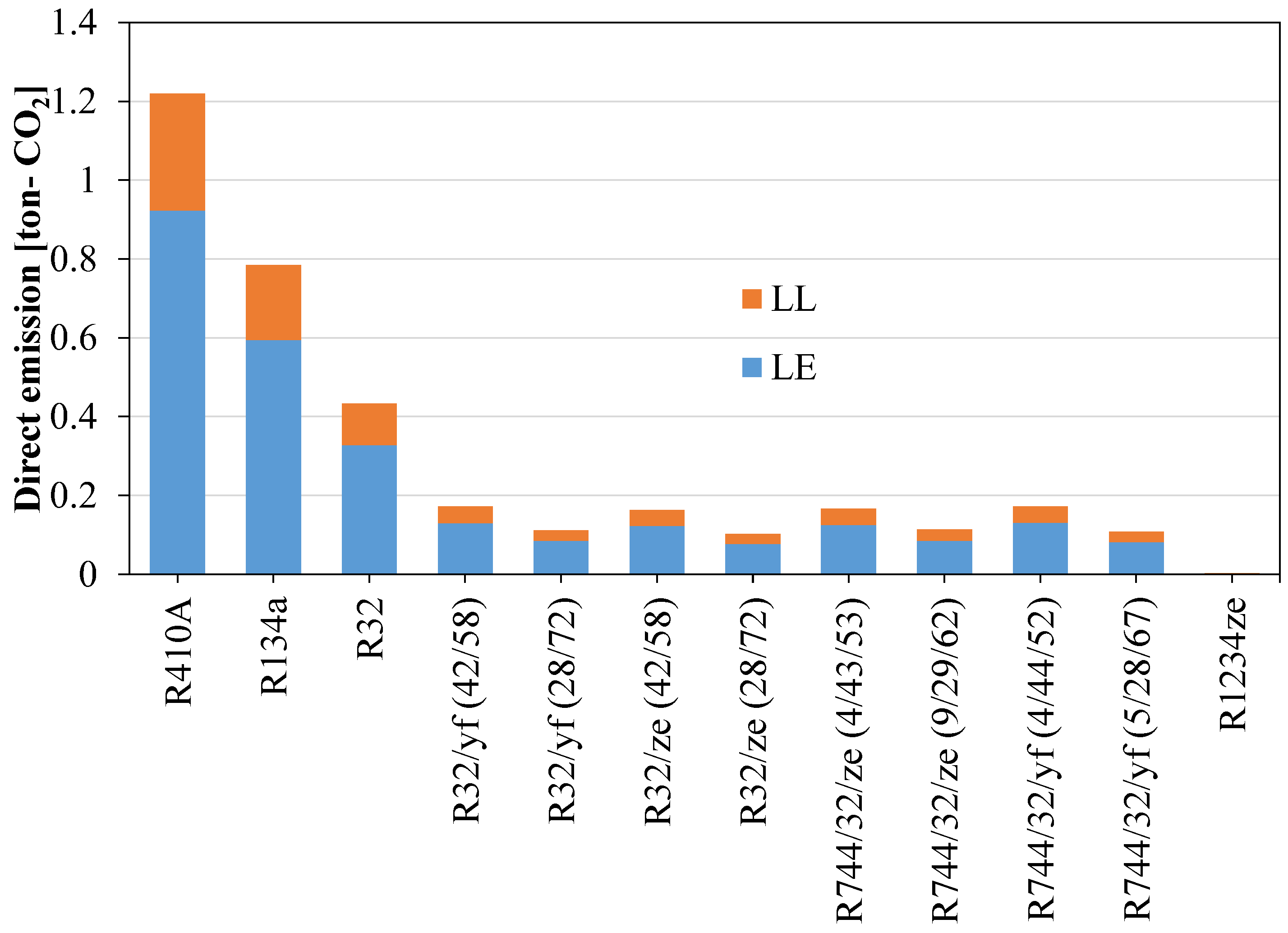
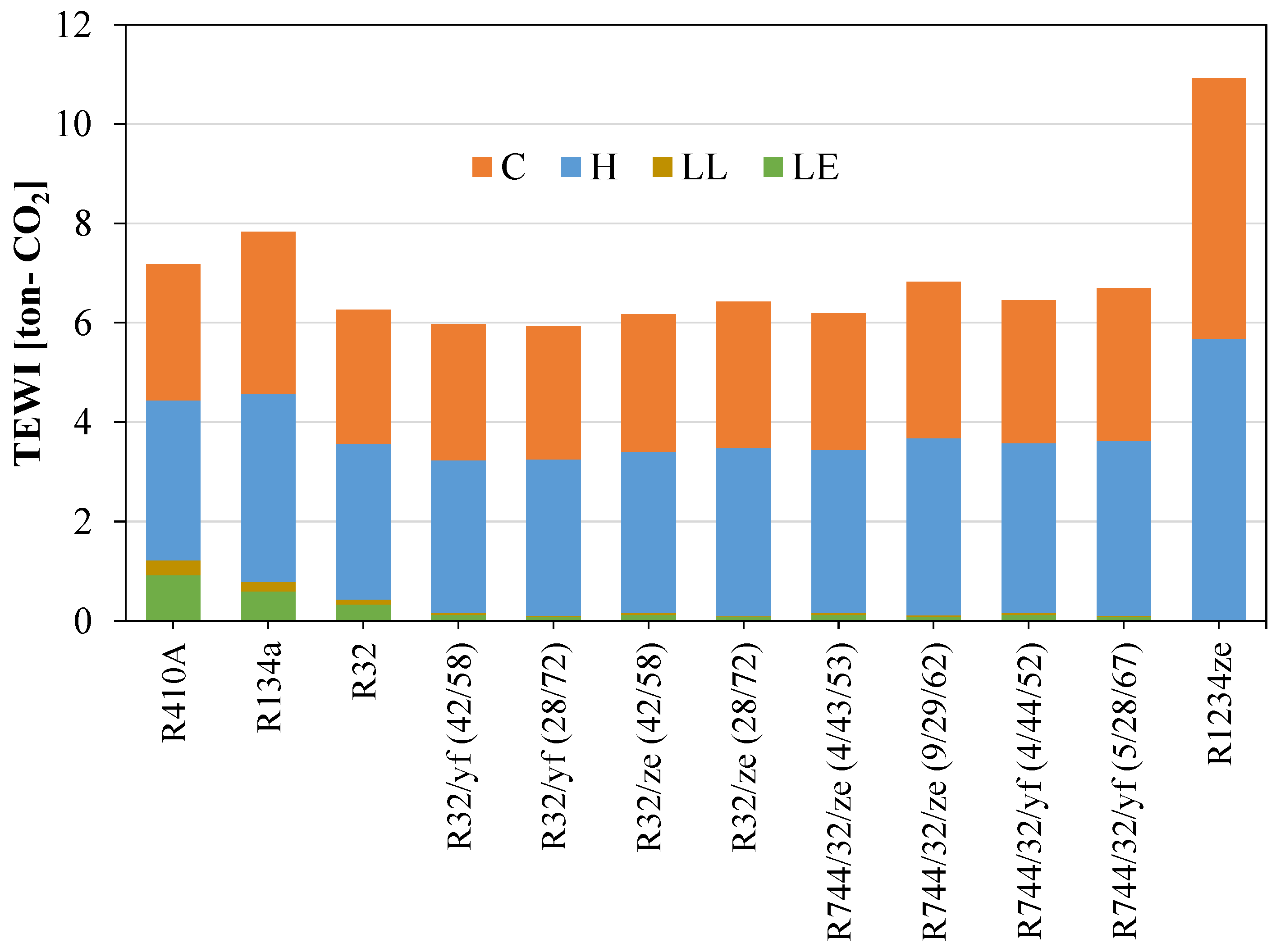
Figure 13 shows that the direct equivalent emissions for the newly proposed blend are significantly lower than for the widely used refrigerants R410A and R134a. Direct emissions can be reduced considerably if low-GWP blends are considered as an alternative. But for the TEWI, the R1234ze(E) shows a very high value because of its low system performance. The TEWI can be reduced by mixing HFOs with other HFCs. Figure 14 shows the TEWI values for conventional and newly developed blended refrigerants.
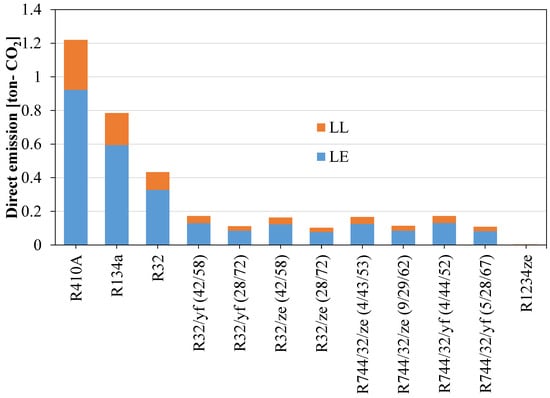
Figure 13.
Direct equivalent CO2 emissions for refrigerants when used in domestic applications.
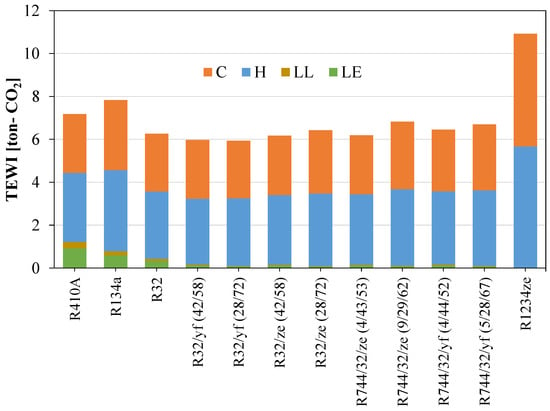
Figure 14.
TEWI for different refrigerants considered for domestic applications.
4. Technical Difficulties and Remedies to Use Low-GWP Refrigerants
- ➢
- Lack of thermophysical property data (experimental) for the mixtures to select/study different binary or ternary mixtures.
- ➢
- Most of the low-GWP refrigerant mixtures are non-azeotropic, so it has higher temperature glide, which avoids the pinch point formation in the condenser and evaporators during the two-phase flow.
- ➢
- Irreversible loss as well as pressure drops in the condenser and evaporator increases due to the lower volumetric capacity of the mixture refrigerants [74].
- ➢
- The lower volumetric capacity of these refrigerant mixtures requires a higher volumetric flow rate to maintain a similar capacity resulting in higher compressor speed and, thus irreversible losses.
- ➢
- Large non-linear variation of two-phase enthalpy with temperature during phase change in some zeotropic mixtures.
- ➢
- Lack of compatibility data of refrigerant mixtures and lubricant oil.
- ➢
- Changes in the temperature glide of the zeotropic mixture due to possible leakage and thus altering the mixing ratio. Therefore, refilling cannot guarantee that the new mixture has the same proportion as it had before the leakage.
The following measures can be taken to solve the above problems; it is noted that some difficulties can be made advantageous for the system.
- ➢
- When the temperature glide of the mixture is comparable with the temperature change in the heat sink and the heat source of a counter-flow heat exchanger, the irreversibility of heat transfer can be reduced [74,155,156,157].
- ➢
- When the refrigerant temperature changes in parallel with the heat source/sink fluids, that allows the ideal Lorentz cycle [156] and helps to reduce the mean temperature difference and irreversible losses in the condenser and evaporator. Kondou et al. [152] and Wang et al. [158] observed a negative effect on the heat transfer coefficient due to mass transport phenomena during the evaporation and condensation of the zeotropic refrigerant mixtures. High turbulence and good mixing in the heat exchanger can neutralize this negative effect.
- ➢
- By choosing the proper compressor size and suitable lubricant oil, the irreversible loss can be reduced for a new mixture.
- ➢
- Tubes with larger diameters and branches of refrigerant circuits in the heat exchangers can be employed to reduce the pressure drop.
- ➢
- The compressor efficiency curve for the mixtures, the solubility with lubricant oil, and their effect on the isentropic efficiency data are needed to find the best combination.
- ➢
- Research and technological development is required for a compressor when it uses blend refrigerants.
5. Conclusions
Refrigerants having low GWP and higher energy performance are required to reduce both direct and indirect contributions to environmental issues. However, most of the newer zero ODP, low-GWP alternatives suffer from one or more undesirable characteristics, such as greater flammability, toxicity, or lower volumetric capacity than the widely used HFC refrigerants. Based on the literature review, it can be understood that HFC and HFO mixtures (R32/R1234yf, R32/R1234ze, and R32/R1123) can be good substitutes for replacing the currently used refrigerants. The addition of R744 in the binary mixture increases the volumetric flow without increasing the GWP. This addition, i.e., ternary mixtures, offers improved system performance, but the increase in the temperature glide of the mixture is possible. The use of counterflow heat exchangers with an appropriate length can be advantageous when the temperature difference between the heat exchange media matches the temperature glide. In this study, R744/R32/R1234ze(E) and R744/R32/R1234yf were found to be promising ternary mixtures. A simple modification of the system can compensate for the temperature glide to switch the system to the next generation of low-GWP refrigerants.
Author Contributions
Conceptualization and methodology, K.U. and B.B.S.; formal analysis and investigation, writing and draft prepations, K.U.; editing and supervision, B.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project low-grade heat powered cooling system-a green technology towards low carbon society. Bangladesh Climate Change Trust (BCCT-581).
Data Availability Statement
The sources are cited carefully. Also REFPROP database are used to prepare our own data.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| COP | Coefficient of performance (–) |
| H | Enthalpy (kJ/kg) |
| Δh | Enthalpy change during evaporation (kJ/kg) |
| L | Irreversibly low (W) |
| Q | Cooling/heating load (kW) |
| S | Entropy (J/kg/K) |
| T | Temperature (°C or K) |
| Subscripts | |
| COMPR | Compressor |
| COND | Condenser |
| EVA | Evaporator |
| EXP | Expansion valve |
| H | Heating |
| inv/INV | Inverter |
| PD | Pressure drop |
| R | Refrigerant |
| R | Cooling |
| V | Vapor |
| W | Water |
| Greek symbols | |
| η | Efficiency (–) |
| ρ | Density at evaporator output (kg/m3) |
References
- Calm, J.M.; Didion, D.A. Trade-offs in refrigerant selections: Past, present, and future. Int. J. Refrig. 1998, 21, 308–321. [Google Scholar] [CrossRef]
- UNEP. Report of the Technology and Economic Assessment Panel. Montreal Protocol On Substances that Deplete the Ozone Layer; UNEP: Nairobi, Kenya, 2016. [Google Scholar]
- Benhadid-Dib, S.; Benzaoui, A. Refrigerants and their environmental impact Substitution of hydro chlorofluorocarbon HCFC and HFC hydro fluorocarbon. Search for an adequate refrigerant. Energy Procedia 2012, 18, 1611–1623. [Google Scholar] [CrossRef]
- Hewitt, N.J.; McMullan, J.T. The replacement of CFCS in refrigeration equipment by environmentally benign alternatives. Appl. Therm. Eng. 1997, 17, 955–972. [Google Scholar] [CrossRef]
- Billiard, F.; Lucas, L. Fluorocarbons and global warming. Rev Générale Therm. 1998, 37, 417–423. [Google Scholar] [CrossRef]
- McMullan, J.T. Refrigeration and the environment—Issues and strategies for the future. Int. J. Refrig. 2002, 25, 89–99. [Google Scholar] [CrossRef]
- Kim, K.H.; Shon, Z.H.; Nguyen, H.T.; Jeon, E.C. A review of major chlorofluorocarbons and their halocarbon alternatives in the air. Atmos. Environ. 2011, 45, 1369–1382. [Google Scholar] [CrossRef]
- Calm, J.M. Refrigerant Transitions … Again. In Proceedings of the ASHRAE-NIST Refrigrants Conference, Gaithersburg, MD, USA, 29–30 October 2012; pp. 1–16. [Google Scholar]
- Aprea, C.; Greco, A.; Maiorino, A. An experimental evaluation of the greenhouse effect in the substitution of R134a with CO2. Energy 2012, 45, 753–761. [Google Scholar] [CrossRef]
- Powell, R.L. CFC phase-out: Have we met the challenge? J. Fluor. Chem. 2002, 114, 237–250. [Google Scholar] [CrossRef]
- Park, K.-J.; Seo, T.; Jung, D. Performance of alternative refrigerants for residential air-conditioning applications. Appl. Energy 2007, 84, 985–991. [Google Scholar] [CrossRef]
- Chen, J.; Yu, J. Performance of a new refrigeration cycle using refrigerant mixture R32/R134a for residential air-conditioner applications. Energy Build 2008, 40, 2022–2027. [Google Scholar] [CrossRef]
- Park, K.J.; Shim, Y.B.; Jung, D. Performance of R433A for replacing HCFC22 used in residential air-conditioners and heat pumps. Appl. Energy 2008, 85, 896–900. [Google Scholar] [CrossRef]
- Park, K.J.; Shim, Y.B.; Jung, D. A “drop-in” refrigerant R431A for replacing HCFC22 in residential air-conditioners and heat pumps. Energy Convers. Manag. 2009, 50, 1671–1675. [Google Scholar] [CrossRef]
- Devotta, S.; Waghmare, A.V.; Sawant, N.N.; Domkundwar, B.M. Alternatives to HCFC-22 for air conditioners. Appl. Therm. Eng. 2001, 21, 703–715. [Google Scholar] [CrossRef]
- Joudi, K.A.; Al-Amir, Q.R. Experimental Assessment of residential split type air-conditioning systems using alternative refrigerants to R-22 at high ambient temperatures. Energy Convers. Manag. 2014, 86, 496–506. [Google Scholar] [CrossRef]
- Qifang, Y.; Jiangping, C.; Zhijiu, C. Experimental investigation of R407C and R410A flow through electronic expansion valve. Energy Convers. Manag. 2007, 48, 1624–1630. [Google Scholar] [CrossRef]
- Karagoz, S.; Yilmaz, M.; Comakli, O.; Ozyurt, O. R134a and various mixtures of R22/R134a as an alternative to R22 in vapour compression heat pumps. Energy Convers. Manag. 2004, 45, 181–196. [Google Scholar] [CrossRef]
- Yun, R.; Heo, J.H.; Kim, Y. Evaporative heat transfer and pressure drop of R410A in microchannels R410A. Int. J. Refrig. 2006, 29, 92–100. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Navarro-Esbrí, J.; Makhnatch, P.; Molés, F. Refrigerant R32 as lower GWP working fluid in residential air conditioning systems in Europe and the USA. Renew. Sustain. Energy Rev. 2017, 80, 1031–1042. [Google Scholar] [CrossRef]
- Spatz, M.W.; Motta, S.F.Y.; Yana Motta, S.F. An evaluation of options for replacing HCFC-22 in medium temperature refrigeration systems. Int. J. Refrig. 2004, 27, 475–483. [Google Scholar] [CrossRef]
- R-32, Next-Generation Refrigerant|Benefits of Daikin Technology|Daikin Global n.d. Available online: http://www.daikin.com/about/why_daikin/benefits/r-32/ (accessed on 4 July 2017).
- Brown, J.S.; Yana-Motta, S.F.; Domanski, P.A. Comparitive analysis of an automotive air conditioning systems operating with CO2 and R134a. Int. J. Refrig. 2002, 25, 19–32. [Google Scholar] [CrossRef]
- Vaghela, J.K. Comparative Evaluation of an Automobile Air—Conditioning System Using R134a and Its Alternative Refrigerants. Energy Procedia 2017, 109, 153–160. [Google Scholar] [CrossRef]
- Minjares, R. Refrigerants for Light-Duty Passenger Vehicle Air Conditioning Systems Technical Assessment of Alternatives to HFC-134a; International Council of Clean Transport: Hague, The Netherlands, 2011. [Google Scholar]
- Ravikumar, T.S.; Lal, D.M. On-road performance analysis of R134a/R600a/R290 refrigerant mixture in an automobile air-conditioning system with mineral oil as lubricant. Energy Convers. Manag. 2009, 50, 1891–1901. [Google Scholar] [CrossRef]
- Bolaji, B.O. Experimental study of R152a and R32 to replace R134a in a domestic refrigerator. Energy 2010, 35, 3793–3798. [Google Scholar] [CrossRef]
- An Mota-Babiloni, A.; Belman-Flores, J.M.; Makhnatch, P.; Navarro-Esbrí, J.; Barroso-Maldonado, J.M. Experimental exergy analysis of R513A to replace R134a in a small capacity refrigeration system. Energy 2018, 162, 99–110. [Google Scholar] [CrossRef]
- Tiwari, A.; Gupta, R.C. Recent development of domestic refrigerator—A review. Int. J. Eng. Sci. Technol. 2011, 3, 4233–4239. [Google Scholar]
- Sciance, F. The Transition from HFC-134a to a Low-GWP Refrigerant in Mobile Air Conditioners HFO-1234yf; General Motors Public Policy Center: Detroit, MI, USA, 2013; pp. 1–15. [Google Scholar]
- Navarro-Esbrí, J.; Mendoza-Miranda, J.M.; Mota-Babiloni, A.; Barragán-Cervera, A.; Belman-Flores, J.M. Experimental analysis of R1234yf as a drop-in replacement for R134a in a vapor compression system. Int. J. Refrig. 2013, 36, 870–880. [Google Scholar] [CrossRef]
- Zilio, C.; Brown, J.S.; Schiochet, G.; Cavallini, A. The refrigerant R1234yf in air conditioning systems. Energy 2011, 36, 6110–6120. [Google Scholar] [CrossRef]
- De Chazournes, L.B. Kyoto Protocol to the United Nations Framework Convention on Climate Change; United Nations Audiovisual Library of International Law: Maastricht, The Netherlands, 1998. [Google Scholar]
- European Environment Agency. Company Reporting for Regulation (EU) No 517/2014 on Fluorinated Greenhouse Gases; European Environment Agency: Copenhagen, Denmark, 2016. [Google Scholar]
- EU MAC Directive. Directive 2006/40/EC of the European Parliament and the Council of the European Union; Official Journal of the European Union: Maastricht, The Netherlands, 2006; L 161. [Google Scholar]
- EPA. 40 CFR Part 82—Protection of stratospheric ozone: Change of listing status for certain substitutes under the significant new alternative policy program, Final Rule. Fed. Regist. 1990, 80, 1–91. [Google Scholar]
- Pham, H.; Rajendran, R. R32 And HFOs As Low-GWP Refrigerants For Air Conditioning. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 24–28 May 2012; pp. 1–10. [Google Scholar]
- UNFCC. United Nations/Framework Convention on Climate Change. Paris Agreement (21st Conf Parties) 2015. p. 27. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 20 August 2022).
- Fukushima, M.; Hashimoto, M. Next Generation Low-GWP Refrigerants “AMOLEA-TM.”; Reports of the Research Laboratory; Asahi Glass Co., Ltd.: Tokyo, Japan, 2015. [Google Scholar]
- Meno, A. Laws and Regulation for Fluorocarbons in Japan; Ministry of Economy, Trade and Industry, Japan: Tokyo, Japan, 2015. [Google Scholar]
- Uddin, K.; Arakaki, S.; Saha, B.B. Thermodynamic analysis of low-GWP blends to replace R410A for residential building air conditioning applications. Environ. Sci. Pollut. Res. 2021, 28, 2934–2947. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Thu, K.; Saha, B.B. Environmental assessment and characteristics of next generation refrigerants. Evergr. Jt. J. Nov. Carbon. Resour. Sci. Green. Asia Strateg. 2018, 5, 58–66. [Google Scholar] [CrossRef]
- Minor, B.H.; Herrmann, D.; Gravell, R. Flammability characteristics of HFO-1234yf. Process. Saf. Prog. 2010, 29, 150–154. [Google Scholar] [CrossRef]
- Honeywell SolsticeTM yf Refrigerants. Honeywell Technical Bulletin. 2012. Available online: https://www.honeywell-refrigerants.com/europe/wp-content/uploads/2013/03/honeywell-solstice-yf-technical-bulletin.pdf (accessed on 12 December 2021).
- Feng, B.; Yang, Z.; Zhai, R. Experimental study on the influence of the flame retardants on the flammability of R1234yf. Energy 2018, 143, 212–218. [Google Scholar] [CrossRef]
- Minor, B.; Spatz, M. HFO-1234yf Low GWP Refrigerant Update. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 14–17 July 2008; pp. 1–8. [Google Scholar]
- Tanaka, K.; Higashi, Y. Thermodynamic properties of HFO-1234yf (2,3,3,3-tetrafluoropropene). Int. J. Refrig. 2010, 33, 474–479. [Google Scholar] [CrossRef]
- Akasaka, R.; Tanaka, K.; Higashi, Y. Thermodynamic property modeling for 2,3,3,3-tetrafluoropropene (HFO-1234yf). Int. J. Refrig. 2010, 33, 52–60. [Google Scholar] [CrossRef]
- Lai, N.A.; Vrabec, J.; Raabe, G.; Fischer, J.; Wendland, M. Description of HFO-1234yf with BACKONE equation of state. Fluid Phase Equilib. 2011, 305, 204–211. [Google Scholar] [CrossRef]
- Richter, M.; McLinden, M.O.; Lemmon, E.W. Thermodynamic properties of 2,3,3,3-tetrafluoroprop-1-ene (R1234yf): Vapor pressure and p- rho- T Measurements and an Equation of State. J. Chem. Eng. Data 2011, 56, 3254–3264. [Google Scholar] [CrossRef]
- SAE-CRP1234; Industry Evaluation of Low Global Warning Potential Refrigerant HFO-1234yf. SAE International: Warrendale, PA, USA, 2009.
- Lee, Y.; Jung, D. A brief performance comparison of R1234yf and R134a in a bench tester for automobile applications. Appl. Therm. Eng. 2012, 35, 240–242. [Google Scholar] [CrossRef]
- Spatz, M.; Minor, B. HFO-1234yf Low GWP Refrigerant: A Global Sustainable Solution for Mobile Air Conditioning. In Proceedings of the SAE Alternate Refrigerant Systems Symposium, Scottsdale, AZ, USA, 10 June 2008; pp. 1–26. [Google Scholar]
- Del Col, D.; Torresin, D.; Cavallini, A. Heat transfer and pressure drop during condensation of the low GWP refrigerant R1234yf. Int. J. Refrig. 2010, 33, 1307–1318. [Google Scholar] [CrossRef]
- Koban, M. HFO-1234yf Low GWP Refrigerant—Information for Manufacturing and Service Facilities. 2010. [Google Scholar]
- Jarall, S. Study of refrigeration system with HFO-1234yf as a working fluid. Int. J. Refrig. 2012, 35, 1668–1677. [Google Scholar] [CrossRef]
- Qi, Z. Performance improvement potentials of R1234yf mobile air conditioning system. Int. J. Refrig. 2015, 58, 35–40. [Google Scholar] [CrossRef]
- Kojima, H.; Fukuda, S.; Kondou, C.; Takata, N.; Koyama, S. Comparative Assessment of Heat Pump Cycle Operated with R32/R1234ze(E) and R32/R1234yf mixtures. In Proceedings of the International Refrigeration and Air Conditioning Conference, Yokohama, Japan, 16–22 August 2015; pp. 1–8. [Google Scholar]
- Barve, A.; Cremaschi, L. Drop-in Performance of Low GWP Refrigerants in a Heat Pump System for Residential Applications. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 16–19 July 2012; pp. 1–9. [Google Scholar]
- Kenji, U.; Kazuki, W.; Yokoyama, A.; Akimasa, S.A.; Ueda, K.; Wajima, K.; Yokoyama, A.; As, A. Low GWP refrigerant application for the centrifugal chiller—Study for application of HFO-1234ze(E). Trans Jpn. Soc Refrig. Air Cond. Eng. 2011, 28, 503–508. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Tian, T. Flammability of Trans-1, 3, 3, 3-tetrafluoroprop-1-ene and its binary blends. Energy 2015, 91, 386–392. [Google Scholar] [CrossRef]
- Higashi, Y.; Tanaka, K.; Ichikawa, T. Critical Parameters and Saturated Densities in the Critical Region for trans-1,3,3,3-Tetrafluoropropene (HFO-1234ze(E)). J. Chem. Eng. Data 2010, 55, 1594–1597. [Google Scholar] [CrossRef]
- Brown, J.S.; Zilio, C.; Cavallini, A. Thermodynamic properties of eight fluorinated olefins. Int. J. Refrig. 2010, 33, 235–241. [Google Scholar] [CrossRef]
- Fukuda, S.; Kondou, C.; Takata, N.; Koyama, S. Low GWP refrigerants R1234ze(E) and R1234ze(Z) for high temperature heat pumps. Int. J. Refrig. 2014, 40, 161–173. [Google Scholar] [CrossRef]
- Koyama, S.; Fukuda, S.; Osafune, K.; Akasaka, R. Development of low GWP refrigerants suitable for heat pump systems. In Proceedings of the JRAIA International Symposium, Kobe, Japan, 3–4 April 2012. [Google Scholar]
- Brown, J.S.; Zilio, C.; Cavallini, A. The fluorinated olefin R-1234ze(Z) as a high-temperature heat pumping refrigerant. Int. J. Refrig. 2009, 32, 1412–1422. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Ge, Z.; Duan, Y.; Yang, Z. Thermodynamic performance analyses and optimization of subcritical and transcritical organic Rankine cycles using R1234ze(E) for 100â(E)“200°C heat sources. Energy Convers. Manag. 2017, 149, 140–154. [Google Scholar] [CrossRef]
- Koyama, S.; Takata, N.; Fukuda, S. An experimental study on heat pump cycle using zeotropic binary refrigerant of HFO-1234ze(E) and HFC-32. In Proceedings of the 10th IEA Heat Pump Conference, Tokyo, Japan, 14–16 September 2011; pp. 1–10. [Google Scholar]
- Koyama, S.; Takata, N.; Fukuda, S. Drop-in Experiments on Heat Pump Cycle Using HFO-1234ze(E) and Its Mixtures with HFC-32. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 9–12 July 2010; pp. 1–7. [Google Scholar]
- Kabeel, A.E.; Khalil, A.; Bassuoni, M.M.; Raslan, M.S. Comparative experimental study of low GWP alternative for R134a in a walk-in cold room. Int. J. Refrig. 2016, 69, 303–312. [Google Scholar] [CrossRef]
- Lai, N.A. Equations of state for HFO-1234ze(E) and their application in the study on refrigeration cycle. Int. J. Refrig. 2014, 43, 194–202. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Navarro-Esbrí, J.; Barragán, Á.; Molés, F.; Peris, B. Drop-in energy performance evaluation of R1234yf and R1234ze(E) in a vapor compression system as R134a replacements. Appl. Therm. Eng. 2014, 71, 259–265. [Google Scholar] [CrossRef]
- An Mota-Babiloni, A.; Navarro-Esbrí, J.; Barrag An-Cervera, A.; Mol, F.; Peris, B. Drop-in analysis of an internal heat exchanger in a vapour compression system using R1234ze(E) and R450A as alternatives for R134a. Energy 2015, 90, 1636–1644. [Google Scholar] [CrossRef]
- Fukuda, S.; Kojima, H.; Kondou, C.; Takata, N.; Koyama, S. Comparative assessment on irreversible losses in heat pumps using R744/R32/R1234yf and R744/R32/R1234ze(E). Sci. Technol. Built Environ. 2016, 22, 1118–1127. [Google Scholar] [CrossRef]
- Yamamoto, S.; Koyama, S. Performance Evaluation of Heat Pump Cycle Using Non-Azeotropic Refrigerant Mixture R32/R1234ze(E); Kyushu University: Fukuoka, Japan, 2014. [Google Scholar]
- Higashi, Y.; Ashizawa, M.; Kabata, Y.; Majima, T.; Uematsu, M.; Watanabe, K. Measurements of vapor pressure, vapor-liquid coexistence curve and critical parameters of Refrigerant 152a. Trans Jpn. Soc. Mech. Eng. Ser. B 1987, 53, 1379–1385. [Google Scholar] [CrossRef][Green Version]
- Tamatsu, T.; Sato, H.; Watanabe, K. An equation of state for 1,1-difluoroethane (HFC 152a). Int. J. Refrig. 1993, 16, 347–352. [Google Scholar] [CrossRef]
- Van Poolen, L.J.; Holcomb, C.D.; Niesen, V.G. Critical temperature and density from liquid-vapor coexistence data: Application to refrigerants R32, R124, and R152a. Fluid Phase Equilib. 1997, 129, 105–111. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013 The Physical Science Basis Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Bitzer Group of Companies. Refrigerant Report, 15th ed.; Bitzer Group of Companies: Sindelfingen, Germany, 2014. [Google Scholar]
- Cabello, R.; Sánchez, D.; Llopis, R.; Arauzo, I.; Torrella, E. Experimental comparison between R152a and R134a working in a refrigeration facility equipped with a hermetic compressor. Int. J. Refrig. 2015, 60, 92–105. [Google Scholar] [CrossRef]
- Park, K.-J.; Jung, D. Performance of alternative refrigerant R430A on domestic water purifiers. Energy Convers. Manag. 2009, 50, 3045–3050. [Google Scholar] [CrossRef]
- ANSI/ASHRAE-34-2007; Designation and Safety Classification of Refrigerants. ASHRAE: Atlanta, GA, USA, 2008; Volume 4723.
- Cabello, R.; Sánchez, D.; Llopis, R.; Catalán, J.; Nebot-Andrés, L.; Torrella, E. Energy evaluation of R152a as drop in replacement for R134a in cascade refrigeration plants. Appl. Therm. Eng. 2017, 110, 972–984. [Google Scholar] [CrossRef]
- Pearson, A. Carbon dioxide—New uses for an old refrigerant. Int. J. Refrig. 2005, 28, 1140–1148. [Google Scholar] [CrossRef]
- Austin, B.T.; Sumathy, K. Transcritical carbon dioxide heat pump systems: A review. Renew. Sustain. Energy Rev. 2011, 15, 4013–4029. [Google Scholar] [CrossRef]
- Bolaji, B.O.; Huan, Z. Ozone depletion and global warming: Case for the use of natural refrigerant—A review. Renew. Sustain. Energy Rev. 2013, 18, 49–54. [Google Scholar] [CrossRef]
- Weber, C.; Favrat, D. Conventional and advanced CO2 based district energy systems. Energy 2010, 35, 5070–5081. [Google Scholar] [CrossRef]
- Maina, P.; Huan, Z. A review of carbon dioxide as a refrigerant in refrigeration technology. S. Afr. J. Sci. 2015, 111, 1–10. [Google Scholar] [CrossRef]
- Lorentzen, G. Revival of carbon dioxide as a refrigerant. Int. J. Refrig. 1994, 17, 292–301. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Tian, H. A review of transcritical carbon dioxide heat pump and refrigeration cycles. Energy 2013, 55, 156–172. [Google Scholar] [CrossRef]
- Lorentzen, G.; Pettersen, J. A new, efficient and environmentally benign system for car air-conditioning. Int. J. Refrig. 1993, 16, 4–12. [Google Scholar] [CrossRef]
- Koyama, K.; Xue, J.; Kuwahara, K. Performance Prediction Method of CO2 Cycle for Air Cooling. Trans. Jpn. Soc. Refrig. Air Cond. Eng. 2009, 26, 325–333. [Google Scholar] [CrossRef]
- Xue, J.; Koyama, S.; Kuwahara, K. Performance Prediction of a R744 Transcritical Cycle for Air Conditioning. In Proceedings of the International Symposium Next-generation Air Conditioning Refrigeration Technology, Tokyo, Japan, 17–19 February 2010; pp. 17–19. [Google Scholar]
- Jing-Yang, M.; Jiang-Ping, C.; Zhi-Jiu, C. System design and analysis of trans-critical carbon-dioxide automotive air-conditioning system. J. Zhejiang Univ. Sci. 2003, 4, 305–308. [Google Scholar]
- Girotto, S.; Minetto, S.; Neksa, P. Commercial refrigeration system using CO2 as the refrigerant. Int. J. Refrig. 2004, 27, 717–723. [Google Scholar] [CrossRef]
- He, Y.; Deng, J.; Zheng, L.; Zhang, Z. Performance optimization of a transcritical CO2 refrigeration system using a controlled ejector. Int. J. Refrig. 2016, 75, 250–261. [Google Scholar] [CrossRef]
- Pitarch, M.; Navarro-Peris, E.; Gonzalvez, J.; Corberan, J.M. Analysis and optimisation of different two-stage transcritical carbon dioxide cycles for heating applications. Int. J. Refrig. 2016, 70, 235–2342. [Google Scholar] [CrossRef]
- Chen, G.; Volovyk, O.; Zhu, D.; Ierin, V.; Shestopalov, K. Theoretical analysis and optimization of a hybrid CO2 transcritical mechanical compression—Ejector cooling cycle. Int. J. Refrig. 2017, 74, 86–94. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Celik, A.; Radermacher, R. Performance of CO2 cycles with a two-stage compressor. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 12–14 July 2004; pp. 1–8. [Google Scholar]
- Nekså, P. CO2 heat pump systems. Int. J. Refrig. 2002, 25, 421–427. [Google Scholar] [CrossRef]
- Dawo, F.; Fleischmann, J.; Kaufmann, F.; Schifflechner, C.; Eyerer, S.; Wieland, C.; Spliethoff, H. R1224yd(Z), R1233zd(E) and R1336mzz(Z) as replacements for R245fa: Experimental performance, interaction with lubricants and environmental impact. Appl. Energy 2021, 288, 116661. [Google Scholar] [CrossRef]
- Sakoda, N.; Higashi, Y.; Akasaka, R. Measurements of PvT Properties, Vapor Pressures, Saturated Densities, and Critical Parameters for trans-1,1,1,4,4,4-Hexafluoro-2-butene (R1336mzz(E)). J. Chem. Eng. Data 2021, 66, 734–739. [Google Scholar] [CrossRef]
- Kedzierski, M.A.; Lin, L. Pool boiling of HFO-1336mzz(Z) on a reentrant cavity surface. Int. J. Refrig. 2019, 104, 476–483. [Google Scholar] [CrossRef]
- Kontomaris, K. Zero-ODP, low-GWP, non-flammable working fluids for high temperature heat pumps. In Proceedings of the ASHRAE 2014 Annual Conference, Seattle, WA, USA, 23 July 2014. [Google Scholar]
- Lampugnani, G.; Zgliczynski, M. R290 as a Substitute of R502 and R22 in Commercial Refrigeration and Air Conditioning. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 23 July 1996; pp. 83–88. [Google Scholar]
- Granryd, E. Hydrocarbons as refrigerants—An overview. Int. J. Refrig. 2001, 24, 15–24. [Google Scholar] [CrossRef]
- Halimic, E.; Ross, D.; Agnew, B.; Anderson, A.; Potts, I. A comparison of the operating performance of alternative refrigerants. Appl. Therm. Eng. 2003, 23, 1441–1451. [Google Scholar] [CrossRef]
- Bjerre, P.; Larsen, P. Evaluation of N-butane as a potential refrigerant for household compressors. In Proceedings of the International Compressor Engineering Conference, Purdue, IN, USA, 17–20 July 2006; pp. 1–6. [Google Scholar]
- Palm, B. Hydrocarbons as refrigerants in small heat pump and refrigeration systems—A review. Int. J. Refrig. 2008, 31, 552–563. [Google Scholar] [CrossRef]
- Wongwises, S.; Chimres, N. Experimental study of hydrocarbon mixtures to replace HFC-134a in a domestic refrigerator. Energy Convers. Manag. 2005, 46, 85–100. [Google Scholar] [CrossRef]
- Corberán, J.M.; Segurado, J.; Colbourne, D.; Gonzálvez, J. Review of standards for the use of hydrocarbon refrigerants in A/C, heat pump and refrigeration equipment. Int. J. Refrig. 2008, 31, 748–756. [Google Scholar] [CrossRef]
- Suzuki, M. Application of adsorption cooling systems to automobiles. Heat Recover. Syst. ClIP 1993, 13, 335–340. [Google Scholar] [CrossRef]
- Zhang, L. Design and testing of an automobile waste heat adsorption cooling system. Appl. Therm. Eng. 2000, 20, 103–114. [Google Scholar] [CrossRef]
- Wang, R.; Oliveira, R. Adsorption refrigeration—An efficient way to make good use of waste heat and solar energy. Prog. Energy. Combust. Sci. 2006, 32, 424–458. [Google Scholar] [CrossRef]
- Abdullah, M.O.; Tan, I.A.W.; Lim, L.S. Automobile adsorption air-conditioning system using oil palm biomass-based activated carbon: A review. Renew. Sustain. Energy Rev. 2011, 15, 2061–2072. [Google Scholar] [CrossRef]
- Wang, R.Z.; Li, Y. Perspectives for natural working fluids in China. Int. J. Refrig. 2007, 30, 568–581. [Google Scholar] [CrossRef]
- Critoph, R.E. Performance estimation of convective thermal wave adsorption cycles. Appl. Therm. Eng. 1996, 16, 429–437. [Google Scholar] [CrossRef]
- Balaras, C.A.; Grossman, G.; Henning, H.-M.; Ferreira, C.A.I.; Podesser, E.; Wang, L.; Wiemken, E. Solar air conditioning in Europe—An overview. Renew. Sustain. Energy Rev. 2007, 11, 299–314. [Google Scholar] [CrossRef]
- Sumathy, K.; Huang, Z.C.; Li, Z.F. Solar absorption cooling with low grade heat source—A strategy of development in south China. Sol. Energy 2002, 72, 155–165. [Google Scholar] [CrossRef]
- Wang, R.Z.; Xia, Z.Z.; Wang, L.W.; Lu, Z.S.; Li, S.L.; Li, T.X.; Wu, J.Y.; He, S. Heat transfer design in adsorption refrigeration systems for efficient use of low-grade thermal energy. Energy 2011, 36, 5425–5439. [Google Scholar] [CrossRef]
- Thu, K.; Kim, Y.-D.D.; Myat, A.; Chun, W.G.; Ng, K.C. Entropy generation analysis of an adsorption cooling cycle. Int. J. Heat Mass. Transf. 2013, 60, 143–150. [Google Scholar] [CrossRef]
- Ng, K.C.; Thu, K.; Saha, B.B.; Chakraborty, A. Study on a waste heat-driven adsorption cooling cum desalination cycle. Int. J. Refrig. 2012, 35, 685–693. [Google Scholar] [CrossRef]
- Myat, A.; Thu, K.; Kim, Y.D.; Chakraborty, A.; Chun, W.G.; Ng, K.C. A second law analysis and entropy generation minimization of an absorption chiller. Appl. Therm. Eng. 2011, 31, 2405–2413. [Google Scholar] [CrossRef]
- Bella, B.; Kaemmer, N. An assessment of low GWP refrigerants in different applications. In Proceedings of the 23rd IIR Congress of Refrigeration, Prague, Czech Republic, 26–28 August 2011; p. 8. [Google Scholar]
- Kamiaka, T.; Dang, C.; Hihara, E. Vapor-liquid equilibrium measurements for binary mixtures of R1234yf with R32, R125, and R134a. Int. J. Refrig. 2013, 36, 965–971. [Google Scholar] [CrossRef]
- Koyama, S.; Takata, N.; Fukuda, S.; Akasaka, R. Measurement of vapor-liquid equilibrium of HFO1234ze(E)/HFC32. In Proceedings of the JSRAE Annual Conference, Tokyo, Japan, 14-16 September 2010. [Google Scholar]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Reference Fluid Thermodynamic and Transport Properties (REFPROP), Version 9.1; NIST: Gaithersburg, MD, USA, 2013.
- Mota-Babiloni, A.; Navarro-Esbrí, J.; Molés, F.; Cervera, Á.B.; Peris, B.; Verdú, G. A review of refrigerant R1234ze(E) recent investigations. Appl. Therm. Eng. 2016, 95, 211–222. [Google Scholar] [CrossRef]
- Wang, X.; Amrane, K. AHRI Low Global Warming Potential Alternative Refrigerants Evaluation Program (Low-GWP AREP)—Summary of Phase I Testing Results. In Proceedings of the 15th International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 14–17 July 2014; pp. 1–10. [Google Scholar]
- Koyama, S.; Takata, N.; Matsuo, Y.; Yoshitake, D.; Fukuda, S. Possibility to introduce HFO-1234ze(E) and its mixture with HFC-32 as low-GWP alternatives for heat pump/refrigeration systems SYSTEMS. In Proceedings of the 2010 International Symposium on Next-generation Air Conditioning and Refrigeration Technology, Tokyo, Japan, 17–19 February 2010; pp. 1–10. [Google Scholar]
- Akasaka, R. Thermodynamic property models for the difluoromethane (R-32)+trans-1,3,3,3-tetrafluoropropene (R-1234ze(E)) and difluoromethane+2,3,3,3-tetrafluoropropene (R-1234yf) mixtures. Fluid Phase Equilib. 2013, 358, 98–104. [Google Scholar] [CrossRef]
- Kojima, H.; Arakaki, S.; Fukuda, S.; Takata, N.; Kondou, C.; Koyama, S. Comparative Study on Heat Pump Cycle Using R32/R1234yf and R744/R32/R1234yf. In Proceedings of the 8th Asian Conference on Refrigeration and Air-Conditioning, Taipei, Taiwan, 15–17 May 2016; pp. 8–11. [Google Scholar]
- Fukuda, S.; Takata, N.; Koyama, S. The Circulation Composition Characteristic of the Zeotropic Mixture R1234ze ( E )/ R32 in a Heat Pump Cycle. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 16–19 July 2012; pp. 1–8. [Google Scholar]
- Fukuda, S.; Kondou, C.; Takata, N.; Koyama, S. Evaluation of High-Temperature Heat Pump Cycle Performance Using Low-Gwp Refrigerants. In Proceedings of the 4th IIR Conference on Thermophysical Properties and Transfer Processes of Refrigerants, Delft, The Netherlands, 17–19 June 2013; pp. 1–8. [Google Scholar]
- Alabdulkarem, A.; Eldeeb, R.; Hwang, Y.; Aute, V.; Radermacher, R. Testing, simulation and soft-optimization of R410A low-GWP alternatives in heat pump system. Int. J. Refrig. 2015, 60, 106–117. [Google Scholar] [CrossRef]
- Mani, K.; Selladurai, V. Experimental analysis of a new refrigerant mixture as drop-in replacement for CFC12 and HFC134a. Int. J. Therm. Sci. 2008, 47, 1490–1495. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, D.Y.; Kim, Y. Performance characteristics of a small-capacity directly cooled refrigerator using R290/R600a (55/45). Int. J. Refrig. 2008, 31, 734–741. [Google Scholar] [CrossRef]
- Mohanraj, M.; Jayaraj, S.; Muraleedharan, C. Environment friendly alternatives to halogenated refrigerants-A review. Int. J. Greenh. Gas. Control. 2009, 3, 108–119. [Google Scholar] [CrossRef]
- Jung, D.; Kim, C.B.; Song, K.; Park, B. Testing of propane/isobutane mixture in domestic refrigerators. Int. J. Refrig. 2000, 23, 517–527. [Google Scholar] [CrossRef]
- Akasaka, R.; Tanaka, K.; Higashi, Y. Measurements of saturated densities and critical parameters for the binary mixture of 2,3,3,3-tetrafluoropropene (R-1234yf) + difluoromethane (R-32). Int. J. Refrig. 2013, 36, 1341–1346. [Google Scholar] [CrossRef]
- Tanaka, T.; Hidekazu, O.; Katsuya, U.; Jun, I.; Otsuka, T.; Nogami, T.; Dobashi, R. Development of a new low-GWP refrigerant composed of HFO-1123 (trifluoroethylene). In Proceedings of the AIChE Annual Meeting, Atlanta, GA, USA, 18 November 2014. [Google Scholar]
- Higashi, Y.; Akasaka, R. Measurements of Thermodynamic Properties for R1123 and R1123 + R32 Mixture. In Proceedings of the International Compressor Engineering Conference, Purdue, IN, USA, 11–14 July 2016; pp. 1–10. [Google Scholar]
- Richardson, R.N.; Butterworth, J.S. The performance of propane/isobutane mixtures in a vapour-compression refrigeration system. Int. J. Refrig. 1995, 18, 58–62. [Google Scholar] [CrossRef]
- Dalkilic, A.S.; Wongwises, S. A performance comparison of vapour-compression refrigeration system using various alternative refrigerants. Int. Commun. Heat Mass. Transf. 2010, 37, 1340–1349. [Google Scholar] [CrossRef]
- Mohanraj, M.; Jayaraj, S.; Muraleedharan, C.; Chandrasekar, P. Experimental investigation of R290/R600a mixture as an alternative to R134a in a domestic refrigerator. Int. J. Therm. Sci. 2009, 48, 103106–103142. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, J.M.; Kim, M.S. Cooling performance of several CO2/propane mixtures and glide matching with secondary heat transfer fluid. Int. J. Refrig. 2008, 31, 800–806. [Google Scholar] [CrossRef]
- Tian, Q.; Cai, D.; Ren, L.; Tang, W.; Xie, Y.; He, G.; Liu, F. An experimental investigation of refrigerant mixture R32/R290 as drop-in replacement for HFC410A in household air conditioners. Int. J. Refrig. 2015, 57, 216–228. [Google Scholar] [CrossRef]
- Mohanraj, M.; Muraleedharan, C.; Jayaraj, S. A review on recent developments in new refrigerant mixtures for vapour compression-based refrigeration, air-conditioning and heat pump units. Int. J. Energy Res. 2011, 35, 647–669. [Google Scholar] [CrossRef]
- Fukuda, S.; Kondou, C.; Takata, N.; Koyama, S. Cycle performance of low GWP refrigerant mixtures R-32/1234ze(E) and R-744/1234ze(E). Proceedings of 7th Asian Conference on Refrigeration and Air Conditioning, Jeju, Korea, 18–21 May 2014. [Google Scholar]
- Kojima, H.; Koyama, S. Performance Evaluation of Low GWP HFO-R1234ze (E) and R1234y Based Refrigerant Mixtures for Heat Pump Cycle; Kyushu University: Fukuoka, Japan, 2016. [Google Scholar]
- Kondou, C.; Mishima, F.; Koyama, S. Condensation and evaporation of R32/R1234ze(E) and R744/R32/R1234ze(E) flow in horizontal microfin tubes. Sci. Technol. Built Environ. 2015, 21, 564–577. [Google Scholar] [CrossRef]
- Maczek, K.; Muller, J.; Wojtas, K.; Domanski, P.A. Ternary Zeotropic Mixture with CO2 Component for R-22 Heat pump application. In Proceedings of the CLIMA Conference, Brussels, Belgium, 30 August 1997. [Google Scholar]
- Hakkaki-fard, A.L.I.; Aidoun, Z.; Ouzzane, M. Air-source heat pumps with refrigerant mixtures for cold climates. In Proceedings of the 11th IEA Heat Pump Conference, Montreal, Canada, 12–16 May 2014; pp. 1–11. [Google Scholar]
- Jakobs, R.; Kruse, H. The use of non-azeotropic refrigerant mixtures in heat pumps for energy saving. Int. J. Refrig. 1979, 2, 29–32. [Google Scholar] [CrossRef]
- Kruse, H. The advantages non-azeotropic refrigerant mixtures for heat pump application. Int. J. Refrig. 1981, 4, 119–125. [Google Scholar] [CrossRef]
- Swinney, J.; Jones, W.; Wilson, J. The impact of mixed non-azeotropic working fluids on refrigeration system performance. Int. J. Refrig. 1998, 21, 607–616. [Google Scholar] [CrossRef]
- Wang, L.; Dang, C.; Hihara, E. Experimental and Theoretical Study on Condensation Heat Transfer of Nonazeotropic Refrigerant Mixtures R1234yf/R32 Inside a Horizontal Smooth Tube. In Proceedings of the International Refrigeration and Air Conditioning Conference, Purdue, IN, USA, 16–19 July 2012. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).