Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review
Abstract
:1. Introduction
2. Pretreatment of Hemp Biomass
3. Pretreatment of Corn Stover
4. Pretreatment of Rice Straw
5. Pretreatment of Wheat Straw
6. Pretreatment of Sugarcane Bagasse
7. Pretreatment of Other Biomasses
8. Ethanol Production Overview
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ClientEarth. Communications Fossil Fuels and Climate Change: The Facts|ClientEarth. Available online: https://www.clientearth.org/latest/latest-updates/stories/fossil-fuels-and-climate-change-the-facts/ (accessed on 16 June 2022).
- Wuebbles, D.J.; Jain, A.K. Concerns about Climate Change and the Role of Fossil Fuel Use. Fuel Process. Technol. 2001, 71, 8. [Google Scholar] [CrossRef]
- Bhanumati, P.; de Haan, M.; Tebrake, J.W. Greenhouse Emissions Rise to Record, Erasing Drop During Pandemic—IMF Blog. Available online: https://blogs.imf.org/2022/06/30/greenhouse-emissions-rise-to-record-erasing-drop-during-pandemic/ (accessed on 25 July 2022).
- British Petroleum Statistical Review of World Energy; 2021; Volume 70. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 26 July 2022).
- Energy Use and Efficiency in Pest Control, Including Pesticide Production, Use, and Management Options—Farm Energy. Available online: https://farm-energy.extension.org/energy-use-and-efficiency-in-pest-control-including-pesticide-production-use-and-management-options/ (accessed on 26 July 2022).
- The Mirage of Industrial Agriculture: Fossil Fuels, Groundwater Irrigation, and Feedlots on the High Plains—NiCHE. Available online: https://niche-canada.org/2020/10/27/the-mirage-of-industrial-agriculture-fossil-fuels-groundwater-irrigation-and-feedlots-on-the-high-plains/ (accessed on 26 July 2022).
- Agriculture, Modern|Encyclopedia.Com. Available online: https://www.encyclopedia.com/science/news-wires-white-papers-and-books/agriculture-modern (accessed on 26 July 2022).
- USDA. Industrial Hemp in the United States. Available online: https://www.ers.usda.gov/webdocs/publications/41740/15853_ages001ec_1_.pdf?v=0 (accessed on 9 June 2022).
- Climate Policy Watcher Fossil Fuel Use in Agriculture—Plant Physiology. Available online: https://www.climate-policy-watcher.org/plant-physiology/fossil-fuel-use-in-agriculture.html (accessed on 15 June 2022).
- European Environment Agency. Agriculture and Climate Change—European Environment Agency. Available online: https://www.eea.europa.eu/signals/signals-2015/articles/agriculture-and-climate-change (accessed on 26 July 2022).
- Lal, R.; Follett, R.F.; Kimble, J.M. Achieving Soil Carbon Sequestration in the United States: A Challenge to the Policy Makers. Soil Sci. 2003, 168, 827–845. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). By the Numbers: GHG Livestock Emissions. Available online: https://www.fao.org/news/story/en/item/197623/icode/ (accessed on 26 July 2022).
- World Resources Institute. World Greenhouse Gas Emissions: 2018|World Resources Institute. Available online: https://www.wri.org/data/world-greenhouse-gas-emissions-2018 (accessed on 15 June 2022).
- European Environment Agency EEA. Greenhouse Gases—Data Viewer—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/data/data-viewers/greenhouse-gases-viewer/ (accessed on 25 July 2022).
- U.S. Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks|US EPA. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks (accessed on 25 July 2022).
- Adejumo, I.O.; Adebiyi, O.A. Agricultural Solid Wastes: Causes, Effects, and Effective Management. In Strategies of Sustainable Solid Waste Management; Saleh, H.M., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83962-560-2. [Google Scholar]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the Evolution, Approaches and Perspectives on Alternative Uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Ignaciuk, A.; Mason-D’Croz, D. Modelling Adaptation to Climate Change in Agriculture; OECD Food, Agriculture and Fisheries Papers, No. 70; OECD Publishing: Paris, France, 2014. [Google Scholar]
- Ahmed, A.T.M.F.; Islam, M.Z.; Mahmud, M.S.; Sarker, M.E.; Islam, M.R. Hemp as a Potential Raw Material toward a Sustainable World: A Review. Heliyon 2022, 8, e08753. [Google Scholar] [CrossRef] [PubMed]
- Baldos, U.L.C.; Fuglie, K.O.; Hertel, T.W. The Research Cost of Adapting Agriculture to Climate Change: A Global Analysis to 2050. Agric. Econ. 2020, 51, 207–220. [Google Scholar] [CrossRef]
- Nowak, A.; Krukowski, A.; Różańska-Boczula, M. Assessment of Sustainability in Agriculture of the European Union Countries. Agronomy 2019, 9, 890. [Google Scholar] [CrossRef]
- Pant, K. Effects of Agriculture on Climate Change: A Cross Country Study of Factors Affecting Carbon Emissions. J. Agric. Environ. 2009, 10, 8. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). Renewable Energy Explained—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/energyexplained/renewable-sources/ (accessed on 16 June 2022).
- Turgeon, A.; Morse, E. Biomass Energy|National Geographic Society. Available online: https://education.nationalgeographic.org/resource/biomass-energy (accessed on 25 July 2022).
- European Biomass Industry Association. Environmental Benefits of Biomass—European Biomass Industry Association. Available online: https://www.eubia.org/cms/wiki-biomass/employment-potential-in-figures/environmental-benefits/ (accessed on 25 July 2022).
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Britannica, T.E. Wheat|Production, Types, Nutrition, Uses, & Facts|Britannica. Britannica. 2021. Available online: https://www.britannica.com/plant/wheat (accessed on 2 September 2022).
- U.S. Department of Agriculture. Wheat 2021 World Production. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=0410000&sel_year=2021&rankby=Production (accessed on 2 September 2022).
- Mcgrath, C. United States Department of Agriculture|Foreign Agricultural Service—China. 2019 Hemp Annual Report. 2020, CH20200018. pp. 1–12. Available online: https://www.fas.usda.gov/data/china-2019-hemp-annual-report (accessed on 2 September 2022).
- European Commission Hemp Production in the EU. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/hemp_en (accessed on 2 September 2022).
- Honig, L. National Hemp Report Executive Summary. 2022. Available online: https://www.nass.usda.gov/Newsroom/Executive_Briefings/2022/02-17-2022.pdf (accessed on 2 September 2022).
- The Editors of Encyclopaedia Britannica. Corn|History, Cultivation, Uses, & Description|Britannica. Britannica. 2022. Available online: https://www.britannica.com/plant/corn-plant (accessed on 2 September 2022).
- Capehart, T.; Proper, S. Corn Is America’s Largest Crop in 2019|USDA. Available online: https://www.usda.gov/media/blog/2019/07/29/corn-americas-largest-crop-2019 (accessed on 2 September 2022).
- Rice Production by Country|World Agricultural Production 2022/2023. Available online: http://www.worldagriculturalproduction.com/crops/rice.aspx (accessed on 3 September 2022).
- USDA. ERS Rice Sector at a Glance. Available online: https://www.ers.usda.gov/topics/crops/rice/rice-sector-at-a-glance/ (accessed on 2 September 2022).
- Figueroa-Rodríguez, K.A.; Hernández-Rosas, F.; Figueroa-Sandoval, B.; Velasco-Velasco, J.; Rivera, N.A. What Has Been the Focus of Sugarcane Research? A Bibliometric Overview. Int. J. Environ. Res. Public Health 2019, 16, 3326. [Google Scholar] [CrossRef]
- Houghton, R.A. Biomass. Encycl. Ecol. 2008, 448–453. [Google Scholar] [CrossRef]
- Semhaoui, I.; Maugard, T.; Zarguili, I.; Rezzoug, S.A.; Zhao, J.M.Q.; Toyir, J.; Nawdali, M.; Maache-Rezzoug, Z. Eco-Friendly Process Combining Acid-Catalyst and Thermomechanical Pretreatment for Improving Enzymatic Hydrolysis of Hemp Hurds. Bioresour. Technol. 2018, 257, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.; Senn, T. Energy Self-Sufficient Production of Bioethanol from a Mixture of Hemp Straw and Triticale Seeds: Life-Cycle Analysis. Biomass Bioenergy 2016, 95, 99–108. [Google Scholar] [CrossRef]
- Paul, S.K.; Chakraborty, S. Microwave-Assisted Ionic Liquid-Mediated Rapid Catalytic Conversion of Non-Edible Lignocellulosic Sunn Hemp Fibres to Biofuels. Bioresour. Technol. 2018, 253, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Teghammar, A.; Karimi, K.; Sárvári Horváth, I.; Taherzadeh, M.J. Enhanced Biogas Production from Rice Straw, Triticale Straw and Softwood Spruce by NMMO Pretreatment. Biomass Bioenergy 2012, 36, 116–120. [Google Scholar] [CrossRef]
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing Cellulose Accessibility of Corn Stover by Deep Eutectic Solvent Pretreatment for Butanol Fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Improving Ethanol Yields with Deacetylated and Two-Stage Pretreated Corn Stover and Sugarcane Bagasse by Blending Commercial Xylose-Fermenting and Wild Type Saccharomyces Yeast. Bioresour. Technol. 2019, 282, 103–109. [Google Scholar] [CrossRef]
- Öhgren, K.; Galbe, M.; Zacchi, G. Optimization of Steam Pretreatment of SO2-Impregnated Corn Stover for Fuel Ethanol Production. Appl. Biochem. Biotechnol. 2005, 121–124, 1055–1067. [Google Scholar] [CrossRef]
- Rezende, C.A.; De Lima, M.; Maziero, P.; Deazevedo, E.; Garcia, W.; Polikarpov, I. Chemical and Morphological Characterization of Sugarcane Bagasse Submitted to a Delignification Process for Enhanced Enzymatic Digestibility. Biotechnol. Biofuels 2011, 4, 54. [Google Scholar] [CrossRef]
- Maache-Rezzoug, Z.; Pierre, G.; Nouviaire, A.; Maugard, T.; Rezzoug, S.A. Optimizing Thermomechanical Pretreatment Conditions to Enhance Enzymatic Hydrolysis of Wheat Straw by Response Surface Methodology. Biomass Bioenergy 2011, 35, 3129–3138. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an Alternative Fuel from Agricultural, Industrial and Urban Residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). Biomass Explained—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/energyexplained/biomass/ (accessed on 17 June 2022).
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Rajesh Banu, J.; Yoon, J.J.; Kant Bhatia, S.; Yang, Y.H.; Varjani, S.; Kim, S.H. Recent Advances in Commercial Biorefineries for Lignocellulosic Ethanol Production: Current Status, Challenges and Future Perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.-Y. Lignin Plays a Negative Role in the Biochemical Process of Producing Lignocellulosic Biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Amit, K.; Nakachew, M.; Yilkal, B.; Mukesh, Y. A Review of Factors Affecting Enzymatic Hydrolysis of Pretreated Lignocellulosic Biomass. Res. J. Chem. Environ. 2018, 22, 62–67. [Google Scholar]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A.S. Targeted Pre-Treatment of Hemp Bast Fibres for Optimal Performance in Biocomposite Materials: A Review. Ind. Crops Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef]
- Sausserde, R.; Adamovics, A. Industrial Hemp for Biomass Production. J. Agric. Eng. 2013, 44, 10–13. [Google Scholar] [CrossRef]
- Das, L.; Li, W.; Dodge, L.A.; Stevens, J.C.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Comparative Evaluation of Industrial Hemp Cultivars: Agronomical Practices, Feedstock Characterization, and Potential for Biofuels and Bioproducts. ACS Sustain. Chem. Eng. 2020, 8, 6200–6210. [Google Scholar] [CrossRef]
- Sánchez, J.; Curt, M.D.; Robert, N.; Fernández, J. Biomass Resources. In The Role of Bioenergy in the Bioeconomy; Academic Press: Cambridge, MA, USA, 2019; pp. 25–111. [Google Scholar] [CrossRef]
- Anukam, A.; Berghel, J. Biomass Pretreatment and Characterization: A Review. In Biomass Pretreatment and Characterization: A Review; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Tabil, L.; Adapa, P.; Kashaninejad, M. Biomass Feedstock Pre-Processing—Part 1: Pre-Treatment. Biofuel’s Eng. Process Technol. 2011, 18, 411–439. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. High Ethanol Concentration (77 g/L) of Industrial Hemp Biomass Achieved through Optimizing the Relationship between Ethanol Yield/Concentration and Solid Loading. ACS Omega 2020, 5, 21913–21921. [Google Scholar] [CrossRef]
- Wawro, A.; Batog, J.; Gieparda, W. Chemical and Enzymatic Treatment of Hemp Biomass for Bioethanol Production. Appl. Sci. 2019, 9, 5348. [Google Scholar] [CrossRef]
- Marcolongo, L.; La Cara, F.; Ionata, E. Hemp Waste Valorization through Enzymatic Hydrolysis for Biofuels and Biochemicals Production. Chem. Eng. Trans. 2021, 86, 127–132. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. Conversion of Liquid Hot Water, Acid and Alkali Pretreated Industrial Hemp Biomasses to Bioethanol. Bioresour. Technol. 2020, 309, 123383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y.; Zhang, M.; Wang, D. Effects of Post-Washing on Pretreated Biomass and Hydrolysis of the Mixture of Acetic Acid and Sodium Hydroxide Pretreated Biomass and Their Mixed Filtrate. Bioresour. Technol. 2021, 339, 125605. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.M.Q.; Chi, K.; Catchmark, J.M. Structural and Physico-Chemical Characterization of Industrial Hemp Hurd: Impacts of Chemical Pretreatments and Mechanical Refining. Ind. Crops Prod. 2021, 171, 113818. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ilyas, R.A.; Nurazzi, N.M.; Rani, M.S.A.; Atikah, M.S.N.; Shazleen, S.S. Chemical Pretreatment of Lignocellulosic Biomass for the Production of Bioproducts: An Overview. Appl. Sci. Eng. Prog. 2021, 14, 588–605. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An Overview of Key Pretreatment Processes for Biological Conversion of Lignocellulosic Biomass to Bioethanol. 3 Biotech 2015, 5, 597. [Google Scholar] [CrossRef]
- Xie, C.; Gong, W.; Yang, Q.; Zhu, Z.; Yan, L.; Hu, Z.; Peng, Y. White-Rot Fungi Pretreatment Combined with Alkaline/Oxidative Pretreatment to Improve Enzymatic Saccharification of Industrial Hemp. Bioresour. Technol. 2017, 243, 188–195. [Google Scholar] [CrossRef]
- Siqueira, G.; Arantes, V.; Saddler, J.N.; Ferraz, A.; Milagres, A.M.F. Limitation of Cellulose Accessibility and Unproductive Binding of Cellulases by Pretreated Sugarcane Bagasse Lignin. Biotechnol. Biofuels 2017, 10, 176. [Google Scholar] [CrossRef]
- European Commission. EU Plant Variety Database (v.3.4). Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchVariety&ctl_type=A&species_id=261&variety_name=&listed_in=0&show_current=on&show_deleted= (accessed on 12 August 2022).
- Fírvida, I.; Del Río, P.G.; Gullón, P.; Gullón, B.; Garrote, G.; Romaní, A. Alternative Lime Pretreatment of Corn Stover for Second-Generation Bioethanol Production. Agronomy 2021, 11, 155. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, X.; Chen, S.; Yu, J.; Zhai, R.; Xu, Z.; Jin, M. Densifying Lignocellulosic Biomass with Alkaline Chemicals (DLC) Pretreatment Unlocks Highly Fermentable Sugars for Bioethanol Production from Corn Stover. Green Chem. 2021, 23, 4828–4839. [Google Scholar] [CrossRef]
- Bohn, L.R.; Dresch, A.P.; Cavali, M.; Vargas, A.C.G.; Führ, J.F.; Tironi, S.P.; Fogolari, O.; Mibielli, G.M.; Alves, S.L., Jr.; Bender, J.P. Alkaline Pretreatment and Enzymatic Hydrolysis of Corn Stover for Bioethanol Production. Res. Soc. Dev. 2021, 10, e149101118914. [Google Scholar] [CrossRef]
- Kamal, S.; Rehman, S.; Rehman, K.; Ghaffar, A.; Bibi, I.; Ahmed, T.; Maqsood, S.; Nazish, N.; Iqbal, H.M.N. Sustainable and Optimized Bioethanol Production Using Mix Microbial Consortium of Saccharomyces Cerevisiae and Candida Cantarelli. Fuel 2022, 314, 122763. [Google Scholar] [CrossRef]
- Hamdy, A.; Elhafez, S.A.; Hamad, H.; Ali, R. The Interplay of Autoclaving with Oxalate as Pretreatment Technique in the View of Bioethanol Production Based on Corn Stover. Polymers 2021, 13, 3762. [Google Scholar] [CrossRef] [PubMed]
- Zohri, A.-N.A.; El-Motaleb, A.; Ramadan, M.; Zohri, A.A.; Ramadan, A.M.; El-Tabakh, M.M.; Al-Tantawy, K. Key Factors Affecting the Efficiency of Ethanol Fermentation Using Beet Molasses. Egypt. Sugar J. 2015, 8, 27–52. [Google Scholar]
- Sehnem, N.T.; Machado, Â.S.; Matte, C.R.; De Morais, M.A.; Ayub, M.A.Z. Second-Generation Ethanol Production by Wickerhamomyces Anomalus Strain Adapted to Furfural, 5-Hydroxymethylfurfural (HMF), and High Osmotic Pressure. An. Acad. Bras. Cienc. 2020, 92, e20181030. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Alam, M.; Amin, M.; Das, N. Comparative Study of the Nutritive Values of the Different Varieties of Rice Straw. Bangladesh J. Anim. Sci. 1970, 39, 75–82. [Google Scholar] [CrossRef]
- Nandal, P.; Sharma, S.; Arora, A. Bioprospecting Non-Conventional Yeasts for Ethanol Production from Rice Straw Hydrolysate and Their Inhibitor Tolerance. Renew. Energy 2020, 147, 1694–1703. [Google Scholar] [CrossRef]
- Areepak, C.; Jiradechakorn, T.; Chuetor, S.; Phalakornkule, C.; Sriariyanun, M.; Raita, M.; Champreda, V.; Laosiripojana, N. Improvement of Lignocellulosic Pretreatment Efficiency by Combined Chemo—Mechanical Pretreatment for Energy Consumption Reduction and Biofuel Production. Renew. Energy 2022, 182, 1094–1102. [Google Scholar] [CrossRef]
- Chang, K.L.; Liu, C.H.; Phitsuwan, P.; Ratanakhanokchai, K.; Lin, Y.C.; Di Dong, C.; Lin, M.H.; Yang, G.C.C. Enhancement of Biological Pretreatment on Biomass by an Ionic Liquid or Surfactant. Catalysts 2021, 11, 1274. [Google Scholar] [CrossRef]
- Chuetor, S.; Panakkal, E.J.; Ruensodsai, T.; Cheenkachorn, K.; Kirdponpattara, S.; Cheng, Y.S.; Sriariyanun, M. Improvement of Enzymatic Saccharification and Ethanol Production from Rice Straw Using Recycled Ionic Liquid: The Effect of Anti-Solvent Mixture. Bioengineering 2022, 9, 115. [Google Scholar] [CrossRef]
- Molaverdi, M.; Mirmohamadsadeghi, S.; Karimi, K.; Aghbashlo, M.; Tabatabaei, M. Efficient Ethanol Production from Rice Straw through Cellulose Restructuring and High Solids Loading Fermentation by Mucor Indicus. J. Clean. Prod. 2022, 339, 130702. [Google Scholar] [CrossRef]
- Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A.; Shraddha. Laccase: Microbial Sources, Production, Purification, and Potential Biotechnological Applications. Enzyme Res. 2011, 2011, 11. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-Methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Srokowski, E.M.; Woodhouse, K.A. 2.20 Decellularized Scaffolds. Compr. Biomater. II 2017, 2, 452–470. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of Cellulose with Ionic Liquids and Its Application: A Mini-Review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Krysztof, M.; Olejnik, K.; Kulpinski, P.; Stanislawska, A.; Khadzhynova, S. Regenerated Cellulose from N-Methylmorpholine N-Oxide Solutions as a Coating Agent for Paper Materials. Cellulose 2018, 25, 3595–3607. [Google Scholar] [CrossRef]
- Nijland, J.G.; Driessen, A.J.M. Engineering of Pentose Transport in Saccharomyces Cerevisiae for Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 7, 464. [Google Scholar] [CrossRef]
- Martinez-Jimenez, F.D.; Neitzel, T.; Biazi, L.E.; Pereira, I.O.; dos Santos, L.V.; da Costa, A.C.; Ienczak, J.L. Exploiting the Non-Conventional Yeast Spathaspora Passalidarum as a Platform for Hemicellulosic Hydrolysate Conversion into Bioproducts: A Mini Review. Bioenergy Res. 2021, 14, 689–708. [Google Scholar] [CrossRef]
- Ruchala, J.; Kurylenko, O.O.; Dmytruk, K.V.; Sibirny, A.A. Construction of Advanced Producers of First- and Second-Generation Ethanol in Saccharomyces Cerevisiae and Selected Species of Non-Conventional Yeasts (Scheffersomyces Stipitis, Ogataea Polymorpha). J. Ind. Microbiol. Biotechnol. 2020, 47, 109–132. [Google Scholar] [CrossRef]
- Ishizaki, H.; Hasumi, K. Ethanol Production from Biomass. Res. Approaches Sustain. Biomass Syst. 2014, 243–258. [Google Scholar] [CrossRef]
- Agbogbo, F.K.; Coward-Kelly, G.; Torry-Smith, M.; Wenger, K.S. Fermentation of Glucose/Xylose Mixtures Using Pichia Stipitis. Process. Biochem. 2006, 41, 2333–2336. [Google Scholar] [CrossRef]
- Tufail, T.; Saeed, F.; Imran, M.; Arshad, M.U.; Anjum, F.M.; Afzaal, M.; Ain, H.B.U.; Shahbaz, M.; Gondal, T.A.; Hussain, S. Biochemical Characterization of Wheat Straw Cell Wall with Special Reference to Bioactive Profile. Int. J. Food Prop. 2018, 21, 1303–1310. [Google Scholar] [CrossRef]
- Ziaei-Rad, Z.; Fooladi, J.; Pazouki, M.; Gummadi, S.N. Lignocellulosic Biomass Pre-Treatment Using Low-Cost Ionic Liquid for Bioethanol Production: An Economically Viable Method for Wheat Straw Fractionation. Biomass Bioenergy 2021, 151, 106140. [Google Scholar] [CrossRef]
- Xian, X.; Fang, L.; Zhou, Y.; Li, B.; Zheng, X.; Liu, Y.; Lin, X. Integrated Bioprocess for Cellulosic Ethanol Production from Wheat Straw: New Ternary Deep-Eutectic-Solvent Pretreatment, Enzymatic Saccharification, and Fermentation. Fermentation 2022, 8, 371. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Zhang, B.; Yang, Y.; Song, Y.; Zhang, F.; Liu, B.; Zhou, Y.; Yi, Y.; Shan, Y.; et al. Integrating Enzymatic Hydrolysis into Subcritical Water Pretreatment Optimization for Bioethanol Production from Wheat Straw. Sci. Total Environ. 2021, 770, 145321. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of Microwave and NaOH Pretreatments of Wheat Straw for Enhancing Biofuel Yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Shah, A.A.; Seehar, T.H.; Sharma, K.; Toor, S.S. Biomass Pretreatment Technologies. Hydrocarb. Biorefinery Sustain. Process. Biomass Hydrocarb. Biofuels 2022, 203–228. [Google Scholar] [CrossRef]
- Zhu, Z.; Macquarrie, D.J.; Simister, R.; Gomez, L.D.; McQueen-Mason, S.J. Microwave Assisted Chemical Pretreatment of Miscanthus under Different Temperature Regimes. Sustain. Chem. Process. 2015, 3, 15. [Google Scholar] [CrossRef]
- Bezerra, T.L.; Ragauskas, A.J. A Review of Sugarcane Bagasse for Second-Generation Bioethanol and Biopower Production. Biofuels Bioprod. Biorefining 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Shen, G.; Yuan, X.; Chen, S.; Liu, S.; Jin, M. High Titer Cellulosic Ethanol Production from Sugarcane Bagasse via DLCA Pretreatment and Process Development without Washing/Detoxifying Pretreated Biomass. Renew. Energy 2022, 186, 904–913. [Google Scholar] [CrossRef]
- Bittencourt, G.A.; da Barreto, E.S.; Brandão, R.L.; Baêta, B.E.L.; Gurgel, L.V.A. Fractionation of Sugarcane Bagasse Using Hydrothermal and Advanced Oxidative Pretreatments for Bioethanol and Biogas Production in Lignocellulose Biorefineries. Bioresour. Technol. 2019, 292, 121963. [Google Scholar] [CrossRef] [PubMed]
- Khongchamnan, P.; Suriyachai, N.; Kreetachat, T.; Laosiripojana, N.; Weerasai, K.; Champreda, V.; Suwannahong, K.; Sakulthaew, C.; Chokejaroenrat, C.; Imman, S. Optimization of Liquid Hot Water Pretreatment and Fermentation for Ethanol Production from Sugarcane Bagasse Using Saccharomyces Cerevisiae. Catalysts 2022, 12, 463. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic Hydrolysis of Biomass at High-Solids Loadings—A Review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Duque, A.; Doménech, P.; Álvarez, C.; Ballesteros, M.; Manzanares, P. Study of the Bioprocess Conditions to Produce Bioethanol from Barley Straw Pretreated by Combined Soda and Enzyme-Catalyzed Extrusion. Renew. Energy 2020, 158, 263–270. [Google Scholar] [CrossRef]
- Álvarez, C.; González, A.; Ballesteros, I.; Negro, M.J. Production of Xylooligosaccharides, Bioethanol, and Lignin from Structural Components of Barley Straw Pretreated with a Steam Explosion. Bioresour. Technol. 2021, 342, 125953. [Google Scholar] [CrossRef]
- Meng, F.; Li, N.; Yang, H.; Shi, Z.; Zhao, P.; Yang, J. Investigation of Hydrogen Peroxide-Acetic Acid Pretreatment to Enhance the Enzymatic Digestibility of Bamboo Residues. Bioresour. Technol. 2022, 344, 126162. [Google Scholar] [CrossRef]
- Azeez, M.A.; Orege, J.I. Bamboo, Its Chemical Modification and Products. Bamboo Curr. Futur. Prospect. 2018, 1, 25–48. [Google Scholar] [CrossRef]
- Rochón, E.; Cabrera, M.N.; Scutari, V.; Cagno, M.; Guibaud, A.; Martínez, S.; Böthig, S.; Guchin, N.; Ferrari, M.D.; Lareo, C. Co-Production of Bioethanol and Xylosaccharides from Steam-Exploded Eucalyptus Sawdust Using High Solid Loads in Enzymatic Hydrolysis: Effect of Alkaline Impregnation. Ind. Crops Prod. 2022, 175, 114253. [Google Scholar] [CrossRef]
- Jesús RANGEL, M.; Hornus, M.; Felissia, F.E.; Area, M.C. Hydrothermal Treatment of Eucalyptus Sawdust for a Forest Biorefinery. Cellul. Chem. Technol. 2016, 50, 521–528. [Google Scholar]
- Díaz, M.J.; Moya, M.; Castro, E. Bioethanol Production from Steam-Exploded Barley Straw by Co-Fermentation with Escherichia Coli SL100. Agron. 2022, 12, 874. [Google Scholar] [CrossRef]
- Acevedo-García, V.; Padilla-Rascón, C.; Díaz, M.J.; Moya, M.; Castro, E. Fermentable Sugars Production from Acid-Catalysed Steam Exploded Barley Straw. Chem. Eng. Trans. 2018, 70, 1939–1944. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Microwave-Assisted Dilute Acid Pretreatment in Bioethanol Production from Wheat and Rye Stillages. Biomass Bioenergy 2020, 136, 105528. [Google Scholar] [CrossRef]
- Zanuttini, M.; Citroni, M.; Marzocchi, V.; Inalbon, C. Alkali Impregnation of Hardwood Chips. Tappi J. 2005, 4, 28–30. [Google Scholar]


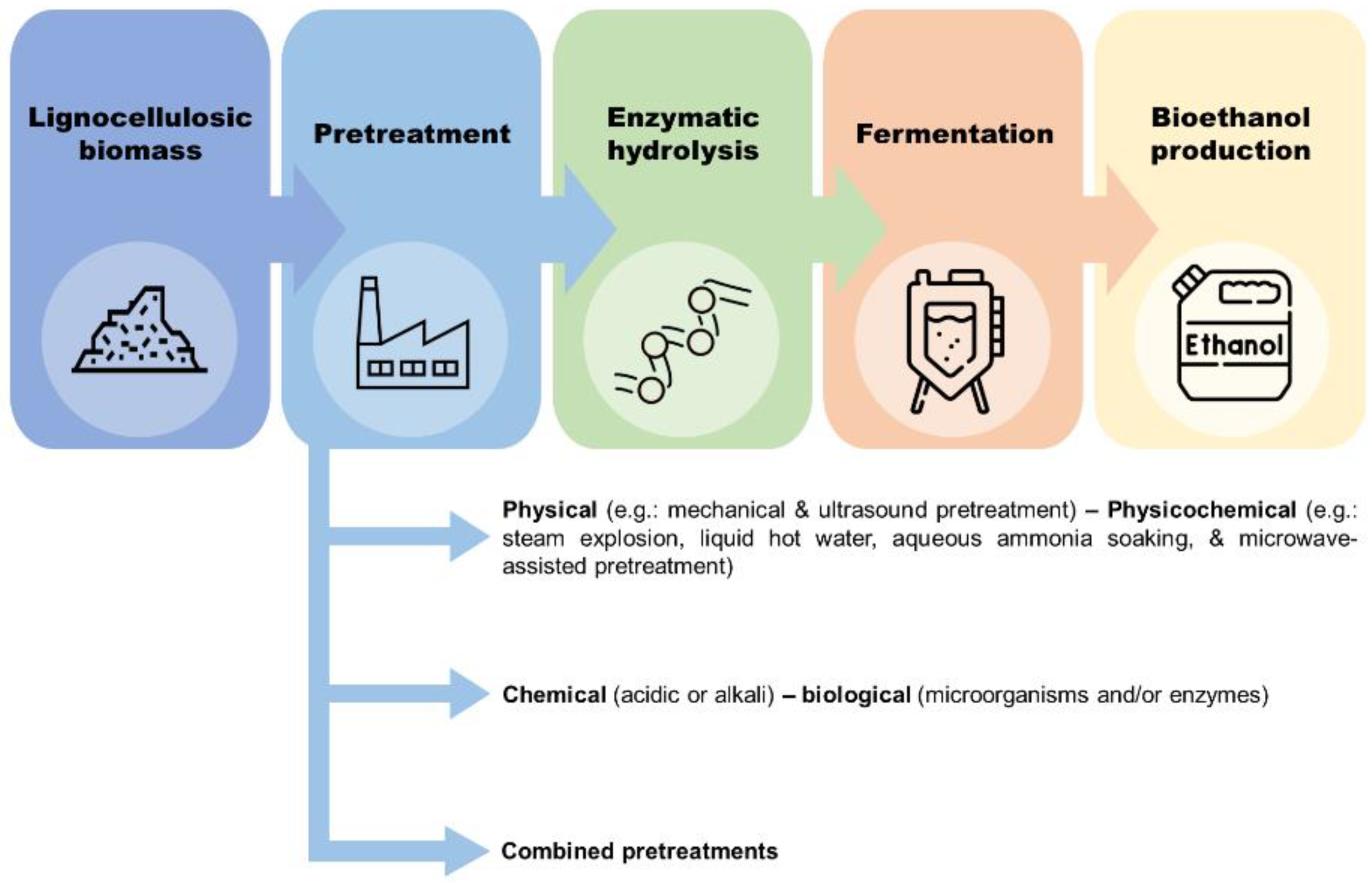
| Biomass | Composition (%) | References | ||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||
| Hemp | 40.1–75.6 | 10.1–14.2 | 10.3–14.6 | [6,37,38,39,40] |
| Rice straw | 28.0–34.7 | 20.0–25.2 | 17.0–19.0 | [26,41] |
| Corn stalks | 31.0–39.0 | 16.5–35.0 | 16.6–25.4 | [26,42,43,44] |
| Sugarcane bagasse | 25.0–45.0 | 28.0–32.0 | 15.0–21.0 | [26,45] |
| Wheat straw | 31.0–39.0 | 23.0–43.0 | 12.0–22.0 | [26,46,47] |
| Raw Material | Biomass Preparation | Pretreatment | Composition (Treated; % db) | Saccharification | Reducing Sugars | Fermentation | Bioethanol | References |
|---|---|---|---|---|---|---|---|---|
| Tygra hemp biomass (Cellulose: 50.82% Hemicellulose: 27.79% Lignin: 14.68%) |
| Chemical: 2% NaOH, 90 °C, 5 h | Cellulose: 62.7% Hemicellulose: 20.16%, Lignin: 15.12% |
| Total RS: 2311 mg/g | S. cerevisiae 37 °C, 900 rpm, pH 4.8, 96 h 1 N NaOH or 1 N HCl, | 6.5 g/L (13 g/100 g of biomass) | [60] |
| Hemp stems (Cellulose: 40.66% Hemicellulose: 13.25% Lignin: 15.74%) | Drying (49 °C, 72 h) Grinding (< 2 mm) | Chemical: 1% NaOH, solid-liquid ratio 1:10
| Cellulose: 77.54 % Hemicellulose: 8.72% Lignin: 11.05 % |
| N.D. 1 | S. cerevisiae (1 mL) SSF (focus point of quadratic equation): solid loading 31%, 37 °C, 96 h, 140 rpm | 76.92 g/L | [59] |
| Industrial hemp biomass (Cellulose: 40% Hemicellulose: 18% Lignin: 16%) |
| Chemical:NaOH (0.05 M, pH 12.71, solid-liquid ratio 1/10, 190 °C, 40 min) or HOAc (1 M, pH 2.36)
| Cellulose: 73.61% Hemicellulose: 12.2% Lignin: 7.51% | Enzymatic hydrolysis: 2.5–10% solid loading, pH 4.8, 100 μL cellulase/g biomass, 50 μL hemicellulase/g biomass, 160 rpm, 49 °C, 72 h | Focus point (II-VW): Glucose: 43.88 g/L Xylose: 12.13 g/L | N.P. 2 | N.D. 1 | [63] |
| Hemp woody core (Cellulose: 37.3% Hemicellulose: 19.79% Lignin: 12.35%) | Preculture (P. eryngii, PDA, 28 °C, 7 days) Cutting (400–800 μm) Air-drying | Biological:
| N.D. | Enzymatic hydrolysis: cellulase: 30 FPU/g, citric acid buffer (pH 4.8), 0.01% sodium azide, 50 °C, 48 h | RS (Bio-chem): 372 mg/g | N.P. | N.D. | [67] |
| Helena hemp stems (Cellulose: 40.13% Hemicellulose: 12.53% Lignin: 14.56%) | Oven-drying (49 °C, 72 h) Grinding (< 2 mm) | Three treatments were done: acidic, alkaline, and LHW Chemical: 1% NaOH, solid-liquid ratio 1:10, 170 °C, 30 min
| After alkaline PT Cellulose: 73.9% Hemicellulose: 7.59% Lignin: 9.21% | Enzymatic hydrolysis: Cellic® CTec3 (30 FPU/g), NS22244 (140 FXU/g), solid loading 5%, 50 °C, 72 h, 140 rpm | Glucose: 40.08 g/L | Incubation 1 g Ethanol Red® yeast, 38 °C, 160 rpm, 25 min. SSF: 37 °C, 140 rpm | 20.1 g/L | [62] |
| Hemp hurd (Cellulose: 45.66% Hemicellulose: 24.57% Lignin: 21.67%) | Decortication (aqueous decort. sol’n, 75–90 °C), incubation 1 h Injection during incubation (34% H2O2) Alkaline agent (30 g, incubation 5 min) Rinsing + milling (2 mm) | Chemical: 1.4% acidified NaClO2 (70 °C, 5 h) and 4% NaOH (70 °C, 2 h, ×2)
| HHH: Hemicellulose: 32.73% LHH: Lignin: 17.99% AHH: Cellulose: 78.03% | N.P. 2 | N.D. 1 | N.P. | N.D. | [64] |
| Raw Materials | Biomass Preparation | Pretreatment | Composition (Treated; % db) | Saccharification | Reducing Sugars | Fermentation | Bioethanol | References |
|---|---|---|---|---|---|---|---|---|
| Corn stover feedstock (Cellulose: 37.57% Hemicellulose: 24.37% Lignin: 17.99%) |
| Chemical: liquid-solid ratio 12 g/g, lime (0.1–0.4 g/gbiomass) Optimization by RSM (Optimal: 121 °C, 1 h, lime loading 0.1 g/g)
| Cellulose: 45% Hemicellulose: 20.8% Lignin: 14.7% | Enzymatic hydrolysis: Celluclast 1.5 L (15 FPU/g), β-glucosidase, 35 °C, pH 5, citric acid buffer 0.05 N, 150 rpm, liquid-solid ratio 20 g/g, β-glucosidase-to-cellulase ratio 5 IU/FPU, 120 rpm, 96 h | Glucose: > 15 g/L (24 h) | S. cerevisiae preculture (32 °C, 24 h, 200 rpm) SSF Centrifugation Filtration | 28.73 g/L | [70] |
| Corn stover (Cellulose: 38.5% Hemicellulose: 21.6% Lignin: 20.4%) | Pelletization | Chemical: 0.1 g/g Ca(OH)2 Thermal: autoclave, 121 °C, 35% solid loading | Cellulose: 36.7% Hemicellulose: 14.3% | Enzymatic hydrolysis: Cellic® CTec 2 cellulase, 20% solid loading, 50 °C, pH 4.8, 250 rpm | Glucose: 81.2 g/L Xylose: 31.3 g/L | S. cerevisiae preculture (30 °C, 150 rpm) Fermentation: 30 °C, 150 rpm | 70.6 g/L | [71] |
| Corn stover (Cellulose: 35.7% Hemicellulose: 21.5% Lignin: 20.1%) |
| Two treatments: autoclave-assisted (AA) and ultrasonic-assisted (UA) Chemical (AA): 2% oxalic acid (1:20 w/v), autoclave (120 °C, 60 min, 310 kPa) Vacuum filtration Washing with hot water until neutral pH Oven-drying (50 °C, 12 h) Optimization by RSM (Optimal conditions: 140 °C, 60 min, 3% oxalic acid, S-L ratio 1:30) | After AA: Cellulose: 56.6% Hemicellulose: 12.7% Lignin: 10.9% | Enzymatic hydrolysis: Cellulase (Novozymes), 20 FPU/g, loading 0.4 g, substrate-buffer ratio 1:25, sodium acetate (0.05 M, pH 5), 50 °C, 100 rpm, 96 h | Glucose: 7.49 g/L | Preculture of S. cerevisiae (YPD, 30 °C, 150 rpm, 36 h) Batch fermentation: 5% yeast inoculum (v/v), 30 °C, 72 h | 3.6 g/L | [74] |
| Raw Materials | Biomass Preparation | Pretreatment | Composition (Treated; %db) | Saccharification | Reducing Sugars | Fermentation | Bioethanol | References |
| Rice straw (Cellulose: 24.1% Hemicellulose: 10.9% Lignin: 24.7%) |
| Chemical: NaOH and KOH (5%), 3 h, 25°C
Mechanical: Centrifugal millng (size = 0.25 mm), 25 °C, 2 min | Soaking, 5% NaOH Cellulose: 43.54% Hemicellulose: 8.62% Lignin 8.37% | Enzymatic hydrolysis: Cellic® CTec 2 (Novozymes) 10 FPU/g, 5% solid loading, 0.05 M sodium acetate buffer, pH 5, 5% sodium azide, 50 °C, 72 h, 200 rpm | Glucose: 0.52–0.63 g/g | N.P. 2 | N.D. 1 | [79] |
| Rice straw (Cellulose: 31.73% Hemicellulose: 23.21% Lignin: 15.79%) |
| Three pretreatments: Laccase only, Laccase + [AMIM]Cl, or Laccase + TritonX-100 Chemical Laccase + TX: Triton X-100 (0.5 g/L), 0.1 M citrate buffer, pH 4.5, 50 °C, 150 rpm, substrate concentration 10%, 24 h
| Laccase + tx Cellulose: 40.83% Hemicellulose: 19.66% Lignin: 10.77% | Enzymatic hydrolysis: beta-glucosidase (40 CBU/g), cellulase complex (50 FPU/g), 2.5% rice straw, 0.1 M sodium citrate buffer, pH 4.8, 0.02% sodium azide, 50 °C, 150 rpm, 72 h | N.D. | N.P. | N.D. | [80] |
| Rice straw (Cellulose: 40.2% Hemicellulose: 12.5% Lignin: 16.7%) |
| Two treatments: NMMO and Phosphoric acid (PA) Chemical NMMO: NNMO (85%, ratio 1:9), 120 °C, 3 h
| After NMMO: Cellulose: 43.5% Hemicellulose: 11.2% Lignin: 13.2% | Enzymatic hydrolysis: Cellic® HTec, Cellic® CTec2 (Novozymes), 20 FPU/g, 15% solid loading, 72 h, 45 °C, sodium citrate buffer (pH 4.8, 0.05 M) | NMMO: Glucose: >100 g/L | Preculture M. indicus (32 °C, 24 h) Inoculation + incubation fungal spores (48 h, 32 °C) Spore suspension (anaerobic fermentation24 h, 32 °C) SSF: 5 g/L M. indicus, 37 °C, anaerobic | NMMO: 44.2 g/L | [82] |
| Rice straw |
| Chemical: [EMIM]Ac Optimization by RSM (Optimal: 5% loading ratio, 128.4 °C, 71.83 min)
| N.D. 1 | Enzymatic hydrolysis: Celluclast 1.5 L (20 FPU/g), 40 μL sodium azide, 2.5% biomass, 72 h, 45 °C, 0.05 M citrate buffer, pH 4.7, 200 rpm | Total RS: 57.3 mg/ 100 mg biomass | S. cerevisiae inoculum (1 mL), 30 °C, 72 h, 150 rpm | 5.9 g/L | [81] |
| Rice straw |
| Chemical: 1% NaOH, 10% solid loading Washing until neutral pH | N.D. 1 | Saccharification: Accellerase®1500 or Celluclast (24–48 h) | Total sugars: 21.66 g/L | Unconventional yeast precult. (MGYP, 30 °C, 72 h) Fermentation: 30 °C, 48 h | 3.32 g/L (P. stipitis NCIM349, 48 h) | [78] |
| Raw Materials | Biomass Preparation | Pretreatment | Composition (Treated; % db) | Saccharification | Reducing Sugars | Fermentation | Bioethanol | References |
| Wheat straw (Cellulose: 36.97% Hemicellulose: 20.17 % Lignin: 27.66%) |
| Thermomechanical: Subcritical water treatment
| Cellulose: 62.89% Hemicellulose: 1.80 % Lignin: 36.47% | Enzymatic hydrolysis: 5 cellulase mixes 20 FPU/g, β-glucosidases 1 U/g, 50 °C, 120 rpm, 96 h, 15% solid loading | Glucose: 80.81 g/L | S. cerevisiae preculture (YPD, 30 °C, 150 rpm, 24 h,) SHF: 10% inoculum, 30 °C, 48 h | 37 g/L (64 h) | [96] |
| Wheat straw (Cellulose: 41.72% Hemicellulose: 19.98% Lignin: 17.83%) |
| Physicochemical: MW+ NaOH
| Cellulose: 74.15% Hemicellulose: 5.44% Lignin: 7.66% | Microbial hydrolysis: (Bacillus sp. BMP01, 30 °C, 14 days) | Total RS: 718 mg/gWS | S. cerevisiae and Zymomonas mobilis precultures (YPG, 30 °C, 24 h) Fermentation (108 h, 120 rpm, 30 °C) | Ethanol yield: 68.2% (96 h) | [97] |
| Wheat straw (Cellulose: 35.69% Hemicellulose: 29.68% Lignin: 18.8%) |
| Chemical: 8 g [TEA][H2SO4] melted at 130 °C, 2 g water (1:5 ratio), 130 °C, 3 h | Cellulose: 53.52% Hemicellulose: 10.55% Lignin: 3.73% | Enzymatic hydrolysis: Cellulase CelluMax GFL (Novozymes), 0.05 M sodium citrate, pH 4.8, solid-liquid ratio 1:20, 28 FPU/g residue biomass, 0.2% sodium azide, 72 h, 50 °C, 200 rpm
| Glucose: 35 g/L Xylose: 6 g/L | S. cerevisiae preculture (YPD) Fermentation: 3 g inoculum, 30 °C, 96 h | 43.1 g/L | [94] |
| Wheat straw (Cellulose: 33.02% Hemicellulose: 17.26% Lignin: 18.58%) | Washing (DI water) Drying, crushing Sieving (60-mesh sieve) | Chemical: DES (solid-liquid ratio 1:15, 1 h, 120 °C) Vacuum filtration Washing | Cellulose: 68.29% Hemicellulose: 3.94% Lignin: 8.09% | Enzymatic hydrolysis: Celluclast 2 L (Novozymes), 50 °C,150 rpm, 11.4 FPU/g | Glucose: 48.05 g/L Xylose: 2.26 g/L | SHF: Optimization by RSM (Optimal: 18 h, Angel Yeast 10% v/v, 40 °C, 120 rpm) | 15.42 g/L | [95] |
| Raw Materials | Biomass Preparation | Pretreatment | Composition (Treated; % db) | Saccharification | Reducing Sugars | Fermentation | Bioethanol | References |
|---|---|---|---|---|---|---|---|---|
| Sugarcane bagasse | Milling (3 mm) | Chemical: Sulfuric Acid (0.5 g water/g dry biomass) Pelletization (200 mm) Air drying (RT) Autoclave (Optimal: 120 °C, 60 min, solid loading 33.3%, 0.05 g/g acid dosage) | N.D. | Low solid loding hydrolysis: Cellic CTec2 (Sigma Aldrich), 10 mg/gglucan, 50 °C, 250 rpm, pH 4.8, 18 h Enzymatic hydrolysis: 30% solid loading, 72 h | Glucose: 95.81 g/L Xylose: 79.73 g/L | S.cerevisiae preculture (YPX, 30 °C, 250 rpm) Fed-Batch and Enzyme (FBE)-SSCF: 34 °C, 150 rpm, 120 h, pH 5.5–6, solid loading 30% | 77.51 g/L | [101] |
| Sugarcane bagasse (Cellulose: 33.1% Hemicellulose: 19.2% Lignin: 29.2%) | Oven-drying (50 °C, 24 h) Grinding (0.061–0.25 mm) Oven-dried (105 °C, 5 h) | Physicochemical: Optimization by RSM (Optimal: 0.05 M H2SO4, 160 °C, 60 min) | Cellulose: 29.4% Hemicellulose: 0.59% Lignin: 23.5% | - Enzymatic hydrolysis: Cellic CTec2 (Novozymes) 5% substrate w/v (25 FPU/g), 50 mM sodium citrate buffer pH 4.8, 1% sodium azide, 150 rpm, 50 °C, 72 h | Glucose yield: 96.86% | S. cerevisiae preculture (30 °C, 24 h in agar) then YPD, 35 °C, 150 rpm SSF: pH 4.8, 6.25% remaining solid residue | 19.9 g/L (72 h) | [103] |
| Sugarcane bagasse (Cellulose: 48.7% Hemicellulose: 21.14% Lignin: 24.81%) |
| Hydrothermal: 183 °C, 41 min, liquid-solid ratio 3.94 mL/g, 11.7 bar Pressing (9 tons pressure) Chemical: Oven-drying (55 °C, 12 h) Grinding (0.42 mm) NaOH (aq, 0.2 mol/L), liquid-solid ratio 10 mL/g, 2800 rpm, 30 s Advanced chemical: Optimization by DoE (30 g, 0.3 gH2O2/gSB, 5 mol/L NaOH, pH 11.6, 150 rpm, 25 °C, liquid-solid ratio 9.67 mL/g, 8 h) Blending (2800 rpm, 30 s) | Advanced chemical: Cellulose: 78.91% Hemicellulose: 7.13% Lignin: 17.6% | Enzymatic hydrolysis: Residual solid fraction (1 g), 0.05 M citrate buffer, pH 4.8, 0.02% sodium azide, 15% Cellic HTec2 and 85% Cellic CTec 2 (cellulase 10 FPU/g, β-glucosidase 52.21 U/g), liquid-solid ratio 10 mL/g, 72 h, 150 rpm, 50°C | Overall Glucose Yield: 60.9% | S. cerevisiae strains: two commercial yeasts (mills), one yeast (distillery), 34 °C, 150 rpm | 37 g/L (LBCM1047 strain) 190.8 Lethanol per ton of pretreated biomass | [102] |
| Barley straw | 32.0–43.0 | 20.0–33.0 | 6.0–20.0 | [26,105,106] |
| Bamboo residue | 40.0–73.83 | 12.49–30 | 10.0–33.0 | [107,108] |
| Eucalyptus sawdust | 43.0–54.1 | 11.0–18.4 | 21.0–34.0 | [26,109,110] |
| Raw Materials | Biomass Preparation | Pretreatment | Composition (Treated; %db) | Saccharification | Reducing Sugars | Fermentation | Bioethanol | References |
| Barley Straw (Cellulose as glucose: 42.27% Hemicellulose as xylose: 30.23% Lignin: 16.26%) |
| Physico-chemical: Steam explosion (Optimal conditions: 160 °C, 30 min, 2.88 w/v phosphoric acid concentration)
| Composition of water-insoluble solid fraction (WIS): Glucose: 65.11% Xylose: 3.72% Lignin: 12.62% | Enzymatic hydrolysis (for SHCF): 50 °C, 150 rpm, pH 4.8, 15 FPU/g substrate enzyme loading, 72 h | Glucose yield: 90% | Co-fermentation: E. coli SL100, 37 °C, 150 rpm, pH 6.5 | Maximum attainable yield (YEmax) = 89.1% | [112] |
| Rye stillage (Cellulose: 16.8% Hemicellulose: 29.62% Lignin: 15.57%) | N.P.2 | Physico-chemical: Microwave (Optimal conditions: 300 W, 0.2 M H2SO4, 54 PSI, 15 min) | N.D.1 | Enzymatic hydrolysis: Cellic® CTec2, 1 FPU/g, 50 °C, pH 5.5, 70 rpm, 24 h | 156 mg/g | S. cerevisiae (2 g/L) 35 °C, 72 h | ≈ 16 g/L | [113] |
| Barley straw (Cellulose: 32.9% Hemicellulose: 27.2% Lignin: 16.8%) |
| Physico-chemical: Steam Explosion (particle size 6–10 mm, 180 °C, 30 min, 10 bar) | WIS fraction: Cellulose: 55.8% Hemicellulose: 10.3% Lignin: 30.7% | Enzymatic hydrolysis (for PSSF): Cellic® CTec2, 30 FPU/g enzyme loading, 8 h, 50 °C, 20% w/v solid concentration | N.D.1 | S. cerevisiae (0.25 g/L inoculum) Fermentation (for PSSF): 35 °C, 48 h
| ≈ 50 g/L | [105] |
| Barley straw (Cellulose: 33.2% Hemicellulose: 20.9% Lignin: 18.8%) |
| Thermo-mechanical/Chemical: Sequential alkaline extrusion; 7.2% w/v NaOH, 2.5% w/v H2SO4, biomass flow 2 kg/h, 100 °C, 120 rpm, NaOH-catalyzed extrusion 3 h Oven-drying of part of extrudate 45 °C, 2 h | Cellulose: 32.61% Hemicellulose: 18.56% Lignin: 20.82% | Bioextrusion: 0.9 kg/h dry extrudate, Cellic® CTec2, 0.02 M citrate buffer, pH 4.8, 7.5 FPU/g enzyme loading, 0.7 L/h, liquid-solid ratio 3 LSSCF | Glucose: 30.94% IF, 3.35% SF Xylose: 10.72% IF, 6.58% SF Arabinose: 1.88% IF, 1.24% SF | Fermentation (LSSCF): Yeast (1 g/L), 30 °C, 150 rpm, 72 h. | 38 g/L | [111] |
| P. amarus bamboo residues (Cellulose: 40.94% Hemicellulose: 18.48% Lignin: 32.41%) | Grinding | Chemical: Hydrogen peroxide-acetic acid (HPAC); HPAC solution 1:1 v/v, 0.5% w/w sulfuric acid, 60 °C, 2 h
| Cellulose: ≈65% Hemicellulose: ≈18% Lignin: 9.28% | Enzymatic hydrolysis: 5% substrate concentration w/v, 10 FPU/g enzyme loading, 50 °C, pH 4.8, 150 rpm, 72 h | Glucose: 79.31% Xylose: 85.53% | Fermentation: 35 °C, 90 rpm, 24 h | ≈13 g/L | [107] |
| Eucalyptus sawdust (Cellulose: 43.6% Hemicellulose: 11.1% Lignin: 30.5%) |
| Chemical: NaOH impregnation for some samples (1:4 solid-liquid ratio), 30 min, conditioning (23 °C, 20 h) Filter pressing (20 MPa) Solar oven-drying: 48 h Physio-chemical: Steam Explosion: 10 min, 200 °C Filter pressing (20 MPa) Washing and filter pressing | Solid fraction; (-) NaOH impregnation Cellulose: 60.1% Hemicellulose: N.D. Lignin: 46.4% | Enzymatic hydrolysis (for SSF): Cellic® CTec 2, 27% solid loading, 25 FPU/g enzyme loading, 96 h, pH 4.8, 50 °C, 150 rpm | Enzymatic hydrolyzates: (-) NaOH impregnation Glucose: 134 g/L | Fermentation (for SSF): 27% solid suspension, 30 °C, 100 rpm, 48 h | 75.6 g/L | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hage, M.; Rajha, H.N.; Maache-Rezzoug, Z.; Koubaa, M.; Louka, N. Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review. Energies 2022, 15, 6912. https://doi.org/10.3390/en15196912
El Hage M, Rajha HN, Maache-Rezzoug Z, Koubaa M, Louka N. Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review. Energies. 2022; 15(19):6912. https://doi.org/10.3390/en15196912
Chicago/Turabian StyleEl Hage, Maria, Hiba N. Rajha, Zoulikha Maache-Rezzoug, Mohamed Koubaa, and Nicolas Louka. 2022. "Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review" Energies 15, no. 19: 6912. https://doi.org/10.3390/en15196912
APA StyleEl Hage, M., Rajha, H. N., Maache-Rezzoug, Z., Koubaa, M., & Louka, N. (2022). Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review. Energies, 15(19), 6912. https://doi.org/10.3390/en15196912









