A Review on the Molecular Modeling of Argyrodite Electrolytes for All-Solid-State Lithium Batteries
Abstract
1. Introduction
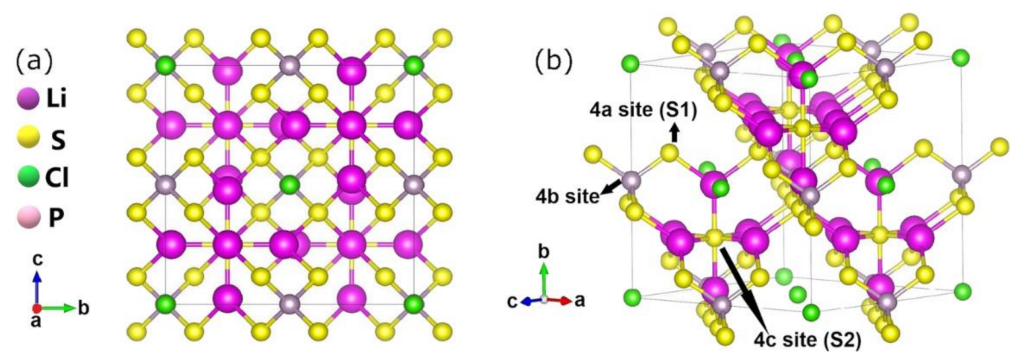
2. Modeling of Argyrodite Materials
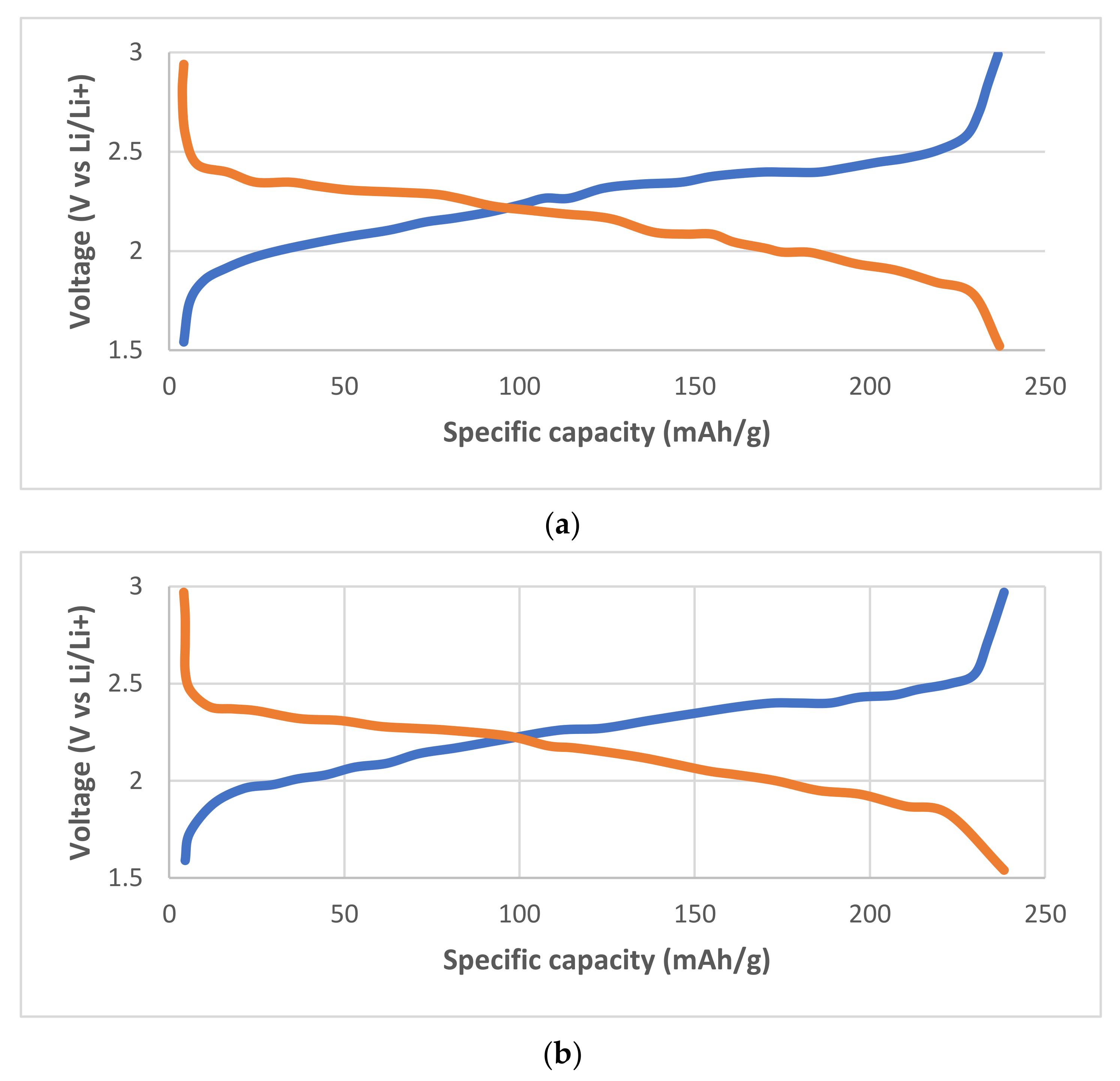
3. Single Halides Argyrodites
4. Mixed Halide Argyrodites
5. Ionic Conductivity of Li5PS4Cl2
Li5PS4Cl2 Collective Diffusion Coefficient
6. Li6PS5Cl Ionic Conductivity
6.1. Li6PS5Cl Pure Diffusion
6.2. Li6PS5Cl: Disorder Effect
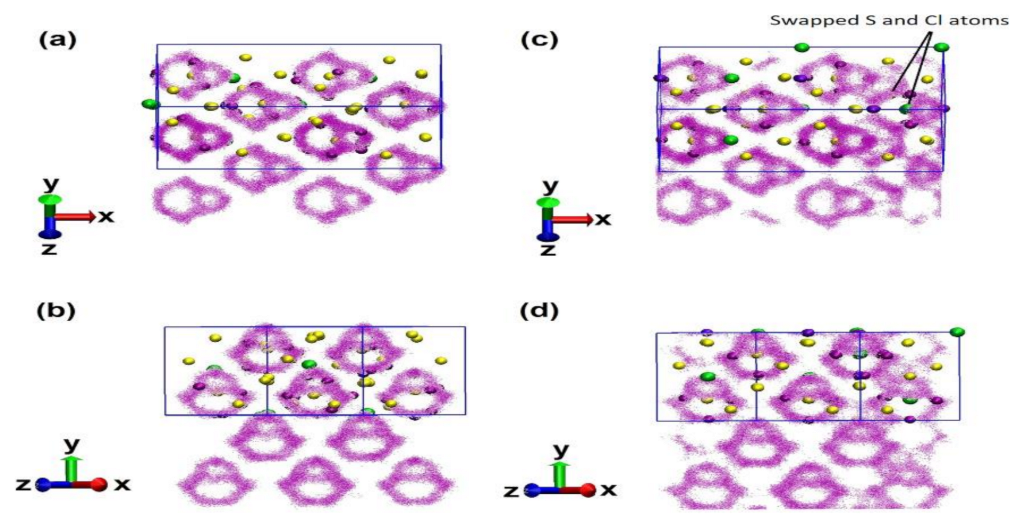
6.3. Li6PS5Cl: Li-Ions Vacancies and S–Cl Disorder
7. Methods of Argyrodite Electrolyte Modeling
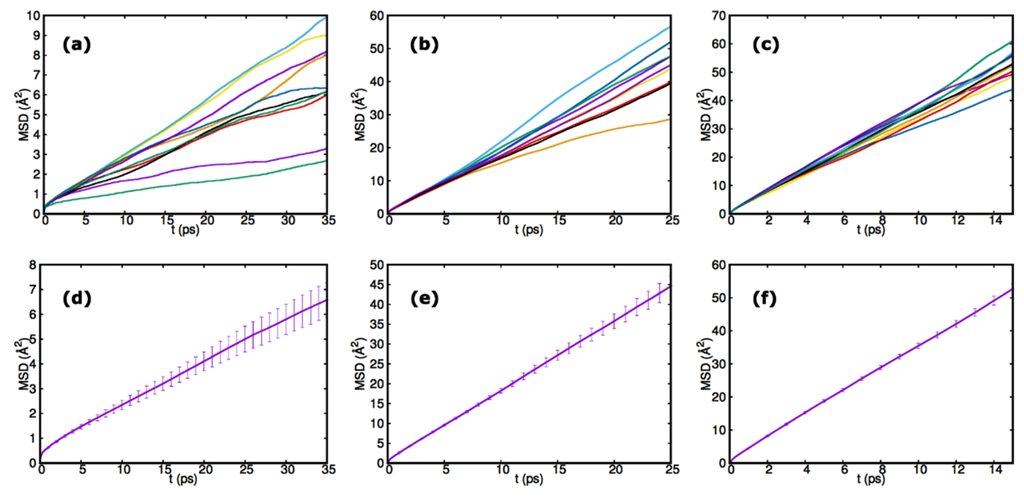
7.1. Ab Initio MD Simulations
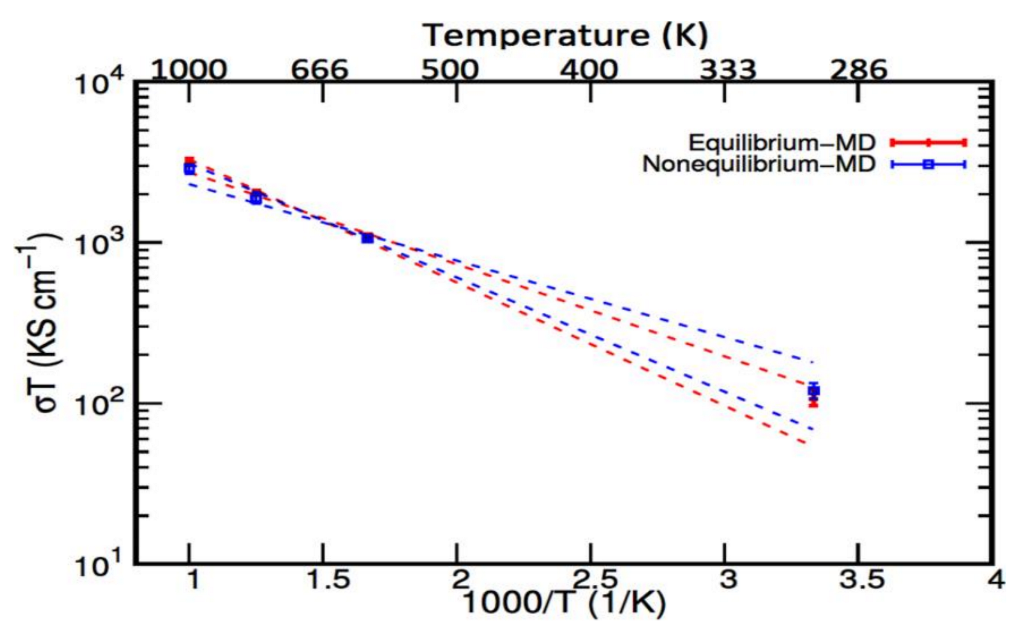
7.2. Equilibrium and Nonequilibrium Models of Motion
7.3. Machine Learning (ML) Models
8. Discussion
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Description |
| activation energy | |
| n | band index |
| kB | Boltzmann’s constant |
| Brillioun zone sampling factor | |
| Lithium center-of-mass coefficient of diffusion | |
| ci | color charge/label |
| color current | |
| Fc | color field |
| α | Nosé–Hoover thermostat couples of atoms |
| diffusion pre-exponential factor | |
| ith displacement of N lithium-ions over a period | |
| e | elementary electron charge |
| ensemble average | |
| Q1, Q2 and Q3 | friction coefficients |
| inter-atomic force on atom | |
| n | ion density of Li |
| σ | ionic conductivity of the material |
| mass of atom | |
| momentum | |
| g | number of degrees of freedom |
| N | number of lithium-ions subject to a color field |
| partial densities states | |
| position | |
| ith position of lithium-ion at time t | |
| coefficient of self-diffusion | |
| smearing factor | |
| T | target temperature |
| Z | valence of Li |
| velocity of th Li-ion | |
| k | wave vector |
| weighing factor | |
| Å | Angstrom |
| AI–EMD/EMD | Ab initio equilibrium molecular dynamics |
| AI–NEMD/NEMD | Ab initio nonequilibrium molecular dynamics |
| ASSB | All-solid-state batteries |
| ASSLB | All-solid-state lithium battery |
| DC | Direct current |
| DFT | Density functional theory |
| DME | Dimethoxy ethane |
| EC | Electronic conductivity |
| EIS | Electrochemical impedance spectroscopy |
| GGA | Generalized gradient approximation |
| GPW | Gaussian and plane-wave |
| IC | Ionic conductivity |
| IL | Ionic liquid |
| LIB | Lithium-ion batteries |
| MD | Molecular dynamics |
| ML | Machine learning |
| MSD | Mean square displacement |
| PBE | Perdew–Burke–Ernzerhof |
| Ry | Rydberg |
| SEM | Scanning electron microscope |
| SS | Stainless steel |
| SSB | Solid-state batteries |
| SSE | Solid-state electrolyte |
| THF | Tetrahydrofuran |
| VASP | Vienna Ab initio Simulation Package |
| XRD | X-ray diffraction |
| Symbol | Description |
References
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design principles for solid-state lithium superionic conductors. Nat. Mater. 2015, 14, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.; Bartsch, T.; de Biasi, L.; Kim, A.-Y.; Janek, J.; Hartmann, P.; Brezesinski, T. Impact of Cathode Material Particle Size on the Capacity of Bulk-Type All-Solid-State Batteries. ACS Energy Lett. 2018, 3, 992–996. [Google Scholar] [CrossRef]
- Richards, W.D.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ceder, G. Interface Stability in Solid-State Batteries. Chem. Mater. 2015, 28, 266–273. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Y.; Lotsch, B.; Hu, Y.-S.; Li, H.; Janek, J.; Nazar, L.F.; Nan, C.-W.; Maier, J.; Armand, M.; et al. New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 2018, 11, 1945–1976. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Xin, S.; You, Y.; Wang, S.; Gao, H.-C.; Yin, Y.-X.; Guo, Y.-G. Solid-State Lithium Metal Batteries Promoted by Nanotechnology: Progress and Prospects. ACS Energy Lett. 2017, 2, 1385–1394. [Google Scholar] [CrossRef]
- Yu, C.; van Eijck, L.; Ganapathy, S.; Wagemaker, M. Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li-ion solid state batteries. Electrochim. Acta 2016, 215, 93–99. [Google Scholar] [CrossRef]
- Zhou, L.; Park, K.H.; Sun, X.; Lalère, F.; Adermann, T.; Hartmann, P.; Nazar, L.F. Solvent-Engineered Design of Argyrodite Li6PS5X (X = Cl, Br, I) Solid Electrolytes with High Ionic Conductivity. ACS Energy Lett. 2019, 4, 265–270. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Wang, Z.; Santhanagopalan, D.; Zhang, W.; Wang, F.; Xin, H.L.; He, K.; Li, J.; Dudney, N.; Meng, Y.S. In Situ STEM-EELS Observation of Nanoscale Interfacial Phenomena in All-Solid-State Batteries. Nano Lett. 2016, 16, 3760–3767. [Google Scholar] [CrossRef]
- Zhang, W.; Weber, D.A.; Weigand, H.; Arlt, T.; Manke, I.; Schröder, D.; Koerver, R.; Leichtweiss, T.; Hartmann, P.; Zeier, W.G.; et al. Interfacial Processes and Influence of Composite Cathode Microstructure Controlling the Performance of All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 17835–17845. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, H.; Li, W.; Dolocan, A.; Manthiram, A. Interfacial Chemistry in Solid-State Batteries: Formation of Interphase and Its Consequences. J. Am. Chem. Soc. 2017, 140, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Leichtweiss, T.; Krüger, D.; Sann, J.; Janek, J. Interphase formation on lithium solid electrolytes—An in situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ion. 2015, 278, 98–105. [Google Scholar] [CrossRef]
- Seo, D.-H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhu, Y.; Epstein, A.; Mo, Y. Statistical variances of diffusional properties from ab initio molecular dynamics simulations. NPJ Comput. Mater. 2018, 4, 18. [Google Scholar] [CrossRef]
- Baktash, A.; Reid, J.C.; Yuan, Q.; Roman, T.; Searles, D.J. Shaping the Future of Solid-State Electrolytes through Computational Modeling. Adv. Mater. 2020, 32, e1908041. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, Z.; Chu, I.-H.; Ong, S.P. Data-Driven First-Principles Methods for the Study and Design of Alkali Superionic Conductors. Chem. Mater. 2016, 29, 281–288. [Google Scholar] [CrossRef]
- Aeberhard, P.C.; Williams, S.R.; Evans, D.J.; Refson, K.; David, W.I. Ab initio Nonequilibrium Molecular Dynamics in the Solid Superionic Conductor LiBH4. Phys. Rev. Lett. 2012, 108, 095901. [Google Scholar] [CrossRef]
- Baktash, A.; Reid, J.C.; Roman, T.; Searles, D.J. Diffusion of lithium ions in Lithium-argyrodite solid-state. NPJ Comput. Mater. 2020, 6, 162. [Google Scholar] [CrossRef]
- Rayavarapu, P.R.; Sharma, N.; Peterson, V.K.; Adams, S. Variation in structure and Li+-ion migration in argyrodite-type Li6PS5X (X = Cl, Br, I) solid electrolytes. J. Solid State Electrochem. 2012, 16, 1807–1813. [Google Scholar] [CrossRef]
- De Klerk, N.J.; Rosłoń, I.; Wagemaker, M. Diffusion Mechanism of Li Argyrodite Solid Electrolytes for Li-ion Batteries and Prediction of Optimized Halogen Doping: The Effect of Li Vacancies, Halogens, and Halogen Disorder. Chem. Mater. 2016, 28, 7955–7963. [Google Scholar] [CrossRef]
- Sun, X.; Sun, Y.; Cao, F.; Li, X.; Sun, S.; Liu, T.; Wu, J. Preparation, characterization and ionic conductivity studies of composite sulfide solid electrolyte. J. Alloy. Compd. 2017, 727, 1136–1141. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, D.Y.; Choi, Y.E.; Nam, Y.J.; Han, L.; Kim, J.Y.; Xin, H.; Lin, F.; Oh, S.M.; Jung, Y.S. Solution-Processable Glass LiI-Li4SnS4 Superionic Conductors for All-Solid-State Li-Ion Batteries. Adv. Mater. 2015, 28, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; El Kazzi, M.; Villevieille, C. Surface and morphological investigation of the electrode/electrolyte properties in an all-solid-state battery using a Li2S-P2S5 solid electrolyte. J. Electroceram. 2017, 38, 207–214. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Zhang, S.; Xia, Y.; Xia, X.; Wu, J.; Tu, J. Rational coating of Li7P3S11 solid electrolyte on MoS2 electrode for all-solid-state lithium ion batteries. J. Power Sources 2017, 374, 107–112. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, G. Theoretical design of solid electrolytes with superb ionic conductivity: Alloying effect on Li+ transportation in cubic Li6PA5X chalcogenides. J. Mater. Chem. A 2017, 5, 1846–21857. [Google Scholar] [CrossRef]
- Deiseroth, H.-J.; Maier, J.; Weichert, K.; Nickel, V.; Kong, S.-T.; Reiner, C. Li7PS6 and Li6PS5X (X: Cl, Br, I): Possible Three-dimensional Diffusion Pathways for Lithium Ions and Temperature Dependence of the Ionic Conductivity by Impedance Measurements. Z. Für Anorg. Und Allg. Chem. 2011, 637, 1287–1294. [Google Scholar] [CrossRef]
- Kraft, M.A.; Culver, S.P.; Calderon, M.; Böcher, F.; Krauskopf, T.; Senyshyn, A.; Dietrich, C.; Zevalkink, A.; Janek, J.; Zeier, W.G. Influence of Lattice Polarizability on the Ionic Conductivity in the Lithium Superionic Argyrodites Li6PS5X (X = Cl, Br, I). J. Am. Chem. Soc. 2017, 139, 10909–10918. [Google Scholar] [CrossRef]
- Epp, V.; Gün, O.; Deiseroth, H.J.; Wilkening, M. Highly Mobile Ions: Low-Temperature NMR Directly Probes Extremely Fast Li+ Hopping in Argyrodite-Type Li6PS5Br. J. Phys. Chem. 2013, 4, 2118–2123. [Google Scholar] [CrossRef]
- Rao, R.P.; Sharma, N.; Peterson, V.; Adams, S. Formation and conductivity studies of lithium argyrodite solid electrolytes using in-situ neutron diffraction. Solid State Ion. 2013, 230, 72–76. [Google Scholar] [CrossRef]
- Boulineau, S.; Courty, M.; Tarascon, J.-M.; Viallet, V. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X = Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 2012, 221, 1–5. [Google Scholar] [CrossRef]
- Yubuchi, S.; Teragawa, S.; Aso, K.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Preparation of High Lithium-ion Conducting Li6PS5Cl Solid Electrolyte from Ethanol Solution for All-Solid-State Preparation of High Lithium-ion Conducting. J. Power Sources 2015, 293, 941–945. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Culver, S.P.; Koerver, R.; Krauskopf, T.; Zeier, W.G. Designing Ionic Conductors: The Interplay between Structural Phenomena and Interfaces in Thiophosphate-Based Solid-State Batteries. Chem. Mater. 2018, 30, 4179–4192. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef]
- Ni, J.E.; Case, E.D.; Sakamoto, J.S.; Rangasamy, E.; Wolfenstine, J.B. Room temperature elastic moduli and Vickers hardness of hot-pressed LLZO cubic garnet. J. Mater. Sci. 2012, 47, 7978–7985. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2S-GeS2-P2S5 System. J. Electrochem. Soc. 2001, 148, A742–A746. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Kuhn, A.; Gerbig, O.; Zhu, C.; Falkenberg, F.; Maier, J.; Lotsch, B.V. A New Ultrafast Superionic Li-Conductor: Ion Dynamics in Li11Si2PS12 and Comparison with other Tetragonal LGPS-type Electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 14669–14674. [Google Scholar] [CrossRef]
- Bron, P.; Johansson, S.; Zick, K.; Schmedt auf der Günne, J.; Dehnen, S.; Roling, B. Li10SnP2S12: An Affordable Lithium Superionic Conductor. J. Am. Chem. Soc. 2013, 135, 15694–15697. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Yamane, H.; Shibata, M.; Shimane, Y.; Junke, T.; Seino, Y.; Adams, S.; Minami, K.; Hayashi, A.; Tatsumisago, M. Crystal Structure of a Superionic Conductor, Li7P3S11. Solid State Ion. 2007, 178, 1163−1167. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2013, 7, 627–631. [Google Scholar] [CrossRef]
- Chu, I.-H.; Nguyen, H.; Hy, S.; Lin, Y.-C.; Wang, Z.; Xu, Z.; Deng, Z.; Meng, Y.S.; Ong, S.P. Correction to Insights into the Performance Limits of the Li7P3S11 Superionic Conductor: A Combined First-Principles and Experimental Study. ACS Appl. Mater. Interfaces 2018, 10, 7843–7853. [Google Scholar] [CrossRef]
- Deiseroth, H.-J.; Kong, S.-T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids with an Unusually High Li+ Mobility. Angew. Chem. Int. Ed. 2008, 47, 755–758. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; Van Eck, E.R.H.; Wang, H.; Basak, S.; Li, Z.; Wagemaker, M. Accessing the Bottleneck in All-Solid State Batteries, Lithium-Ion Transport over the Solid-Electrolyte-Electrode Interface. Nat. Commun. 2017, 8, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Sedlmaier, S.J.; Dietrich, C.; Zeier, W.G.; Janek, J. Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 2018, 318, 102–112. [Google Scholar] [CrossRef]
- Chen, M.; Rao, R.P.; Adams, S. High capacity all-solid-state Cu–Li2S/Li6PS5Br/In batteries. Solid State Ion. 2014, 262, 183–187. [Google Scholar] [CrossRef]
- Chen, M.; Rao, R.P.; Adams, S. The unusual role of Li6PS5Br in all-solid-state CuS/Li6PS5Br/In–Li batteries. Solid State Ion. 2014, 268, 300–304. [Google Scholar] [CrossRef]
- Chen, M.; Yin, X.; Reddy, M.V.; Adams, S. All-Solid-State MoS2/Li6PS5Br/In-Li Batteries as a Novel Type of Li/S Battery. J. Mater. Chem. A 2015, 3, 10698–10702. [Google Scholar] [CrossRef]
- Chen, M.; Adams, S. High performance all-solid-state lithium/sulfur batteries using lithium argyrodite electrolyte. J. Solid State Electrochem. 2014, 19, 697–702. [Google Scholar] [CrossRef]
- Auvergniot, J.; Cassel, A.; Ledeuil, J.B.; Viallet, V.; Seznec, V.; Dedryvère, R. Interface Stability of Argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in Bulk All-Solid-State Batteries. Chem. Mater. 2017, 29, 3883–3890. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; De Klerk, N.J.; Roslon, I.; van Eck, E.R.; Kentgens, A.P.; Wagemaker, M. Unravelling Li-ion Transport from Picoseconds to Seconds: Bulk versus Interfaces in an Argyrodite Li6PS5Cl-Li2S All-Solid-State Li-Ion Battery. J. Am. Chem. Soc. 2016, 138, 11192–11201. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Oh, D.Y.; Park, K.H.; Choi, Y.E.; Nam, Y.J.; Lee, H.A.; Lee, S.-M.; Jung, Y.S. Infiltration of Solution-Processable Solid Electrolytes into Conventional Li-Ion-Battery Electrodes for All-Solid-State Li-Ion Batteries. Nano Lett. 2017, 17, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Sedlmaier, S.J.; Indris, S.; Dietrich, C.; Yavuz, M.; Dräger, C.; von Seggern, F.; Sommer, H.; Janek, J. Li4PS4I: A Li+ Superionic Conductor Synthesized by a Solvent-Based Soft Chemistry Approach. Chem. Mater. 2017, 29, 1830–1835. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, W.; Payzant, E.A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous High Ionic Conductivity of Nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, E.; Liu, Z.; Gobet, M.; Pilar, K.; Sahu, G.; Zhou, W.; Wu, H.; Greenbaum, S.; Liang, C. An Iodide-based Li7P2S8I Superionic Conductor. J. Am. Chem. Soc. 2015, 137, 1384–1387. [Google Scholar] [CrossRef]
- Ito, S.; Nakakita, M.; Aihara, Y.; Uehara, T.; Machida, N. A Synthesis of Crystalline Li7P3S11 Solid Electrolyte from 1,2-dimethoxyethane Solvent. J. Power Sources 2014, 271, 342–345. [Google Scholar] [CrossRef]
- Yubuchi, S.; Uematsu, M.; Deguchi, M.; Hayashi, A.; Tatsumisago, M. Lithium-Ion-Conducting Argyrodite-Type Li6PS5X (X = Cl, Br, I) Solid Electrolytes Prepared by a Liquid-Phase Technique Using Ethanol as a Solvent. ACS Appl. Energy Mater. 2018, 1, 3622–3629. [Google Scholar] [CrossRef]
- Pecher, O.; Kong, S.T.; Goebel, T.; Nickel, V.; Weichert, K.; Reiner, C.; Deiseroth, H.J.; Maier, J.; Haarmann, F.; Zahn, D. Atomistic Characterisation of Li+ Mobility and Conductivity in Li7−xPS6−xIx Argyrodites from Molecular Dynamics Simulations, Solid-State NMR, and Impedance Spectroscopy. Chem.-Eur. J. 2010, 16, 8347–8354. [Google Scholar] [CrossRef]
- Evans, D.J.; Morriss, G.P. Statistical Mechanics of Nonequilibrium Liquids; ANU E Press: Canberra, Australia, 2007. [Google Scholar]
- Zhu, Z.; Chu, I.H.; Deng, Z.; Ong, S.P. Role of Na+ interstitials and dopants in enhancing the Na+ conductivity of the cubic Na3PS4 superionic conductor. Chem. Mat. 2015, 27, 8318–8325. [Google Scholar] [CrossRef]
- Marcolongo, A.; Marzari, N. Ionic correlations and failure of Nernst-Einstein relation in solid-state electrolytes. Phys. Rev. Mater. 2017, 1, 025402. [Google Scholar] [CrossRef]
- Keil, F.J.; Krishna, R.; Coppens, M.-O. Modeling of Diffusion in Zeolites. Rev. Chem. Eng. 2000, 16, 71–197. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Preparation of lithium ion conductive Li6PS5Cl solid electrolyte from solution for the fabrication of composite cathode of all-solid-state lithium battery. J. Sol-Gel Sci. Technol. 2018, 89, 303–309. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; Hageman, J.; van Eijck, L.; van Eck, E.R.H.; Zhang, L.; Schwietert, T.; Basak, S.; Kelder, E.M.; Wagemaker, M. Facile Synthesis toward the Optimal Structure-Conductivity Characteristics of the Argyrodite Li6PS5Cl Solid-State Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 33296–33306. [Google Scholar] [CrossRef]
- Rao, R.P.; Adams, S. Studies of lithium argyrodite solid electrolytes for all-solid-state batteries. Phys. Status Solidi A 2011, 208, 1804–1807. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Shen, Y.; Li, L.; Nan, C.W. High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes Prepared via Optimized Sintering Processes for All-Solid-State Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, G.; Fang, T.; Jiang, K.; Liu, X. Structural Reorganization of Ionic Liquid Electrolyte by a Rapid Charge/Discharge Circle. J. Phys. Chem. Lett. 2021, 12, 2273–2278. [Google Scholar] [CrossRef]
- Lynden-Bell, R.M. Gas–Liquid interfaces of room temperature ionic liquids. Mol. Phys. 2003, 101, 2625–2633. [Google Scholar] [CrossRef]
- Lynden-Bell, R.M.; Kohanoff, J.; Del Popolo, M.G. Simulation of interfaces between room temperature ionic liquids and other liquids. Faraday Discuss. 2004, 129, 57–67. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Ionic Liquids at Electrified Interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. CP2K: Atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for abinitio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169−11186. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- VandeVondele, J.; Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef]
- Lippert, G.; Hutter, J.; Parrinello, M. A hybrid Gaussian and plane wave density functional scheme. Mol. Phys. 1997, 92, 477–487. [Google Scholar] [CrossRef]
- Evans, D.J.; Morriss, O. Non-Newtonian molecular dynamics. Comput. Phys. Rep. 1984, 1, 297–343. [Google Scholar] [CrossRef]
- Wheeler, D.R.; Newman, J. Molecular dynamics simulations of multicomponent diffusion. 2. Nonequilibrium method. J. Phys. Chem. B 2004, 108, 18362–18367. [Google Scholar] [CrossRef]
- Arya, G.; Chang, H.-C.; Maginn, E. A critical comparison of equilibrium, non-equilibrium and boundary-driven molecular dynamics techniques for studying transport in microporous materials. J. Chem. Phys. 2001, 115, 8112–8124. [Google Scholar] [CrossRef]
- Brańka, A.C. Nosé-Hoover chain method for nonequilibrium molecular dynamics simulation. Phys. Rev. E 2000, 61, 4769–4773. [Google Scholar] [CrossRef] [PubMed]
- Beedle, A.E.M.; Mora, M.; Davis, C.T.; Snijders, A.P.; Stirnemann, G.; Garcia-Manyes, S. Forcing the reversibility of a mechanochemical reaction. Nat. Commun. 2018, 9, 3155. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, N.; Lepley, N.; Du, Y. A Computer Modeling Study of Lithium Phosphate and Thiophosphate Electrolyte Materials. ECS Meet. Abstr. 2010, 1, 517. [Google Scholar] [CrossRef]
- Grasselli, F.; Baroni, S. Topological quantization and gauge invariance of charge transport in liquid insulators. Nat. Phys. 2019, 15, 967–972. [Google Scholar] [CrossRef]
- Electronic Design. 2020. Available online: https://www.electronicdesign.com/markets/article/21210395/machine-learning-boosts-electrolyte-search (accessed on 21 August 2022).
- Gallo-Bueno, A.; Reynaud, M.; Casas-Cabanas, M.; Carrasco, J. Unsupervised machine learning to classify crystal structures according to their structural distortion: A case study on Li-argyrodite solid-state electrolytes. Energy AI 2022, 9, 100159. [Google Scholar] [CrossRef]
- Zhao, Q.; Avdeev, M.; Chen, L.; Shi, S. Machine learning prediction of activation energy in cubic Li-argyrodites with hierarchically encoding crystal structure-based (HECS) descriptors. Sci. Bull. 2021, 66, 1401–1408. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Duan, X.; Peng, L.; Jia, H.; Zhang, Y.; Shan, B.; Xie, J. Design and synthesis of room temperature stable Li-argyrodite superionic conductors via cation doping. J. Mater. Chem. A 2019, 7, 2717–2722. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. A Mathematical Model of Stress Generation and Fracture in Lithium Manganese Oxide. J. Electrochem. Soc. 2006, 153, A1019. [Google Scholar] [CrossRef]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in Li-ion battery electrodes. Electrochim. Acta 2015, 185, 28–33. [Google Scholar] [CrossRef]
- Golmon, S.; Maute, K.; Lee, S.-H.; Dunn, M.L. Stress generation in silicon particles during lithium insertion. Appl. Phys. Lett. 2010, 97, 033111. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Z.; Niu, H.; Yin, Y.; Kuai, C.; Wang, J.; Shao, C.; Guo, Y. Machine-Learning-Accelerated Catalytic Activity Predictions of Transition Metal Phthalocyanine Dual-Metal-Site Catalysts for CO2 Reduction. J. Phys. Chem. Lett. 2021, 12, 6111–6118. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R. Modeling Mismatch Strain Induced Self-Folding of Bilayer Gel Structures. Int. J. Appl. Mech. 2016, 8, 1640004. [Google Scholar] [CrossRef]
- Huang, R.; Zheng, S.; Liu, Z.; Ng, T.Y. Recent Advances of the Constitutive Models of Smart Materials—Hydrogels and Shape Memory Polymers. Int. J. Appl. Mech. 2020, 12, 2050014. [Google Scholar] [CrossRef]
- Brassart, L.; Suo, Z. Reactive flow in large-deformation electrodes of lithium-ion batteries. Int. J. Appl. Mech. 2012, 4, 1250023. [Google Scholar] [CrossRef]
- Bhandakkar, T.K.; Gao, H. Cohesive modeling of crack nucleation under diffusion induced stresses in a thin strip: Implications on the critical size for flaw tolerant battery electrodes. Int. J. Solids Struct. 2010, 47, 1424–1434. [Google Scholar] [CrossRef]
- Christensen, J. Modeling Diffusion-Induced Stress in Li-Ion Cells with Porous Electrodes. J. Electrochem. Soc. 2010, 157, A366–A380. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Verbrugge, M.W. Diffusion-Induced Stress, Interfacial Charge Transfer, and Criteria for Avoiding Crack Initiation of Electrode Particles. J. Electrochem. Soc. 2010, 157, A508–A516. [Google Scholar] [CrossRef]
- Deshpande, R.; Cheng, Y.-T.; Verbrugge, M.W. Modeling diffusion-induced stress in nanowire electrode structures. J. Power Sources 2010, 195, 5081–5088. [Google Scholar] [CrossRef]
- Garcia, R.E.; Chiang, Y.-M.; Carter, W.C.; Limthongkul, P.; Bishop, C. Microstructural Modeling and Design of Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A255–A263. [Google Scholar] [CrossRef]
- Renganathan, S.; Sikha, G.; Santhanagopalan, S.; White, R.E. Theoretical Analysis of Stresses in a Lithium Ion Cell. J. Electrochem. Soc. 2010, 157, A155. [Google Scholar] [CrossRef]
| Phase | Ionic Conductivity (IC) (mScm−1) | Activation Energy (Ea) (eV) | Reference |
|---|---|---|---|
| Li3.25Ge0.25P0.75S4 | 2.2 | 0.21 | [1,37] |
| Li10GeP2S12 | 12 | 0.25 | [1,3,38] and derivatives [39,40] |
| Li9.54Si1.74P1.44S11.7 | 25 | 0.24 | [41] |
| Li7P3S11 | 17 | 0.18 | [1,42,43,44] |
| Li6PS5X (X = Cl, Br) | ∼1 | 0.3−0.4 | [31,45,46] |
| S/N | Material | IC (mS·cm−1) | EC (mS·cm−1) |
|---|---|---|---|
| 1. | Li5.5PS4.5Cl1.5 | 3.9 | 1.4 × 10−5 |
| 2. | Li5.75PS4.75Cl1.25 | 3.0 | 2.6 × 10−5 |
| 3. | Li6PS5Br | 1.9 | 4.4 × 10−6 |
| 4. | Li6PS5Cl0.25Br0.75 | 3.4 | 1.1 × 10−5 |
| 5. | Li6PS5Cl0.5Br0.5 | 3.9 | 1.4 × 10−5 |
| 6. | Li6PS5Cl0.75Br0.25 | 3.2 | 3.7 × 10−6 |
| 7. | Li6PS5Cl | 2.4 | 5.1 × 10−6 |
| Temp. (K) | AI–EMD | AI–NEMD | ||
|---|---|---|---|---|
| Ds (cm2 s−1) | σ (S·cm−1) | Ds (cm2 s−1) | σ (S·cm−1) | |
| 300 | 2.9 × 10−6 | 0.35 | 3.3 × 10−6 | 0.4 |
| 600 | 2.9 × 10−5 | 1.80 | 2.9 × 10−5 | 1.8 |
| 800 | 5.6 × 10−5 | 2.50 | 5.2 × 10−5 | 2.4 |
| 1000 | 8.9 × 10−5 | 3.20 | 8.9 × 10−5 | 2.9 |
| Material | IC (S·cm−1) | Reference |
|---|---|---|
| Li6PS5Cl | 1.4 × 10−5 | Experiment [32] |
| 3.3 × 10−5 | Experiment [20] | |
| 6 × 10−5–3 × 10−4 *a | AI–NEMD [19] | |
| 6 × 10−5 | Experiment [65] | |
| 0.29 | Computation (MSD) [21] | |
| 0.05 (0.16) | Computation (Jump) [21] | |
| 2 × 10−6 | Computation (MSD) [17] | |
| Li5.6PS4.8Cl1.2 | 1.1 × 10−3 | Experiment [66] |
| Li5.75PS4.75Cl1.25 | 6 × 10−2–1.2 × 10−1 *a | [19] |
| Li6PS5Cl with chlorine and sulfur disorder | 1.9 × 10−3 | Experiment [67] |
| 4.96 × 10−3 | Experiment [7] | |
| 3.38 × 10−3 | Experiment [68] | |
| 0.26 | Computation (MSD) [21] | |
| 0.89 (1.29) | Computation (Jump) 21] | |
| 6 × 10−3–0.1 *a | AI–EMD [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoola, O.M.; Buldum, A.; Farhad, S.; Ojo, S.A. A Review on the Molecular Modeling of Argyrodite Electrolytes for All-Solid-State Lithium Batteries. Energies 2022, 15, 7288. https://doi.org/10.3390/en15197288
Ayoola OM, Buldum A, Farhad S, Ojo SA. A Review on the Molecular Modeling of Argyrodite Electrolytes for All-Solid-State Lithium Batteries. Energies. 2022; 15(19):7288. https://doi.org/10.3390/en15197288
Chicago/Turabian StyleAyoola, Oluwasegun M., Alper Buldum, Siamak Farhad, and Sammy A. Ojo. 2022. "A Review on the Molecular Modeling of Argyrodite Electrolytes for All-Solid-State Lithium Batteries" Energies 15, no. 19: 7288. https://doi.org/10.3390/en15197288
APA StyleAyoola, O. M., Buldum, A., Farhad, S., & Ojo, S. A. (2022). A Review on the Molecular Modeling of Argyrodite Electrolytes for All-Solid-State Lithium Batteries. Energies, 15(19), 7288. https://doi.org/10.3390/en15197288









